Abstract
Infection of the brain by lentiviruses, including human immunodeficiency virus (HIV) and feline immunodeficiency virus (FIV), causes inflammation and results in neurodegeneration. Molecular diversity within the lentivirus envelope gene has been implicated in the regulation of cell tropism and the host response to infection. Here, we examine the hypothesis that envelope sequence diversity modulates the expression of host molecules implicated in lentivirus-induced brain disease, including matrix metalloproteinases (MMP) and related transcription factors. Infection of primary macrophages by chimeric HIV clones containing brain-derived envelope fragments from patients with HIV-associated dementia (HAD) or nondemented AIDS patients (HIV-ND) showed that MMP-2 and -9 levels in conditioned media were significantly higher for the HAD clones. Similarly, STAT-1 and JAK-1 levels were higher in macrophages infected by HAD clones. Infections of primary feline macrophages by the neurovirulent FIV strain (V1CSF), the less neurovirulent strain (Petaluma), and a chimera containing the V1CSF envelope in a Petaluma background (FIV-Ch) revealed that MMP-2 and -9 levels were significantly higher in conditioned media from V1CSF- and FIV-Ch-infected macrophages, which was associated with increased intracellular STAT-1 and JAK-1 levels. The STAT-1 inhibitor fludarabine significantly reduced MMP-2 expression, but not MMP-9 expression, in FIV-infected macrophages. Analysis of MMP mRNA and protein levels in brain samples from HIV-infected persons or FIV-infected cats showed that MMP-2 and -9 levels were significantly increased in lentivirus-infected brains compared to those of uninfected controls. Elevated MMP expression was accompanied by significant increases in STAT-1 and JAK-1 mRNA and protein levels in the same brain samples. The present findings indicate that two lentiviruses, HIV and FIV, have common mechanisms of MMP-2 and -9 induction, which is modulated in part by envelope sequence diversity and the STAT-1/JAK-1 signaling pathway.
Lentiviruses, including human immunodeficiency virus (HIV) and feline immunodeficiency virus (FIV), are associated with immunological and neurological impairment in their respective hosts (17, 70). HIV and FIV share many properties, including structural organization, life cycle, cell tropism, and a common mechanism of infection involving the chemokine receptors (72). Both HIV and FIV are neurotropic, infecting the central nervous system (CNS) and causing primary neurological disease that manifests as motor dysfunction, behavioral abnormalities, and neuronal loss (36, 46, 49). The pathogenesis of lentivirus-induced neurological disease remains unclear, although several mechanisms that are common to both FIV and HIV have been proposed to explain neuronal damage in the absence of productive infection of neurons. These mechanisms include the inherent toxicity of viral proteins and the excess release of host molecules by infected and activated brain macrophages, such as cytokines, excitotoxic amino acids, and free oxygen radicals (19, 32, 48). Hence, FIV has been proposed as a potential animal model for HIV infection of the CNS and the development of HIV-associated dementia (HAD) (22, 49).
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that function primarily in degrading components of the extracellular matrix (20, 75). Recently, elevated expression of MMPs in the CNS following lentivirus infection has suggested a role for these enzymes in lentiviral neuropathogenesis (3, 9, 63), possibly through their ability to promote breakdown in blood-brain barrier (BBB) integrity and cell death (59, 75). Numerous factors that regulate MMP transcription are also elevated during lentivirus infection of the CNS, such as the cytokines tumor necrosis factor alpha (TNF-α) (50, 71) and alpha interferon (IFN-α) (28, 57) and the β-chemokines RANTES and MIP-1α (60). Induction of several MMPs by mediators of inflammation or viral proteins involves activation of specific transcription factors, such as AP-1 and NF-κB (4, 30). The signal transducer and activator of transcription (STAT)/Janus kinase (JAK) signaling pathway, which plays an important role in mediating the biological effects of several cytokine receptors (64), has also been shown to regulate MMP gene expression (27). It has recently been demonstrated that chemokine receptors, like the receptors for other cytokines, regulate a variety of cell functions through activation of specific signal transduction pathways, especially the STAT/JAK pathway (58, 69, 73). In addition, chemokine receptor-mediated signaling has been shown to influence MMP-2 and -9 expression in microglial cells (10, 66) and induce neuronal damage (76, 77), suggesting a role for STATs in these processes.
The HIV-1 gp120 envelope protein activates several transcription factors, including STAT-1 (62), and alters host cell signaling through its interaction with chemokine receptors (38, 51, 77). Viral envelope gene variability was also reported to influence the occurrence of neurological disease in several retroviral systems (33, 45, 67). Previous studies have demonstrated that HIV-1 strains derived from AIDS patients with dementia differ from viruses derived from nondemented patients primarily in the V3 sequences of the gp120 envelope protein (29, 55). In addition to conferring enhanced ability to replicate in microglial cells (65), the V3 region of the HIV envelope has been shown to influence the release of neurotoxic molecules following infection of macrophages (11, 24, 26, 56). Thus, it is conceivable that specific sequences in the envelope gene of neurovirulent lentiviruses may influence the pattern of MMP expression in infected cells in a manner analogous to that reported for other molecules implicated in neurodegeneration.
In the present study, we examined the hypothesis that a mechanism common to lentiviruses was responsible for the induction of MMP expression in the brain. Furthermore, the role of envelope diversity and the STAT/JAK signaling pathway in modulating this process was investigated in relation to MMP production. Our results indicated that infection with HIV or FIV increased STAT-1 and MMP expression in both brain and macrophages. In addition, HIV and FIV envelope sequences associated with neurological disease induced MMP expression to a greater extent than sequences not associated with neurological disease, through a mechanism mediated in part by the STAT/JAK signaling pathway.
MATERIALS AND METHODS
Cell culture.
U937 cells (ATCC) were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS). CrFK cells (ATCC) were cultured in minimum essential medium (MEM) with 10% horse serum. Human peripheral blood mononuclear cells (PBMC) were obtained from healthy donors as previously described (54), initially stimulated with 5 μg of concanavalin A/ml, and maintained in RPMI with 15% FCS and 100 IU of interleukin-2 (IL-2)/ml. Primary macrophages were isolated from PBMC by adherence and cultured in RPMI with 20% FCS as previously reported (54). Feline PBMC and macrophages were obtained from specific-pathogen-free adult cats and were prepared in the same manner as the human cells. All cell cultures were supplemented with 100 μg of streptomycin/ml and 0.25 μg of amphotericin/ml.
Viruses.
FIV strains used in this study included two primary isolates, cerebrospinal fluid-derived V1CSF (52) and blood-derived Petaluma (gift from N. C. Pedersen), that underwent fewer than 10 in vitro passages prior to the present experiments. Although both strains are neurotropic, V1CSF was previously shown to exhibit greater neurovirulence than Petaluma (52). Molecular clones of HIV-1 were generated as described previously (54) by exchanging sequences containing the V3 loop of the pNL4-3 envelope gene with equivalent sequences from viruses derived from the brains of demented (HAD) or nondemented (HIV-ND) HIV-infected patients. The Petaluma molecular clone, pFIV-34TF10, was obtained from the NIH AIDS Reagent program (#1236). HIV and FIV viral stocks were prepared from supernatants from infected PBMC cultures, and titers were determined by limiting dilution as previously described (56).
Construction of FIV chimeras.
