Abstract
Synaptic dysfunction and degeneration is likely the key pathophysiology for the progression of cognitive decline in various dementia disorders. Synaptic status can be monitored by measuring synaptic proteins in CSF. In this study, both known and new synaptic proteins were investigated and compared as potential biomarkers of synaptic dysfunction, particularly in the context of Alzheimer's disease (AD).
Seventeen synaptic proteins were quantified in CSF using two different targeted mass spectrometry assays in the prospective Swedish BioFINDER-2 study. The study included 958 individuals, characterized as having mild cognitive impairment (MCI, n = 205), AD dementia (n = 149) and a spectrum of other neurodegenerative diseases (n = 171), in addition to cognitively unimpaired individuals (CU, n = 443). Synaptic protein levels were compared between diagnostic groups and their associations with cognitive decline and key neuroimaging measures (amyloid-β-PET, tau-PET and cortical thickness) were assessed.
Among the 17 synaptic proteins examined, 14 were specifically elevated in the AD continuum. SNAP-25, 14-3-3 zeta/delta, β-synuclein, and neurogranin exhibited the highest discriminatory accuracy in differentiating AD dementia from controls (areas under the curve = 0.81–0.93). SNAP-25 and 14-3-3 zeta/delta also had the strongest associations with tau-PET, amyloid-β-PET and cortical thickness at baseline and were associated with longitudinal changes in these imaging biomarkers [β(standard error, SE) = −0.056(0.0006) to 0.058(0.005), P < 0.0001]. SNAP-25 was the strongest predictor of progression to AD dementia in non-demented individuals (hazard ratio = 2.11). In contrast, neuronal pentraxins were decreased in all neurodegenerative diseases (except for Parkinson's disease), and NPTX2 showed the strongest associations with subsequent cognitive decline [longitudinal Mini-Mental State Examination: β(SE) = 0.57(0.1), P ≤ 0.0001; and mPACC: β(SE) = 0.095(0.024), P ≤ 0.001] across the AD continuum. Interestingly, utilizing a ratio of the proteins that displayed higher levels in AD, such as SNAP-25 or 14-3-3 zeta/delta, over NPTX2 improved the biomarkers' associations with cognitive decline and brain atrophy.
We found 14-3-3 zeta/delta and SNAP-25 to be especially promising as synaptic biomarkers of pathophysiological changes in AD. Neuronal pentraxins were identified as general indicators of neurodegeneration and associated with cognitive decline across various neurodegenerative dementias. Cognitive decline and brain atrophy were best predicted by ratios of SNAP-25/NPTX2 and 14-3-3 zeta/delta/NPTX2.
Keywords: synaptic pathology, mass spectrometry, biomarkers, cognition
Nilsson et al. explore potential synaptic CSF biomarkers of cognitive decline and find that certain proteins including SNAP-25 and 14-3-3 zeta/delta are elevated in Alzheimer’s disease, whereas neuronal pentraxins are reduced across dementias. The ratios of these proteins can be used to predict cognitive decline and brain atrophy.
Introduction
The increasing prevalence of dementia is a major global challenge. The most common cause is Alzheimer's disease (AD), which has been predicted to affect 100 million people by 2050.1 The World Health Organization has specified improved early diagnostics as a key area in the global action plan against dementia since it is of high importance for optimal disease management and treatment.2 Furthermore, the boundaries between the different causes of dementia are often clinically unclear, and the presence of mixed pathology is common. With the advent of disease-modifying treatments now within reach, it is of utmost importance to have early and accurate diagnostic tools that assess multiple disease domains, for which fluid biomarkers will likely be of great value. In AD, recent improvements include the incorporation in the diagnostic framework of PET targeting the underlying disease pathologies of amyloid-β (Aβ)3 and tau,4 as well as CSF analysis of the Aβ42/40 ratio and phosphorylated tau (p-tau) forms,5 providing high diagnostic accuracy.6 However, there is still a need for additional biomarkers that target other pathophysiological processes beyond Aβ and tau, especially when it comes to improving the prediction of cognitive decline and tightening associations between biomarkers and measures of cognitive function. Furthermore, exploring the relationship between distinct classes of mechanistic pathways can expand the understanding of dementia and assist in the identification of novel drug target candidates.
Part of the central pathophysiology of dementia is the dysfunction of synapses, followed by their degeneration. In fact, in clinicopathological AD studies, measures of synaptic degeneration better correlate with antemortem cognitive function than the underlying Aβ and tau pathologies, even though synaptic degeneration has also mechanistically been linked as a downstream effect of such pathologies.7 This strong link between synaptic dysfunction and cognitive decline highlights the potential use of synaptic biomarkers as relevant outcome measures in interventional studies. Over the past 30 years, since their first detection in CSF,8 various synaptic proteins have been studied as potential biomarkers, and several methods for their quantification have been developed, such as SNAP-25 and neurogranin, which are among the most prominent.9 Recently, we developed two in-house mass spectrometric assays to quantify synaptic proteins in the CSF and to study synaptic dysfunction in dementia.10,11 The first method utilizes an immunoprecipitation step to target the low-abundance proteins SNAP-25 and synaptotagmin-1,10 both found to be increased in the CSF of AD patients. The second method includes a panel of 15 synaptic proteins which were selected based on an exploratory proteomics study12 and encompasses neurogranin, the activating protein 2 subunit complex beta (AP2B1), complexin-2, rab GDP dissociation inhibitor alpha (GDI-1), phosphatidylethanolamine-binding protein 1 (PEBP-1) and several members of the protein families of the 14-3-3s, syntaxins, synucleins and neuronal pentraxins (NPTXs).11 Using this panel, we showed, in a smaller AD dataset, that the concentrations of β-synuclein, gamma-synuclein, neurogranin, PEBP-1 and 14-3-3 proteins are higher in the CSF of AD patients compared with cognitively unimpaired (CU) individuals. At the same time, the NPTX proteins are found at lower levels in AD patients compared with CU individuals, while the concentrations of complexin-2, GDI-1 and AP2B1 seem to remain unchanged.11
The main objective of this study was to leverage the two methods to study these synaptic CSF biomarker candidates in the Swedish BioFINDER-2 study, which is a prospective and longitudinal cohort study, including a deeply-characterized sample covering the AD continuum as well as a broad range of non-AD neurodegenerative diseases. Together, the two methods cover several synaptic proteins with diverse functions and localizations at the synapse, hopefully allowing for a comprehensive overview when studying synaptic dysfunction in dementia. The aim was to determine when CSF levels of synaptic proteins become abnormal and investigate their potential clinical utility and discriminatory ability between diagnoses as well as their associations with clinical deterioration over time and core AD pathologies, including Aβ-plaque pathology (determined by Aβ-PET), tau-tangle pathology (determined by tau-PET) and neurodegeneration (determined by volumetric MRI).
Materials and methods
Study population (Swedish BioFINDER-2 study)
The prospective Swedish BioFINDER-2 study (NCT03174938)13 included patients with mild cognitive impairment (MCI), AD dementia (ADD) and a spectrum of other neurodegenerative diseases as well as CU individuals. The patients with AD fulfilled the Diagnostic and Statistical Manual of Mental Disorders (5th edition) criteria for AD14 and were required to be Aβ-positive (Aβ+) as previously described.15 The CU and MCI participants were required to be fluent in Swedish, be 40 years of age or older and have a Mini-Mental State Examination (MMSE) score between 27 and 30 for CU participants and between 24 and 30 for MCI. MCI was classified as previously described.16 Further subdivision into Aβ+ and Aβ-negative (Aβ−) members of the CU group (Aβ−/Aβ+ CU) and participants with MCI (Aβ−/Aβ+ MCI) was performed based on the CSF Aβ1-42/1-40 ratio.17 Inclusion criteria for the other neurodegenerative diseases included fulfilment of criteria for behavioural variant frontotemporal dementia (bvFTD),14 dementia with Lewy bodies (DLB),18 Parkinson's disease (PD),19 PD with dementia (PDD),14 subcortical vascular dementia (VaD),14 progressive supranuclear palsy (PSP),20 multiple system atrophy (MSA)21 or primary progressive aphasia (PPA).22 Of the PPA patients, five presented with semantic variant PPA and three with non-fluent variant PPA. Due to small inclusion numbers for PPA (n = 7), bvFTD (n = 23), PSP (n = 24) and MSA (n = 13), these patients were merged into bvFTD/PPA (frontotemporal disorders) and PSP/MSA (atypical parkinsonism) groups. All non-AD patients had negative AD core biomarkers (CSF Aβ1–42/1–40 ratio and p-tau181), except for four participants (DLB, n = 1; PD, n = 1; and VaD, n = 2). Participants were recruited at Skåne University Hospital between April 2017 to September 2019. The Regional Ethical Committee in Lund, Sweden provided ethical approval. The patient demographics are listed in Table 1. For full details on the diagnostic criteria, refer to Palmqvist et al.15
Table 1.
