Abstract
Purpose of review
In an attempt to reduce waiting list mortality in liver transplantation, less-than-ideal quality donor livers from extended criteria donors are increasingly accepted. Predicting the outcome of these organs remains a challenge. Machine perfusion provides the unique possibility to assess donor liver viability pretransplantation and predict postreperfusion organ function.
Recent findings
Assessing liver viability during hypothermic machine perfusion remains challenging, as the liver is not metabolically active. Nevertheless, the levels of flavin mononucleotide, transaminases, lactate dehydrogenase, glucose and pH in the perfusate have proven to be predictors of liver viability. During normothermic machine perfusion, the liver is metabolically active and in addition to the perfusate levels of pH, transaminases, glucose and lactate, the production of bile is a crucial criterion for hepatocyte viability. Cholangiocyte viability can be determined by analyzing bile composition. The differences between perfusate and bile levels of pH, bicarbonate and glucose are good predictors of freedom from ischemic cholangiopathy.
Summary
Although consensus is lacking regarding precise cut-off values during machine perfusion, there is general consensus on the importance of evaluating both hepatocyte and cholangiocyte compartments. The challenge is to reach consensus for increased organ utilization, while at the same time pushing the boundaries by expanding the possibilities for viability testing.
Keywords: ex-situ machine perfusion, liver transplantation, liver viability assessment
INTRODUCTION
Liver transplantation is a lifesaving treatment for patients with end-stage liver disease, acute liver failure and primary liver cancer. Unfortunately, with increasing rates of liver disease, the number of transplantable organs available is unable to meet demand, and mortality on the waiting list is high [1]. The shortage of donor livers, in combination with altering organ donor demographics, such as ageing population and increased obesity, has led to the increased utilization of livers from extended criteria donors (ECD) of suboptimal quality [2,3]. ECD livers provide an increased risk of developing early allograft dysfunction (EAD), primary nonfunction (PNF), or posttransplant ischemic cholangiopathy [4–11]. Predicting the postreperfusion viability of ECD livers prior to transplantation is one of the most challenging calls to make in organ transplantation. For recipient safety reasons, high-risk donor livers are, therefore, often declined for transplantation [12].
Ex-situ machine perfusion now provides a unique opportunity to assess ECD liver function before transplantation. Viability assessment has mainly been described during normothermic machine perfusion (NMP). However, there are new approaches for assessment during hypothermic machine perfusion (HMP). To add value to known donor and recipient risk factors for worse posttransplant outcome, reliable criteria to determine donor liver viability and predict save use need to be established. This article reviews currently used and promising new viability criteria during ex-situ liver machine perfusion.
Box 1.
no caption available
VIABILITY ASSESSMENT
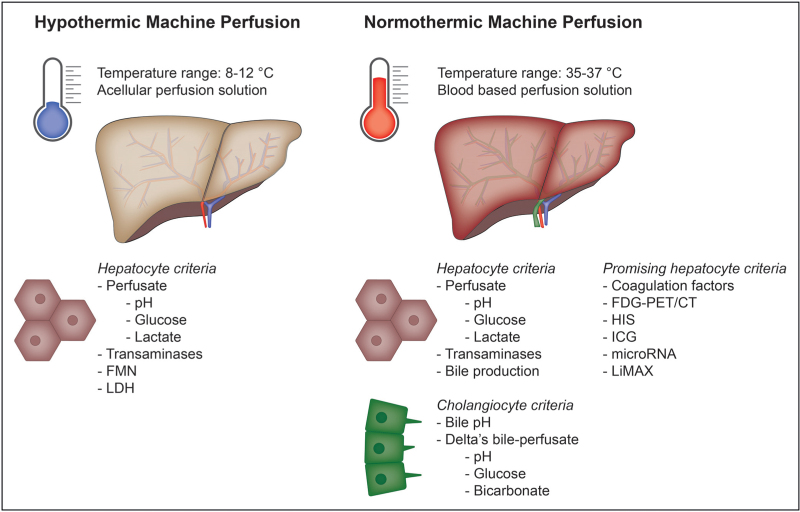
For long-term graft survival, both liver synthetic and detoxification functions and biliary integrity have to be preserved. Therefore, viability criteria have been chosen to test the hepatocyte and cholangiocyte compartment. Most viability criteria can only be assessed during NMP, but some tests are also possible during HMP (Fig. 1). Table 1 provides an overview of the viability criteria currently used in clinical practice, which are further discussed below.
FIGURE 1.
Viability criteria during hypothermic and normothermic machine perfusion. FDG, 18F-fluorodeoxyglucose; FMN, flavin mononucleotide; HIS, hyperspectral imaging; ICG, indocyanine green; LDH, lactate dehydrogenase; LiMAx, liver maximum capacity.
