Abstract
Purpose
The optimal treatment for gastroesophageal junction adenocarcinoma (GEJA) remains controversial. We evaluated the treatment patterns and outcomes of patients with locally advanced GEJA according to the histological type.
Materials and Methods
We conducted a single-institution retrospective cohort study of patients with locally advanced GEJA who underwent curative-intent surgical resection between 2010 and 2020. Perioperative therapies as well as clinicopathologic, surgical, and survival data were collected. The results of endoscopy and histopathological examinations were assessed for Siewert and Lauren classifications.
Results
Among the 58 patients included in this study, 44 (76%) were clinical stage III, and all received neoadjuvant therapy (72% chemoradiation, 41% chemotherapy, 14% both chemoradiation and chemotherapy). Tumor locations were evenly distributed by Siewert Classification (33% Siewert-I, 40% Siewert-II, and 28% Siewert-III). Esophagogastrectomy (EG) was performed for 47 (81%) patients and total gastrectomy (TG) for 11 (19%) patients. All TG patients received D2 lymphadenectomy compared to 10 (21%) EG patients. Histopathological examination showed the presence of 64% intestinal-type and 36% diffuse-type histology. The frequencies of diffuse-type histology were similar among Siewert groups (37% Siewert-I, 36% Siewert-II, and 33% Siewert-III). Regardless of Siewert type and compared to intestinal-type, diffuse histology was associated with increased intraabdominal recurrence rates (P=0.03) and decreased overall survival (hazard ratio, 2.33; P=0.02). With a median follow-up of 31.2 months, 29 (50%) patients had a recurrence, and the median overall survival was 50.5 months.
Conclusions
Present in equal proportions among Siewert types of esophageal and gastric cancer, a diffuse-type histology was associated with high intraabdominal recurrence rates and poor survival. Histopathological evaluation should be considered in addition to anatomic location in the determination of multimodal GEJA treatment strategies.
Keywords: Esophagogastric junction adenocarcinoma, Esophageal cancer, Siewert-Stein classification, Gastric cancer, Histology evaluation
INTRODUCTION
The incidence of gastroesophageal junction adenocarcinoma (GEJA) is increasing worldwide [1]. GEJAs represent a spectrum of histologically and molecularly diverse tumors defined by their anatomical location between the esophagus and the stomach. The pretreatment determination of GEJA, of either esophageal or gastric origin, has traditionally led to two divergent perioperative treatment paths. Multimodal treatment strategies for GEJA include curative-intent radical surgery and often involve either neoadjuvant chemoradiation following the guidelines of the CROSS Trial [2] for esophageal adenocarcinoma (EAC) or perioperative chemotherapy based on the FLOT4 Trial [3] recommendations for gastric adenocarcinoma (GC). However, in real-world surgical practice, accurate anatomical classification of GEJA remains difficult, and the selection of patient-specific management poses significant challenges [4].
The management of GEJA varies significantly between Eastern and Western countries, and multiple classification systems exist [5]. The most internationally utilized system for anatomical classification of GEJA is the Siewert classification [6]. It divides GEJA into 3 subtypes based on the epicenter of the tumor in relation to the gastroesophageal junction (GEJ) primarily by endoscopic evaluation and secondarily supported by cross-sectional imaging. Siewert type I tumors have epicenters located 1–5 cm above the GEJ and are associated with Barrett’s esophagus. Type III tumors arises from the gastric cardia 2 cm distal to the GEJ and infiltrate the GEJ. Type II tumors are located within 1 cm proximal and 2 cm distal of the GEJ and are considered true cancers of the GEJ. These tumors are the most difficult to classify with significant debate regarding their management.
Over the years, the changing guidelines of the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) staging system reflect the ambiguity of GEJA classification and the inconsistencies in stage-specific survival outcomes. The 8th and latest edition of the AJCC TNM staging system realigned the staging and management of Siewert type I and II with EAC and Siewert type III with GC. Previously, the 6th edition considered Siewert type II as GC, and the 7th edition grouped all three Siewert types as EAC. As considerable debate persists regarding whether to manage these patients as EAC or GC, the histology and genomics of GEJA may support a re-alignment of GEJA tumors with GC. Quante et al. recently suggested that EACs derive their origin from gastric cells [7]. To better understand the impact of non-anatomical factors on the outcomes of patients with GEJA, we evaluated the real-life work-up, treatment, and outcomes of patients with GEJA who underwent curative-intent surgical resection at a high-volume tertiary referral center.
MATERIALS AND METHODS
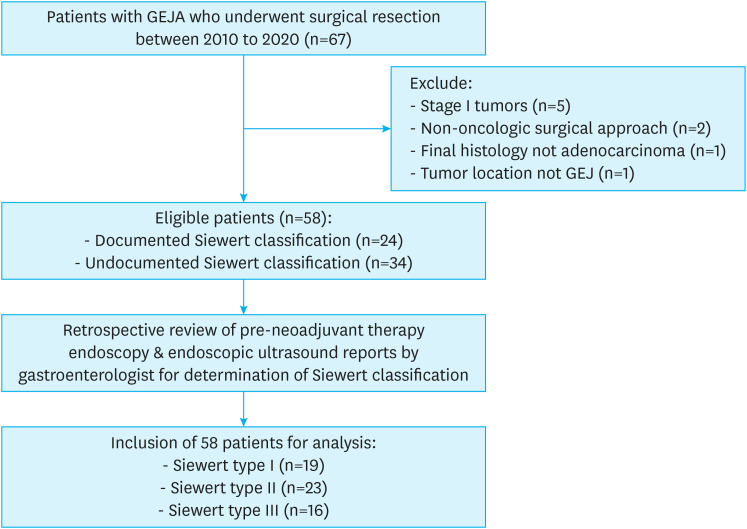
We performed a retrospective review of a prospectively maintained upper gastrointestinal cancer database at the City of Hope National Medical Center, Duarte, USA with ethical approval provided by the Internal Review Board (IRB) of the Center (IRB approval number: 20645). Patients with locally advanced GEJA who underwent curative intent surgical resection between 2010 and 2020 were included in the study. Patients with early-stage disease (T1-2, N0) who underwent resection without neoadjuvant therapy (NT) and patients who underwent a non-oncologic resection (proximal gastrectomy) were excluded from this study (Fig. 1).
