Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating motor neuron disease (MND) that shares a common clinical, genetic and pathologic spectrum with frontotemporal dementia (FTD). It is highly heterogeneous in its presentation and features. Up to 50% of patients with MND develop cognitive-behavioural symptoms during the course of the disease, meeting criteria for FTD in 10%–15% of cases. In the absence of a precise biomarker, neuropathology is still a valuable tool to understand disease nosology, reach a definite diagnostic confirmation and help define specific subgroups of patients with common phenotypic, genetic and biomarker profiles. However, few neuropathological series have been published, and the frequency of frontotemporal lobar degeneration (FTLD) in MND is difficult to estimate.
In this work we describe a large clinicopathological series of MND patients, analysing the frequency of concurrent FTLD changes and trying to define specific subgroups of patients based on their clinical, genetic and pathological characteristics.
We performed an observational, retrospective, multicentre case study. We included all cases meeting neuropathological criteria for MND from the Neurological Tissue Bank of the FRCB-IDIBAPS-Hospital Clínic Barcelona Biobank between 1994 and 2022, regardless of their last clinical diagnosis. While brain donation is encouraged in all patients, it is performed in very few, and representativeness of the cohort might not be precise for all patients with MND. We retrospectively reviewed clinical and neuropathological data and describe the main clinical, genetic and pathogenic features, comparing neuropathologic groups between MND with and without FTLD changes and aiming to define specific subgroups.
We included brain samples from 124 patients, 44 of whom (35.5%) had FTLD neuropathologic features (i.e. FTLD-MND). Pathologic TDP-43 aggregates were present in 93.6% of the cohort and were more extensive (higher Brettschneider stage) in those with concurrent FTLD (P < 0.001). Motor symptom onset was more frequent in the bulbar region in FTLD-MND cases than in those with isolated MND (P = 0.023), with no differences in survival. We observed a better clinicopathological correlation in the MND group than in the FTLD-MND group (93.8% versus 61.4%; P < 0.001). Pathogenic genetic variants were more common in the FTLD-MND group, especially C9orf72.
We describe a frequency of FTLD of 35.5% in our series of neuropathologically confirmed cases of MND. The FTLD-MND spectrum is highly heterogeneous in all aspects, especially in patients with FTLD, in whom it is particularly difficult to define specific subgroups. In the absence of definite biomarkers, neuropathology remains a valuable tool for a definite diagnosis, increasing our knowledge in disease nosology.
Keywords: amyotrophic lateral sclerosis, motor neuron disease, frontotemporal dementia, neuropathology
Carbayo et al. study the clinical and pathological features of a large series of patients with amyotrophic lateral sclerosis. They describe a high frequency of concurrent FTLD pathological features and highlight the marked heterogeneity within the disease and its main characteristics.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder characterized by progressive muscle weakness and atrophy due to progressive degeneration of motor neurons in the brain and spinal cord, leading to respiratory insufficiency and death within a mean of 3 to 5 years.1 Cognitive and behavioural impairment is recognized as part of the disease and present in approximately 50% of patients, while 10%–20% fulfil clinical diagnostic criteria for any of the clinical variants of frontotemporal dementia (FTD), especially the behavioural variant (bvFTD).2,3 Furthermore, around 12% of patients with FTD develop clinical motor neuron impairment, and up to 40% show minor clinical or neurophysiological motor signs.4 Hence, motor neuron disease (MND) and FTD are now considered to be part of the same disease spectrum. At neuropathological examination, most ALS patients and up to half of FTD patients present pathological cytoplasmic neuronal aggregates of TAR DNA-binding protein 43 (TDP-43) in several brain and spinal cord regions.5 The two diseases also share a common genetic background, with mutations in genes such as hexanucleotide expansion in chromosome 9 open reading frame 72 (C9orf72), tank-binding kinase 1 (TBK1) and TAR DNA-binding protein (TARDBP) as some of the most commonly seen mutations as causative for the disease.
The clinical presentation of ALS is highly heterogeneous considering the variability in phenotype and disease course, and it becomes even more so with the addition of cognitive-behavioural symptoms, especially with the added possibility of an underlying causative genetic mutation and the nature of protein aggregates. A definite biomarker for the diagnosis of ALS is lacking, and diagnosis currently relies on sets of consensus criteria based on clinical features, updated over time to increase sensitivity.6,7 It is, therefore, necessary to define and characterize specific subgroups of patients with common and relatively homogeneous phenotypic and genetic characteristics that correlate with a biomarker profile and neuropathological features. Our still very limited knowledge of the pathophysiology of the disease hinders the design of future novel molecular targeted treatments. In this context, neuropathology remains the current reference for a definite diagnosis of the frontotemporal lobar degeneration (FTLD)-MND spectrum and is invaluable to understanding the molecular basis of the disease and its pathophysiology. Nevertheless, few clinicopathological series have been published so far,8-11 making it difficult to estimate the frequency of FTLD in MND.
In this work, we describe a clinico-neuropathological series of 124 patients with MND with or without concurrent FTLD. We describe their main clinical, pathological and genetic features, compare the two groups (MND versus MND-FTLD) and discuss the clinicopathological correlation.
Materials and methods
Case selection
We performed an observational, retrospective, multicentre, retrospective cohort study. We selected all cases meeting neuropathological criteria for MND from the Neurological Tissue Bank of the FRCB-IDIBAPS-Hospital Clínic Barcelona Biobank register between January 1994 and November 2022, irrespective of their last clinical diagnosis. These cases included brain donations from 13 hospitals and tertiary care centres in the province of Barcelona, Spain.
The Neurological Tissue Bank is a brain tissue repository for all neurodegenerative diseases and receives altruistic donations from patients with a wide range of neurodegenerative conditions. While brain donation is encouraged in all patients, especially in tertiary care hospitals, this takes place in very few cases. Considering a stable incidence of MND among several populations (2–3 cases/100 000 population/year),12 between 1994 and 2022 we estimated an expected number of 3080 ALS cases in the province of Barcelona (population of 5.5 million). The patients included in our series (n = 124) therefore represent approximately 4% of the total ALS cases. Cases with motor neuron loss in the context of advanced stages of other neurodegenerative disorders were excluded. Other exclusion criteria were lack of adequate clinical information and incomplete or equivocal clinical data. Patients were divided into two groups depending on whether or not they had concurrent FTLD pathology changes.
Neuropathological work-up
Cases were systematically studied as part of the routine neuropathological diagnostic practice. Post-mortem neuropathological studies were performed at the Neurological Tissue Bank of the Biobanc-Hospital Clínic-IDIBAPS, as previously reported according to standardized protocols and following BrainNet Europe II recommendations (www.brainnet-europe.org).13 A minimum of 25 representative brain areas were embedded in paraffin, cut at 5 µm and stained with haematoxylin and eosin and Luxol Fast Blue in selected brain areas. Immunohistochemistry was performed using antibodies anti-βA4, anti-pTau, anti-RD3 and anti-RD4 Tau, anti-α-synuclein, anti α-internexin, anti-FUS and anti-TDP-43, pTDP-43, anti-ubiquitin, anti-p62, anti-transportin and anti-TAF15. Immunoreaction was visualized using the EnVision+ system peroxidase procedure (DAKO). Antibodies used for immunohistochemistry and their pretreatments are listed in Supplementary Table 1.
