Abstract
Background
Patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) are usually asymptomatic and seek treatments that improve survival but have a low risk of adverse events. Darolutamide, a structurally distinct androgen receptor inhibitor (ARi), significantly reduced the risk of metastasis and death versus placebo in ARAMIS. We assessed the extended safety and tolerability of darolutamide and the time-course profile of treatment-emergent adverse events (TEAEs) related to ARis and androgen-suppressive treatment.
Patients and Methods
Patients with nmCRPC were randomized 2:1 to darolutamide (n = 955) or placebo (n = 554). After trial unblinding, patients could receive open-label darolutamide. Tolerability and TEAEs were assessed every 16 weeks. Time interval–specific new and cumulative event rates were determined during the first 24 months of the double-blind period.
Results
Darolutamide remained well tolerated during the double-blind and open-label periods, with 98.8% of patients receiving the full planned dose. The incidence of TEAEs of interest in the darolutamide group was low and ≤2% different from that in the placebo group, except for fatigue. When incidences were adjusted for exposure time, there were minimal differences between the darolutamide double-blind and double-blind plus open-label periods. The rate of initial onset and cumulative incidence of grade 3/4 TEAEs and serious TEAEs were similar for darolutamide and placebo groups over 24 months.
Conclusion
Extended treatment with darolutamide was well tolerated and no new safety signals were observed. Most ARi-associated and androgen-suppressive treatment–related TEAEs occurred at low incidences with darolutamide, were similar to placebo, and showed minimal increase over time with continued treatment.
Trial number
ClinicalTrials.gov identifier NCT02200614
Keywords: darolutamide, nonmetastatic castration-resistant prostate cancer, safety, tolerability, adverse events, androgen receptor inhibitor
This report examines darolutamide tolerability from extended follow-up for both the double-blind and open-label treatment periods of the phase III ARAMIS trial and characterizes the onset and occurrence over time of treatment-emergent adverse events of interest, including those commonly associated with androgen receptor inhibitor therapy and androgen-suppressive treatment.
Implications for Practice.
Results of this extended safety and tolerability analysis from the phase III ARAMIS trial demonstrate that darolutamide was consistently well tolerated during the double-blind and open-label treatment periods. Discontinuations due to treatment-emergent adverse events (TEAEs) were similar in the darolutamide and placebo groups during the double-blind period. Cumulative incidences of most TEAEs commonly associated with androgen receptor inhibitors and androgen-suppressive treatment were low and similar for darolutamide and placebo. When adjusted for longer exposure with darolutamide, incidences of TEAEs showed minimal difference versus placebo. These findings can assist in treatment selection discussions between patients and physicians.
Introduction
Patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) are often free of cancer-related symptoms but may receive prolonged treatment with an approved androgen receptor inhibitor (ARi).1-3 Therefore, it is important that ARi treatment for nmCRPC not only improves survival but also has a low risk of adverse events and drug-drug interactions to avoid changes to patients’ lifestyle. Second-generation ARis (darolutamide, apalutamide, and enzalutamide) offer an extended survival benefit for patients with nmCRPC, but differences exist in their adverse event profiles that may impact patients’ daily activities.1,4-7 Adverse events commonly associated with ARi therapy include fatigue, falls, fractures, rash, mental impairment, and hypertension. These adverse events may reduce treatment compliance and may require dose modifications that have the potential to negatively impact treatment efficacy.3-5 In addition, androgen-deprivation therapy (ADT), the standard of care for nmCRPC and used in combination with ARi therapy, is associated with adverse events such as hot flushes, anemia, gynecomastia, erectile dysfunction, cardiac disorders, diabetes, weight gain, and dyslipidemia.1,3
Darolutamide is a structurally distinct and highly potent ARi that significantly improved metastasis-free survival (MFS) by approximately 2 years (median, 40.4 vs. 18.4 months; hazard ratio [HR], 0.41; 95% CI, 0.34-0.50; P < .001) and reduced the risk of death by 31% (HR, 0.69; 95% CI, 0.53-0.88; P = .003) versus placebo in patients with nmCRPC in the phase III ARAMIS study.4,5,8 Patients receiving darolutamide had a significant delay in time to deterioration in health-related quality of life compared with patients receiving placebo, particularly related to urinary and bowel symptoms.9 In addition, darolutamide was associated with fewer prostate cancer–related locally invasive procedures and with similar incidences of urinary and bowel treatment-emergent adverse events (TEAEs) compared with placebo.10 A preplanned subgroup analysis of ARAMIS found similar benefit of darolutamide among patients with a prostate-specific antigen (PSA) doubling time (PSADT) of ≤6 months and those with a PSADT of 6-10 months, with reduced risks of metastasis or death versus placebo of 59% and 62%, respectively.11 In the phase III ARASENS study of patients with metastatic hormone-sensitive prostate cancer (mHSPC), darolutamide in combination with ADT and docetaxel significantly reduced the risk of death by 32.5% compared with placebo, ADT, and docetaxel.12
Darolutamide has demonstrated a consistently favorable safety profile in patients with nmCRPC, mHSPC, and metastatic CRPC from early-phase studies.4,5,12-15 In the ARAMIS study that included patients with nmCRPC, the incidence of most TEAEs commonly associated with ARi therapy showed ≤2% difference between darolutamide and placebo, except for fatigue (13.2% vs. 8.3%).4,5 The similarities in incidences of TEAEs, especially those related to the central nervous system, may be the result of low blood-brain barrier penetration of darolutamide.8,16,17 Darolutamide also has a low potential for clinically relevant drug-drug interactions.18
This report examines darolutamide tolerability from extended follow-up for both the double-blind and open-label treatment periods of ARAMIS and characterizes the onset and occurrence over time of TEAEs of interest, including those commonly associated with ARi therapy and androgen-suppressive treatment.
