Animal models are often used to test the effectiveness of a drug or procedure before proceeding to clinical trials. One reason for use of animal models is that they allow researchers to focus on particular pathological processes without the confounding effects of other injuries and treatments. However, it is essential that their results are valid and precise. Biased or imprecise results from animal experiments may result in clinical trials of biologically inert or even harmful substances, thus exposing patients to unnecessary risk and wasting scarce research resources. Moreover, if animal experiments fail to inform medical research then the animals suffer unnecessarily.
The Italian pathologist Pietro Croce criticised vivisection on scientific grounds. He argued that results from animal experiments cannot be applied to humans because of the biological differences between animals and humans and because the results of animal experiments are too dependent on the type of animal model used.1 Croce's arguments were based on insights into zoology and pathophysiology. In this paper, we make some methodological observations on animal experiments. Our observations were made in the context of a systematic review of all available randomised controlled trials of fluid resuscitation in animal models of uncontrolled bleeding. We conducted this review because we wanted to assess the scientific basis for fluid resuscitation. A previous systematic review of randomised trials of fluid resuscitation in bleeding trauma patients had provided no evidence that fluid resuscitation improved outcome.2
Summary points
New drugs and procedures are usually tested in animals before conducting clinical trials
Validity of animal experiments is essential for human health care and fundamental to animal welfare
A systematic review of animal experiments on fluid resuscitation found that most studies were underpowered and provided little information on possible bias
Systematic reviews of animal experiments allow a more objective appraisal of the evidence and reduce the chance of false negatives results
Systematic reviews across species would help determine whether the results could be generalised to humans
Systematic review of fluid resuscitation in uncontrolled haemorrhage
We did a systematic review of randomised controlled trials of the timing or volume of fluid administration in animal models of uncontrolled haemorrhage. Details of the review methods, search strategy, and included trials are available on bmj.com. The combined electronic search strategies identified 3193 potentially eligible reports. Two reviewers examined each of these records and 104 reports were retrieved in full. From these, we identified 44 randomised controlled trials meeting the inclusion criteria. The 44 trials included a total of 2039 experimental animals (1772 rats, 251 pigs, and 16 sheep). Mortality data were reported in 42 trials, of which 31 were in rats, 10 in pigs, and one in sheep. In most of the rat experiments uncontrolled bleeding was induced by resecting the tail. Three trials in large animals (pigs and sheep) could not be included in the meta-analysis because they did not include a no fluid resuscitation group: one compared early and late resuscitation and two compared different blood pressure resuscitation targets. Three trials in rats could not be included in the meta-analysis: one compared early and late fluid resuscitation, one compared different blood pressure resuscitation targets, and one presented time to death data only.
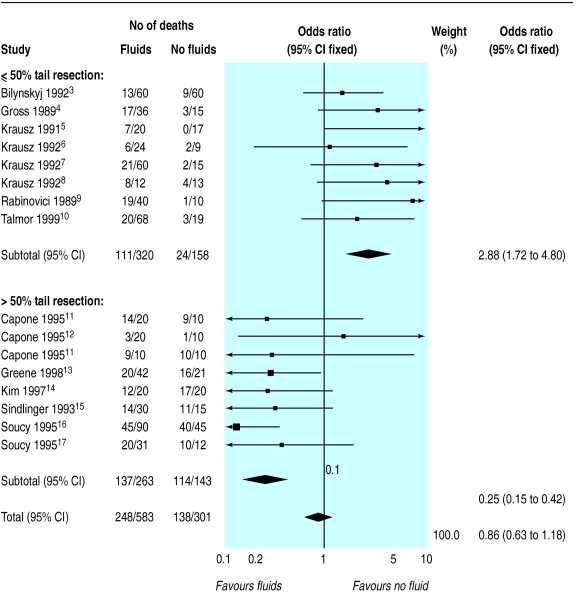
The pooled odds ratio (fixed effect) for death in large animals (pigs and sheep) with fluid resuscitation was 0.63 (95% confidence interval 0.15 to 2.61) but there was statistical heterogeneity (χ2 =16.84, df=7, P=0.018). The pooled odds ratio (fixed effect) for death in small animals with fluid resuscitation was 1.14 (0.65 to 2.02). Again, there was substantial heterogeneity (χ2 =93.40, df=27, P<0.0001). When the meta-analysis was stratified according to how uncontrolled bleeding was induced, a large amount of the heterogeneity was accounted for. Figure 1 shows the results of meta-analysis of the 16 randomised controlled trials of fluid resuscitation in rats in which bleeding was induced by resecting the tail. The meta-analysis is stratified according to where the tail was cut. Fluid resuscitation seems to be harmful (odds ratio=2.88, 95% confidence interval 1.72 to 4.80) with less than 50% tail resection (χ2 =5.57, df=7, P=0.59) but beneficial (odds ratio=0.25, 0.15 to 0.42) with greater than 50% tail resection (χ2 =6.14, df=7, P=0.52).
