Abstract
Objective
The study compared the morphometric changes of the cervical spinal cord using dynamic magnetic resonance imaging (MRI) in patients with cervical spondylotic myelopathy (CSM) and assessed the correlation with kinematic changes, cord cross-sectional area (CSA), and high signal intensity (SI) on T2-weighted imaging (T2WI).
Methods
Patients with CSM were evaluated through dynamic MRI for sagittal and axial CSA changes of the cervical cord, cerebrospinal fluid (CSF) reserve ratio, degree of cord impingement, cord compression rate, range of motion (ROM), and severity of SI on T2WI. The degree of cord impingement was evaluated using the Muhle grading system. Clinical outcomes were assessed using Japanese Orthopaedic Association scoring and Nurick grade.
Results
The study included 191 patients (113 males) with a mean age of 55.34 ± 12.09 years. The lowest sagittal CSF reserve ratio and cord occupation rate were observed during extension. Cord impingement and SI change were more prevalent in extension-positioned MRI. There was no difference between ROM on dynamic radiographs and dynamic MRI. Preoperative cervical ROM was greater in patients with intensely high SI change.
Conclusion
Dynamic MRI is useful for evaluating neck movement. Patients with high SI had greater ROM before surgery but worse outcomes after. Neck extension exacerbated cervical stenosis and cord compression compared to flexion, and cervical spinal motion contributed to the severity of CSM. Cervical spinal motion should be carefully evaluated, particularly in hyperextension, to prevent worsening of CSM.
Keywords: Cervical myelopathy, Dynamic MRI, Cervical motion, Signal intensity, Anterior decompression, Posterior decompression
INTRODUCTION
Magnetic resonance imaging (MRI) is essential to diagnose cervical spondylotic myelopathy (CSM), as it can accurately determine the relationship between the disc and the spinal cord (SC) and the presence of intramedullary signal change. High signal intensity (SI) on T2-weighted imaging (T2WI) has been regarded as an important prognostic factor and was found to correlate with the severity of CSM [1-5]. Studies have demonstrated a high signal change in T2WI, cord compression, and neurological outcomes with only static MRI [3,4,6,7]. Additionally, some pathologies can be found to reflect dynamic strain of the cervical spine when invisible cases using static imaging [8].
Dynamic mechanical factors are reported as the cause of the occurrence and exacerbation of cervical myelopathy [5,9,10]. Dynamic MRI is beginning to be clinically applied for the diagnosis and treatment of cervical spine diseases, especially CSM, which is reported to cause and worsen symptoms through cervical motion [5,11]. However, there is still no consensus on the significance of changes in SI severity and dynamic MRI findings. Furthermore, the impact of dynamic factors on the clinical outcomes of cervical myelopathy needs to be better understood.
In this study, we investigated prospectively dynamic MRI in preoperative neck flexion-extension positions in cervical myelopathic patients. We compared the morphometric changes of cord compression on dynamic MRI, changes in the SI on T2WI, and parameters of the cervical spine in patients with CSM. The objective of the study was to analyze the relationship between preoperative intramedullary signal change on MRI, dynamic factors, and surgical outcomes.
MATERIALS AND METHODS
1. Patient Populations
This prospective cohort study included 233 patients with CSM caused by cervical disc protrusion, bony spur, or ossification of the posterior longitudinal ligament (OPLL) who underwent surgery at Yongin Severance Hospital and Inje University Sanggye Paik Hospital between June 2018 and December 2022. Among these patients, 32 patients were excluded due to congenital anomaly, infection, trauma, tumor, previous history of cervical fusion, or ankylosing spondylitis (Fig. 1). This study was approved by the Institutional Review Board of Yonsei College of Medicine, Yongin Severance (IRB No. 9-2023-0222). A diagnosis of CSM was assigned with radiological confirmation by MRI, and the diagnosis was determined when one or more upper motor neuron domains were involved (e.g., spasticity, hyperreflexia, positive Babinski sign), based on neurological examination [3].
Fig. 1.
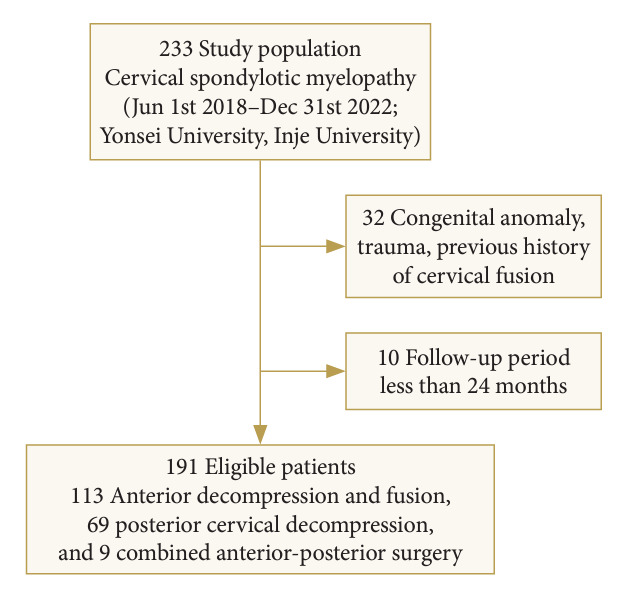
Flow chart of patient selection.
2. Radiologic Assessment
1) Plain radiographs
Preoperative lateral standing plain radiographs were obtained in neutral, flexion, and extension positions. The following parameters were evaluated: C2–7 angle (the angle created by a line parallel to the inferior end plate of the C2 body and a line parallel to that of the C7 body was measured on neutral, flexion, and extension position). The C2–7 angle was measured in the neutral position, flexion position, and extension position. Cervical range of motion (ROM) was measured as the difference of C2–7 angle values between the extension and flexion positions. The dynamic parameter of C2–7 ROM was calculated using the following formula: C2–7 ROM (°)= (extension C2–7 Cobb lordotic angle)–(flexion C2–7 Cobb lordotic angle) [12].
2) Patient positioning and MRI protocol
All patients underwent high-resolution MRI using the 3.0T Signa MRI unit (GE HealthCare, Chicago, IL, USA) or 3.0T Skyra unit (Siemens, Munich, Germany). For cervical dynamic MRI, the patient’s neck was positioned in extension and flexion using a custom-made cushion placed under the head and shoulders (Fig. 2). A routine neutral examination was first performed in the supine position. The patients were tolerable to the neck extension and flexion position under the observation of a physician. There was no standard predetermined flexion-extension position to avoid neurologic problems. Neutral MRI obtained T1- and T2-weighted sequences in sagittal and axial views; only T2-weighted images were obtained by dynamic MRI.