Patients and Methods
Study Design
ARAMIS was a prospective, randomized, double-blind, global, phase III study (NCT02200614) of darolutamide versus placebo plus ADT in patients with nmCRPC. The study methodology has been previously reported.4,5 Key inclusion criteria required patients to be aged 18 years or older and have histologically or cytologically confirmed prostate adenocarcinoma with a baseline PSA level ≥2 ng/mL, PSADT ≤10 months, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were excluded if they had a history of metastatic disease or evidence of distant metastases on conventional imaging. The study protocol was approved by the institutional review board at each participating site prior to study initiation. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice, and all patients provided written informed consent to participate.
Patients meeting eligibility criteria were randomly assigned 2:1 to oral darolutamide 600 mg twice daily or matched placebo, while continuing ADT. Randomization was stratified by PSADT (≤6 months or >6 months) and use of osteoclast-targeted therapy (yes or no). Patients continued treatment until protocol-defined progression, intolerable TEAEs, or withdrawal of consent.
Study Analyses and Safety Assessments
The primary analysis of MFS was performed using the data cutoff date of September 3, 2018, after a median follow-up of 17.9 months. Unblinding occurred on November 30, 2018, at which time patients receiving darolutamide could continue to receive the drug during an extended open-label period. The final analysis of overall survival was conducted using the data cutoff date of November 15, 2019, after a median follow-up of 29.0 months. The occurrence or worsening of TEAEs and laboratory safety assessments were obtained at every scheduled study visit (days 1, 15, and 29; at 16 weeks; and every 16 weeks thereafter). The severity of TEAEs was determined according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03, and the seriousness and relatedness of TEAEs to study treatment were assessed by the investigator at each visit. TEAEs of interest were defined as those commonly associated with ARi therapy (fatigue, falls, fractures, rash, mental impairment, and hypertension) and other hormone treatment–related adverse events (hot flushes, gynecomastia, erectile dysfunction, anemia, memory impairment, cardiac disorders, diabetes, weight gain, and dyslipidemia). Falls included Medical Dictionary for Regulatory Activities (MedDRA) version 20.0 preferred terms of fall and accident, and fractures combined the following terms: any fractures and dislocations, limb fractures and dislocations, pelvic fractures and dislocations, skull fractures, facial bone fractures and dislocations, spinal fractures and dislocations, and thoracic cage fractures and dislocations. Rash was defined as MedDRA labeling group (MLG) terms of rash and skin erythema and the preferred term of dermatitis. Hot flushes included MLG terms of vasodilation and flushing. Mental impairment, coronary artery disorders, and heart failure were defined by MedDRA high-level group terms.
Statistical Analyses
Descriptive analyses were performed for safety data. To adjust for differences in the study treatment duration between treatment groups, exposure-adjusted incidence rates (EAIRs) were calculated for TEAEs of special interest as the total number of patients with a given event divided by the total treatment duration in years, expressed per 100 patient-years. Cumulative incidences of TEAEs of interest were analyzed using Kaplan-Meier estimates during the double-blind period. The observation period was truncated at 24 months to ensure that at least 10% of the population was at risk for adverse events in each treatment group. During the double-blind period, time interval–specific new event rates of TEAEs of interest occurring in >2% of patients were determined for the time period between consecutive study visits.
Results
The ARAMIS study included 955 patients randomized to darolutamide and 554 patients randomized to placebo. One patient did not receive darolutamide and was not evaluated for safety. As previously reported, treatment groups were balanced for baseline demographic and clinical characteristics.4
During the double-blind period, the median (range) duration of treatment was longer in patients randomized to darolutamide (18.5 [0-48] months) compared with those receiving placebo (11.6 [0-45] months). Patients who continued to receive darolutamide in the open-label period had an additional median 7.3 months of treatment, with a total median (range) duration of 25.8 (0-59) months during the double-blind plus open-label periods. Among patients randomized to placebo who crossed over to darolutamide during the open-label period, the median (range) duration of darolutamide treatment was 11.0 (1-12) months. Nearly half (48.8%) of patients in the darolutamide double-blind plus open-label periods were still receiving darolutamide at the final cutoff date (November 15, 2019).