MND was defined as a loss of motor neurons and gliosis in primary motor cortex and/or signs of corticospinal tract degeneration at the level of the spinal cord (upper motor neuron, UMN), in the nuclei of the hypoglossus nerve in the medulla oblongata and/or in the anterior horn of the spinal cord at any level (lower motor neuron, LMN), or both. All cases were staged following the criteria proposed by Brettschneider14 for ALS, according to which, presence of TDP-43 aggregates was categorized following a semiquantitative rating scale (0, not detectable or ≤2 aggregates per region; +, mild; ++, moderate; +++, severe/numerous). Presence of any pathologic TDP-43 aggregates in the region of the highest-ranked stage defined the final neuropathologic Brettschneider stage. The presence of glial/oligodendroglial TDP-43 aggregates was recorded but was not graded separately. FTLD was defined as a macro- or microscopic frontotemporal lobar degeneration pattern with neuronal loss, gliosis and/or a superficial laminar sclerosis in the frontal and/or temporal lobes.15 FTLD TDP subtype classification was performed based on TDP-43 or pTDP-43 immunohistochemistry following current recommendations.16 Pathological subtypes of FTLD-FUS were classified according to current recommendations.17
Specific immunostains were performed retroactively and reviewed thoroughly for cases studied prior to the description of TDP-435 and FUS18 protein aggregates or the specific pathology associated with C9orf72 expansion.19
Concurrent pathologies were categorized and staged according to respective current criteria: neurofibrillary pathology was staged according to Braak criteria,20 amyloid-β phases were evaluated according to Thal criteria21 and the neuritic plaque score was assessed according to the Consortium to Establish a Registry for Alzheimer Disease criteria.22 Argyrophilic grain disease (AGD) was staged according to Saito criteria.23
Clinical classification
Medical records were retrospectively reviewed by the neurologists responsible for the care of patients during life, and a form with the requested clinical information was filled in. The demographic variables and clinical features recorded were sex, age at onset of each individual motor and cognitive symptom, and age at death.
Clinical diagnosis of MND was made according to the revised diagnostic criteria of El Escorial, meeting criteria for definite, probable, and probable laboratory-supported ALS.6,7 Patients clinically diagnosed with FTD or other cognitive predominant neurodegenerative diseases were not systematically screened for MND. Neurophysiological studies and specific evaluation by MND specialists were only performed when considered necessary by the treating neurologist.
Patients’ motor phenotypes were categorized as progressive muscular atrophy (PMA) when only LMN signs were present, primary lateral sclerosis (PLS) when only UMN signs were present and ALS when both signs were evident. Clinical and semiological variables included were the region of motor symptoms onset (spinal/bulbar), site of onset (bulbar/upper or lower limbs/proximal or distal), clinical diagnosis at death or last visit and presence of bulbar symptoms such as dysarthria and dysphagia.
We recorded cognitive and behavioural symptoms and FTD diagnosis according to the expert opinion of the treating neurologist. Neuropsychological testing has been performed systematically in all cases since 2015 but was previously assessed only when patients or relatives reported cognitive or behavioural symptoms or when these were suspected by the neurologist. Clinical diagnosis of FTD was made according to current criteria for FTD and its variants [bvFTD, semantic variant (sv-PPA) or non-fluent variants (nfv-PPA) of primary progressive aphasia (PPA)].24-26 Patients with cognitive or behavioural impairment who did not fulfil the criteria for FTD were classified as ALS-ci/bi.24 Clinical diagnosis of progressive supranuclear palsy (PSP),27 corticobasal degeneration (CBD),28 Alzheimer’s disease (AD)29 and Lewy body dementia (LBD)30 was made following current criteria. Family history of ALS, FTD and other neurodegenerative diseases was also recorded.
Genetic analysis
DNA was extracted from fresh-frozen cerebellum using the QIAamp DNA Mini kit for DNA purification from tissues (Qiagen Co.) following the manufacturer’s instructions. In post-mortem tissue, we performed systematic screening for potential C9orf72 expansion mutation carriers searching for ubiquitin/p62-positive inclusions in the cerebellum and hippocampus as surrogate and as previously reported.19 The C9orf72 repeat was confirmed in suspected cases by repeat-primed PCR and fragment-length analysis. Other mutations were not identified by systematic screening of all patients but were identified in the framework of previous studies or by specific protocols in highly specialized units. In mutation carriers, information concerning other affected family members was not available.
Statistical analysis
Statistical analysis was done using the Statistical Package for Social Sciences (version 27.0, SPSSInc). Comparisons between neuropathologic groups (MND versus FTLD-MND) and clinical, demographic, and genetic data were performed by chi-square or Fisher tests for categorical data and Student’s t-test or Kruskal–Wallis test for ordinal and continuous data. Survival analysis was performed using the Kaplan–Meier method. Statistical significance was set at P < 0.05 for all analyses.
Ethics
This study was conducted with the approval of the Ethics Committee of the Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. All individuals were brain donors and they or their relatives provided informed consent for the use of brain tissue for diagnostic and research purposes at the Neurological Tissue Bank of the Biobanc-Hospital Clínic-FRCB-IDIBAPS.
Results
Study cohort description and patient selection
One-hundred and twenty-four patients fulfilled the inclusion criteria, 55.65% of whom were males (n = 69). Mean age at death and brain donation was 66.35 years [standard deviation (SD) 12.55]. Primary neuropathological diagnosis was isolated MND in 64.4% (n = 80) and FTLD-MND in the remaining 35.5% of individuals (n = 44). Of the 124 patients, 102 (82.26%) were referred by neurologists from third-level healthcare centres, and the others were referred from lower complexity facilities. Patients with MND were more frequently referred from third-level hospitals than patients with FTLD-MND (P < 0.001). Characteristics of the study cohort are reported in Table 1. No differences were found between neuropathological groups regarding the main demographic characteristics.
Table 1.