Genomic DNA from feline PBMC persistently infected with V1CSF was amplified using an Expand Long Template PCR kit (Boehringer Mannheim) for 1 cycle (94°C for 1 min), 30 cycles (94°C for 1 min, 50°C for 1 min, and 68°C for 5 min), and 1 cycle (68°C for 10 min) with primers 5′-TTA GGG TAC CTG GAA TAA CAG-3′ and 5′-TCG TAA ACA GTC CCT AGT CCA TAA-3′. This protocol generated a product that spanned the V1CSF genome from the leader region of the env gene (nucleotide [nt] 6393) to the U3 element of the 3′ long terminal repeat (nt 9181) and contained both a flanking 5′ Acc65I restriction site (5′-GGTAC-3′) and the 3′ NdeI site that is conserved among several FIV strains. PCR products were gel purified using a Concert Gel Extraction kit (Gibco) and digested with Acc65I and NdeI to yield a 2,500-bp fragment comprised of the V1CSF env gene. The fragment including the surface unit of the envelope was sequenced in both directions using multiple primers and an ABI automated sequencer. The Acc65I-NdeI V1CSF fragment (nt 6393 to 8906) was cloned into a 34TF10 shuttle vector that was prepared by digesting the pFIV-34TF10 molecular clone with SphI and NdeI and ligating the resulting fragment (nt 3451 to 8906) into a Bluescript cloning vector. Subsequently, a BspRI-NdeI fragment (nt 5328 to 8906) was exchanged between the shuttle vector containing the V1CSF env sequences and pFIV-34TF10 to yield V1CSF-Petaluma chimeras.
Transfection.
CrFK cells (4 × 105) were seeded in six-well plates and cultured for 24 h to achieve 50% confluency. For each chimera, 2 μg of plasmid DNA was mixed with 10 μl of Lipofectin reagent (Gibco) in 2 ml of Opti-MEM medium (Gibco) and transfected into CrFK cells by incubation for 8 h. Cells were washed with phosphate-buffered saline, cultured in MEM containing 10% horse serum for 3 days, and cocultured with feline PBMC (2 × 106) in RPMI containing 15% FCS and IL-2 for 48 h. Cocultured PBMC were removed, and viral replication was assessed by reverse transcriptase (RT) assay and RT-PCR analysis of viral RNA in culture supernatants over a 2-week period. FIV chimeras were passaged in feline PBMC, and viral stocks were prepared as described above.
Cell treatments and infection.
U937 cells and feline macrophages were seeded at 106 cells/ml in AIM V serum-free medium (Gibco) and incubated in the presence or absence of IFN-α (Sigma), fludarabine (Sigma), and RANTES (NIH AIDS Reagent program, #3045) for 24 h prior to the collection of cells and conditioned media. For infection with HIV or FIV, primary macrophages were inoculated with 200 μl of viral stock, incubated for 2 h at 37°C, washed twice, and cultured in serum-free medium until supernatants and cells were harvested. Human macrophages were infected with ND or HAD HIV strains at titers of 104.5 50% tissue culture infective doses (TCID50)/106 cells. Feline macrophages were infected with 102.0 or 103.5 TCID50/106 cells of V1CSF, Petaluma, or chimeric FIV strains. Fludarabine was added to FIV-infected macrophage cultures 24 h before samples were collected. For all samples, cells were counted and assessed for viability by trypan blue staining and culture supernatants were cleared by centrifugation at the time of collection.
RT assay.
RT activity in culture supernatants was measured using a protocol described previously (61). Briefly, 10 μl of culture supernatant was cleared of cellular debris by high-speed centrifugation and incubated with 40 μl of reaction cocktail containing [α-32P]TTP for 2 h at 37°C. Samples were blotted on DE81 Ion Exchange Chromatography Paper (Whatman International, Ltd.) and washed three times for 5 min in 2× SSC and twice for 5 min in 95% ethanol (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). RT levels were measured by liquid scintillation counting. All assays were performed in duplicate and repeated a minimum of two times.
RT-PCR.
Total cellular RNA was isolated from tissue or cultured cells with TRIzol reagent (Gibco) and was DNase treated for 1 h at 37°C. cDNA was prepared using a First Strand cDNA Synthesis kit (Boehringer Mannheim) and amplified by 1 cycle (95°C for 1 min), 30 cycles (95°C for 1 min, 55°C for 1 min, and 72°C for 2 min) and 1 cycle (72°C for 10 min) with the following primers: 5′-GGC ATC CAG GTT ATC GGG GA-3′ and 5′-GGC CCT GTC ACT CCT GAG AT-3′ (MMP-2), 5′-GTC GTG CGT GTC CAA AGG CA-3′ and 5′-TGG ACG ATG CCT GCA ACG TG-3′ (MMP-9), 5′-CCG GGA AGG GGC CAT CAC AT-3′ and 5′-CCA CTA TCC GGG ACA TCT CAT CAA AC-3′ (STAT-1), 5′-GAG GTG CAG AAG GGC CGC TAC AGT C-3′ and 5′-TCA CGG GCC AGG AGG AGG TTT TTA-3′ (JAK-1), and 5′-AAG CCT GTA GCC CAT GTT GTA GC-3′ and 5′-GAA GAC CCC TCC CAG ATA GAT G-3′ (TNF-α). Equal amounts of template cDNA were assessed by amplification of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) using primers 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ and 5′-CGG AGT CAA CGG ATT TGG TCG-3′ at an annealing temperature of 50°C. FIV infection of feline cells was confirmed by amplification of the V1CSF pol gene, as described previously (21). RNA levels were compared by densitometric analysis of Southern blots by using Scion Image computer imaging software (35) and equalized to the corresponding GAPDH RNA level for statistical analysis. RNA samples amplified in the absence of RT served as controls to ensure the absence of contaminating DNA, and experiments were performed to ensure amplification was within the linear range.
Western blot analysis.
Cultured cells or brain tissue, extracted in buffer containing 10 mM Tris, pH 7.4, 10 mM NaCl, 3 mM MgCl2, and 0.5% NP-40, was cleared by centrifugation, and protein levels were quantified using a Bradford assay (BIO-RAD, Mississauga, Ontario, Canada). Equal amounts of protein from each sample (20 μg), determined by Coomassie blue staining and detection of housekeeping proteins, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and blocked with 1% casein in TBST (25 mM Tris-buffered saline and 0.05% Tween 20). Primary antibodies were diluted 1:1,000 in TBST containing 0.5% casein and incubated with membranes for 2 h at room temperature. Membranes were washed and incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch Lab Inc., Westgrove, Pa.) diluted 1:5,000 in 0.5% casein-TBST. Immunoreactive proteins were detected by chemiluminescence (Amersham, Arlington Heights, Ill.), and protein abundance was measured by densitometry. Monoclonal antibodies against STAT-1, JAK-1, and P-TYR were obtained from Transduction Laboratories (Lexington, Ky.). Antibodies to MMP-2 and -9 were obtained from Oncogene Research Products (Cambridge, Mass.) and British Biotech (Oxford, United Kingdom), respectively. Standard curves were generated by Western blotting and densitometric analysis using serial protein dilutions to ensure that detection was within the linear range.
Gelatin zymography.
MMP levels in conditioned media were measured by zymography as previously described (42). Volumes of serum-free conditioned media were equalized to the number of cells present in each culture at the time of harvest and separated by electrophoresis on a sodium dodecyl sulfate–15% polyacrylamide gel that was copolymerized with 1 mg of gelatin/ml. Gels were agitated for 1 h in renaturing buffer (50 mM Tris-HCl, 5 mM CaCl2, 2.5% Triton X-100) to restore enzymatic activity and were incubated for 24 h at 37°C in buffer lacking detergent. Gelatinase activity on gels stained with Coomassie blue was detectable as unstained bands representing areas of gelatin digestion. Stained gels were dried, and MMP abundance was determined by densitometry. Standard curves were generated by zymography and densitometric analysis using different sample volumes to ensure that detection was within the linear range.
Human and feline brain tissue.
