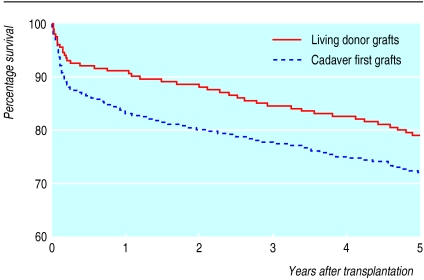
Since the first successful transplant of a kidney from one twin to another in 1954, renal transplantation has moved from being at the cutting edge to being a mature technology. Registry data show that the current survival rates for grafts from cadavers are around 88% and 60% at one and 10 years after transplantation, respectively, while comparable rates for grafts from living donors are in excess of 95% and 70% (fig 1).1,3 These rates have shown steady improvements over the past 10 years, with the one year survival rates for grafts from cadavers and living donors improving by around 5% in that time.1,3 One year patient survival after transplantation is steady at 95%, with 87% alive at five years. In the medium term (one to three years) after transplantation, clinical outcomes are now so good that it is difficult to improve the survival of the patients or the grafts. Rejection rates have fallen over the years, and rejection is now an uncommon cause of early loss of a graft.
Figure 1.
Kaplan-Meier analysis of survival of cadaveric and living donor grafts in renal transplants in the United Kingdom. Data reproduced by permission of UK Transplant1,2
Major issues that now need to be resolved include the inadequate supply of donor organs, the side effects of treatment, the epidemic of cardiovascular disease in patients who have received renal transplants, and equity of access to transplantation. This paper discusses the recent progress in the field of renal transplantation and considers what work is needed to tackle the current issues facing transplant specialists.
Recent developments
The outcome of renal transplantation has steadily improved—survival one year after transplantation is >88% for cadaveric grafts and 95% for grafts from living donors
Renal transplantation improves survival in all age groups and for all underlying renal pathologies
Grafts from living related donors and patients' spouses and partners are being used more and more
Laparoscopic nephrectomy minimises morbidity in living renal donors
Interleukin 2 receptor antibodies reduce early rejection rates
Chronic allograft nephropathy may be reduced with mycophenolate mofetil
“Tailored immunosuppression” aims to minimise transplant related morbidity
Methods
Topics to include in this article were chosen by searching Medline for articles published between 1999 to 2001 with the keywords “kidney” and “transplant”, by discussing renal transplantation with clinical colleagues, and by reviewing articles in specialist journals and abstracts from conferences.
New approaches to increasing organ donation
In December 2001, 6101 patients were waiting for a renal transplant in the United Kingdom.1 Waiting lists continue to grow by about 3% per year.1 The reasons for this increase in recent years have been well documented,4 but two are particularly important. Improved road safety and a lack of neurosurgical facilities have led to fewer organ donors becoming available in recent years. During the same period, the incidence of renal failure in our ageing, more ethnically diverse population has increased, resulting in more patients being considered for transplantation.
Cadaver donation
Several steps have been taken to improve the rate of organ donation from cadavers. The first has been to improve the organisation of transplant coordinators. This step has drawn on a programme of changes made to the coordinator service in Spain, which involved heavy investment and gave outstanding results. The programme began in 1989 and included the appointment of a coordinator to every hospital; the result was an increase in the transplant rate to 33.6 per million population within 10 years. In contrast, the number of organ donors in the United Kingdom and Germany remained static, at 13 per million, over this period.5
A second initiative uses increasing amounts of investment in neurosurgical facilities. This might increase the numbers of potential organ donors to be admitted, and pilots will soon begin under the auspices of UK Transplant.
Launched in 1994, the NHS organ donor register is a third step that is intended to promote organ donation in the United Kingdom. This register allows individuals to register their willingness to be considered as an organ donor. To date, nine million people have registered, often when they renewed their passport or driving licence. However, the usefulness of the register has been questioned, and there is no evidence that shows that donation rates have improved since its launch.
Three systems of organ donation are used in current practice worldwide: “opting in,” “opting out,” and “required request” (box B1). In practice, in all three systems, the wishes of the potential donor's next of kin remain paramount.
Box 1.