Fig. 1. CONSORT diagram of the study cohort. Classification of patients with locally advanced GEJA who underwent curative intent surgical resection.
GEJA = gastroesophageal junction adenocarcinoma.
Multimodal patient treatment strategies
All study patients received NT (chemoradiation, chemotherapy, or both) before surgery, and surgical resection entailed either Ivor-Lewis esophagogastrectomy (EG) or total gastrectomy (TG), with most cases utilizing a minimally invasive robotic approach. Surgical lymphadenectomy was either a 2-field lymphadenectomy (encompassing periesophageal, subcarinal, superior mediastinal, diaphragmatic, paracardial, lesser curvature, and celiac axis lymph nodes) or a D2 dissection (stations 1-7, 8a, 9, 12a, 11 p/d) [8]. Surgical lymphadenectomy was based on the surgical approach; a 2-field lymphadenectomy was performed for EG while D2 dissection was performed for TG. D2 dissection was additionally performed for select patients with EG and type II/III tumors involving the cardia or concern for celiac nodal disease.
NT regimens were primarily determined by tumor location utilizing a Siewert type classification. The majority of Siewert type I/II patients were managed like esophageal cancer patients and received neoadjuvant chemoradiation as recommended by the CROSS trial, and Siewert type III patients were managed like gastric cancer patients and received perioperative chemotherapy as per the FLOT4 trial guidelines. NT regimen variability was observed where select Siewert type II patients with tumors located in the cardia or with celiac nodal disease received perioperative chemotherapy instead of neoadjuvant chemoradiation. Additionally, select patients received full dose, oxaliplatin-based perioperative chemotherapy in addition to neoadjuvant chemoradiation. Chemoradiation was typically trimodal therapy with weekly carboplatin/paclitaxel and 50.4 Gy of radiotherapy, and neoadjuvant chemotherapy regimens were typically leucovorin, fluorouracil, and oxaliplatin (FOLFOX) or fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT).
Demographics and clinicopathologic data
We performed a retrospective review of the demographic and clinicopathologic data. Postoperative complications occurring within 30 days of surgery were recorded using the Clavien-Dindo classification system [9]. Clinical and post-neoadjuvant pathologic staging was performed according to the 8th edition of the Union for International Cancer Control (UICC)/AJCC classification system. Siewert types I and II were staged as EAC and Siewert type III as GC.
The Siewert classification of patient tumors was ascertained from medical record documentation or by secondary review of pretreatment upper endoscopy and endoscopic ultrasonography (EUS) images and reports by an experienced gastroenterologist at our institution.
The Lauren classification (intestinal-, diffuse-, or mixed-type) was determined from histopathology reports or by secondary review of archived histopathology slides by an experienced pathologist at our institution.
The tumor regression score (TRS), as defined by the College of American Pathologists, was retrieved from the surgical pathology reports and used to measure local treatment response to preoperative therapy. TRS was determined by a pathologist on a scale of 0–3 according to previously defined histopathologic features: TRS 0, pathologically complete response; TRS 1, complete response; TRS 2, partial response, and TRS 3, poor or no response to NT.
Statistical analysis
Statistical analysis was performed using Stata 14.2 software (StataCorp LLC, College Station, TX, USA). Student’s t-test was used for continuous variables, while Pearson’s χ2 test was used for categorical variables. Survival curves were compared using the Kaplan-Meier method and the log-rank test. Univariate analysis was performed using the Cox proportional hazards model. Statistical significance was set at P<0.05. Overall survival (OS) was calculated from the date of operation to death from any cause. Recurrence-free survival (RFS) was calculated from the date of operation to the date of tumor recurrence. For RFS, patients who died without known tumor recurrence were censored at the date of their last documented follow-up.
RESULTS
Patient characteristics, preoperative staging, and therapies
Our study included 58 patients with locally advanced GEJA who underwent curative intent surgery after receiving NT between 2010 and 2020. A minority of patients (41%) had pretreatment documentation of a Siewert classification. After a secondary review of endoscopy/EUS findings for the remaining 34 patients in this study, we found an even distribution of GEJA by Siewert Classification, with 19 (32.8%) Siewert type I, 23 (39.7%) Siewert type II, and 16 (27.6%) Siewert type III patients. Table 1 shows clinicopathologic factors, NT regimens, and perioperative outcomes with respect to the Siewert classification of all patients.