Fig. 2.
Positions of the cervical spine for the dynamic magnetic resonance imaging (MRI). During cervical dynamic MRI, the patient’s neck was alternately positioned in extension and flexion using a custom-made cushion under the head and shoulders. Flexion-positioned MRI (A), neutral-positioned MRI (B), and extension-positioned MRI (C).
3) Signal intensity grading
Increased SI referred to a high-intensity area compared with the adjacent isointensity portion of the SC in both sagittal and axial planes. We defined the increased SI at the narrowest level of the SC as “grade 0 (G0)” if no intramedullary high SI appeared on the T2WI, as “grade 1 (G1)” if there was a predominantly faint and indistinct border, and as “grade 2 (G2)” if there was a predominantly intense and well-defined border [2,3].
4) Cervical stenosis grading
Cervical stenosis was evaluated using the classification of Muhle et al. [8], which has 4 stages: stage 0, normal width of the spinal canal, and no signs of anterior and posterior subarachnoid space narrowing; stage 1, partial obliteration of the anterior or posterior subarachnoid space or of both; stage 2, complete obliteration of the anterior or posterior subarachnoid space or of both; and stage 3, anterior or posterior cord impingement or both. Muhle grade was determined by evaluating multi-positional MRI at the most compressed lesion. The degree of cervical stenosis was evaluated using Muhle classification on T2WI in 3 kinetic positions: neutral, flexion, and extension (axial and sagittal).
5) Cross-sectional area
The cross-sectional area (CSA) of the SC was measured at the greatest compressed levels. The CSA was obtained at the affected level during flexion, neutral position, and extension (Fig. 3). The CSA of the SC and canal on the midsagittal plane was measured at the area between a line crossing the SC at the lower endplate of C2 and the lower endplate of C7. A comparison of morphometric parameters at compression levels in all 3 positions on axial T2WI was performed, including the SC area, cerebrospinal fluid (CSF) area, and CSF reserve ratio (CSF/CSF+ SC) [9]. The degree of SC compression changes in the cervical flexion-extension state was compared and analyzed to measure the CSA using software (ZeTTA PACS, TaeYoung Soft Co., Ltd., Gwacheon, Korea). CSA measurements were conducted 2 times, and the mean was used to minimize intraobserver differences.
Fig. 3.
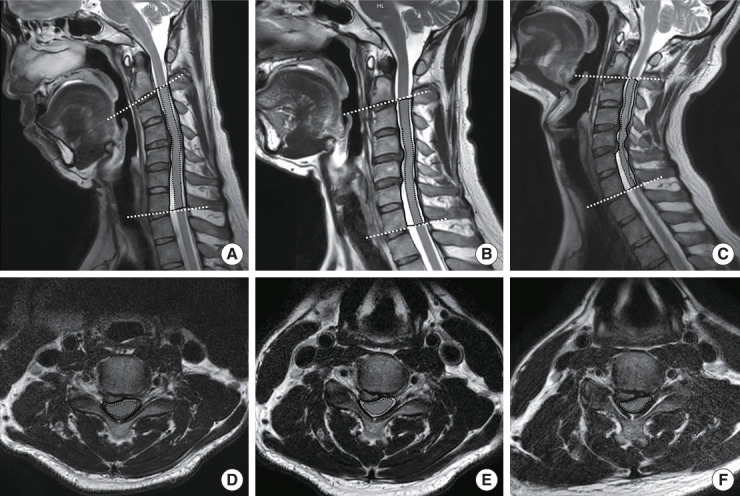
Measurement technique of morphometric parameters on T2-weighted magnetic resonance imaging (MRI) during flexion, neutral, and extension. Flexion MRI in sagittal section (A), neutral MRI in sagittal section (B), extension MRI in sagittal section (C), flexion MRI in axial section at C4–5 (D), neutral MRI in axial section at C4–5 (E), and extension MRI in axial section at C4–5 (F). Black dashed line indicates the SC area, black solid line indicates the SC plus CSF area of compression level, and white dashed line indicates the C2–7 angle. CSF, cerebrospinal fluid; SC, spinal cord.
6) Diameter
On axial and sagittal T2WI MRI, the SC occupation rate in the dural sac was calculated using the following formula: (diameter of the SC)/(diameter of the dural sac)× 100 [13]. The measurements were performed at the level of maximum SC compression. The level with the smallest SC area was selected for patients with multisegmental involvement.
3. Assessment of Clinical Outcomes
Clinical outcomes were assessed using the Japanese Orthopaedic Association (JOA) scoring system and Nurick grade. Data were obtained for all patients preoperatively and for a minimum of 24 months postoperatively. The recovery ratio was calculated using the following formula:
JOA recovery ratio (%)= (postoperative JOA score–preoperative JOA score)/[17 (full score)–preoperative JOA score]
4. Treatments
The surgical treatments were determined based on various factors such as the number of affected levels, patient comorbidities, cervical alignment, instability, occupying ratio, and surgeon preference. Various surgical approaches such as anterior, posterior, or combined operations were chosen to decompress affected levels. Follow-up examinations were conducted at 3-month, 6-month, 12-month, or 24-month intervals after the surgery to investigate the clinical correlation between postoperative neurological outcomes and radiographic imaging findings.
5. Statistical Analysis
All data are expressed as mean ± standard deviation or percentage. For the demographic data, the means of continuous and categorical data were compared by Student t-test and chi-square test, respectively. Repeated measures analysis of variances with a post hoc Scheffé test was used to compare the mean results on neutral, flexion, and extension MRI at the compression levels. Radiological parameters measured by 2 observers were analyzed using the Cohen κ coefficient for categorical variables and intraclass correlations (ICCs; 2-way mixed model with consistency agreement; 95% confidence interval) for continuous variables. Cohen κ coefficient and ICC values were categorized as poor agreement (0.00–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), good agreement (0.61–0.80), and very good agreement (0.81–1.0) [14]. Patients were classified based on the severity of SI on T2WI in a neutral position, such as none, faint, or intense SI. The stage of stenosis based on the classification of Muhle et al. [8] was considered a quantitative variable. All statistical analyses were performed using MedCalc ver. 22.014 (MedCalc, Mariakerke, Belgium), and p-values < 0.05 were considered to indicate statistical significance.
RESULTS
Clinical and radiographic data were recorded from 191 patients (113 males and 78 females) who underwent anterior decompression with fusion (n = 113), posterior cervical decompression (n = 69), including cervical laminoplasty (n = 43) or laminectomy with fusion (n = 26), and combined anterior-posterior surgery (n = 9) for CSM and completed at least 24 months of follow-up. The average follow-up period was 49.8 ± 13.4 months (25–64 months). The mean age at the time of surgery was 55.34 ± 12.09 years (range, 29–79 years). Baseline patient characteristics, demographics, and surgical information are listed in Table 1.