Current systems of organ donation
- A voluntary system of organ donation in which the hospital's staff approach the potential donor's next of kin about organ donation, with no expectation of consent
- People are encouraged to register their willingness to donate organs, such as by carrying an organ donor card or registering on the NHS organ donor registry
- Potential organ donors are presumed to consent to organ donation, unless they have specifically registered their wish not to donate
- The hospital's staff approach the potential donor's next of kin about organ donation, expecting to receive consent
- In the United States, doctors in charge of potential donors have to get someone to speak to the family about organ donation, although there is no expectation that donation will occur as a result
The adoption of an opting out system as a means of increasing organ donation remains controversial. This option has little political support, despite being supported by the British Medical Association and by surveys that show that most people in the United Kingdom would favour a system of “presumed consent.” After Belgium introduced such a system in 1982, the numbers of organ donors increased considerably.6 Germany and Italy introduced similar legislation in 1997 and 2000, respectively. The opting out system will continue to be a subject for debate.
The use of “marginal donors” is also a subject of debate (box B2). Grafts from very old donors are associated with reduced functioning of the nephron mass, increased susceptibility to cold ischaemic injury, and impaired survival of grafts in the long term.7 Similarly, very young donors are associated with inadequate nephron mass and a higher incidence of technical failure compared with adults.8 Some doctors have advocated the use of dual organ transplantation from such donors, although such a policy would inevitably further reduce the total number of patients who could receive transplants.9,10 In practice, most units continue to broaden their definition of acceptable donors, but they ensure that potential recipients are aware of and accept the possibility that they may receive suboptimal organs.
Box 2.
Marginal donors
- Extremes of age (usually <14 years or >65 years)
- Prolonged cold or warm ischaemia
- Technical problems with organ retrieval (such as vascular injury)
- Diabetic donors
- Hypertensive donors (especially if subarachnoid haemorrhage)
- Donors with impaired renal function
- Donors with primary brain tumour
- Donors with prolonged hypotension or poor physiology before brain stem death
- Donors with primary renal disease
- Unfavourable results from pretransplant biopsy of the donor kidney
Living donation
Enthusiasm for transplants with kidneys from living donors varies, but the shortage of kidneys and the excellent survival rates for such grafts has driven the development of such programmes. In Norway, for example, 38% of transplants use kidneys from biologically or emotionally related donors; as a result, Norway is almost alone in seeing a reduction in the numbers of patients on its waiting list for transplants.11 In the United Kingdom only 20% of transplants use kidneys from living donors.1 This low number, and the need to establish standards for organ donation, led a joint working party of the British Transplantation Society and the Renal Association to publish guidelines for transplanting kidneys from living donors in January 2000.12
The better survival of grafts from living donors than grafts from cadavers reflects the high quality of the donor organs and the optimal circumstances under which they are retrieved. A genetic relationship means that the tissue match between the graft from a living donor and the recipient is often good. However, a good match is less important with a living donor, and many transplant units now promote transplants of kidneys between spouses and partners.1,3,12 Initiatives such as these mean that living donation is likely to be responsible for most of the increases in the numbers of available donor organs for the foreseeable future.
For living donors, nephrectomy is a painful and unnecessary procedure. The technique of laparoscopic nephrectomy has received much attention, with excellent results reported from several centres in the United States.13,14 Postoperative pain and inpatient stay are shorter and wound size smaller with laparoscopic nephrectomy than open nephrectomy, the former producing only a small increase in warm ischaemic time and, to date, few complications. The surgery is technically demanding, however, and damage to the donor kidney may transfer morbidity from the donor to the recipient. A controlled trial comparing the open and laparoscopic techniques is needed before the use of laparoscopic nephrectomy can be promoted uncritically.14
Non-heart beating donation
Most kidneys are retrieved from patients who are brain stem dead, but whose circulation and ventilation are supported until the organ is removed. In contrast, a smaller number of organs are retrieved from donors without an active circulation—non-heart beating donors. In these cases rapid organ retrieval is needed to minimise damage secondary to warm ischaemia. Research in this area is bedevilled by differing case mixes, with not all centres agreeing on the definition of a non-heart beating donor. However, data from the United States, the United Kingdom, Europe, and Japan indicate that carefully selected kidneys can give excellent graft function, approaching that of grafts from cadavers.15–17 This technique needs staff to be immediately available to retrieve organs from non-heart beating donors; it is labour intensive and needs considerable commitment of resource. However, the transplant rate may be increased by 20%-40% if such donors are used.15,17 For these reasons, central funding will soon be available to increase the use of this technique in the United Kingdom.