Table 1. Clinicopathologic factors, neoadjuvant therapy, and perioperative outcomes with respect to Siewert classification.
| Variable | All (n=58) | Siewert I (n=19) | Siewert II (n=23) | Siewert III (n=16) | P-value | ||
|---|---|---|---|---|---|---|---|
| Age (yr) | 64 (39–81) | 68 (39–81) | 64 (47–81) | 62 (45–72) | 0.115 | ||
| Sex | 0.490 | ||||||
| Female | 15 (25.9) | 6 (31.6) | 4 (17.4) | 5 (31.3) | |||
| Male | 43 (74.1) | 13 (68.4) | 19 (82.6) | 11 (68.8) | |||
| BMI (kg/m2) | 25.6 (14.3–40.8) | 26.9 (19.2–40.8) | 26.4 (19.5–39.8) | 25.4 (14.3–33) | 0.380 | ||
| Ethnicity‡ | 0.083 | ||||||
| White | 41 (70.7) | 14 (73.7) | 19 (82.6) | 8 (50.0) | |||
| Hispanic | 5 (8.6) | 0 (0.0) | 1 (4.3) | 4 (25.0) | |||
| Asian | 9 (15.5) | 2 (10.5) | 3 (13.0) | 4 (25.0) | |||
| Black/African American | 1 (1.7) | 1 (5.3) | 0 (0.0) | 0 (0.0) | |||
| American Indian | 1 (1.7) | 1 (5.3) | 0 (0.0) | 0 (0.0) | |||
| History of Barrett’s esophagus | 22 (37.9) | 11 (57.9) | 7 (30.4) | 4 (25.0) | 0.086 | ||
| History of Helicobacter pylori | 4 (6.9) | 0 (0.0) | 1 (4.3) | 3 (18.8) | 0.076 | ||
| Documented Siewert classification | 24 (41.4) | 4 (21.1) | 13 (56.5) | 7 (43.8) | 0.066 | ||
| Clinical stage | 0.340 | ||||||
| II | 4 (6.9) | 1 (5.3) | 2 (8.7) | 1 (6.3) | |||
| IIA | 2 | 0 | 1 | 1 | |||
| IIB | 2 | 1 | 1 | 0 | |||
| III | 44 (75.9) | 12 (63.2) | 18 (78.3) | 14 (87.5) | |||
| IVA | 10 (17.2) | 6 (31.6) | 3 (13.0) | 1 (6.3) | |||
| Neoadjuvant therapy | |||||||
| Chemoradiation | 42 (72.4) | 18 (94.7) | 17 (73.9) | 7 (43.8) | 0.003 | ||
| Chemotherapy | 24 (41.4) | 1 (5.3) | 10 (43.5) | 13 (81.3) | <0.001 | ||
| Both† | 8 (13.8) | 0 (0.0) | 4 (17.4) | 4 (25.0) | 0.083 | ||
| Surgical approach | <0.001 | ||||||
| Esophagogastrectomy | 47 (81.0) | 18 (94.7) | 23 (100.0) | 6 (37.5) | |||
| Total gastrectomy | 11 (19.0) | 1 (5.3) | 0 (0.0) | 10 (62.5) | |||
| D2 LN dissection performed | 21 (36.2) | 1 (5.3) | 9 (39.1) | 11 (68.8) | <0.001 | ||
| Pathologic LNs | |||||||
| Positive status | 30 (51.7) | 9 (47.4) | 12 (52.2) | 9 (56.3) | 0.870 | ||
| Total number harvested | 30 [22–38] | 26 [17–32] | 29 [23–34] | 37 [30–50] | 0.027 | ||
| Pathologic stage | 0.742 | ||||||
| I | 23 (39.7) | 8 (42.1) | 8 (34.8) | 7 (43.8) | |||
| II | 9 (15.5) | 2 (10.5) | 3 (13.0) | 4 (25.0) | |||
| III | 22 (37.9) | 7 (36.8) | 10 (43.5) | 5 (31.3) | |||
| IIIA | - | 2 | 3 | - | |||
| IIIB | - | 5 | 7 | - | |||
| IVA | 4 (6.9) | 2 (10.5) | 2 (8.7) | 0 (0.0) | |||
| Histologic type | 0.975 | ||||||
| Intestinal | 36 (64.3) | 12 (63.2) | 14 (63.6) | 10 (66.7) | |||
| Diffuse | 20 (35.7) | 7 (36.8) | 8 (36.4) | 5 (33.3) | |||
| Unknown | 2 | 0 | 1 | 1 | |||
| Pathologic grade | 0.038 | ||||||
| Moderately differentiated* | 19 (33.9) | 2 (10.5) | 9 (39.1) | 8 (50.0) | |||
| Poorly differentiated | 39 (67.2) | 17 (89.5) | 14 (60.9) | 8 (50.0) | |||
| Tumor regression score§ | 0.256 | ||||||
| Complete response | 12 (20.7) | 4 (21.1) | 4 (17.4) | 4 (25.0) | |||
| Near complete response | 12 (20.7) | 5 (26.3) | 4 (17.4) | 3 (18.8) | |||
| Partial response | 25 (43.1) | 8 (42.1) | 13 (56.5) | 4 (25.0) | |||
| Poor or no response | 8 (13.8) | 1 (5.3) | 2 (8.7) | 5 (31.3) | |||
| Recurrence | |||||||
| Any | 29 (50.0) | 9 (47.4) | 15 (65.2) | 5 (31.3) | 0.109 | ||
| Intrathoracic | 14 (24.1) | 3 (15.8) | 10 (43.5) | 1 (6.3) | 0.016 | ||
| Intraabdominal | 20 (34.5) | 5 (26.3) | 10 (43.5) | 5 (31.3) | 0.482 | ||
| Local | 3 (5.2) | 1 (5.3) | 2 (8.7) | 0 (0.0) | 0.483 | ||
| Bone | 4 (6.9) | 2 (10.5) | 1 (4.3) | 1 (6.3) | 0.729 | ||
| Brain | 2 (3.4) | 2 (10.5) | 0 (0.0) | 0 (0.0) | 0.119 | ||
Values are presented as number (%), median (range), or median [interquartile range]. Bold styled P-values indicate statistically significant.