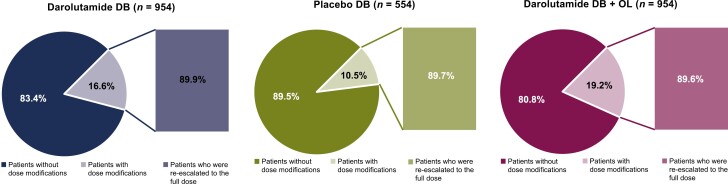
Almost all patients were able to receive the full planned dose of darolutamide during the double-blind and double-blind plus open-label periods (98.8%). At least one dose modification (interruption, delay, or reduction) was required for 16.6% of patients receiving darolutamide in the double-blind period, 10.5% of patients receiving placebo in the double-blind period, and 19.2% of patients receiving darolutamide in the double-blind plus open-label periods (Fig. 1). TEAEs were reported as the most common primary reason (darolutamide double-blind, 81.9%; placebo double-blind, 72.1%) for a dose modification event. Across all treatment groups, 90% of patients who had dose modifications were re-escalated to the full dose of darolutamide or placebo equivalent.
Figure 1.
Dose modifications and re-escalation to full dose of darolutamide or placebo equivalent. The safety population excluded one patient in the darolutamide group who did not receive any dose of study drug. Abbreviations: DB: double-blind; OL: open-label.
Darolutamide was consistently well tolerated during the double-blind and open-label treatment periods. A small increase in the incidence of any-grade, serious, and grade 3/4 TEAEs was observed between the darolutamide double-blind period and the extended treatment in the double-blind plus open-label periods (Table 1). Incidences of these TEAEs were lower in the placebo double-blind group. Discontinuations due to TEAEs remained comparable between darolutamide and placebo groups in the double-blind period (8.9% vs. 8.7%) and increased only slightly during the extended open-label period of darolutamide treatment (10.5%). The most common drug-related TEAEs that led to discontinuation of darolutamide were laboratory changes (increased transaminases, increased blood creatinine, and decreased neutrophil count; n = 5), gastrointestinal events (n = 3), and vascular events (n = 3), including deep vein thrombosis, hypotension, and peripheral ischemia (Supplementary Table S1). Discontinuations associated with drug-related central nervous system TEAEs occurred in one patient in the darolutamide group and 3 patients in the placebo group during the double-blind period. During the additional 7.3 months of darolutamide treatment in the open-label period, one additional patient had a drug-related TEAE (myalgia) that led to treatment discontinuation. Discontinuations due to disease progression were lower in the darolutamide group versus the placebo group during the double-blind period (12.5% vs. 25.3%) and remained similar (12.6%) with extended darolutamide treatment in the double-blind and open-label periods.
Table 1.
TEAEs during the double-blind and open-label periods.
| TEAEs, n (%) | Darolutamide DB (n = 954) | Placebo DB (n = 554) | Darolutamide DB + OL (n = 954) |
|---|---|---|---|
| Any TEAE | 818 (85.7) | 439 (79.2) | 857 (89.8) |
| Serious TEAE | 249 (26.1) | 121 (21.8) | 306 (32.1) |
| Grade: 3 or 4a | 251 (26.3) | 120 (21.7) | 303 (31.8) |
| TEAE leading to permanent discontinuation of study drug | 85 (8.9) | 48 (8.7) | 100 (10.5) |
aTEAEs were assessed according to National Cancer Institute CTCAE version 4.03 and reported for the worst grade.
Abbreviations: CTCAE: Common Terminology Criteria for Adverse Events; DB: double-blind; OL: open-label; TEAE: treatment-emergent adverse event.
