Abstract
Objective:
To determine the feasibility, efficacy, and safety of early cold stored platelet transfusion compared with standard care resuscitation in patients with hemorrhagic shock.
Background:
Data demonstrating the safety and efficacy of early cold stored platelet transfusion are lacking following severe injury.
Methods:
A phase 2, multicenter, randomized, open label, clinical trial was performed at 5 US trauma centers. Injured patients at risk of large volume blood transfusion and the need for hemorrhage control procedures were enrolled and randomized. The intervention was the early transfusion of a single apheresis cold stored platelet unit, stored for up to 14 days versus standard care resuscitation. The primary outcome was feasibility and the principal clinical outcome for efficacy and safety was 24-hour mortality.
Results:
Mortality at 24 hours was 5.9% in patients who were randomized to early cold stored platelet transfusion compared with 10.2% in the standard care arm (difference, −4.3%; 95% CI, −12.8% to 3.5%; P=0.26). No significant differences were found for any of the prespecified ancillary outcomes. Rates of arterial and/or venous thromboembolism and adverse events did not differ across treatment groups.
Conclusions and Relevance:
In severely injured patients, early cold stored platelet transfusion is feasible, safe and did not result in a significant lower rate of 24-hour mortality. Early cold stored platelet transfusion did not result in a higher incidence of arterial and/or venous thrombotic complications or adverse events. The storage age of the cold stored platelet product was not associated with significant outcome differences.
Trial Registration:
ClinicalTrials.gov identifier: NCT 04667468.
Keywords: cold stored platelets, hemorrhage, randomized, traumatic injury
Traumatic injury remains a leading cause of mortality for individuals 45 years and younger and hemorrhage remains the leading cause of potentially preventable death postinjury.1–4 Despite improvements in trauma resuscitation,5–8 the highest rate of mortality from hemorrhage occurs during the first hours after arrival to definitive care,9,10 underscoring the importance of early interventions which provide benefit.11–15
Early platelet transfusion is an essential component of trauma resuscitation and is associated with attenuation of endothelial barrier disruption, improved hemostasis, and attributable survival in severely injured patients.7,16–21 The storage temperature employed for platelets may affect hemostatic function and can impact the platelet supply due to shelf-life constraints.22–24 Cold stored platelets (1–6°C) demonstrate improved hemostatic capabilities, may have a lower risk of bacterial contamination, and can be utilized for urgent transfusion for the acutely bleeding patient.25–30 Recent Food and Drug Administration (FDA) guidance allows for the use of cold stored platelets with 14 days of storage to treat active bleeding when conventional room temperature platelets are not available, or their use is not practical.31 Despite this recent guidance from the FDA, there exists no robust clinical trial evidence demonstrating the safety and efficacy of cold stored platelets (CSPs) in the severely injured patient population at risk of hemorrhage.
The Cold Stored Platelet for Hemorrhagic Shock (CriSP-HS) trial aimed to characterize the safety and efficacy of incorporating early CSP transfusion in injured patients at risk of hemorrhagic shock as compared with standard care trauma resuscitation. We enrolled patients at risk of hemorrhagic shock and allocated patients to early CSP transfusion and concomitant standard care resuscitation or standard care resuscitation alone. We hypothesized that early CSP transfusion would be safe and associated with clinical outcome benefits.
METHODS
Trial Design
The CriSP-HS study was a phase 2, multicenter, open label, randomized clinical trial that compared outcomes in patients at risk for hemorrhagic shock receiving CSP transfusion and concomitant standard care resuscitation versus standard care resuscitation alone. A total of 422 patients who arrived at one of 5 participating level 1 trauma centers were screened for inclusion criteria and assessed for eligibility. We enrolled 200 patients from June 23, 2022, through September 7, 2023. The intervention arm received a single apheresis unit of urgent release CSPs which was transfused as soon as feasible, while concomitant standard care resuscitation was occurring. Resuscitation in the standard care arm followed standard procedures at each participating site. We did not alter any other aspects of a care besides the administration of early CSPs. Patients in the standard care arm were not required to receive platelet transfusion unless indicated by standard resuscitation procedures at the enrolling site. The transfusion of CSPs was not standard care for sites preceding or during enrollment of the trial; room temperature platelets were utilized as standard care when required. The trial protocol can be found as a Supplemental Data File (Supplemental Digital Content 1, http://links.lww.com/SLA/F93).
The US FDA (Investigational New Drug 19467), the Office of Human Research Oversight of the US Department of Defense, and the Human Research Protection Office at the University of Pittsburgh approved the clinical trial. An external data and safety monitoring board oversaw the trial. The single institutional review board (IRB) at the University of Pittsburgh, with review and acknowledgment from local site IRBs, approved an exception from informed consent to enroll participants. This approval included community consultation and public disclosure/notification. We notified enrolled participants and/or their legally authorized representatives as soon as feasible and obtained consent for continued participation.32 The study followed the Consolidated Standard of Reporting Trials (CONSORT, Supplemental Digital Content 2, http://links.lww.com/SLA/F94 and Supplemental Digital Content 3, http://links.lww.com/SLA/F95) reporting guideline.
Study Patient Population
We selected inclusion criteria to include a severely injured population of patients at risk of hemorrhagic shock and large volume transfusion. Injured patients arriving at a participating trauma center were eligible for enrollment if they met Assessment of Blood Consumption (ABC) criteria33,34 (2 or more of the following): (1) hypotension (systolic blood pressure≤90 mm Hg); (2) penetrating mechanism of injury; (3) positive Focused Assessment for the Sonography of Trauma (FAST) examination; (4) Heart rate ≥ 120 and who within 60 minutes required a hemorrhage control procedure in the operating room or interventional radiology suite. Patients were eligible if they met ABC criteria qualifying vital signs in the prehospital or in-hospital phase of care. A FAST examination that was deferred due to the expedient transport to the operating room was considered as meeting one of the ABC criteria.33 Exclusion criteria included ages less than 15 years or more than 90 years, fall from standing, penetrating brain injury, >5 minutes of cardiopulmonary resuscitation (CPR), known prisoners or known pregnancy, isolated drowning or hanging victims, burns >20% total body surface area, objection to the study voiced by subject or family or wearing a “No CriSP” opt-out bracelet.
Randomization and Matching
We generated a 1:1 ratio random allocation sequence to either CSP arm or standard care arm with variable block sizes of 4 to 6, stratified by site, using a computer random-number generator. Predefined randomization assignment envelopes were maintained within easy access in the trauma bay or emergency department. Randomization was performed by a member of the study team with randomization cards in sealed envelopes after eligibility was confirmed at the individual patient level and in real-time. Clinical providers were unable to be masked to treatment assignment. Arm assignment was concealed to outcome assessors.
Intervention and Comparison Arms
After meeting all inclusion and no exclusion criteria, patients randomized to the CSP arm received a single apheresis cold stored platelet unit prepared following all FDA approved guidelines as soon as feasible.31 CSP units were refrigerated within 4 hours of donation and were stored in a monitored refrigerator at 1 to 6°C for up to 14 days from the date of donation. The CSP unit was transfused as soon as feasible in the trauma bay, during transport, or in the operating theater or interventional radiology suite. Participating site standard care resuscitation was performed concomitantly.
Patients who met all inclusion criteria and no exclusion criteria and who were randomized to the standard care arm received the site’s standard care resuscitation. Standard care arm patients were not required to received room temperature platelet transfusion.
Outcomes
The primary outcome for the trial was feasibility, including: the proportion of patients able to be randomized, the proportion of eligible patients enrolled, the proportion of patients enrolled with adherence to the study protocol and the proportion of patients for whom the principal clinical outcome of 24-hour mortality was obtained.
