Abstract
Abstract
Interferon-based therapies, such as ropeginterferon alfa-2b have emerged as promising disease-modifying agents for myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET). Current ET treatments aim to normalize hematological parameters and reduce the thrombotic risk, but they do not modify the natural history of the disease and hence, have no impact on disease progression. Ropeginterferon alfa-2b (trade name BESREMi®), a novel, monopegylated interferon alfa-2b with an extended administration interval, has demonstrated a robust and sustained efficacy in polycythemia vera (PV) patients. Given the similarities in disease pathophysiology and treatment goals, ropeginterferon alfa-2b holds promise as a treatment option for ET. The ROP-ET trial is a prospective, multicenter, single-arm phase III study that includes patients with ET who are intolerant or resistant to, and/or are ineligible for current therapies, such as hydroxyurea (HU), anagrelide (ANA), busulfan (BUS) and pipobroman, leaving these patients with limited treatment options. The primary endpoint is a composite response of hematologic parameters and disease-related symptoms, according to modified European LeukemiaNet (ELN) criteria. Secondary endpoints include improvements in symptoms and quality of life, molecular response and the safety profile of ropeginterferon alfa-2b. Over a 3-year period the trial assesses longer term outcomes, particularly the effects on allele burden and clinical outcomes, such as disease-related symptoms, vascular events and disease progression. No prospective clinical trial data exist for ropeginterferon alfa-2b in the planned ET study population and this study will provide new findings that may contribute to advancing the treatment landscape for ET patients with limited alternatives.
Trial registration
EU Clinical Trials Register; EudraCT, 2023-505160-12-00; Registered on October 30, 2023.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00277-024-05665-4.
Keywords: Myeloproliferative neoplasms (MPNs), Essential thrombocythemia (ET), Ropeginterferon alfa-2b, ROP-ET, Phase III, Disease modification
Introduction
Recognized for their potential for disease modification, interferon-based therapies have gained recognition in myeloproliferative neoplasms (MPNs), a group of rare blood cancers [1]. Pegylated interferon alfa has shown good tolerability and efficacy in achieving hematologic remission in polycythemia vera (PV) patients [2–7] and to reduce or eliminate the need for phlebotomy [5, 7]. In addition to its ability to induce hematologic responses, pegylated interferon alfa triggers molecular remission by suppressing JAK2-driver mutation-carrying cells [2, 5, 7]. Robust and durable results were observed after long-term treatment [8, 9], coinciding with significantly less fluctuations in response and improved event-free survival compared with hydroxyurea (HU) or best available treatment, with risk events defined as thromboembolic events, disease progression, or mortality. Furthermore, some studies showed that sustained treatment with interferon alfa is associated with normalization of the bone marrow histopathology [10] and decreased risk of myelofibrosis, as compared to HU or phlebotomy alone [11]. This reflects the disease modifying activity of interferons through selective targeting of JAK2-mutated hematopoietic stem cells to induce exit from quiescence and promote terminal myeloid differentiation, resulting in the depletion of JAK2-mutant cells [12, 13].
Ropeginterferon alfa-2b, a unique formulation of pegylated interferon, is characterized by its favorable safety profile, long-term tolerability and convenient dosing schedules. Ropeginterferon alfa-2b was approved under the tradename BESREMi® in 2019 in Europe and in 2021 in US for the treatment of PV. Given the overlapping disease pathophysiology, clinical symptoms and treatment goals, ropeginterferon alfa-2b has emerged as a promising treatment option for other MPNs, especially essential thrombocythemia (ET) [14–16].
Approved treatments for ET, such as hydroxyurea (HU) and anagrelide (ANA), efficiently normalize hematological parameters, specifically platelet (HU and ANA) and white blood cell counts (HU), aiming to reduce the risk of vascular events [17, 18]. Due to teratogenic, carcinogenic, and/or potential leukemogenic risks HU and ANA may have limited suitability for specific patient subgroups. HU carries a high risk of non-melanoma skin cancer, a concern amplified by its often decades-long treatment course and patients with cardiovascular diseases or risk factors should be cautious with ANA due to potential cardiac side effects [19]. Alkylating agents such as busulfan (BUS) and pipobroman are effective cytoreductive options with availability in only a limited number of European countries and, owing to their leukemogenic potential, these agents are primarily considered secondary choices for elderly ET patients who are unresponsive or intolerant to other cytoreductive agents [20]. Importantly, none of these treatments possesses disease-modifying capabilities, thereby not addressing the risk of disease progression to secondary myelofibrosis or acute myeloid leukemia. Furthermore, resistance and/or intolerance to current treatments can occur in approximately 20–25% of patients with ET [21, 22]. Additionally, specific patient groups, such as younger patients are ineligible for available therapies due to contraindications because of potential teratogenic, carcinogenic, and leukemogenic risks (including HU, ANA, BUS). As a result, a significant portion of patients with ET are unable to receive available therapies, whether due to treatment failures, contraindications, or other safety concerns and are left with no approved treatment alternatives.
Therefore, the aim of this multicenter, prospective, single arm phase III trial is to provide evidence for ropeginterferon alfa-2b in the effective and safe management of ET patients lacking treatment options due to intolerance, resistance, and/or contraindications. The primary objective of this trial is to evaluate the capability of ropeginterferon alfa-2b to control abnormal hematologic parameters, disease-related symptoms and vascular risk, while controlling malignant mutation-carrying clones, thereby potentially slowing the neoplastic progression of the disease.