Figure 1.
Meta-analysis of 16 randomised controlled trials of fluid resuscitation in rats with uncontrolled haemorrhage by tail resection. *Capone11 reported two trials
Are the individual experiments valid?
In clinical trials, systematic error can arise from problems with the study design, especially if allocation of treatment is inadequately concealed.18Bias is avoided by ensuring strict randomisation with well concealed treatment allocation. The extent to which inadequate concealment of allocation might introduce bias in animal experiments is uncertain. However, it is easy to imagine how bias could arise. For example, weaker animals may be easier to catch than healthy animals, and this could result in systematic differences between the intervention and control groups on baseline prognostic factors. Of the 44 randomised controlled trials meeting the inclusion criteria, only two described how the animals were divided into treatment groups; both of these trials used alternation.
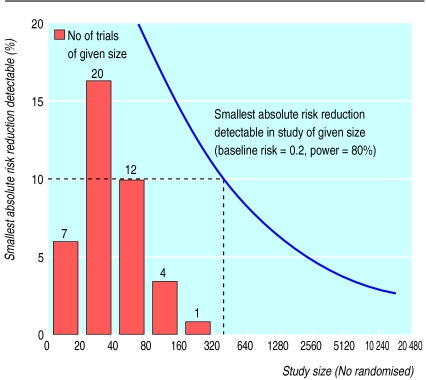
Random error in clinical trials is minimised by increasing the number of randomised participants.19 However, animal researchers are encouraged to reduce the number of experimental animals to a minimum. Indeed, the need to use the minimum number of animals to obtain valid results is embodied in the Animals (Scientific Procedures) Act 1986 and European legislation.20 As a result, some animal experiments are underpowered and provide little reliable information. All of the animal experiments in our systematic review were small (fig 2). The average number of animals per trial was 46 (2039/44), and the largest trial included only 207 animals (rats). None of the trials would have been large enough to detect reliably a 10% absolute difference (halving) in the risk of death between the intervention and comparison groups. Moreover, many of the trials included several different fluid resuscitation groups, which we combined for our analyses. The average number of experimental animals per treatment group was only 13 (160 groups). If, as was the case in most trials, the aim was to compare the effects of different fluid resuscitation regimens, the studies had little power.
Figure 2.
Trial size and smallest absolute risk reduction detectable
Has all the evidence been assessed?
Although each individual animal experiment provides little reliable information on the effectiveness of fluid resuscitation, each contributes to the total body of evidence. Any inferences should be based on all the evidence.21A 1996 narrative review of fluid resuscitation in animal experiments included only nine of the 24 trials (38%) that were available at that time.22
Systematic reviews and meta-analyses of animal experiments are uncommon. About 1 in 1000 Medline records pertaining to human research is tagged as a meta-analysis compared with 1 in 10 000 records pertaining to animal research. In his book The Principles of Humane Experimental Technique, William Russell proposed the principle of reduction—that is, the use of methods to “reduce the number of animals needed to obtain information of a given amount and precision.”23 Meta-analyses of the results of previous animal experiments would increase the precision of estimates of treatment effects and therefore reduce the number of animals needed in future experiments.