Our prespecified principal outcome for assessing safety and efficacy for the trial was 24-hour mortality. Additional secondary outcomes included 3-hour mortality, in-hospital mortality, 30-day mortality, death from adjudicated hemorrhage, acute respiratory distress syndrome, time to hemostasis, incidence of arterial or venous thromboembolic events, laboratory coagulation assessment, and 24-hour blood component transfusion requirements.
Achievement of hemostasis was defined when a patient received a single unit or less of blood transfusion in any 60-minute period in the first 4 hours from enrollment. Those patients who did not reach this blood transfusion nadir were considered to not have achieved hemostasis.
Prespecified subgroup analyzes for the 24-hour mortality outcome included: (1) patients who did or did not require in-hospital blood transfusion; (2) patients who did or did not require massive transfusion (≥10 U blood in first 24 hours); (3) patients with and without significant traumatic brain injury (head Abbreviated Injury Score—AIS >2); (4) patients who arrived from the scene of injury versus from a referring hospital; and (5) patients who received CSPs with an age since donation of ≤7 days as compared with 8 to 14 days.
Statistical Analysis
The feasibility of enrollment was evaluated by determining the proportion of patients in each feasibility category. These proportions were estimated directly as the observed ratio of numbers of patients, with 95% CIs being calculated.
The principal intent-to-treat safety and efficacy analysis compared 24-hour mortality across the CSP and standard care arms using a 2-sided Z-test for proportions. The Kaplan-Meier curves were generated for each treatment group and a log-rank test was used to compare the distribution of the cumulative proportion. A logistic regression model was used to assess the independent impact of the CSP intervention on 24-hour survival after controlling for site of enrollment and the potential effects of baseline characteristics with imbalance between treatment groups.
Analyzes to test for the homogeneity of the treatment effect were carried out for the 4 prespecified randomized subgroups. Regression models appropriate for the outcome variable were used to test for the homogeneity of the treatment effect. For the prespecified CSP age less than or equal to 7 days versus 8 to 14 days old analyzes, outcomes were compared across each age subgroup. Since the CSP age was not randomly assigned, a propensity score was generated as an indicator of the age of the CSP (≤7 vs 8–14 days) and multivariable regression models were used to assess the independent relationship of age of the CSP product on 24-hour mortality. All other secondary outcomes were also compared across the CSP product age groups.
We estimated there would be 80% power to detect a 14.2% difference using a mortality estimate of 23% in the control arm, a type I error rate of 0.05, a 2-sided alternative hypothesis, and 1 df with a sample size of 100 patients per treatment arm.
RESULTS
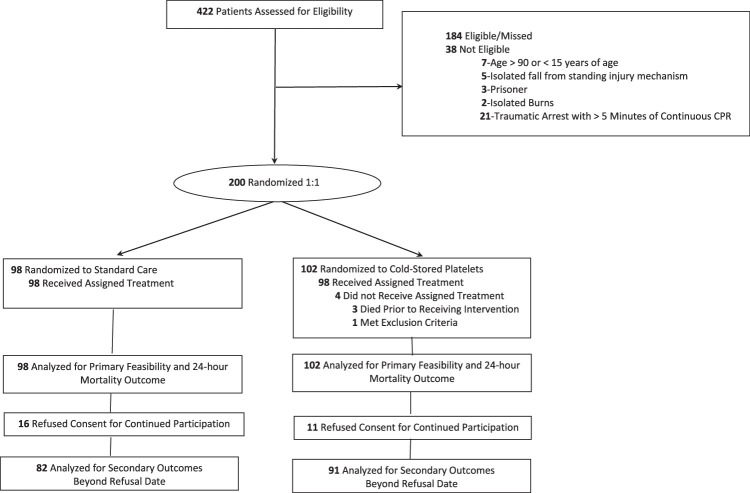
A total of 422 patients were assessed for trial eligibility, of which 384 met all inclusion and no exclusion criteria, with 200 patients being randomized, constituting the intent-to-treat cohort. There were 184 patients who met all inclusion criteria but were unable to be randomized due to staff or product unavailability (Fig. 1). Of the 200 patients in the intent-to-treat cohort, 102 were randomized to the early CSP arm, and 98 were randomized to the standard care arm. The primary and 24-hour mortality outcomes were available for 102 (100%) patients in the CSP arm and 98 (100%) patients in the standard care arm. Eleven patients in the CSP arm and 16 patients in the standard care arm refused consent for continued participation from secondary outcome comparisons. All events before refusal of consent for continued participation were included in the analyzes. We estimated a 24-month enrollment period for the study. We completed the study after reaching our target enrollment of 200 patients after 15 months.
FIGURE 1.
Flow of patients in the cold stored platelets in hemorrhagic shock (CRISP-HS) trial. Screening, randomization, and follow-up of the study participants. All data and events before refusal of consent for continued participation were included in clinical outcome comparisons.
Enrolled patients had an average age of 34±13 years, were 85.0% male, were injured via a penetrating mechanism 78.5% of the time, had a median Injury Severity Score (ISS) of 17 (interquartile range, 9–28) and had an all-cause mortality of 8.0% within 24 hours postinjury. Surgeons performed operative intervention in 97% of cases, while 3% required interventional radiology hemorrhage control procedures.
The assigned treatment regimen was provided to the enrolled patient 98.0% of the time. For the CSP randomized arm, 98 (96.1%) of the 102 patients received the early CSP transfusion. In the standard care arm, 98 (100%) of the 98 of patients received standard care resuscitation with 47 (48%) patients receiving room temperature platelet transfusion. The CSP and standard care arms were similar in demographics, injury characteristics and shock severity (Table 1). When complete blood count of enrolled patients was compared, the groups were also similar (Supplemental Digital Content 4, Table 1S, http://links.lww.com/SLA/F96).
TABLE 1.