BMI = body mass index; LN = lymph node.
*Includes one patient with well-differentiated pathologic grade.
†Refers to patients receiving both neoadjuvant chemoradiotherapy and full-dose perioperative chemotherapy.
‡A patient in the Siewert I group is missing ethnicity data.
§A patient in the Siewert I group is missing tumor regression score data.
At the time of surgery, the median patient age was 63.5 years and 43 (74%) were men. Age, sex, and body mass index (BMI) were similar for all Siewert groups. Approximately 80% of Siewert I/II patients were identified as non-Hispanic white (NWH) compared to Siewert type III patients being 50% NHW, 25% Hispanic and 25% Asian. There was a trend towards higher rates of Barrett’s esophagus for Siewert type I/II cases, while a history of Helicobacter pylori infections was more common for Siewert type III patients. The majority of patients were clinical stage III (76%). Notably, 10 patients had clinical stage IVA (cT3N2M0) as per the AJCC (8th edition) guidelines for EAC. Although all patients received NT, there were significant differences between Siewert groups in the type of regimens received (Table 1). Eighteen (95%) Siewert type I patients received chemoradiation, and 13 (81%) Siewert type III cases received chemotherapy. Interestingly, 8 patients in our study received both modalities.
Surgical approach
Forty-seven (81%) patients underwent EG, while 11 (19%) underwent TG (Table 1). As expected, significantly more Siewert type III patients (69%) underwent TG and D2 lymph node (LN) dissection compared to Siewert type I/II (44%) cases. A surgical oncologist was involved in all cases with D2 lymphadenectomy including those performed during an EG for Siewert type I/II tumors. As part of the growing robotic surgery practice at our center, 81% of surgical interventions were completed by a 2-field robotic approach (transthoracic and transabdominal), with 5 other cases that began robotically but were converted to open surgery for a conversion rate of 8.6%. Comparing EG and TG surgeries, the median duration of hospital stay was similar between these two surgical approaches (8 vs. 6 days, respectively), but postoperative complications were more frequent in the EG group than the TG group (72% vs. 36%, respectively; P=0.024) (Supplementary Table 1).
Pathologic findings
Based on surgical pathology findings, 30 (52%) patients had positive LNs, and the median number of harvested LNs was 30 (range, 22–38) (Table 2). Positive LN status was equivalent among the 3 Siewert groups, but Siewert type III cases had significantly more LNs harvested than Siewert type I and II patients (median 37, 26, and 29, respectively; P=0.027), likely due to their higher rate of D2 LN dissection. The pathologic stage was similar between Siewert groups, and 42% of patients had a TRS score signifying a complete or near complete local treatment response to preoperative therapy. The differences in tumor grade and the similar proportion of diffuse-type histology amongst all three Siewert groups were notable. Poorly differentiated histology was found in 89% of Siewert I, 67% of Siewert II, and 50% of Siewert III (P=0.038) cases. Overall, more intestinal-type histology was observed compared to diffuse-type tumors. Surprisingly, the proportion of diffuse-type histology was similar between the various Siewert groups (37% for Siewert type I, 36% for Siewert type II, and 33% for Siewert type III).
Table 2. Univariate analysis of potential variables associated with overall survival.
| Variable | Hazard radio | 95% confidence interval | Log-rank P-value | |
|---|---|---|---|---|
| Age (≥66 vs. <66 years) | 0.76 | 0.36–1.61 | 0.477 | |
| Sex (male vs. female) | 0.92 | 0.39–2.16 | 0.840 | |
| Siewert classification | 0.757 | |||
| II vs. I | 1.43 | 0.56–3.69 | ||
| III vs. I | 1.19 | 0.45–3.15 | ||
| III vs. II | 0.83 | 0.33–2.10 | ||
| Clinical stage (III/IV vs. II) | 1.09 | 0.33–3.60 | 0.377 | |
| Chemoradiation (yes vs. no) | 0.68 | 030–1.53 | 0.355 | |
| Chemotherapy (yes vs. no) | 1.46 | 0.69–3.10 | 0.322 | |
| D2 LN dissection (yes vs. no) | 1.26 | 0.57–2.78 | 0.564 | |
| Surgical approach (EG vs. TG) | 0.94 | 0.36–2.50 | 0.917 | |
| LN involvement (present vs. absent) | 3.89 | 1.68–9.01 | 0.007 | |
| Pathologic stage (III/IV vs. I/II) | 4.92 | 2.20–11.04 | <0.001 | |
| Pathologic grade (poorly differentiated vs. moderately differentiated) | 1.08 | 0.71–1.63 | 0.740 | |
| Histologic type (diffuse vs. intestinal) | 2.33 | 1.10–4.90 | 0.021 | |
| Tumor regression score (partial/poor vs. near complete/complete) | 3.15 | 1.32–7.54 | 0.012 | |
| Recurrence (yes vs. no) | 3.19 | 1.35–7.53 | 0.005 | |
Bold styled values indicate statistically significant.