Table 1.
Patient demographics
| Characteristic | Value |
|---|---|
| Age (yr) | 55.34 (29–79) |
| Sex, male:female | 113:78 |
| Preoperative clinical assessment | |
| JOA | 11.07 ± 2.41 (5–15) |
| Nurick | 2.56 ± 0.98 (1–5) |
| 2-Year postoperative clinical assessment | |
| JOA | 14.89 ± 1.75 (9–17) |
| Recovery rate (%) | 85.49 ± 13.21 (40–100) |
| Nurick | 1.63 ± 0.87 (0–4) |
| Most compressed levels | |
| C3–4 | 36 (18.8) |
| C4–5 | 50 (26.2) |
| C5–6 | 77 (40.3) |
| C6–7 | 28 (14.7) |
| Procedure | |
| Anterior | 113 (59.16) |
| Posterior | 69 (36.13) |
| Combined anterior-posterior | 9 (4.71) |
| Pathology | |
| Disc | 141 (73.8) |
| Osteophyte | 12 (6.3) |
| OLF | 9 (4.7) |
| OPLL | 29 (15.2) |
Values are presented as mean±standard deviation (range) or number (%).
JOA, Japanese Orthopaedic Association; OLF, ossification of ligament flavum; OPLL, ossification of the posterior longitudinal ligament.
1. Radiological Findings According to Neck Motion
The sagittal CSA of the cervical cord was smaller when the neck was extended (516.95 ± 106.20 mm2) compared with when it was in a neutral position (534.04 ± 86.96 mm2) or flexed (560.70 ± 90.53 mm2). Similarly, the sagittal CSA of CSF was smaller in the extension position (294.53 ± 101.02 mm2) compared with the neutral (353.50 ± 106.61 mm2) and flexion positions (349.43 ± 123.80 mm2). The sagittal CSF reserve ratio also showed significant differences between flexion-extension neck motion and the neutral posture (p = 0.004). However, there were no significant changes in the axial CSA of the SC, CSF area, or CSF reserve ratio during neck movement (Table 2).
Table 2.
Radiological findings according to neck motion
| Variable | Flexion | Neutral | Extension | p-value |
|---|---|---|---|---|
| Sagittal CSA at compression | ||||
| SC (mm2) | 560.70 ± 90.53 | 534.04 ± 86.96 | 516.95 ± 106.20 | < 0.001* |
| CSF area (mm2) | 349.43 ± 123.80 | 353.50 ± 106.61 | 294.53 ± 101.02 | < 0.001* |
| CSF reserve ratio (%) | 37.63 ± 9.63 | 39.34 ± 8.71 | 35.89 ± 9.06 | 0.004* |
| Axial CSA at compression | ||||
| SC (mm2) | 68.95 ± 15.18 | 67.60 ± 15.44 | 68.03 ± 15.92 | 0.637 |
| CSF area (mm2) | 79.77 ± 33.89 | 77.33 ± 30.78 | 76.51 ± 35.26 | 0.594 |
| CSF reserve ratio (%) | 51.55 ± 13.08 | 51.60 ± 13.37 | 50.50 ± 14.31 | 0.759 |
| Sagittal diameter at compression | ||||
| SC (mm) | 5.04 ± 1.29 | 4.70 ± 1.26 | 4.59 ± 1.55 | 0.006* |
| Dural sac (mm) | 6.45 ± 0.72 | 6.33 ± 0.69 | 6.61 ± 0.82 | 0.002* |
| Cord occupation rate (%) | 78.02 ± 17.31 | 74.36 ± 18.07 | 69.07 ± 20.62 | < 0.001* |
| Axial diameter at compression | ||||
| SC (mm) | 5.97 ± 1.02 | 5.67 ± 1.05 | 5.48 ± 1.49 | 0.015* |
| Dural sac (mm) | 10.31 ± 1.96 | 10.19 ± 1.97 | 10.04 ± 2.25 | 0.472 |
| Cord occupation rate (%) | 59.49 ± 12.43 | 55.60 ± 9.61 | 56.64 ± 16.39 | 0.096 |
Values are presented as mean±standard deviation.
CSA, cross-sectional area; SC, spinal cord; CSF, cerebrospinal fluid.
p<0.05, statistically significant differences.
In addition, the diameter of the sagittal SC and cord occupation rate at the compression level were significantly different depending on neck movement, with the lowest diameter observed during the extended posture. The sagittal cord occupation rate was lowest during extension (69.07% ± 20.62%) posture compared with neutral (74.36% ± 18.07%) and flexion (78.02% ± 17.31%) posture (p < 0.001). The smallest diameter of the axial SC was observed in the extension posture compared with flexion and neutral postures (p = 0.015). During neck motion, there was no difference in the diameter of the dural sac, and the axial cord occupancy rate was also similar (Table 2).
2. Signal Intensity, Compression Level, and Cervical Stenosis With Neck Motion
The severity of intramedullary high SI on T2WI changed with neck movement. Out of 191 patients, 70 (36.65%) showed no intramedullary SI (G0) on sagittal T2WI in a neutral position, 47 patients had faint intramedullary SI (G1), and 74 had intense SI change (G2). However, in the extension posture, 3 of 70 patients who had no SI in the neutral position exhibited faint SI (G1). The percentage of high SI (G1 and G2) increased in the extension posture (124 of 191, 64.92%) compared with the neutral posture (121 of 191, 63.35%), while that of the high SI decreased in the flexion posture (118 of 191, 61.78%) (Fig. 4). Our results showed that 4.3% of patients with no SI in a neutral position displayed SI changes in an extension MRI, and 6.4% of patients with SI in a neutral position displayed no SI in a flexion MRI. Compared with the neutral position, the extension-positioned MRI increased compression levels in 122 of 191 (63.87%) patients. On the other hand, flexion-positioned MRI decreased compression levels in 16 of 191 patients (8.38%). Moreover, upon analysis of Muhle classification, the grade of cervical stenosis increased in the extension posture compared with the neutral and flexion postures (Table 3).
Fig. 4.
