Abstract
Background:
Jin’s three needle (JTN) is a commonly utilized treatment for ischemic stroke in China. Mirror therapy (MT) is also gradually transitioning from treating limb discomfort to restoring motor function in the damaged limb. Investigations into the 2 treatments’ mechanisms of action are still ongoing. We used functional magnetic resonance imaging (fMRI) technique in this study to examine the effects of JTN combined with mirror therapy MT on brain function in patients with upper limb dysfunction in ischemic stroke, as well as potential central mechanisms. The goal was to provide a solid evidence-based medical basis to support the continued use of JTN combination MT.
Methods:
This study will be a single-blind, randomized, and controlled experiment. Randomization was used to assign 20 patients who met the study’s eligibility requirements to the JTN + MT treatment group or the JTN control group. Each intervention will last for 4 weeks, with 6 days of treatment per week. The JTN acupuncture points are 3 temporal acupuncture points on the opposite side of the wounded limb, 3 hand acupuncture points on the injured upper limb, 3 shoulder acupuncture points, Renzhong and Baihui, The (JTN + MT) group simultaneously takes MT for 30 minutes. fMRI of the brain using BOLD and T1-weighted images was done both before and after therapy. Brain areas exhibiting changes in regional homogeneity during the pre and posttreatment periods were analyzed.
Results:
By the end of the treatment course, Jin three-needle therapy plus MT activated more relevant brain functional regions and increased cerebral blood oxygen perfusion than Jin three-needle therapy alone (P <.05).
Conclusion:
In patients with upper limb impairment following an ischemic stroke, JTN with MT may improve brain function reconstruction in the relevant areas.
Keywords: ischemic stroke, Jin’s three needle, mirror therapy, ReHo, upper limb and hand motor dysfunction
1. Introduction
Stroke is a set of disorders that significantly impair quality of life and endanger human health due to their high frequency, death, disability, and recurrence of ischemia or hemorrhagic brain injury. The most recent epidemiological report on ischemic strokes was released in 2016, and it states that there are 70 million ischemic stroke patients in China, 2 million new cases, and 1.65 million ischemic stroke deaths annually.[1] Of the patients who survive, approximately 75% had limb impairment to varying degrees,[2] and 69% had hand and upper extremity dysfunction.[3] The extent to which upper extremity dysfunction is recovered plays a significant role in the patient’s quality of survival.[4]
Jin’s three-needle (JTN) therapy was established via repeated clinical practice and methodical experimental study. It is the culmination of more than 50 years of clinical practice by Professor Jin Rui and summarizes the clinical experience of notable acupuncturists from previous generations. Compared to medication, the therapy is no taxing on the liver and kidneys, is straightforward and simple to use, and is effective in treating limb dysfunction in ischemic stroke patients.[5–7] The fundamental working principle of mirror therapy (MT) is planar mirror imaging. A therapeutic tool that combines a rehabilitation program through visual illusion, visual feedback, and virtual reality, ask the patient to imagine that the motor function of the affected limb is completely normal after seeing the mirror image of the movement of the healthy limb, and perform the same movements as the healthy upper limb. With impressive clinical outcomes, the technique was first utilized to treat phantom limb discomfort before progressively being applied to unilateral limb movement abnormalities following an ischemic stroke.[8,9]
The utilization of functional magnetic resonance imaging (fMRI) has grown in popularity as a novel, noninvasive, visible, and real-time technique to investigate the key mechanisms underlying acupuncture research. The existence of acupoint specificity and the correlation between acupuncture, acupoints, and the brain were first confirmed by numerous Block Design fMRI-based acupuncture studies in the past.[10,11] However, these experiments do not accurately reflect the actual clinical acupuncture treatment process, and when compared to resting-state cerebral functional magnetic resonance imaging (Rs-fMRI), Rs-fMRI is able to more accurately simulate the human physiological state and reflect the complex functional activity of the brain.
In this study, we used JTN combined with MT to treat upper limb dysfunction in ischemic stroke patients. Using resting-state fMRI, we observed changes in regional homogeneity (ReHo) of abnormal brain regions before and after the treatment to look into the main mechanism underlying the therapy’s impact on brain function.
2. Methods/design
2.1. General information
The subjects of this study were all outpatients and ward inpatients of the Department of Acupuncture and Rehabilitation, Shenzhen Hospital, Guangzhou University of Chinese Medicine, between January 1, 2018 and January 1, 2020, aged between 40 and 65 years. Twenty healthy subjects of similar age, gender, and educational level were recruited. In total, 20 patients with left-sided upper limb motor dysfunction following an ischemic stroke that met the inclusion criteria were gathered for this study. Ten patients will be assigned to the treatment group and 10 patients to the control group in a 1:1 randomization process. Seven male and 3 female patients with a mean age of 55.90 ± 5.63 years and a mean illness duration of 51.90 ± 22.04 days were in the treatment group; 8 male and 2 female patients with a mean age of 57.70 ± 6.38 years and a mean illness duration of 58.90 ± 21.33 days were in the control group. This research was authorized by the Guangzhou University of Chinese Medicine Ethics Committee and registered with the China Clinical Trials Registry.
2.2. Inclusion criteria
The following are among the requirements for inclusion: matching the ischemic stroke diagnostic standards[12]; matching the TCM ischemic stroke diagnostic standards[13]; verified by head CT or MRI, with lesions seen in the basal ganglia or left radiological crown; the initial clinical instance of an ischemic stroke happened between 2 and 6 months ago, with a total of 2 ischemic stroke occurrences; a person or female between the ages of 40 and 65; the following criteria must be met: upper limb dysfunction; no internal metal objects or pacemakers; a cumulative limb impairment score of ≥10 on the clinical Neurological Deficit Scale.[14] Brunnstrom stage[15] of flaccid (Brunnstrom I–II) or spastic (Brunnstrom III–IV). Steady vital signs, consciousness, and a normal Kinesthetic Visual Imagery Questionnaire test result[16]; gave their signed, informed consent to take part.
2.3. Exclusion criteria
The following are among the exclusion criteria: transient ischemic attack; upper limb dysfunction due to brain tumors, brain trauma, and other diseases; hematencephalon or necessitated craniotomy; severe spastic deformation on upper limbs (Ashworth spasticity rating[17] ≥ grade 3); pregnant and lactating women; severe complications of the heart, liver, kidney, hematopoietic system, and endocrine system or with a severe mental illness that interferes with treatment-seeking, such as schizophrenia, addiction, and dissociative disorders, and individuals who are deemed clinically inappropriate for acupuncture therapy.
2.4. Experiment summary
Prior to the experiment, the recruited volunteers were asked to urinate and defecate. The first Rs-fMRI scan prior to treatment was carried out following a 10-minute rest. After that, the treatment group received JTN therapy together with MT, and the control group received JTN therapy. Each group’s treatment lasted for 30 minutes. At the conclusion of the first treatment and once more at the end of the session, Rs-fMRI scans were carried out. Subsequently, the Rs-fMRI and every functional score for both groups were statistically assessed using ReHo analysis before and after therapy.