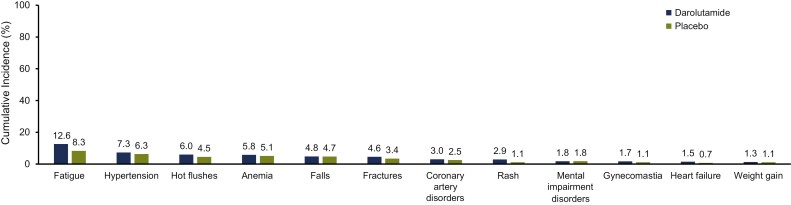
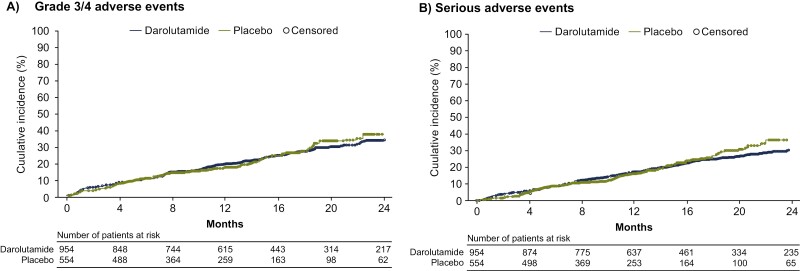
During the first 24 months of the double-blind period, cumulative incidences of most ARi-associated and androgen-suppressive treatment–related TEAEs were low and similar for the darolutamide and placebo groups (Fig. 2). Fatigue was the only TEAE with an incidence >10% in the darolutamide group at 24 months (12.6% vs. 8.3% in the placebo group). The other central nervous system–related adverse event commonly associated with hormone treatment, memory impairment, had a lower cumulative incidence in the darolutamide group versus the placebo group (0.7% vs. 1.6%). The cumulative incidences of diabetes (0.9%), dyslipidemia (0.3%), and erectile dysfunction (0.1%) were ≤1% in the darolutamide double-blind period and similar to the placebo double-blind period (1.1%, 0.2%, and 0.2%, respectively). Consistent with these findings, Kaplan-Meier curves of most TEAEs of interest showed a low incidence with darolutamide and similar onset and cumulative incidence compared with placebo (Supplementary Fig. S1). The rate of initial onset and cumulative incidences of grade 3/4 TEAEs and serious TEAEs were similar for darolutamide and placebo groups over 24 months (Fig. 3).
Figure 2.
Cumulative incidence of treatment-emergent adverse events associated with androgen receptor inhibitors and hormone treatments (>1%) at 24 months during the double-blind treatment period. Acute myocardial infarction occurred in >1% of patients assigned to darolutamide (1.2% vs. 0.7% in patients assigned to placebo) and is included under coronary artery disorders.
Figure 3.
Cumulative incidences of grade 3/4 treatment-emergent adverse events (A) and serious treatment-emergent adverse events (B) over 24 months.
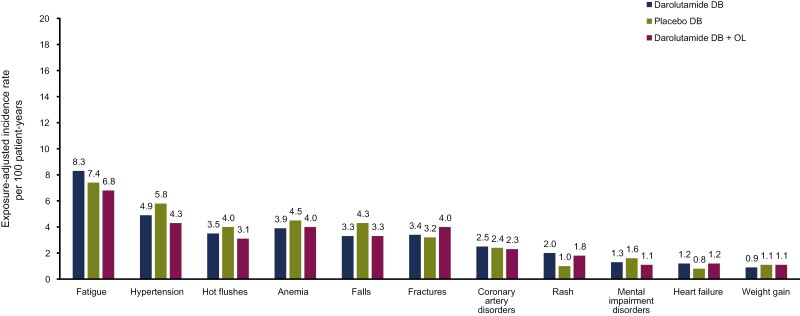
When the duration of therapy was considered for the double-blind and open-label periods, EAIRs for TEAEs commonly associated with ARi therapy and androgen-suppressive treatment showed minimal differences between the darolutamide double-blind and double-blind plus open-label periods and the placebo double-blind period, with the exception of rash (Fig. 4).
Figure 4.
Exposure-adjusted incidence rates for treatment-emergent adverse events of interest during the double-blind and open-label periods. Abbreviations: DB: double-blind; OL: open-label.
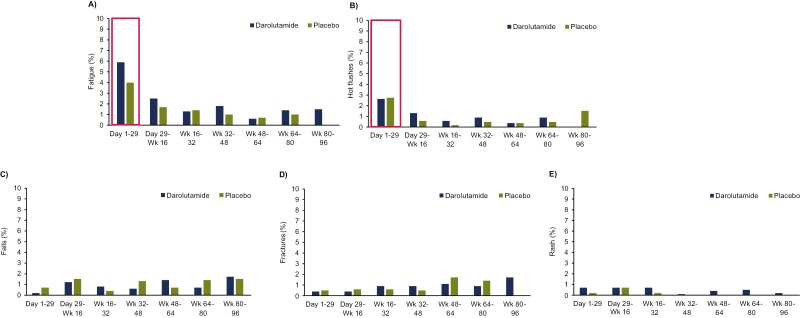
Time interval–specific analyses of TEAEs of interest revealed that the highest new event rates of fatigue and hot flushes occurred during the first month of darolutamide treatment (Fig. 5). In patients who had fatigue during the first 24 months (up to week 96), almost one-half experienced fatigue onset during the first month of treatment in both groups (darolutamide, 5.9%; placebo, 4.0%). Most events of falls and fractures occurred after the first month of treatment, and rash mostly occurred in the first 4 months of treatment and showed minimal increase over time (Fig. 5). New onset of hypertension, coronary artery disorders, and anemia was not time specific for darolutamide or placebo (Supplementary Fig. S2) and cumulative incidences of other hormone treatment–related TEAEs (gynecomastia, heart failure, and weight gain) were ≤2% and too low to provide meaningful time interval–specific analyses. Mental impairment rarely occurred, with incidences <1% in each treatment group per time interval.