Autopsied human brain tissue from donors with defined neuropathologies was obtained from HIV-infected patients (n = 5) and HIV-seronegative controls (n = 6) from the AIDS Brain Banks at St. Paul's Hospital, Vancouver, British Columbia, Canada, and Johns Hopkins University, Baltimore, Md. Uninfected controls included patients diagnosed with ischemic stroke (n = 3), septic encephalopathy (n = 1), acute myelogenous leukemia (n = 1), or anoxic encephalopathy (n = 1). All HIV-infected patients were AIDS defined at death and were diagnosed with multifocal necrotizing leukoencephalopathy (n = 1), HIV encephalitis (n = 3), or cytomegalovirus encephalitis (n = 1). Feline brain samples were collected from adult specific-pathogen-free cats that were uninfected, or infected experimentally with V1CSF, as described previously (52). All FIV-infected felines from which brain samples were obtained exhibited neurological impairment that included ataxia, aggressivity, and reduced motor activity and neuropathological changes that included gliosis, perivascular cuffing, and neuronal loss (52).
Statistical analysis.
Statistical analyses were performed using Instat, Graphpad, for both parametric and nonparametric comparisons. P values of less than 0.05 were considered significant. All experiments were repeated a minimum of two times using different sample preparations to ensure reproducibility of results.
RESULTS
MMP and STAT protein detection by zymography and Western blotting.
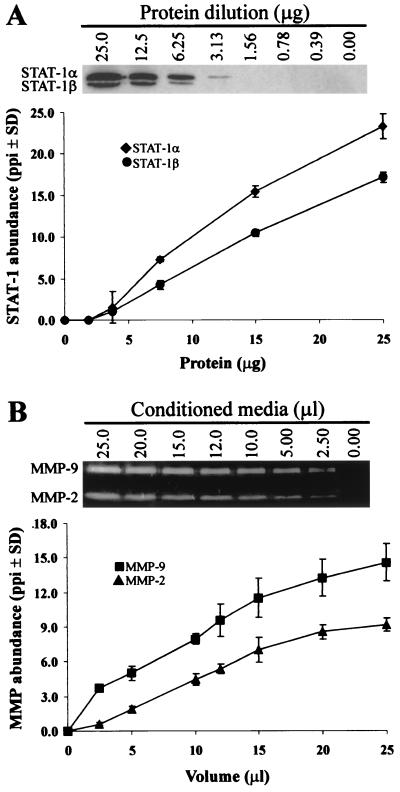
To ensure that protein levels in conditioned media and cell lysates could be compared semiquantitatively by zymography and Western blot analysis, standard curves were generated by densitometry using each method (Fig. 1). A linear relationship was obtained between protein abundance and pixel density following Western blot analysis of STAT-1 levels in serial dilutions of total cellular protein from HIV-infected human macrophages (Fig. 1A). Moreover, both the STAT-1α (91 kDa) and STAT-1β (84 kDa) isoforms were found to fall within the linear range of detection of the Western blot protocol. In a similar manner, MMP-2 and -9 abundance, detected by gelatin zymography in conditioned media from HIV-infected macrophages, varied linearly with sample volume (Fig. 1B). Similar results were obtained using protein derived from HIV-infected brain tissue (data not shown), demonstrating that the abundance of MMPs and STAT-1 could be compared accurately using semiquantitative techniques.
FIG. 1.
Detection of STAT-1 by Western blotting and MMPs by gelatin zymography. (A) Western blot analysis of serial dilutions of protein from HIV-infected human macrophages detected both of the STAT-1 isoforms, STAT-1α and STAT-1β. Protein abundance was assessed by densitometry and equalized to the corresponding level of housekeeping protein detected in each sample. Similar results were obtained using FIV-infected feline brain and macrophages. Data are expressed as pixels per square inch (ppi) and represent the mean ± standard deviation (SD) of two experiments. (B) MMP-2 and -9 abundance was measured by gelatin zymography using conditioned medium from HIV-infected human (shown) and FIV-infected feline macrophages. Coomassie-stained gels were converted to grey-scale, and protein levels were quantified by densitometry and equalized to the number of cells in each sample well at the time of harvest. Data represent the mean ± standard deviation (SD) of three experiments.
Lentiviral infection induces expression of MMPs in primary macrophages.
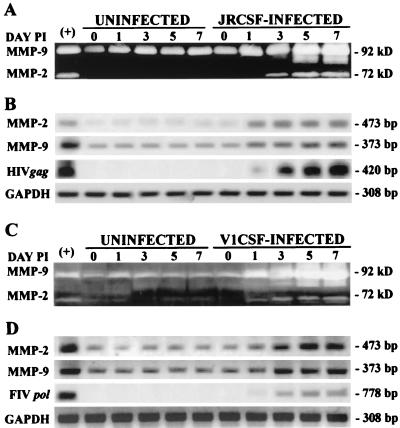
Previously, increased MMP expression has been demonstrated in the cerebrospinal fluid (CSF) of AIDS patients with HAD (9, 63). Since macrophages and microglia are the principal CNS cell types infected by lentiviruses (14, 25), we investigated MMP expression in primary human and feline macrophages following infection with CSF-derived strains of HIV and FIV. JRCSF, an infectious molecular clone of HIV-1 derived from a patient with HAD, was found to induce the expression of MMP-2 and -9 protein (Fig. 2A) and mRNA (Fig. 2B) levels. Similarly, the neurovirulent FIV isolate, V1CSF, induced MMP-2 and -9 expression in primary feline cultures (Fig. 2C and D). Moreover, MMP expression was elevated early after infection by either virus and increased with viral replication over a 1-week time course (Fig. 2B and D). These findings demonstrated that lentiviral strains associated with CNS infection induced concurrent increases in MMP protein and mRNA expression in macrophages and suggested a potential mechanism common to the pathogenesis of HIV and FIV.
FIG. 2.
Infection with CNS-derived HIV and FIV strains increases MMP expression in primary macrophages. MMP-2 and -9 protein (A, C) and RNA (B, D) levels were assessed by gelatin zymography and semiquantitative RT-PCR at days 0, 1, 3, 5, and 7 postinfection using conditioned media from uninfected and JRCSF-infected human macrophages (A, B) and uninfected and V1CSF-infected feline macrophages (C, D). Infection with either virus induced concurrent increases in MMP-2 and -9 protein and mRNA expression that increased with viral replication. Viral replication was measured by RT-PCR amplification of the HIV-1 gag gene or the FIV pol gene using RNA from human and feline PBMC infected with HIV or FIV as positive controls (+). Conditioned medium and RNA from stimulated human macrophages served as controls for detection of MMP expression (+). Amplification of GAPDH was used to ensure equal template loading.
Properties and replication of lentivirus envelope chimeras.
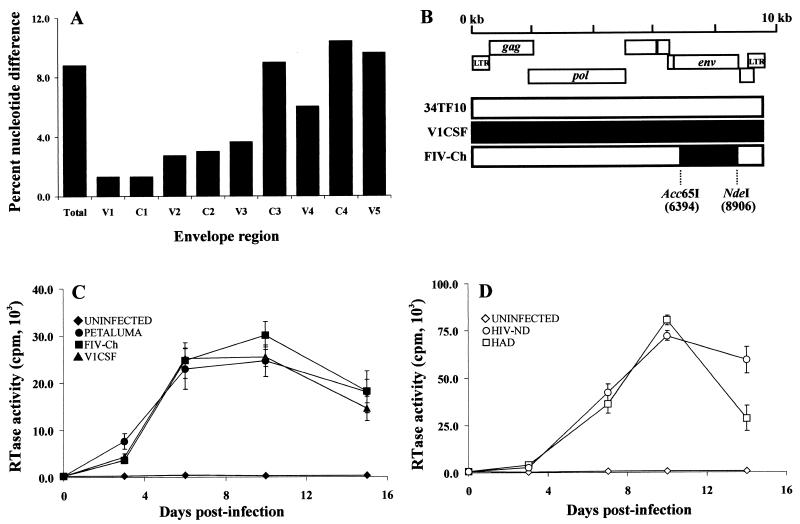
Sequence diversity in lentiviral envelope genes has been shown to influence the expression of potential neurotoxins (11, 56). Comparison of the V1CSF and Petaluma envelope surface unit (gp100) sequences revealed diversity ranging from 2 to 10%, depending on the individual domain, with the sequences spanning the C3 to V5 regions exhibiting the greatest diversity (Fig. 3A). To assess the role of envelope variability in MMP expression, an FIV chimera (FIV-Ch) was constructed by cloning envelope sequences from V1CSF into a genetic background based on a molecular clone (pFIV-34TF10) of the less neurovirulent FIV strain, Petaluma (Fig. 3B). The FIV chimera was found to replicate in feline PBMC as efficiently as the V1CSF and Petaluma parent viruses (Fig. 3C), but unlike the infectious 34TF10 clone, neither the chimera nor V1CSF replicated in CrFK cells (data not shown). Similarly, we investigated HIV chimeras that contained envelope (C2V3) sequences derived from the brains of demented (HAD) and nondemented (HIV-ND) HIV-infected patients in a T-cell-tropic HIV-1 molecular clone (pNL4-3) background. These clones, which had been previously shown to be macrophage tropic (56), differ in the extent to which they induced neuronal injury (56) and possess envelope sequence diversity analogous to that observed between V1CSF and Petaluma (54). Both HAD and HIV-ND chimeras replicated with equal efficiency in primary human PBMC cultures (Fig. 3D).