Table 1.
Viability criteria used in the clinical practice
| Reference | Number of livers | DCD/DBD | Viability criteria | Outcomes |
| HMP | ||||
| Eden et al.[16] (2023) | Perfused: not specified Transplanted: 158 |
Not specified | FMN at 30 min of HOPE (<6000 A.U.) NADH at 30 min of HOPE (<8.000 A.U.) |
89% 1-year graft survival 7 PNF 11 IC 53 AS 9 bile leakage |
| Patrono et al.[17] (2020) | Perfused: 50 Transplanted: 50 |
0/50 | Perfusate lactate, AST, ALT, LDH, glucose, and pH during DHOPE | 1 graft loss 13 EAD |
| Schlegel et al.[15] (2020) | Perfused: 50 Transplanted: 50 |
32/18 | Perfusate, tissue and mitochondria during HOPE: FMN at 30 min (<8000 A.U.) NADH (<10 000 A.U.) |
7 graft loss (unspecified) |
| Muller et al.[14] (2019) | Perfused: 54 Transplanted: 54 |
35/19 | FMN within 30 min of HOPE | 7 graft loss 4 PNF 1 IC |
| NMP | ||||
| Van Leeuwen et al.[33] (2022) | Perfused: 54 Transplanted: 34 |
53/1 | After 2.5 h of NMP - Lactate <1.7 mmol/l - Perfusate pH 7.35–7.45 - Bile production >10 ml, of which ≥4 ml in the last hour - Bile pH > 7.45 - Delta pH bile and perfusate >0.10 - Delta sodium bicarbonate bile and perfusate >5.0 - Delta glucose bile and perfusate <−5.0 |
94% 1-year graft survival 1 NAS 12 AS 4 bile leakage |
| Seidita et al.[31] (2022) | Perfused: 19 Transplanted: 17 |
3/16 | - Lactate clearance normalization or at least halving of lactates at end of perfusion - pH > 7.3 - Bile production - Vascular flow HA and PV |
94% 1-year graft survival 1 EAD |
| Quintini et al.[29] (2022) | Perfused: 21 Transplanted: 15 |
13/8 | Within 6 h of NMP, ≥2 of the following - Perfusate lactate <4.5 mmol/l or decrease of 60% from peak in first 4 h - Bile production >2 mL/h - Stable HA and PV flow (>0.05 ml/min/g and >0.4 ml/min/g) - Macroscopic homogenous perfusion and soft consistency |
7 EAD 1 IC |
| Zhang et al.[36] (2020) | Perfused: 4 Transplanted: 4 Retrospect |
3/1 | Within 4 h of NMP - Perfusate lactate <2.5 mmol/l - Bile production - Stable HA and PV flow (>150 ml/min and >500 ml/min) - Perfusate pH > 7.3 |
100% 6-months graft survival 1 EAD 1 AS |
| Reiling et al.[30] (2020) | Perfused: 10 Transplanted: 10 |
5/5 | After 4 h of NMP - Perfusate lactate <2 mmol/l within 2 h - Metabolism of glucose, evidenced by decreasing trend by 4 h - Physiological pH without continuous need for sodium bicarbonate - Stable HA and PV flows - Homogenous graft perfusion with soft parenchyma consistency - Bile production (no lower limit) |
100% 6-months graft survival 5 EAD 1 AS 1 anastomotic leak |
| Mergental et al.[25] (2020) | Perfused: 31 Transplanted: 22 |
14/17 | Within 4 h of NMP - Perfusate lactate ≤2.5 mmol/l And ≥2 of the following criteria - Evidence of bile production - Perfusate pH ≥7.30 - Metabolism of glucose - Stable HA and PV flows (≥150 ml/min and ≥500 ml/min) - Homogenous perfusion with soft consistency of the parenchyma |
86.4% 1-year graft survival 7 EAD 4 NAS 2 AS |
| Cardini et al.[22] (2020) | Perfused: 34 Transplanted: 25 |
4/30 | After 2 h of NMP - Rapid decrease and maintenance of lactate levels (first 2 h of NMP) - Bile output and biliary pH - Maintaining a physiological perfusate pH without sodium bicarbonate - Warning signals: exceptionally high or sharp incline of AST, ALT and LDH |
88% graft survival at 20 months 7 AS 3 bile leakage |
| Bral et al.[21] (2019) | Perfused: 46 Transplanted: 43 |
10/33 | - Lactate level at start perfusion - Lactate clearance - Necessity of bicarbonate pH correction - Bile production |
100% 3-month graft survival 11 EAD 2 NAS 6 AS |
| Watson et al.[35] (2018) | Perfused: 47 Transplanted: 22 |
35/12 | - Peak lactate fall ≥4.4 mmol/l/kg/h - ALT <600 iU/l at 2 h - Perfusate pH > 7.2 with ≤30 mmol/l bicarbonate supplementation - Maximum bile pH > 7.5 - Bile glucose concentration ≤3 mmol/l or ≥10 mmol less than perfusate glucose - Falling glucose beyond 2 h or perfusate glucose under 10 mmol/l with subsequent fall during challenge with 2.5 g glucose |
1 PNF 1 EAD 4 IC |
ALT, alanine transaminase; AS, anastomotic biliary strictures; AST, aspartate aminotransferase; DBD, donation after brain death; DCD, donation after circulatory death; DHOPE, dual hypothermic oxygenated machine perfusion; EAD, early allograft dysfunction; HA, hepatic artery; HMP, hypothermic machine perfusion; HOPE, hypothermic oxygenated machine perfusion; IC, ischemic cholangiopathy; LDH, lactate dehydrogenase; NADH, nicotine adenine dinucleotide reduced; NAS, nonanastomotic biliary strictures; NMP, normothermic machine perfusion; PNF, primary nonfunction; PV, portal vein.