Methods
Study design and objectives
The ROP-ET trial is a multicenter, prospective, single-arm phase III trial designed to assess the use of ropeginterferon alfa-2b in the treatment of ET patients, who are intolerant, resistant, and/or not eligible for other cytoreductive treatments. The estimated total study duration per patient is a maximum of 36 months. This includes a 12-month period for primary analysis and a further 24 months to gather long-term data on efficacy and safety. Participating centers are listed in Table S1 (see Supplementary Information). The primary objective of the study is to assess the disease response rates of ropeginterferon alfa-2b in ET patients, defined by the modified ELN criteria as durable (for at least 3 months) peripheral blood count remission (platelets (PLTs) ≤ 400 × 109/L and white blood cells (WBC) < 10 × 109/L), absence of hemorrhagic or thrombotic events and disease progression, durable improvement and non-progression in disease-related signs, and durable symptoms improvement based on the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS). The secondary objectives include to further assess the efficacy of ropeginterferon alfa-2b in terms of disease response, symptom improvement, vascular events, disease progression, and quality of life (QoL). Additionally, the study aims to evaluate the efficacy of ropeginterferon alfa-2b with regard to disease modification, defined by a sustained decline in mutant allele burden of the driver mutations JAK2, CALR, or MPL. Safety and tolerability of ropeginterferon alfa-2b is planned to be assessed in the study population throughout the entire study duration.
Study population
The inclusion and exclusion criteria for this study are outlined in Table 1. Major eligibility criteria include male or female patients aged 18 or older diagnosed with ET according to WHO 2016 criteria [23]. The diagnosis is to be confirmed by a bone marrow biopsy not more than 5 years old. The study focusses on patients who require cytoreductive treatment but are intolerant, resistant to, and/or ineligible for all locally approved cytoreductive therapies for the treatment of ET, such as HU, ANA, BUS, and pipobroman. Resistance or intolerance to HU should be documented according to the modified ELN criteria [24]. Resistance or intolerance to ANA, BUS, or pipobroman is defined based on the non-responder status according to the primary efficacy endpoint of the study protocol or by the presence of treatment-related toxicities. Patients are considered ineligible for HU, ANA, BUS, or pipobroman due to contraindications according to locally available product information or due to investigator-assessed benefit-risk concerns. Additionally, patients must be interferon-treatment naive, and if they have received prior cytoreductive treatment, a washout period of at least 14 days or longer is required.
Table 1.
Eligibility criteria for ROP-ET study
| Inclusion criteria | Exclusion criteria |
|---|---|
|
1. Informed consent 2. Age ≥ 18 years 3. ET diagnosis (WHO 2016 criteria) with a bone marrow biopsy test result not more than 5 years old 4. Need for cytoreductive treatment and documented resistance/intolerance to, and/or ineligibility for all cytoreductive therapies locally registered for the treatment of ET in the EU (i.e., HU, ANA, BUS, and pipobroman) § 5. If patient received any prior cytoreductive treatment for ET, the washout period between the last dose of treatment and the first dose of study drug must be at least 14 days, or longer. (If washout period was not completed at first day of patients screening, washout may be done after obtaining ICF during the 28-day screening phase) 6. Interferon treatment-naive 7. Adequate hepatic function defined as bilirubin ≤ 1.5 x ULN, prothrombin time (INR) ≤ 1.5 x ULN, albumin > 3.5 g/dL, alanine aminotransferase ≤ 2.0 x ULN, aspartate aminotransferase ≤ 2.0 x ULN at screening 8. HADS score 0–7 on both subscales 9. Patient with HADS score of 8–10 inclusive on either, or both, of the subscales may be eligible following psychiatric assessment that excludes clinical significance of the observed symptoms in the context of potential treatment with an interferon alfa. |
1. Any patient requiring a legally authorized representative 2. Any hypersensitivity to interferon alfa or to any of the drug excipients 3. Pre-existing thyroid disease, if not in remission or not controlled with conventional treatment 4. Existence of, or history of severe psychiatric disorders, particularly severe depression, suicidal ideation or suicide attempt 5. Severe cardiovascular disease (i.e. uncontrolled hypertension, congestive heart failure (≥ NYHA class 2), serious cardiac arrhythmia, significant coronary artery stenosis, unstable angina) or recent stroke or myocardial infarction or pulmonary hypertension 6. Patients with diabetes mellitus that cannot be effectively controlled by medicinal products 7. History or presence of autoimmune disease (excluding well-controlled Hashimoto’s disease) 8. Immunosuppressed transplant recipients 9. Concomitant treatment with telbivudine 10. Decompensated cirrhosis of the liver (Child-Pugh B or C) 11. End stage renal disease (GFR < 15 mL/min) 12. Symptomatic splenomegaly (per the investigator’s judgement) 13. Patients with any other significant medical conditions that, in the opinion of the investigator, would compromise the results of the study or may impair compliance with the requirements of the protocol¶ 14. Use of any investigational drug < 4 weeks prior to the first dose of study drug, or ongoing effects/symptoms due to prior administration of any investigational agent 15. HADS score of 11 or higher on either, or both, of the subscales, and /or development or worsening of the clinically significant depression or suicidal thoughts 16. Pregnant patients, breastfeeding patients or females of childbearing potential not willing to comply with contraceptive requirements |
|
§ Patients resistant/intolerant to HU must have documented resistance/intolerance as defined by modified ELN criteria [24], whereby at least one of the following criteria is met: • Platelet count > 600 × 109/L at ≥ 2 g/day (or ≥ 2.5 g/day if patient body weight > 80 kg) or maximally tolerated dose if < 2 g/day or at maximum dose per local practice after at least 3 months of HU • Platelet count > 400 × 109/L and WBC count < 2.5 × 109/L at any dose and any duration of HU • Platelet count > 400 × 109/L and Hb < 10 g/dL at any dose and any duration of HU • Presence of HU-related toxicities at any dose and any duration of therapy (e.g. leg ulcers, mucocutaneous manifestations, pneumonitis, or HU-related fever) Patients resistant/intolerant to ANA, BUS or pipobroman must meet one of the following criteria: • Patient designated as non-responder according to the primary efficacy endpoint of this protocol (modified ELN criteria) after at least 3 months of treatment with the recommended dosing defined in SmPC or local practice • Presence of treatment-related toxicities at any dose and any duration of therapy Patients ineligible for HU, ANA, BUS or pipobroman with contraindications as defined by locally available SmPC or designated as such by investigator due to benefit-risk concerns |
¶ including but not limited to: • History of any malignancy within 5 years (except stage 0 chronic lymphocytic leukemia, basal cell, squamous cell, and superficial melanoma) • Infections with systemic manifestations (e.g. bacterial, fungal, or HIV, except HBV and/or HCV, at screening) • Evidence of severe retinopathy (e.g. cytomegalovirus retinitis, macular degeneration) or clinically relevant ophthalmological disorder (due to diabetes mellitus or hypertension) • History of alcohol or drug abuse within the last year |
ANA Anagrelide; BUS, Busulfan, ET Essential Thrombocythemia, GFR Glomerular Filtration Rate, HADS Hospital Anxiety and Depression Scale, HBV Hepatitis B virus, HCV Hepatitis C virus, Hb Hemoglobin, HIV Human immunodeficiency virus, HU Hydroxyurea, ICF Informed consent form, INR International Normalized Ratio, NYHA New York Heart Association, SmPC Summary of Product Characteristics, ULN Upper Limit of Normal, WBC White Blood Cell
Study drug
Ropeginterferon alfa-2b will be administered as a subcutaneous injection every two weeks for up to 36 months at a dose of 125 µg per injection. The dose may be adjusted to 250 or 500 µg every two weeks only if optimal hematologic response is not achieved after 3 months or after 6 months, respectively. Treatment may be interrupted, or the dose reduced in case of drug-related toxicity or intolerance. After 12 months, dosing frequency may be adjusted to every four weeks at the discretion of the investigator. Low-dose aspirin (75–100 mg/day) may be given, unless contraindicated.