FIG. 3.
Sequence and tropism of FIV. (A) Comparison of the V1CSF and Petaluma envelope sequences, revealing diversity ranging from 2 to 10%, depending on the individual domain. (B) The FIV envelope gene (env) encoding both the surface unit and transmembrane proteins was removed from the FIV-34TF10 molecular clone to form an env-deleted background vector. The equivalent surface unit-transmembrane region from the V1CSF FIV strain, amplified and cloned from genomic DNA isolated from V1CSF-infected feline PBMC, was inserted into the 34TF10 env-deleted vector. Resulting chimeras were transfected into CrFK cells, and infectious virus was harvested following coculture with primary feline PBMC. (C, D) RT activity in culture supernatants from FIV-infected feline (C) and HIV-infected human (D) PBMC at days 3, 7, 10, and 14 postinfection. The parent FIV strains, V1CSF or Petaluma, and the FIV env chimera replicated with equal efficiency following infection of feline PBMC. Human cells were infected with HIV-1 clones expressing brain-derived HAD (n = 4) and HIV-ND (n = 4) env sequences. Mean RT activity for each group is shown and did not differ between groups. Uninfected feline and human macrophages served as controls. Data represent the mean ± standard deviation of two experiments.
MMP and STAT/JAK protein expression is increased following infection with lentiviruses expressing envelope sequences associated with neurological disease.
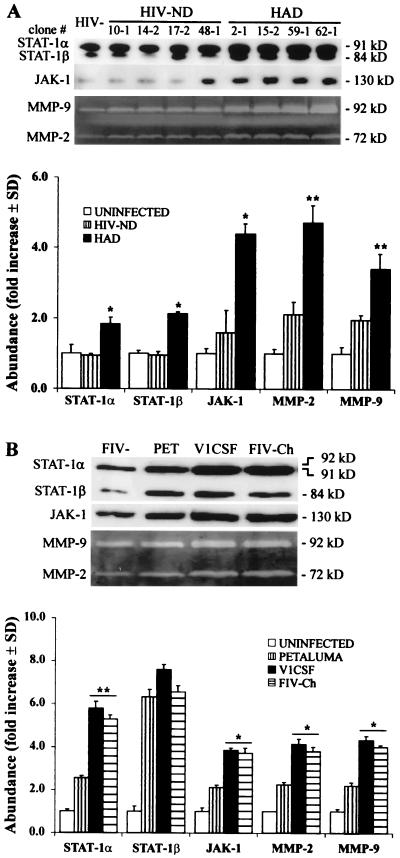
To determine if sequence diversity in the lentivirus envelope gene could account for the differences observed in host cell gene expression, human and feline macrophages were infected with the HIV and FIV envelope chimeras. STAT-1α (P < 0.002), STAT-1β (P < 0.01), and JAK-1 (P < 0.01) expression was increased in human macrophages infected with HAD HIV chimeras compared to uninfected cultures and macrophages infected with HIV-ND clones (Fig. 4A). In contrast, expression of these proteins in macrophages infected with HIV-ND clones did not differ significantly from uninfected cultures. Although MMP expression was increased in the HIV-ND-infected cells compared to that in the uninfected controls (P < 0.05), MMP-2 and -9 levels were significantly greater in conditioned media from human macrophages infected with HAD clones compared to those in HIV-ND-infected cultures (Fig. 4A).
FIG. 4.
env sequences from neurovirulent HIV-1 (A) and FIV (B) strains induce STAT/JAK and MMP expression. (A) STAT-1 and JAK-1 expression was increased in primary human macrophages infected with HAD HIV-1 clones compared to HIV-ND-infected and uninfected cells. Similarly, MMP-2 and -9 protein levels in conditioned media from human macrophages were significantly greater following infection with HAD HIV strains compared to those in HIV-ND clones. (B) Infection of feline macrophages with V1CSF, Petaluma, or chimeric (FIV-Ch) FIV strains revealed that STAT-1α and JAK-1 levels were higher in macrophages infected by V1CSF and FIV-Ch compared to Petaluma-infected and uninfected cells. MMP-2 and -9 levels were also significantly higher in conditioned media from feline macrophages infected with V1CSF or FIV-Ch. Data are expressed as fold increases over uninfected controls and represent the mean ± standard deviation of three experiments. Significant differences between neurovirulent and nonneurovirulent viruses are indicated (Tukey-Kramer test; ∗, P < 0.01; ∗∗, P < 0.001).
Western blot analysis revealed increased expression of STAT-1α (P < 0.001) and JAK-1 (P < 0.01) in feline macrophages infected with either FIV strain relative to that in uninfected controls, but STAT-1α and JAK-1 levels were significantly greater in cultures infected with V1CSF compared to those in Petaluma-infected macrophages (Fig. 4B). The FIV chimera (FIV-Ch) containing the V1CSF envelope induced STAT-1α and JAK-1 expression following infection of feline macrophages to the same extent as V1CSF, exceeding the levels induced by the less neurovirulent Petaluma strain that constituted the genetic background of the chimera (Fig. 4B). Like HIV, FIV infection increased STAT-1β expression (P < 0.001), but no difference was observed between viral strains. Conditioned media from feline macrophages infected with any of the FIV strains showed higher MMP levels compared to uninfected cultures (Fig. 4B), but levels of both MMP-2 and -9 were significantly greater in macrophages infected with V1CSF than those in Petaluma-infected cells. Similarly, feline macrophages infected with the FIV chimera exhibited MMP-2 and -9 protein levels higher than those in Petaluma-infected cultures (Fig. 4B). These results demonstrated that lentiviral strains associated with neurological disease concurrently induced higher levels of MMP and STAT/JAK expression than nonneurovirulent strains and implicated the lentiviral envelope as a determinant in this phenomenon.
MMP-2 expression is regulated by the STAT/JAK signaling pathway.