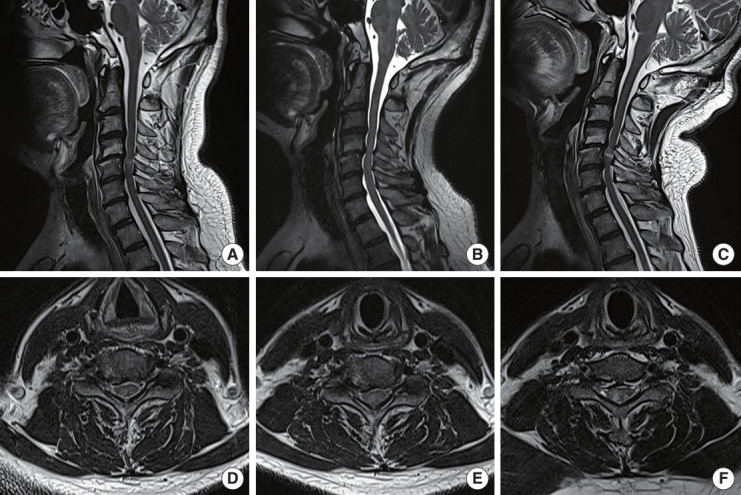
Increase of SI grade and cervical stenosis during extension. A 53-year-old male was diagnosed with cervical spondylotic myelopathy due to a herniated cervical disc at C4–5–6. (A) Flexion-positioned magnetic resonance imaging (MRI) showed intramedullary faint SI (G1) at C4–5 and no effacement of subarachnoid space. (B) Neutral-positioned MRI showed complete obliteration of the anterior subarachnoid space (Muhle grade 2). (C) Extension-positioned MRI showed severe cord impingement at C4–5–6 (Muhle grade 3) and intense intramedullary SI (G2). (D) Flexion-positioned axial view showed cord compression, but the subarachnoid space was not obliterated. (E) On the neutral axial T2-weighted imaging (T2WI), effacement on the right anterior subarachnoid space was noted. (F) On extension-positioned axial T2WI, intense intramedullary SI (G2) was observed.
Table 3.
Change of signal intensity and cervical stenosis according to neck motion
| Variable | Flexion | Neutral | Extension |
|---|---|---|---|
| SI grade | |||
| G0 | 73 (38.22) | 70 (36.65) | 67 (35.08) |
| G1 | 44 (23.04) | 47 (24.61) | 50 (26.18) |
| G2 | 74 (38.74) | 74 (38.74) | 74 (38.74) |
| Muhle grade | |||
| G0 | 8 (4.19) | 0 (0) | 0 (0) |
| G1 | 76 (39.79) | 90 (47.12) | 36 (18.85) |
| G2 | 71 (37.17) | 64 (33.51) | 68 (35.60) |
| G3 | 36 (18.85) | 37 (19.37) | 87 (45.55) |
Values are presented as number (%).
SI, signal intensity; G, grade.
3. Radiological and Clinical Findings According to the Severity of Signal Intensity
Patients who had intensely high SI on T2WI before surgery had significantly lower preoperative JOA scores and recovery ratios (10.09% ± 2.33% and 79.82% ± 15.26%, respectively) compared with those who did not have SI changes (12.09% ± 2.13% and 92.93% ± 6.35%, respectively). The mean preoperative cervical ROM was more prominent in patients with intensely high SI change compared with those with no SI, with values of 45.04° ± 18.90° in SI G2, 35.05° ± 15.63° in SI G1, and 30.34° ± 12.31° in SI G0 (p < 0.001) (Table 4). Moreover, there was no difference between ROM on dynamic radiographs and dynamic MRI (p = 0.3296).
Table 4.
Radiological and clinical findings according to signal intensity
| SI grade | G0 (n = 70) | G1 (n = 47) | G2 (n = 74) | p-value |
|---|---|---|---|---|
| No. of compression lesions | 3.61 ± 0.99 | 3.51 ± 1.09 | 3.36 ± 0.97 | 0.319 |
| Preop JOA | 12.09 ± 2.13 | 11.11 ± 2.32 | 10.09 ± 2.33 | < 0.001* |
| Postop JOA | 15.89 ± 1.02 | 14.64 ± 1.65 | 14.11 ± 1.92 | < 0.001* |
| Recovery rate (%) | 92.93 ± 6.35 | 83.35 ± 12.24 | 79.82 ± 15.26 | < 0.001* |
| ROM on dynamic x-ray | 27.68 ± 9.99 | 33.88 ± 15.89 | 43.61 ± 14.43 | < 0.001* |
| ROM on MRI | 30.34 ± 12.31 | 35.05 ± 15.63 | 45.04 ± 18.90 | < 0.001* |
Values are presented as mean±standard deviation.
SI, signal intensity; JOA, Japanese Orthopaedic Association; ROM, range of motion; MRI, magnetic resonance imaging.
p<0.05, statistically significant differences.
4. Interobserver Reliability
For the CSA assessment, interobserver reliability was classified as “very good agreement” (ICC between 0.85 and 0.95) at the compressed level and in the neutral, flexion, and extension positions (Table 5). The interobserver reliability for cord and dural sac diameters was classified as “very good agreement” (ICC between 0.81 and 0.94) at the compressed level and in the neutral, flexion, and extension positions. Details are presented in Table 5. The interobserver reliability was found to be very good, with a Cohen κ coefficient of 0.99 for the intramedullary SI grade on sagittal T2WI and 0.99 for the detection of the Muhle grade of cervical stenosis.
Table 5.
Interobserver reliability of the radiological parameters
| Variable | Flexion | Neutral | Extension |
|---|---|---|---|
| Sagittal CSA of SC | 0.95 (0.94–0.96) | 0.87 (0.83–0.91) | 0.85 (0.81–0.89) |
| Sagittal CSA of CSF | 0.93 (0.92–0.95) | 0.91 (0.87–0.93) | 0.87 (0.80–0.91) |
| Axial CSA of SC | 0.95 (0.93–0.96) | 0.90 (0.88–0.93) | 0.91 (0.87–0.92) |
| Axial CSA of CSF | 0.92 (0.89–0.94) | 0.91 (0.88–0.93) | 0.94 (0.92–0.95) |
| Sagittal diameter of SC | 0.94 (0.92–0.95) | 0.88 (0.84–0.91) | 0.87 (0.83–0.90) |
| Sagittal diameter of dural sac | 0.81 (0.75–0.86) | 0.82 (0.75–0.86) | 0.81 (0.76–0.86) |
| Axial diameter of SC | 0.93 (0.91–0.95) | 0.87 (0.83–0.90) | 0.89 (0.86–0.92) |
| Axial diameter of dural sac | 0.89 (0.86–0.92) | 0.85 (0.80–0.89) | 0.90 (0.87–0.93) |
Values are presented as intraclass confidence correlation (95% confidence interval).