2.5. Interventions
2.5.1. Western medication therapy
Patients in all groups received regular medication regimens based on the 2014 Chinese Diagnostic Guidelines for acute ischemic stroke.[18] Regulation of blood glucose and blood lipids; avoidance of platelet aggregation; use of tailored treatment to stabilize blood pressure below 135/85 mm Hg or within the normal range give neurotrophic drugs: cerebroprotein hydrolysate (Xiuzheng Pharmaceutical Group Co., Ltd., State Drug Administration H22024371) 60 mg/d, divided into 3 oral doses; bayaspirin (Jilin Hengjin Pharmaceutical Co., Ltd., State Pharmacopoeia H20046238) 0.1 g/d, once daily; symptomatic treatment, prevention, and control of complications; supplemented with necessary nutritional support.
2.5.2. Treatment group
Using JTN combination with MT:
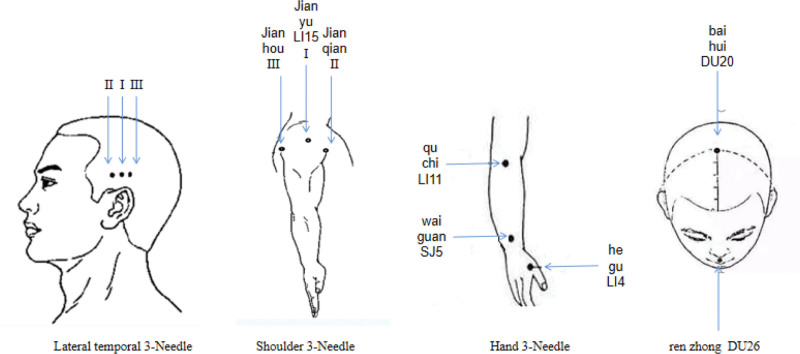
JTN acupuncture points[19]: Take 3 stitches from the contralateral temporal side of the affected limb (located at the tip of the ear straight up into the hairline 2 inches for the temporal I, in the same horizontal line before and after each side open an inch for the temporal II, temporal III); 3 shoulder stitches for the affected upper limb (jianyu LI15, 1 inch above the anterior axillary crease, 1.5 inches above the posterior axillary crease); hand 3 needles (Quchi LI11, Waiguan SJ05, Hegu LI04); renzhong DU26¸baihui DU20 (see Fig. 1, which demonstrates the location of JTN acupoints).
Figure 1.
The location of selected Jin’s three-needle points.
MT: The patient is seated near the table, and a 35 cm by 35 cm vertical mirror is positioned between the 2 upper limbs, with the afflicted upper limb positioned behind the nonreflective side of the mirror and the healthy upper limb facing the reflective side. The patient was told to visualize motion in the injured limb while seeing the motion imaging of the healthy upper limb. As much as feasible, the afflicted limb was expected to perform the same movements as the healthy limb; the therapist would help with any actions the patient was unable to perform on his own. Six upper extremity motions (shoulder flexion, elbow flexion and extension, forearm rotation, wrist flexion and extension, finger extension and grip, and thumb abduction) were required of the patient on the healthy side. To reach maximum joint range of motion, each exercise needed to be performed for 5 minutes, and the training needed to be done for 30 minutes every day (see Fig. 2, which demonstrates the operation of MT).
Figure 2.
Demonstration of mirror therapy movements.
2.5.3. Control group
Only JTN was used, and the acupuncture points were the same as those of the treatment group.
2.5.4. Treatment methods
Both groups were treated by the same acupuncturist, using 0.32 mm × 25 mm or 0.32 mm × 50 mm Hua Tuo brand stainless steel disposable acupuncture needles (Suzhou Hua Tuo Medical Instrument Factory), and the needles were inserted after routine skin disinfection with 75% alcohol. The acupuncture technique is adjusted to the tolerance of each patient by the method of flat tonic and flat diarrhea, to achieve the feeling of “getting qi” (such as soreness, numbness, heaviness, fullness, etc), without obvious pain. The needles were left in place for 30 minutes, during which the treatment group performed mirror image rehabilitation therapy on the healthy upper limb. During the acupuncture in both groups, the acupuncture points were performed 9 times at 10-minute intervals to strengthen the needle sensation.
2.5.5. MT considerations
When the patient receives their first treatment, they should be given enough information to comprehend the history, objectives, and potential adverse effects of the mirror treatment. Additionally, the patient should be guided to shift his gaze to the image of the healthy upper limb and the healthy upper limb in the mirror by the rehabilitation therapist before beginning the exercise. This will help the patient imagine that the affected upper limb is performing the rehabilitation exercise. The rehabilitation therapist should also explain and demonstrate the movements to the patient and give instructions to try to imitate them.
2.5.6. Course of treatment
Acupuncture was administered to patients in both groups for 4 weeks (28 days) in a row, with a 1-day weekly break for a total of 6 treatment days. Needles were left in the body once a day for 30 minutes.
2.6. Rs-fMRI scan
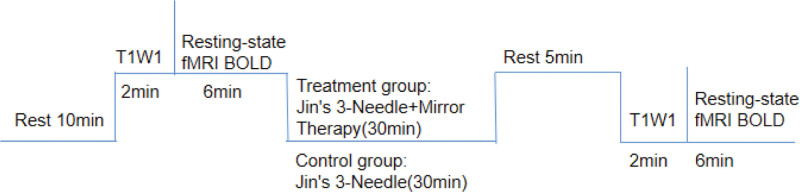
Subjects were scanned using a Siemens 3.0T MRI machine with an 8-channel head coil for T1-weighted images and brain oxygen level-dependent (BOLD) images in order to collect resting-state fMRI data. In order to reduce active or passive movements during the scan, subjects were instructed to wear earplugs (Aearo, Indianapolis), close their eyes, relax, breathe deeply, try not to think but not to fall asleep, avoid audio-visual interference, and have their head immobilized by a foam headrest.
Scanning parameters: T1-weighted images scans were imaged using MP-RAGE sequence thin-layer scanning with specific parameter settings: repetition time = 1900 ms, echo time = 2.53 ms, inversion time = 900 ms, flip angle 9°, image field of view = 250 mm × 250 mm, sampling matrix = 256 × 256, thickness 1 mm, resolution 1 mm × 1 mm × 1 mm; resting-state fMRI uses an echo planar imaging (EPI) sequence, specific parameters: TR/echo time = 3000/30, tilt angle 90°, field of view = 192 mm × 192 mm, sampling matrix = 128 × 128, number of layers (transverse bit) 36, thickness 3 mm, resolution 1.5 mm × 1.5 mm × 3.75 mm (see Fig. 3, which demonstrates the process of magnetic resonance scanning).
Figure 3.
Flowchart of the experimental procedure. T1WI = transverse T1-weighted image; BOLD = blood-oxygen-level dependent data.