Publication bias may be as potent a threat to validity in systematic reviews of animal experiments as it is in systematic reviews of clinical trials. We contacted the authors of included trials to ask about unpublished studies but none were identified. However, it would be surprising if there were no unpublished trials meeting our inclusion criteria. Prospective registration of animal experiments at inception may help to avoid the problem of publication bias.24In the United Kingdom, the Animals (Scientific Procedures) Act 1986 regulates “any experimental or other scientific procedure applied to a protected animal which may have the effect of causing that animal pain, suffering, distress, or lasting harm.” Researchers must have a project licence from the Home Office before conducting any animal research, and the licence application describes the experimental protocol. These data could be used for prospective registration of all animal experiments.
Systematic reviews of animal models could, like ours, include a range of animal species and models. If the results were consistent across species and models this would indicate that they might also apply in humans. Since the primary aim of animal experimentation is to inform human experimentation, this would be valuable information.
We found substantial statistical heterogeneity in our meta-analysis, making it impossible to interpret the odds ratios. Investigation of heterogeneity is essential and can increase the scientific and clinical relevance of their results. In our meta-analysis, stratification according to how uncontrolled bleeding was induced accounted for a large amount of the heterogeneity, but these results need to be interpreted with caution. Meta-analytic subgroup analyses are akin to subgroup analyses within trials and are prone to bias. Although we specified in our protocol that the analyses would be stratified according to the animal model used, we did not specify that we would stratify according to where the tail was cut. Nevertheless, the meta-analysis provides an insight into model dependency that could be taken into account in future animal experiments and when considering whether the results can be generalised to humans.
Implications for human health
Animal experiments can inform human health care only if their results are valid and can be generalised. However, little information is available on the methodological determinants of bias in animal experiments, and in our example the sample sizes were too small to obtain precise estimates of the effects of the interventions. Systematic reviews of animal experiments would help to ensure that animal experiments do not set out to answer questions that have already been answered, reduce bias and increase precision, and provide reassurance about whether the results can be generalised. Prospective registration of animal experiments would help to avoid publication bias. In a recent editorial, Smith promoted the three Rs of animal research first suggested by William Russell: replacement, reduction, and refinement.25 On methodological grounds, animal experimentation would better contribute to human health care if we promoted registration, randomisation, and systematic reviews.
Supplementary Material
Acknowledgments
We thank Sir Iain Chalmers for his comments on the manuscript and the authors of the included trials who responded to our requests for further information.
Footnotes
Funding: None.
Competing interests: None declared.
The methods of the systematic review and details of included trials appear on bmj.com
References
- 1.Croce P. Vivisection or science? An investigation into testing drugs and safeguarding health. London: Zed Books; 1999. [Google Scholar]
- 2.Roberts I, Evans A, Bunn F, Kwan I, Crowhurst E. Normalising the blood pressure in bleeding trauma patients may be harmful. Lancet. 2001;357:385–387. doi: 10.1016/S0140-6736(00)03653-9. [DOI] [PubMed] [Google Scholar]
- 3.Bilynskyj MC, Errington ML, Velasco IT, Silva R. Effect of hypertonic sodium chloride (7.5%) on uncontrolled hemorrhage in rats and its interaction with different anesthetic procedures. Circulatory Shock. 1992;36:68–73. [PubMed] [Google Scholar]