CSA, cross-sectional area; SC, spinal cord; CSF, cerebrospinal fluid.
DISCUSSION
Several previous researchers used quantitative MRI to measure the SC’s transverse area or the diameters of compressed lesions [9,15,16]. They reported that the spinal canal becomes narrower when the neck is extended [15-17]. However, no previous prospective research analyzed changes in the CSAs of the SC and subarachnoid space or changes in intramedullary SI in the sagittal plane using dynamic MRI. The present study compared the morphometric measurements of the SC in kinematic motion among patients with CSM and investigated the relationship between cervical motion, myelopathic symptoms, and the severity of SI. The results revealed that the morphometric SC was more compressed during neck extension than during neck flexion or neutral positions. The sagittal plane of T2WI on magnetic resonance was more effective than axial T2WI in detecting changes in the CSA of the cord and CSF reserve ratio. The diameter of the cord became smaller during neck extension on axial and sagittal MRI. The severity of SI on T2WI was more prominent in extension-positioned MRI than in flexion-positioned MRI. Additionally, patients with a higher grade of SI had a more comprehensive range of neck motion before the surgery and experienced an unfavorable recovery after surgery.
Hyperextension of the neck causes canal narrowing by inducing buckling ligamentum flavum and the laminae, and the cord impinges anteriorly against a disc or bony spur. They will induce a higher intrinsic pressure, increasing axial tension and potential ischemic injury [18]. This “pincer effect” repeatedly aggravates SC compression, leading from mild myelopathic symptoms to severe myelopathy [19]. A previous report showed the narrowing of the spinal canal by 17%–29% in extension MRI, but canal diameter measurements were performed in only 2 cases [17]. We measured the CSA on T2WI, and our results showed 8.57% narrowing of the CSA of the spinal canal on the sagittal plane on T2WI MR by neck extension but no change of the spinal canal on the axial plane during extension position. During flexion, the cervical SC is stretched and more anterior in the canal [20]. Uchida et al. reported increased SC compression during neck flexion in patients with cervical myelopathy [4,21,22]. In contrast to previous reports, flexion-positioned MRI may reflect a state without SC compression, as the spinal canal is enlarged by the neck flexion position [23]. Watanabe et al. [24] measured the SC pressure at the C5–6 level in 20 patients with CSM. The authors found that compressive forces to the dura at the stenotic level were low in neutral and flexion positions but increased in neck extension. The pressure increased to 23.6 ± 7.5 mmHg with neck extension and decreased to 5.3 ± 2.7 mmHg with flexion. In this study, we found that the spinal canal sagittal CSA increased by 2.54% in a flexion position compared with a neutral position as analyzed on MRI. In cadaveric studies, it was determined that flexion stretched the cervical SC while extension loosened it [25]. Thus, the subarachnoid space should be considered a buffer for cord compression rather than the spinal canal. Muhle et al. reported shortening of the subarachnoid space during neck extension [8]. In this study, we measured the CSF reserve as a subarachnoid space, including values of both SC and CSF during neck motion. We observed a significant difference in the CSF reserve ratio between flexion and extension, with a smaller ratio in extension than in flexion. The sagittal plane of T2WI was more effective than axial T2WI in detecting changes in the CSA of the cord and CSF reserve ratio.
Signal change on T2WI at the level of cord compression is an important prognostic factor that also correlates with the severity of CSM [1,23]. Some pathologies may not be visible on static MRI. Zeitoun et al. [20,26] stated that extension-positioned MRI did not help to identify intramedullary SI change due to significant cervical canal stenosis and cord compression. Flexion-positioned MRI permits better intramedullary high SI visualization on a T2-weighted sequence [20,26]. Our present findings are not in agreement with these previous observations. There is a correlation between the severity of cord compression and the SI change, as reported in the literature [2,17,27]. Patients with more advanced spondylosis had significantly more stenosis at dynamic positions compared with those with less advanced disease [8,11,13]. We showed a significant increase in cord impingement in extension (87 of 191 patients, 45.5%) versus flexion (36 of 191 patients, 18.8%) in CSM, which is consistent with the findings of other studies [8]. We observed an increase in the prevalence of intramedullary SI change in extension (64.92%) versus flexion (61.78%). We found that 4.3% of patients with no SI in a neutral position changed into SI change in an extension MRI and 6.4% of patients with SI in a neutral position changed into no SI in a flexion MRI. Based on our results, extension-positioned MRI provides a more reliable evaluation of high intramedullary SI than the neutral and flexion positions.
The increase in SI in the neck extension position has several possible explanations. The intramedullary signal changes on T2WI were presumed to indicate myelomalacia or cord gliosis secondary to long-standing compression of the SC [2,28,29]. Previous experimental studies support the notion that chronic compression of the SC leads to diminished blood flow, and that ischemia to the cord is the pathophysiological mechanism of cervical compressive myelopathy [30,31]. The presence of a high SI lesion on T2WI was observed in patients with a more constricted or narrow SC, and the intensity of the signal change increased over time [2]. In the early stage, an intramedullary SI change on an MRI reflects cord edema. In the intermediate stage, a signal change reflects cystic necrosis of the central gray matter after prolonged cord edema [32]. Ramanauskas et al. [32] reported that, in the early and intermediate stages, the SC exhibited high SI on T2WI, whereas at a later stage, the SC manifested low SI on T1-weighted imaging (T1WI) and high SI on T2WI. In our study, the extension-positioned MRI presented a narrower CSA of the compressed cord and a lower CSF reserve ratio than the flexion- or neutral-positioned MRI, and compression of the SC aggravates ischemia of the cord. In reversible intramedullary signal changes, SC compression is aggravated during neck extension, causing the cord SI change to intensify; conversely, during neck flexion, SC compression improves slightly, and the resulting intramedullary SI change could mask the spinal SI change. An intramedullary SI change might become irreversible if this phenomenon occurs repeatedly over time, such as a low SI on a T1WI MRI. Patients with preoperatively low SI on T1WI MRI, that is, a snake eye appearance, had poor neurological outcomes after decompression [5,23].
Dynamic MRI can reveal detailed compression levels, but static MRI might not show cord compression [9,33]. We found that compression levels increased by 63.87% when patients underwent an extension-positioned MRI. SC compression might be observed in asymptomatic patients on neutral MRI, and not every compression level is clinically significant [27,34]. The increase in compression levels found in extension MRI should be considered when determining whether surgical treatment and surgical level expansion are necessary. As it is difficult to statistically show the influence of dynamic MRI on surgical decision-making in this study, we have included example cases in the supplementary figures (Supplementary Figs. 1–3). In the future, we plan to investigate how changes in dynamic MRI caused by neck movement affect changes in surgical treatment strategies. A multicenter expert opinion study is needed to investigate the clinical relevance and effectiveness of dynamic magnetic resonsnce images.