Baseline Characteristics by Treatment Arm*
| n/N (%) | ||
|---|---|---|
| Characteristics | Standard care (N=98) | Cold stored platelets (N=102) |
| Age [mean (SD)] | 35.3 (14.4) | 34.4 (13.5) |
| Male sex | 78/98 (80) | 85/102 (83) |
| Race | ||
| White | 18/98 (18) | 28/102 (28) |
| Black | 51/98 (52) | 47/102 (47) |
| Other | 13/98 (13) | 13/102 (13) |
| Hispanic | 13/98 (13) | 20/102 (20) |
| SBP [median (IQR)] (mm Hg) | ||
| Initial | 108.5 (84.5, 129.5) | 99 (83, 140) |
| Highest | 122 (102, 144) | 127 (98, 148) |
| Lowest | 93.5 (78, 117.5) | 90 (78, 127) |
| HR [median (IQR)] (beats/min) | ||
| Initial | 106 (86, 123) | 112 (88, 130.5) |
| Highest | 111.5 (93, 130) | 116 (96.5, 135) |
| Lowest | 95 (81.5, 117) | 101 (75, 123) |
| Transport mode | ||
| Ground EMS | 66/98 (67) | 71/102 (70) |
| Air EMS | 24/98 (24) | 20/102 (20) |
| Does not apply† | 5/98 (5) | 4/102 (4) |
| Transferred from | ||
| Scene of accident/injury | 65/98 (66) | 73/102 (72) |
| Home | 4/98 (4) | 3/102 (3) |
| Other hospital | 26/98 (26) | 19/102 (19) |
| Mechanism of injury blunt | 23/98 (23) | 12/102 (12) |
| Fall | 5/98 (5) | 2/102 (2) |
| MVC occupant ejected | 3/98 (3) | 1/102 (1) |
| MVC occupant not ejected | 6/98 (6) | 3/102 (3) |
| MVC motorcycle | 5/98 (5) | 3/102 (3) |
| MVCpedestrian | 1/98 (1) | 2/102 (2) |
| Struck by or against | 3/98 (3) | 1/102 (1) |
| Mechanism of injury penetrating | 76/98 (77) | 90/102 (88) |
| Firearm | 53/98 (54) | 62 /102 (61) |
| Impalement | 1/98 (1) | 1/102 (1) |
| Stabbing | 20/98 (20) | 26/102 (25) |
| Other | 2/98 (2) | 1/102 (1) |
| AIS [median (IQR)] | ||
| Head and Neck AIS | 0, (0, 0) | 0, (0, 0) |
| Face AIS | 0, (0, 0) | 0, (0, 0) |
| Chest AIS | 1.5 (0, 3) | 0 (0, 3) |
| Abdomen AIS | 3 (0, 4) | 2 (0, 4) |
| External AIS | 1 (0, 1) | 1 (0, 1) |
| Extremity AIS | 0 (0, 3) | 0 (0, 3) |
| ISS‡ [median (IQR)] | 17.5 (10, 29) | 17 (9, 27) |
| TBI | 9/98 (9) | 6/102 (6) |
| Prehospital advanced airway§ | 16/98 (16) | 12/102 (12) |
| Supraglottic airway | 2/98 (2) | 1/102 (1) |
| Endotracheal intubation | 14/98 (14) | 11/102 (11) |
| Prehospital crystalloid/colloid fluids§ | 42/98 (43) | 36/102 (35) |
| Crystalloid/colloid volume [median (IQR)] (mL) | 1000 (300, 1000) | 700 (300, 2000) |
| Prehospital blood product§ | ||
| Whole blood | 6/98 (6) | 6/102 (6) |
| Whole blood volume [median (IQR)] (U) | 1 (1, 1) | 1.5 (1, 2) |
| Plasma | 14/98 (14) | 9/102 (9) |
| Plasma volume [median (IQR)] (U) | 2 (1, 2) | 2 (2, 2) |
| Red blood cell | 22/98 (22) | 14/102 (14) |
| Red blood cell volume [median (IQR)] (U) | 2 (1, 3) | 2 (1, 4) |
| Platelets | 1/98 (1) | 0/102 (0) |
AIS indicates Abbreviated Injury Score; ED, emergency department; EMS, Emergency Medical Services; HR, heart rate; IQR, interquartile range; ISS, Injury Severity Score; MVC, motor vehicle crash; RR, respiratory rate; SBP, systolic blood pressure; TBI, traumatic brain injury.
No statistically significant differences were observed between baseline characteristics.
Not delivered to the ED by EMS.
The score range was 0 to 75. A score >15 indicates major trauma.
Provided by EMS during transport or at an outside ED before transfer.
For the primary feasibility outcome, the proportion of eligible patients who could be randomized was 91%, (95% CI, 88.3%–93.7%), with 52.1%, (95% CI, 47.1%–57.1%) being randomized and enrolled in the trial. The proportion of enrolled patients with adherence to study protocol was 98%, (95% CI, 96.1%–99.9%). Finally, the proportion of enrolled patients where the status of the principle clinical outcome of 24-hour mortality was known/available was 100%.
At 24 hours after arrival, 6 deaths occurred in the CSP arm and 10 deaths in the standard care arm. Patients randomized to early CSP compared with standard care did not significantly differ in the rate of 24-hour mortality (5.9% vs 10.2%; difference, -4.3%; 95% CI, −12.8% to 3.5%; P=0.26). Kaplan-Meier survival curves demonstrate early separation in curves which did not reach statistical significance (log-rank χ2=1.19, P=0.27; Fig. 2). In a binary logistic regression model controlling for site, the assignment to the CSP arm did not significantly change the odds of 24-hour mortality (adjusted odds ratio 0.56; 95% CI, 0.18–1.56; P=0.27).
FIGURE 2.
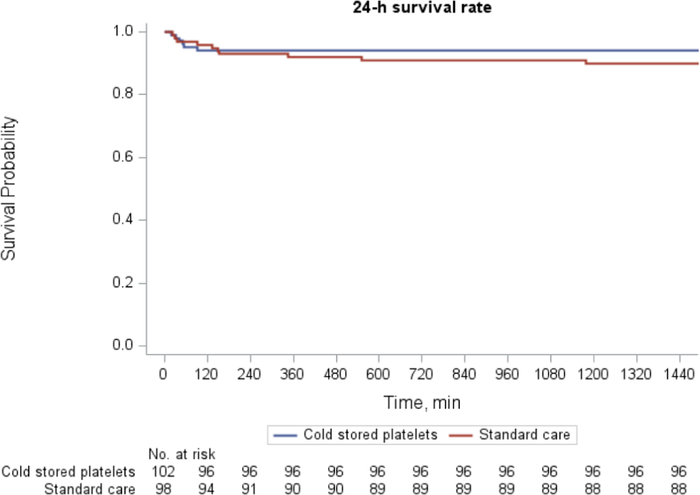
Kaplan-Meier survival analysis at 24-hour postrandomization.
Mortality at 24 hours in the 4 prespecified randomized subgroups is depicted in Figure 3. No heterogeneity of treatment effect across any of the randomized subgroups was found.
FIGURE 3.
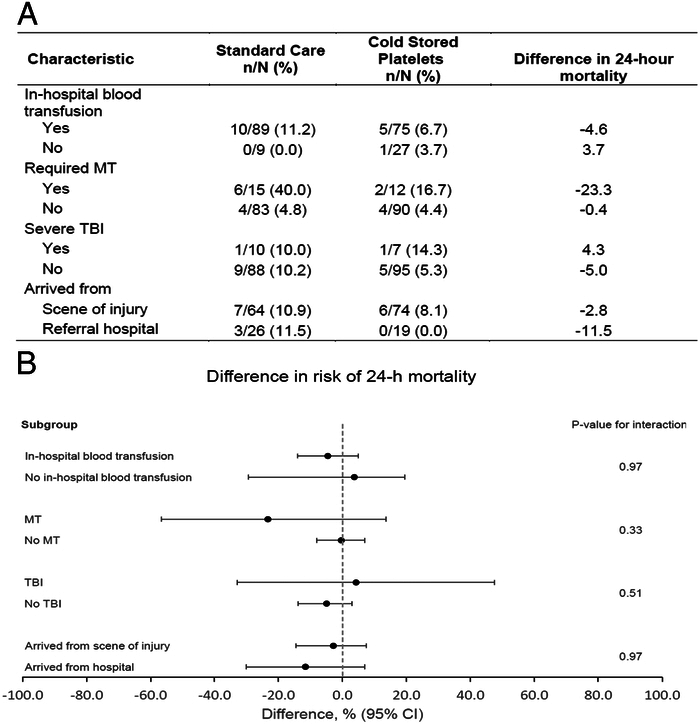
Subgroup analysis for 24-hour mortality. A, Differences in 24-hour mortality in the 4 prespecified subgroups. B, Twenty-four hour mortality differences with CIs. The black circles represent the difference in 24-hour mortality and horizontal bars represent the 95% CI. The difference was computed as 24-hour mortality for CSP minus 24-hour mortality for SC. The 95% CIs are exact (Clopper-Pearson) confidence limits. The gray dashed vertical line represents a difference in 24-hour mortality of 0, indicating no difference in 24-hour mortality between CSP and SC treatment arms. P-values are for the interaction term between treatment arms and subgroup in a logistic regression model with 24-hour mortality as the outcome. CSP indicates cold stored platelets; SC, standard care; MT, massive transfusion; TBI, traumatic brain injury.