VIABILITY ASSESSMENT DURING HYPOTHERMIC MACHINE PERFUSION
At 4–10 °C, metabolic activity is significantly reduced to 6–15% of normal, posing challenges for real-time assessment of metabolism and function [13]. Nevertheless, several perfusate analyses can be performed at 7–10 °C, at which HMP is typically performed.
Flavin mononucleotide
Flavin mononucleotide (FMN) can be assessed during both NMP and HMP. FMN, typically bound to mitochondrial complex I under physiological conditions, is released from damaged complex I during ischemia and subsequent reoxygenation. The Zurich group analyzed FMN in 54 livers during oxygenated-HMP and demonstrated a strong correlation between FMN release, coagulation factors levels and peak transaminases post-transplantation, and showed predictive value for EAD [14]. In 2020, the group form Zurich measured FMN in the perfusate of 50 livers, with elevated concentrations of FMN in the perfusate correlating with a high rate of graft loss [15]. The authors advise against transplantation if FMN concentration exceeds 8000AU after 30 minutes of oxygenated-HMP. In their most recent work, which includes all perfusions from previous work, they suggest a FMN threshold of 6000AU and a NADH threshold of 8000AU after 30 minutes of perfusion [16]. Confirmation of these single center observations in other centers is eagerly awaited.
Perfusate analysis
Patrono et al.[17] have analyzed perfusate in 50 DBD livers during oxygenated-HMP and assessed levels of aspartate aminotransferase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), glucose, lactate and pH at 90 min of perfusion in relation to postoperative outcomes [17]. All parameters, except lactate, correlated with EAD. However, macrovesicular steatosis was the only independent predictor of EAD, as all perfusate parameters were closely correlated to the severity of steatosis.
VIABILITY ASSESSMENT DURING NORMOTHERMIC MACHINE PERFUSION: HEPATOCYTE VIABILITY
NMP is typically performed at 35–37 °C, enabling assessment of a metabolically active liver. The criteria for hepatocyte viability are based on perfusate analysis, perfusion parameters and bile production.
Lactate levels
During NMP, three phases in lactate dynamics can be typically identified; initially increasing, peaking at 1 h, followed by a rapid clearance within 2 h, ending in a stable state characterized by consistently low lactate levels during the remainder of the perfusion [18,19]. Nondecreasing lactate levels in the perfusate are generally considered a sign of poor graft function. As shown in Table 1, all clinical studies incorporated perfusate lactate clearance [20–36]. It is noteworthy that cut-off values range between 1.7 and 4.5 mmol/l, and lactate is measured at different time points during NMP. Watson et al.[35] integrated liver weight in the equation for lactate clearance, leading to a more accurate estimation of clearance per gram liver tissue. Seidita et al.[31], Cardini et al.[22] and Bral et al.[21] state that lactate should decrease over time, without further specification. In addition to the varying cut-off values, controversy exists on whether lactate clearance is an accurate marker of graft viability. The Zurich group showed lactate clearance up to 24 h of NMP in four explant cirrhotic livers, questioning the predictive value of lactate clearance in early assessment [37▪].