Clinical, neuropathological and genetic features of the neuropathologically confirmed MND series
| Total | FTLD-MND | MND | P-value | |
|---|---|---|---|---|
| n = 124 | n = 44 (35.5%) | n = 80 (64.4%) | ||
| Demographic features | ||||
| Male (%)/female | 69 (55.6)/55 | 28 (63.6)/16 | 41 (51.2)/39 | ns (0.18) |
| Patients from third level hospital (%) | 102 (82.3) | 28 (63.6) | 74 (92.5) | <0.001 |
| Motor symptoms (%) | 114 (91.9) | 34 (77.3) | 80 (100) | <0.001 |
| Motor onset age, years (SD) | 62.58 (13.2) | 64.55 (12.2) | 61.72 (13.6) | ns (0.32) |
| Mean MND duration, months (SD) | 37.15 (28.8) | 34.84 (32.9) | 38.20 (27.3) | ns (0.59) |
| Bulbar onset (%) | 31 (27.7) | 14 (42.4) | 17 (21.5) | 0.023 |
| Age at death, years (SD) | 66.35 (12.6) | 68.64 (10.4) | 65.09 (13.5) | ns (0.11) |
| Cognitive symptoms (%) | 48 (38.7) | 39 (88.6) | 9 (11.3) | <0.001 |
| Clinical FTD diagnosis (%) | 35 (28.2) | 30 (68.2) | 5 (6.3) | <0.001 |
| Clinical diagnosis | ||||
| Last diagnosis before death | <0.001 | |||
| FTD (%) | 5 (4.0) | 5a (11.4) | 0 | – |
| FTD-MND (%) | 32 (25.8) | 27b (61.4) | 5 (6.3) | – |
| MND (%) | 82 (66.1) | 7 (15.9) | 75 (93.8) | – |
| Other non-motor neurodegenerative (%) | 5 (4.0) | 5 (11.4) | 0 | – |
| Diagnostic accuracy (matching clinic-pathological diagnosis), (%) | 102/124 (82.3) | 27/44 (61.4) | 75/80 (93.8) | <0.001 |
| Neuropathology | ||||
| Brain weight (g), mean (SD) | 1239.67 (147.6) | 1194.5 (138.2) | 1264.2 (147.6) | 0.012 |
| Protein deposit in motor neurons | 0.007 | |||
| TDP43 (%) | 104 (83.9) | 31 (70.5) | 73 (91.3) | – |
| TDP43—C9 pathology (%) | 12 (9.7) | 10 (22.7) | 2 (2.5) | <0.001 |
| FUS-FET (%) | 5 (4.0) | 2 (4.5) | 3 (3.8) | – |
| PrPsc (VPSPr) (%) | 2 (1.6) | 1 (2.3) | 1 (1.3) | – |
| No inclusions (%) | 1 (0.8) | 0 | 1 (1.3) | – |
| Brettschneider stage 1/2/3/4 (median) | 13/27/21/43 (3) | 2/2/3/27 (4) | 11/25/18/16 (2) | <0.001 |
| No UMN loss (%) | 6 (4.8) | 3 (7) | 3 (3.8) | ns (0.34) |
| Genetic mutations, n (%) | 18 (14.5%) | 14 (31.8) | 4 (5.0) | <0.001 |
| C9orf72 (%) | 12 (9.7%) | 10 (22.7) | 2 (2.5) | <0.001 |
| TARDBP (%) | 1 (0.8%) | 0 | 1 | – |
| VCP (%) | 1 (0.8%) | 0 | 1 | – |
| TBK1 (%) | 1 (0.8%) | 1 | 0 | – |
| SQSTM1 (%) | 2 (1.6%) | 2 | 0 | – |
| Taf15 (%) | 1 (0.8%) | 1 | 0 | – |
A definite diagnosis was made according to neuropathologic characteristics and the comparison between neuropathologic groups. bvFTD = behavioural variant-frontotemporal dementia; CJD = Creutzfeldt-Jakob disease; FTD = frontotemporal dementia; FTLD = frontotemporal lobar degeneration; MND = motor neuron disease; nfv-PPA = non-fluent variant-primary progressive aphasia; ns = not significant; PrPsc = prion protein abnormal isoform; SD = standard deviation; sv-PPA = semantic variant-primary progressive aphasia; UMN = upper motor neuron.
aCases of FTD included two with sv-PPA and three with bvFTD.
bCases of FTD in this group included two with nfv-PPA and 24 with bvFTD.
Neuropathology
Mean brain weight was significantly lower in patients with FTLD-MND neuropathology (1194.5 g in FTLD-MND versus 1264.2 g in MND; P = 0.012).
In terms of protein deposition, we found TDP-43 protein aggregates in 93.55% of patients (n = 116), 12 of whom (9.68% of the total) had concurrent specific C9orf72 mutation pathology with ubiquitin- and p62-positive, TDP-43-negative neuronal cytoplasmic and intranuclear inclusions containing dipeptide-repeat proteins, most abundant in the cerebellum, hippocampus and neocortex, as previously described.19,31,32 Five patients (4.03%) showed FET (FUS) protein aggregates. Two of these five patients had concurrent FTLD pathology. In two other patients (2%), MND was related to prion pathology (variably protease sensitive prionopathy, VPSPr), one with the typical FTLD pattern.33 Finally, one patient had extensive motor neuron loss but no identifiable protein inclusions. No differences were found between MND or FTLD-MND in terms of type of protein deposition other than those related to C9orf72 pathology.
We stratified the distribution of TDP-43 pathology according to the Brettschneider score for ALS (Fig. 1 and Table 1). The score was not strictly applicable in 12 individuals because despite the presence of TDP43 aggregates in the anterior temporal lobe and/or hippocampus (i.e. stage 4), they lacked protein deposits in other brain regions required for stages 2 or 3. These patients were no different from the others regarding the frequency of cognitive symptoms (P = 0.124), age at onset (P = 0.778) or death (P = 0.378). Only two patients exceeded 80 years of age at the time of death.
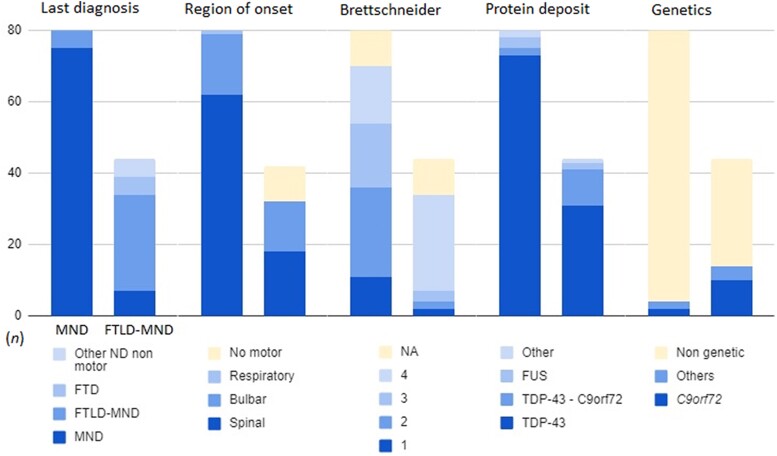
Figure 1.
Motor neuron disease (MND) and frontotemporal lobar degeneration (FTLD)-MND distribution of the cohort’s main clinical, neuropathologic and genetic features. The bar plot visually represents the distribution of the cohort’s main features. The columns represent the absolute count (n) of patients in both neuropathological groups for every feature: MND on the left (n = 80) and FTLD on the right (n= 44). Note the global heterogeneity among both groups in every characteristic analysed, especially in the FTLD-MND group.
Regarding protein deposit within FTLD pathology (n = 44), 40 cases had TDP-43 aggregates and four did not. In the former group, distribution of TDP-43 within frontal cortical layers followed a type A FTLD pattern in 4 cases, a type B pattern in 21 cases and type C in 2 cases. In the remaining 13 cases, the pattern was unclassifiable or showed a mixture of types A/B. In the group with FTLD pathology but no TDP-43 aggregates, we found two FTLD cases associated with FUS-pathology [one in the form of basophilic inclusion body disease (BIBD) and the other with features of atypical FTLD-U (aFTLD-U)], one case associated with prion disease (VPSPr) and one FTLD-MND case with Tau inclusions.