LN = lymph node; EG = esophagogastrectomy; TG = total gastrectomy.
Recurrence and survival
At a median follow-up of 31.2 months, half of the patients (28/56) had disease recurrence (Supplementary Fig. 1B). Initial recurrence patterns were similar among the various Siewert groups, with most recurrences occurring intra-abdominally rather than in the chest. However, Siewert type II patients had the highest rate of intrathoracic recurrences than the other Siewert types (P=0.016) (Table 1).
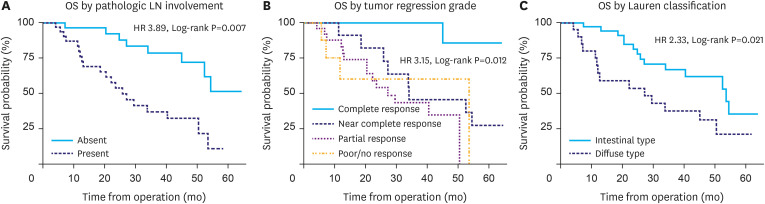
The median OS was 50.5 months (Supplementary Fig. 1), and patients with recurrence had a median post-recurrence survival of 14.3 months. Table 2 shows the univariate analysis performed to determine the clinicopathologic variables associated with OS. Age, sex, Siewert classification, NT modalities including chemoradiation and chemotherapy, surgical approach, and D2 LN dissection were not associated with survival (Supplementary Fig. 1A). No association was found between clinical stage and survival. In contrast, higher pathologic stage significantly correlated with worse OS (P<0.001; Supplementary Fig. 1C and D). Pathologic LN involvement and poorer pathologic response to NT were significantly associated with decreased OS (P=0.007 and P=0.012, respectively; Fig. 2A and B). Additionally, while pathologic grade was not associated with survival, diffuse-type histology had worse OS than that associated with intestinal-type (P=0.021; Fig. 2C).
Fig. 2. Kaplan-Meier survival curves. Curves for OS (A), with and without positive pathologic LNs (B), and treatment response by tumor regression grade (C) diffuse- vs. intestinal-type histology.
OS = overall survival; LN = lymph node; HR = hazard ratio.
Importantly, Lauren histopathology was associated with patient stage, pathologic grade, and clinical outcomes. Compared to intestinal-type, patients with diffuse-type histology had significantly higher pathologic stage (P=0.008) and frequency of poorly differentiated tumors (P=0.004; Table 3). Histologic type also correlated with pathologic treatment response to neoadjuvant chemotherapy but not chemoradiation or a combination of both. Diffuse-type histology had a significantly reduced treatment response to neoadjuvant chemotherapy than intestinal-type (P=0.037; Supplementary Table 2). A trend towards higher frequency of positive pathologic LN status (P=0.066) and a higher total number of positive LNs (P=0.058) for patients with diffuse- rather than intestinal-type histology was seen but was not statistically significant. Notably, there was no significant difference in the frequency of D2 dissection performed for the 2 histologic types (Table 3).
Table 3. Perioperative factors, pathologic data and recurrence outcomes by histologic type.
| Variable | Intestinal (n=36) | Diffuse (n=20) | P-value | |
|---|---|---|---|---|
| D2 LN dissection performed | 15 (41.7) | 6 (30) | 0.390 | |
| Pathologic lymph nodes | ||||
| Positive status | 16 (44.4) | 14 (70) | 0.066 | |
| Total number harvested | 31 [14–47] | 28 [17–51] | 0.549 | |
| Total number positive | 1 (0–12) | 3 (0–17) | 0.058 | |
| Positive surgical margin | 1 (2.8) | 2 (10) | 0.250 | |
| Pathologic stage | 0.008 | |||
| I/II | 24 (66.7) | 6 (30) | ||
| III/IV | 12 (33.3) | 14 (70) | ||
| Pathologic grade† | 0.004 | |||
| Moderately differentiated* | 15 (41.7) | 1 (5.0) | ||
| Poorly differentiated | 20 (55.6) | 18 (90.0) | ||
| Tumor regression score‡ | 0.910 | |||
| Complete response | 8 (22.2) | 3 (15.0) | ||
| Near complete response | 7 (19.4) | 4 (20.0) | ||
| Partial response | 15 (41.7) | 10 (50.0) | ||
| Poor or no response | 5 (13.9) | 3 (15.0) | ||
| Recurrence | ||||
| All | 15 (41.7) | 14 (70.0) | 0.042 | |
| Intrathoracic | 8 (22.2) | 6 (30.0) | 0.520 | |
| Intraabdominal | 8 (22.2) | 12 (60.0) | 0.005 | |
| Lymph nodes | 5 (13.9) | 8 (40.0) | 0.027 | |
| Peritoneum | 4 (11.1) | 6 (30.0) | 0.077 | |
| Liver | 1 (2.8) | 4 (20.0) | 0.030 | |
| Local | 3 (8.3) | 0 (0.0) | 0.184 | |
| Bone | 1 (2.8) | 3 (15.0) | 0.089 | |
| Brain | 1 (2.8) | 1 (5.0) | 0.668 | |
Values are presented as number (%), median (range), or median [interquartile range]. Bold styled P-values indicate statistically significant.
LN = lymph node.
*Includes one patient with well-differentiated pathologic grade.
†One patient in each group (diffuse and intestinal) missing pathologic grade data.