Study assessments
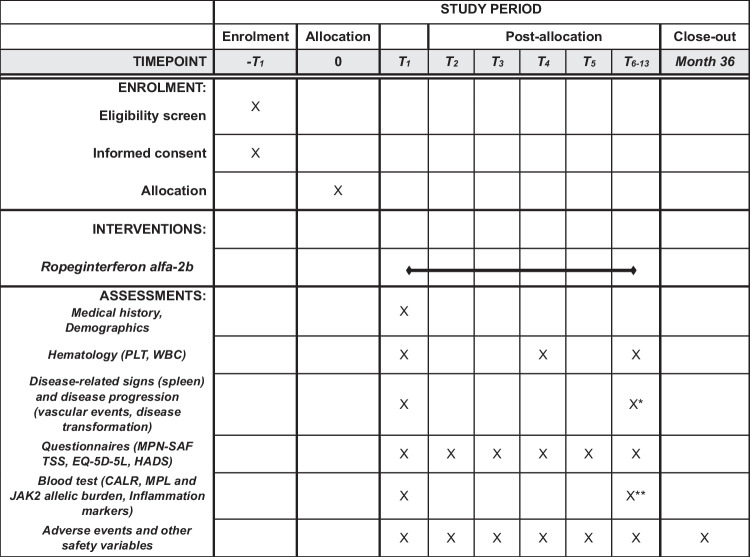
The estimated total study duration per patient is maximum 36 months, which includes 12 months for primary analysis. Detailed study procedures can be found in the SPIRIT figure (Fig. 1). Patient visits will be scheduled every two weeks in the first two months, followed by visits every 3 months until month 12, and visits every 6 months thereafter. Disease response assessments will be performed every 3 months in the first year of treatment, and every 6 months in the second and third year of treatment. Primary efficacy is evaluated at 12 months and includes assessments of peripheral blood counts (PLT and WBC; performed by a central laboratory), ET-related hemorrhagic or thrombotic events, disease progression (i.e. transformation into PV, post-ET myelofibrosis, myelodysplastic syndrome or acute leukemia), disease-related signs (splenomegaly) and MPN-SAF TSS. Quantitative measurements of JAK2, CALR, and MPL allelic burden will be undertaken by a central laboratory every 6 months. The assessment of safety will include monitoring of vital signs, clinical safety laboratory tests, physical examinations, ECG evaluation, ECOG performance status, ocular examination, and adverse events.
Fig. 1.
Schedule of enrolment and assessments (SPIRIT 2013 Figure). T1: Baseline assessments, start of treatment, T2: 2 wks after treatment start, T3: 4 wks after treatment start, T4: 6 wks after treatment start, T5: 8 wks after treatment start, T6-13: 3, 6, 9,12, 18, 24, 30, 36 months after treatment start; *except T6; **except T6 and T8. Abbreviations: CALR, Calreticulin; EQ-5D-5L, EuroQol 5-Dimensions 5-Levels; HADS, Hospital Anxiety and Depression Scale; JAK2, Janus Kinase 2; MPL, Myeloproliferative Leukemia Virus Oncogene; MPN-SAF TSS, Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; PLT, Platelet; WBC, White Blood Cell
Outcomes
The primary efficacy endpoint of the study will assess the rate of disease response at month 12, utilizing modified criteria established by a consensus of the working group comprising the European LeukemiaNet (ELN) and International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) [25]. Disease response for the primary efficacy endpoint will be defined as follows: (i) durable peripheral blood count remission (PLTs ≤ 400 × 109/L and WBCs < 10 × 109/L) lasting for at least 3 months, (ii) absence of hemorrhagic or thrombotic events and disease progression, (iii) durable improvement or non-progression in disease-related signs, and (iv) durable symptom improvement or maintenance of non-progression based on the MPN-SAF TSS. Progression of disease-related signs is defined as the conversion from asymptomatic to symptomatic splenomegaly or a clinically relevant progression of spleen size at the discretion of the investigator. The definition of symptom improvement or maintenance of non-progression is based on changes from baseline MPN-SAF TSS with changes defined as follows: for baseline scores ≥ 20, a 10-point score reduction; for baseline scores inclusive of 15–19, a 5-point score reduction; for baseline scores inclusive of 10–14, a score decrease to ≤ 10 points; and for a baseline score < 10, the score stays < 10. Secondary endpoints include response rates according to the modified ELN criteria at months 9, 18, 24, 30, and 36, longitudinal changes in response rates over 12 months, additional response analyses (e.g. time and duration of first disease response or first peripheral blood count remission response), occurrence of thromboembolic and bleeding events and disease progression, assessment of QoL and symptomatic improvement (assessed using the EQ-5D-5 L questionnaire and the MPN-SAF TSS, respectively), change of inflammation markers (CRP, pentraxin 3, GRO-α, EGF) and cytokines (BLC, M-CSF, eotaxin-2, and TIMP-1) and change of JAK2, CALR, or MPL allelic burden over time. As an exploratory aspect of the study, patients have the option to participate in a sub-study focused on neutrophil extracellular traps (NETs) marker testing. The purpose of this sub-study is to investigate the impact of ropeginterferon alfa-2b on plasma NETs and its association with thrombotic events, and the measurement will include the assessment of circulating cell-free DNA (as a non-specific marker of NETs) and citH3-DNA complexes (as a specific marker of NETs).