Figure 5.
Time interval–specific new event rates of fatigue (A), hot flushes (B), falls (C), fractures (D), and rash (E). Abbreviation: Wk: week.
Discussion
Treatment safety and tolerability are important considerations for patients with nmCRPC, who are relatively asymptomatic during this stage of their prostate cancer journey.3,19 Patients are most impacted by adverse events that can influence treatment, and thus, physicians prefer treatments with limited adverse events, particularly cognitive problems, fatigue, falls, and fractures.3,20,21 For this patient population, assessing patient preference values regarding benefits and risks of adding a new line of therapy will hopefully optimize both survival and quality of life.
The results of the current analyses of the phase III ARAMIS study are consistent with previous reports4,5,13-15 and indicate that darolutamide remained well tolerated with extended treatment at the recommended dose of 600 mg twice daily. There was no substantial increase in the incidence of TEAEs over prolonged treatment and no new safety signals were observed during the combined double-blind and open-label periods of darolutamide treatment. Importantly, almost all patients with nmCRPC were able to receive the full planned dose of darolutamide, with low rates of dose modifications and discontinuations due to TEAEs.
The analysis of cumulative incidence and time-course profiles of TEAEs associated with ARi and hormone therapy revealed that most TEAEs of interest occurred at a low incidence with darolutamide and with similar incidence to that of placebo. Fatigue, hypertension, hot flushes, falls, fractures, rash, anemia, and coronary artery disorders showed minimal increase with continued darolutamide treatment. In addition, the expected increases in incidence between the darolutamide double-blind and double-blind plus open-label periods largely disappeared when adverse events were adjusted for longer exposure.
Time interval–specific analyses allowed examination of the onset and time-course profile of these TEAEs to better inform patients and clinicians of their occurrence. Most TEAEs of interest showed a similar onset and cumulative incidence between darolutamide and placebo groups. Fatigue and hot flushes occurred early during treatment and increased minimally over time. The incidence of rash was low, occurring mostly during the first 4 months of treatment with darolutamide, and almost all events were grade 1 or 2. New onset of hypertension, anemia, and coronary artery disorders were not time specific for either treatment group, whereas falls and fractures tended to occur after the first month of therapy.
A few shortcomings of the analyses presented herein are worth mentioning. These analyses were conducted post hoc and are descriptive in nature. No statistical comparisons were made between treatment groups for the incidences or time-course profiles of TEAEs. Moreover, comparative phase III studies of second-generation ARi therapy have not been conducted, and therefore, real-world experience comparing ARis is of great clinical interest. Recently published matching-adjusted indirect comparisons and meta-analyses found significant differences in safety and tolerability between darolutamide, apalutamide, and enzalutamide, as well as abiraterone, showing a lower risk of adverse events with darolutamide compared with the other agents.19,22 Importantly, the favorable safety profile of darolutamide does not decrease its efficacy, as these meta-analyses found no significant difference in HRs for MFS or overall survival among nmCRPC treatments.
Conclusion
The favorable safety profile of darolutamide was confirmed during extended treatment with darolutamide. The incidences of most TEAEs commonly associated with ARi therapy were low and comparable to placebo. The time-course profile of these TEAEs showed a similar onset and cumulative incidence over time compared with placebo. Understanding and communicating both efficacy and safety profiles for various oncolytic drug choices within a therapeutic class are consistent with the tenets of joint decision-making between the patient and the treating physician.
Supplementary Material
Acknowledgments
Jorge Ortiz (MD), Anja Schmall (MD, PhD), and Sumita Rai (MD) of Bayer provided review of the manuscript. Medical writing and editorial support in the development of this manuscript was provided by Michelle McDermott (PharmD) and Lauren Gallagher (RPh, PhD) of Luna, an OPEN Health team, OPEN Health Communications, with financial support from Bayer HealthCare. The authors retained full editorial control over the content of the manuscript and the decision to publish.
Contributor Information
Neal D Shore, Carolina Urologic Research Center, Myrtle Beach, SC, USA.
Christian Gratzke, Department of Urology, University Hospital Freiburg, Freiburg, Germany.
Susan Feyerabend, Studienpraxis Urologie, Nürtingen, Germany.
Patrick Werbrouck, Department of Urology, AZ Groeninge, Kortrijk, Belgium.
Joan Carles, Vall d’Hebron Institute of Oncology, Hospital Universitari Vall d’Hebron, Barcelona, Spain.