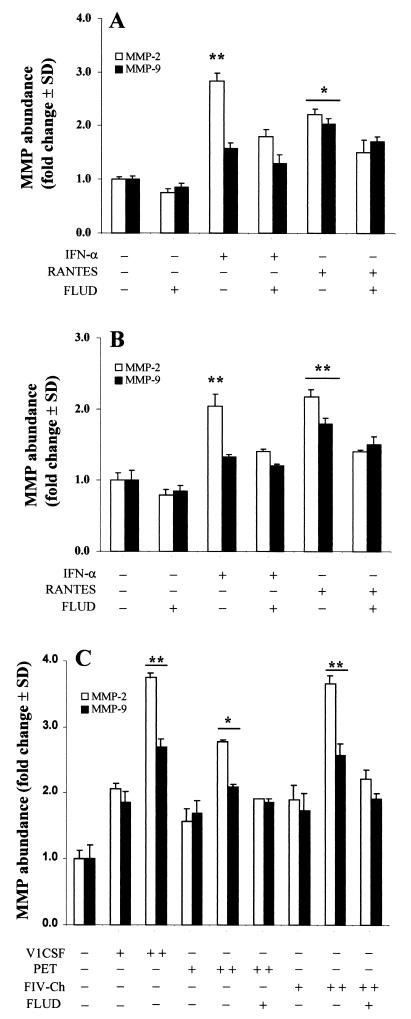
Since STAT-1 and JAK-1 levels were elevated in conjunction with MMP-2 and -9, following infection of macrophages with neurovirulent strains of HIV and FIV, we investigated MMP expression in the context of the STAT/JAK signaling pathway. Treatment with IFN-α, which is known to induce STAT-1 (1, 12), increased MMP-2 expression in both human U937 monocytes (P < 0.02) (Fig. 5A) and primary feline macrophages (P < 0.01) (Fig. 5B). This effect was partially attenuated by incubation with the STAT-1 inhibitor, fludarabine (15), which decreased MMP-2 levels by 40 and 31% in IFN-treated human and feline macrophage cultures, respectively. Although MMP-9 expression was also increased by IFN-α, it was comparatively lower than MMP-2 and was not significantly affected by fludarabine treatment. Similarly, treatment of human (Fig. 5A) and feline (Fig. 5B) macrophages with RANTES, a β-chemokine receptor ligand that has also been shown to activate STAT-1 (10), increased MMP-2 and -9 expression in both U937 cells (Fig. 5A) and feline macrophages (Fig. 5B). As with IFN-α, fludarabine partially inhibited MMP-2 expression in cultures treated with RANTES (approximately 35% in both cell lines), but did not significantly decrease MMP-9 levels.
FIG. 5.
Regulation of MMP-2 and -9 levels in human U937 monocytoid cells (A) and primary feline macrophages (B, C) by the STAT/JAK signaling pathway. Uninfected U937 (A) or feline macrophage (B) cultures were incubated in the presence (+) or absence (−) of IFN-α (100 IU/ml), RANTES (50 ng/ml), or fludarabine (FLUD; 50 μM) for 24 h. IFN-α and RANTES significantly increased MMP-2 and -9 expression in both human (A) and feline (B) cultures compared to those in untreated controls. Fludarabine partially attenuated this effect. (C) Conditioned media from feline macrophages, infected with V1CSF, Petaluma (Pet), or chimeric (FIV-Ch) FIV strains at titers of 102 TCID50/106 cells (+) or 103.5 TCID50/106 cells (++), exhibited increased MMP-2 and -9 levels. MMP levels were dependent on the viral strain, with V1CSF and FIV-Ch showing higher levels than those in Petaluma or uninfected controls at day 3 postinfection. Parallel cultures infected with Pet or FIV-Ch viruses, incubated with fludarabine for 24 h, disclosed significant reductions in MMP levels, especially MMP-2. Data are expressed as fold increases over uninfected controls and represent the mean ± standard deviation of three experiments. Significant differences relative to control cultures are indicated (Tukey-Kramer test; ∗, P < 0.05; ∗∗, P < 0.01).
To determine the extent to which STAT-1 and JAK-1 participated in the increased MMP expression observed following lentiviral infection, primary feline macrophages were infected with V1CSF, Petaluma, or chimeric FIV strains. As shown in Fig. 4, all three viruses induced MMP-2 and -9 expression that was dependent on viral titer, although MMP expression was greater in cultures infected with V1CSF and the FIV chimera-expressing V1CSF envelope sequences than in cultures infected with Petaluma (Fig. 5C). MMP-2 expression in macrophages infected with either chimeric FIV or Petaluma was significantly decreased by fludarabine treatment; however, a greater degree of inhibition was observed with the chimera (42%) than with Petaluma (25%). As with cytokine-treated feline macrophage cultures, fludarabine did not significantly decrease MMP-9 levels in FIV-infected cultures or completely abrogate MMP-2 expression. Taken together, these results suggested that increased MMP expression following lentivirus infection was modulated, in part, by the STAT/JAK signaling pathway.
Lentivirus infection increases the expression of MMPs and STAT/JAK proteins in brain.
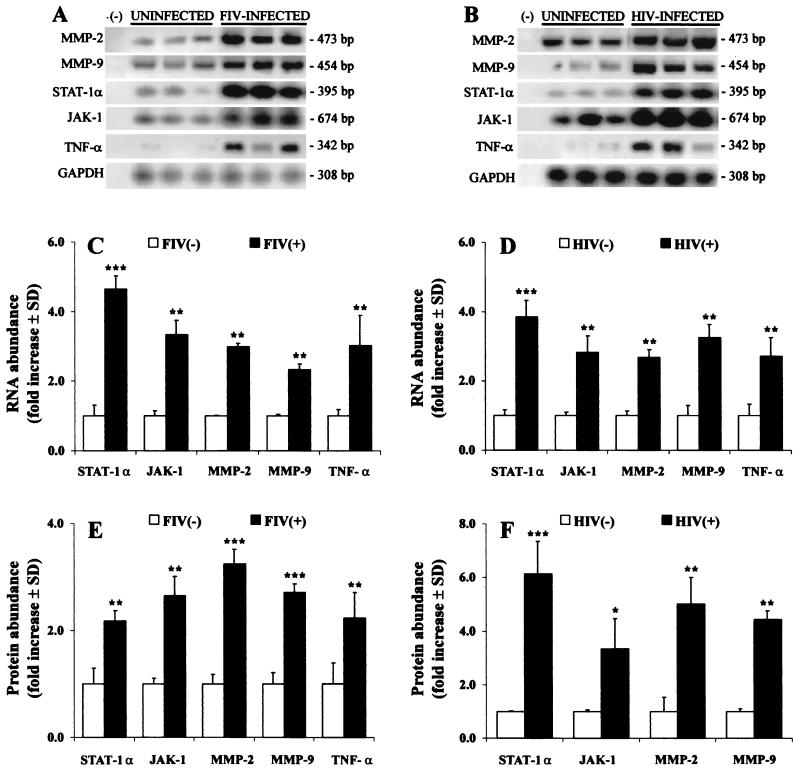
To establish that the above findings reflected in vivo events in the brain, we assessed MMP and STAT-JAK expression in brain tissue from HIV-infected patients, FIV-infected felines, and uninfected feline and human controls. RT-PCR analysis revealed that MMP-2 and -9, STAT-1α, and JAK-1 mRNA (Fig. 6A and C) and protein (Fig. 6E) levels were increased in FIV-infected felines (P < 0.005) compared to those in controls. Similarly, MMP-2 and -9 mRNA levels were significantly elevated in HIV-infected human brain tissue relative to those in controls, concurrent with increased STAT-1 and JAK-1 mRNA levels (Fig. 6B and D). HIV-infected brains also exhibited increased MMP-2 and -9, STAT-1α, and JAK-1 protein levels compared to those in the uninfected controls (Fig. 6F). To confirm that increased STAT-1 abundance was associated with activation of the signaling molecule, immunoblot detection of phosphorylated STAT-1α (92 kDa) was performed using feline brain tissue; the level of phosphorylated STAT-1α was increased in FIV-infected brain tissue, compared to that in uninfected controls (data not shown). Expression of TNF-α was also elevated in both HIV- and FIV-infected brain tissues (P < 0.01). Taken together, these results indicated that lentivirus infection of the CNS increased the expression of both MMPs and host molecules associated with their regulation.
FIG. 6.