Adverse events are graded according to Common Terminology Criteria for Adverse Events (CTCAE; version 5.0) and captured throughout the study.
Sample size
Based on previous studies using ELN response criteria for disease response assessment [4, 26] and the proposed inclusion and exclusion criteria, a response rate of at least 40% for ropeginterferon alfa-2b in the overall population is anticipated. To achieve a level of precision of 10%, the estimated sample size for the primary endpoint analysis is 93 patients. Assuming a 20% dropout rate, a total of 117 patients will be enrolled in the study to ensure that 93 patients remain evaluable for primary endpoint assessment at 12 months. The two subgroups of interest are patients below the age of 45 and cytoreductive treatment resistant/intolerant patients. Assuming 40% response rate and approximately 47 patients evaluable for primary endpoint assessment at 12 months in each subgroup, level of precision will be 13.5%.
Statistical analysis methods
The statistical analysis of this clinical trial will adhere to the guidelines outlined in ICH E9, Statistical Principles for Clinical Trials [27]. A finalized statistical analysis plan will be developed prior to study start (i.e. inclusion of the first patient). For continuous data, the descriptive statistics include the number of cases, mean, standard deviation, median, lower quartile (Q1), upper quartile (Q3), minimum, and maximum. Statistical analyses will be performed using Statistical Analysis System (SAS®) software, version 9.4 or higher (SAS Institute, NC, USA).
The primary efficacy endpoint is the durable disease response rate at month 12. An interim analysis of the 12-month primary endpoint is planned to be performed after the last patient had reached month 12. The number and percentage of patients with a durable disease response at month 12 will be calculated, and the 95% confidence interval for the rate will be estimated using the Clopper-Pearson method. The median and 95% confidence interval of the time-to-event end points will be estimated using the Kaplan–Meier method. For response assessment, patients prematurely discontinuing due to safety or efficacy reasons before the time-point of interest will be considered non-responders. Subgroups of interest are predefined and include patients below the age of 45 and cytoreductive treatment resistant/intolerant. Additional subgroup and sensitivity analyses will have exploratory character and will be defined in all details in the statistical analysis plan. All safety data will be analyzed descriptively. Adverse events will be categorized by system organ classes and preferred terms as defined by the MedDRA dictionary and will be graded using CTCAE v.5.0. Descriptive statistics will be calculated for the number of occurrences, the number of patients, and the incidence of various types of adverse events, summarised by its seriousness, severity, and relationship to the drug.
Discussion
Interferons are well established in the management of patients with PV and are commonly used off-label in the treatment of other MPNs, especially ET [28–34]. Current guidelines recommend interferon alfa as first- and second-line treatment in high-risk ET patients as well as in younger patients, including those who are pregnant or planning pregnancy [35–37]. Meta-analyses of large cohorts of patients with ET reveal that interferon alfa exhibit high clinical efficacy with an overall response rate (ORR) exceeding 80% and with approximately 60% achieving complete hematologic remission (CHR) [38, 39]. In addition, treatment of ET with interferon alfa induces a molecular response in 42% of patients (95% CI: 31–52%) [39] and a significant improvement in myelofibrosis-free survival [40], suggesting that, much like in PV, interferons hold the potential for disease modification in patients with ET.
In patients with ET switching from other cytoreductive therapies, clinical evidence confirms the efficacy and safety of pegylated interferon as second- or third-line therapy option, as summarized in Table 2. Toxicity-related discontinuation rates ranged from 11 to 28% in studies reporting discontinuation rate due to adverse events among ET patients [4, 26, 41–43]. Overall, an ORR in the ranges of 69–100% and 54–78% were observed based on hematologic response and composite hematologic and symptoms response, respectively. Of note, the variability in response rates to interferon for ET arises from using various formulations (peg-IFN-α-2a/b, ropeg-IFN-α-2b), broad inclusion criteria (new and pretreated patients) and varied response criteria definitions. While providing promising clinical evidence for ropeginterferon alfa-2b in treating patients with ET, including those who have undergone and/or failed prior cytoreductive therapies, available studies, however, have several limitations. Most are from single or a few academic centers, involve small participant numbers, have short follow-up periods (up to 2 years), or are retrospective in design. Assessments of clinical outcomes such as vascular events, disease progression, symptom relief, and improvements in quality of life were not frequently conducted in these studies. Yacoub et al. reported that in response to interferon therapy patients experienced significant improvements in MPN-related symptoms, notably in fatigue, dizziness, numbness and tingling, and weight loss [4]. Furthermore, the impact of interferon on mutational burden was assessed in only a few studies [2, 4, 26, 43] with observed molecular response rates of 37% for JAK2V617F and 42% for CALR allele burden [2, 43] and longitudinal evaluations showing a reduction in JAK2V617F variant allele frequency from 30.5 to 8.3% over 4 years and 18.1% after 8 years [26]. Of note, these analyses are mostly based on results from a subset of patients only, depending on sample availability and baseline mutational status.
Table 2.