Advances in immunosuppression
Research into immunosuppression after transplantation has been relatively stagnant for many years. Recently, however, great progress has been made in the options available.
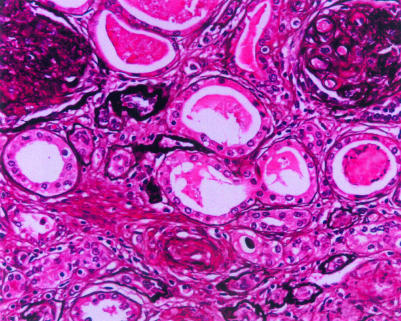
Tacrolimus and mycophenolate mofetil—Although most centres still use treatments based on steroids and ciclosporin, tacrolimus and mycophenolate mofetil have emerged as effective and well tolerated options for inducing and maintaining immunosuppression.18,19 Hypertension, hyperuricaemia, and cosmetic side effects all seem to be less severe with these newer agents, while rejection rates are equivalent and lipid profiles are significantly improved.18,19 Mycophenolate mofetil exerts effects on B cells and T cells, and recent data suggest that it may also reduce the incidence of chronic allograft nephropathy—a poorly understood condition that is a major contributor to graft loss (fig 2).20 If mycophenolate mofetil does reduce the incidence of chronic allograft nephropathy, it would be a major therapeutic advance. However, cost issues have restricted the use of mycophenolate mofetil in the United Kingdom, and long term follow up of patients is needed to determine whether the levels of immunosuppression produced by mycophenolate mofetil are associated with long term problems related to infection and malignancy.
Figure 2.
Light microscopic appearances of chronic allograft nephropathy characterised by vascular obliteration, membranoproliferative glomerular changes, and tubular atrophy
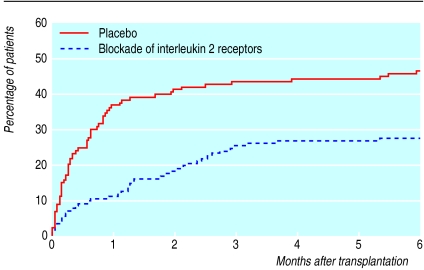
Anti-interleukin 2 receptor antibodies—Another major advance in the area of induction immunosuppression is the development of the anti-interleukin 2 receptor antibodies (basiliximab and daclizumab). These drugs have produced impressive reductions in the rates of early rejection in adult and paediatric transplants (fig 3).21,22 Once again, cost issues have restricted the use of these promising drugs.
Figure 3.
Cumulative probability of rejection after induction immunosuppression with interleukin 2 receptor antibodies and placebo21,22
Sirolimus—Sirolimus is another immunosuppressant that has entered the clinical arena in the United States and, more recently, in Europe. Impressive reductions in the rates of early rejection and hypertension with sirolimus compared with alternative agents need to be balanced against adverse changes in lipid profiles and a lack of data on long term outcome.23
Other agents—A number of other immunosuppressive agents are nearly ready for clinical use (for example, FTY720, RAD, monoclonal antibodies, leflunomide, and brequinar sodium).24
Ciclosporin—Almost 20 years after ciclosporin's introduction, clinicians are still learning how to use this drug. Recent studies have shown that it is better to adjust the dose of ciclosporin according to blood levels two hours after treatment (C2 monitoring) than trough levels. Preliminary data show that this new technique produces reductions in rejection rates and side effects.25 The advantage of this technique needs to be compared with the claims of alternative immunosuppressive agents.