Egils Vjaters, Urological Center, Pauls Stradiņš Clinical University Hospital, Riga, Latvia.
Teuvo L J Tammela, Department of Urology, Tampere University Hospital and Tampere University, Tampere, Finland.
David Morris, Urology Associates, PC, Nashville, TN, USA.
Jeanny B Aragon-Ching, Inova Schar Cancer Institute, Fairfax, VA, USA.
Raoul S Concepcion, Urology Associates, PC, Nashville, TN, USA.
Urban Emmenegger, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada; Odette Cancer Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Neil Fleshner, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Markus Grabbert, Department of Urology, University Hospital Freiburg, Freiburg, Germany.
Vilnis Lietuvietis, Urology Clinic, Department of Surgery, Riga East Clinical University Hospital, Riga, Latvia.
Hakim Mahammedi, Medical Oncology, Jean Perrin Center, Clermont-Ferrand, France.
Felipe M Cruz, Núcleo de Ensino e Pesquisa da Rede São Camilo, São Paulo, Brazil.
Adriano Paula, Oncologic Surgery, Hospital Araújo Jorge, Goiânia, Brazil.
Christopher Pieczonka, Associated Medical Professionals, Syracuse, NY, USA.
Antti Rannikko, Helsinki University Hospital Comprehensive Cancer Center, Helsinki, Finland.
Martin Richardet, Oncologic Institute of Córdoba, Sanatorio Aconcagua, Córdoba, Argentina.
Glauco Silveira, Centro Oncológico do Triângulo, Uberlândia, Brazil.
Iris Kuss, Bayer AG, Berlin, Germany.
Marie-Aude Le Berre, Bayer HealthCare SAS, Loos, France.
Frank Verholen, Bayer Consumer Care AG, Basel, Switzerland.
Toni Sarapohja, Orion Pharma, Espoo, Finland.
Matthew R Smith, Massachusetts General Hospital Cancer Center, Boston, MA, USA.
Karim Fizazi, Institut Gustave Roussy, University of Paris-Saclay, Villejuif, France.
Funding
This work was supported by Bayer AG and Orion Pharma.
Conflict of Interest
Neal D. Shore: Consulting or advisory role: AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb/Sanofi, CG Oncology, Clarity Pharmaceuticals, Clovis Oncology, Dendreon, Exact Imaging, Exact Sciences, FerGene, Ferring, Foundation Medicine, Genesis Cancer Care, Genzyme, Invitae, Janssen Scientific Affairs, Lantheus Medical Imaging, Lilly, Mdxhealth, Medivation/Astellas Pharma, Merck, Myovant Sciences, Myriad Genetics, Nymox, Pacific Edge Biotechnology, PeerView, Pfizer, Phosphorus, Photocure, Propella Therapeutics, Sanofi, Sema4, Sesen Bio, Specialty Networks, Telix Pharmaceuticals, Tempus, Tolmar, UroGen Pharma, Vaxiion; speakers’ bureau: Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, Dendreon, Foundation Medicine, Guardant Health, Janssen, Merck, Pfizer; expert testimony: Ferring; other relationship: Alessa Therapeutics, Photocure; research funding: AbbVie, Advantagene, Amgen, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Boston Scientific, CG Oncology, Clovis Oncology, Dendreon, DisperSol, Endocyte, Exact Imaging, Exelixis, Ferring, FKD Therapies, FORMA Therapeutics, Foundation Medicine, Genentech, Guardant Health, Invitae, Istari Oncology, Janssen, Jiangsu Yahong Meditech, Mdxhealth, Medivation, Merck, MT Group, Myovant Sciences, Myriad Genetics, Novartis, Nymox, OncoCell MDx, ORIC Pharmaceuticals, Pacific Edge, Palette Life Sciences, Pfizer, Plexxikon, POINT Biopharma, Propella Therapeutics, RhoVac, Sanofi, Seattle Genetics, Sesen Bio, Steba Biotech, Theralase, Tolmar, UroGen Pharma, Urotronic, US Biotest, Vaxiion, Veru, Zenflow. Christian Gratzke: No conflicts. Susan Feyerabend: Advisory boards: Astellas, Aventis, Bayer, Janssen; honorarium: Janssen; travel, accommodation, expenses: Aventis. Patrick Werbrouck: No conflicts. Joan Carles: Consulting and scientific advisory board attendee role: Amgen, Astellas, Bayer, Bristol-Myers Squibb, Johnson & Johnson, Merck Sharp & Dohme, Pfizer, Sanofi; speakers’ bureau: Asofarma, Astellas, Bayer, Johnson & Johnson, Sanofi. Egils Vjaters: No conflicts. Teuvo L. Tammela: Consultant: Astellas Pharma; investigator: Bayer, Laboratórios Pfizer Ltda. David Morris: Consulting or advisory role: Astellas Pharma, AstraZeneca, Bayer, Decipher