Previous studies showed that the anteroposterior diameter of the dural sac and SC is shorter during extension than flexion [13,35]. Machino et al. [13] confirmed that the anteroposterior diameter of the dural sac and SC in patients with CSM is significantly shorter than that in asymptomatic subjects. This study has shown that the sagittal spinal canal diameter and cord occupancy rate differ significantly during neck motion. The compressed cord diameter decreases in the extension position rather than in flexion. Accurately assessing the difference in diameters on the axial view can be challenging in cases of severe cord compression.
Stretch and shear forces are a leading cause of myelopathy, supported by evidence from various experimental models, including neural injury, tethered cord syndrome, and diffuse axonal injury [11]. Studies have shown that segmental instability and mobility of the cervical spine play a significant role in the onset and prognosis of CSM [8,11]. However, there is limited data on the relationship between cervical motion, high SI, and the severity of myelopathy symptoms. There are several grading systems for SI changes in the SC, but most focus on 2 intensity types: faint/fuzzy or intense/well-defined [3,29]. In the present study, there was a correlation between greater neck ROM and more severe SI changes. With respect to neurological outcomes based on the severity of SI, the patients with no signal changes on T2WI had greater improvement in the JOA recovery ratio than patients with faint or intense SI changes on T2WI.
Dynamic MRI has benefits in identifying missed pathologies not visible on static MRI, such as changes in compressed levels and SI grade. In the following cases, we recommend routine or additional dynamic MRI for more benefits. Dynamic MRI has the potential to enable an early diagnosis when patients exhibit signs of myelopathy without severe cord compression or SI changes in static MRI by allowing the clinicians to assess changes in SC compression according to neck movement. Dynamic MRI can be helpful in planning the proper surgical position, and surgical decompression can be performed carefully when a severely compressed lesion is fully understood. Surgeons need to prescribe dynamic MRI to evaluate compression lesions and determine the appropriate surgical approach and levels. Dynamic MRI can also be helpful in assessing severe spondylosis in elderly patients with cervical myelopathy to determine which of the multilevel compression lesions caused by a bony spur or disc protrusion is the most compressed. On the other hand, dynamic MRI has the potential risk of symptom exacerbation, such as developing weakness in the upper or lower extremities, spasticity, or gait disturbance. As the potential benefits of dynamic MRI must be weighed against the risks of symptom worsening, dynamic MRI should be performed with careful consideration in patients with severe compressive myelopathy who have disc protrusions, osteophyte formation, hypertrophied ligamentum flavum, cervical canal stenosis, or segmental instability. In addition, it is crucial to consider the cost-effectiveness and time requirements of dynamic MRI. In Korea, a dynamic MRI can cost an additional USD 300 and take an extra 20 minutes compared with a routine cervical MRI.
In clinical practice, it is essential to avoid neck hyperextension exercises, which can exacerbate cervical conduction abnormalities and lead to severe cervical myelopathy [36]. Before conducting a dynamic MRI, it is recommended that the patient’s neck flexion and extension motion be practiced. If patients develop paralysis of their upper or lower extremities, worsening of neurological deficits, or intolerable numbness in both hands during the neck motion trial, a dynamic MRI would not be indicated because it dangerously exacerbates the symptoms. Also, patients with intense SI on static MRI must carefully perform flexion-and extension-positioned MRIs. Dynamic MRI should only be conducted during the daytime, and the patient should be closely monitored by the doctor throughout the test. We received permission from the patients before we conducted dynamic MRIs, and they made it clear that they would call for a halt to the procedure immediately if any symptoms, such as numbness or weakness, worsened. During our study period, 2 patients had to discontinue the dynamic MRI due to claustrophobia or panic symptoms, even after being given sedatives. However, none of the patients experienced neurological deficits that worsened during the dynamic MRI.
Dynamic MRI is a diagnostic tool that can reveal pathological phenomena that might not be visible in static MRI. However, it can worsen neurological deficits due to severe SC compression in the extended posture. Therefore, it should never be used in cases of traumatic instability, and it is contraindicated in cases of traumatic spinal hematoma or spontaneous spinal epidural hematoma.
This study had several limitations. The study included a small number of patients and did not consider other cervical alignment parameters, such as T1 slope, TIA, neck tilt, C2–7 sagittal vertical axis, or rotational motion. We included surgical cases and did not evaluate nonoperative cases. We focused on the degree of morphological cord compression visible on MRI using quantitative measurements in cervical myelopathic patients who needed surgery. Moreover, we only used T2-weighted images for data measurements as T1WI was not performed on dynamic motion due to high costs and the national insurance policy. The degrees of neck motion were not standardized for dynamic MRI due to pain or neurological deficits limiting neck motion. Additionally, the study was performed using dynamic supine MRI, which does not resemble physiological status in the upright position. However, our results showed no significant difference in ROM between cervical dynamic x-ray and dynamic MRI. We plan to perform further research to investigate the numerical value of signal change on T1WI and T2WI in a large number of patients with cervical myelopathy. Nonetheless, our current findings may be helpful in considering the decision of surgical levels and approaches for patients with CSM.
CONCLUSION
Dynamic MRI is a valuable tool for evaluating the physiological state during neck movement. We found that dynamic MRI identified the severity of cord compression and the prevalence of SI change and detected missed lesions more effectively than static MRI. The severity of intramedullary high SI is more prominent in an extended position. Patients with a higher grade of SI had a wider range of neck motion before surgery and experienced a less favorable recovery after surgery. The degree of cervical stenosis and cord compression on neck extension increased, and cervical spinal motion contributed to the severity of CSM, particularly in a hyperextension posture, which may help avoid aggravating myelopathic symptoms.
Acknowledgments
The authors would like to thank Hyun Kyung Park for their contributions in drafting and revising the manuscript for important intellectual content. The authors also wish to thank all the subjects who participated in the study, as well as the support staff and the research coordinator.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2021-0136).
Author Contribution
Conceptualization: JJS, SJY, TWK, WJJ, JS, YH; Data curation: JJS, SJY, TWK, JYS, MHL, JS, YH; Formal analysis: JJS, SJY, TWK, JYS, WJJ, JS, YH; Funding acquisition: JS, YH; Methodology: JJS, SJY, TWK, JYS, WJJ, JS, YH; Project administration: JJS, TWK, JS, YH; Visualization: JJS, JS, YH; Writing – original draft: JJS, JS; Writing – review & editing: JJS, JS, YH.