Representative MMP, TNF-α, and STAT/JAK mRNA and protein levels in brain tissue from HIV-infected persons (A, B) or FIV-infected felines (C, D) with uninfected controls. RT-PCR showed increased MMP-2 and -9, STAT-1, JAK-1, and TNF-α mRNA levels in FIV-infected feline (n = 4) (A, C) and HIV-infected human (n = 5) (B, D) brain tissue compared to brain tissue from uninfected felines (n = 4) and human controls (n = 6). Amplification of GAPDH was used to ensure equal template loading. Gelatin zymography and Western blot analysis revealed concurrent increases in STAT-1α, JAK-1, MMP-2, and MMP-9 in FIV-infected (E) and HIV-infected (F) brains compared to uninfected controls. In contrast to macrophages, only the STAT-1α monomer was detected in brain tissue. Data are expressed as fold increases over uninfected controls and represent the mean ± standard deviation of three experiments. Significant differences relative to uninfected controls are indicated (Tukey-Kramer test; ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
DISCUSSION
The aim of this study was to investigate the relationship between lentiviral envelope diversity and the expression of several host molecules with potential roles in lentivirus neuropathogenesis. Using infectious molecular clones of HIV and FIV, we have shown that differences in the envelope sequences influence the extent to which the STAT/JAK signaling pathway is induced following lentivirus infection. Furthermore, elevated STAT-1 and JAK-1 levels were accompanied by concomitant increases in MMP-2 and -9 expression. Cytokine and virus-induced MMP-2 production in primary macrophages was attenuated by the STAT-1 inhibitor fludarabine, suggesting a role for this transcription factor in regulating MMP expression. These in vitro findings were supported by similar in vivo findings of increased MMP and STAT/JAK mRNA and protein levels in lentivirus-infected brain tissue. Thus, increased MMP expression, modulated by the STAT/JAK signaling pathway, is a property exhibited by at least two lentiviruses that cause neurological disease.
Although independent studies have reported that HIV infection is associated with changes in MMP levels (9, 63) and STAT/JAK (47), a relationship between MMP expression and the STAT/JAK signaling pathway has not been previously demonstrated in the context of lentivirus infection. However, other retroviruses that cause CNS disease have been shown to upregulate expression of these molecules. For example, infection with human T-lymphotrophic virus type 1 is associated with increased levels of MMP-3 and -9 (18), as well as elevated STAT-1 and -5 expression and activation (39, 41, 68). Because cytokines extensively regulate MMP transcription (40) and STATs are fundamental to cytokine receptor signal transduction (12), it is plausible that the STAT/JAK signaling pathway could play a role in MMP expression. In support of this concept, we found that the STAT-1 inhibitor, fludarabine, attenuated the increased MMP-2 expression detected in macrophages following lentivirus infection or treatment with cytokines known to activate the STAT/JAK signaling pathway. Although increased expression of STAT/JAK proteins has been associated with increased cell signaling activity (31), activation of STATs requires tyrosine phosphorylation through the upstream activity of tyrosine kinases, such as JAKs. In keeping with this requirement, we observed increased JAK-1 expression in lentivirus-infected macrophages and brain and increased abundance of the phosphorylated form of STAT-1. Taken together, these findings suggest that infection by both HIV and FIV is accompanied by increased STAT-1 activity that results in increased MMP expression.
In this study, we have focused on STAT-1 because it was previously shown to be activated following HIV infection (5) and has been implicated in HIV gp120-induced gene expression in microglia (62). However, the failure of fludarabine to significantly affect MMP-9 levels, or to completely inhibit MMP-2 expression, suggests that other transcription factors also mediate lentivirus-induced MMP expression. The cellular responses elicited by lentiviral envelope proteins are highly diverse and not limited to a single signaling pathway. For example, both NF-κB and AP-1 are activated by HIV proteins (6, 51) and have also been shown to transcriptionally regulate MMP gene expression (2, 30, 74). In addition, the ability of fludarabine to inhibit STAT expression is STAT-1-specific; therefore, other STATs that are known to be activated by lentiviruses, such as STAT-3 (5), would not be affected by fludarabine treatment. It is also conceivable that STAT-1 functions in cooperation with other signaling molecules to regulate MMP expression, possibly acting as a modulating factor to enhance the effect of other mediators of MMP transcription. For example, IFNs and TNF-α cooperate to induce the expression of many gene products during inflammation, a portion of which is mediated by synergism between the transcription factors, STAT-1 and NF-κB (43). Similarly, regulation of MMP-1 expression by oncostatin M requires the activation and cooperation of both the mitogen-activated protein kinase and STAT/JAK signaling pathways to achieve maximal transcriptional activity (27).
Previous reports have demonstrated that the HIV gp120 envelope protein alone was sufficient to induce both STAT-1 (62) and MMP-2 expression (34). Since gp120 has not been shown to directly transactivate gene expression, this process is likely receptor mediated. A common feature of lentivirus infection is the use of chemokine receptors as coreceptors for cell entry (72). Recently, ligand binding of both CC and CXC chemokine receptors has been shown to activate multiple-signal transduction pathways, including the STAT/JAK pathway (73). Furthermore, signaling through the chemokine receptors induces MMP expression (10) and initiates a cascade of events culminating in neuronal injury (69, 76, 77). Thus, interaction between chemokine receptors and viral envelope proteins resulting in activation of intracellular transcription factors, such as STATs, represents a plausible mechanism by which lentiviruses upregulate MMP expression. This concept is supported by our finding that RANTES, a β-chemokine known to interact with several CC receptors, including CCR1, CCR3, and CCR5, induced MMP-2 expression in primary human and feline macrophages through a mechanism that was partially attenuated by inhibition of STAT-1.
The profile of MMP expression following HIV infection of the CNS has been shown to vary with the clinical status of the patient (9). Our findings that MMP and STAT-JAK expression was greater following infection with HIV and FIV clones expressing neurovirulent envelope sequences compared to less neurovirulent sequences implicate envelope diversity in this phenomenon. Previously, differences in envelope sequences have been shown to influence ligand-receptor interactions and modulate downstream signaling pathways, including those mediated by CD4 and chemokine receptors (44, 76). Furthermore, the induction of potential neurotoxins, such as quinolinic acid (11), nitric oxide (26), and TNF-α (24), by different HIV-1 strains has been shown to depend on envelope sequences, including the V3 hypervariable region. Since distinct HIV envelope sequences are associated with the clinical expression of HIV dementia (29, 55), envelope-dependent induction of potential toxins, such as MMPs, may influence disease development.
Although the events in the neurodegenerative cascade induced by lentiviruses are uncertain, an increase in the number of activated macrophages in the brain is considered to play a critical role (15, 25, 37). As has been shown in simian immunodeficiency virus infection, activated macrophages represent the most likely source of the increased MMP expression detected in infected brain (3). Similarly, we have observed increased STAT-1 and MMP immunoreactivity in cells resembling microglia and macrophages in immunocytochemical studies of HIV- and FIV-infected brains (data not shown). Increased BBB permeability is associated with the development of HAD (53) and is one of several mechanisms proposed to account for this influx of monocyte-derived cells (9, 34, 63). This is supported by studies demonstrating that collagen type IV, a primary constituent of basal membranes in the BBB, is reduced in HIV-infected brain (7). Type IV collagen is also a substrate of MMP-2 and -9; therefore, our finding that expression of these enzymes was elevated in HIV- and FIV-infected brain and in association with neurovirulent lentiviruses is consistent with this mechanism. Alternatively, MMPs produced in the brain may act directly to alter neuronal function, development, and survival, as suggested in other neurological diseases (59, 75). For example, MMP-2 modulates chloride current (13) and hence may influence excitotoxicity caused by neurotransmitters, such as a glutamate, or other macrophage-derived molecules, which have been implicated in lentivirus neuropathogenesis (11, 21). In addition, degradation of laminin, a substrate for MMP-2 and -9, has been shown to result in neuronal death (8).