Demographics
| – | Aβ− CU (n = 330) | Aβ+ CU (n = 113) | Aβ+ MCI (n = 122) | ADD (n = 149) | Aβ− MCI (n = 83) | PD (n = 48) | PSP/MSA (n = 37) | bvFTD/PPA (n = 30) | VaD (n = 20) | DLB (n = 26) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex female, n (%) | 149 (45.2%) | 55 (48.7%) | 61 (50.0%) | 70 (47.0%) | 51 (61.4%) | 30 (62.5%) | 18 (48.6%) | 15 (50.0%) | 14 (70.0%) | 23 (88.5%) | <0.001 |
| Age | 63.4 (11.9) | 70.4 (9.0) | 71.4 (7.6) | 73.2 (7.0) | 68.9 (9.2) | 69.4 (10.4) | 67.3 (8.7) | 68.6 (8.6) | 74.2 (5.9) | 74.4 (5.4) | <0.001 |
| APOE ε4, n (%) | 111 (34.2%) | 81 (71.7%) | 92 (76.0%) | 108 (72.5%) | 18 (21.7%) | 20 (41.7%) | 10 (27.8%) | 10 (33.3%) | 7 (35.0%) | 11 (42.3%) | <0.001 |
| MMSE | 29.0 (1.2) | 28.7 (1.4) | 26.7 (1.9) | 20.3 (4.3) | 27.4 (1.9) | 27.7 (3.3) | 26.21(3.2) | 23.5 (4.4) | 23.1 (3.8) | 22.0 (5.9) | <0.001 |
| Aβ42/40 ratio | 0.110 (0.012) | 0.053 (0.013) | 0.049 (0.013) | 0.044 (0.011) | 0.109 (0.013) | 0.096 (0.020) | 0.094 (0.017) | 0.096 (0.022) | 0.087 (0.033) | 0.083 (0.030) | <0.001 |
| P-tau181, pg/ml | 16.2 (5.4) | 26.7 (10.4) | 31.1 (14.0) | 41.45 (18.8) | 18.1 (7.8) | 17.3 (5.9) | 13.6 (3.7) | 17.6 (5.5) | 18.1 (6.8) | 18.1 (6.7) | <0.001 |
| T-tau, pg/ml | 192.0 (63.8) | 288.5 (95.6) | 325.3 (128.4) | 419.5 (167.9) | 226.9 (117.1) | 207.6 (69.8) | 177.5 (51.5) | 244.7 (70.3) | 214.7 (72.9) | 212.4 (69.9) | <0.001 |
Continuous data are shown as mean (standard deviation). Demographic factors, clinical characteristics, and CSF biomarkers were compared using ANOVA and chi-squared goodness of fit test. Of the primary progressive aphasia (PPA) patients, five presented with semantic variant PPA and three with non-fluent variant PPA. Due to small inclusion numbers for PPA (n = 7), behavioural variant of frontotemporal dementia (bvFTD; n = 23), progressive supranuclear palsy (PSP; n = 24) and multiple system atrophy (MSA; n = 13), the patients were merged into bvFTD/PPA and PSP/MSA groups. Aβ = amyloid-β; ADD = Alzheimer’s disease dementia; CU = cognitively unimpaired; DLB = dementia with Lewy bodies; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; p-tau = phosphorylated tau; t-tau = total tau; PD = Parkinson's disease; PDD = Parkinson's disease with dementia; VaD = subcortical vascular dementia.
CSF sampling and analysis
The collection of lumbar CSF samples was performed according to a standardized protocol developed by an international expert group.23 Following collection, the samples were centrifuged (2000g, +4°C, 10 min), aliquoted in polypropylene tubes and stored at −80°C. CSF Aβ1–42/1–40 ratio, phosphorylated tau at Thr181 (p-tau181), and total tau (t-tau) were determined on a Cobase 601 analyser using the NeuroToolKit (Roche Diagnostics). The Aβ status (positive/negative) was determined based on the CSF Aβ1–42/1–40 ratio, using a cut-off value of 0.08.24 The p-tau181 status (positive/negative) was determined using a cut-off of 27 pg/ml.25
Neuropsychological tests
A neuropsychological evaluation was performed at baseline and then yearly for participants with cognitive impairment and every 2 years for CU participants. The main measures of interest for the study were the MMSE score to assess global cognition26 and a modified composite score of the Preclinical Alzheimer's Cognitive Composite 5 (PACC5)27,28 as a measure to capture early cognitive decline (mPACC). The tests included were the MMSE, ADAS-cog delayed recall, Symbol Digit Test and Category Fluency of animals. All tests were z-scored based on the mean and standard deviation (SD) of CU Aβ− participants over 50 years old and then averaged to generate the mPACC score as described previously.29 For longitudinal analyses, regardless of diagnosis, 830 and 622 participants had follow-up neuropsychological tests and clinical diagnoses on average 2.53 (0.95) (range 0.40–4.76) and 2.61 (0.99) (range 0.37–4.76) years after baseline for MMSE and mPACC, respectively.
Liquid chromatography-tandem mass spectrometry analysis
For details on sample preparation, refer to previously published work.11 In brief, a mixture of heavy peptide standards was added to 100 µl CSF; the samples were then subjected to reduction of cysteine disulfides, alkylation and trypsin digestion. Quantitation was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a micro-flow LC-MS/MS system (6495 Triple Quadrupole LC/MS system, Agilent Technologies) equipped with a Hypersil Gold reversed-phase column (100×2.1 mm, particle size 1.9 µm, Thermo Fisher Scientific). A panel of 15 synaptic proteins was measured: neurogranin, AP2B1, complexin-2, GDI-1, PEBP-1, 14-3-3 epsilon, 14-3-3 theta, 14-3-3 zeta/delta, syntaxin-1B, syntaxin-7, β-synuclein, gamma-synuclein, NPTX1, NPTX2 and NPTXR. For detailed settings, refer to Supplementary Table 1A. Quality control samples, consisting of CSF pools, were injected periodically to monitor assay performance.
For SNAP-25 and synaptotagmin-1 measurements, an in-house assay was used consisting of enrichment with immunoprecipitation (KingFisher™ Flex System) of 200 µl CSF, followed by digestion, the addition of heavy isotope-labelled standards and quantitation with liquid chromatography/selected reaction monitoring mass spectrometry (LC-SRM/MS) (Agilent 6490 QQQ MS). For details on sample preparation and settings, refer to previously published work30,31 and Supplementary Table 1B, respectively.
MRI and PET acquisition and processing
MRI was performed on a Siemens 3T MAGNETOM Prisma scanner (Siemens Medical Solutions). Structural T1-weighted MRI images were acquired from a magnetization-prepared rapid gradient echo (MPRAGE) sequence with 1 mm isotropic voxels. PET images were acquired on digital GE Discovery MI scanners. For Aβ-PET, image acquisition was performed 90–110 min post injection of ∼185 MBq 18F-flutemetamol. Note that patients with dementia did not undergo Aβ-PET. For tau-PET, acquisition was carried out 70–90 min post injection of ∼370 MBq 18F-RO948 and was available for all participants. Image processing was done as described previously.32 Briefly, PET images were attenuation corrected, motion corrected, summed and registered to the closest T1-weighted MRI processed through the longitudinal pipeline of FreeSurfer version 6.0. Standardized uptake value ratio (SUVR) images were created using the inferior cerebellar grey matter as the reference region for 18F-RO948 and the cerebellum for 18F-flutemetamol.
For Aβ, the region of interest (ROI) was the average SUVR from a neocortical global region (prefrontal, lateral temporal, parietal, anterior cingulate and posterior cingulate/precuneus). For tau, a temporal meta-ROI comprised of the average bilateral entorhinal, amygdala, fusiform, parahippocampal, inferior and middle temporal cortex SUVR was used.33 As a measure of neurodegeneration, the average cortical thickness from an AD signature of temporal regions34 (bilateral entorhinal, inferior and middle temporal and fusiform cortex) was used. For tau-PET and cortical thickness, longitudinal data regardless of diagnosis were available for 638 and 626 participants, respectively, for an average follow-up of 2.4 (SD 0.9) years.
Data processing and statistical analysis
Skyline 20.1 (MacCoss Lab Software) was utilized to analyse the mass spectrometric data. For the statistical analyses, R version 3.6.1 (R Foundation for Statistical Computing) was used. Group comparisons of continuous and categorical demographics values were performed using ANOVA and chi-squared goodness-of-fit tests, respectively. The group-wise comparisons of the biomarkers were all assessed in linear regression models, with respective biomarkers as an outcome and adjusted for sex and age as well as for multiple comparisons with false rate discovery (FDR) correction. Standardization of relative peptide levels was performed by z-scoring biomarker values based on the controls (Aβ− CU). Forty subjects had missing SNAP-25 levels due to technical problems (seven CU−, seven CU+, seven MCI+, 10 AD, five MCI−, one PD, one PSP/MSA and two VaD). Following group comparison analyses, all subsequent analyses excluded subjects missing SNAP-25 to ensure that all analyses included the same participants.
The receiver operating characteristic curve (ROC) area under the curve (AUC) was used to evaluate diagnostic performance, and the DeLong test was performed to compare AUCs. For these analyses, the NPTX2 levels were inverted so that all coefficients were in the same direction. To determine which combination of biomarkers enabled the best discrimination in the logistic regression, the Multi-Model-Inference R package version 1.43.17, which generates models with the best combinations of biomarker ranking based on Akaike Information Criteria (AIC), was utilized. The model with the lowest AIC represented the best model fit (change lower than two points implied a similar fit) and was compared to subsequent models to retain the most parsimonious models. Note that when performing the analyses, biomarker inclusion was limited to the best performers to minimize the risk of random false-positive findings. ANOVA and the Delong test were used to compare the best model to subsequent models. Furthermore, to avoid collinearity due to dependent predictors, the analyses of synaptic biomarkers and their ratios were performed separately.