No group differences were found in 3-hour mortality (difference, −1.3%, 95% CI, −9.1% to 6.3%; P= 0.72), in-hospital mortality (difference, −2.7; 95% CI, −13.8% to 8.2%; P= 0.62) or 30-day mortality (difference, −2.7; 95% CI, −13.8% to 8.2%; P=0.62) (Table 2). No significant differences were found in the incidence of death due to hemorrhage (difference −0.5%; 95% CI, −8.7% to 7.4%; P=0.89), the incidence of ARDS (difference 2.8%; 95% CI, −4.0% to 9.8%; P=0.36) or in the incidence of arterial or venous thromboembolic events (difference, −0.8%; 95% CI, −10.2% to 8.3%; P=0.86). There were no differences in the achievement of hemostasis (difference, 0.3; 95% CI, −6.8 to 7.4; P=0.94) or time to hemostasis (Supplemental Digital Content 4, Figure 1S, http://links.lww.com/SLA/F96) across the groups. There were no significant group differences in coagulation measurements or differences in 24-hour blood transfusion requirements. There were no documented transfusion/allergic reactions in either arm of the trial.
TABLE 2.
Clinical Outcomes by Treatment Group
| Unadjusted analysis | Adjusted analysis | |||||
|---|---|---|---|---|---|---|
| Clinical outcome | Standard Care (N=98) [n (%)] | Cold stored platelets (N=102) [n (%)] | Difference* (95% CI) | P | OR/difference† (95% CI) | P |
| Principle clinical outcome | ||||||
| 24-h mortality | 10 (10.2) | 6 (5.9) | −4.3% (−12.8, 3.5) | 0.26 | 0.6 (0.2, 1.6) | 0.27 |
| Secondary clinical outcome | ||||||
| 3-h mortality | 7 (7.1) | 6 (5.9) | −1.3% (−9.1, 6.3) | 0.72 | 0.9 (0.3, 2.9) | 0.86 |
| In-hospital mortality‡ | 13/82 (15.8) | 12/91 (13.2) | −2.7% (−13.9, 8.2) | 0.62 | 0.8 (0.3, 1.9) | 0.62 |
| 30-day mortality ‡ § | 13/82 (16) | 12/91 (13) | −2.7% (−13.9, 8.2) | 0.62 | 0.8 (0.3, 1.9) | 0.62 |
| Mortality from hemorrhage | 7 (7.1) | 7 (6.9) | −0.3% (−8.8, 7.4) | 0.89 | 1.0 (0.3, 3.1) | 0.99 |
| ARDS | 3/93 † (3.2) | 6/100†(6.0) | 2.8% (−4.0, 9.8) | 0.36 | 2.2 (0.6, 11.0) | 0.27 |
| Allergic/transfusion reaction | 0 (0) | 0 (0) | - | - | ||
| Thromboembolic events | 10/93†(10.8) | 10/100† (10.0) | −0.8% (−10.2, 8.3) | 0.86 | 0.9 (0.4, 2.4) | 0.85 |
| Achieve hemostasis | 91/98 (92.9) | 95/102 (93.1) | 0.3 (−6.8, 7.4) | 0.94 | 1.1 (0.4, 3.2) | 0.90 |
| Initial rapid thromboelastography measurements∥ ¶ [median (IQR)] | ||||||
| Activated clotting time (sec) | 105 (97, 113) | 113 (105, 113) | 8.0 (−8.0, 8.0) | 0.79 | 0.5 (−11.2, 5.5) | 0.49 |
| K-time (min) | 1.9 (1.3, 2.3) | 2 (1.4, 2.2) | 0.1 (−0.5, 0.5) | 0.97 | −0.2 (−0.7, 0.3) | 0.39 |
| Alpha angle (deg.) | 69 (66, 73) | 72 (67, 73) | 3.0 (−2.0, 5.0) | 0.56 | 1.4 (−2.2, 5.0) | 0.43 |
| Maximal amplitude (mm) | 56.4 (51.1, 62.2) | 57.7 (48.9, 63.6) | 1.3 (−2.4, 3.4) | 0.55 | 0.1 (−3.2, 3.5) | 0.94 |
| LY30 (%) | 0.10 (0, 1.30) | 0.35 (0, 1.25) | 0.3 (0, 0.2) | 0.27 | −0.5 (−1.8, 0.9) | 0.49 |
| 24-h rapid thromboelastography measurements∥ # [median (IQR)] | ||||||
| Activated clotting time (sec) | 121 (105, 121) | 113 (97, 121) | −8.0 (−16.0, 8.0) | 0.45 | −3.7 (−16.9, 9.5) | 0.56 |
| K-time (min) | 1.6 (1.2, 2.6) | 2.0 (1.4. 2.3) | 0.4 (−0.6, 0.9) | 0.70 | 0.1 (−0.8, 0.9) | 0.90 |
| Alpha angle (deg.) | 73 (70, 76) | 73 (69, 75) | 0.0 (−7.0, 4.0) | 0.67 | 0.3 (−6.0, 6.5) | 0.93 |
| Maximal amplitude (mm) | 60.2 (56.6, 64.2) | 57.4 (52.4, 64.6) | −2.8 (−6.0, −0.4) | 0.09 | −2.5 (−6.2, 1.2) | 0.18 |
| LY30 (%) | 0.3 (0, 2.1) | 0.8 (0, 2.0) | 0.6 (−0.2, 0.7) | 0.75 | −0.1 (−0.8, 0.7) | 0.89 |
| Initial coagulation measurements | ||||||
| Prothrombin time (sec)** | 12.6 (11.6, 14.2) | 12.9 (11.2, 4.3) | 0.3 (−1.0, 0.6) | 0.58 | −0.1 (−0.8, 0.6) | 0.74 |
| International normalized ratio†† | 1.1 (1.1, 1.2) | 1.1 (1.0, 1.2) | 0.0 (−0.1, 0.0) | 0.46 | 0.0 (−0.1, 0.1) | 0.73 |
| 24-h coagulation measurements | ||||||
| Prothrombin time (sec)‡‡ | 13.9 (12.2, 15.8) | 14.5 (12.6, 7.2) | 0.6 (−0.6, 1.8) | 0.29 | 0.4 (−0.6, 1.4) | 0.82 |
| International normalized ratio§§ | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.4) | 0.0 (−0.1, 0.1) | 0.54 | 0.0 (−0.1, 0.1) | 0.53 |
| 24-h transfusion requirements | ||||||
| Red cells (U) | 5.5 (3, 9) | 6 (2, 10) | 0.5 (−1.0, 1.0) | 0.94 | 0.1 (−2.6, 2.8) | 0.95 |
| Plasma (U) | 5 (3, 9) | 4 (2, 9) | −1.0 (−1.0, 1.0) | 0.75 | 0.2 (−2.3, 2.7) | 0.85 |
| Platelets (U) | 1 (1, 3) | 1 (1, 3) | 0.0 (0.0, 0.0) | 0.86 | 0.1 (−0.9, 1.1) | 0.79 |
| Whole cells (U) | 1 (1, 2) | 1 (1, 2) | 0.0 (0.0, 1.0) | 0.86 | −0.4 (−1.9, 1.1) | 0.56 |
| Total (U) | 10 (5, 18) | 9.5 (5, 19) | −0.5 (−3.0, 2.0) | 0.92 | 0.1 (−5.3, 5.5) | 0.98 |
| Total (mL) | 3089 (1547, 5333) | 2881 (1456, 5876) | −208 (−754, 754) | 0.97 | −8.3 (−1619, 1603) | 0.99 |
OR indicates odds ratio.
