Abstract
We have previously reported that Foot-and-mouth disease virus (FMDV), which is virulent for cattle and swine, can utilize the integrin αvβ3 as a receptor on cultured cells. Since those studies were performed with the human integrin, we have molecularly cloned the bovine homolog of the integrin αvβ3 and have compared the two receptors for utilization by FMDV. Both the αv and β3 subunits of the bovine integrin have high degrees of amino acid sequence similarity to their corresponding human subunits in the ectodomains (96%) and essentially identical transmembrane and cytoplasmic domains. Within the putative ligand-binding domains, the bovine and human αv subunits have a 98.8% amino acid sequence similarity while there is only a 93% similarity between the β3 subunits of these two species. COS cell cultures, which are not susceptible to FMDV infection, become susceptible if cotransfected with αv and β3 subunit cDNAs from a bovine or human source. Cultures cotransfected with the bovine αvβ3 subunit cDNAs and infected with FMDV synthesize greater amounts of viral proteins than do infected cultures cotransfected with the human integrin subunits. Cells cotransfected with a bovine αv subunit and a human β3 subunit synthesize viral proteins at levels equivalent to those in cells expressing both human subunits. However, cells cotransfected with the human αv and the bovine β3 subunits synthesize amounts of viral proteins equivalent to those in cells expressing both bovine subunits, indicating that the bovine β3 subunit is responsible for the increased effectiveness of this receptor. By engineering chimeric bovine-human β3 subunits, we have shown that this increase in receptor efficiency is due to sequences encoding the C-terminal one-third of the subunit ectodomain, which contains a highly structured cysteine-rich repeat region. We postulate that amino acid sequence differences within this region may be responsible for structural differences between the human and bovine β3 subunit, leading to more efficient utilization of the bovine receptor by this bovine pathogen.
Foot-and-mouth disease virus (FMDV), an Aphthovirus in the Picornaviridae family, is the cause of foot-and-mouth disease, a highly infectious disease of domestic livestock. The virus initiates infection by binding to its cellular receptor via an arginine-glycine-aspartic acid (RGD) sequence found within a surface protrusion consisting of the loop between the βG and βH strands (G-H loop) of the capsid protein VP1 (1, 6, 23, 42, 45). While FMDV can utilize other receptors on cultured cells, such as the Fc receptor (7, 44) or heparan sulfate (3, 25, 36, 47), these receptors do not require the RGD sequence (43, 47). We have demonstrated that antibodies to the integrin receptor αvβ3 can inhibit adsorption and plaque formation by FMDV (11). Furthermore, we have also shown that the virus, which is virulent for cattle, can infect only cells expressing this integrin receptor and that site-directed mutants of these viruses lacking an RGD sequence are not capable of infecting cells expressing αvβ3 (45, 47).
Integrins are heterodimeric molecules, consisting of α and β subunits which interact noncovalently at the cell surface and have a wide species distribution (35). They are involved in extracellular matrix and cell-cell interactions and also serve as signal-transducing receptors (29). A total of 16 α and 8 β subunits have been described, giving rise to 22 different integrins, each with its own ligand-binding specificity, and 7 of which, including αvβ3, bind to their natural ligands via an RGD sequence (22, 35). Electron microscopic visualization of integrins reveals a globular structure, presumably the ligand-binding region combining elements of both subunits with two stalk-like structures extending to the cell surface (16, 49).
The αvβ3 integrin is one of two receptors within the class of integrins called cytoadhesins (29). The β3 subunit is found only complexed with one other subunit, αIIb, while the αv subunit can complex with four additional β subunits (β1, β5, β6, and β8) (35). Although αvβ3 was originally called the vitronectin receptor, it can bind to other ligands (33). While it is clear that both the α and β subunits of integrins structurally contribute to ligand binding (22, 34), there are specific regions of the αv (41, 57) and β3 (13, 19, 39, 56, 61, 62) subunits that have been identified as directly interacting with ligands. At least two other picornaviruses can utilize αvβ3 to initiate infection, coxsackievirus A9 (CAV9) (53) and echovirus 9 (48). In addition, human adenovirus utilizes integrins αvβ3 and αvβ5 to facilitate internalization (64); two hantaviruses, which cause different human disease syndromes, utilize αvβ3 and αIIbβ3 to mediate cellular entry (26, 27); and human parechovirus 1 (formerly echovirus 22) may utilize the αvβ1 integrin as a receptor (50).
While previous studies have shown that FMDV can utilize the human (47) and simian (11) homologs of the αvβ3 integrin to infect cells, this virus does not cause disease in humans (4). Therefore, we have characterized the bovine homolog of this integrin to determine how it interacts with this bovine pathogen. These studies demonstrate that FMDV utilizes the bovine integrin with much greater efficiency than it utilizes the human integrin, and we show that this increased efficiency is due to bovine sequences located within the C-terminal one-third of the β3 subunit ectodomain.