Supplementary Materials
Supplementary Figs. 1-3 can be found via https://doi.org/10.14245/ns.2448166.083.
Multilevel cord compression during extension. A 73-year-old male was diagnosed with cervical spondylotic myelopathy due to a herniated cervical disc and bony spur at C5–6–7. (A) Flexion-positioned magnetic resonance imaging (MRI) showed cord compression decrease at C5–6–7 and no effacement of the subarachnoid space. (B) Neutral-positioned MRI showed partial obliteration of the anterior subarachnoid space at C5–6–7. (C) Extension-positioned MRI showed severe cord impingement at C3–4–5–6–7 and complete cord compression at C3–4–5. (D) We performed cervical selective laminoplasty and fusion C3–4–5–6–7, and his upper and lower weakness improved after the surgery.
Mild cord compression in neutral-positioned magnetic resonance imaging (MRI). A 55-year-old female was diagnosed with cervical spondylotic myelopathy due to a herniated cervical disc and bony spur at C5–6. (A) Flexion-positioned MRI showed mild cord compression decrease and no effacement of the subarachnoid space at C5–6. (B) Neutral-positioned MRI showed a partial anterior subarachnoid space at C5–6. (C) Extension-positioned MRI showed severe cord impingement at C5–6 and intense intramedullary SI (G2). (D) We performed anterior cervical discectomy and fusion C5–6, and she experienced an improvement in her arm and leg weakness and numbness.
A pathological lesion with no visible compression lesion in neutral-positioned magnetic resonance imaging (MRI). A 57-year-old male had undergone anterior cervical discectomy and fusion (ACDF) with a standalone cage on C3–4 10 years ago and had upper extremities weakness and ataxic gait aggravation one year previously. He was diagnosed with cervical spondylotic myelopathy, but no cord compression was found. (A) Flexion-positioned MRI showed SI at C3–4 and C5–6 but no cord compression. (B) Neutral-positioned MRI did not show any cord compression. (C) Extension-positioned MRI showed severe cord impingement at C4–5 and an increase in the intramedullary SI (G2) at C5–6. (D) We performed ACDF C4–5–6, and he showed improved gait disturbance and weakness in the upper extremities after surgery.
REFERENCES
- 1.Shin JJ, Jeon H, Lee JJ, et al. Predictors of neurologic outcome after surgery for cervical ossification of the posterior longitudinal ligament differ based on myelopathy severity: a multicenter study. J Neurosurg Spine. 2021;34:749–58. doi: 10.3171/2020.8.SPINE20504. [DOI] [PubMed] [Google Scholar]
- 2.Kim TH, Ha Y, Shin JJ, et al. Signal intensity ratio on magnetic resonance imaging as a prognostic factor in patients with cervical compressive myelopathy. Medicine (Baltimore) 2016;95:e4649. doi: 10.1097/MD.0000000000004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin JJ, Jin BH, Kim KS, et al. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir (Wien) 2010;152:1687–94. doi: 10.1007/s00701-010-0692-8. [DOI] [PubMed] [Google Scholar]
- 4.Uchida K, Nakajima H, Sato R, et al. Cervical spondylotic myelopathy associated with kyphosis or sagittal sigmoid alignment: outcome after anterior or posterior decompression. J Neurosurg Spine. 2009;11:521–8. doi: 10.3171/2009.2.SPINE08385. [DOI] [PubMed] [Google Scholar]
- 5.Joaquim AF, Baum GR, Tan LA, et al. Dynamic cord compression causing cervical myelopathy. Neurospine. 2019;16:448–53. doi: 10.14245/ns.1938020.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YJ, Cho PG, Kim KN, et al. Risk factors of unplanned readmission after anterior cervical discectomy and fusion: a systematic review and meta-analysis. Yonsei Med J. 2022;63:842–9. doi: 10.3349/ymj.2022.63.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa F, Anania CD, Agrillo U, et al. Cervical spondylotic myelopathy: from the World Federation of Neurosurgical Societies (WFNS) to the Italian Neurosurgical Society (SINch) Recommendations. Neurospine. 2023;20:415–29. doi: 10.14245/ns.2244996.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhle C, Metzner J, Weinert D, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol. 1998;19:1763–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Tykocki T, du Plessis J, Wynne-Jones G. Analysis of morphometric parameters in cervical canal stenosis on neutral and dynamic magnetic resonance imaging. World Neurosurg. 2018;114:e317–22. doi: 10.1016/j.wneu.2018.02.179. [DOI] [PubMed] [Google Scholar]
- 10.Mattei TA, Goulart CR, Milano JB, et al. Cervical spondylotic myelopathy: pathophysiology, diagnosis, and surgical techniques. ISRN Neurol. 2011;2011:463729. doi: 10.5402/2011/463729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson FC, Geddes JF, Vaccaro AR, et al. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101–13. discussion1101-13. [PubMed] [Google Scholar]
- 12.Shin JJ, Kim KR, Son DW, et al. Radiological changes in adjacent and index levels after cervical disc arthroplasty. Yonsei Med J. 2022;63:72–81. doi: 10.3349/ymj.2022.63.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machino M, Ito K, Kato F, et al. Kinetic changes in the spinal cord occupation rate of dural sac in cervical spondylotic myelopathy. J Orthop. 2021;24:222–6. doi: 10.1016/j.jor.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 15.Karpova A, Arun R, Davis AM, et al. Reliability of quantitative magnetic resonance imaging methods in the assessment of spinal canal stenosis and cord compression in cervical myelopathy. Spine (Phila Pa 1976) 2013;38:245–52. doi: 10.1097/BRS.0b013e3182672307. [DOI] [PubMed] [Google Scholar]
- 16.Funaba M, Imajo Y, Suzuki H, et al. Impact of various MRI signal intensity changes on radiological parameters, the neurological status, and surgical outcomes in degenerative cervical myelopathy. Clin Neurol Neurosurg. 2021;207:106802. doi: 10.1016/j.clineuro.2021.106802. [DOI] [PubMed] [Google Scholar]
- 17.Guppy KH, Hawk M, Chakrabarti I, et al. The use of flexion-extension magnetic resonance imaging for evaluating signal intensity changes of the cervical spinal cord. J Neurosurg Spine. 2009;10:366–73. doi: 10.3171/2009.1.SPINE08567. [DOI] [PubMed] [Google Scholar]