Most patients showed involvement in both UMN and LMN. However, we found a variable degree—and even absence—of neuronal degeneration in either the UMN or LMN. Six patients had isolated LMN disease (LMNd) (4.8%). Three of the six also showed a typical FTLD pattern and one had isolated UMN disease (0.8%). Figure 2 illustrates the main neuropathologic features of the MND-FTLD spectrum.
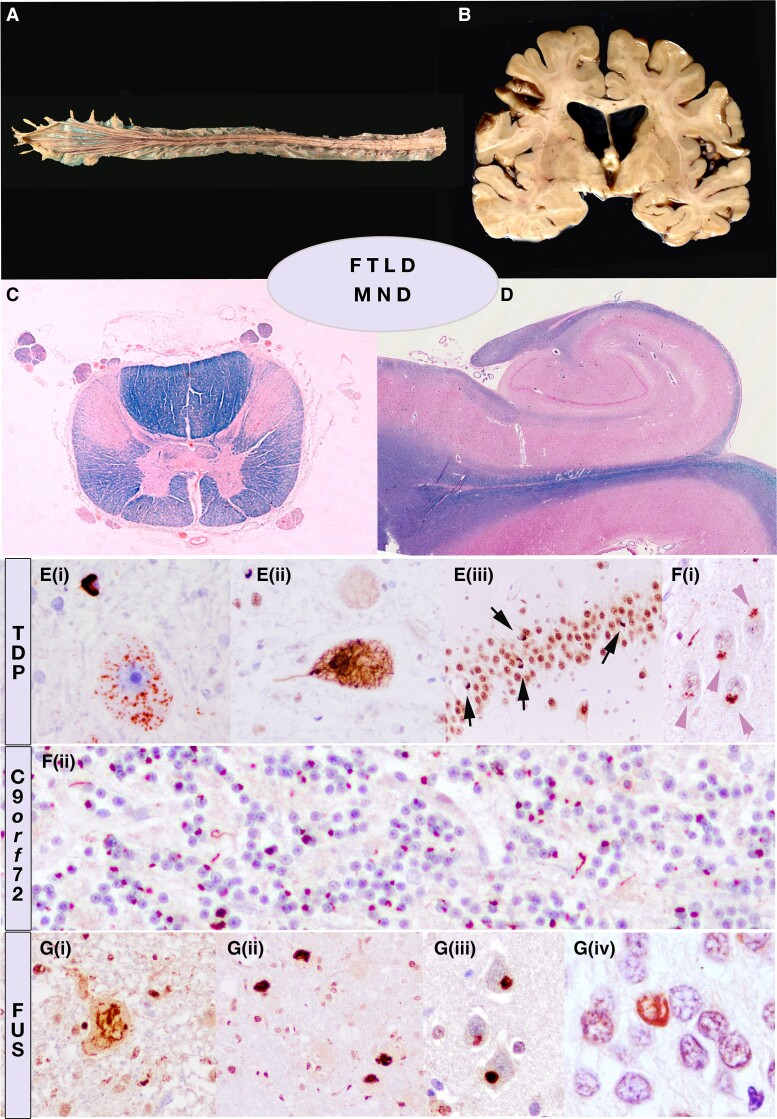
Figure 2.
Representation of the neuropathological overlap between motor neuron disease (MND) and frontotemporal lobar degeneration (FTLD). Gross examination (A and B), histological overview (C and D) (Klüver-Barrera/Luxol Fast Blue) and immunohistochemistry. Macroscopic thinning of anterior roots of the spinal cord in amyotrophic lateral sclerosis (ALS) (A) may be associated with a narrowing of gyri in fronto-temporal regions and ventricular enlargement (B). The major neuropathological features of ALS are a secondary degeneration of the corticospinal tracts due to the loss of upper motor neurons and extensive loss of lower motor neurons in the brainstem and/or anterior horn cells. (C) Cross-section through the thoracic spinal cord shows prominent degeneration of the lateral and anterior corticospinal tract and atrophy of the anterior horns (Klüver-Barrera; blue colour highlights myelin sheaths). (D) Representation of normal hippocampus with preserved neuronal densities in all sectors. (E) Accumulation of pTDP43 protein in motor neurons in fine-granular cytoplasmic or mesh-like threads in MND (i and ii), or as small compact cytoplasmic inclusion in the granular neurons of the dentate gyrus (iii). (F) The intronic expansion in C9orf72 may manifest as MND-FTLD continuum and shares neuropathological features consistent of small ubiquitin and p62positive star-like inclusions (representing dipeptide repeats, DPR) in cortical neurons (i) or granular cytoplasmic inclusions in granule cells of the cerebellar cortex (ii), independently of TPD43 pathology and clinicopathological phenotype, which is determined by TDP but not by DPR. (G) FUS pathology may also be observed in ALS as fibrillar inclusions (i) or more compact (ii) and basophilic on haemotoxylin and eosin stained sections (not shown), as well as in FTLD, either in the neuronal intermediate filament inclusion disease (NIFID) phenotype (iii) or atypical FTLD (aFTLD). [G(iv)] Vermiform nuclear inclusion in a granule cell of the dentate gyrus of the hippocampus.
Clinical characteristics according to the neuropathologic groups: MND versus FTLD-MND
Motor symptoms
In the FTLD-MND neuropathologic group, 10 subjects (9.06%) never reported motor neuron symptoms during life despite neuropathological motor neuron loss and corticospinal tract degeneration. They received a late clinical diagnosis of FTD (three bvFTD, two PPA) and other neurodegenerative diseases (two CBD, one PSP, one AD and one LBD). In contrast, all patients in the ‘isolated MND’ group presented motor neuron symptoms, these being more frequent than in the FTLD-MND group (P < 0.001).
Among the whole group, when motor neuron symptoms were present (n = 114), onset was in the bulbar region in 31 (27.7%) patients. This was more frequently observed in the FTLD-MND group (42.4%, n = 14) than in the group with MND only (21.5%, n = 17), (P = 0.023). The rest of the cohort (72.3%, n = 83) had spinal onset, in which distal upper limb impairment was significantly more frequent in the FTLD-MND group than in the MND-only group (57.9% versus 21%, respectively) (P = 0.003). Of the remaining three patients, one presented with respiratory onset; however, this information was not available for the other two patients. Patients with a bulbar onset were older than those with spinal onset at the time of disease debut (68.37 versus 61.09 years, P = 0.011) and at death (69.97 versus 64.62 years, P = 0.024), but no differences were found in terms of disease duration or survival (30.26 months for bulbar onset versus 37.71 months for spinal onset, P = 0.21).
The initial motor phenotype was ALS in 86 patients (69.35%), LMNd in 14 (11.29%), PLS in four (3.23%) and progressive bulbar palsy in six (4.84%). This information was not available for four patients (3.23%). No differences were found between MND or FTLD-MND patients regarding motor phenotype distribution.