Biosciences, Dendreon, Janssen Oncology, Myovant Sciences, Myriad Genetics, Pfizer/EMD Serono, UroGen Pharma, UroGRO; speakers’ bureau: AstraZeneca, Astellas/Medivation, Clovis Oncology, Janssen, Merck; research funding: Merck. Jeanny B. Aragon-Ching: Consulting or advisory role: AZD, Bayer, Exelixis, Immunomedics, Janssen Oncology, Merck, Pfizer/EMD Serono, Pfizer/Myovant; speaker honorarium: Astellas/Seagen, Bristol-Myers Squibb, Pfizer/EMD Serono. Raoul S. Concepcion: Speakers’ bureau: Amgen, Astellas, Pfizer, Sun Pharma; consultant: AZ, Blue Earth, Curium, Dendreon, GoPath, Immunity Bio, Janssen, Sun Pharma. Urban Emmenegger: Consulting or advisory role: Amgen Canada, Astellas Pharma Canada, AstraZeneca Canada, Bayer, Janssen Biotech, Knight Inc Canada, Merck, Novartis, Pfizer Canada Inc; research support: Clovis Oncology, Merck, Novartis, Point Biopharma. Neil Fleshner: Grants/sponsorship support: Astellas, Bayer, Janssen; honorarium/consulting fees/speaker fees: AbbVie, Astellas, Bayer, Janssen, Sanofi; and is the co-founder of Point Biopharma and Verity. Markus Grabbert: Consulting or advisory role: Ipsen, MSD; travel, accommodation, expenses: Astellas, Janssen, MSD; honoraria: Bristol-Myers Squibb, Janssen, Medac, MSD. Vilnis Lietuvietis: No conflicts. Hakim Mahammedi: Consulting or advisory role: 3A, Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Ipsen, Janssen, Merck/Pfizer, MSD, Novartis, Pfizer; travel, accommodation, expenses: Astellas, Ipsen, Janssen, Pfizer. Felipe M. Cruz: No conflicts. Adriano Paula: No conflicts. Christopher Pieczonka: Employment: Associated Medical Professionals of New York; consulting or advisory role: AstraZeneca, Bayer, Bristol-Myers Squibb, Dendreon, Janssen Oncology, Merck, Pfizer/Astellas, Sun Pharma, Tolmar; leadership: Associated Medical Professionals of New York; speakers’ bureau: Astellas Pharma, Bayer, Dendreon, Janssen Oncology, Pfizer, Merck, Myovant Sciences, Sun Pharma, Tolmar; stock ownership: US Urology Partners; honoraria: AstraZeneca, Bayer, Bristol-Myers Squibb, Dendreon, Janssen, Merck, Myovant Sciences, Pfizer/Astellas, Sun Pharma, Tolmar; research funding: Advantagene, Astellas Pharma, AstraZeneca, Bayer, Dendreon, Innocrin Pharma, Janssen Oncology, Merck, Pfizer. Antti Rannikko: Board member: the Ida Montin Foundation and Orion Research Foundation; advisory board member: Bayer, Janssen, Orion Pharma; stockholder and clinical advisor: Aqsens Health; clinical investigator: Astellas Pharma Inc., Bayer, Janssen, Orion Pharma, RhoVac; receiving competitive state research funding: HUS Helsinki University Hospital, Finnish Cancer Organizations, the Jane and Aatos Erkko Foundation. Martin Richardet: No conflicts. Glauco Silveira: No conflicts. Iris Kuss: Employment: Bayer. Marie-Aude Le Berre: Employment: Bayer. Frank Verholen: Employment: Bayer. Toni Sarapohja: Employment: Orion Pharma. Matthew R. Smith: Consulting or advisory role: Amgen, Astellas Pharma, Bayer, Janssen Oncology, Lilly, Novartis, Pfizer; research funding: Bayer (Inst), ESSA (Inst), Janssen Oncology (Inst), Lilly (Inst), ORIC Pharmaceuticals (Inst). Karim Fizazi: Honoraria: Astellas Pharma (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Janssen (Inst), Sanofi (Inst); consulting or advisory role: Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer, Clovis Oncology (Inst), ESSA (Inst), Novartis (Inst), Janssen Oncology (Inst), Pfizer (Inst), Orion Pharma, Sanofi (Inst); travel, accommodation, expenses: AstraZeneca, Janssen, MSD.
Author Contributions
Conception/design: All authors. Provision of study material or patients: N.D.S., C.G., S.F., P.W., J.C., E.V., T.L.T., D.M., J.B.A.-C., R.S.C., U.E., N.F., M.G., V.L., H.M., F.M.C., A.P., C.P., A.R., M.R., G.S., M.R.S., K.F. Collection and/or assembly of data: N.D.S., C.G., S.F., P.W., J.C., E.V., T.L.T., D.M., J.B.A.-C., R.S.C., U.E., N.F., M.G., V.L., H.M., F.M.C., A.P., C.P., A.R., M.R., G.S., M.A.L.B., M.R.S., K.F. Data analysis and interpretation: All authors. Manuscript writing and final approval of manuscript: All authors.