Clinical studies with pegylated interferon alfa in ET patients with prior cytoreductive therapies
| Reference | Study design; follow-up | Treatment* | n of ET patients | Patient population | Previous treatment | Outcomes | Molecular response | Outcome definition | Discontinuation due to toxicity, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| [41] |
Prospective Single center FU: NA |
Peg-IFN alpha 2b 3 µg/kg/wk |
11 | New diagnosis and previously treated |
Cytoreduction naive: 4/11 Cytoreduction treated: 7/11 |
ORR: 100% (CHR: 100%, PHR: 0%) |
NA |
CHR: platelet count < 400 × 109/L, no TE events PHR: platelet count 400–600 × 109/L, no TE events |
2 (18.2%) |
| [44] |
Retrospective Multicenter FU: 1.4 years |
Peg-IFN alpha 2a 80 µg/wk |
46 | New diagnosis and previously treated | NA | ORR: 78% (CR: 63%; PR: 15%) | NA | 2009 ELN response criteria | Not reported separately for ET patients |
| [45] |
Retrospective Single center FU: 3.8 years |
Peg-IFN alpha 2a 45 µg/wk |
20 | New diagnosis and previously treated | NA | ORR: 65% (CR: 25%, PR: 20%, CR/PR: 20%) | NA | 2013 ELN/IWG-MRT response criteria | Not reported separately for ET patients |
| [46] |
Prospective Single center FU: 2.3 years |
Peg-IFN alpha 2b 2 µg/kg/wk | 13 | New diagnosis and previously treated | NA | ORR: 69% (CHR: 54%; PHR: 15%) | NA |
CHR: platelet count < 440 × 109/L PHR: >50% reduction of platelet count without achieving normal levels |
Not reported separately for ET patients |
| [42] |
Prospective Multicenter FU: 2 years |
Peg-IFN alpha 2b 50 µg/wk |
36 | High-Risk ET, New diagnosis and previously treated |
Cytoreduction naive: 28/36 Cytoreduction treated: 8/36 |
ORR: 75% (CHR: 67%, PHR: 8%) | NA |
CHR: platelet count < 450 × 109/L PHR: platelet count 450–600 × 109/L |
10 (28%) |
| [2] |
Prospective Single center FU: 7 years |
Peg-IFN alpha 2a 90 µg/wk |
40 | New diagnosis and previously treated | NA | ORR: 80% (CHR: 73%; PHR: 3%) |
Baseline: JAK2V617F n = 19 (48%); median allelic burden: 23% ORR: 37% (CMR: 9%, PMR: 17%) |
CHR/PHR based on 2009 ELN response criteria CMR: defined as JAK2V617F ULD, PMR as reductions in baseline allele burden of > 50% |
Not reported separately for ET patients |
| [47] |
Prospective Multicenter FU: 2 years |
Peg-IFN alpha 2b 0.5 µg/kg/wk |
21 | New diagnosis and previously treated | NA | CHR: 33% | NA | CHR: platelet count < 400 × 109/L if previous TE events or platelet count < 600 × 109/L if no previous TE events | Not reported separately for ET patients |
| [26] |
Retrospective Multicenter FU: 2.3 years |
Peg-IFN alpha 2a and b no information on dosing |
127 | New diagnosis and previously treated |
Cytoreductive naive: 75/127 Cytoreductive treated: 52/127 |
ORR: 89% (CHR: 54%, PHR: 35%) |
Baseline: JAK2V617F (51%); median allelic burden: 30.5% allelic burden reduced to 8.3% after 4 years, and 18.1% after 8 years (based on n = 14) |
CHR/PHR based on 2009 ELN response criteria | 16% |
| [43] |
Prospective Observational Multicenter FU: 12 years |
Peg-IFN alpha 124 µg/wk |
31 | New diagnosis and previously treated; CALRmut+ |
Cytoreductive naive: 6/31 Cytoreductive treated: 25/31 |
ORR: 100% (CHR: 77%, PHR: 23%) |
Baseline: CALRmut (n = 31) median allelic burden: 41% (baseline) reduced to 26% ORR: 42%; (CMR: 6%, PMR 36%) |
CHR/PHR based on 2009 ELN response criteria CMR: defined as CALR ULD, PMR as reductions in baseline allele burden of > 50% |
19% |
| [4] |
Prospective Multicenter FU: 1.6 years |
Peg-IFN alpha 2a 45 µg/wk |
65 | High risk ET, HU resistant/intolerant | Cytoreductive treated:65/65 | ORR: 69% (CR 43%,26% PR) |
Baseline: JAK2V617F n = 31 (48%), median allele burden: 29% median absolute reduction in JAK2V617F VAF was 6% (range: -84–47%) in patients achieving a CR vs. 4% (range: -18–56%) in patients with PR or no response |
2009 ELN response criteria | 7 (11%) |
* Interferon doses were modified based on patients’ individualized response and tolerance, only the starting or median/mean doses are displayed
CHR Complete hematologic response, CMR Complete molecular response, CR Complete remission, ELN European Leukemia Network, ET Essential thrombocytemia, FU Median or mean follow-up period (in years), IFN Interferon, IWG-MRT International Working Group for Myeloproliferative Neoplasms Research and Treatment, NA Not available, ORR Overall response rate, PHR Partial hematologic response, PR Partial remission, PMR Partial molecular response, TE Thromboembolic events, ULD Under the level of detection, VAF Variant allele frequency
Larger, prospective studies are warranted in cohorts of patients who require cytoreductive therapy but lack alternative treatment options, focusing particularly on the impact of interferon alfa on molecular response and disease modification, validating preliminary findings and elucidating potential clinical significance. Ropeginterferon alfa-2b is currently being studied in ongoing trials for the treatment of ET: The Surpass-ET trial (NCT04285086) focuses on high-risk ET patients that are resistant or intolerant to HU [48], while the Exceed-ET trial (NCT05482971) includes treatment-naive as well HU and ANA pretreated patients. In addition to enrolling cytoreductive treatment resistant and intolerant patients, the ROP-ET trial distinctively includes those who are ineligible or contraindicated for approved therapies, thereby addressing a critical gap in the currently available care and treatment of ET patients. Ongoing trials, similar to the ROP-ET trial, plan the primary endpoint analysis after 12 months. Additionally, for the Exceed-ET trial there is an extension of up to 3 years for responding patients [49], while the ROP-ET trial is designed with a 3-year treatment duration for all enrolled patients. As demonstrated in pivotal trials that contributed to the approval of ropeginterferon alfa-2b for PV [9, 50] as well as a recently published clinical trial [6], response to pegylated interferon alfa deepens with longer treatment duration and hence, a significant treatment duration is necessary to achieve disease response, particularly on a molecular level. The planned treatment duration in the ROP-ET trial allows to evaluate long-term outcomes and a comprehensive assessment of the treatment impact on clinical and molecular response. Maintaining patients on treatment for a sufficient time to achieve possible disease modification necessitates a dosing regimen that is well-tolerated. Notably, the highest treatment-related discontinuation rates for pegylated interferon alfa occur during the initial treatment phase [2, 9, 26]. Our dosing strategy, therefore, adopts a low starting dose (125 µg) that is anticipated to be an effective and well-tolerated maintenance dose for the majority of patients, as evidenced by platelet response [7] and long-term tolerability [8]. If the desired disease response (CHR) is not achieved, an individualized dose escalation to 250 or 500 µg can be employed, reinforcing that these higher doses are alternative strategies for poor responders and not a target for all. This low-dose run-in concept has led to the approval of ropeginterferon alfa-2b by EMA and FDA and is confirmed by recently published study results from other investigators, ensuring improved drug tolerability (with discontinuation rates at or below 10%), while achieving a timely and favorable therapeutic response [7, 50, 51].