Cognitive and behavioural symptoms
Cognitive or behavioural impairment during clinical follow-up was observed in 38.71% of patients (n = 48). We found that 39 of the 124 cases (31.45% of the overall cohort) met the clinical criteria for FTD (35 bvFTD, 4 PPA). The proportion of patients with cognitive or behavioural symptoms was higher in the FTLD-MND neuropathological group (88.6%, n = 39) than in the MND group (13.3%, n = 9) (P < 0.001). Furthermore, in the FTLD-MND group, 67.4% of patients received a clinical diagnosis of FTD (n = 29), significantly more than those in the MND group (6.3%, n = 5) (P < 0.001).
In the group of patients with isolated MND but no FTLD pathology, five patients who had received a clinical diagnosis of FTD-MND (bvFTD) showed no matching FTLD features in neuropathology. However, they displayed other neuropathologic changes that could explain the cognitive-behavioural impairments: (i) one had prion disease with a spinal onset FTD-MND phenotype and a disease duration of 6 years; (ii) one patient had prominent cognitive and behavioural symptoms with extensive AD pathology (stage VI of Braak); and (iii) three patients had an FTD-MND related genetic variant (C9orf72, VCP and TARDBP). The patient with a C9orf72 mutation presented extensive extramotor TDP-43 pathology (Brettschneider stage 4) and showed associated neurofibrillary pathology (Braak III) with amyloid-β deposits (Thal stage III). The patient with a TARDBP mutation13 presented mild cognitive impairment but prominent behavioural symptoms meeting the criteria for bvFTD. This patient’s neuropathological examination showed extramotor TDP-43 pathology (Brettschneider stage 4) with abundant TDP-43 inclusions and gliosis in the amygdala and, to a lesser extent, in the thalamus and hippocampal dentate gyrus. Lastly, the VCP mutation carrier (Individual 13), who died at the age of 82, presented with cognitive and behavioural impairment that met bvFTD criteria, while neuropathology revealed restricted MND (Brettschneider stage I) but additional AGD (Saito I) and neurofibrillar pathology (Braak stage II).
Four patients, all from the FTLD-MND pathology group, presented language impairment as a main clinical feature (i.e. PPA). Two of the four never presented motor symptoms and their final clinical diagnosis was sv-PPA; the other two had a nfv-PPA combined with MND.
Clinicopathological diagnostic correlation
Table 1 and Fig. 1 show the distribution of the final clinical diagnosis or last diagnosis before death.
When comparing the correspondence between clinical and neuropathological diagnoses, we found that patients with MND pathology were more accurately diagnosed (93.8%, n = 75) than patients with FTLD-MND (61.4%, n = 27) (P < 0.001).
In the FTLD-MND neuropathological group, we found 17 patients with non-concordant diagnoses. They included five patients with FTD, five patients with other neurodegenerative diseases, with no evident motor neuron impairment during life, observed only at neuropathologic examination, and seven patients with MND, in whom cognitive/behavioural symptoms were never referred or detected during the course of the disease.
Among patients with isolated MND pathology, we recorded five individuals with non-correspondent clinico-pathological diagnoses, as they met the clinical criteria for bvFTD but had no matching FTLD pathology (see ‘Neuropathology’ section).
In two patients, a prion disease pathology was diagnosed only after neuropathologic examination. Both these patients presented clinical features of MND with progressive asymmetric limb weakness and atrophy, with additional UMN signs on physical examination, hence meeting the diagnostic criteria for definite ALS. Both had concurrent prominent cognitive-behavioural symptoms and were ultimately diagnosed with FTD-MND. Disease duration was 4 and 6 years, respectively, and both patients died of aspiration pneumonia. Neuropathological features in both cases were a spongiform encephalopathy with pathological prion protein deposits consistent with the rare subtype ‘variable protease sensitive prionopathy’, involving cortical and subcortical areas and particularly the upper and lower motor systems.33
Survival analysis
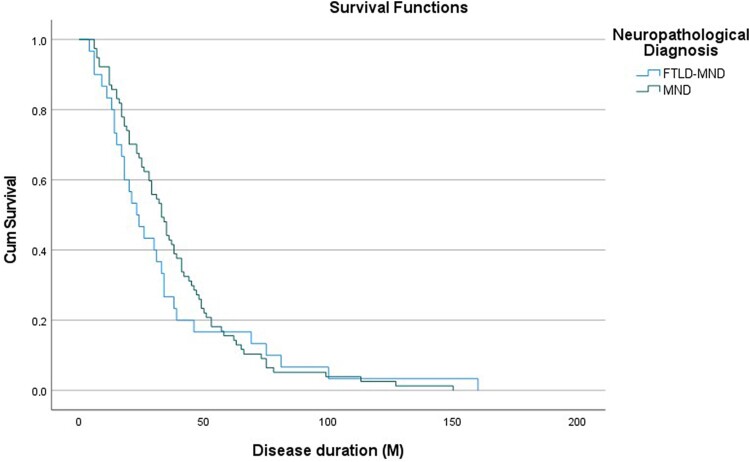
For survival analysis due to MND, we excluded those patients who had no motor symptoms during life. When evaluating survival separately for the neuropathological (i.e. FTLD-MND versus MND) or clinical diagnoses (i.e. FTD-MND versus clinical MND), we found no statistically significant differences in the survival rate (P = 0.64 and P = 0.45, respectively), with both groups showing a similar disease duration (Fig. 3). There was no difference in survival when patients were stratified by region of onset, presence of bulbar symptoms or detection of a pathogenic mutation.
Figure 3.
Survival analysis. Comparison of survival analysis between neuropathological groups [i.e. frontotemporal lobar degeneration (FTLD)-motor neuron disease (MND) versus MND]. There was no difference between groups (P = 0.64). M = months.
Genetics
Regarding genetics, a genetic variant related to ALS or FTD was found in 14.5% (n = 18) patients, 14 from the FTLD-MND neuropathological group and four from the MND group. These variants included 12 C9orf72 expansions, two sequestosome-1 (SQSTM1), one TATA-box binding protein associated factor 15 (TAF15), one TARDBP, one TBK1 and one valosin containing protein (VCP), as described in previous work.34 The distribution of mutations in the two groups is shown in Table 1 and Fig. 1.
Detection of a pathogenic genetic variant was more frequently associated with FTLD-MND pathology (31.8% of the FTLD-MND group versus 5% in MND pathology, P < 0.001), and C9orf72 repeat expansion mutation was independently associated with FTLD-MND pathology (P < 0.001).
Clinically, among the patients with FTLD-MND pathology and a mutation (n = 14), nine presented as FTD-MND and one as MND with no cognitive symptoms (C9orf72). These patients never presented motor neuron symptoms (two patients with a C9orf72 repeat expansion mutations and the two SQSTM1 mutation carriers) but were diagnosed with FTD (n = 2), AD (n = 1) or CBD (n = 1). Among the patients with isolated MND pathology and a mutation (n = 4), one presented as ALS and three presented as cognitive/behavioural symptoms that met clinical FTD criteria. Despite the lack of classical FTLD pathological features, these patients had other pathological findings that could justify their symptoms (see ‘Neuropathology’ section).
Family history
Eleven (8.87%) patients presented a positive family history of ALS (n = 5), FTD (n = 5) or both (n = 1). Five of the 11 had an identified ALS-related mutation (four C9orf72 and one VCP), and 25 additional patients (20.16%) reported a positive family history of other neurodegenerative diseases, including AD, Parkinson’s disease, LBD and other unspecified cognitive impairments.