MATERIALS AND METHODS
cDNA cloning.
Single-stranded cDNA was reverse transcribed from bovine lung poly(A)+ RNA (Clontech) using Superscript II reverse transcriptase (Life Technologies) and an oligo(dT)18 primer. The bovine αv cDNA was generated in two pieces by a 30-cycle PCR using Taq polymerase (Boehringer Mannheim) and the following sets of primers, which introduce a silent mutation creating a BamHI restriction endonuclease site that could be used to recreate complete αv coding sequences: 5′CGCGCACCCCGGCGATGGCT3′ plus 5′CCATCGGATCCGCGATCCATG3′, and 5′GGATCCGATGGCAAACTCCAGGAG3′ plus 5′GGAATTCCTTAAGTTTCTGAGTTTCCTTCACC 3′. The resulting PCR products were ligated into the vector pCR2.1 (Invitrogen) and sequenced. Plasmid DNA containing the 3′ piece of bovine αv cDNA was ligated to the 5′ bovine αv cDNA utilizing this synthetic BamHI site. The resulting full-length αv cDNA fragment was inserted into the vector pcDNA3.1/Zeo(−) (Invitrogen) to create pBovαvZEO.
The cDNA encoding the mature bovine β3 subunit was generated by a 30-cycle PCR using Advantage KlenTaq polymerase mix (Clontech) and the following primers, which introduce a silent mutation creating an MluI site following the predicted N-terminal signal peptide cleavage site: 5′CCACGCGTGGTGTGAGCTCCTG3′ plus 5′GGATCCTAAGGCCCCGGTACGTGATATTG3′. To generate a signal peptide sequence for use with the bovine β3 coding region, human β3 cDNA (β3/pIAP58) (14, 40, 47) was used as a template for 15 cycles of PCR amplification with Advantage KlenTaq polymerase mix and the following primers, which also introduce a silent mutation creating an MluI site in frame with the bovine open reading frame: 5′CAGATGCGAGCGCGGCCGC3′ plus 5′CGGGATCCTTAAGTGCCCCGGTACGTGATATTG3′. The PCR products were inserted into the pCR-Blunt II-TOPO vector (Invitrogen) and sequenced. The cDNA encoding mature bovine β3 was ligated to the human signal peptide sequence and inserted into pcDNA3.1/Zeo(−) to create pBovβ3ZEO. The human αv-encoding plasmid, pHumαvZEO, has been described previously (47). The human β3-encoding plasmid, β3/pIAP58 (14, 40, 47), was inserted into pcDNA3.1/Zeo(−) to generate pHumβ3ZEO.
Generation of chimeric β3 subunits.
Chimeric cDNAs for the β3 integrin were created using a KpnI site shared by pBovβ3ZEO and pHumβ3ZEO at codon 136. Plasmid phkbβ3 contained the first 136 codons of the human β3 subunit and the remaining codons from the bovine β3 subunit. Plasmid pbkhβ3 was the inverse chimera and contained bovine sequences to codon 136 and human sequences for the remainder of the subunit. The chimeric cDNAs phsbβ3 and pbshβ3 were created using a similar strategy and a SmaI site at codon 488 of pBovβ3ZEO and pHumβ3ZEO. The resulting constructs were sequenced around the restriction sites to ensure their identity.
Sequencing.
Automated sequencing was performed on an Applied Biosystems 370A sequencer with an XL upgrade, using the ABI Prism Big Dye terminator cycle-sequencing ready reaction kit (Perkin-Elmer) as described by the manufacturer. Sequence analysis was done using the Lasergene analysis software package (DNASTAR Inc.). The nucleotide sequences for the human αv and β3 subunits were from GenBank (accession numbers M14648 [60] and M35999 [24], respectively).
Coupled in vitro transcription-translation.
Plasmid DNA (1 μg) was used in either a wheat germ extract or rabbit reticulocyte lysate TNT Quick coupled transcription-translation system (Promega) in the presence of [35S]methionine (Amersham) as described by the manufacturer. The resulting protein products were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 7.5% gel.
Viruses and cells.
FMDV type A12, strain 119ab, was derived from the infectious cDNA clone pRMC35 (52). An antigenic variant of type A12, harboring the VP1 sequence present in a bovine tongue tissue-propagated type A12 (vRM-SSP), has been described previously (51). The cattle-virulent variant derived from infectious cDNA containing capsid sequences isolated from a vaccine seed stock of type O1Campos (vCRM8) has also been described previously (54). COS-1 cells were maintained on Dulbecco's minimal essential medium (Life Technologies Inc.) containing 10% calf serum, an additional 2 mM l-glutamine, 1 mM sodium pyruvate, 10 U of penicillin G per ml, 10 U of streptomycin sulfate per ml, and 0.25 μg of amphotericin B per ml.