Reducing side effects and comorbidity after transplantation
Cardiovascular disease—Most transplant patients die of cardiovascular disease, and attention is directed increasingly towards reducing the cardiovascular risk factors in the dialysis and transplant populations.26 Targets for blood pressure in such patients are being reduced, and most transplant units now aim well below the 145/85 mm Hg recommended for the general population. For dialysis and transplant patients, inappropriate lipid concentrations should be treated aggressively—as if for secondary prevention—and drugs that interfere with the renin-angiotensin axis may produce specific benefits.27
Osteoporosis—Osteoporosis occurs often after renal transplantation, and the high incidence has prompted a number of trials of anti-osteoporosis drugs in patients undergoing renal transplant.28 Most bone loss seems to occur in the first year after transplantation, so treatment needs to be started early. A recent survey of patients from transplant centres in the United Kingdom showed that the risk of fracture depended on pretransplant and post-transplant variables, but the risk was highest in postmenopausal women.29 Steroid sparing immunosuppression may be important in improving long term outcome of bone mineral density in transplant patients.
Side effects of immunosuppression—The availability of newer immunosuppressive agents should allow doctors to use a “tailored” approach to immunosuppression, rather than the current “one size fits all” model. The higher incidence of post-transplantation diabetes in black patients compared with other patient groups suggests that tacrolimus, cyclosporin, and high doses of steroids should be avoided in these patients. For patients with an adverse lipid profile, doctors might favour tacrolimus over ciclosporin. Elderly patients need less intensive immunosuppression, while paediatric patients and those undergoing repeat transplants may need more. Particular issues may arise for non-compliant or poor patients, and steroid sparing regimens may be preferred in patients prone to diabetes.
Improving access to transplantation
Transplantation must be available to all, regardless of colour, creed, sex, or social status. In the United Kingdom, the rules for allocating organs to patients are fine tuned at regular intervals by a working party of UK Transplant, with the emphasis being placed on the quality of tissue matching. Similar systems exist for transplantation in other countries: one notable European collaboration is the Eurotransplant organisation, which coordinates organ transplantation across Austria, Belgium, Germany, Luxembourg, the Netherlands, and Slovenia.
Allocating organs according to regularly updated and predetermined criteria has the advantage of being objective and associated with optimal short term and long term graft survival rates.1 A recent analysis also showed an economic advantage for an allocation policy based on HLA matching.30 Other criteria for organ allocation include the age of the recipient, the age difference between the donor and recipient, the time since the patient was placed on the transplant list, and the level of sensitisation of the recipient by pre-formed antibodies.
Current issues include the possibility of adjusting the matching criteria to ensure that groups with a particular imbalance between donor supply and demand are less disadvantaged. Such groups include recipients with blood group B and those from minority ethnic groups (who often have blood group B); in the latter group tissue matching with the donor pool, which mainly consists of white donors, is also less good.
Ethical issues in transplantation
Renal transplantation has evolved under an ethical spotlight, and many difficult issues need to be resolved. One argument revolves around essentially practical principles: rates of graft survival are low in certain groups (including patients with diabetes and patients with focal segmental glomerulosclerosis)—should transplantation for patients in these groups be restricted? An important recent paper showed that transplantation improves life expectancy and quality of life in all groups of patients, regardless of the cause of renal failure; in fact, the relative benefit was greatest in some “high risk” groups of patients, such as those with diabetes.31 Therefore, no logical grounds support the restriction of access to transplantation for such groups. Similarly, although it has been argued that patients should not receive a transplant until dialysis has been established, recent evidence shows that pre-emptive transplantation is associated with lower morbidity and improved long term outcome.32
Other topical ethical issues include the use of conditional organ donation, in which the donor imposes conditions regarding potential recipients (this is banned in the United Kingdom), the use of non-altruistic (paid) organ donation,33 and the potential risks and benefits of xenotransplantation.34
Additional educational resources
Selected review articles
Gonin MJ. Maintenance immunosuppression: new agents and persistent dilemmas. Adv Renal Replacement Ther 2000;7:95-116.
Kasiske BL. Cardiovascular disease after renal transplantation. Semin Nephrol 2000;20:164-75.
Miranda B, Matesanz R. International issues in transplantation. Setting the scene and flagging the most urgent and controversial issues. Ann N Y Acad Sci 1998;862:129-43.