Difference in proportions (difference) or medians (Wilcoxon rank-sum test).
Adjusted for study site.
Excluding 27 subjects who refused participation past 24-hour endpoint (16 in standard care arm and 11 in cold stored platelets arm).
Patients with attempted follow-up without documented mortality at 30-days and with verified in-hospital survival considered alive (standard care, n=10; cold stored platelet, n=14).
Thromboelastography measurements provide viscoelastic properties of a blood sample. Activated clotting time is the time in seconds between initiation of the test and the initial fibrin formation and is increased with factor deficiency or severe hemodilution. The α-angle is the slope of the tracing that represents the rate of clot formation, decreasing with hypofibrinogenemia or platelet deficiency. K-time is the time in minutes needed to reach 20 mm clot strength and is generally increased with hypofibrinogenemia or platelet deficiency. The maximal amplitude is the greatest amplitude of the tracing and reflects platelet contribution to clot strength. LY30 is the percent amplitude reduction at 30 minutes after the maximal amplitude and when elevated reflects a state of hyperfibrinolysis.
Unavailable in 25 patients in the standard care group (n=73) and 36 patients in the cold stored platelet group (n=66).
Unavailable in 52 patients in the standard care group (n=46) and 55 patients in the cold stored platelet group (n=47).
Unavailable in 35 patients in the standard care group (n=63) and 44 patients in the cold stored platelet group (n=58).
Unavailable in 35 patients in the standard care group (n=63) and 44 patients in the cold stored platelet group (n=58).
Unavailable in 47 patients in the standard care group (n=51) and 61 patients in the cold stored platelet group (n=41).
Unavailable in 47 patients in the standard care group (n=51) and 61 patients in the cold stored platelet group (n=41).
The total number of adverse events and serious adverse events was similar across CSP and standard care arms. The number of serious adverse events with any relatedness was also similar across the randomized groups (Table 3).
TABLE 3.
Trial Adverse Events
| Standard care (N=98) | Cold stored platelets (N=102) | |
|---|---|---|
| Adverse events total | 68 | 61 |
| Serious adverse events total | 52 | 49 |
| Individual serious adverse events with any relatedness | ||
| Coagulopathy | 0 | 1 |
| Pneumonia/VAP | 1 | 1 |
| Arterial thrombosis | 1 | 1 |
| Deep vein thrombosis | 0 | 2 |
| Pulmonary embolism | 2 | 3 |
| Transfusion-associated cardiac overload | 0 | 1 |
| Serious adverse events with any relatedness total | 4 | 9 |
Adverse events were at the discretion of the treating physician. An adverse event was any adverse reaction that was thought to be related in any magnitude to the trial regimen. Serious adverse events were defined as any adverse reaction that resulted in death, was life-threatening, resulted in prolongation of existing hospitalization or resulted in persistent or significant disability or incapacity. Severity of an adverse event and relatedness was adjudicated by the site investigator. Individual adverse events listed include all designations other than “definitely not related” that were designated as Serious. All relatedness and severity determinations occurred during trial execution. Event relatedness and severity determinations were to be assessed independently of the unblinded intervention. All pulmonary emboli, deep vein thromboses, and arterial thromboses, irrespective of relatedness were also compared as a formal secondary outcome.
There was no association between 24-hour mortality and the storage age of the cold stored platelet product transfused after accounting for confounders via propensity score adjustment (adjusted odds ratio, 1.4; 95% CI, 0.12–17.6; P=0.77; Supplemental Digital Content 4, Table 2S, http://links.lww.com/SLA/F96) When other secondary outcomes including laboratory measurements were compared across the CSP age groups, no significant differences were found (Table 4).
TABLE 4.
Trial Clinical Outcomes by Age of Cold Stored Platelet Product
| Cold stored platelets shelf time | ||||
|---|---|---|---|---|
| Clinical outcome | ≤7 d (n=41) | 8–14 d (n=57) | Difference* (95% CI) | P |
| Principal clinical outcome | ||||
| 24-h mortality | 1 (2.4) | 2 (3.5) | −1.1% (−10.1,10.1) | 0.78 |
| Secondary clinical outcome | ||||
| 3-h mortality | 1 (2.4) | 2 (3.5) | −1.1% (−10.1, 10.1) | 0.78 |
| In-hospital mortality | 4/39 (10.3) | 5/49 (10.2) | 0.1% (−13.5,15.7) | 0.99 |
| 30-day mortality † ‡ | 4/39 (10.3) | 5/49 (10.2) | 0.1% (−13.5, 15.7) | 0.99 |
| Mortality from hemorrhage† | 3/40 (7.5) | 2/56 (3.6) | 3.9% (−6.2, 16.9) | 0.65 |
| ARDS† | 3/40 (7.5) | 3/56 (5.4) | 2.1% (−8.7, 15.6) | 0.69 |
| Allergic/transfusion reaction | 0 (0) | 0 (0) | − | |
| Thromboembolic events† | 4/40 (10.0) | 6/56 (10.7) | −0.7% (−0.1, 0.2) | 0.91 |
| Initial rapid thromboelastography measurements§ ∥ [median (IQR)] | ||||
| Activated clotting time (sec) | 113 (105,117) | 89 (89, 113) | −24 (−31, 0) | 0.06 |
| K-time (min) | 1.9 (1.45, 2.25) | 1.4 (1.2, 2.0) | −0.5 (−1, 0.4) | 0.14 |
| Alpha angle (deg.) | 70.5 (65, 73) | 73 (67, 76) | 2.5 (−2, 11) | 0.18 |
| Maximal amplitude (mm) | 57 (47.2, 62.0) | 59.4 (50.9, 64.7) | 2.4 (−6.6, 2) | 0.27 |
| LY30 (%) | 0.3 (0, 1.9) | 0.6 (0, 1.2) | 0.3 (−0.6, 0.3) | 0.84 |
| 24-h rapid thromboelastography measurements§ ¶ [median (IQR)] | ||||
| Activated clotting time (sec) | 113 (113, 121) | 101 (97, 113) | −12 (−47, 16) | 0.41 |
| K-time (min) | 1.3 (1.1, 2.3) | 2.1 (1.9, 2.2) | 0.8 (−2.0, 1.2) | 0.33 |
| Alpha angle (deg.) | 76 (69, 77) | 71 (67, 73) | −5 (−12, 12) | 0.31 |
| Maximal amplitude (mm) | 57 (51.2, 64.6) | 57.3 (53, 65) | 0.3 (−6.4, −3) | 0.41 |
| LY30 (%) | 0.8 (0, 2.1) | 0.7 (0, 1.8) | −0.1 (−0.9, 1) | 0.78 |
| Initial coagulation measurements | ||||
| Prothrombin time (sec)# | 12.5 (10.8, 13.8) | 13 (11.6, 14.5) | 0.5 (−1.8, 0.5) | 0.25 |
| International normalized ratio** | 1.1 (1.1, 1.2) | 1.1 (1.0, 1.3) | 0 (−0.1, 0) | 0.12 |
| 24-h coagulation measurements | ||||
| Prothrombin time (sec)†† | 14.3 (12.8, 17.1) | 14.4 (12.8, 17.2) | 0.1 (−2.7, 1.5) | 0.64 |
| International normalized ratio‡‡ | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.4) | 0.1 (−0.2, 0.1) | 0.39 |
| Transfusion requirements within 24 h | ||||
| Red cells (U) | 6 (3, 11) | 6 (2, 9) | 0 (−2, 3) | 0.71 |
| Plasma (U) | 4.5 (2.5, 11.5) | 4 (2, 8.5) | −0.5 (−1, 3) | 0.52 |
| Platelets (U) | 2 (1, 4.5) | 1 (1, 2) | −1 (−1, 2) | 0.08 |
| Whole blood (U) | 1.5 (1, 3) | 1 (1, 2) | −0.5 (−3, 0) | 0.44 |
| Total (U) | 11 (5, 21) | 9.5 (4.5, 19) | −1.5 (−3, 6) | 0.60 |
| Total (mL) | 3,432 (1,599, 6,201) | 2,881 (1,411, 5,612) | −551 (−845, 1,833) | 0.56 |
OR indicates odds ratio.