‡One patient in the intestinal group is missing tumor regression score data.
Lastly, patients with diffuse-type GEJA were significantly more likely to have a recurrence than those with intestinal-type (P=0.042; Table 3). The recurrence pattern also strongly correlated with histologic type, where 60% of patients with diffuse-type histology had intra-abdominal recurrences compared to 22% for intestinal-type (P=0.005). The most common location for intra-abdominal recurrence was intra-abdominal lymph nodes, followed by the peritoneum and liver. Notably, recurrence detection was primarily performed with computed tomography (CT) and positron emission tomography (PET) scan imaging, both of which have low sensitivity for peritoneal metastases.
DISCUSSION
Optimization of curative intent multimodal management of patients with GEJA requires accurate pretreatment assessment of the tumor. While the ambiguity of its anatomical characterization as being gastric or esophageal continue to challenge standardization of our practices, nonanatomic factors may contribute to improved treatment planning and patient outcomes. Our real-world study of patients with GEJA who underwent curative-intent resection identified higher-than-expected rates of diffuse-type histology in Siewert type I and II tumors that were comparable to the rates found in Siewert type III tumors. Regardless of Siewert classification, tumors with diffuse-type histology were less likely to respond to neoadjuvant chemotherapy and were associated with high rates of intra-abdominal recurrence and poor survival compared to tumors with intestinal-type histology.
The Lauren classification system that categorizes GC into 2 distinct histologic subtypes with biological differences has been shown to significantly impact the outcomes of patients with GC [10]. Compared to tumors with intestinal-type histology, diffuse-type tumors demonstrate higher rates of disease recurrence, shorter time to disease progression, and poorer OS [11]. Additionally, tumor histologic type correlates with recurrence patterns, and the peritoneum has previously been shown to be the most common recurrence site for tumors with diffuse-type histology [12]. Our study demonstrates that compared to intestinal-type, GEJA with diffuse-type histology was associated with increased overall and intra-abdominal recurrence rates, and correlated with significantly shorter median OS (27.2 vs. 53.6 months; hazard ratio, 2.33; 95% confidence interval, 1.10–4.90; P=0.021).
While clinical TNM-staging and Siewert classification are the main determinants of the therapeutic strategy for GEJA, clinical TNM-staging often has poor prognostic accuracy [13] and the Siewert classification is not always assessed. In our study, only 41% of patients had pretreatment Siewert classification documented. This is likely due to challenges associated with endoscopic assignment of Siewert classification [14]. Frequently, the GEJ is obliterated by tumor, so that the tumor epicenter in relation to the GEJ cannot be assessed. When both GEJ obliteration and a hiatal hernia occur together, the diaphragmatic impression may be misconstrued as the GEJ resulting in erroneous estimation of the anatomical GEJ. Lastly, tumor obstruction of the GEJ may inhibit passage of the endoscope into the stomach that can lead to a default and incorrect diagnosis of EAC.
In addition to being prognostic, the histologic type of the tumor has been correlated with treatment response. Tumors with a diffuse-type histology are associated with lower response rates to systemic chemotherapy compared to tumors displaying intestinal-type histology [15]. In our study, no diffuse-type tumors had a complete or near complete response, while 55% of intestinal-type did. Although the impact of tumor histologic type on chemoradiation response is less defined, the presence of signet ring cells has been correlated with resistance [16]. However, routine reporting of Lauren type histology is not standard practice for type I and II GEJA since Lauren type was developed for GC. In this study, slightly more than half of the patients had their pretreatment tumor histologic type documented (21% type I, 52% type II, and 88% type III). Upon secondary review of tumor pathology, diffuse-type histology was surprisingly equally distributed among all three Siewert subtypes (37% type I, 36% type II, and 33% for type III). The impact of tumor histology type on treatment response and the presence of diffuse-type histology in all Siewert subtypes supports the incorporation of tumor histologic type in individualized treatment strategies for patients with GEJA.
Surgical resection with negative margins and appropriate lymphadenectomy remains central to curative intent therapy in GEJA. As a diffuse-type tumor histology is a risk factor for positive surgical margins, the use of intraoperative frozen sections and more extensive resections to ensure negative margins have been suggested [17]. When clearance of the esophageal margin is of concern, a careful gross examination of the margin with representative sampling for microscopic examination can be helpful. However, the cells of tumors with diffuse-type histology often mimic inflammatory cells and can lead to false negative findings. In our study, 10% of patients with tumors displaying a diffuse-type histology had positive esophageal margins compared to 2% with for tumors with an intestinal-type histology. In addition to margin status, patients with GEJA and nodal involvement have decreased locoregional recurrences with more extensive abdominal lymphadenectomies [18]. As tumors with a diffuse-type histology are associated with increased intra-abdominal recurrences, their presence may indicate the need for an abdominal lymphadenectomy that is not standard in the current management of Siewert type I/II tumors.
The clinically observed biological differences between tumors with diffuse and intestinal-like histology are driven by differences in their underlying genomics. The Cancer Genome Atlas network categorized GC into four molecular subtypes with implications for treatment response and survival, namely Epstein-Barr virus-positive tumors, microsatellite instable (MSI) tumors, genomically stable (GS) tumors, and tumors with chromosomal instability (CIN) [19]. The GS subtype, which is enriched in the diffuse-type histology and defined by alterations in cellular adhesion pathways, has the worse prognosis and demonstrates poor response to both chemotherapy and immunotherapy [20]. The MSI and CIN subtypes are enriched in the intestinal-type histology. Although the MSI subtype demonstrates good response to immunotherapy, the CIN subtype has even worse response to immunotherapy than GS [21]. However, compared to the GS subtype, CIN tumors are more likely to respond to chemotherapy and have higher levels of potentially targetable mutations [22]. These molecular differences may account for differing responses to systemic therapies between tumors of differing histologic types.