Glucose levels
The liver is a key metabolic organ with a major role in glucose metabolism, including glycogenolysis, gluconeogenesis and glycolysis [38]. Glycogenolysis, an ATP-independent process, continues during static cold storage (SCS) driven by the lack of ATP, which is demonstrated by increasing glucose levels at the beginning of NMP. In viable livers, high levels of glucose should prevent glycogenolysis and trigger glycogenesis, and as a result, glucose levels will decrease. Normal levels of glucose during NMP can reflect minimal ischemia, but it can also point to glycogen exhaustion or extensive liver injury [35]. A glucose challenge test can rule out liver injury: a viable liver metabolizes glucose and levels in the perfusate will drop [25,39,40]. Only Watson et al.[35] included a glucose challenge in their viability criteria. Both Reiling et al.[30] and Mergental et al.[25] included a decreasing glucose trend.
Acid–base homeostasis
The liver is an important regulator of acid–base homeostasis. Healthy livers tend to have better pH regulation and stabilization. All groups except Quintini et al.[29], have included a near physiological pH, from 7.20 to 7.45, as viability criterion. Watson et al.[35] described a NMP procedure of a liver requiring more than 30 mmol/l bicarbonate, much more than any other livers, and this liver developed PNF. Therefore, they incorporated a maximum of 30 mmol/l bicarbonate bolus support. pH levels are influenced by perfusate composition, addition of sodium bicarbonate and partial pressure of CO2 and therefore pH is best used in combination with other criteria to determine viability.
Transaminases
Liver transaminases synthesize and break down amino acids and convert energy storage molecules. Damaged hepatocytes release transaminases into the perfusate because of increased membrane permeability. Perfusate transaminase levels, influenced by factors like donor age, steatosis and ischemia time, are nonspecific and require normalization to liver weight and perfusate volume for accurate assessment [41,42]. AST also rises from hemolysis in the perfusion circuit, and therefore ALT is considered more liver-specific [35,39,43]. As shown in Table 1, only Watson et al.[35] stated a cut-off value for ALT of less than 6000 IU/l at 2 h of NMP. Furthermore, Cardini et al.[22] stated exceptionally high or sharp increase of AST and ALT, without further specification, as a warning signal.
Perfusion parameters
In liver perfusion, flow in the hepatic artery and portal vein is determined by perfusion pressure and vascular resistance. Prolonged ischemia can damage the microcirculation of the liver, leading to increased vascular resistance and reduced perfusion at fixed pressure, resulting in impaired function [44,45]. Steatotic livers, with narrower sinusoids, exhibit lower flow, leading to secondary hypoxia and reperfusion injury [46]. Elevated vascular resistance during machine perfusion indicates liver injury and later liver dysfunction [45]. Whereas many groups suggest necessity for stable hepatic artery and portal vein flows [20,28,30,31], some added specific target flows [25–27,29], all livers reached the target flows. Quintini et al.[29] further specified target flow per unit liver weight, but even here, all livers met the target flows. This indicates that currently flow measurements alone cannot distinguish between viable and nonviable livers.
Bile production
Bile production is considered one of the higher levels of liver functions, as it requires considerable ATP content. Bile is produced by hepatocytes, and cholangiocytes lining the bile duct alter the composition of bile. Therefore, bile production is associated with hepatocyte viability, whereas bile composition is associated with cholangiocyte viability. All studies in Table 1 have listed production of bile as a viability marker [21,22,25,29–31,33,35,36], demonstrating consensus on the negative implications of absence of bile production. Only two groups have stated a specific cut-off value for bile production. Quintini et al.[29] defined a production of more than 2 ml/h as a minimum, Van Leeuwen et al.[33] defined a production of more than 10 ml after 2.5 h of NMP, of which at least 4 ml in the last hour. However, graft loss has been described despite proper bile production, and successful transplantations have been reported without proper bile production [35,36,39,47]. As an example, Zhang et al.[36] transplanted four livers that did not meet the Groningen group criteria, yet all showed immediate function. It should be noted that malposition of the biliary drain may lead to false-negative absence of bile production.
VIABILITY ASSESSMENT DURING NORMOTHERMIC MACHINE PERFUSION: CHOLANGIOCYTE VIABILITY
As cholangiocytes play a crucial role in modifying bile composition by re-absorbing solutes and secreting water and bicarbonate, the viability of the cholangiocytes can be assessed by analyzing the composition of bile.