Associations between the CSF levels of synaptic proteins (standardized) and neocortical Aβ-PET SUVR, tau-PET SUVR in the temporal meta-ROI, cortical thickness in the temporal AD (only cross-sectional data) and cognition (MMSE and mPACC) cross-sectionally and longitudinally were tested using linear models and linear mixed models, respectively. All models were adjusted for sex, age and diagnosis at baseline, whereas models of cognition also included adjustment for years of education. Separate analysis of cross-sectional and longitudinal data was performed to maximize data inclusion. All linear mixed models had random intercepts and slopes, and the interaction between time and the synaptic marker is reported. Cox-proportional hazard regression models were used with age and sex as covariates to assess the association of the baseline concentrations of the synaptic proteins (standardized) and conversion to AD dementia during longitudinal follow-up, and hazard ratios are reported. The outcome for the model was time to diagnosis, the patients were censored at their last follow-up visit and the NPTX2 levels were inverted. FDR correction was performed for multiple comparisons for all models, where all P-values reported are FDR-corrected.
Results
The BioFINDER-2 study included 958 participants, of whom 330 (34%) were controls (Aβ− CU), 113 (12%) had preclinical AD (Aβ+ CU), 122 (13%) had prodromal AD (Aβ+ MCI), 149 (16%) had ADD and 244 (25%) had various other neurodegenerative diseases (Table 1). The non-AD neurodegenerative disease groups included bvFTD/PPA, DLB, PD, VaD and PSP/MSA. The mean age was 68.4 (SD 10.4) years, 49.3% were women and the mean years of education was 12.5 (SD 3.8).
Diagnostic group comparisons of synaptic biomarkers
All synaptic biomarkers, except for the NPTX proteins, were found at higher levels in CSF of ADD patients compared with controls (Aβ− CU) [β(standard error, SE) = −0.37(0.11) to −2.51(0.14), P < 0.01; Supplementary Fig. 1 and Supplementary Table 2]. Of the synaptic proteins, SNAP-25, 14-3-3 zeta/delta, β-synuclein and neurogranin were the top-performing biomarkers with the largest concentration increases in ADD compared with controls (Aβ− CU). The standardized CSF concentrations by diagnostic group are shown in Fig. 1.
Figure 1.
CSF concentrations of SNAP-25, 14-3-3 zeta/delta (ζ/d), β-synuclein, neurogranin and NPTX2 (one representative peptide for each protein) across diagnostic groups. For visualization purposes only, P-values for comparisons, including the control or Alzheimer’s disease dementia (ADD) groups, are shown due to space constraints. All P-values from the group comparisons are shown in Supplementary Table 2. The bottom and top hinges of the box plot denote the 25th and 75th percentiles, respectively, while the medians are represented by vertical lines, and the whiskers extend to the most extreme points within the 1.5× interquartile range of the 25th and 75th percentiles. AD = Alzheimer’s disease; Aβ = amyloid-β; Aβ− CU = controls; Aβ+ CU = preclinical AD; Aβ+ MCI = prodromal AD; bvFTD/PPA = behavioural variant frontotemporal dementia/primary progressive aphasia; CU = cognitively unimpaired; DLB = dementia with Lewy bodies; MCI = mild cognitive impairment; non-AD = non-AD neurodegenerative diseases; PD = Parkinson's disease; PSP/MSA = progressive supranuclear palsy/multiple system atrophy; VaD = subcortical vascular dementia. P-values: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
Most of the proteins, except NPTXs and syntaxin-1B, were also already found to be increased in CSF of preclinical AD (Aβ+ CU) participants when compared with controls (Aβ− CU) [β(SE) = −0.26(0.12) to −1.07(0.15), P < 0.05], and they (except syntaxin-7) were also increased in prodromal AD (Aβ+ MCI) when compared with controls (Aβ− CU) [β(SE) = −0.41(0.12) to −1.40(0.15), P < 0.01]. In addition, 14-3-3 was further increased in prodromal AD (Aβ+ MCI) when compared with preclinical AD (Aβ+ CU) [β(SE) = −0.42(0.17), P = 0.032], which was not the case for the other proteins. Finally, the levels of 14-3-3 zeta/delta, SNAP-25, β-synuclein, neurogranin, 14-3-3 epsilon, gamma-synuclein, GDI-1 and PEBP-1 were further increased in ADD compared with prodromal AD (Aβ+ MCI) [β(SE) = −0.38(0.16) to −1.11(0.16), P < 0.01].
In summary, SNAP-25, 14-3-3 zeta/delta, β-synuclein and neurogranin were clearly increased in all stages of AD when compared with controls, and 14-3-3 zeta/delta increased further when moving from preclinical AD to prodromal AD, and all of these four proteins increased further when moving from prodromal AD to ADD (Fig. 1).
When comparing non-AD neurodegenerative diseases with controls (Aβ− CU), most synaptic proteins were unchanged. The CSF concentrations of SNAP-25, neurogranin and β-synuclein were found to be lower [β(SE) = 0.68(0.25) to 1.01(0.25), P < 0.05] in the PSP/MSA group compared with controls (Aβ− CU). Additionally, 14-3-3 zeta/delta were found to be present at higher concentrations in bvFTD/PPA compared with controls (Aβ− CU) [β(SE) = −0.63(0.26), P = 0.030].
When it comes to the NPTXs, the CSF concentrations of NPTX1, NPTX2 and NPTXR were found to be lower in ADD as well as in the non-AD neurodegenerative groups, except PD, when compared with controls (Aβ− CU) [NPTX2: β(SE) = 0.57(0.10) to 1.13(0.17), P < 0.01; NPTX1: β(SE) = 0.56(0.21) to 0.97(0.17) (except AD), P < 0.05; NPTXR: β(SE) = 0.35(0.11) to 1.21(0.18), P < 0.01]. However, the NPTXs showed no stepwise decrease along the AD continuum, i.e. no differences were found between preclinical AD, prodromal AD and ADD. Furthermore, only NPTX2 was found at lower concentrations at the prodromal stage of AD (Aβ+ MCI) when compared with controls (Aβ− CU) [β(SE) = 0.42(0.11), P = 0.00031].
Given that SNAP-25, 14-3-3 zeta/delta, β-synuclein and neurogranin were the proteins with the most significant increases across the AD continuum and compared with the non-AD neurodegenerative disease group, the main results from subsequent analyses focus on these four proteins. NPTX2, which was most clearly decreased in both AD and most other neurodegenerative diseases, is also highlighted. Detailed results for all proteins quantified can be found in the Supplementary material.
Discriminatory accuracy of synaptic biomarkers
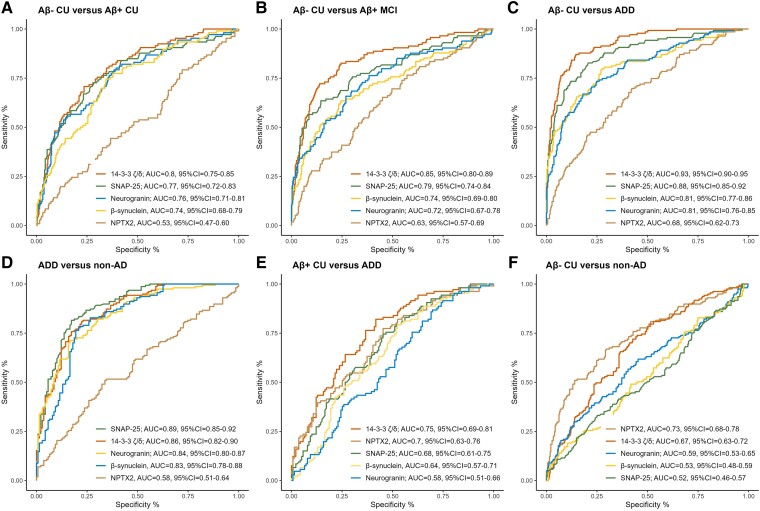
Next, the ability of the synaptic proteins to differentiate the different diagnostic groups from controls (Aβ− CU) was evaluated (Fig. 2). Overall, 14-3-3 zeta/delta exhibited the highest AUCs (AUC = 0.80–0.95) across the AD continuum (Fig. 2A–C), showing significantly better diagnostic performance than the other synaptic proteins at both the prodromal AD (Aβ+ MCI) and AD dementia stages (DeLong, P < 0.001). Furthermore, 14-3-3 zeta/delta exhibited better diagnostic performance than NPTX2 even at the preclinical AD (Aβ+ CU) stage (DeLong, P ≤ 0.0001). The protein exhibiting the second highest AUCs across the AD continuum was SNAP-25, i.e. when comparing preclinical AD, prodromal AD and ADD dementia to controls (AUC = 0.77–0.88).
Figure 2.
Diagnostic accuracy of SNAP-25, neurogranin, 14-3-3 zeta/delta (ζ/d), β-synuclein and NPTX2. Receiver operating curves calculated for (A) controls (amyloid-β-negative cognitively unimpaired, Aβ− CU) versus preclinical Alzheimer’s disease (AD) (amyloid-β-negative cognitively unimpaired, Aβ+ CU), (B) Aβ− CU versus prodromal AD (Aβ+ MCI), (C) Aβ− CU versus Alzheimer's disease dementia (ADD), (D) ADD versus non-AD neurodegenerative disease (non-AD), (E) Aβ+ CU versus ADD and (F) Aβ− CU versus non-AD for the five synaptic proteins. For the analysis, the NPTX2 levels were inverted. CI = confidence interval.
When differentiating ADD from all other non-AD neurodegenerative diseases, SNAP-25 was instead the protein demonstrating the highest AUCs [AUC = 0.89, 95% confidence interval (CI) = 0.85–0.92; Fig. 2D], which were significantly higher than those of β-synuclein, neurogranin and NPTX2 (DeLong, P < 0.01).