Difference in proportions or medians (Wilcoxon rank-sum test).
Excluding subjects who refused participation past 24-hour endpoint.
Patients with attempted follow-up without documented mortality at 30-days and with verified in-hospital survival considered alive (≤7 days, n=9 ; 8–14 days, n=5).
Thromboelastography measurements provide viscoelastic properties of a blood sample. Activated clotting time is the time in seconds between initiation of the test and the initial fibrin formation and is increased with factor deficiency or severe hemodilution. The α-angle is the slope of the tracing that represents the rate of clot formation, decreasing with hypofibrinogenemia or platelet deficiency. K-time is the time in minutes needed to reach 20 mm clot strength and is generally increased with hypofibrinogenemia or platelet deficiency. The maximal amplitude is the greatest amplitude of the tracing and reflects platelet contribution to clot strength. LY30 is the percent amplitude reduction at 30 minutes after the maximal amplitude and when elevated reflects a state of hyperfibrinolysis.
Unavailable in 12 patients in the ≤7 days group (n=29) and 21 patients in the 8 to 14 days group (n=36).
Unavailable in 22 patients in the ≤7 days group (n=19) and 30 patients in the 8 to 14 days group (n=27).
Unavailable in 20 patients in the ≤7 days group (n=21) and 21 patients in the 8 to 14 days group (n=36).
Unavailable in 20 patients in the ≤7 days group (n=21) and 21 patients in the 8 to 14 days group (n=36).
Unavailable in 24 patients in the ≤7 days group (n=17) and 34 patients in the 8 to 14 days group (n=23).
Unavailable in 24 patients in the ≤7 days group (n=17) and 34 patients in the 8 to 14 days group (n=23).
To verify any outcomes differences in those patients who actually received platelet transfusion across the randomized groups, we performed similar comparisons as was done for the intent-to-treat analysis. There remained no significant differences across the platelet transfused randomized groups (Table 3S, Supplemental Digital Content 4, http://links.lww.com/SLA/F96).
Finally, when the time to first platelet transfusion was compared across the randomized arms for those patients who received platelets, patients randomized to CSP, due to the urgent release capabilities of the product, received their platelet transfusion significantly earlier than those in the standard care arm from the time of randomization [median time (IQR) to first platelet 31 (16, 53) vs 87 (45, 143) minutes, P<0.01).
DISCUSSION
Resuscitation strategies for the severely injured patient at risk of hemorrhage continues to evolve, with a focus on initiation of early blood product transfusion and hemostatic adjuncts which have been shown to improve outcomes.5,6,8,11,14,15 Early platelet transfusion for traumatic bleeding has been demonstrated to be associated with lower mortality and improved hemostasis.7,16,18,19,35 In 2019, the military was afforded an FDA variance allowing the use of CSPs out to 14 days for combat situations.36 In June, 2023, The FDA approved cold stored platelet use for bleeding when standard room temperature platelets are not available, or their use is not practical for the civilian population.31 The results of the current randomized, multicenter, phase 2, clinical trial provides timely and vital evidence for the feasibility and safety of early cold stored platelet transfusion in patients with hemorrhage. The design of the trial attempted to characterize the early transfusion of a platelet product that can be provided early, in an urgent release fashion, as well as the safety and efficacy of cold storage. For the 200 patients enrolled in the trial, those who received early cold stored platelets had a lower 24-hour mortality rate difference that did not reach statistical significance. Importantly, the study did not find a higher rate of arterial or venous thromboembolism or a higher incidence of other adverse events. The current results also provide evidence suggesting the cold stored platelet product stored out to 14 days may be safe for the treatment of hemorrhage.
The ability to safely transfuse cold stored platelets for the bleeding patient can dramatically augment the platelet supply and represents the most efficient use of a precious resource. Demand for blood products can exceed the available inventory. Cold storage of platelets increases their shelf-life currently to 14 days while lowering the risk of bacterial contamination, expanding the available inventory for patient care.24,28,30,37 CSPs have been demonstrated to have improved hemostatic capabilities.26,27,38 Incorporation of CSPs into the available armamentarium for the treatment of injury-related hemorrhage has the potential to provide an essential blood component for urgent transfusion with similar or more robust hemostatic capabilities.22–24,30 Cold storage also can improve accessibility in rural and austere environments including combat and mass casualty situations that otherwise would have limited platelet transfusion capabilities due to shelf-life restrictions.2,3,39
Room temperature platelets became the principal platelet product available for transfusion since the early 1970’s due to their longer survival in circulation based upon radioactive labeling experiments.40–42 The age of room temperature stored platelets has also been previously demonstrated to affect their hemostatic function.43–46 The current results demonstrate similar outcomes and coagulation measurements across patients who received cold stored platelets within ≤7 days of donation relative to 8 to 14 days since donation. The current results also provide preliminary evidence that the urgent release capability of a cold stored platelet product may promote a shorter time to first platelet transfusion for severely injured patients.
Strength and Limitations
The multicenter study has strengths including generalizability across multiple trauma centers and geographic regions and being randomized at the level of the patient. Patients with a wide spectrum of injury characteristics were enrolled. The study characterized the safety and efficacy of the storage temperature of the platelet products, but also the incorporation of early platelet transfusion into trauma resuscitation practice as the standard care arm was not required to receive room temperature platelet transfusion. The results represent the first randomized evidence assessing the safety and efficacy of CSPs in clinical practice following injury. The adverse event monitoring and recording was robust and demonstrated the safety of the transfusion product. Although the current results focus on patients with severe injury and concomitant hemorrhage, these results may be applicable to other types of bleeding diatheses. Other clinical trials characterizing the efficacy and safety of CSPs in both injury-related populations and noninjury cohorts are currently underway and will further expand our understanding and the potential benefits attributable to CSP transfusion.47,48
Limitations of the trial include its phase 2 design and smaller sample size. The intervention could not be blinded resulting in potential bias. There was a greater than expected rate of penetrating mechanism of injuries enrolled and overall mortality was lower than estimated.49 The trial was powered based upon previous clinical trial data which included a larger proportion of blunt injured patients with a higher rate of mortality.5,6,8 In addition to a lower overall mortality for the cohort, the current inclusion criteria utilized resulted in a cohort of patients with a lower than expected incidence of severe hemorrhage and coagulopathy. Not all patients in the standard care arm received platelet transfusion based upon standard practice at the enrolling sites and this represents a potential confounding difference across comparison groups for the intent-to-treat analysis. There may be participating site differences which cannot be controlled for by model adjustment alone. There were differences in enrollment rates across participating sites. The current trial utilized high volume, level 1, trauma centers and the current results may not by applicable to trauma centers with different attributes and capabilities. Missing data limits the ability to draw conclusions from laboratory measurements.