Bile composition
Low levels of biliary glucose, high levels of bicarbonate and an alkalotic pH are indications of viable cholangiocytes [24,48]. A biliary pH of more than 7.45 was defined by Cardini et al.[22] and Van Leeuwen et al.[33]. In the cohort of Watson et al.[35], 3 out of 16 transplanted livers were unable to achieve a biliary pH greater than 7.40, and developed ischemic cholangiopathy. In addition to low pH, these livers also had similar glucose levels in bile and perfusate, and a low biliary bicarbonate. Therefore, Watson et al.[35] stated that the difference between bile and perfusate glucose should at least be 10 mmol/l, and Van Leeuwen et al.[33] suggested a delta of at least 5 mmol/l. In addition to the biliary pH and the delta glucose, Van Leeuwen et al.[33] also included a delta pH and a delta bicarbonate. Importantly, the VITTAL trial showed that meeting hepatocellular viability criteria is not sufficient to predict ischemic biliary damage [25]. All transplanted livers met the hepatocyte criteria, but nevertheless, 45% of the recipients developed irregularities in the bile ducts, with 18% requiring retransplantation for ischemic cholangiopathy, suggesting nonviable cholangiocytes and bile ducts, despite viable liver parenchyma.
PROMISING VIABILITY CRITERIA
In addition, clinically implemented viability criteria, new criteria, such as coagulation factors and imaging techniques have been studied in preclinical setting.
Coagulation factors
The liver is crucial for regulating coagulation and fibrinolysis [49]. NMP circuits are heparinized, yet production of coagulation factors can serve as a viability marker. The long-term NMP study of Eshmuminov et al.[40] revealed significantly higher coagulation factor-V levels in the perfusate of functioning versus nonfunctioning livers at 48 h of perfusion, although this difference disappeared thereafter. Weissenbacher et al.[50] showed significantly higher levels of von Willebrand factor antigen in the perfusate of EAD livers. Van den Boom et al.[51▪] measured the international normalized ratio (INR) during NMP. Addition of fibrinogen and/or polybrene, which neutralizes the anticoagulant effects of heparin, was necessary to measure INR in perfusate samples. INR at 150 min or at the end of NMP did not correlate with hepatocyte viability criteria, suggesting that measuring INR may have added value for determining viability.
Imaging techniques
Orita et al. performed a 18F-fluorodeoxyglucose (FDG)-PET/CT to assess viability in two discarded human livers after 1 week of NMP [52]. With FDG-PET/CT, the glucose metabolism can be followed: glucose is taken up by the GLUT-2-transporter, metabolized into FDG-6P and dephosphorylated back to FDG, which then leaves the cells and returns to the circulation [53]. Homogenous FDG uptake was observed in all livers and therefore indicated intact transport, metabolism, and excretion of glucose. Fodor et al.[54] utilized hyperspectral imaging (HIS) to analyze the liver parenchyma, monitoring oxygen saturation levels (StO2), tissue hemoglobin index (THI), near-infrared perfusion (NIR), and tissue water indices (TWI). HIS was performed during SCS and at 1, 6, 12 h, and end NMP. During NMP, the StO2, THI and NIR perfusion indices significantly increased, whereas the TWI drastically decreased. A significantly higher THI was seen in discarded livers compared with transplanted livers end NMP. Kneifel et al. also used HIS during NMP at 1, 2 and 4 h of perfusion and showed that StO2 and THI predicted lactate values at 1 and 2 h of NMP, THI also predicted lactate values at 4 h [55▪]. Lau et al.[56▪▪] analyzed the indocyanine green (ICG) plasma-disappearance-rate (PDR) and the ICG-perfusion with a near-infrared camera during 7 days of NMP. The ICG-PDR was significantly higher on day 0 in grafts that survived at least 7 days of NMP. ICG perfusion, the distribution of ICG in the liver during NMP, was significantly different at day 0 between long-surviving and short-surviving grafts.
MicroRNA
Matton et al.[57] investigated microRNAs as sensitive, specific, and stable markers for cell function, stress, and injury during NMP. Elevated miRNA-122 was significantly associated with liver injury, although there was no threshold value identified for irreparable damage [58–60].
Liver maximum capacity test
Our group employed the liver maximum capacity (LiMAx) test to assess liver function during NMP [61]. This is a clinically validated substrate-based cytochromal breath test, which measures 13CO2 production. The advantage is that all livers can be subjected to an equivalent dose, making direct comparison between results possible. Schurink et al. demonstrated a significant correlation between LiMAx values and lactate clearance, and significant inverse correlation between LiMAx values and histological injury. However, no correlation was found between LiMAx values and microRNA-122 or FMN values in the perfusate.