2.7. Image preprocessing
To ensure the reliability of statistical results, image preprocessing was carried out using the Rs-fMRI Data Processing Assistant (DPARSFA2.3) software[20] running on the MATLAB R2016 version (Mathworks, Inc., Natick) platform for data preprocessing, functional image postprocessing, and statistics. The original files from the scans should be arranged before preprocessing. All subjects in the control, treatment, and healthy groups should have their resting functional and 3D structural files classed separately, and their files named with “sub + number.” Subsequently, the original DICOM file obtained from the MRI scanner was transformed to NIFTI format for the data format. The conversion of the DICOM format, the removal of 10 time points prior to picture scanning, time correction, head motion correction, spatial normalization, and spatial smoothing are the main steps in the preprocessing procedure. Subjects whose rotation angle or 3D translation was >1.5° or 1.5 mm had their data eliminated. In order to process the data using DPARSFA software, the data were first normalized using the standard template provided by the Montreal Neurological Institute (MNI). Next, the data were smoothed using a 3D Gaussian filter to increase the signal-to-noise ratio of the images. Lastly, the smoothed images were subjected to pixel-based statistical analysis to determine the Kendall consistency coefficient values for each voxel in order to determine the initiated functional areas (also known as separate Kendall consistency coefficient maps or ReHo maps). Finally, the initiated functional areas were fused with the anatomical images to develop the Landmark stereo images.[20–22]
2.8. Statistical analysis of data
Employing the MATLAB-based REST1.8 (resting-State fMRI data analysis Toolkit 1.8, REST1.8, http://www.restfmri.net/forum/) software,[23] created by Beijing Normal University’s Cognitive Neuroscience and Learning Laboratory. The ReHo files of the treatment group group and the control group and the normal group were subjected to one-sample t tests, respectively, and the ReHo plots of the respective one-sample t tests of the treatment group and the control group were obtained; a two-sample t test was applied to the treatment and control groups with Rest1.8 software, and the subjects’ age, sex, years of education, and weight were removed as covariates, and the concatenation of the significant brain areas in each of the 2 groups by one-sample t test was used as the mask to derive the difference brain area maps of ReHo in the treatment and control groups, multiple comparison correction was performed using the AlphaSim function within the REST software, where full width at half maximum = 6, α = 0.05, by Monte Carlo simulation, thus determining the threshold size of the cluster; the specific anatomical site of the brain region corresponding to the Montreal Neurological Institute coordinates was identified using the viewer of the REST software and the results were presented in the form of images. When consecutive voxels’ size was >54 and their corrected P-value was <.05, clusters were deemed statistically significant, as per the cluster threshold previously mentioned.[24].
3. Results
3.1. Changes in brain areas in each group after the end of the first treatment
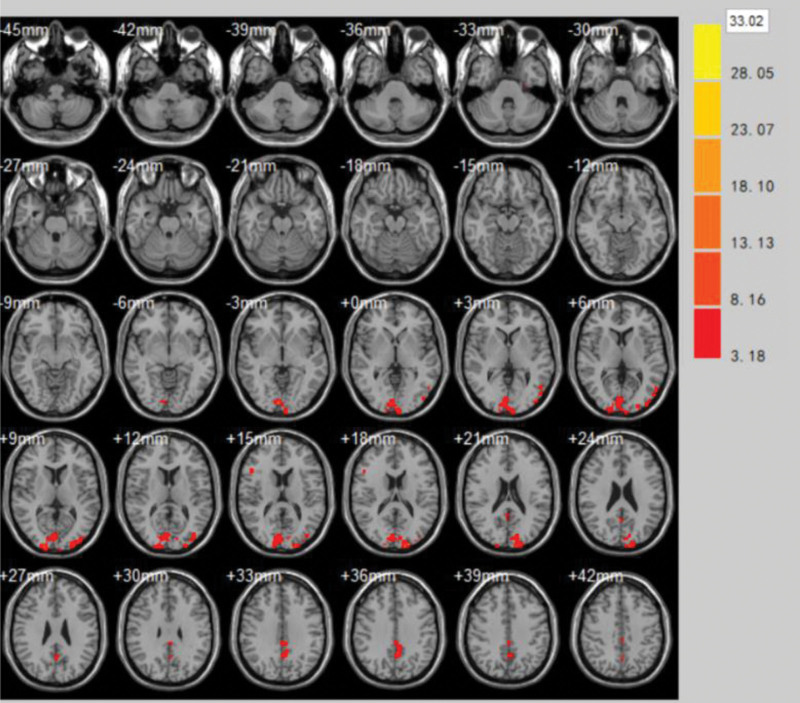
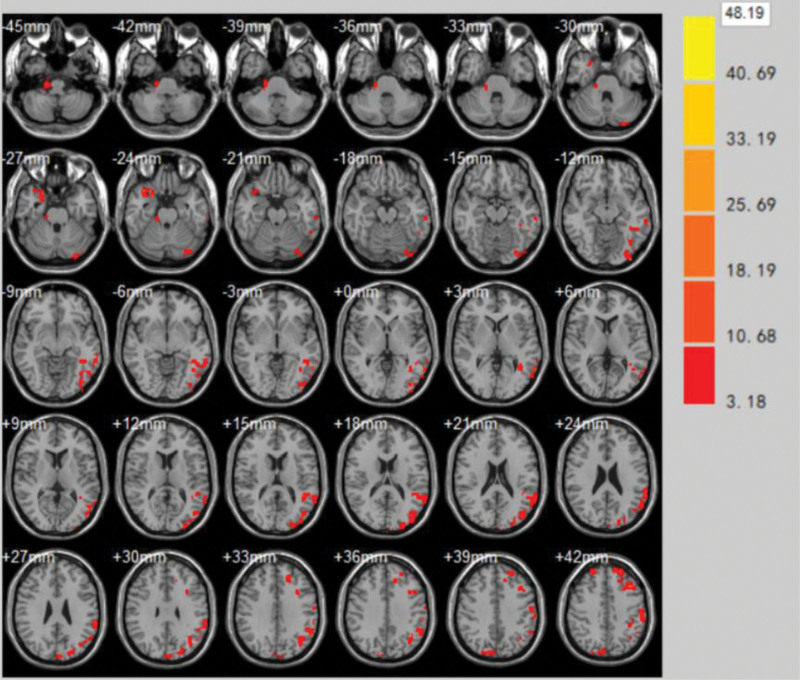
(1)The brain areas with significantly enhanced ReHo values at the end of the first treatment in the treatment group compared to the pretreatment period were Supramarginal Gyrus, Cuneus, Superior Temporal Gyrus, Middle temporal gyrus, Inferior temporal gyrus (volume of voxel cluster >54, P < .05). Table 1 demonstrates the brain areas with altered ReHo values of brain function effects immediately after the first treatment in the treatment group compared to those before treatment. Figure 4 demonstrates the brain areas with changes in ReHo values immediately after the first treatment in the treatment group compared to those before treatment.
Table 1.
Brain areas with altered ReHo values of brain function effects immediately after the first treatment in the treatment group compared to those before treatment.
| Brain zone | Brodmann subregion | MNI | Voxel cluster | T | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Supramarginal gyrus | BA40 | −45 | −74 | 7 | 75 | 1.5103 |
| Cuneus | – | −13 | −91 | 20 | 105 | 4.0221 |
| Superior temporal gyrus | BA22 | 64 | −9 | −4 | 106 | 7.9133 |
| Middle temporal gyrus | BA21 | −48 | −63 | 4 | 94 | 1.1004 |
| Inferior temporal gyrus | BA20 | −44 | −61 | −16 | 94 | 2.1531 |
BA = Brodmann area, MNI = Montreal Neurological Institute, ReHo = regional homogeneity.
Figure 4.
Brain areas with changes in ReHo values immediately after the first treatment in the treatment group compared to those before treatment. ReHo = regional homogeneity.