Discussion
We describe an extensive series of clinically well-documented patients who had neuropathologically confirmed MND, with or without associated FTLD, and discuss clinicopathological correlations and discrepancies. We recorded a surprisingly high frequency of concomitant FTLD and identified various neuropathological subgroups. Our findings indicate there are several molecular pathology patterns with specifically altered pathological mechanisms that cannot be identified or predicted in clinical practice.
In our cohort, about one-third (35.5%) of patients with MND had concurrent neuropathological features of FTLD (i.e. FTLD-MND pathology), exceeding previous pathological8,9,11 and clinical reports.35-37 Large population-based series performing systematic cognitive testing have shown that up to 50% of patients with ALS develop cognitive and behavioural impairment,2,3,38 meeting clinical criteria for FTD in 10%–15%,35,36 with a significant impact on clinical management.39,40 Such findings indicate that the previous concept that ALS is a pure motor disease is definitely changing and expanding to a motor-behavioural-cognitive disease continuum.
On one hand, the higher frequency of FTLD in MND found in our study may partially be explained by the absence of reported motor symptoms in 10 of 124 patients (8%). These patients had a late clinical diagnosis of FTD-‘only’ or other neurodegenerative diseases but showed motor neuron involvement in neuropathology, leading to a final diagnosis of FTLD-MND. Some of these patients might have developed minimal motor signs that passed unnoticed, as there is neuropathologic9,41 and electrophysiologic evidence of motor system dysfunction in patients with FTD but no evident muscular atrophy or weakness.4
On the other hand, seven patients received a clinical diagnosis of MND but cognitive or behavioural symptoms were not recorded, even though FTLD pathology was found at post-mortem. This may have been due to a lack of a systematic cognitive evaluation at early disease stages and difficulty in their recognition when physical and articulatory impairments are extensive,39,40 suggesting the frequency of FTD in MND is underdiagnosed.
Even though clinicopathological concordance was higher in the MND pathology group, it was not perfect, as five patients showed prominent cognitive-behavioural symptoms that met bvFTD criteria (and were thus clinically diagnosed with FTD-ALS), despite not having corresponding FTLD neuropathology; alternative pathologies, however, might explain the symptoms. Both co-pathologies13,42 and predominant amygdalar impairment43,44 in ALS may induce cognitive-behavioural impairment and mimic an FTD phenotype. In three of these patients in our study, pathogenic genetic variants may also have contributed to frontotemporal dysfunction beyond the microscopic level.
Neuropathology is currently one of the most reliable diagnostic methods for both disease phenotypes, and our results may provide a more precise estimate of the real frequency of FTLD in MND and highlight these underrecognized features at both motor and cognitive-behavioural ends of the spectrum. This broader phenotypic horizon of the disease should not only prompt the search for new reliable diagnostic tools and biomarkers for early diagnosis and better characterization of the disease continuum and its nosology but also encourage closer collaboration between motor neuron and cognitive or memory units.
Another objective of our study was to identify possible subgroups of patients based on neuropathological, clinical and/or genetic features. ALS is clinically characterized by the concomitant presence of UMN and LMN signs that progress over time. However, diagnosis and classification can be challenging due to the highly variable presentation regarding the degree of involvement of UMN and/or LMN, the region of onset and the possible convergence of cognitive symptoms.45,46
In most patients in our series, as expected, we found neuropathological evidence of neuronal loss of both the UMN and LMN systems. However, we found six patients with neuronal loss and TDP-43 inclusions restricted to the hypoglossal nucleus and/or anterior horns of the medulla. They showed no neuropathological signs of UMN loss or corticospinal tract degeneration, and three of them had concurrent FTLD pathology. Also, clinically, they presented phenotypically as LMNd (i.e. PMA), with associated FTD in those with the corresponding pathology. Moreover, one of these patients had a genetic pathogenic variant in a gene linked to ALS (TBK1).
Patients with no UMN signs in physical examination would not meet ‘El Escorial ALS diagnostic criteria’.6 The recently published ‘Gold Coast Criteria for ALS’7 allow the categorization of similar patients, who have no evident UMN signs during life. However, despite their higher sensitivity, these criteria have lower specificity, and patients with no UMN signs are particularly more liable to misdiagnosis.39 Considering cognitive impairment and FTD diagnosis as an additional supportive criterion might be of help in confirming a definite ALS or FTD-MND diagnosis.47
The appearance of cognitive symptoms adds to the clinical heterogeneity and makes it difficult to establish distinctive clinical categories. Beyond the more frequent bulbar onset and distal upper limb weakness and atrophy in patients with clinical and neuropathological FTLD,36 we found little difference with patients in our series who had no cognitive impairment in terms of their motor neuron phenotype or even survival. The irruption of motor symptoms seems to be the main conditioning factor in prognosis9 but documenting cognitive and behavioural impairment can be essential due to the distinct implications in clinical management.35,48
Concerning the neuropathological features, the most widely represented pathology is TDP-43, presenting in the form of cytoplasmic neuronal and glial aggregates in both MND and FTLD-MND.42 Its extension, however, can vary along the continuum of the FTD-ALS spectrum, both in density and in anatomical and cytological location. Brettschneider et al.14 proposed a staging classification system based on sequential dissemination of TDP-43 in ALS and FTLD, where higher stages mostly correlate with more cognitive impairment according to our experience.13 Nevertheless, we found that 12 patients had a similar cognitive behavioural impairment profile, showing different distribution patterns. In these cases, the Brettschneider staging system was not strictly applicable, and thus they remained unclassifiable. Recent works using a disease progression model and data analysis suggest a model of distribution and dissemination patterns of TDP-43 in the FTLD-MND spectrum and also in limbic age-related TDP-43 encephalopathy49 that may differ from the proposed patterns. Other distributions, such as predominantly pallido-nigro-luysian involvement, have also been described.50
Although TDP-43 is the most predominant component of neuronal cytoplasmic inclusions, proteins other than TDP-43, such as FUS, may underly both FTLD and MND. While these aggregates are often related to mutations of the FUS gene in ALS patients, these are not usually apparent in FTLD. The simultaneous expression of other FET family protein inclusions (TAF15 and Trn1) may help to differentiate mutation carriers.51,52 However, some ALS-FUS cases also lack mutations and co-expression of TAF15 and Trn1 in the neuronal inclusions that behave neuropathologically similarly to sporadic FTLD-FUS/FET cases.53 Here we present two additional cases with FTLD-MND-FUS with similar neuropathological features but no FUS mutations, suggesting a shared pathophysiological mechanism for FTLD-MND and MND related to FUS/FET pathology.
The heterogeneity we observed within the FTLD-MND spectrum was also reflected in the genetics, especially when FTLD was present, with several mutations underlying the same spectrum of disease. The main mutation we identified was C9orf72 expansion (Table 1), which had a high degree of correspondence with specific neuropathological alterations.19,31,32 However, detection of a genetic variant is not predictive of any distinctive clinical features, and even in cases with a previous family history of the disease, we are sometimes unable to find an underlying causative mutation. This might change in the foreseeable future, as genetic and molecular diagnoses are on the rise, paving the way for novel molecular-targeted treatments.