When comparing ADD to preclinical AD (Aβ+ CU) instead of controls (Aβ− CU), the diagnostic performance of all markers was lower, as expected, but 14-3-3 zeta/delta showed acceptable discrimination with an AUC of 0.75 (95%CI = 0.69–0.81; Fig. 2E), which was significantly better than β-synuclein, neurogranin and SNAP-25 (DeLong, P < 0.01).
When differentiating non-AD neurodegenerative diseases from controls (Aβ− CU), NPTX2 provided the best discrimination (AUC = 0.73, 95%CI = 0.68–0.78; Fig. 2F), which was significantly better than β-synuclein, neurogranin and SNAP-25 (Delong, P ≤ 0.0001) but not 14-3-3 zeta/delta. The performance of NPTX2 in this context was comparable to its performance when discriminating ADD from controls (Aβ− CU) (AUC = 0.68, 95%CI = 0.62–0.73). Results for all proteins can be found in Supplementary Table 3.
Associations of synaptic markers with AD pathology and atrophy
Associations between the different synaptic markers and AD pathology measured with neuroimaging, i.e. Aβ-plaque pathology load (measured with Aβ-PET SUVR), tau-tangle pathology (measured with tau-PET SUVR) as well as cortical thickness (measured with volumetric MRI) were assessed both cross-sectionally and longitudinally. The strongest associations with Aβ-plaque pathology load and tau-tangle pathology across the AD continuum, i.e. in preclinical AD, prodromal AD and ADD were seen consistently with 14-3-3 zeta/delta and SNAP-25 (Supplementary Table 4A). Cross-sectionally, higher levels of 14-3-3 zeta/delta and SNAP-25 were related to a higher load of tau-tangle pathology [β(SE) = 0.40(0.03) and β(SE) = 0.41(0.03), respectively, both P ≤ 0.0001] as well as more Aβ-plaque pathology [β(SE) = 0.22(0.02), P = 0.011 and β(SE) = 0.31(0.02), P ≤ 0.0001, respectively]. The next strongest associations with baseline tau- and Aβ-PET were seen for β-synuclein and NPTX2 but with lower magnitudes [tau-PET: β(SE) = −0.23 to 0.26(0.03), P ≤ 0.0001 and Aβ PET: β(SE) = −0.18(0.02), P = 0.025 (only NPTX2)]. When it comes to cross-sectional associations with cortical thickness, NTPX2 exhibited the strongest association [β(SE) = 0.34(0.01), P ≤ 0.0001], followed by 14-3-3 zeta/delta [β(SE) = −0.13(0.01), P = 0.023].
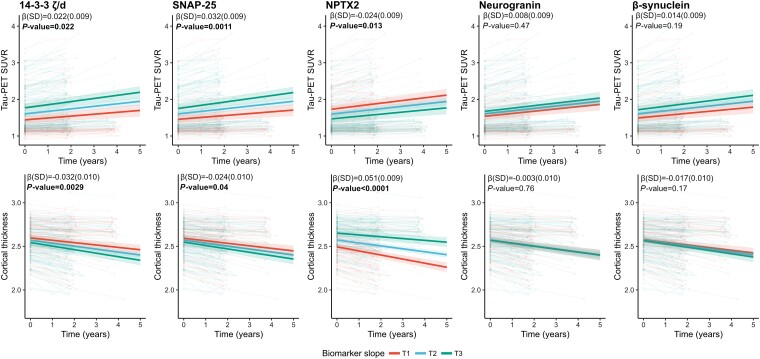
Looking at longitudinal increases in tau-tangle pathology or brain atrophy [average 2.3 (0.9 SD) years, n = 248 participants for tau-PET and n = 260 for cortical thickness], higher baseline levels of 14-3-3 zeta/delta or SNAP-25 and lower baseline levels of NPTX2 were most strongly associated with a greater increase in tau-PET SUVR [β(SE) = 0.022(0.009), β(SE) = 0.032(0.009) and β(SE) = −0.024(0.009), respectively, all P < 0.05] and decrease in cortical thickness over time [β(SE) = −0.032(0.010), β(SE) = −0.024(0.010) and β(SE) = 0.051(0.009), respectively, all P < 0.05] (Fig. 3). In longitudinal analyses, no associations were found for β-synuclein or neurogranin.
Figure 3.
Associations with Alzheimer’s disease pathology and atrophy for SNAP-25, neurogranin, 14-3-3 zeta/delta (ζ/d), β-synuclein and NPTX2 across the Alzheimer’s disease continuum. Associations of tau-PET, amyloid-β (Aβ)-PET and thickness [Alzheimer’s disease (AD) signature—MRI] cross-sectionally and longitudinally with individual biomarkers (standardized concentrations) were examined using linear models and linear mixed-effects models (with random slopes and intercept), respectively, that included age, diagnosis at baseline and sex as covariates. False rate discovery correction was performed for multiple comparisons. Only participants with longitudinal data were kept for the linear mixed-effect models. For longitudinal tau, 611 data points from 248 participants were included, with an average of 2.28 years of follow-up (SD 0.92). For longitudinal cortical thickness, 649 data points from 260 participants were included, with an average of 2.26 years of follow-up (SD 0.91). Statistical models were performed with continuous values for the synaptic protein and tertiles were created only for visualization purposes. SUVR = standardized uptake value ratio.
In contrast, looking at associations between CSF biomarkers of synaptic pathology and neuroimaging across the non-AD neurodegenerative diseases, no associations (P > 0.05; Supplementary Table 4B) were found with either tau-PET or cortical thickness for cross-sectional analyses as well as when studying the prediction of subsequent longitudinal change. The exception was for lower baseline levels of NPTX2, which was found to be associated with cross-sectional cortical thickness [β(SE) = 0.26(0.01), P = 0.00012]. Similarly, no associations (P > 0.05; Supplementary Table 4C) were found in controls (Aβ− CU). All statistical details with associations with PET and MRI can be found in Supplementary Tables 4A–C.
Associations of synaptic markers with cognitive decline and clinical progression
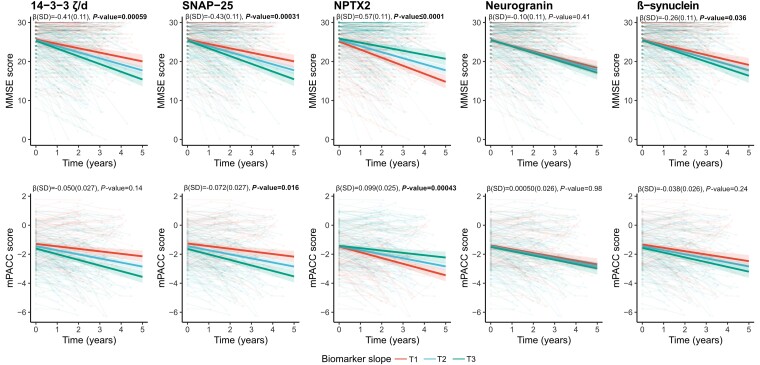
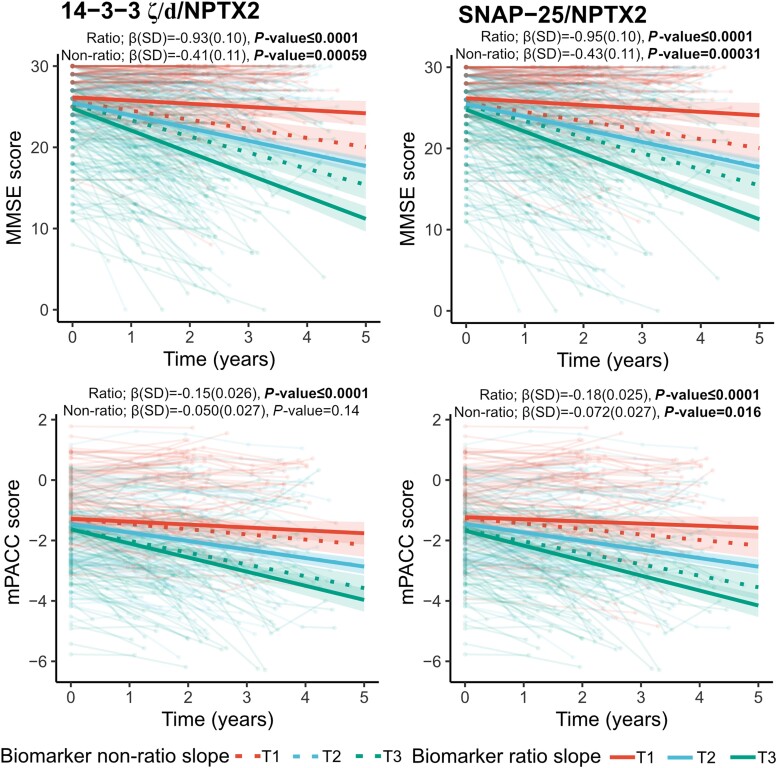
Associations between CSF biomarkers of synaptic pathology and cognitive decline were assessed across the AD continuum, i.e. in preclinical AD, prodromal AD and ADD (Supplementary Table 5A). Cross-sectional analyses revealed that only higher levels of 14-3-3 zeta/delta [β(SE) = −0.16(0.053), P = 0.013] were found to be associated with lower performance on mPACC, but no synaptic protein showed any association with MMSE. When studying subsequent longitudinal change in MMSE [average follow-up 2.61 (0.94 SD) years, n = 336 participants], it was found that lower baseline concentrations of NPTX2 [β(SE) = 0.57(0.11), P ≤ 0.0001] and higher concentrations of SNAP-25, 14-3-3 zeta/delta and β-synuclein [β(SE) = −0.26(0.11) to −0.43(0.11), P < 0.05] were associated with more rapid cognitive decline on MMSE over time (Fig. 4). When studying subsequent longitudinal change in mPACC [average follow-up 2.66 (0.98 SD) years, n = 242 participants], only baseline NPTX2 [β(SE) = 0.099(0.025), P = 0.00043] and SNAP-25 [β(SE) = −0.072(0.027), P = 0.016] were associated with more rapid cognitive decline (Fig. 4).