- 18.Dolan RT, Butler JS, O’Byrne JM, et al. Mechanical and cellular processes driving cervical myelopathy. World J Orthop. 2016;7:20–9. doi: 10.5312/wjo.v7.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Z, Tung NTC, Makino H, et al. Assessment of cervical myelopathy risk in ossification of the posterior longitudinal ligament patients with spinal cord compression based on segmental dynamic versus static factors. Neurospine. 2023;20:651–61. doi: 10.14245/ns.2346124.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Zeitoun D, Rangel A, et al. Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: about a prospective study of fifty patients. Spine (Phila Pa 1976) 2011;36:E1134–9. doi: 10.1097/BRS.0b013e3181f822c7. [DOI] [PubMed] [Google Scholar]
- 21.Yoshii T, Yamada T, Hirai T, et al. Dynamic changes in spinal cord compression by cervical ossification of the posterior longitudinal ligament evaluated by kinematic computed tomography myelography. Spine (Phila Pa 1976) 2014;39:113–9. doi: 10.1097/BRS.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto T, Funaba M, Imajo Y, et al. The impact of anterior spondylolisthesis and kyphotic alignment on dynamic changes in spinal cord compression and neurological status in cervical spondylotic myelopathy: a radiological analysis involving kinematic CT myelography and multimodal spinal cord evoked potentials. Spine (Phila Pa 1976) 2021;46:72–9. doi: 10.1097/BRS.0000000000003735. [DOI] [PubMed] [Google Scholar]
- 23.Seki S, Kawaguchi Y, Nakano M, et al. Clinical significance of high intramedullary signal on T2-weighted cervical flexion-extension magnetic resonance imaging in cervical myelopathy. J Orthop Sci. 2015;20:973–7. doi: 10.1007/s00776-015-0757-x. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe Y, Unayama K. Dural pressure measurements at the stenotic level in cervical spondylotic myelopathy. Spine J. 2002;2:35. [Google Scholar]
- 25.Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis. A study on fresh cadaver material. J Neurosurg. 1966;25:45–56. doi: 10.3171/jns.1966.25.1.0045. [DOI] [PubMed] [Google Scholar]
- 26.Zeitoun D, El Hajj F, Sariali E, et al. Evaluation of spinal cord compression and hyperintense intramedullary lesions on T2-weighted sequences in patients with cervical spondylotic myelopathy using flexion-extension MRI protocol. Spine J. 2015;15:668–74. doi: 10.1016/j.spinee.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim CH, Chung CK, Kim KJ, et al. Cervical extension magnetic resonance imaging in evaluating cervical spondylotic myelopathy. Acta Neurochir (Wien) 2014;156:259–66. doi: 10.1007/s00701-013-1951-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohshio I, Hatayama A, Kaneda K, et al. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976) 1993;18:1140–9. doi: 10.1097/00007632-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Wei L, Wei Y, Tian Y, et al. Does three-grade classification of T2-weighted increased signal intensity reflect the severity of myelopathy and surgical outcomes in patients with cervical compressive myelopathy? A systematic review and meta-analysis. Neurosurg Rev. 2020;43:967–76. doi: 10.1007/s10143-019-01106-3. [DOI] [PubMed] [Google Scholar]
- 30.Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg. 1975;43:9–17. doi: 10.3171/jns.1975.43.1.0009. [DOI] [PubMed] [Google Scholar]
- 31.Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24 Suppl 2:132–8. doi: 10.1007/s00586-014-3264-4. [DOI] [PubMed] [Google Scholar]
- 32.Ramanauskas WL, Wilner HI, Metes JJ, et al. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989;13:399–404. doi: 10.1097/00004728-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Lee JB, Park JH, Lee JJ, et al. Influence of dynamic neck motion on the clinical usefulness of multi-positional MRI in cervical degenerative spondylosis. Eur Spine J. 2021;30:1542–50. doi: 10.1007/s00586-021-06760-0. [DOI] [PubMed] [Google Scholar]
- 34.Oshima Y, Seichi A, Takeshita K, et al. Natural course and prognostic factors in patients with mild cervical spondylotic myelopathy with increased signal intensity on T2-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2012;37:1909–13. doi: 10.1097/BRS.0b013e318259a65b. [DOI] [PubMed] [Google Scholar]
- 35.Dalbayrak S, Yaman O, Firidin MN, et al. The contribution of cervical dynamic magnetic resonance imaging to the surgical treatment of cervical spondylotic myelopathy. Turk Neurosurg. 2015;25:36–42. doi: 10.5137/1019-5149.JTN.9082-13.1. [DOI] [PubMed] [Google Scholar]
- 36.Barrey C, Roussouly P, Perrin G, et al. Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J. 2011;20 Suppl 5(Suppl 5):626–33. doi: 10.1007/s00586-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multilevel cord compression during extension. A 73-year-old male was diagnosed with cervical spondylotic myelopathy due to a herniated cervical disc and bony spur at C5–6–7. (A) Flexion-positioned magnetic resonance imaging (MRI) showed cord compression decrease at C5–6–7 and no effacement of the subarachnoid space. (B) Neutral-positioned MRI showed partial obliteration of the anterior subarachnoid space at C5–6–7. (C) Extension-positioned MRI showed severe cord impingement at C3–4–5–6–7 and complete cord compression at C3–4–5. (D) We performed cervical selective laminoplasty and fusion C3–4–5–6–7, and his upper and lower weakness improved after the surgery.
Mild cord compression in neutral-positioned magnetic resonance imaging (MRI). A 55-year-old female was diagnosed with cervical spondylotic myelopathy due to a herniated cervical disc and bony spur at C5–6. (A) Flexion-positioned MRI showed mild cord compression decrease and no effacement of the subarachnoid space at C5–6. (B) Neutral-positioned MRI showed a partial anterior subarachnoid space at C5–6. (C) Extension-positioned MRI showed severe cord impingement at C5–6 and intense intramedullary SI (G2). (D) We performed anterior cervical discectomy and fusion C5–6, and she experienced an improvement in her arm and leg weakness and numbness.
A pathological lesion with no visible compression lesion in neutral-positioned magnetic resonance imaging (MRI). A 57-year-old male had undergone anterior cervical discectomy and fusion (ACDF) with a standalone cage on C3–4 10 years ago and had upper extremities weakness and ataxic gait aggravation one year previously. He was diagnosed with cervical spondylotic myelopathy, but no cord compression was found. (A) Flexion-positioned MRI showed SI at C3–4 and C5–6 but no cord compression. (B) Neutral-positioned MRI did not show any cord compression. (C) Extension-positioned MRI showed severe cord impingement at C4–5 and an increase in the intramedullary SI (G2) at C5–6. (D) We performed ACDF C4–5–6, and he showed improved gait disturbance and weakness in the upper extremities after surgery.