Our study has some limitations. First, as a brain bank series, although brain donation is encouraged for all patients, only a small percentage accept. Therefore, the series might not be fully representative of the whole population and may be subject to a selection bias. As this work does not intend to be an epidemiological study, the results should be interpreted with caution. Second, due to its retrospective nature, we included patients who were evaluated before the acceptance of formal FTD-ALS clinical criteria, and cognitive and behavioural impairment was assessed according to the opinion of the treating neurologist. However, we did not find any difference when we analysed patient subgroups before and after the publication of the criteria. While the lack of formal and systematic neuropsychological testing might underestimate subtle cognitive alterations, there was a good clinicopathological correlation in our series, and the retrospective application of these criteria has been proven to be fairly sensitive and specific.54 Further prospective studies with a thorough registry of motor, cognitive-behavioural and ancillary testing should be conducted, as they are likely to detect subtle or masked cognitive/behavioural alterations. Finally, despite the presence of specific neuropathological changes that are highly specific to C9orf72 mutations, genetic screening was not systematically performed for other mutations and was only performed under clinical criteria. Therefore, additional genetic cases might have been missed.
Our study reflects the heterogeneity of the MND-FTLD disease spectrum and the difficulties in defining distinct subgroups based on clinical presentation and phenotype, neuropathology and genetics, especially in those patients with mixed FTLD-MND features. In particular, some patients may have underlying proteinopathies other than TDP-43, others can present cognitive impairment with a neuropathological substrate other than classical FTLD or may have ‘asymptomatic’ MND. With the advent of protein-targeted therapies in neurodegeneration, it is of utmost importance to develop early disease-specific biomarkers related to altered pathophysiological mechanisms. In the meantime, neuropathology remains a valuable tool for defining the nosology and molecular pathology of the MND-FTLD disease spectrum.
Supplementary Material
Acknowledgements
We would like to dedicate this work to Dr Jesús Pradas Orozco as a token of our deep admiration and gratitude for being a constant source of inspiration and guidance in the field of MND. His wisdom and insights have been a guiding light, and his dedication to pushing the boundaries of knowledge has been a true inspiration. With our utmost respect and appreciation.
We are indebted to the Biobanc-Hospital Clinic-FRCB-IDIBAPS for samples and data procurement. The authors also thank the brain donors and their families for their generous donation for research.
Also, we thank Carolyn Newey for her help with language supervision and correction, which greatly improved the quality of our work.
Contributor Information
Álvaro Carbayo, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Sergi Borrego-Écija, Alzheimer’s Disease and Other Cognitive Disorders Unit, Neurology Department, Hospital Clínic, Institut d’Investigacions Biomediques August Pi i Sunyer, University of Barcelona, Barcelona 08036, Spain.
Janina Turon-Sans, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Elena Cortés-Vicente, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Laura Molina-Porcel, Alzheimer’s Disease and Other Cognitive Disorders Unit, Neurology Department, Hospital Clínic, Institut d’Investigacions Biomediques August Pi i Sunyer, University of Barcelona, Barcelona 08036, Spain; Neurological Tissue Bank, Biobanc-Hospital Clínic-FRCB-IDIBAPS, Barcelona 08036, Spain.
Jordi Gascón-Bayarri, Department of Neurology, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona 08907, Spain.
Miguel Ángel Rubio, Neuromuscular Unit, Department of Neurology, Hospital del Mar, Barcelona 08003, Spain.
Mónica Povedano, Department of Neurology, Motor Neuron Unit, Instituto de Investigación Biomédica de Bellvitge (IDIBELL), Bellvitge University Hospital, Hospitalet de Llobregat, Barcelona 08907, Spain.
Josep Gámez, GMA Clinic, Neurology Department, European Reference Network On Rare Neuromuscular Diseases (ERN EURO-NMD), Barcelona 08029, Spain.
Javier Sotoca, Neuromuscular Diseases Unit, Department of Neurology, Hospital Vall d'Hebron, Universitat Autònoma de Barcelona, Barcelona, 08035, Spain.
Raúl Juntas-Morales, Neuromuscular Diseases Unit, Department of Neurology, Hospital Vall d'Hebron, Universitat Autònoma de Barcelona, Barcelona, 08035, Spain.
Miriam Almendrote, Neurology Department, Hospital Germans Trias i Pujol, Badalona 08916, Spain.
Marta Marquié, Ace Alzheimer Center Barcelona, Universitat Internacional de Catalunya (UIC), Barcelona 08028, Spain.
Raquel Sánchez-Valle, Alzheimer’s Disease and Other Cognitive Disorders Unit, Neurology Department, Hospital Clínic, Institut d’Investigacions Biomediques August Pi i Sunyer, University of Barcelona, Barcelona 08036, Spain.
Ignacio Illán-Gala, Sant Pau Memory Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Madrid 28029, Spain.
Oriol Dols-Icardo, Sant Pau Memory Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Madrid 28029, Spain.
Sara Rubio-Guerra, Sant Pau Memory Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain; Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), Madrid 28029, Spain.
Sara Bernal, Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain; Genetics Department, Hospital de la Santa Creu i Sant Pau, Barcelona 08025, Spain.
Marta Caballero-Ávila, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Ana Vesperinas, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Ellen Gelpi, Neurological Tissue Bank, Biobanc-Hospital Clínic-FRCB-IDIBAPS, Barcelona 08036, Spain; Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna 1090, Austria.
Ricard Rojas-García, Neuromuscular Diseases Unit, Department of Neurology, Hospital de la Santa Creu i Sant Pau, Biomedical Research Institute (IIB Sant Pau) Sant Pau, Barcelona 08025, Spain; Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona 08025, Spain; Biomedical Network Research Centre on Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid 28029, Spain.
Data availability
All data remain available at Hospital de la Santa Creu i Sant Pau upon request to the corresponding author.