Chronic inflammation is a hallmark characteristic of MPNs and plays a significant role in disease progression [52–54]. In addition, inflammatory cytokines in MPN patients are involved in the interplay between inflammation and thrombosis, the so-called MPN thromboinflammation. JAK2V617F, the most prevalent mutation in ET, has been shown to activate not only erythrocytes and platelets, but also granulocytes. Specifically, activation of neutrophils leads to an increased NETs generation [55, 56], which are extracellular net-like structures containing DNA, proteases and enzymes, such as myeloperoxidase and neutrophil elastase. Data regarding NETosis in MPNs and its association with thrombotic events are conflicting [57] and no comprehensive analysis is available investigating the impact of interferon alfa therapy on NETosis in a large cohort of ET patients [55, 56, 58, 59]. Hence, the ROP-ET trial is designed to assess the effects of ropeginterferon alfa-2b on cytokine profiles and NET markers in ET patients with the goal to explore and better understand the relationship between these markers and thrombotic events.
In summary, the ROP-ET trial is a multicenter, prospective, single-arm phase III trial in ET patients with highly limited treatment options for whom ropeginterferon alfa-2b represents a promising cytoreductive therapy with proven long-term safety and tolerability. The primary objective is to assess the safety of ropeginterferon alfa-2b in ET patients with resistance, intolerance and/or ineligibility to currently available therapies and to demonstrate its ability to regulate abnormal hematologic parameters and disease symptoms while depleting malignant driver mutation-carrying clones, thereby altering the natural progression of the disease.
Supplementary information
Below is the link to the electronic supplementary material.
(DOCX 37.2 KB (38)
(PDF 189 KB)
Acknowledgements
This study is supported by research funding from AOP Health. Special thanks to the patients and their families, to study support staff, and to Jakob Ackerl and Olha Horna for operational support, and Szabolcs Biro for contributions to the protocol development, all of whom are funded by AOP Health. We thank Radka Štěpánová, ANOVA s.r.o. for statistical analyses.
Author contributions
CK, KK, MU developed the trial protocol. JJK, FFM, HKAA, AAL, EB, MBe, MBre, VBA, OC, AMC, CDD, VDS, KD, JGT, MG, SG, PG, VGG, FH, AI, CT, CJ, SK, MTK, MCL, JM, ZGN, FEN, FP, VP, AJR, TS, SSm, ET, AW and BXC contribute to the conduct of the trial. SS wrote the manuscript. CK, FFM, JJK, KK, MU, VE provided input and critical reviews. All authors read and approved the final manuscript.
Funding
This study is sponsored by AOP Health, Leopold-Ungar-Platz 2, 1190 Vienna, Austria.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Trial Status
Protocol Version 3.0 dated 26 Sep 2023. The first subject was enrolled on 04 Dec 2023.
Ethics approval and consent to participate
List of participating centers (Table S1).
Consent for publication
Not applicable.
Competing interests
CK, KK, MU, SS, VE are employees of AOP Health.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haifa Kathrin Al-Ali, Alberto Alvarez-Larrán, Eloise Beggiato, Maria Bieniaszewska, Massimo Breccia, Veronika Buxhofer-Ausch, Olga Cerna, Ana-Manuela Crisan, Catalin Doru Danaila, Valerio De Stefano, Konstanze Döhner, Joanna Gora-Tybor, Martin Griesshammer, Sebastian Grosicki, Paola Guglielmelli, Valentin García-Gutierrez, Florian H. Heidel, Arpád Illés, Ciprian Tomuleasa, Chloe James, Steffen Koschmieder, Maria-Theresa Krauth, Mihaela-Cornelia Lazaroiu, Jiri Mayer, Zsolt György Nagy, Franck-Emmanuel Nicolini, Francesca Palandri, Vassiliki Pappa, Andreas Johannes Reiter, Tomasz Sacha, Stefan Schmidt, Evangelos Terpos, Albert Wölfler and Blanca Xicoy Cirici contributed equally to this work and are listed alphabetically.