In this study, we present evidence that upregulation of MMP and STAT/JAK expression is a potential mechanism in the neuropathogenesis of both feline and primate lentiviruses, supporting the concept that evolutionarily distinct lentiviruses retain conserved mechanisms of infection and disease induction. It is likely that MMPs produced by macrophages other than MMP-2 and -9, such as MMP-7 and -12 (75), participate in the cascade of cellular events that causes neurodegeneration by acting on their respective substrates and modulating the activity of other toxic molecules (40). Characterization of these MMPs and the processes by which they are regulated will be the focus of future studies.
ACKNOWLEDGMENTS
We thank V. W. Yong and B. Chesebro for helpful discussions.
These studies were supported by AHFMR and MRC. J.B.J. is a recipient of an MHRC studentship. C.P. is a recipient of an MRC scholarship.
REFERENCES
- 1.Beadling C, Guschin D, Witthuhn B A, Ziemiecki A, Ihle J N, Kerr I M, Cantrell D A. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13:5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benbow U, Brinckerhoff C E. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 3.Berman N E, Marcario J K, Yong C, Raghavan R, Raymond L A, Joag S V, Narayan O, Cheney P D. Microglial activation and neurological symptoms in the SIV model of neuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiol Dis. 1999;6:486–498. doi: 10.1006/nbdi.1999.0261. [DOI] [PubMed] [Google Scholar]
- 4.Borden P, Heller R A. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997;7:159–178. doi: 10.1615/critreveukargeneexpr.v7.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 5.Bovolenta C, Camorali L, Lorini A L, Ghezzi S, Vicenzi E, Lazzarin A, Poli G. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood. 1999;94:4202–4209. [PubMed] [Google Scholar]
- 6.Briant L, Coudronniere N, Robert-Hebmann V, Benkirane M, Devaux C. Binding of HIV-1 virions or gp120-anti-gp120 immune complexes to HIV-1-infected quiescent peripheral blood mononuclear cells reveals latent infection. J Immunol. 1996;156:3994–4004. [PubMed] [Google Scholar]
- 7.Buttner A, Mehraein P, Weis S. Vascular changes in the cerebral cortex in HIV-1 infection. II. An immunohistochemical and lectinhistochemical investigation. Acta Neuropathol. 1996;92:35–41. doi: 10.1007/s004010050486. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z L, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 9.Conant K, McArthur J C, Griffin D E, Sjulson L, Wahl L M, Irani D N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Cross A K, Woodroofe M N. Chemokine modulation of matrix metalloproteinase and TIMP production in adult rat brain microglia and a human microglial cell line in vitro. Glia. 1999;28:183–189. [PubMed] [Google Scholar]
- 11.Cunningham A L, Naif H, Saksena N, Lynch G, Chang J, Li S, Jozwiak R, Alali M, Wang B, Fear W, Sloane A, Pemberton L, Brew B. HIV infection of macrophages and pathogenesis of AIDS dementia complex: interaction of the host cell and viral genotype. J Leukoc Biol. 1997;62:117–125. doi: 10.1002/jlb.62.1.117. [DOI] [PubMed] [Google Scholar]
- 12.Darnell J E, Jr, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 13.Duszyk M, Shu Y, Sawicki G, Radomski A, Man S F, Radomski M W. Inhibition of matrix metalloproteinase MMP-2 activates chloride current in human airway epithelial cells. Can J Physiol Pharmacol. 1999;77:529–535. [PubMed] [Google Scholar]
- 14.Eilbott D J, Peress N, Burger H, LaNeve D, Orenstein J, Gendelman H E, Seidman R, Weiser B. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc Natl Acad Sci USA. 1989;86:3337–3341. doi: 10.1073/pnas.86.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein L G, Gendelman H E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 16.Frank D A, Mahajan S, Ritz J. Fludarabine-induced immunosuppression is associated with inhibition of STAT1 signaling. Nat Med. 1999;5:444–447. doi: 10.1038/7445. [DOI] [PubMed] [Google Scholar]
- 17.Georgsson G. Neuropathologic aspects of lentiviral infections. Ann N Y Acad Sci. 1994;724:50–67. doi: 10.1111/j.1749-6632.1994.tb38895.x. [DOI] [PubMed] [Google Scholar]
- 18.Giraudon P, Buart S, Bernard A, Belin M F. Cytokines secreted by glial cells infected with HTLV-I modulate the expression of matrix metalloproteinases (MMPs) and their natural inhibitor (TIMPs): possible involvement in neurodegenerative processes. Mol Psychiatry. 1997;2:107–110. doi: 10.1038/sj.mp.4000218. [DOI] [PubMed] [Google Scholar]
- 19.Giulian D, Vaca K, Noonan C A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 20.Goetzl E J, Banda M J, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- 21.Gruol D L, Yu N, Parsons K L, Billaud J N, Elder J H, Phillips T R. Neurotoxic effects of feline immunodeficiency virus, FIV-PPR. J Neurovirol. 1998;4:415–425. doi: 10.3109/13550289809114540. [DOI] [PubMed] [Google Scholar]
- 22.Henriksen S J, Prospero-Garcia O, Phillips T R, Fox H S, Bloom F E, Elder J H. Feline immunodeficiency virus as a model for study of lentivirus infection of the central nervous system. Curr Top Microbiol Immunol. 1995;202:167–186. doi: 10.1007/978-3-642-79657-9_12. [DOI] [PubMed] [Google Scholar]
- 23.Johnston J, Power C. Productive infection of human peripheral blood mononuclear cells by feline immunodeficiency virus: implications for vector development. J Virol. 1999;73:2491–2498. doi: 10.1128/jvi.73.3.2491-2498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna K V, Yu X F, Ford D H, Ratner L, Hildreth J K, Markham R B. Differences among HIV-1 variants in their ability to elicit secretion of TNF-alpha. J Immunol. 2000;164:1408–1415. doi: 10.4049/jimmunol.164.3.1408. [DOI] [PubMed] [Google Scholar]
- 25.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 26.Kong L Y, Wilson B C, McMillian M K, Bing G, Hudson P M, Hong J S. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 1996;172:77–83. doi: 10.1006/cimm.1996.0217. [DOI] [PubMed] [Google Scholar]
- 27.Korzus E, Nagase H, Rydell R, Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J Biol Chem. 1997;272:1188–1196. doi: 10.1074/jbc.272.2.1188. [DOI] [PubMed] [Google Scholar]
- 28.Krivine A, Force G, Servan J, Cabee A, Rozenberg F, Dighiero L, Marguet F, Lebon P. Measuring HIV-1 RNA and interferon-alpha in the cerebrospinal fluid of AIDS patients: insights into the pathogenesis of AIDS dementia complex. J Neurovirol. 1999;5:500–506. doi: 10.3109/13550289909045379. [DOI] [PubMed] [Google Scholar]
- 29.Kuiken C L, Goudsmit J, Weiller G F, Armstrong J S, Hartman S, Portegies P, Dekker J, Cornelissen M. Differences in human immunodeficiency virus type 1 V3 sequences from patients with and without AIDS dementia complex. J Gen Virol. 1995;76:175–180. doi: 10.1099/0022-1317-76-1-175. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Dhawan S, Mukhopadhyay A, Aggarwal B B. Human immunodeficiency virus-1-tat induces matrix metalloproteinase-9 in monocytes through protein tyrosine phosphatase-mediated activation of nuclear transcription factor NF-kappaB. FEBS Lett. 1999;462:140–144. doi: 10.1016/s0014-5793(99)01487-8. [DOI] [PubMed] [Google Scholar]
- 31.Lehtonen A, Matikainen S, Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol. 1997;159:794–803. [PubMed] [Google Scholar]
- 32.Lipton S A. Neuronal injury associated with HIV-1 and potential treatment with calcium-channel and NMDA antagonists. Dev Neurosci. 1994;16:145–151. doi: 10.1159/000112101. [DOI] [PubMed] [Google Scholar]
- 33.Mankowski J L, Flaherty M T, Spelman J P, Hauer D A, Didier P J, Amedee A M, Murphey-Corb M, Kirstein L M, Munoz A, Clements J E, Zink M C. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall D C, Wyss-Coray T, Abraham C R. Induction of matrix metalloproteinase-2 in human immunodeficiency virus-1 glycoprotein 120 transgenic mouse brains. Neurosci Lett. 1998;254:97–100. doi: 10.1016/s0304-3940(98)00674-0. [DOI] [PubMed] [Google Scholar]