Figure 4.
Associations with cognitive measures (MMSE and m PACC scores) for SNAP-25, neurogranin, 14-3-3 zeta/delta (ζ/d), β-synuclein and NPTX2 across the Alzheimer’s disease continuum. Associations of cognition (MMSE and mPACC scores) longitudinally with individual biomarkers (standardized concentrations) were examined using linear mixed-effects models (with random slopes and intercept), respectively, that included age, sex, diagnosis and years of education as covariates across the Alzheimer’s disease (AD) continuum (preclinical AD, prodromal AD and AD dementia). False rate discovery correction was performed for multiple comparisons. Only participants with longitudinal data were kept for the linear mixed-effect models. 1069 data points from 336 participants were included for longitudinal MMSE, with an average of 2.61 (0.94 SD) years of follow-up. For longitudinal mPACC, 738 data points from 242 participants were included, with an average of 2.66 (0.98 SD) years of follow-up. Statistical models were performed with continuous values for the synaptic protein, and tertiles were created only for visualization purposes. MMSE = Mini-Mental State Examination; mPACC = modified Preclinical Alzheimer's Cognitive Composite.
In contrast, when assessing associations between CSF biomarkers of synaptic pathology and cognitive decline across the non-AD neurodegenerative diseases, no associations (P > 0.05; Supplementary Table 5B) were found with either MMSE or mPACC for cross-sectional analyses as well as when studying the prediction of subsequent longitudinal change. The exception was for lower baseline levels of NPTX2, which was found to be associated with baseline mPACC [β(SE) = 0.22(0.08), P = 0.04] and longitudinal change in MMSE [β(SE) = 0.39(0.12), P = 0.0087]. Similarly, no associations (P > 0.05; Supplementary Table 5C) were found in controls (Aβ− CU) except for 14-3-3 zeta/delta and mPACC [β(SE) = −0.13(0.04), P = 0.010] in the cross-sectional analysis.
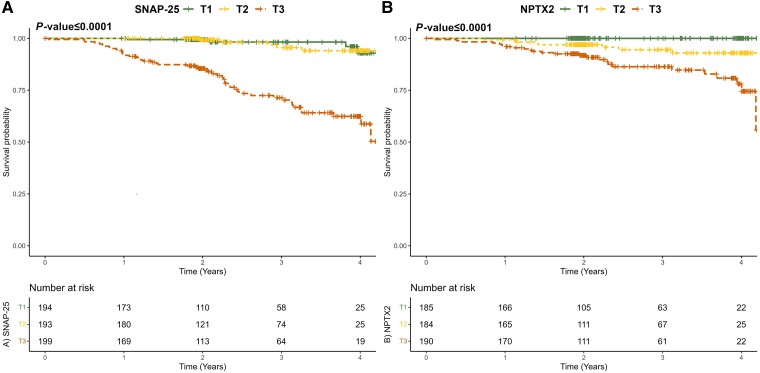
Follow-up diagnosis over the same period with an average of 2.4 (1.18 SD) years was available from 586 study participants who were non-demented (CU or MCI) at baseline, of whom 59 (10%) progressed to ADD. Cox regression analysis showed that SNAP-25, 14-3-3 zeta/delta, β-synuclein, neurogranin and NPTX2 were independent predictors of progression to an ADD diagnosis [hazard ratio = 1.42–2.11, P < 0.05; Supplementary Table 6A]. SNAP-25 concentration was the strongest predictor, with a hazard ratio of 2.11, indicating a substantial increase in the likelihood of progression to ADD, with higher concentrations of baseline SNAP-25 in non-demented individuals (Fig. 5A). Replicating the analyses focusing on the progression to a diagnosis of MCI due to AD from non-demented (CU) at baseline [17 (4%) progressors of 425 participants, mean follow-up = 2.42 (1.17 SD) years], similar results were observed with SNAP-25 concentration as the strongest predictor, with a hazard ratio of 2.79 (Supplementary Table 6B). Focusing on the non-AD neurodegenerative diseases, 34 non-demented (6%, CU and MCI) of 561 study participants [average follow-up of 2.42 (1.19 SD) years] progressed to a dementia diagnosis. For this case, Cox regression analysis revealed NPTX2 to be an independent predictor of progression to a dementia diagnosis in non-AD neurodegenerative diseases, with a hazard ratio of 6.03 (inverted levels, P ≤ 0.0001), indicating a substantial increase in the likelihood of progression, with lower concentrations of baseline NPTX2 in non-demented individuals (Fig. 5B).
Figure 5.
Associations of clinical progression with SNAP-25 and NPTX2 levels. (A) SNAP-25 levels in 586 participants who were cognitively unimpaired or had mild cognitive impairment (MCI) at baseline were included, with an average of 2.4 (1.2 SD) years of follow-up, of whom 59 (10%) progressed to Alzheimer’s disease dementia (ADD). Statistical models were performed with continuous values for the synaptic protein, and tertiles were created only for visualization purposes. (B) NPTX2 levels in 561 participants who were cognitively unimpaired or had MCI at baseline were included, with an average of 2.4 (1.2 SD) years of follow-up, of whom 34 (6%) progressed to non-AD dementia. Statistical models were performed with continuous values for the synaptic protein, and tertiles were created only for visualization purposes.
Utility of combining synaptic proteins in ratios
Lastly, to investigate the potential utility of combining synaptic biomarkers for the quantification of synaptic pathology, the ratio of SNAP-25, 14-3-3 zeta/delta, β-synuclein, or neurogranin over NPTX2 was also explored for all previous analyses. The rationale was to create ratios of key proteins with increased concentrations along the AD continuum to the key protein with a decreased concentration in AD and non-AD (NPTX2). Interestingly, the results improved, and the most notable changes were related to associations with cognitive function in the AD continuum. Across all analyses, the ratios SNAP-25/NPTX2 and 14-3-3 zeta/delta/NPTX2 yielded the strongest associations, and the main focus in the following section will then be on these two ratios. Both for cross-sectional and longitudinal associations with MMSE and mPACC, using the ratio SNAP-25/NPTX2 or 14-3-3 zeta/delta/NPTX2 yielded much stronger associations [MMSE: β(SE) = −0.75(0.18) to −0.95(0.10), P < 0.001; mPACC: β(SE) = −0.15(0.026) to −0.28(0.055), P ≤ 0.0001], with coefficients from regressions models being almost twice as high with the ratios compared with SNAP-25 or 14-3-3 zeta/delta alone (Fig. 6 and Supplementary Table 5A). In non-AD neurodegenerative disease, the ratios SNAP-25/NPTX2 and 14-3-3 zeta/delta/NPTX2 were associated with both lower MMSE scores at baseline and faster decline over time [β(SE) = −0.63(0.13) to −1.01(0.25), P < 0.01; see Supplementary Table 5B for all statistics], associations which were not seen with the standalone synaptic measures. In addition, 14-3-3 zeta/delta/NPTX2 displayed both weak cross-sectional and longitudinal associations in the control (Aβ− CU) group with mPACC [β(SE) = −0.15(0.04) to −0.05(0.01), P < 0.01]
Figure 6.
Associations with cognitive measures (MMSE and mPACC scores) for SNAP-25/NPTX2 and 14-3-3 zeta/delta (ζ/d)/NPTX2 across the Alzheimer’s disease continuum. Associations of cognition (MMSE and mPACC scores) longitudinally with individual biomarkers (standardized concentrations) were examined using linear mixed-effects models (with random slopes and intercept), respectively, that included age, sex, diagnosis and years of education as covariates across the Alzheimer’s disease (AD) continuum (preclinical AD, prodromal AD and AD dementia). Dotted lines indicate the biomarker's original (non-ratio) associations. False rate discovery correction was performed for multiple comparisons. Only participants with longitudinal data were kept for the linear mixed-effect models. For longitudinal MMSE, 1069 data points from 336 participants were included, with an average of 2.61 (0.94 SD) years of follow-up. For longitudinal mPACC, 738 data points from 242 participants were included, with an average of 2.66 (0.98 SD) years of follow-up. Statistical models were performed with continuous values for the synaptic protein, and tertiles were created only for visualization purposes. MMSE = Mini-Mental State Examination; mPACC = modified Preclinical Alzheimer's Cognitive Composite; SD = standard deviation.
The associations with cortical thickness and the PET measures of Aβ and tau in the AD continuum also clearly improved (Supplementary Table 4A). In both cross-sectional and longitudinal analyses, all associations improved with the SNAP-25/NPTX2 and 14-3-3 zeta/delta/NPTX2 ratios. The main difference in using the ratios was more evident in cross-sectional associations with cortical thickness in the non-AD neurodegenerative diseases and the controls (Aβ− CU): the synaptic ratios were associated with thinner cortex in both groups, respectively [14-3-3 zeta/delta/NPTX2: β(SE) = −0.36(0.01) and −0.31(0.01), P < 0.001]. These associations were not seen in these groups with the standalone synaptic markers.