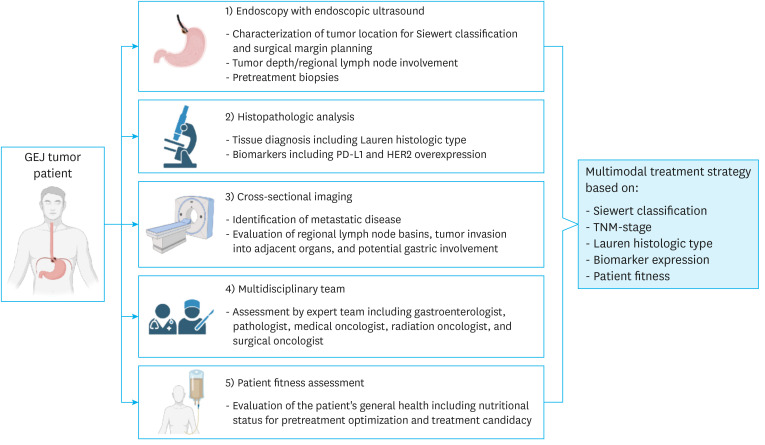
Comprehensive, centralized care of patients with GEJA at high-volume centers has been shown to improve outcomes [23]. At our institution, GC, EAC, and GEJAs are managed by a team of experts from the divisions of gastroenterology, pathology, radiology, radiation oncology, surgical oncology, thoracic oncology and medical oncology. Centralized care can facilitate the standardization and optimization of GEJA management through quality control measures to ensure completion of proper preoperative assessments (depicted in Fig. 3) and providing guidance for appropriate multimodal management.
Fig. 3. Flowchart depicting the elements required for a comprehensive GEJA work-up including: 1) EGD/EUS for anatomic localization (Siewert classification and extent of proximal esophageal and distal gastric involvement), evaluation of tumor depth and regional LNs for TNM staging, and pretreatment biopsies; 2) evaluation of preoperative biopsies for Lauren histologic type, PD-L1 (CPS), MSI, and HER2 overexpression status; 3) CT scan ± PET scan of the chest, abdomen, and pelvis for evaluation of regional LN basins and metastatic disease for TMN staging, tumor invasion into adjacent organs, and gastric wall thickening indicating possible involvement; 4) evaluation by a multidisciplinary team; and 5) assessment of patient fitness and nutritional status for pretreatment optimization and determination of treatment candidacy.
GEJA = gastroesophageal junction adenocarcinoma; EGD = esophagogastroduodenoscopy; EUS = endoscopic ultrasonography; LN = lymph node; TNM = tumor, node, metastasis; PD-L1 = programmed cell death ligand 1; CPS = combined positive score; MSI = microsatellite instable; HER2 = human epidermal growth factor receptor 2; CT = computed tomography; PET = positron emission tomography; GEJ = gastroesophageal junction.
This study has some limitations. First, it is a single institution retrospective study with biases inherent in tertiary referral centers that often receive more advanced cases. Additionally, it is underpowered to demonstrate differences in outcomes between perioperative chemotherapy and neoadjuvant chemoradiation based on histologic or anatomical classifications. Further, genomics, programmed cell death ligand 1, and MSI status may have impacted outcomes and were not included in the current analysis.
Nevertheless, our study is a comprehensive analysis of patients with GEJA and includes a thorough evaluation of anatomical tumor location and histologic type by experienced clinicians. To our knowledge, this is the first study to highlight the high frequency of tumors with a diffuse-type histology in Siewert type I/II patients and its impact on recurrence patterns and survival outcomes for GEJA of all Siewert types. Future studies are warranted to determine whether incorporation of tumor histologic type into multimodal treatment strategy selection can improve the prognosis for GEJA.
In conclusion, diffuse-type GEJA is equally present throughout the GEJ and is associated with high rates of intra-abdominal recurrences and poor overall survival. Pretreatment assessment of Lauren histology may identify a subset of Siewert I/II tumors with diffuse-type histology that resemble GC [24] and may benefit more from GC-based surgical approaches and perioperative therapies rather than EAC-directed treatment strategies. Histologic and genomic characterization rather than anatomical location may better define GEJA and should be considered to optimize the multimodal management of patients with GEJA.
ACKNOWLEDGMENTS
Research reported in this publication included work performed at the City of Hope Research Pathology Informatics Shared Resource supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Funding: This study was funded by the Department of Surgery, Start-up Grant for Support of the Gastroesophageal Cancer Program Development; and the SU2C Gastric Cancer Interception Award # SU2C-AACR-30-20–237459.
Conflict of Interest: Dr. Woo is a scientific consultant for Johnson & Johnson Ethicon and serves on the advisory board of Imugene. Dr. Fong is a scientific consultant for Medtronics, Covidien, Xdemics, Vergent Biosciences, Eureka Biologics, and Imugene; receives royalties from Merck and Imugene; has a research study agreement with Imugene; a member of Dr. Fong’s family is President and CEO of XDemics. There are no disclosures for the remaining authors.