Useful websites
Background information for patients and links for transplant professionals
National Kidney Federation (www.kidney.org.uk/Medical-Info/transplant.html)
Addenbrooke's NHS Trust transplant unit (www.cambridgetransplant.org.uk/links/linksprofessional.htm)
Provides links to transplant information for professionals
Current statistics, activity data, and publication lists
UK Transplant (www.uktransplant.org.uk)
United States Renal Data System (www.usrds.org/)
NHS Organ Donor Register (www.uktransplant.org.uk.d1.asp)
Information about the NHS Organ Donor Register, including how to join.
British Organ Donor Society (www.argonet.co.uk/body/)
A patient orientated site
United Network for Organ Sharing (www.patients.unos.org/)
An American perspective on organ transplantation
Acknowledgments
I thank Dr S Sampson for providing figure 2.
Footnotes
Competing interests: None declared.
References
- 1.Transplant Activity Report 2000. www.uktransplant.org.uk/b2d.asp (accessed 22 Feb 2002).
- 2.UK Transplant. Renal transplant audit 1990-98. Bristol: UK Transplant; 2001. [Google Scholar]
- 3.United States Renal Data System. Annual data report 2001. Minneapolis: USRDS; 2001. www.usrds.org/adr.htm (accessed 18 Jan 2002). [Google Scholar]
- 4.Briggs JD, Crombie A, Fabre J, Major E, Thorogood J, Veitch PS. Organ donation in the UK: a survey by a British Transplantation Society working party. Nephrol Dial Transplant. 1997;12:2251–2257. doi: 10.1093/ndt/12.11.2251. [DOI] [PubMed] [Google Scholar]
- 5.Miranda B, Fernandez Lucas M, de Felipe C, Naya M, Gonzalez-Posada JM, Matesanz R. Organ donation in Spain. Nephrol Dial Transplant. 1999;14(suppl 3):15–21. doi: 10.1093/ndt/14.suppl_3.15. [DOI] [PubMed] [Google Scholar]
- 6.Michielsen P. Presumed consent to organ donation: 10 years' experience in Belgium. J R Soc Med. 1996;89:663–666. doi: 10.1177/014107689608901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takemoto S, Terasaki PI. Donor and recipient age. In: Terasaki PI, editor. Clinical transplants 1988. Los Angeles: UCLA Immunogenetics Center; 1988. pp. 345–355. [PubMed] [Google Scholar]
- 8.Breshnahan BA, McBride MA, Cherikh WS, Hariharan S. Risk factors for renal allograft survival from pediatric cadaver donors: an analysis of United Network for Organ Sharing data. Transplantation. 2001;72:256–261. doi: 10.1097/00007890-200107270-00016. [DOI] [PubMed] [Google Scholar]
- 9.Jerius JT, Taylor RJ, Murillo D, Leone JP. Double renal transplants from marginal donors: 2 year results. J Urol. 2000;163:423–425. [PubMed] [Google Scholar]
- 10.Lee CM, Carter JT, Weinstein RJ, Pease HM, Scandling JD, Pavalakis M, et al. Dual kidney transplantation: older donors for older recipients. J Am Coll Surg. 1999;189:82–92. doi: 10.1016/s1072-7515(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen A. Living renal donors: the Norwegian experience. Transplant Proc. 1996;28:3581. [PubMed] [Google Scholar]
- 12.Working Party of the British Transplantation Society and the Renal Association. United Kingdom guidelines for living donor kidney transplantation. Cambridge: British Transplantation Society and the Renal Association; 2000. www.cambridge-transplant.org.uk/program/renal/guidelines.htm (accessed 18 Jan 2002). [Google Scholar]
- 13.Kim FJ, Ratner LE, Kavoussi LR. Renal transplantation: live donor nephrectomy. Urol Clin North Am. 2000;27:777–785. doi: 10.1016/s0094-0143(05)70125-7. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson ML, Veitch PS. Laparoscopic live-donor nephrectomy. Nephrol Dial Transplant. 2000;15:1124–1126. doi: 10.1093/ndt/15.8.1124. [DOI] [PubMed] [Google Scholar]