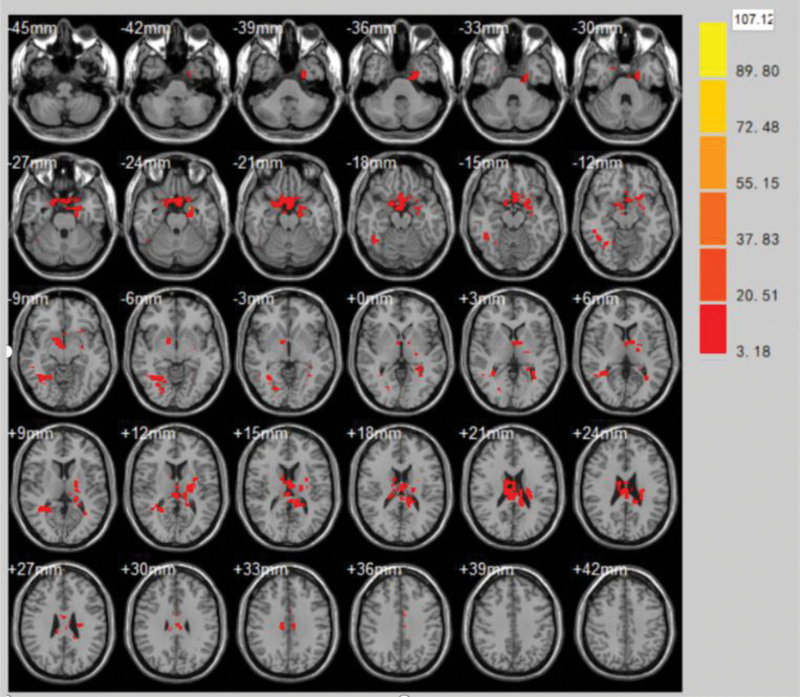
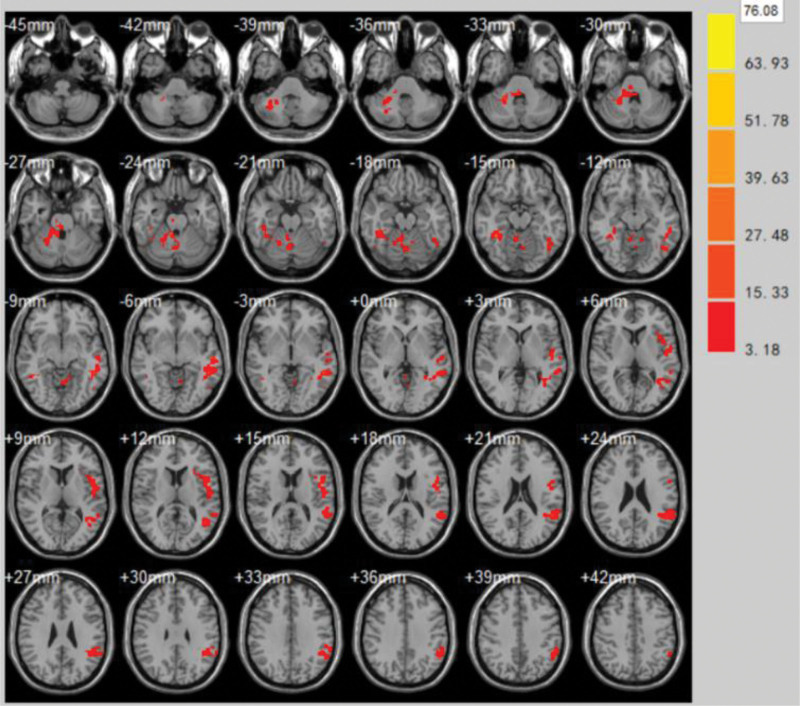
(2)The areas with significantly enhanced ReHo values at the end of the first treatment in the control group compared to the pretreatment period were Caudate nucleus, Parahippocampal Gyrus, Postcentral gyrus, Cingulate Gyrus, Parietal Lobe (volume of voxel cluster >54, P < .05). Table 2 demonstrates the brain areas with changes in ReHo values in the control group immediately after the first treatment compared to the pretreatment brain function effect. Figure 5 demonstrates the brain areas with changes in ReHo values in the control group immediately after the first treatment compared to the pretreatment brain function effect.
Table 2.
Brain areas with changes in ReHo values in the control group immediately after the first treatment compared to the pretreatment brain function effect.
| Brain zone | Brodmann subregion | MNI | Voxel cluster | T | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Caudate nucleus | – | 9 | 0 | −9 | 96 | 4.8503 |
| Parahippocampa gyrus | BA34, BA35 | −26 | −45 | 3 | 105 | 2.5541 |
| Postcentral gyrus | BA1, BA2 | 29 | −70 | 57 | 95 | 1.4526 |
| Cingulate gyrus | BA30 | −2 | −3 | 34 | 72 | 4.7441 |
| Parietal lobe | BA32 | −13 | −70 | 61 | 87 | 1.8737 |
BA = Brodmann area, MNI = Montreal Neurological Institute, ReHo = regional homogeneity.
Figure 5.
Brain areas with changes in ReHo values in the control group immediately after the first treatment compared to the pretreatment brain function effect. ReHo = regional homogeneity.
3.2. Changes in brain areas in each group at the end of the treatment course
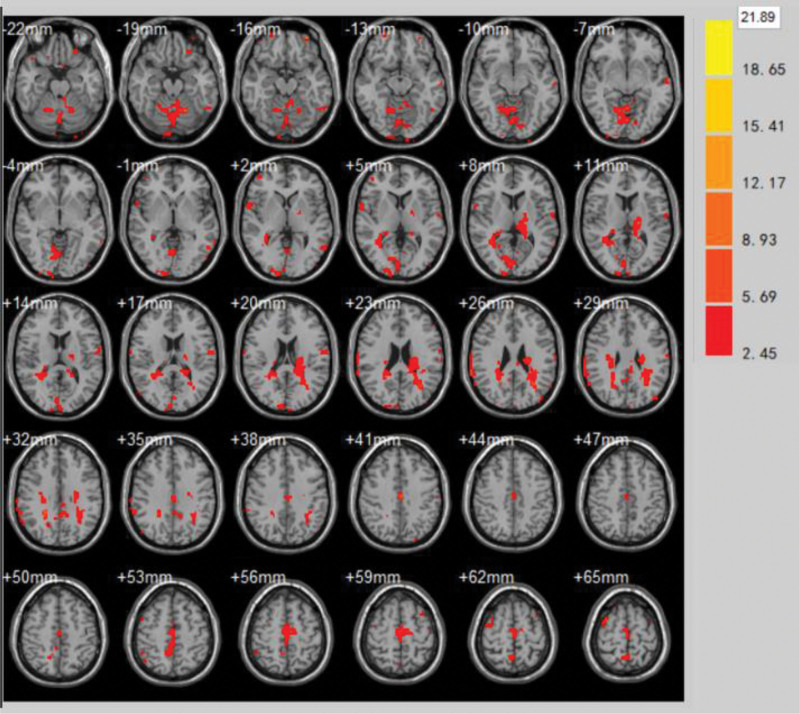
The brain areas with significantly enhanced ReHo values after the last treatment at the end of the treatment course compared to the first treatment were Superior Parietal Lobule, Superior Frontal Gyrus, Middle Frontal Gyrus, Postcentral Gyrus, Precentral Gyrus, Supramarginal Gyrus (volume of voxel cluster >54, P < .05). Table 3 demonstrates the brain areas with altered functional brain effect ReHo values at the end of the treatment course compared to the first treatment in the treatment group. Figure 6 demonstrates the brain areas with altered brain function effect ReHo values after the last treatment compared to the first treatment at the end of the treatment group.