DISCUSSION AND FUTURE PERSPECTIVES
In this review, we have summarized the emerging consensus on parameters that indicate liver viability during NMP. Viability assessment during HMP could prove to be an important contribution to the field. During NMP lactate clearance, pH stabilization and bile production are uniformly considered as biomarkers of adequate liver function prior to acceptance. There is also growing agreement that besides hepatocellular criteria, cholangiocyte criteria should be considered to prevent biliary ischemic complications post-transplant. In both domains, no consensus was found on specific cut-off values, which might be hampered by considerable differences in perfusion protocols, acceptance criteria and recipient populations. In most viability assessment studies, livers were transplanted into recipients with relatively low Model for End-Stage Liver Disease (MELD) scores. This raises the question if these tested ECD livers are equally suitable for recipients that require a retransplantation and recipients with high MELD scores, as these patients are more at risk for complications and graft loss. So far, recipient factors have not been combined with viability assessment, opposed to the risk scores that were developed recently to balance donor and recipient risks (e.g. UK-DCD score, Balance of Risk score, ET-DRI score).
Considering the differences in length of the viability assessment protocol, the 2.5 h decision moment described by Van Leeuwen et al.[33] is swift but proved to be a safe decision moment, without any PNF in their cohort. In their series, about two of three of all tested livers were ultimately transplanted. The short assessment period might, however, be too strict for slowly recovering livers, discarding potentially viable organs meeting the criteria shortly after the decision moment. Other protocols take up to 6 h to reach a decision moment, which might yield a higher ECD liver utilization rate. This indicates that there is potential to further explore the lower limits of organ viability and even push the boundaries more. In addition, organ perfusion and viability assessment can lead to a better understanding of essential hepatobiliary injury and repair processes, opening doors for regenerative medicine applications and potentially repair currently declined organs.
CONCLUSION
In conclusion, current viability assessment protocols during ex-situ liver perfusion enable well tolerated use of ECD livers. Efforts should be taken to reach consensus on requirements for acceptance to increase wider application of these organs and ultimately reduce waitlist mortality.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Nasralla D, Coussios CC, Mergental H, et al. Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature 2018; 557:50–56. [DOI] [PubMed] [Google Scholar]
- 2.Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult liver allocation in eurotransplant. Transplantation 2017; 101:1542–1550. [DOI] [PubMed] [Google Scholar]
- 3.Barshes NR, Horwitz IB, Franzini L, et al. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant 2007; 7:1265–1270. [DOI] [PubMed] [Google Scholar]
- 4.Merion RM, Goodrich NP, Feng S. How can we define expanded criteria for liver donors? J Hepatol 2006; 45:484–488. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel A, Kalisvaart M, Scalera I, et al. The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol 2018; 68:456–464. [DOI] [PubMed] [Google Scholar]
- 6.Linares I, Hamar M, Selzner N, Selzner M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation 2019; 103:78–90. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer DP, Paul A, Gallinat A, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int 2015; 35:156–163. [DOI] [PubMed] [Google Scholar]
- 8.Foley DP, Fernandez LA, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg 2011; 253:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blok JJ, Detry O, Putter H, et al. Eurotransplant Liver Intestine Advisory Committee. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl 2016; 22:1107–1114. [DOI] [PubMed] [Google Scholar]
- 10.de Vries Y, von Meijenfeldt FA, Porte RJ. Posttransplant cholangiopathy: classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis 2018; 1864 (4 Pt B):1507–1515. [DOI] [PubMed] [Google Scholar]
- 11.Meurisse N, Vanden Bussche S, Jochmans I, et al. Outcomes of liver transplantations using donations after circulatory death: a single-center experience. Transplant Proc 2012; 44:2868–2873. [DOI] [PubMed] [Google Scholar]
- 12.Israni AK, Zaun D, Rosendale JD, et al. OPTN/SRTR 2019 Annual Data Report: deceased organ donors. Am J Transplant 2021; 21: (Suppl 2): 521–558. [DOI] [PubMed] [Google Scholar]
- 13.Michenfelder JD, Theye RA. Hypothermia: effect on canine brain and whole-body metabolism. Anesthesiology 1968; 29:1107–1112. [DOI] [PubMed] [Google Scholar]