References
- 1.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 2.Masarova L, Patel KP, Newberry KJ, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. 2017;4(4):e165–e175. doi: 10.1016/S2352-3026(17)30030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen TA, Hansen DL, Ocias LF, et al. Long-term efficacy and safety of recombinant interferon alpha-2 vs. hydroxyurea in polycythemia vera: preliminary results from the three-year analysis of the Daliah Trial - a randomized controlled phase III clinical trial. Blood. 2018;132(no Suppl 1):pp580. doi: 10.1182/blood-2018-99-111255. [DOI] [Google Scholar]
- 4.Yacoub A, Mascarenhas J, Kosiorek H, et al. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood. 2019;134(18):1498–1509. doi: 10.1182/blood.2019000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196–e208. doi: 10.1016/S2352-3026(19)30236-4. [DOI] [PubMed] [Google Scholar]
- 6.Mascarenhas J, Kosiorek HE, Prchal JT, et al. A randomized phase 3 trial of interferon-alpha vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139(19):2931–2941. doi: 10.1182/blood.2021012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbui T, Vannucchi AM, De Stefano V et al (2023) Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid 2(6). 10.1056/EVIDoa2200335 [DOI] [PubMed]
- 8.Gisslinger H, Klade C, Georgiev P, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. 2023 doi: 10.1038/s41375-023-02008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiladjian JJ, Klade C, Georgiev P, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia. 2022;36(5):1408–1411. doi: 10.1038/s41375-022-01528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasselbalch HC, Holmstrom MO. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin Immunopathol. 2019;41(1):5–19. doi: 10.1007/s00281-018-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Zeinah G, Silver RT, Abu-Zeinah K, et al. Normal life expectancy for polycythemia vera (PV) patients is possible. Leukemia. 2021;36(2):569–572. doi: 10.1038/s41375-021-01447-3. [DOI] [PubMed] [Google Scholar]
- 12.How J, Garcia JS, Mullally A. Biology and therapeutic targeting of molecular mechanisms in MPNs. Blood. 2023;141(16):1922–1933. doi: 10.1182/blood.2022017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verger E, Soret-Dulphy J, Maslah N, et al. Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo. Blood Cancer J. 2018;8(10):94. doi: 10.1038/s41408-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.How J, Hobbs G. Interferons as the first choice of cytoreduction in essential thrombocythemia and polycythemia vera. J Natl Compr Canc Netw. 2022;20(9):1063–1068. doi: 10.6004/jnccn.2022.7026. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey AL, Green AC, Harrison CN. Essential thrombocythemia: challenges in clinical practice and future prospects. Blood. 2023;141(16):1943–1953. doi: 10.1182/blood.2022017625. [DOI] [PubMed] [Google Scholar]
- 16.Masarova L, Verstovsek S. Emerging drugs for essential thrombocythemia. Expert Opin Emerg Drugs. 2019;24(2):93–105. doi: 10.1080/14728214.2019.1615437. [DOI] [PubMed] [Google Scholar]
- 17.Birgegard G, Besses C, Griesshammer M, et al. Treatment of essential thrombocythemia in Europe: a prospective long-term observational study of 3649 high-risk patients in the evaluation of anagrelide efficacy and long-term safety study. Haematologica. 2018;103(1):51–60. doi: 10.3324/haematol.2017.174672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood. 2013;121(10):1720–1728. doi: 10.1182/blood-2012-07-443770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018;8(1):2. doi: 10.1038/s41408-017-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol. 2014;93(12):2037–2043. doi: 10.1007/s00277-014-2152-7. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Boluda JC, Alvarez-Larran A, Gomez M, et al. Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br J Haematol. 2011;152(1):81–88. doi: 10.1111/j.1365-2141.2010.08430.x. [DOI] [PubMed] [Google Scholar]
- 22.Birgegard G. The use of anagrelide in myeloproliferative neoplasms, with focus on essential thrombocythemia. Curr Hematol Malig Rep. 2016;11(5):348–355. doi: 10.1007/s11899-016-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 24.Barosi G, Besses C, Birgegard G, et al. A unified definition of clinical resistance/intolerance to hydroxyurea in essential thrombocythemia: results of a consensus process by an international working group. Leukemia. 2007;21(2):277–280. doi: 10.1038/sj.leu.2404473. [DOI] [PubMed] [Google Scholar]
- 25.Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778–4781. doi: 10.1182/blood-2013-01-478891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stegelmann F, Teichmann LL, Heidel FH, et al. Clinicohematologic and molecular response of essential thrombocythemia patients treated with pegylated interferon-alpha: a multi-center study of the German Study Group-Myeloproliferative Neoplasms (GSG-MPN) Leukemia. 2023;37(4):924–928. doi: 10.1038/s41375-023-01837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ICH Harmonised Tripartite Guideline (1999) Statistical principles for clinical trials, international conference on harmonisation E9 Expert Working Group Stat Med 18(15):1905–1942. https://www.ncbi.nlm.nih.gov/pubmed/10532877 [PubMed]
- 28.Gilreath JA, Tashi T, Kim SJ, et al. Compassionate use of ropeginterferon-alfa-2b/P1101 for treatment of high risk polycythemia vera and essential thrombocythemia patients previously controlled on pegylated interferon-alfa-2a/Pegasys®. Blood. 2018;132(Supplement 1):5459–5459. doi: 10.1182/blood-2018-99-116852. [DOI] [Google Scholar]
- 29.How J, Hobbs G. Real-world experience of ropeginterferon in myeloproliferative neoplasm patients. Blood. 2022;140(Supplement 1):12284–12285. doi: 10.1182/blood-2022-157076. [DOI] [Google Scholar]
- 30.Huang CE, Wu YY, Hsu CC, et al. Real-world experience with ropeginterferon-alpha 2b (Besremi) in philadelphia-negative myeloproliferative neoplasms. J Formos Med Assoc. 2021;120(2):863–873. doi: 10.1016/j.jfma.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Novak W, Annamária C, Crazzolara R, et al. Severe complications in JAK2 V617F positive pediatric patients with myeloproliferative neoplasms. Hemasphere. 2022;6(Suppl):936–937. doi: 10.1097/01.HS9.0000852292.38263.b8. [DOI] [Google Scholar]