CONCLUSIONS
Cold stored platelets can be provided early, without evidence of a higher rate of arterial or venous thromboembolism, adverse events, or clinical outcome differences based upon age of the platelet product out to 14 days following donation. Early cold stored platelet transfusion of one apheresis unit did result in a lower rate of 24-hour mortality which did not reach statistical significance. There were no significant differences in all other clinical outcomes. Definitive phase 3 clinical trials are needed for definitive comparison as the current results are not appropriately powered for clinical outcomes. The current results provide important safety information and further impetus to characterize CSPs following severe traumatic injury and demonstrates the need the characterize the safety and efficacy of CSPs in non–injury-related populations. The incorporation of urgent release cold stored platelets into trauma resuscitation practice in patients at risk of hemorrhagic shock is feasible and safe. Cold stored platelets have the potential to expand the available platelet supply to treat bleeding.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all the research personnel and providers at all enrolling sites, who were essential for the successful execution of the trial.
Footnotes
The CRISP-HS study group collaborators: University of Pittsburgh: LITES ClinicalCoordinating Center: Meghan Buck, Chelsea Peet, NatalieRogers, Alexandra Merti, Elizabeth Gimbel, Leah Agostino, Dawn Renee Weinman,Alexandra Correll, Barbara Early-Young; LITES Data Coordinating Center:Laurie Silfies, Angela Pattison, Melody Macey-Kalcevic, Tina Panthalukaran,Lisa Over. University of Mississippi: Sarah M. Meagher, Tamara Brocks,Lanise Lacey, Christy G. Barrick, and the Surgical ICU nurse practitioner staff;University of California, San Francisco: Brenda Nunez-Garcia, MarcelaMatheus, Christopher Lee, Deanna Lee, Isabel Arango, Celine (Yu) Chou, SuzannaChak, Seif Elmankabadi, Jennifer Reid, Kent Garber, Cheyenne Sonntag, ArielKnight, Eireen Malari; University of Texas Health Science Center at Houston:Iqra Rehman, Victoria Herrick, Thet Thet Khin, Kendra Tyner,and Rhonda Hobbes; Baylor College of Medicine: Andrea AlmaguerJuarez, Damian Jimenez Trejo; University of Southern California: MonicaD. Wong, Susan M. DeNunzio, Doris Roldan.
Authors' contribution: S.W., E.Z., and B.R.-R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J.L.S., F.X.G., S.R.W., L.E.V., A.M.H., M.D.N., C.M.L., and M.H.Y. Acquisition, analysis, or interpretation of data: J.L.S., F.X.G., S.R.W., M.E.K., L.Z.K., B.A.C., C.T.W., K.I., E.T.L., J.E.D., J.C., A.E.K., M.K.H., C.P.S., E.E.F., Y.B., J.H., and S.M.-S. Drafting of the manuscript: J.L.S., F.X.G., S.R.W., L.E.V., A.M.H., E.T.L., M.D.N., C.M.L., and M.H.Y. Critical revision of the manuscript for important intellectual content: J.L.S., F.X.G., S.R.W., P.C.S., M.E.K., L.Z.K., B.A.C., C.T.W., K.I., J.E.D., J.C., A.E.K., M.K.H., C.P.S., E.E.F., Y.B., J.H., S.M.-S., E.V.Z., and B.L.R.-R. Statistical analysis: S.R.W., E.V.Z., B.L.R.-R., and J.L.S. Obtained funding: J.L.S., F.X.G., S.R.W., L.E.V., and A.M.H. Administrative, technical, or material support: L.E.V., A.M.H., E.T.L., and P.C.S.
Supported by the Congressionally Directed Medical Research Program (CDMRP) and the US Army Medical Research Acquisition Activity (USAMRAA) under Contract No. W81XWH-16-D-0024, Task Order W81XWH-19-F-0494.
J.L.S. reports grants from the DoD; F.X.G. reports grants from the DoD; S.R.W. reports grants from the DoD; L.Z.K. reports grants and consulting fees from University of Maryland/BARDA, personal fees from Gamma Diagnostics, Coagulant Therapeutics, Haemonetics, other from Cerus, outside the submitted work; A.E.K. reports being founder of CaptureDx; J.C. reports participates on the steering committee for Faraday Pharmaceuticals; M.D.N. has received grants from National Institutes of Health, Department of Defense, DARPA, Haemonetics, Alexion and Instrumentation Laboratories, honoraria for lectures from Haemonetics and Takeda, support for attending meetings and/or travel from Takeda; participates on a Data Safety Monitoring Board or Advisory Board from NHLBI CONNECTS Steering Committee; is the Chief Medical Officer, Haima Therapeutics, and has Patents planned, issued or pending (US Patent 11,408.844; US Patent 9.072,760), outside the submitted work; P.C.S. reports personal fees from Hemanext, Cerus, participates in advisory board for Octapharma,and Haima, and is the Co-Founder and Chief Medical Officer of Kalocyte, outside the submitted work. The remaining authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Jason L. Sperry, Email: sperryjl@upmc.edu.
Francis X. Guyette, Email: guyefx@upmc.edu.
Bedda L. Rosario-Rivera, Email: rosarioriverabl7@upmc.edu.
Matthew E. Kutcher, Email: mkutcher@umc.edu.
Lucy Z. Kornblith, Email: lucy.kornblith@ucsf.edu.
Bryan A. Cotton, Email: bryan.a.cotton@uth.tmc.edu.
Chad T. Wilson, Email: chad.wilson@bcm.edu.
Kenji Inaba, Email: kenji.inaba@med.usc.edu.
Eva V. Zadorozny, Email: evaz@pitt.edu.
Laura E. Vincent, Email: vincentl3@upmc.edu.
Ashley M. Harner, Email: rymanam@upmc.edu.
Emily T. Love, Email: kellye17@upmc.edu.
Joseph E. Doherty, Email: jdoherty@umc.edu.
Joseph Cuschieri, Email: joseph.cuschieri@ucsf.edu.
Aaron E. Kornblith, Email: aaron.kornblith@ucsf.edu.
Erin E. Fox, Email: erin.e.fox@uth.tmc.edu.
Yu Bai, Email: yu.bai@uth.tmc.edu.
Marcus K. Hoffman, Email: marcus.hoffman@bcm.edu.
Catherine P. Seger, Email: catherine.seger@bcm.edu.
Jay Hudgins, Email: dococt168@gmail.com.
Sheila Mallett-Smith, Email: sheilasmith103115@gmail.com.
Matthew D. Neal, Email: nealm2@upmc.edu.
Christine M. Leeper, Email: leepercm@upmc.edu.
Philip C. Spinella, Email: spinella@pitt.edu.
Mark H. Yazer, Email: yazermh@upmc.edu.
Stephen R. Wisniewski, Email: stevewis@pitt.edu.