- 4.Gross D, Landau EH, Klin B, Krausz MM. Quantitative measurement of bleeding following hypertonic saline therapy in ‘uncontrolled’ hemorrhagic shock. J Trauma. 1989;29:79–83. doi: 10.1097/00005373-198901000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Krausz MM, Kablan M, Rabinovici R, Klin B, Sherman Y, Gross D. Effect of injured vessel size on bleeding following hypertonic saline infusion in uncontrolled hemorrhagic shock in anesthetised rats. Circulatory Shock. 1991;35:9–13. [PubMed] [Google Scholar]
- 6.Krausz MM, Horn Y, Gross D. The combined effect of small volume hypertonic saline and normal saline solutions in uncontrolled hemorrhagic shock. Surg Gynecol Obstet. 1992;174:363–368. [PubMed] [Google Scholar]
- 7.Krausz MM, Landau EH, Klin B, Gross D. Hypertonic saline treatment of uncontrolled hemorrhagic shock at different periods from bleeding. Arch Surg. 1992;127:93–96. doi: 10.1001/archsurg.1992.01420010107017. [DOI] [PubMed] [Google Scholar]
- 8.Krausz MM, Bar-Ziv M, Rabinovici R, Gross D. “Scoop and run” or stabilize hemorrhagic shock with normal saline or small-volume hypertonic saline? J Trauma. 1992;33:6–10. doi: 10.1097/00005373-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Rabinovici R, Gross D, Krausz MM. Infusion of small volume of 7.5 per cent sodium chloride in 6.0 per cent dextran 70 for the treatment of uncontrolled hemorrhagic shock. Surg Gynecol Obstet. 1989;169:137–142. [PubMed] [Google Scholar]
- 10.Talmor D, Merkind V, Artru AA, Shapiro O, Geva D, Roytblat L, et al. Treatment to support blood pressure increases bleeding and/or decreases survival in a rat model of closed head trauma combined with uncontrolled hemorrhage. Anesth Analg. 1999;89:950–956. doi: 10.1097/00000539-199910000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Capone AC, Safar P, Stezoski W, Tisherman S, Peitzman AB. Improved outcome with fluid restriction in treatment of uncontrolled hemorrhagic shock. J Am Coll Surg. 1995;180:49–56. [PubMed] [Google Scholar]
- 12.Capone AC, Safar P, Stezoski SW, Peitzman A, Tisherman S. Uncontrolled hemorrhagic shock outcome model in rats. Resuscitation. 1995;29:143–152. doi: 10.1016/0300-9572(95)00829-i. [DOI] [PubMed] [Google Scholar]
- 13.Greene SP, Soucy DM, Song WC, Barber AE, Hagedorn FN, Illner HP, et al. Early isotonic saline resuscitation from uncontrolled hemorrhage in rats. Surgery. 1998;124:568–574. [PubMed] [Google Scholar]
- 14.Kim S, Stezoski SW, Safar P, Capone A, Tisherman S. Hypothermia and minimal fluid resuscitation increases survival after uncontrolled hemorrhagic shock in rats. J Trauma. 1997;42:213–222. doi: 10.1097/00005373-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Sindlinger JF, Soucy DM, Greene SP, Barber AE, Illner H, Shires GT. The effects of isotonic saline volume resuscitation in uncontrolled hemorrhage. Surg Gynecol Obstet. 1993;177:545–550. [PubMed] [Google Scholar]
- 16.Soucy DM, Rudé, Hsia WC, Hagedorn FN, Illner H, Shires GT. The effects of varying fluid volume and rate of resuscitation during uncontrolled hemorrhage. J Trauma. 1999;46:209–214. doi: 10.1097/00005373-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Soucy DM, Sindlinger JF, Greene SP, Barber AE, Illner HP, Shires GT. Isotonic saline resuscitation in uncontrolled hemorrhage under various anesthetic conditions. Ann Surg. 1995;222:89–93. doi: 10.1097/00000658-199507000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 19.Peto R, Collins R, Gray R. Large-scale randomised evidence: large simple trials and overviews of trials. J Clin Epidemiol. 1995;48:23–40. doi: 10.1016/0895-4356(94)00150-o. [DOI] [PubMed] [Google Scholar]
- 20.Guidance on the operation of the Animals (Scientific Procedures) Act 1986. www.homeoffice.gov.uk/animact/aspag1.htm (accessed August 2001).
- 21.Chalmers I. Using systematic reviews and registers of on-going trials for scientific and ethical trial design, monitoring, and reporting. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in healthcare: meta-analyses in context. London: BMJ Books; 2001. pp. 429–443. [Google Scholar]
- 22.Dries DJ. Hypotensive resuscitation. Shock. 1996;6:311–316. doi: 10.1097/00024382-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen; 1959. [Google Scholar]
- 24.Horton R, Smith R. Time to register randomised trials. Lancet. 1999;354:1138–1139. doi: 10.1016/S0140-6736(99)00328-1. [DOI] [PubMed] [Google Scholar]
- 25.Smith R. Animal research: the need for a middle ground. BMJ. 2001;322:248–249. doi: 10.1136/bmj.322.7281.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.