Table 3.
Brain areas with altered functional brain effect ReHo values at the end of the treatment course compared to the first treatment in the treatment group.
| Brain zone | Brodmann subregion | MNI | Voxel cluster | T | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Superior parietal lobule | BA5, BA7 | −39 | −60 | 51 | 73 | 11.55 |
| Superior frontal gyrus | BA11 | −4 | 42 | 51 | 81 | 5.43 |
| Middle frontal gyrus | BA10 | −34 | 22 | 42 | 105 | 5.11 |
| Postcentral gyrus | BA3 | −58 | −28 | 42 | 72 | 4.31 |
| Precentral gyrus | BA4 | −59 | −14 | 39 | 102 | 10.8 |
| Supramarginal gyrus | BA40 | −32 | −44 | 54 | 98 | 5.05 |
BA = Brodmann area, MNI = Montreal Neurological Institute, ReHo = regional homogeneity.
Figure 6.
Brain areas with altered brain function effect ReHo values after the last treatment compared to the first treatment at the end of the treatment group. ReHo = regional homogeneity.
The brain areas with significantly enhanced ReHo values after the last treatment at the end of the control course compared to the first treatment were Cerebellum Anterior Lobe, Superior Temporal Gyrus, Temporal Mid Gyrus, Precentral Gyrus, Parietal Lobe (volume of voxel cluster >54, P < .05). Table 4 demonstrates the brain areas with altered brain function effect ReHo values at the end of the treatment compared to the first treatment in the control group. Figure 7 demonstrates the brain areas with altered brain function effect ReHo values at the end of the treatment compared to the first treatment in the control group.
Table 4.
Brain areas with altered brain function effect ReHo values at the end of the treatment compared to the first treatment in the control group.
| Brain zone | Brodmann subregion | MNI | Voxel cluster | T | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Cerebellum anterior lobe | – | 23 | −51 | −30 | 104 | 8.54 |
| Superior temporal gyrus | BA22 | −51 | −3 | 8 | 113 | 4.23 |
| Temporal mid gyrus | BA21 | 37 | −42 | −15 | 106 | 7.67 |
| Precentral gyrus | BA4 | −44 | −2 | 21 | 113 | 3.06 |
| Parietal lobe | BA5, BA7 | −44 | 24 | 20 | 117 | 1.37 |
BA = Brodmann area, MNI = Montreal Neurological Institute, ReHo = regional homogeneity.
Figure 7.
Brain areas with altered brain function effect ReHo values at the end of the treatment compared to the first treatment in the control group. ReHo = regional homogeneity.
The brain areas with significantly enhanced ReHo values at the end of the treatment course compared to the end of the control group were Corona radiata, Caudate nucleus, Cingulate Gyrus, Supramarginal Gyrus, Temporal Gyrus, Precentral Gyrus, Middle Frontal Gyrus (volume of voxel cluster >54, P < .05). Table 5 demonstrates the change in ReHo values of brain function effects at the end of the last treatment in the treatment group vs the last treatment in the control group.Figure 8 demonstrates the brain areas with changes in ReHo values at the end of treatment compared to the end of treatment in the control group.
Table 5.
Change in ReHo values of brain function effects at the end of the last treatment in the treatment group versus the last treatment in the control group.
| Brain xone | L/R | Brodmann subregion | MNI | Voxel cluster | T | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Corona radiata | L | – | −22 | −22 | 21 | 105 | 3.98 |
| Caudate nucleus | L | – | −30 | −40 | 26 | 112 | 2.68 |
| Cingulate gyrus | L | BA30 | −5 | −19 | 58 | 89 | 2.70 |
| Supramarginal gyrus | L | BA40 | −42 | −81 | 29 | 87 | 2.64 |
| Inferior temporal gyrus | L | BA20 | 58 | −6 | −30 | 71 | 3.34 |
| R | −52 | −6 | −32 | 89 | 2.53 | ||
| Precentral gyrus | L | BA4 | 69 | −8 | 20 | 72 | 2.72 |
| R | −60 | −10 | 20 | 119 | 3.71 | ||
| Middle frontal gyrus | R | – | 45 | 52 | 3 | 101 | 1.87 |
BA = Brodmann area, MNI = Montreal Neurological Institute, ReHo = regional homogeneity.
Figure 8.
Brain areas with changes in ReHo values at the end of treatment compared to the end of treatment in the control group. ReHo = regional homogeneity.
4. Discussion
A balanced diet and a normal, healthy lifestyle have been neglected by individuals in recent years as their standard of living has increased and their pace of life has quickened, which has led to an annual increase in the incidence of ischemic stroke that is occurring at a younger age.[25] Only 30% of patients who suffer an ischemic stroke recover fully from their motor and sensory abnormalities, with upper limb dysfunction accounting for 80% of these cases.[26] Large tertiary care general hospitals in China are currently treating this disease mostly with active rehabilitation training derived from Western medicine, which instructs patients on how to execute functional activities on the damaged limbs in order to cause dissociation motions. As an alternative, acupuncture is used at traditional Chinese hospitals, which are predominately based on traditional Chinese medicine, to passively stimulate muscles and nerves in order to ease stiffness in the afflicted limbs. Although it is frequently challenging to achieve rapid benefits with a single rehabilitation treatment, a review of prior clinical literature and relevant experimental experiments indicated that both forms of rehabilitation treatments can yield definitive results.[27–31] The formation of new central nerve limb movement conduction pathways can be facilitated by acupuncture stimulation, but this is not the same as purposeful and targeted training or proper limb movement guidance to promote normal functional patterns as the primary focus and inhibit abnormal joint muscle movement patterns, which will eventually affect the further functional rehabilitation of the affected limbs. Despite this, acupuncture alone is not a substitute for modern Western rehabilitation. This not only extends the time it takes for patients to recuperate, but it also inevitably raises the financial, social, and psychological strain on them. To support the prompt functional rehabilitation of the damaged limbs, it is crucial to judiciously choose a number of very efficient rehabilitation treatments.