Similarly, the ratios improved the discrimination between certain diagnostic groups for all four synaptic proteins, most notably when comparing preclinical AD (Aβ+ CU) with ADD (Supplementary Table 3). In this comparison, the ratios of either 14-3-3 zeta/delta/NPTX2 (AUC = 0.87, 95%CI = 0.83–0.92) or SNAP-25/NPTX2 (AUC = 0.86, 95%CI = 0.82–0.91) were had the highest AUCs, which were significantly higher than the AUCs obtained when using 14-3-3 zeta/delta, SNAP-25 or NPTX2 alone (AUCs = 0.68–0.75, DeLong, P < 0.001). Similarly, improvements in AUCs were also seen when differentiating controls from prodromal AD (Aβ+ CU) or ADD as well as separating controls (Aβ− CU) from non-AD neurodegenerative diseases. No major improvements were observed in predicting progression utilizing the ratios compared with the single biomarkers (Supplementary Tables 6A–C).
Comparison with current AD core biomarkers, p-tau181 and t-tau
To investigate the potential utility of the synaptic biomarkers compared with current AD core biomarkers, p-tau181 and t-tau were also explored for all previous analyses. In the cross-sectional analyses, associations were only seen with the synaptic ratios and not with p-tau181 and t-tau [MMSE: both β(SE) = −0.05(0.17), P = 0.91; mPACC: β(SE) = −0.09(0.053) and −0.077(0.054), P > 0.05] (Supplementary Table 5A). For the prediction of subsequent longitudinal change in cognition, associations were found for p-tau181 and t-tau [MMSE: β(SE) = −0.52(0.11) and −0.50(0.11), P ≤ 0.0001; mPACC: β(SE) = −0.083(0.027) and −0.080(0.027), P < 0.01] but at almost half of the magnitude of the best synaptic ratio SNAP-25/NPTX2 [MMSE: β(SE) = −0.95(0.10), P ≤ 0.0001; mPACC: β(SE) = −0.18(0.025), P ≤ 0.0001]. It is important to note that the p-tau181 and t-tau as well as the synaptic protein measures are heavily confounded by diagnosis in the cross-sectional analyses, which was a covariate for cognition. However, even when removing the covariate from the analysis, the ratio of SNAP25/NPTX2 [MMSE: β(SE) = −2.29(0.22), P ≤ 0.0001; mPACC: β(SE) = −0.81(0.077), P ≤ 0.0001) still presented with higher associations than p-tau181 [MMSE: β(SE) = −1.30(0.24), P ≤ 0.0001; mPACC: β(SE) = −0.48(0.087), P ≤ 0.0001] and t-tau [MMSE: β(SE) = −1.27(0.24), P ≤ 0.0001; mPACC: β(SE) = −0.44(0.089), P ≤ 0.0001](data not shown). No associations with cognition and p-tau181 or t-tau were found outside of the AD continuum.
The effects of p-tau181 and total tau in relation to clinical progression were also investigated (Supplementary Tables 6A–C). For progression to AD dementia, p-tau181 and t-tau [hazard ratio = 2.09 (1.77–2.46) and 2.21 (1.82–2.70), P ≤ 0.0001] displayed similar hazard ratios to the best synaptic proteins [SNAP-25/NPTX2: hazard ratio = 2.25(1.90–2.67), P ≤ 0.0001]. However, for progression to an AD MCI diagnosis, p-tau181 and t-tau [hazard ratio = 1.90(1.47–2.45) and 2.08(1.52–2.85), P ≤ 0.0001] were outperformed by SNAP-25 alone [hazard ratio = 2.79(1.82–4.30), P ≤ 0.0001]. Furthermore, for the progression to a non-ADD diagnosis, p-tau181 and t-tau [hazard ratio = 0.67(0.41–1.08) and 0.78(0.50–1.20), P > 0.05] were not found to be significant predictors, unlike NPTX2 [hazard ratio = 6.03(2.91–12.47), P ≤ 0.0001].
Lastly, to further investigate the role of synaptic biomarkers beyond p-tau181 and t-tau in discriminating between different groups, the combination of the top synaptic markers as standalone markers or their ratios with p-tau181 or t-tau in the ROC analyses was explored. Across the models in the AD continuum, the combination of p-tau181 and NPTX2 consistently outperformed all the single biomarkers and the synaptic ratios (AUC = 0.87–0.99; ANOVAs for model fit, all P ≤ 0.0001; DeLong, all P < 0.001). The exception was at early AD stages compared with controls, Aβ− CU versus Aβ+ CU, where NPTX2 addition did not improve the model (AUC = 0.87; ANOVA for model fit, P > 0.05; DeLong, P > 0.05) and p-tau181 alone (AUC = 0.84) constituted the best parsimonious model. Of note, combining p-tau181 with either the ratio SNAP-25/NPTX2 or 14-3-3 zeta/delta/NPTX2 was not better than p-tau181 combined with NPTX2 alone. Furthermore, in controls (Aβ− CU) compared with the non-AD neurodegenerative diseases group, p-tau181 typically did not provide much information, and in such cases, 14-3-3 zeta/delta/NPTX2 was the best parsimonious model (AUC = 0.83; ANOVA for model fit, P > 0.05; DeLong, P > 0.05).
Discussion
This large-scale study of the Swedish BioFINDER-2 cohort enabled the comparisons of multiple potential synaptic biomarkers side by side, revealing that several proteins characterized with in-house mass spectrometric methods have the potential to be biomarkers of synaptic dysfunction in AD. Our findings support our previously published results (that differential patterns of synaptic protein alterations exist across neurodegenerative diseases) but now in a larger, more extensive phenotype dataset, which includes a broad range of non-AD neurodegenerative diseases. One of the main novel findings of this study was that of the 17 synaptic proteins studied, 14 showed higher concentrations in the AD continuum compared with non-AD participants. This indicated that, even if synaptic degeneration is not a unique hallmark of AD, synaptic proteins are particularly increased in AD compared with other neurodegenerative diseases, underscoring synaptic dysfunction as a prominent feature of the disease. However, an exception to this finding was that the NPTXs were lower in the CSF and were found to be non-specific indicators of synaptic dysfunction and cognitive decline across neurodegenerative dementias. By looking at the synaptic proteins with increased levels in AD, no clear indications of differences in either function or localizations such as between pre- or post-synaptic proteins were apparent.
Of special note, among the 14 synaptic proteins quantified as having increased concentrations in the AD continuum, 14-3-3 zeta/delta, SNAP-25, β-synuclein and neurogranin consistently showed the strongest associations across all analyses. 14-3-3 zeta/delta belongs to a synapse-enriched protein family involved with synaptic transmission and plasticity regulation,35 a family emerging as one of the top differentially expressed AD proteins in recent explorative CSF proteomics papers.12,36,37 In our previous targeted studies, 14-3-3 zeta/delta concentration was indicated to be higher in AD compared with controls as well as other neurodegenerative diseases.11,38,39 SNAP-25 and neurogranin, which are, respectively, a pre-synaptic protein involved in vesicle exocytosis and a post-synaptic protein involved in calcium-mediated signalling pathways, are both well-known synaptic biomarkers implicated specifically in AD.9 Higher concentrations of the two proteins have been confirmed by ELISA and mass spectrometric methods in AD patients.30,40-43 Lastly, β-synuclein belongs to the same pre-synaptic family as alpha-synuclein, a protein prominently known as a major component of Lewy bodies and Aβ plaques.44,45 We and others have shown that β-synuclein levels seem to be higher in AD than in controls, with no changes observed in comparison with other neurodegenerative diseases.11,38,39,46,47 The protein is also one of the first suggested synaptic blood biomarkers48 even in preclinical AD.49 In the current study, it was shown that the CSF levels of these four proteins in particular discriminate AD (AUC = 0.81–0.93) from both controls (Aβ− CU) and non-AD, with the best discrimination shown with SNAP-25 and 14-3-3 zeta/delta. Their CSF concentrations also seem to increase stepwise across the AD continuum and provide good discrimination (AUC = 0.74–0.80) at the preclinical stages of AD (Aβ+ CU) compared with controls (Aβ− CU). This increase in synaptic protein concentrations in early AD, i.e. Aβ-positive individuals with no evidence of tau pathology, suggests that the changes may occur due to Aβ-triggered synaptic dysfunction prior to tau accumulation and neurodegeneration.
Higher concentrations of SNAP-25, β-synuclein, 14-3-3 zeta/delta and neurogranin were also performed well in predicting the progression to ADD (from CU and MCI stages), and they were associated with more rapid cognitive decline. In particular, higher CSF concentrations of SNAP-25 doubled the probability of conversion to dementia, and it was the only protein, apart from NPTX2, that showed an association with cognitive decline as measured by both MMSE and mPACC. A previous systematic review supports the suggestion that, among other synaptic biomarkers, CSF concentrations of SNAP-25, together with NPTX2, have a particular association with cognition.50 Our study goes beyond these two markers in investigating many synaptic biomarkers side by side in a large sample and relating them to brain changes. To that effect, only SNAP-25 and 14-3-3 zeta/delta were associated with cortical thickness as well as tau and Aβ-PET imaging, where the strongest associations were found specifically with the underlying pathology of AD and less with atrophy. Higher baseline concentrations of these two proteins were also related to the accumulation of tau tangles, suggesting a close interplay between synaptic dysfunction and the progression of AD pathology.