Data Availability
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the US and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
- 1. Saad F, Bögemann M, Suzuki K, Shore N.. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. 2021;24(2):323-334. 10.1038/s41391-020-00310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mateo J, Fizazi K, Gillessen S, et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019;75(2):285-293. 10.1016/j.eururo.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 3. Tomaszewski EL, Moise P, Krupnick RN, et al. Symptoms and impacts in non-metastatic castration-resistant prostate cancer: qualitative study findings. Patient. 2017;10(5):567-578. 10.1007/s40271-017-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fizazi K, Shore N, Tammela TL, et al. ; ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. 10.1056/NEJMoa1815671 [DOI] [PubMed] [Google Scholar]
- 5. Fizazi K, Shore N, Tammela TL, et al. ; ARAMIS Investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. 10.1056/NEJMoa2001342 [DOI] [PubMed] [Google Scholar]
- 6. Smith MR, Saad F, Chowdhury S, et al. ; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. 10.1038/srep12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith MR, Shore N, Tammela TL, et al. Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the phase 3 ARAMIS trial. Eur J Cancer. 2021;154:138-146. 10.1016/j.ejca.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 10. Shore ND, Stenzl A, Pieczonka C, et al. Impact of darolutamide on local symptoms: pre-planned and post hoc analyses of the ARAMIS trial. BJU Int. 2023;131(4):452-460. 10.1111/bju.15887 [DOI] [PubMed] [Google Scholar]
- 11. Bögemann M, Shore ND, Smith MR, et al. Efficacy and safety of darolutamide in patients with nonmetastatic castration-resistant prostate cancer stratified by prostate-specific antigen doubling time: planned subgroup analysis of the phase 3 ARAMIS trial. Eur Urol. 2023;83(3):212-221. 10.1016/j.eururo.2022.07.018 [DOI] [PubMed] [Google Scholar]
- 12. Smith MR, Hussain M, Saad F, et al. ; ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. 10.1056/NEJMoa2119115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fizazi K, Massard C, Bono P, et al. ; ARADES study group. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15(9):975-985. 10.1016/S1470-2045(14)70240-2 [DOI] [PubMed] [Google Scholar]
- 14. Matsubara N, Mukai H, Hosono A, et al. Phase 1 study of darolutamide (ODM-201): a new-generation androgen receptor antagonist, in Japanese patients with metastatic castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2017;80(6):1063-1072. 10.1007/s00280-017-3417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massard C, Penttinen HM, Vjaters E, et al. Pharmacokinetics, antitumor activity, and safety of ODM-201 in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: an open-label phase 1 study. Eur Urol. 2016;69(5):834-840. 10.1016/j.eururo.2015.09.046 [DOI] [PubMed] [Google Scholar]
- 16. Zurth C, Sandman S, Trummel D, et al. Higher blood–brain barrier penetration of [14C]apalutamide and [14C]enzalutamide compared to [14C]darolutamide in rats using whole-body autoradiography [abstract]. J Clin Oncol. 2019;37(7 suppl):156. 10.1200/JCO.2019.37.7_suppl.156 [DOI] [Google Scholar]
- 17. Williams SCR, Mazibuko N, O’Daly O, et al. Comparison of cerebral blood flow in regions relevant to cognition after enzalutamide, darolutamide, and placebo in healthy volunteers: a randomized crossover trial. Target Oncol. 2023;18(3):403-413. 10.1007/s11523-023-00959-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shore N, Zurth C, Fricke R, et al. Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019;14(5):527-539. 10.1007/s11523-019-00674-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halabi S, Jiang S, Terasawa E, et al. Indirect comparison of darolutamide versus apalutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer. J Urol. 2021;206(2):298-307. 10.1097/JU.0000000000001767 [DOI] [PubMed] [Google Scholar]
- 20. Srinivas S, Mohamed AF, Appukkuttan S, et al. Physician preferences for non-metastatic castration-resistant prostate cancer treatment. BMC Urol. 2020;20(1):73. 10.1186/s12894-020-00631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srinivas S, Mohamed AF, Appukkuttan S, et al. Patient and caregiver benefit-risk preferences for nonmetastatic castration-resistant prostate cancer treatment. Cancer Med. 2020;9(18):6586-6596. 10.1002/cam4.3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L, Paller C, Hong H, et al. Comparison of treatments for nonmetastatic castration-resistant prostate cancer: matching-adjusted indirect comparison and network meta-analysis. J Natl Cancer Inst. 2022;114(2):191-202. 10.1093/jnci/djab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the US and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.