4.1. Analysis of JTN
Professor Jin Rui founded JTN, a brand-new acupuncture and moxibustion academic sects. We selected the temporal 3 acupuncture points, shoulder 3 acupuncture points, and hand 3 acupuncture points from the Governor’s vein, hand-foot Yangming and Shaoyang veins, along with Renzhong and Baihui, based on the theory of traditional Chinese medicine and pertinent experimental studies.[32,33] These points can improve upper limb function, increase muscle strength, reduce muscle tension, and relieve muscle spasm. When combined with the meridian alignment and main treatment efficacy. The “scalp-cortex correspondence theory”[34] states that the “temporal three needles” side-by-side flush area exactly matches the projection area of the sensory and motor areas on the temporal ear. Through acupuncture points in the temporal region, the nerve impulses generated act on the damaged brain area, dilating the blood vessels to establish new lateral branch circulation, activating reversible nerve cells in the brain, and maintaining normal brain function compensation to improve the abnormal dysfunction of the limbs affected by an ischemic stroke.[35–37] In order to gradually restore upper limb motor function, the “three shoulder needles” have been shown to be an efficient means of releasing widespread adhesions of muscles and soft tissues, relieving fascial muscle tension, and improving shoulder joint mobility.[38,39] In order to alleviate joint edema and pain and facilitate the restoration of upper limb function, the combination of “shoulder three needles” and Bobath therapy can balance the muscle tone of the shoulder muscle groups, correct biomechanical imbalances, and lessen alterations and remodeling of the pain center neuroglia.[40] The TCM theory of “treating impotence by taking only Yangming” states that the area through which a meridian passes is the area of the acupuncture point to which the meridian belongs. By selecting the “Hand Three Needles” in conjunction with the “Shoulder Three Needles” co-treatment, 1 can unblock the blood and qi in the upper limbs of the hand 3 Yang meridians, relieve constriction in the muscle bundle, coordinate and balance the tone of the muscles in the active and antagonistic muscles, make it easier to transition from co-movement to separate movement, and alleviate pain, sensory disturbance, and other symptoms. In addition to increasing cerebral blood flow, dilates microvasculature, shrinks infarct lesions, and reduces nerve phagocytosis, Baihui with Renzhong acupuncture point also promotes the activation of glial cells in the brain and thereby protects neurons,[41] inhibits the expression of pro-apoptotic factor Bax, and promotes the expression of apoptotic factor bcl-2 in the semidark zone, preventing further spread of ischemic infarct area to ischemic semidark areas.[42–44]
4.2. Analysis of MT
According to current opinion, 1 important mechanism for the therapeutic effects of MT may be the brain’s mirror neurons.[45] Reactivating the motor program in the damaged brain area is necessary for patients with ischemic stroke limb dysfunction who wish to regain motor function. This process depends on the human brain’s plasticity, and contemporary rehabilitation training can successfully encourage the reconstruction of functional loops in the damaged brain area.[46] By stimulating the neurotransmission pathway, the visual illusion created by MT can prevent or lessen the occurrence of “learned disuse” of the paralyzed limb. Additionally, because the center is partially plastic and visual feedback can alter the cortical electrical activity of the central sensory and motor areas, it can functionally reorganize to compensate for the motor deficit. As a result, there is a solid foundation for depending on visual feedback to meet the goals of rehabilitation therapy. A strong connection is established between movement imitation and motor relearning by supplying visual perception by imitation of visual cues and then creating mirror visual feedback.[47] Research has demonstrated that MT can improve a patient’s prospective motor function by causing functional remodeling in the brain’s motor cortex regions (M1).[48–50] Oouchida et al[51] discovered that the mirror neuron mechanism, elevated excitability of the corticospinal tract, and the consequent easing effect all contributed to the rehabilitation of motor function in the afflicted limb during limb motions observed in the mirror. By bilaterally moving the patient’s limbs, MT can also create stability in the formation of motor movements in space and time. When the limbs move in the same way on both sides of the brain, similar functional response areas are triggered, which can lessen intercortical inhibition in both hemispheres.[52] It is thus evident that MT receives the visually perceived motor image of the healthy limb, processes it through mirror neurons, and then activates the motor system with visual feedback, improving the patient’s limb motor function. This process results in functional remodeling in the injured cerebral motor cortex.
The afflicted hemiplegic arm was able to break free from “learned disuse” and the patient’s total motor function was improved by using JTN in conjunction with MT to activate relevant sensory areas in the brain through visual feedback. Thus, it is essential and beneficial for MT to concentrate on the induction of the 3 mechanisms of central nervous system plasticity building, visual feedback simulation, and mirror neuron activation for patients with ischemic stroke hemiplegia.
4.3. Significance of ReHo analysis
ReHo, one of the Rs-fMRI study methods, is a resting-state functional data analysis method that computes the correlation coefficient between a voxel and multiple adjacent voxel time series, and then assigns the correlation coefficient to each voxel, indicating the local functional intensity of a brain region.[53] Whereas a decrease in ReHo suggests that local neuronal activity is more disordered, an increase in ReHo implies that local brain areas’ neuronal activity tends to be synchronized under the same time sequence.[54] Consequently, the through-complementarity and coordination mechanism of neuronal activity in local brain regions are reflected by the ReHo value rather than the signal strength. In order to evaluate the clinical efficacy of the treatment, we compared the ReHo values before and after the intervention. This allowed us to gather important data regarding the changes in neuronal activity in the localized brain regions affected by an ischemic stroke.
The study’s findings demonstrated that there were significant differences in the BOLD signal values of the entire brain in the resting state between the treatment group and the control group following the first treatment, before and after the final treatment itself at the end of the course of treatment, and when comparing the final treatment between the 2 groups. Additionally, the increase and decrease of ReHo values were not restricted to any 1 area of the brain but were instead widely distributed throughout several brain regions, suggesting that the combined use of the 2 rehabilitation therapies could improve cerebral ischemia site hemodynamic information and enhance cerebral circulatory metabolism. At the conclusion of the treatment cycle, the treatment group’s left hemisphere showed a significant increase in activation in the BA3, BA4, BA5, BA7, BA10, BA11, and BA40 areas when compared to the pretreatment period. When comparing the pretreatment period to the end of the treatment, the control group’s activation in the left hemisphere’s BA4, BA5, BA7, BA21, and BA22 areas was considerably higher. (3) The L radial crown, L caudate nucleus, L cingulate gyrus (BA30), L supramarginal gyrus (BA40), L/R inferior temporal gyrus (BA20), L/R precentral gyrus (BA4), and R middle frontal gyrus showed significantly higher ReHo values in the treatment group at the conclusion of the last treatment session compared to the control group. This suggests that the aforementioned brain regions were significantly activated following treatment. Owing to the lesions in all of the cases included in this analysis being located in the regions of the left radial crown and basal ganglia (caudate nucleus), following an ischemic stroke, the motor function-related basal ganglia regions sustain damage, and the cerebral motor cortex rebuilds a robust motor network that is involved in the compensatory role of movement in patients suffering from ischemic stroke. There is a considerable initiation at the left lesion site compared to the low signal prior to treatment, and this is further supported by the significant initiation of ReHo signal in these regions. Following therapy, basal ganglia ischemic-hypoxic function is improved. Furthermore, in line with the goal of this study, the left brain stimulates a larger spectrum of brain regions than the right brain. At the conclusion of each session and prior to treatment, the before-and-after comparison between the treatment and control groups demonstrated that Jin Sanzhen in conjunction with MT not only targeted the affected limb’s motor function area for activation, but also improved the affected upper limb’s imitation motor learning ability by mobilizing the patient’s auditory-verbal comprehension-executive cognitive information (BA4, BA10, BA11, BA21, BA22), perception-behavior recognition-memory (BA3, BA20, BA30, BA40), and visual-motor somatosensory information (BA5, BA7), as well as deepening the affected upper limb’s recovery of voluntary motor function.
Furthermore, the development of ischemic stroke is not restricted to a rise in the functional impairment of the affected limb; it is also correlated with a steadily worsening cognitive dysfunction. The majority of patients with ischemic stroke already have a long-term blood pressure disorder, and elevated blood pressure is a major contributing factor to ischemic stroke. Hypertensive patients’ brain morphology and cognitive function are already somewhat impaired prior to ischemic stroke, which can lead to depression or anxiety affective disorder later in the course of the disease.[55,56] Activating BA11 and BA30 can modulate mood and affective cognition, thereby reducing the effects of depression or anxiety affective disorder resulting from the progression of the disease.