- 35.Mayne M, Shepel P N, Jiang Y, Geiger J D, Power C. Dysregulation of adenosine A1 receptor-mediated cytokine expression in peripheral blood mononuclear cells from multiple sclerosis patients. Ann Neurol. 1999;45:633–639. doi: 10.1002/1531-8249(199905)45:5<633::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 36.McArthur J C. Neurologic manifestations of AIDS. Medicine (Baltimore) 1987;66:407–437. doi: 10.1097/00005792-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Merrill J E, Chen I S. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- 38.Meucci O, Fatatis A, Simen A A, Bushell T J, Gray P W, Miller R J. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migone T S, Lin J X, Cereseto A, Mulloy J C, O'Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 40.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 41.Nakamura N, Fujii M, Tsukahara T, Arai M, Ohashi T, Wakao H, Kannagi M, Yamamoto N. Human T-cell leukemia virus type 1 Tax protein induces the expression of STAT1 and STAT5 genes in T-cells. Oncogene. 1999;18:2667–2675. doi: 10.1038/sj.onc.1202608. [DOI] [PubMed] [Google Scholar]
- 42.Oh L Y, Larsen P H, Krekoski C A, Edwards D R, Donovan F, Werb Z, Yong V W. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohmori Y, Schreiber R D, Hamilton T A. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 44.Pappas T C, Alagarsamy S, Pollard R B, Nokta M. HIV decreases glutamate transport in SK-N-MC neuroblastoma cells. J Neurovirol. 1998;4:69–79. doi: 10.3109/13550289809113483. [DOI] [PubMed] [Google Scholar]
- 45.Paquette Y, Hanna Z, Savard P, Brousseau R, Robitaille Y, Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci USA. 1989;86:3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedersen C, Thomsen C, Arlien-Soborg P, Praestholm J, Kjaer L, Boesen F, Hansen H S, Nielsen J O. Central nervous system involvement in human immunodeficiency virus disease. A prospective study including neurological examination, computerized tomography, and magnetic resonance imaging. Dan Med Bull. 1991;38:374–379. [PubMed] [Google Scholar]
- 47.Pericle F, Pinto L A, Hicks S, Kirken R A, Sconocchia G, Rusnak J, Dolan M J, Shearer G M, Segal D M. HIV-1 infection induces a selective reduction in STAT5 protein expression. J Immunol. 1998;160:28–31. [PubMed] [Google Scholar]
- 48.Phillips D M, Tan X, Perotti M E, Zacharopoulos V R. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res Hum Retrovir. 1998;14(Suppl. 1):S67–S70. [PubMed] [Google Scholar]
- 49.Phillips T R, Prospero-Garcia O, Puaoi D L, Lerner D L, Fox H S, Olmsted R A, Bloom F E, Henriksen S J, Elder J H. Neurological abnormalities associated with feline immunodeficiency virus infection. J Gen Virol. 1994;75:979–987. doi: 10.1099/0022-1317-75-5-979. [DOI] [PubMed] [Google Scholar]
- 50.Poli A, Pistello M, Carli M A, Abramo F, Mancuso G, Nicoletti E, Bendinelli M. Tumor necrosis factor-alpha and virus expression in the central nervous system of cats infected with feline immunodeficiency virus. J Neurovirol. 1999;5:465–473. doi: 10.3109/13550289909045375. [DOI] [PubMed] [Google Scholar]
- 51.Popik W, Hesselgesser J E, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Power C, Buist R, Johnston J B, Del Bigio M R, Ni W, Dawood M R, Peeling J. Neurovirulence in feline immunodeficiency virus-infected neonatal cats is viral strain specific and dependent on systemic immune suppression. J Virol. 1998;72:9109–9115. doi: 10.1128/jvi.72.11.9109-9115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Power C, Kong P A, Crawford T O, Wesselingh S, Glass J D, McArthur J C, Trapp B D. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34:339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- 54.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Dewey R, Chesebro B. Distinct HIV-1 env sequences are associated with neurotropism and neurovirulence. Curr Top Microbiol Immunol. 1995;202:89–104. doi: 10.1007/978-3-642-79657-9_7. [DOI] [PubMed] [Google Scholar]
- 55.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power C, McArthur J C, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson R T, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rho M B, Wesselingh S, Glass J D, McArthur J C, Choi S, Griffin J, Tyor W R. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Frade J M, Vila-Coro A J, Martin A, Nieto M, Sanchez-Madrid F, Proudfoot A E, Wells T N, Martinez A C, Mellado M. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J Cell Biol. 1999;144:755–765. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg G A. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 60.Sasseville V G, Smith M M, Mackay C R, Pauley D R, Mansfield K G, Ringler D J, Lackner A A. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- 61.Sears J F, Repaske R, Khan A S. Improved Mg2+-based reverse transcriptase assay for detection of primate retroviruses. J Clin Microbiol. 1999;37:1704–1708. doi: 10.1128/jcm.37.6.1704-1708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrikant P, Benos D J, Tang L P, Benveniste E N. HIV glycoprotein 120 enhances intercellular adhesion molecule-1 gene expression in glial cells. Involvement of Janus kinase/signal transducer and activator of transcription and protein kinase C signaling pathways. J Immunol. 1996;156:1307–1314. [PubMed] [Google Scholar]
- 63.Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel F D, Pfister H W. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- 64.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 65.Strizki J M, Albright A V, Sheng H, O'Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stuve O, Chabot S, Jung S S, Williams G, Yong V W. Chemokine-enhanced migration of human peripheral blood mononuclear cells is antagonized by interferon beta-1b through an effect on matrix metalloproteinase-9. J Neuroimmunol. 1997;80:38–46. doi: 10.1016/s0165-5728(97)00134-3. [DOI] [PubMed] [Google Scholar]
- 67.Szurek P F, Yuen P H, Ball J K, Wong P K. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990;64:467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takemoto S, Mulloy J C, Cereseto A, Migone T S, Patel B K, Matsuoka M, Yamaguchi K, Takatsuki K, Kamihira S, White J D, Leonard W J, Waldmann T, Franchini G. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vila-Coro A J, Rodriguez-Frade J M, Martin De Ana A, Moreno-Ortiz M C, Martinez A C, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 70.Vitkovic L, Stover E, Koslow S H. Animal models recapitulate aspects of HIV/CNS disease. AIDS Res Hum Retrovir. 1995;11:753–759. doi: 10.1089/aid.1995.11.753. [DOI] [PubMed] [Google Scholar]
- 71.Wesselingh S L, Power C, Glass J D, Tyor W R, McArthur J C, Farber J M, Griffin J W, Griffin D E. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- 72.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong M, Fish E N. RANTES and MIP-1alpha activate stats in T cells. J Biol Chem. 1998;273:309–314. doi: 10.1074/jbc.273.1.309. [DOI] [PubMed] [Google Scholar]
- 74.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 75.Yong V W, Krekoski C A, Forsyth P A, Bell R, Edwards D R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 76.Zheng J, Ghorpade A, Niemann D, Cotter R L, Thylin M R, Epstein L, Swartz J M, Shepard R B, Liu X, Nukuna A, Gendelman H E. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73:8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng J, Thylin M R, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y C, Gelbard H A, Shepard R B, Swartz J M, Gendelman H E. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]