- 15.Cho YW, Terasaki PI, Cecka JM, Gjertson DW. Transplantation of kidneys from donors whose hearts have stopped beating. N Engl J Med. 1998;338:221–225. doi: 10.1056/NEJM199801223380403. [DOI] [PubMed] [Google Scholar]
- 16.Andrews PA, Compton F, Koffman CG, Bewick M, Chang RWS. Prediction of outcome in non-heart-beating kidney transplantation. Transplant Proc. 2001;33:1121–1124. doi: 10.1016/s0041-1345(00)02456-8. [DOI] [PubMed] [Google Scholar]
- 17.Koostra G. The asystolic, or non-heartbeating, donor. Transplantation. 1997;63:917–921. doi: 10.1097/00007890-199704150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Knoll GA, Bell RC. Tacrolimus versus cyclosporin for immunosuppression in renal transplantation: meta-analysis of randomised trials. BMJ. 1999;318:1104–1107. doi: 10.1136/bmj.318.7191.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215–245. doi: 10.1016/s0162-3109(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 20.Ojo AO, Meier-Kriesche H-U, Hanson JA, Leichtman AB, Cibrik D, Magee JC, et al. Mycophenolate mofetil reduces late allograft loss independent of acute rejection. Transplantation. 2000;69:2405–2409. doi: 10.1097/00007890-200006150-00033. [DOI] [PubMed] [Google Scholar]
- 21.Nashan B, Moore R, Amlot P, Schmidt A-G, Abeywickrama K, Soulillou J-P. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. Lancet. 1997;350:1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 22.Nashan B, Light S, Hardie IR, Lin A, Johnson JR. Reduction of acute renal allograft rejection by daclizumab. Transplantation. 1999;67:110–115. doi: 10.1097/00007890-199901150-00019. [DOI] [PubMed] [Google Scholar]
- 23.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 24.Denton MD, Magee CC, Sayegh M. Immunosuppressive strategies in transplantation. Lancet. 1999;353:1083–1091. doi: 10.1016/S0140-6736(98)07493-5. [DOI] [PubMed] [Google Scholar]
- 25.Levy GA. C2 monitoring strategy for optimising cyclosporin immunosuppression from the Neoral formulation. Biodrugs. 2001;15:279–290. doi: 10.2165/00063030-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Aakus S, Dahl K, Wideroe TE. Cardiovascular morbidity and risk factors in renal transplant recipients. Nephrol Dial Transplant. 1999;14:648–654. doi: 10.1093/ndt/14.3.648. [DOI] [PubMed] [Google Scholar]
- 27.Navis G, de Zeeuw D, de Jong P. ACE-inhibitors: panacea for progressive renal disease? Lancet. 1997;349:1852–1853. doi: 10.1016/s0140-6736(97)22026-x. [DOI] [PubMed] [Google Scholar]
- 28.Arlen DJ, Lambert K, Ioannidis G, Adachi JD. Treatment of established bone loss after renal transplantation with etidronate. Transplantation. 2001;71:669–673. doi: 10.1097/00007890-200103150-00017. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Kwan JTC, McCloskey E, McGee G, Thomms G, Johnson D, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Min Res. 2001;16:1863–1870. doi: 10.1359/jbmr.2001.16.10.1863. [DOI] [PubMed] [Google Scholar]
- 30.Schnitzler MA, Hollenbeak CS, Cohen DS, Woodward RS, Lowell JA, Singer GG, et al. The economic implications of HLA matching in cadaveric renal transplantation. N Engl J Med. 1999;341:1440–1446. doi: 10.1056/NEJM199911043411906. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodon LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 32.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 33.Cameron JS, Hoffenberg R. The ethics of organ transplantation reconsidered: paid organ donation and the use of executed prisoners as donors. Kidney Int. 1999;55:724–732. doi: 10.1046/j.1523-1755.1999.00286.x. [DOI] [PubMed] [Google Scholar]
- 34.Clark MA. This little piggy went to market: the xenotransplantation and xenozoonose debate. J Law Medicine Ethics. 1999;27:137–152. doi: 10.1111/j.1748-720x.1999.tb01446.x. [DOI] [PubMed] [Google Scholar]