- Conceptualization: M.K.M., W.Y.
- Data curation: M.K.M., S.K.M., H.M.C., C.Y.J., M.R., L.J.L., W.Y.
- Formal analysis: M.K.M., C.J., M.L.G., P.I.B., K.J.Y., M.R., L.J.L., F.Y., W.Y.
- Funding acquisition: F.Y., W.Y.
- Investigation: M.K.M., C.J., M.L.G., P.I.B., K.J.Y., M.R., L.J.L., F.Y., W.Y.
- Methodology: M.K.M., M.R., L.J.L., W.Y.
- Project administration: F.Y., W.Y.
- Resources: F.Y., W.Y.
- Software: S.K.M., M.K.M., M.R., L.J.L.
- Supervision: F.Y., W.Y.
- Validation: M.K.M., W.Y.
- Visualization: M.K.M., W.Y.
- Writing - original draft: M.K.M., W.Y.
- Writing - review & editing: S.K.M., M.K.M., H.M.C., C.Y.J., C.J., M.L.G., P.I.B., K.J.Y., M.R., L.J.L., F.Y., W.Y.
SUPPLEMENTARY MATERIALS
Perioperative data and complications by surgical approach
Neoadjuvant treatment response (tumor regression score) by histologic type
Kaplan-Meier survival curves including (A) OS, (B) RFS, (C) OS by clinical stage, (D) OS by pathologic stage, and (E) OS by Siewert classification.
References
- 1.Bartel M, Brahmbhatt B, Bhurwal A. Incidence of gastroesophageal junction cancer continues to rise: analysis of Surveillance, Epidemiology, and End Results (SEER) database. J Clin Oncol. 2019;37:40. [Google Scholar]
- 2.Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 4.Greally M, Agarwal R, Ilson DH. Optimal management of gastroesophageal junction cancer. Cancer. 2019;125:1990–2001. doi: 10.1002/cncr.32066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlth F, Hoelscher AH. History of esophagogastric junction cancer treatment and current surgical management in Western countries. J Gastric Cancer. 2019;19:139–147. doi: 10.5230/jgc.2019.19.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siewert JR, Hölscher AH, Becker K, Gössner W. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg. 1987;58:25–32. [PubMed] [Google Scholar]
- 7.Quante M, Wang TC, Bass AJ. Adenocarcinoma of the oesophagus: is it gastric cancer? Gut. 2023;72:1027–1029. doi: 10.1136/gutjnl-2022-327096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 10.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Chen YC, Fang WL, Wang RF, Liu CA, Yang MH, Lo SS, et al. Clinicopathological variation of Lauren classification in gastric cancer. Pathol Oncol Res. 2016;22:197–202. doi: 10.1007/s12253-015-9996-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren histologic type is the most important factor associated with pattern of recurrence following resection of gastric adenocarcinoma. Ann Surg. 2018;267:105–113. doi: 10.1097/SLA.0000000000002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119–130. doi: 10.21037/acs.2017.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedrazzani C, Bernini M, Giacopuzzi S, Pugliese R, Catalano F, Festini M, et al. Evaluation of Siewert classification in gastro-esophageal junction adenocarcinoma: what is the role of endoscopic ultrasonography? J Surg Oncol. 2005;91:226–231. doi: 10.1002/jso.20302. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Li E, Busuttil RA, Kong JC, Pattison S, Sung JJ, et al. A cohort study and meta-analysis of the evidence for consideration of Lauren subtype when prescribing adjuvant or palliative chemotherapy for gastric cancer. Ther Adv Med Oncol. 2020;12:1758835920930359. doi: 10.1177/1758835920930359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charalampakis N, Nogueras González GM, Elimova E, Wadhwa R, Shiozaki H, Shimodaira Y, et al. The proportion of signet ring cell component in patients with localized gastric adenocarcinoma correlates with the degree of response to pre-operative chemoradiation. Oncology. 2016;90:239–247. doi: 10.1159/000443506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celli R, Barbieri AL, Colunga M, Sinard J, Gibson JA. Optimal intraoperative assessment of gastric margins. Am J Clin Pathol. 2018;150:353–363. doi: 10.1093/ajcp/aqy062. [DOI] [PubMed] [Google Scholar]
- 18.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin Cancer Res. 2017;23:4441–4449. doi: 10.1158/1078-0432.CCR-16-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 22.de Mello RA, Marques AM, Araújo A. HER2 therapies and gastric cancer: a step forward. World J Gastroenterol. 2013;19:6165–6169. doi: 10.3748/wjg.v19.i37.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teh SH, Uong S, Lin TY, Shiraga S, Li Y, Gong IY, et al. Clinical outcomes following regionalization of gastric cancer care in a US integrated health care system. J Clin Oncol. 2021;39:3364–3376. doi: 10.1200/JCO.21.00480. [DOI] [PubMed] [Google Scholar]
- 24.Barra WF, Moreira FC, Pereira Cruz AM, Khayat AS, Calcagno DQ, Carneiro Dos Santos NP, et al. GEJ cancers: gastric or esophageal tumors? searching for the answer according to molecular identity. Oncotarget. 2017;8:104286–104294. doi: 10.18632/oncotarget.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perioperative data and complications by surgical approach
Neoadjuvant treatment response (tumor regression score) by histologic type
Kaplan-Meier survival curves including (A) OS, (B) RFS, (C) OS by clinical stage, (D) OS by pathologic stage, and (E) OS by Siewert classification.