Another group of interesting proteins in the study was the NPTXs, a family of scaffold proteins whose functions involve the recruitment of post-synaptic receptors into synapses and thus modulation of homeostatic synaptic plasticity.51,52 Simultaneously studying NPTX levels in a broad range of non-AD neurodegenerative diseases for the first time, we were able to show that NPTXs, in particular the CSF concentration of NPTX2, seem to be generally lower across neurodegenerative diseases, including AD, DLB, atypical parkinsonism (PSP/MSA), FTD and VaD. The results were in line with those from smaller recent studies that have reported lower CSF concentrations of the pentraxins in AD38,42,53-57 as well as in other neurodegenerative diseases such as atypical parkinsonism,39 DLB38,58,59 and FTLD.38,59,60 In a previous study, lower levels of NPTX2 were also observed in PD compared with controls; the results were not replicated in the current study possibly due to the low sample size (PD, n = 48). Among the synaptic proteins, NPTX2 best discriminated (AUC = 0.73) non-AD patients from controls but was outperformed by almost all other synaptic biomarkers for discriminating AD from controls. We and others have previously shown that NPTX2 seems to have a stronger association with cognitive measurements (MMSE) in AD and other neurodegenerative diseases such as PD than other synaptic biomarkers,11,38,39,42,59 and this was discussed in a recent systematic review of the field.50 In the current study, we confirmed that there is a particularly strong association between baseline pentraxin concentrations and more rapid cognitive decline across the AD continuum as estimated by changes in MMSE as well as mPACC scores. Associations between NPTX2 and AD pathology in the brain, measured with tau-PET and Aβ-PET, were generally lower than for the other synaptic proteins, both at baseline and longitudinally. However, associations between cortical thickness and NPTX2 were stronger, outperforming the other markers. Additionally, lower NPTX2 levels were predictive of a more rapid clinical progression to ADD. Taken together, the findings indicated that NPTX2 is possibly more associated with neurodegeneration in general than with the underlying pathology of AD, since its levels were low in other neurodegenerative diseases and not specifically low in AD. In fact, this decrease could be attributed to reduced neuronal activity, as NPTX2 is known to be an immediate early gene (IEG) produced in response to increased synaptic activity.61 However, alterations in NPTX levels have been suggested to signify the specific disruption of pyramidal neuron-parvalbumin circuits and subsequent excitability homeostasis,62,63 since NPTX2 accumulates prominently at excitatory synapses on parvalbumin-expressing interneurons when perineuronal nets are present.63
Finally, we investigated the potential utility of creating ratios of key proteins (SNAP-25, β-synuclein, 14-3-3 zeta/delta and neurogranin) with increased concentrations along the AD continuum to the main protein (NPTX2) with a decreased concentration in AD and non-AD. Interestingly, as a central novel finding of the study, most results improved, most notably indicating that the utilization of ratios of synaptic proteins to track cognition might be particularly useful. Few studies have explored the use of ratios of synaptic proteins, but in the few that have, similar improvements were reported when utilizing NPTXR and neurogranin.64 In comparing the synaptic biomarkers with current AD core biomarkers (p-tau181 and t-tau), the synaptic proteins and their ratios, especially NPTX2 and SNAP25/NPTX2, presented higher associations with cognitive decline than p-tau181 and t-tau. The combination of p-tau181 with NPTX2 seemed to improve the diagnostic accuracy across the AD continuum, while the ratio of 14-3-3 zeta/delta/NPTX2 should be further investigated for its discriminatory value outside the AD spectrum. Furthermore, in specific cases of predicting progression, SNAP-25 and NPTX2 also seemed to have an advantage over p-tau181 and t-tau, such as for progression to MCI due to AD and to a non-AD dementia diagnosis, respectively. Overall, we found that, of the synaptic biomarkers studied, NPTX2 and its ratios add value beyond p-tau181 and t-tau.
The major strength of this study in comparing potential synaptic pathology biomarkers across neurodegenerative dementias was the use of multiplexed mass spectrometry, which allowed for the simultaneous quantification of several biomarkers. In addition, SNAP-25 and synaptotagmin-1, two potential synaptic biomarkers from the literature, were quantified. This allowed us to study a range of synaptic biomarkers with different functions and localizations, enabling us not only to compare them but also to reinforce the credibility of the results from proteins with similar outcomes, i.e. validating general and specific pathological patterns. Even though only cross-sectional measurements of synaptic proteins were available, 2–4 years of longitudinal cognitive data and brain imaging measures were included. It is important to acknowledge that even though this study was among the largest targeted investigations to focus on so many synaptic proteins in the AD continuum, the sample sizes of the non-AD groups were relatively smaller (n = 20–48 per diagnostic group). Future studies should thus include a longitudinal analysis to confirm the value of the biomarkers to track disease progression and to determine how early in the disease trajectory the changes occur and further investigations are needed to explore the impact and implication of the use of synaptic protein ratios. Additionally, the discriminatory accuracy against a specific non-AD neurodegenerative disease should be interpreted with caution, and future studies should focus on replicating these findings in larger study samples.
Conclusion
Using in-house mass spectrometric measurements of a large panel of synaptic proteins in AD and neurodegenerative diseases, we found the most prominent synaptic biomarker changes in AD, corroborating that synaptic dysfunction is a particularly strong feature of this disease. In contrast, members of the pentraxin family showed alterations across neurodegenerative diseases, suggesting that they may be sensitive biomarkers for general synaptic degeneration. Taken together, our results indicate that several synaptic proteins, specifically NPTX2, SNAP-25, neurogranin, β-synuclein and 14-3-3 zeta/delta, show promise as possible complements to other CSF and imaging markers as diagnostic, prognostic, stage and/or monitoring biomarkers of cognitive decline and synaptic pathology. In particular, the ratios of SNAP-25/NPTX2 and 14-3-3 zeta/delta/NPTX2 emerged as strong contenders for predicting cognitive decline and brain atrophy. The study of synaptic proteins is of great importance as it offers valuable mechanistic insight into the complex overlapping impact of synaptic pathology in the spectrum of neurodegenerative diseases.
Supplementary Material
Acknowledgements
We gratefully acknowledge the participants, their families and the personnel who made this work possible.
Contributor Information
Johanna Nilsson, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden.
Alexa Pichet Binette, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden.
Sebastian Palmqvist, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden; Memory Clinic, Skåne University Hospital, 205 02 Malmö, Sweden.
Wagner S Brum, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Graduate Program in Biological Sciences: Biochemistry, Department of Biochemistry, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre 90035-003, Brazil.
Shorena Janelidze, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden.
Nicholas J Ashton, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Centre for Age-Related Medicine, Stavanger University Hospital, 4011 Stavanger, Norway; Department of Old Age Psychiatry, Maurice Wohl Clinical Neuroscience Institute, King’s College London, London SE5 9RX, UK; NIHR Maudsley Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, London SE5 8AF, UK.
Nicola Spotorno, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden.
Erik Stomrud, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden; Memory Clinic, Skåne University Hospital, 205 02 Malmö, Sweden.
Johan Gobom, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 431 30 Mölndal, Sweden.
Henrik Zetterberg, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 431 30 Mölndal, Sweden; Fluid Biomarker Laboratory, UK Dementia Research Institute at UCL, London WC1E 6BT, UK; Department of Neurodegenerative Disease, UCL Institute of Neurology, London WC1N 3BG, UK; Hong Kong Center for Neurodegenerative Diseases, Hong Kong, China; Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53792, USA.
Ann Brinkmalm, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 431 30 Mölndal, Sweden.
Kaj Blennow, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, S-431 80 Mölndal, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, 431 30 Mölndal, Sweden; Paris Brain Institute, ICM, Pitié-Salpêtrière Hospital, Sorbonne University, 75646 Paris, France; Neurodegenerative Disorder Research Center, Division of Life Sciences and Medicine, and Department of Neurology, Institute on Aging and Brain Disorders, University of Science and Technology of China and First Affiliated Hospital of USTC, Hefei 230036, P.R. China.
Oskar Hansson, Clinical Memory Research Unit, Department of Clinical Sciences, Malmö, Lund University, 211 46 Malmö, Sweden; Memory Clinic, Skåne University Hospital, 205 02 Malmö, Sweden.
Data availability
Pseudonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority and Region Skåne, which should be regulated by a material transfer agreement.
Funding
The work at the University of Gothenburg was supported by the Eivind o Elsa K: son Sylvan Foundation (Eivind och Elsa K:son Sylvan Stiftelse), the Foundation for Gamla Tjänarinnor, Demensfonden and the Märta and Gustaf Ågren Foundation. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union's Horizon Europe research and innovation programme under grant agreement no. 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research, University College London Hospitals Biomedical Research Centre and the UK Dementia Research Institute at UCL (UKDRI-1003). S.P. is supported by the Swedish Research Council (#2018-02052), the Swedish Brain Foundation (#FO2022-0204) and the Swedish Alzheimer’s Foundation (Alzheimerfonden) (#AF-981132). K.B. is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish State under the agreement between the Swedish Government and the County Councils, the ALF-agreement (#ALFGBG-715986) and European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236). J.G. is supported by grants from Alzheimerfonden (#AF-930934), Åhlénsstiftelsen (#K8020071398) and Stiftelsen Gamla Tjänarinnor. Work at Lund University was supported by the Alzheimer’s Association (SG-23-1061717), Swedish Research Council (2022-00775), ERA PerMed (ERAPERMED2021-184), the Knut and Alice Wallenberg foundation (2017-0383), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's disease) at Lund University, the Swedish Alzheimer’s Foundation (Alzheimerfonden) (AF-980907), the Swedish Brain Foundation (FO2021-0293), The Parkinson Foundation of Sweden (1412/22), the Cure Alzheimer's fund, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2022-1259) and the Swedish Federal Government under the ALF agreement (2022-Projekt0080). The precursor of 18F-flutemetamol was sponsored by GE Healthcare. The funding sources had no role in the design and conduct of the study; in the collection, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript.