There are still several issues with the study design. Functional MRI data acquisition studies are limited by the relatively small number of cases included; secondly, the short observation period may cause clinical efficacy to not be fully revealed through functional brain activation images and deviations from the anticipated image processing results; thirdly, patients in the mirror-only rehabilitation therapy group were excluded from the experimental design, which is a unique factor that exists in the real world; this control group was not set up for the time being in order to avoid delaying the patients’ condition and misunderstanding along with the conditions of the institution conducting the clinical trial. After that, in order to understand the mechanism of action of the combination of Jin San Acupuncture and mirror treatment, we must monitor long-term Jin San Acupuncture combined with MT in patients with upper limb dysfunction following an ischemic stroke and increase the sample size.
5. Conclusion
To sum up, we employed Jin Sanzhen plus MT to treat patients with upper limb dysfunction following an ischemic stroke. We also used the ReHo method to analyze Rs-fMRI data in order to compare the brain functional areas of combined rehabilitation therapy and single rehabilitation therapy. Our findings support the notion that, for patients with limited treatment time, Jin Sanzhen plus MT is an effective combination that can better activate the relevant functional areas of motor, somatosensory, visual, auditory, spatial orientation, emotional processing, and cognition.
Acknowledgments
The authors are very grateful to the medical workers and volunteers at the Shenzhen Hospital of Guangzhou University of Traditional Chinese Medicine and the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences for providing guidance on the acquisition and data processing of functional MRI.
Author contributions
Conceptualization: Yunqiu Yang, Qingmao Hu, Xiaojing Long, Shaoyang Cui, Mingzhu Xu, Chunzhi Tang, Chen Yang.
Data curation: Yunqiu Yang, Qingmao Hu, Shaoyang Cui, Chunzhi Tang.
Methodology: Zhen Wang, Qingmao Hu, Xiaojing Long, Shaoyang Cui, Mingzhu Xu, Chunzhi Tang.
Project administration: Guorui Ma.
Software: Qingmao Hu, Xiaojing Long.
Supervision: Yunqiu Yang, Chen Yang.
Validation: Yunqiu Yang.
Visualization: Yunqiu Yang.
Writing – original draft: Yunqiu Yang.
Writing – review & editing: Yunqiu Yang, Zhen Wang, Qingmao Hu, Xiaojing Long, Shaoyang Cui, Chunzhi Tang.
Abbreviations:
- fMRI
- functional magnetic resonance imaging
- JTN
- Jin’s three needle
- MT
- mirror therapy
- ReHo
- regional homogeneity
- T1WI
- T1-weighted images.
This study was supported by grants from the Guangdong Provincial Bureau of Traditional Chinese Medicine Scientific Research Project (No. 20161215); Shenzhen Science and Technology Innovation Commission Science and Technology Program (No. JCYJ20160425151844396); Shenzhen Futian District Health Public Welfare Scientific Research Project (No. FTWS20160034).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Yang Y, Wang Z, Hu Q, Long X, Ma G, Cui S, Xu M, Tang C, Yang C. The short-term effects of Jin’s three needles in conjunction with mirror therapy on brain function in patients with upper limb disability following an ischemic stroke were evaluated using ReHo analysis. Medicine 2024;103:27(e38707).
QH and SC contributed equally to this work.
Contributor Information
Yunqiu Yang, Email: 55245825@qq.com.
Zhen Wang, Email: wangzhen@ziat.com.
Qingmao Hu, Email: huqingmao@siat.com.
Xiaojing Long, Email: longxiaojing@siat.ac.com.
Guorui Ma, Email: maguorui@ziat.com.
Mingzhu Xu, Email: xumingzhu@126.com.
Chen Yang, Email: 7425914@qq.com.
References
- [1].National Health and Family Planning Commission Prevention and Control Engineering Committee. China Stroke Epidemic Report 2015[M]. Beijing: China Union Medical University Press. 2015: 17–18. [Google Scholar]
- [2].Yang Q. Neurology[M]. Beijing:People’s Health Publishing House. 2002:118. [Google Scholar]
- [3].Stig Jorgense H, Nakayama H, Otto Raaschou H, Møller Pedersen P, Houth J, Skyhøj Olsen T. Functional and neurological outcome of stroke and the relation to stroke severity and type, stroke unit treatment, body temperature, age, and other risk factors: the copenhagen stroke study. Top Stroke Rehabil. 2000;6:1–19. [Google Scholar]
- [4].Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke. 2009;40:2123–8. [DOI] [PubMed] [Google Scholar]
- [5].Xu SF, Zhuang LX, Jia C, et al. Effect of “Jin three-needle therapy” on cognitive function and activity of daily living in patients of hemiplegia after stroke: a multi-central randomized controlled study]. Zhongguo Zhen Jiu. 2009;29:689–94. Chinese [PubMed] [Google Scholar]
- [6].Sun K, Shen W, Han DX, et al. Influence of Jinsanzhen therapy on the motor function and activities of daily life of the apoplectic hemiplegia patients. J Tradit Chin Med. 2010;51:524–7. [Google Scholar]
- [7].Lang JY, Zhuang LX, Jia C, et al. Therapeutic effect of Jin’s three-needle therapy combined with rehabilitation therapy for spastic hemiplegia after ischemic stroke. J Guangzhou Univ Tradit Chin Med. 2011;28:369–73. [Google Scholar]
- [8].Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature. 1995;377:489–90. [DOI] [PubMed] [Google Scholar]
- [9].Altschuler EL. Motor rehabilitation in a stroke patient using a mirror. Soc Neurosci. 1998;24:1408. [Google Scholar]
- [10].Shan Y, Wang Z, Zhao Z, et al. An FMRI study of neuronal specificity in acupuncture: the multiacupoint siguan and its sham point. Evid Based Complement Alternat Med. 2014;2014:103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kong S, Tan Q, Liu Y, et al. Specific correlation between the Hegu point (LI4) and the orofacial part: evidence from an fMRI study. Evid Based Complement Alternat Med. 2015;2015:585493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chinese Journal of Neurology. 2018;51:666–82. [Google Scholar]
- [13].State Administration of Traditional Chinese Medicine Emergency Brain Disease Research Collaborative Group 1996. Stroke diagnosis and efficacy assessment criteria. J Beijing Univ TCM. 1996;19:55. [Google Scholar]
- [14].The Fourth National Academic Conference on Cerebrovascular Diseases. Clinical neurological deficit scoring criteria for stroke patients(1995). Chin J Neurol. 1996;29:381–3. [Google Scholar]
- [15].Zhou WJ, Sun QL. Paralysis Rehabilitation Assessment Manual[M].Beijing:People’s Health Publishing House,2006:36–37. [Google Scholar]
- [16].Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: a reliability and construct validity study. J Neurol Phys Ther. 2007;31:20–9. [DOI] [PubMed] [Google Scholar]
- [17].Li JA, Huang XL. Rehabilitation Medicine[M]. Beijing: People’s Health Publishing House. 2016;5:95 [Google Scholar]
- [18].Chinese Medical Association, Neurology Branch, Cerebrovascular Disease Group of the Neurological Branch of the Chinese Medical Association. Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2014. Chin J Neurol. 2015;48:246–57. [Google Scholar]