REFERENCES
- 1. Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378:370–379. [DOI] [PubMed] [Google Scholar]
- 2. Davis JS, Satahoo SS, Butler FK, et al. An analysis of prehospital deaths: Who can we save? J Trauma Acute Care Surg. 2014;77:213–218. [DOI] [PubMed] [Google Scholar]
- 3. Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(suppl 5):S431–S437. [DOI] [PubMed] [Google Scholar]
- 4. Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260:13–21. [DOI] [PubMed] [Google Scholar]
- 5. Guyette FX, Brown JB, Zenati MS, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial. JAMA Surg. 2020;156:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guyette FX, Zenati M, Triulzi DJ, et al. Prehospital low titer group O whole blood is feasible and safe: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2022;92:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–326. [DOI] [PubMed] [Google Scholar]
- 9. Fox EE, Holcomb JB, Wade CE, et al. Earlier endpoints are required for hemorrhagic shock trials among severely injured patients. Shock. 2017;47:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holcomb JB, Moore EE, Sperry JL, et al. Evidence-based and clinically relevant outcomes for hemorrhage control trauma trials. Ann Surg. 2021;273:395–401. [DOI] [PubMed] [Google Scholar]
- 11. Deeb AP, Guyette FX, Daley BJ, et al. Time to early resuscitative intervention association with mortality in trauma patients at risk for hemorrhage. J Trauma Acute Care Surg. 2023;94:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shackelford SA, Del Junco DJ, Powell-Dunford N, et al. Association of prehospital blood product transfusion during medical evacuation of combat casualties in afghanistan with acute and 30-day survival. JAMA. 2017;318:1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qasim Z, Butler FK, Holcomb JB, et al. Selective prehospital advanced resuscitative care - developing a strategy to prevent prehospital deaths from noncompressible torso hemorrhage. Shock. 2022;57:7–14. [DOI] [PubMed] [Google Scholar]
- 14. Gruen RL, Mitra B, Bernard SA, et al. PATCH-Trauma Investigators and the ANZICS Clinical Trials Group. Prehospital Tranexamic acid for severe trauma. N Engl J Med. 2023;389:127–136. [DOI] [PubMed] [Google Scholar]
- 15. Rowell SE, Meier EN, McKnight B, et al. Effect of out-of-hospital tranexamic acid vs placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cardenas JC, Zhang X, Fox EE, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2018;2:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etchill EW, Myers SP, Raval JS, et al. Platelet transfusion in critical care and surgery: evidence-based review of contemporary practice and future directions. Shock. 2017;47:537–549. [DOI] [PubMed] [Google Scholar]
- 18. Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. [DOI] [PubMed] [Google Scholar]
- 19. Holcomb JB, Zarzabal LA, Michalek JE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328. [DOI] [PubMed] [Google Scholar]
- 20. Pidcoke HF, Aden JK, Mora AG, et al. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012;73(suppl 5):S445–S452. [DOI] [PubMed] [Google Scholar]
- 21. Barry M, Pati S. Targeting repair of the vascular endothelium and glycocalyx after traumatic injury with plasma and platelet resuscitation. Matrix Biol Plus. 2022;14:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pidcoke HF, Spinella PC, Ramasubramanian AK, et al. Refrigerated platelets for the treatment of acute bleeding: a review of the literature and reexamination of current standards. Shock. 2014;41(Suppl 1):51–53. [DOI] [PubMed] [Google Scholar]
- 23. Shea SM, Reisz JA, Mihalko EP, et al. Cold-stored platelet hemostatic capacity is maintained for three weeks of storage and associated with taurine metabolism. J Thromb Haemost. 2023;22:1154–1166. [DOI] [PubMed] [Google Scholar]
- 24. Getz TM. Physiology of cold-stored platelets. Transfus Apher Sci. 2019;58:12–15. [DOI] [PubMed] [Google Scholar]
- 25. Lopez E, Srivastava AK, Burchfield J, et al. Platelet-derived- extracellular vesicles promote hemostasis and prevent the development of hemorrhagic shock. Sci Rep. 2019;9:17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair PM, Meledeo MA, Wells AR, et al. Cold-stored platelets have better preserved contractile function in comparison with room temperature-stored platelets over 21 days. Transfusion. 2021;61(Suppl 1):S68–S79. [DOI] [PubMed] [Google Scholar]
- 27. Nair PM, Pandya SG, Dallo SF, et al. Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol. 2017;178:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddoch-Cardenas KM, Peltier GC, Chance TC, et al. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion. 2021;61:178–190. [DOI] [PubMed] [Google Scholar]
- 29. Montgomery RK, Reddoch KM, Evani SJ, et al. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion. 2013;53:1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palavecino EL, Yomtovian RA, Jacobs MR. Bacterial contamination of platelets. Transfus Apher Sci. 2010;42:71–82. [DOI] [PubMed] [Google Scholar]
- 31. US Department of Health and Human Services. Food and Drug Administration. Alternative procedures for the manufacture of cold-stored platelets intended for the treatment of active bleeding when conventional platelets are not available or their use is not practical. 2023. Accessed January 6, 2024. http://www.fda.gov/media/169714/download
- 32. US Department of Health and Human Services. Food and Drug Administration. Guidance for institutional review boards, clinical investigators, and sponsors; exception from informed consent requirements for emergency research. 2013. Accessed January 6, 2024. http://www.fda.gov/media/80554/download
- 33. Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69(suppl 1):S33–S39. [DOI] [PubMed] [Google Scholar]
- 34. Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–352. [DOI] [PubMed] [Google Scholar]
- 35. Neal MD. The great platelet paradox: evolution of platelet contribution to hemostasis, inflammation, and thrombosis after injury. Blood Adv. 2020;4:2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ABC Newsletter. FDA variance for use of cold-stored platelets. 2019. Accessed January 6, 2024. http://iwebb.carterbloodcare.org/Memo/DownloadFile/3981
- 37. Getz TM, Montgomery RK, Bynum JA, et al. Storage of platelets at 4 degrees C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56:1320–1328. [DOI] [PubMed] [Google Scholar]
- 38. Baimukanova G, Miyazawa B, Potter DR, et al. The effects of 22°C and 4°C storage of platelets on vascular endothelial integrity and function. Transfusion. 2016;56(suppl 1):S52–S64. [DOI] [PubMed] [Google Scholar]
- 39. Chang R, Eastridge BJ, Holcomb JB. Remote damage control resuscitation in austere environments. Wilderness Environ Med. 2017;28(suppl):S124–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4°C and 22°C. J Lab Clin Med. 1978;91:618–624. [PubMed] [Google Scholar]
- 41. George CE, Saunders CV, Morrison A, et al. Cold stored platelets in the management of bleeding: is it about bioenergetics? Platelets. 2023;34:2188969. [DOI] [PubMed] [Google Scholar]
- 42. Murphy S, Gardner FH. Platelet preservation. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–1098. [DOI] [PubMed] [Google Scholar]
- 43. Sims C, Salliant N, Worth AJ, et al. Metabolic tracing analysis reveals substrate-specific metabolic deficits in platelet storage lesion. Transfusion. 2017;57:2683–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mittal K, Kaur R. Platelet storage lesion: an update. Asian J Transfus Sci. 2015;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu C, Su Y, Guo W, et al. The platelet storage lesion, what are we working for? J Clin Lab Anal. 2023;38:e24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bennett JS. Shedding new light on the platelet storage lesion. Arterioscler Thromb Vasc Biol. 2016;36:1715–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ClinicalTrials.gov. http://classic.clinicaltrials.gov/ct2/show/NCT04834414?term=spinella&draw=2&rank=2. http://classic.clinicaltrials.gov/ct2/show/NCT04834414?term=spinella&draw=2&rank=2 Chilled platelet study; 2024. Accessed March 13, 2024.
- 48.ClinicalTrials.gov. http://classic.clinicaltrials.gov/ct2/show/NCT04726410?term=sperry&draw=2&rank=6. http://classic.clinicaltrials.gov/ct2/show/NCT04726410?term=sperry&draw=2&rank=6 Cold-stored platelet early intervention in TBI; 2024. Accessed March 13, 2024.
- 49. Donohue JK, Gruen DS, Iyanna N, et al. Mechanism matters: mortality and endothelial cell damage marker differences between blunt and penetrating traumatic injuries across three prehospital clinical trials. Sci Rep. 2024;14:2747. [DOI] [PMC free article] [PubMed] [Google Scholar]