Competing interests
S.P. has acquired research support (for the institution) from ki elements / ADDF. In the past 2 years, he has received consultancy/speaker fees from Bioartic, Biogen, Lilly and Roche. HZ has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. K.B. has served as a consultant, on advisory boards, or data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens. The other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global action plan on the public health response to dementia 2017–2025. https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025.
- 3. Rabinovici GD, Gatsonis C, Apgar C, et al. . Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ossenkoppele R, Rabinovici GD, Smith R, et al. . Discriminative accuracy of [18F] flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattsson N, Zetterberg H, Hansson O, et al. . CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. [DOI] [PubMed] [Google Scholar]
- 6. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27:954–963. [DOI] [PubMed] [Google Scholar]
- 7. Spires-Jones TL, Hyman BT. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidsson P, Puchades M, Blennow K. Identification of synaptic vesicle, pre- and postsynaptic proteins in human cerebrospinal fluid using liquid-phase isoelectric focusing. Article. Electrophoresis. 1999;20:431–437. [DOI] [PubMed] [Google Scholar]
- 9. Camporesi E, Nilsson J, Brinkmalm A, et al. . Fluid biomarkers for synaptic dysfunction and loss. Biomark Insights. 2020;15:1177271920950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira JB, Janelidze S, Ossenkoppele R, et al. . Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain. 2021;144:310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nilsson J, Gobom J, Sjödin S, et al. . Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer's disease. Alzheimers Dement (Amst). 2021;13:e12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tijms BM, Gobom J, Reus L, et al. . Pathophysiological subtypes of Alzheimer's disease based on cerebrospinal fluid proteomics. Brain. 2020;143:3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansson O, Palmqvist S. The Swedish BIOFINDER Study. https://biofinder.se/
- 14. Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21:591–643. [Google Scholar]
- 15. Palmqvist S, Janelidze S, Quiroz YT, et al. . Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmqvist S, Rossi M, Hall S, et al. . Cognitive effects of Lewy body pathology in clinically unimpaired individuals. Nat Med. 2023;29:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jack CR Jr, Bennett DA, Blennow K, et al. . NIA-AA research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKeith IG, Boeve BF, Dickson DW, et al. . Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 20. Höglinger GU, Respondek G, Stamelou M, et al. . Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilman S, Wenning G, Pa L, et al. . Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. . Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansson O, Batrla R, Brix B, et al. . The Alzheimer’s Association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid β and tau. Alzheimers Dement. 2021;17:1575–1582. [DOI] [PubMed] [Google Scholar]
- 24. Quadalti C, Palmqvist S, Hall S, et al. . Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat Med. 2023;29:1964–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blennow K, Shaw LM, Stomrud E, et al. . Predicting clinical decline and conversion to Alzheimer’s disease or dementia using novel Elecsys Aβ (1–42), pTau and tTau CSF immunoassays. Sci Rep. 2019;9:19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 27. Donohue MC, Sperling RA, Salmon DP, et al. . The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papp KV, Rentz DM, Orlovsky I, Sperling RA, Mormino EC. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimers Dement (N Y). 2017;3:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pichet Binette A, Franzmeier N, Spotorno N, et al. . Amyloid-associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer’s disease. Nat Commun. 2022;13:6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brinkmalm A, Brinkmalm G, Honer WG, et al. . SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener. 2014;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohrfelt A, Brinkmalm A, Dumurgier J, et al. . The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer's disease. Alzheimers Res Ther. 2016;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leuzy A, Smith R, Ossenkoppele R, et al. . Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020;77:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho H, Choi JY, Hwang MS, et al. . In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–258. [DOI] [PubMed] [Google Scholar]
- 34. Jack CR Jr, Wiste HJ, Weigand SD, et al. . Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Foote M, Zhou Y. 14-3-3 proteins in neurological disorders. Int J Biochem Mol Biol. 2012;3:152. [PMC free article] [PubMed] [Google Scholar]
- 36. Wisch JK, Butt OH, Gordon BA, et al. . Proteomic clusters underlie heterogeneity in preclinical Alzheimer’s disease progression. Brain. 2022;146(7):2944–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Modeste ES, Ping L, Watson CM, et al. . Quantitative proteomics of cerebrospinal fluid from African Americans and Caucasians reveals shared and divergent changes in Alzheimer's disease. Mol Neurodegener. 2023;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nilsson J, Cousins KA, Gobom J, et al. . Cerebrospinal fluid biomarker panel of synaptic dysfunction in Alzheimer's disease and other neurodegenerative disorders. Alzheimers Dement. 2023;19:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nilsson J, Constantinescu J, Nellgård B, et al. . Cerebrospinal fluid biomarkers of synaptic dysfunction are altered in Parkinson's disease and related disorders. Mov Disord. 2023;38:267–277. [DOI] [PubMed] [Google Scholar]
- 40. Mavroudis IA, Petridis F, Chatzikonstantinou S, Kazis D. A meta-analysis on CSF neurogranin levels for the diagnosis of Alzheimer's disease and mild cognitive impairment. Aging Clin Exp Res. 2020;32:1639–1646. [DOI] [PubMed] [Google Scholar]
- 41. Liu W, Lin H, He X, et al. . Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer’s disease and mild cognitive impairment. Transl Psychiatry. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galasko D, Xiao M, Xu D, et al. . Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement (N Y). 2019;5:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Das S, Goossens J, Jacobs D, et al. . Synaptic biomarkers in the cerebrospinal fluid associate differentially with classical neuronal biomarkers in patients with Alzheimer’s disease and frontotemporal dementia. Alzheimers Res Ther. 2023;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burré J. The synaptic function of α-synuclein. J Parkinsons Dis. 2015;5:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uéda K, Fukushima H, Masliah E, et al. . Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oeckl P, Metzger F, Nagl M, et al. . Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer’s and Creutzfeldt-Jakob disease but no alteration in synucleinopathies. Mol Cell Proteomics. 2016;15:3126–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halbgebauer S, Oeckl P, Steinacker P, et al. . Beta-synuclein in cerebrospinal fluid as an early diagnostic marker of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2021;92:349–356. [DOI] [PubMed] [Google Scholar]
- 48. Oeckl P, Halbgebauer S, Anderl-Straub S, et al. . Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer’s disease. J Proteome Res. 2020;19:1310–1318. [DOI] [PubMed] [Google Scholar]
- 49. Oeckl P, Janelidze S, Halbgebauer S, et al. . Higher plasma β-synuclein indicates early synaptic degeneration in Alzheimer’s disease. Alzheimers Dement. 2023;19:5095–5102. [DOI] [PubMed] [Google Scholar]
- 50. Saunders TS, Gadd DA, Spires-Jones TL, King D, Ritchie C, Muniz-Terrera G. Associations between cerebrospinal fluid markers and cognition in ageing and dementia: A systematic review. Eur J Neurosci. 2022;56:5650–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu D, Hopf C, Reddy R, et al. . Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. [DOI] [PubMed] [Google Scholar]
- 52. Lee S-J, Wei M, Zhang C, et al. . Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J Neurosci. 2017;37:1062–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spellman DS, Wildsmith KR, Honigberg LA, et al. . Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer’s Disease Neuroimaging Initiative (ADNI) CSF. Proteomics Clin Appl. 2015;9(7–8):715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Swanson A, Willette A, Initiative AsDN . Neuronal pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain Behav Immun. 2016;58:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Begcevic I, Tsolaki M, Brinc D, et al. . Neuronal pentraxin receptor-1 is a new cerebrospinal fluid biomarker of Alzheimer’s disease progression. F1000Res. 2018;7:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lim B, Sando SB, Grøntvedt GR, Bråthen G, Diamandis EP. Cerebrospinal fluid neuronal pentraxin receptor as a biomarker of long-term progression of Alzheimer’s disease: A 24-month follow-up study. Neurobiol Aging. 2020;93:97.e1–97.e7. [DOI] [PubMed] [Google Scholar]
- 57. Libiger O, Shaw LM, Watson MH, et al. . Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer's disease. Alzheimers Dement. 2021;17:1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boiten WA, van Steenoven I, Xiao M-F, et al. . Pathologically decreased CSF levels of synaptic marker NPTX2 in DLB are correlated with levels of alpha-synuclein and VGF. Cells. 2020;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bolsewig K, Hok-A-Hin Y, Sepe F, et al. . A combination of neurofilament light, glial fibrillary acidic protein, and neuronal pentraxin-2 discriminates between frontotemporal dementia and other dementias. J Alzheimers Dis. 2022;90:363–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bergström S, Öijerstedt L, Remnestål J, et al. . A panel of CSF proteins separates genetic frontotemporal dementia from presymptomatic mutation carriers: a GENFI study. Mol Neurodegener. 2021;16:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao MF, Xu D, Craig MT, et al. . NPTX2 and cognitive dysfunction in Alzheimer's disease. Elife. 2017;6:e23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chang MC, Park JM, Pelkey KA, et al. . Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dulewicz M, Kulczyńska-Przybik A, Borawska R, Słowik A, Mroczko B. Evaluation of synaptic and axonal dysfunction biomarkers in Alzheimer’s disease and mild cognitive impairment based on CSF and bioinformatic analysis. Int J Mol Sci. 2022;23:10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pseudonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Swedish Ethical Review Authority and Region Skåne, which should be regulated by a material transfer agreement.