- 14.Muller X, Schlegel A, Kron P, et al. Novel real-time prediction of liver graft function during hypothermic oxygenated machine perfusion before liver transplantation. Ann Surg 2019; 270:783–790. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel A, Muller X, Mueller M, et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020; 60:103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eden J, Breuer E, Birrer D, et al. Screening for mitochondrial function before use-routine liver assessment during hypothermic oxygenated perfusion impacts liver utilization. EBioMedicine 2023; 98:104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrono D, Catalano G, Rizza G, et al. Perfusate analysis during dual hypothermic oxygenated machine perfusion of liver grafts: correlations with donor factors and early outcomes. Transplantation 2020; 104:1929–1942. [DOI] [PubMed] [Google Scholar]
- 18.Panconesi R, Flores Carvalho M, Mueller M, et al. Viability assessment in liver transplantation-what is the impact of dynamic organ preservation? Biomedicines 2021; 9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nasralla D, Coussios CC, Mergental H, et al., Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature 2018; 557:50–56. [DOI] [PubMed] [Google Scholar]
- 20.Boteon YL, Laing RW, Schlegel A, et al. Combined hypothermic and normothermic machine perfusion improves functional recovery of extended criteria donor livers. Liver Transpl 2018; 24:1699–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bral M, Dajani K, Leon Izquierdo D, et al. A back-to-base experience of human normothermic ex situ liver perfusion: does the chill kill? Liver Transpl 2019; 25:848–858. [DOI] [PubMed] [Google Scholar]
- 22.Cardini B, Oberhuber R, Fodor M, et al. Clinical implementation of prolonged liver preservation and monitoring through normothermic machine perfusion in liver transplantation. Transplantation 2020; 104:1917–1928. [DOI] [PubMed] [Google Scholar]
- 23.de Vries Y, Matton APM, Nijsten MWN, et al. Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution. Am J Transplant 2019; 19:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matton APM, de Vries Y, Burlage LC, et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation 2019; 103:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mergental H, Laing RW, Kirkham AJ, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun 2020; 11:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 2016; 16:3235–3245. [DOI] [PubMed] [Google Scholar]
- 27.Mergental H, Stephenson BTF, Laing RW, et al. Development of clinical criteria for functional assessment to predict primary nonfunction of high-risk livers using normothermic machine perfusion. Liver Transpl 2018; 24:1453–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavel MC, Reyner E, Molina V, et al. Evolution under normothermic machine perfusion of type 2 donation after cardiac death livers discarded as nontransplantable. J Surg Res 2019; 235:383–394. [DOI] [PubMed] [Google Scholar]
- 29.Quintini C, Del Prete L, Simioni A, et al. Transplantation of declined livers after normothermic perfusion. Surgery 2022; 171:747–756. [DOI] [PubMed] [Google Scholar]
- 30.Reiling J, Butler N, Simpson A, et al. Assessment and transplantation of orphan donor livers: a back-to-base approach to normothermic machine perfusion. Liver Transpl 2020; 26:1618–1628. [DOI] [PubMed] [Google Scholar]
- 31.Seidita A, Longo R, Di Francesco F, et al. The use of normothermic machine perfusion to rescue liver allografts from expanded criteria donors. Updates Surg 2022; 74:193–202. [DOI] [PubMed] [Google Scholar]
- 32.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One 2014; 9:e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Leeuwen OB, Bodewes SB, Lantinga VA, et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am J Transplant 2022; 22:1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Leeuwen OB, de Vries Y, Fujiyoshi M, et al. Transplantation of high-risk donor livers after ex situ resuscitation and assessment using combined hypo- and normothermic machine perfusion: a prospective clinical trial. Ann Surg 2019; 270:906–914. [DOI] [PubMed] [Google Scholar]
- 35.Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant 2018; 18:2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Ju W, Tang Y, et al. First preliminary experience with preservation of liver grafts from extended-criteria donors by normothermic machine perfusion in Asia. Ann Transplant 2020; 25:e921529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37▪.Schuler MJ, Becker D, Mueller M, et al. Observations and findings during the development of a subnormothermic/normothermic long-term ex vivo liver perfusion machine. Artif Organs 2022; 47:317–329. [DOI] [PubMed] [Google Scholar]; This study showed that even ’poor’ livers clear lactate during the first 24 h of machine perfusion, questioning the importance of lactate clearance as a viability marker.