- [19].Zhang DS, Wang SX, Huang Y, Chen XH. Jin’s Three Needle Shorthand Manual[M]. Beijing:Chemical Industry Press.2012:3–10. [Google Scholar]
- [20].Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. [DOI] [PubMed] [Google Scholar]
- [22].Tian L, Ren J, Zang Y. Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage. 2012;60:539–44. [DOI] [PubMed] [Google Scholar]
- [23].Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang YQ, Tang CZ, Cui SY, et al. Effects of JIN’s three-needle combined with mirror therapy on brain function of patients with stroke and upper limb dysfunction. J Tradit Chin Med. 2019;60:675–9. [Google Scholar]
- [25].Sun XQ, Liu XM, Liu ZY, et al. Analysis of population status and stroke survey (stroke screening population prevalence analysis). Med Lab Sci Clinics. 2013;24:59–66. [Google Scholar]
- [26].Tang CZ, Jia J. Advances in the application of occupational therapy for post-stroke hand dysfunction. Chin J Rehabilit Med. 2014;29:1191–5. [Google Scholar]
- [27].Jiang JP, Liu HB, Wang ZS. Clinical analysis of mirror therapy combined with rehabilitation training on stroke patients upper limb motor function. China Med Equip. 2015;12:107–9. [Google Scholar]
- [28].Wang HY, Zhao ZJ, Jiang P, et al. Study on the rehabilitation effect of mirror therapy on lower limb motor dysfunction in stroke patients with hemiplegia. Guizhou Med. 2017;41:603–4. [Google Scholar]
- [29].Liu YY, Meng DY, Zhou Y. Effect of head acupuncture combined with knee joint controlling exercise on walking ability in paints with hemiplegia after cerebral stroke. Modern J Integrat Tradit Chin Western Med. 2016;25:3788–91. [Google Scholar]
- [30].Cui SY, Xu MZ, Wang SH, et al. Effect of acupuncture plus mirror therapy on lower-limb dysfunction in hemiplegia after cerebral infarction. Shanghai J Acup Moxib. 2017;36:9–13. [Google Scholar]
- [31].Yang YQ, Tang CZ, Cui SY, et al. Current status of clinical application of acupuncture combined with mirror therapy in stroke treatment. Lishizhen Med Materia Medica Research. 2018;29:2737–9. [Google Scholar]
- [32].Kong Y, Xu F, Lin X, et al. Effects of the lifting manipulation of scalp acupuncture for raising myodynamia of the affected limbs in hemiplegic patients due to cerebral thrombosis. J Tradit Chin Med. 2005;25:256–9. [PubMed] [Google Scholar]
- [33].Zhao JG, Cao CH, Liu CZ, et al. Effect of acupuncture treatment on spastic states of stroke patients. J Neurol Sci. 2009;276:143–7. [DOI] [PubMed] [Google Scholar]
- [34].Yu ZS. Basic and Clinical Head Acupuncture[M].Beijing:China Medical Science and Technology Press,1992: 64. [Google Scholar]
- [35].Fang Y, Li J, Yang C. Effect of Jin three needles on spasticity and walking ability of patients with post-stroke spastic hemiplegia. J Clin Acupunct Moxibustion. 2019;35(:19–22. [Google Scholar]
- [36].Wang XL, Chen HZ, Zhan QF, et al. 42 cases of spastic hemiplegia after ischemic stroke treated with Jin San acupuncture therapy. Chin J Ethnomed Ethnopharm. 2016;25:112–3. [Google Scholar]
- [37].He J, Zhuang LX, Lin H. A multifactorial analysis study of Jin’s three needle therapy for post-stroke spastic paralysis. J Clin Acupunct Moxibust. 2016;32:6–9. [Google Scholar]
- [38].Zhang JW. Discussion on the correspondence between the three shoulder needles and the treatment of frozen shoulder. J Clin Acupunct Moxibust. 2004;20:7–8. [Google Scholar]
- [39].Yu C, Shen B, Xu SW, et al. 98 cases of spastic paralysis of upper limbs after stroke treated with minimally invasive trans-acupuncture point embedding. Global Tradit Chin Med. 2015;8:222–5. [Google Scholar]
- [40].Shao YL, Tang TC, Zhang P, et al. The efficacy of shoulder triple acupuncture combined with Bobath therapy in the treatment of post-stroke shoulder-hand syndrome. Zhejiang J Integrat Tradit Chin Western Med. 2017;27:584–6. [Google Scholar]
- [41].Luo MJ, Chen L, Xu L, et al. Activation of microglia in the brain of ischemia-reperfused rats after electroacupuncture stimulation of Shuigou and Baihui acupoints. Chin J Tissue Eng Res. 2006;10:180–2. [Google Scholar]
- [42].Li YJ, Fan XN, Wang S, et al. Study on the parameter optimization of acupuncture at Shuigou (GV 26) for the treatment of brain infarction. J Tradit Chin Med. 2009;50:428–31. [Google Scholar]
- [43].Li YJ, Fan XN, Wang S, et al. Influences on MCAO model of rats triggered by applying different acupuncture parameters on shuigou point. Liaoning J Tradit Chin Med. 2012;39:1165–8. [Google Scholar]
- [44].Yu J, Zhang W, Guo WJ, et al. Interventional effect of electro-acupuncture at Renzhong acupoint on neuronal apoptosis and expressions of related genes in rats with middle cerebral artery occlusion. Chin J Clin Rehabilit. 2006;10:90–2. [Google Scholar]
- [45].Sale P, Franceschini M. Action observation and mirror neuron network: a tool for motor stroke rehabilitation. Eur J Phys Rehabil Med. 2012;48:313–8. [PubMed] [Google Scholar]
- [46].Thrane G, Friborg O, Anke A, Indredavik B. A meta-analysis of constraint-induced movement therapy after stroke. J Rehabil Med. 2014;46:833–42. [DOI] [PubMed] [Google Scholar]
- [47].Cacchio A, De Blasis E, De Blasis V, Santilli V, Spacca G. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair. 2009;23:792–9. [DOI] [PubMed] [Google Scholar]
- [48].Wang LJ, Chen LZ, Ou Y, et al. Effects of mirror visual feedback and electromyographic biofeedback on upper extremity function in hemiplegics after stroke. Chin J Rehabilit Theory Pract. 2015;21:202–6. [Google Scholar]
- [49].Nojima I, Mima T, Koganemaru S, Thabit MN, Fukuyama H, Kawamata T. Human motor plasticity induced by mirror visual feedback. J Neurosci. 2012;32:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garrison KA, Winstein CJ, Aziz-Zadeh L. The mirror neuron system: a neural substrate for methods in stroke rehabilitation. Neurorehabil Neural Repair. 2010;24:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Oouchida Y, Izumi S. The mirror neuron system in motor and sensory rehabilitation. Brain Nerve. 2014;66:655–63. Japanese. [PubMed] [Google Scholar]
- [52].Liang TJ, Wu XP, Mo MY. Effect of upper limb rehabilitation robot training on upper limb dexterity recovery in patients with migraine. Chin J Rehabilit Med. 2012;27:254–6. [Google Scholar]
- [53].Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. [DOI] [PubMed] [Google Scholar]
- [54].Liao YH, Tang JS, Wang XY, et al. Regional Homogeneity In Ketamine dependence:a resting-state FMRI study. Chin J Drug Dependence. 2010;19:343–8. [Google Scholar]
- [55].Iulita MF, Girouard H. Treating hypertension to prevent cognitive decline and dementia: re-opening the debate. Adv Exp Med Biol. 2017;956:447–73. [DOI] [PubMed] [Google Scholar]
- [56].Tadic M, Cuspidi C, Hering D. Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc Disord. 2016;16:208. [DOI] [PMC free article] [PubMed] [Google Scholar]