- 32.Okikiolu J, Woodley C, Cadman-Davies L, et al. Real world experience with ropeginterferon alpha-2b (Besremi) in essential thrombocythaemia and polycythaemia vera following exposure to pegylated interferon alfa-2a (Pegasys) Leuk Res Rep. 2023;19:100360. doi: 10.1016/j.lrr.2022.100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podstavková N, Weinbergerová B, Procházková J, et al. Current experience with ropeginterferon Alfa-2b in Ph negative myeloproliferative neoplasm at the Department of Internal Medicine – Haematology and Oncology in Brno. Transfuze Hematol Dnes. 2022;28(4):1–6. doi: 10.48095/cctahd2022prolekare.cz15. [DOI] [Google Scholar]
- 34.Tashi T, Reeves BN, Kim SJ, et al. Real-world experience of ropeginterferon-alfa treatment of PV and ET - Two centers experience. Blood. 2023;142(Supplement 1):6397. doi: 10.1182/blood-2023-191268. [DOI] [Google Scholar]
- 35.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057–1069. doi: 10.1038/s41375-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Larran A, Sant’Antonio E, Harrison C, et al. Unmet clinical needs in the management of CALR-mutated essential thrombocythaemia: a consensus-based proposal from the European LeukemiaNet. Lancet Haematol. 2021;8(9):e658–e665. doi: 10.1016/S2352-3026(21)00204-0. [DOI] [PubMed] [Google Scholar]
- 37.Mesa RA, Jamieson C, Bhatia R, et al. NCCN guidelines insights: Myeloproliferative neoplasms, version 2.2018. J Natl Compr Canc Netw. 2017;15(10):1193–1207. doi: 10.6004/jnccn.2017.0157. [DOI] [PubMed] [Google Scholar]
- 38.Bewersdorf JP, Giri S, Wang R, et al. Interferon alpha therapy in essential thrombocythemia and polycythemia vera-a systematic review and meta-analysis. Leukemia. 2021;35(6):1643–1660. doi: 10.1038/s41375-020-01020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu W, Yang R, Xiao Z, et al. Clinical outcomes of interferon therapy for polycythemia vera and essential thrombocythemia: a systematic review and meta-analysis. Int J Hematol. 2021;114(3):342–354. doi: 10.1007/s12185-021-03171-1. [DOI] [PubMed] [Google Scholar]
- 40.Beauverd Y, Ianotto J-C, Thaw KH et al (2023) Impact of cytoreductive drugs upon outcomes in a contemporary cohort of adolescent and young adults with essential thrombocythemia and polycythemia vera. Retrieved from https://ash.confex.com/ash/2023/webprogram/Paper185108.html. Accessed 27 Nov 2023
- 41.Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol. 2003;51(1):81–86. doi: 10.1007/s00280-002-0533-4. [DOI] [PubMed] [Google Scholar]
- 42.Langer C, Lengfelder E, Thiele J, et al. Pegylated interferon for the treatment of high risk essential thrombocythemia: results of a phase II study. Haematologica. 2005;90(10):1333–1338. [PubMed] [Google Scholar]
- 43.Verger E, Cassinat B, Chauveau A, et al. Clinical and molecular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. Blood. 2015;126(24):2585–2591. doi: 10.1182/blood-2015-07-659060. [DOI] [PubMed] [Google Scholar]
- 44.Gowin K, Thapaliya P, Samuelson J, et al. Experience with pegylated interferon alpha-2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica. 2012;97(10):1570–1573. doi: 10.3324/haematol.2011.061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gowin K, Jain T, Kosiorek H, et al. Pegylated interferon alpha – 2a is clinically effective and tolerable in myeloproliferative neoplasm patients treated off clinical trial. Leuk Res. 2017;54:73–77. doi: 10.1016/j.leukres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer. 2007;110(9):2012–2018. doi: 10.1002/cncr.23018. [DOI] [PubMed] [Google Scholar]
- 47.Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer. 2006;106(11):2397–2405. doi: 10.1002/cncr.21900. [DOI] [PubMed] [Google Scholar]
- 48.Verstovsek S, Komatsu N, Gill H, et al. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022;18(27):2999–3009. doi: 10.2217/fon-2022-0596. [DOI] [PubMed] [Google Scholar]
- 49.Masarova L, Mascarenhas J, Qin A, et al. EXCEED-ET: a single-arm multicenter study to assess the efficacy, safety, and tolerability of ropeginterferon alfa-2b-njft (P1101) in north American adults with essential thrombocythemia. J Clin Oncol. 2023;41(16):TPS7088–TPS7088. doi: 10.1200/JCO.2023.41.16_suppl.TPS7088. [DOI] [Google Scholar]
- 50.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126(15):1762–1769. doi: 10.1182/blood-2015-04-637280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edahiro Y, Ohishi K, Gotoh A, et al. Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. Int J Hematol. 2022;116(2):215–227. doi: 10.1007/s12185-022-03341-9. [DOI] [PubMed] [Google Scholar]
- 52.Ferrer-Marin F, Arroyo AB, Bellosillo B, et al. miR-146a rs2431697 identifies myeloproliferative neoplasm patients with higher secondary myelofibrosis progression risk. Leukemia. 2020;34(10):2648–2659. doi: 10.1038/s41375-020-0767-3. [DOI] [PubMed] [Google Scholar]
- 53.Hasselbalch HC, Bjorn ME. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:102476. doi: 10.1155/2015/102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morales ML, Ferrer-Marin F (2023) Deepening our understanding of the factors affecting landscape of myeloproliferative neoplasms: what do we know about them? Cancers (Basel) 15(4). 10.3390/cancers15041348 [DOI] [PMC free article] [PubMed]
- 55.Guy A, Favre S, Labrouche-Colomer S, et al. High circulating levels of MPO-DNA are associated with thrombosis in patients with MPN. Leukemia. 2019;33(10):2544–2548. doi: 10.1038/s41375-019-0500-2. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt S, Daniliants D, Hiller E, et al. Increased levels of NETosis in myeloproliferative neoplasms are not linked to thrombotic events. Blood Adv. 2021;5(18):3515–3527. doi: 10.1182/bloodadvances.2020004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrer-Marin F, Cuenca-Zamora EJ, Guijarro-Carrillo PJ et al (2021) Emerging role of neutrophils in the thrombosis of chronic myeloproliferative neoplasms. Int J Mol Sci 22(3). 10.3390/ijms22031143 [DOI] [PMC free article] [PubMed]
- 58.Marin Oyarzun CP, Carestia A, Lev PR, et al. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci Rep. 2016;6:38738. doi: 10.1038/srep38738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolach O, Sellar RS, Martinod K et al (2018) Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med 10(436). 10.1126/scitranslmed.aan8292 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 37.2 KB (38)
(PDF 189 KB)
Data Availability Statement
No datasets were generated or analysed during the current study.