- 38.Rui L. Energy metabolism in the liver. Compr Physiol 2014; 4:177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases. Transplantation 2017; 101:1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eshmuminov D, Becker D, Bautista Borrego L, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol 2020; 38:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martins PN, Rizzari MD, Ghinolfi D, et al. Design, analysis, and pitfalls of clinical trials using ex situ liver machine perfusion: the International Liver Transplantation Society Consensus Guidelines. Transplantation 2021; 105:796–815. [DOI] [PubMed] [Google Scholar]
- 42.Thirunavayakalathil MA, Varghese CT, Bharathan VK, et al. Double-blind placebo-controlled randomized trial of N-acetylcysteine infusion following live donor liver transplantation. Hepatol Int 2020; 14:1075–1082. [DOI] [PubMed] [Google Scholar]
- 43.Op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant 2013; 13:1327–1335. [DOI] [PubMed] [Google Scholar]
- 44.Hide D, Ortega-Ribera M, Garcia-Pagan JC, et al. Effects of warm ischemia and reperfusion on the liver microcirculatory phenotype of rats: underlying mechanisms and pharmacological therapy. Sci Rep 2016; 6:22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monbaliu D, Liu Q, Libbrecht L, et al. Preserving the morphology and evaluating the quality of liver grafts by hypothermic machine perfusion: a proof-of-concept study using discarded human livers. Liver Transpl 2012; 18:1495–1507. [DOI] [PubMed] [Google Scholar]
- 46.Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. Why is fatty liver unsuitable for transplantation? Deterioration of mitochondrial ATP synthesis and sinusoidal structure during cold preservation of a liver with steatosis. Transplant Proc 1997; 29:412–415. [DOI] [PubMed] [Google Scholar]
- 47.Ceresa CDL, Nasralla D, Watson CJE, et al. Transient cold storage prior to normothermic liver perfusion may facilitate adoption of a novel technology. Liver Transpl 2019; 25:1503–1513. [DOI] [PubMed] [Google Scholar]
- 48.Brüggenwirth IMA, Porte RJ, Martins PN. Bile composition as a diagnostic and prognostic tool in liver transplantation. Liver Transpl 2020; 26:1177–1187. [DOI] [PubMed] [Google Scholar]
- 49.Karangwa SA, Burlage LC, Adelmeijer J, et al. Activation of fibrinolysis, but not coagulation, during end-ischemic ex situ normothermic machine perfusion of human donor livers. Transplantation 2017; 101:e42–e48. [DOI] [PubMed] [Google Scholar]
- 50.Weissenbacher A, Bogensperger C, Oberhuber R, et al. Perfusate enzymes and platelets indicate early allograft dysfunction after transplantation of normothermically preserved livers. Transplantation 2022; 106:792–805. [DOI] [PubMed] [Google Scholar]
- 51▪.van den Boom BP, Bodewes SB, Lascaris B, et al. The international normalised ratio to monitor coagulation factor production during normothermic machine perfusion of human donor livers. Thromb Res 2023; 228:64–71. [DOI] [PubMed] [Google Scholar]; INR is detectable in treated perfusate samples during NMP with both a coagulation analyzer and a point-of-care device. Measuring INR can possibly provide additional inside on graft viability.
- 52.Orita E, Becker D, Mueller M, et al. FDG-PET/CT: novel method for viability assessment of livers perfused ex vivo. Nucl Med Commun 2021; 42:826–832. [DOI] [PubMed] [Google Scholar]
- 53.Sarikaya I, Schierz JH, Sarikaya A. Liver: glucose metabolism and 18F-fluorodeoxyglucose PET findings in normal parenchyma and diseases. Am J Nucl Med Mol Imaging 2021; 11:233–249. [PMC free article] [PubMed] [Google Scholar]
- 54.Fodor M, Lanser L, Hofmann J, et al. Hyperspectral imaging as a tool for viability assessment during normothermic machine perfusion of human livers: a proof of concept pilot study. Transpl Int 2022; 35:10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Kneifel F, Wagner T, Flammang I, et al. Hyperspectral imaging for viability assessment of human liver allografts during normothermic machine perfusion. Transplant Direct 2022; 8:e1420. [DOI] [PMC free article] [PubMed] [Google Scholar]; HSI is a noninvasive technique and can easily be applied on the liver parenchyma. Oxygen saturation and the tissue haemogobin index can predict lactate during NMP.
- 56▪▪.Lau NS, Ly M, Ewenson K, et al. Indocyanine green: a novel marker for assessment of graft quality during ex situ normothermic machine perfusion of human livers. Artif Organs 2023; 48:472–483. [DOI] [PubMed] [Google Scholar]; ICG PDR can be used as a hepatocyte viability marker during NMP and can make a distinction between long-term and short-term surviving grafts.
- 57.Matton APM, Selten JW, Roest HP, et al. Cell-free microRNAs as early predictors of graft viability during ex vivo normothermic machine perfusion of human donor livers. Clin Transplant 2020; 34:e13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersson P, Gidlöf O, Braun OO, et al. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012; 37:234–238. [DOI] [PubMed] [Google Scholar]
- 59.Roderburg C, Benz F, Vargas Cardenas D, et al. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int 2015; 35:1172–1184. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Antoine DJ, Weemhoff JL, et al. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl 2014; 20:1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schurink IJ, de Haan JE, Willemse J, et al. A proof of concept study on real-time LiMAx CYP1A2 liver function assessment of donor grafts during normothermic machine perfusion. Sci Rep 2021; 11:23444. [DOI] [PMC free article] [PubMed] [Google Scholar]