Funding
This study has been funded by Instituto de Salud Carlos III PI19/01543 and PI23/00845, co-funded by ERDF/ESF, “A way to make Europe”/“Investing in your future”. A.C. and M.C.-Á. are supported by Instituto de Salud Carlos III (Rio Hortega Contract CM21/00057 and CM21/00101, respectively). I.I.-G. is a senior Atlantic Fellow for Equity in Brain Health at the Global Brain Health Institute (GBHI), and receives funding from the GBHI, the Alzheimer’s Association and the Alzheimer Society (GBHI ALZ UK-21-720973 and AACSF-21-850193). I.I.-G. was also supported by Insituto de Salud Carlos III (Juan Rodés Contract JR20/0018) and PI21/00791 from Instituto de Salud Carlos III. E.C.-V. was supported by Insituto de Salud Carlos III (Juan Rodés Contract JR19/00037).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. [DOI] [PubMed] [Google Scholar]
- 2. Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. J Neurol Neurosurg Psychiatry. 2012;83:102–108. [DOI] [PubMed] [Google Scholar]
- 3. Elamin M, Phukan J, Bede P, et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology. 2011;76:1263–1269. [DOI] [PubMed] [Google Scholar]
- 4. Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–2594. [DOI] [PubMed] [Google Scholar]
- 5. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. [DOI] [PubMed] [Google Scholar]
- 6. Brooks BR, Miller RG, Swash M, Munsat TL. El escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord. 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 7. Shefner JM, Al-Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020;131:1975–1978. [DOI] [PubMed] [Google Scholar]
- 8. Nishihira Y, Tan C-F, Onodera O, et al. Sporadic amyotrophic lateral sclerosis: Two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol. 2008;116:169–182. [DOI] [PubMed] [Google Scholar]
- 9. Geser F, Martinez-Lage M, Robinson J, et al. Clinical and pathological Continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009;66:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geser F, Brandmeir NJ, Kwong LK, et al. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. [DOI] [PubMed] [Google Scholar]
- 11. Coan G, Mitchell CS. An assessment of possible neuropathology and clinical relationships in 46 sporadic amyotrophic lateral sclerosis patient autopsies. Neurodegener Dis. 2015;15:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pradas J, Puig T, Rojas-García R, Viguera ML, Gich I, Logroscino G. Amyotrophic lateral sclerosis in catalonia: A population based study. Amyotroph Lateral Scler Front Degener. 2013;14:278–283. [DOI] [PubMed] [Google Scholar]
- 13. Borrego-Écija S, Turon-Sans J, Ximelis T, et al. Cognitive decline in amyotrophic lateral sclerosis: Neuropathological substrate and genetic determinants. Brain Pathol. 2021;31: e12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mackenzie IR, Neumann M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol. 2017;134:79–96. [DOI] [PubMed] [Google Scholar]
- 17. Mackenzie IRA, Munoz DG, Kusaka H, et al. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. 2011;121:207–218. [DOI] [PubMed] [Google Scholar]
- 18. Blair IP, Williams KL, Warraich ST, et al. FUS mutations in amyotrophic lateral sclerosis: Clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2010;81:639–645. [DOI] [PubMed] [Google Scholar]
- 19. Ramos-Campoy O, Ávila-Polo R, Grau-Rivera O, et al. Systematic screening of ubiquitin/p62 aggregates in cerebellar Cortex expands the neuropathological phenotype of the C9orf72 expansion mutation. J Neuropathol Exp Neurol. 2018;77:703–709. [DOI] [PubMed] [Google Scholar]
- 20. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 22. Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–479. [DOI] [PubMed] [Google Scholar]
- 23. Saito Y, Ruberu NN, Sawabe M, et al. Staging of argyrophilic grains: An age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63:911–918. [DOI] [PubMed] [Google Scholar]
- 24. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–146. [DOI] [PubMed] [Google Scholar]
- 25. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Sarraj S, King A, Troakes C, et al. P62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie IRA, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014;127:347–357. [DOI] [PubMed] [Google Scholar]
- 33. Vicente-Pascual M, Rossi M, Gámez J, et al. Variably protease-sensitive prionopathy presenting within ALS/FTD spectrum. Ann Clin Transl Neurol. 2018;5:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dols-Icardo O, García-Redondo A, Rojas-García R, et al. Analysis of known amyotrophic lateral sclerosis and frontotemporal dementia genes reveals a substantial genetic burden in patients manifesting both diseases not carrying the C9orf72 expansion mutation. J Neurol Neurosurg Psychiatry. 2018;89:162–168. [DOI] [PubMed] [Google Scholar]
- 35. Gordon PH, Delgadillo D, Piquard A, et al. The range and clinical impact of cognitive impairment in French patients with ALS: A cross-sectional study of neuropsychological test performance. Amyotroph Lateral Scler. 2011;12:372–378. [DOI] [PubMed] [Google Scholar]
- 36. Montuschi A, Iazzolino B, Calvo A, et al. Cognitive correlates in amyotrophic lateral sclerosis: A population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168–173. [DOI] [PubMed] [Google Scholar]
- 37. Woolley SC, Strong MJ. Frontotemporal dysfunction and dementia in amyotrophic lateral sclerosis. Neurol Clin. 2015;33:787–805. [DOI] [PubMed] [Google Scholar]
- 38. Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Front Degener. 2014;15(1–2):9–14. [DOI] [PubMed] [Google Scholar]
- 39. Cortés-Vicente E, Turon-Sans J, Gelpi E, et al. Distinct clinical features and outcomes in motor neuron disease associated with behavioural variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2018;45(3–4):220–231. [DOI] [PubMed] [Google Scholar]
- 40. Hu WT, Shelnutt M, Wilson A, et al. Behavior matters—Cognitive predictors of survival in amyotrophic lateral sclerosis. PLoS One. 2013;8:e57584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006;63:506. [DOI] [PubMed] [Google Scholar]
- 42. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takeda T, Seilhean D, Le Ber I, et al. Amygdala TDP-43 pathology in frontotemporal lobar degeneration and motor neuron disease. J Neuropathol Exp Neurol. 2017;76:800–812. [DOI] [PubMed] [Google Scholar]
- 44. Makkinejad N, Schneider JA, Yu J, et al. Associations of amygdala volume and shape with transactive response DNA-binding protein 43 (TDP-43) pathology in a community cohort of older adults. Neurobiol Aging. 2019;77:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Al-Chalabi A, Hardiman O, Kiernan MC, Chiò A, Rix-Brooks B, van den Berg LH. Amyotrophic lateral sclerosis: Moving towards a new classification system. Lancet Neurol. 2016;15:1182–1194. [DOI] [PubMed] [Google Scholar]
- 46. Feldman EL, Goutman SA, Petri S, et al. Amyotrophic lateral sclerosis. Lancet. 2022;400:1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agosta F, Al-Chalabi A, Filippi M, et al. The el escorial criteria: Strengths and weaknesses. Amyotroph Lateral Scler Front Degener. 2015;16(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 48. Hu WT, Seelaar H, Josephs KA, et al. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol. 2009;66:1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Young AL, Vogel JW, Robinson JL, et al. Data-driven neuropathological staging and subtyping of TDP-43 proteinopathies. Brain. 2023;146:2975–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miki Y, Mori F, Nunomura J, et al. Sporadic amyotrophic lateral sclerosis with pallido-nigro-luysian degeneration: A TDP-43 immunohistochemical study. Neuropathology. 2010;30:149–153. [DOI] [PubMed] [Google Scholar]
- 51. Mackenzie IRA, Neumann M. Fused in sarcoma neuropathology in neurodegenerative disease. Cold Spring Harb Perspect Med. 2017;7:a024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mackenzie IRA, Ansorge O, Strong M, et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: Two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011;122:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Borrego-Écija S, Cortés-Vicente E, Cervera-Carles L, et al. Does ALS-FUS without FUS mutation represent ALS-FET? Report of three cases. Neuropathol Appl Neurobiol. 2019;45:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Balasa M, Gelpi E, Martín I, et al. Diagnostic accuracy of behavioral variant frontotemporal dementia consortium criteria (FTDC) in a clinicopathological cohort. Neuropathol Appl Neurobiol. 2015;41:882–892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data remain available at Hospital de la Santa Creu i Sant Pau upon request to the corresponding author.