Transient expression of integrin subunits in COS-1 cells.
Cells were plated at a density of 105 cells/well on six-well tissue culture dishes the day before transfection. Transfections were performed using 2.0 μg of each integrin-encoding plasmid mixed with the transfection reagent FuGENE 6 (Roche Molecular Biochemicals) as specified by the manufacturer. After incubation at 37°C overnight, the cells were trypsinized and replated onto 2 wells of a 24-well tissue culture dish. After an additional overnight incubation at 37°C, cells in one well of each transfected condition were infected with FMDV and cells in the other well were analyzed for integrin expression by immunohistochemistry (IHC).
Immunohistochemistry.
Transfected cells were fixed on ice for 5 min with acetone-methanol (50:50). Fixed cells were rehydrated with minimal essential medium containing 0.2% bovine serum albumin, 50 mM HEPES (pH 7.5), and 1% normal horse serum. The cells were reacted with a 1:500 dilution of the anti-αvβ3 monoclonal antibody (MAb) LM609 (MAB1976; Chemicon International Inc.) (17) for 30 min at 37°C. Primary-antibody binding was detected using an alkaline phosphatase avidin-biotin system and biotinylated horse anti-mouse immunoglobulin G (Vectastain Elite ABC kit; Vector Laboratories). Bound phosphatase was visualized using the Vector VIP substrate kit (Vector Laboratories).
Viral replication assays.
Transfected and nontransfected COS-1 cells were infected and analyzed as previously described (47). Briefly, cells were infected with various FMDV serotypes at a multiplicity of infection (MOI) of 10 PFU/cell and labeled overnight, beginning at 4 h after infection, with 50 to 75 μCi of [35S]methionine at 37°C. Cell lysates were prepared in 1% Triton X-100, trichloroacetic acid (TCA)-precipitable counts per minute (cpm) were determined, and radioimmunoprecipitation (RIP) was preformed as previously described (5) using anti-FMDV type A12 MAb 6EE2 (8) for type A12- and vRM-SSP-infected cells and anti-FMDV type O1 MAb 10GA4 (59) for vCRM8-infected cultures. Equal amounts of TCA-precipitable CPM were immunoprecipitated and analyzed by SDS-PAGE using a 10% polyacrylamide gel.
Nucleotide sequence accession numbers.
Nucleotide sequences for the bovine αv and β3 cDNAs have been submitted to GenBank and have been assigned accession numbers AF239958 and AF239959, respectively.
RESULTS
cDNA cloning of the bovine integrin αvβ3 subunits.
The identification of αvβ3 as the receptor for virulent forms of FMDV (47) utilizing human αvβ3 cDNAs led us to examine the available integrin subunit sequences. Interspecies comparisons for both the αv (63) and β3 (18) subunits have shown that there are differences in the deduced amino acid sequences among the species sequenced to date. For this reason, we thought it important to obtain cDNAs encoding αvβ3 from a species that is naturally susceptible to FMDV infection, such as cattle. PCR with cDNA from bovine lung tissue and primers based on known human and mouse integrin sequences (18, 60, 63, 65) generated fragments of the expected sizes for both the αv and β3 subunits. These fragments were ligated and inserted into expression vectors that would allow in vitro transcription-translation analysis and eukaryotic expression. The complete coding sequence for the bovine αv subunit cDNA was 3,144 bp coding for a 1,048-amino acid protein. The encoded protein consists of a 30-residue signal peptide, a 963-residue extracellular ectodomain, a 23-residue transmembrane domain, and a 32-residue cytoplasmic domain. The complete coding sequence for the bovine β3 subunit cDNA was 2,364 bp coding for a 788-amino-acid protein. Since we were unable to generate a fragment which included the authentic bovine β3 signal peptide sequence, we removed the coding region for this peptide from the human β3 cDNA plasmid and ligated it to the remainder of the bovine β3 cDNA as described in Materials and Methods. Thus, the encoded protein consists of the 26-residue human signal peptide, a 692-residue extracellular ectodomain, a 23-residue transmembrane domain, and a 47-residue cytoplasmic domain.
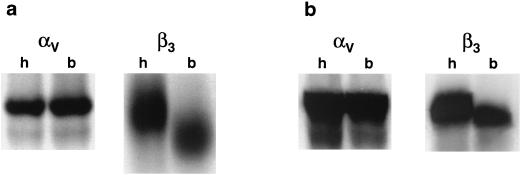
Coupled in vitro transcription-translation reactions were performed using the cDNA constructs encoding both the human and the bovine αv and β3 subunits to determine if the bovine constructs were capable of generating proteins in the same size range as the human subunits. Radiolabeled protein was generated and separated by SDS-PAGE (7.5% polyacrylamide) as described in Materials and Methods. Transcription-translation of the bovine αv subunit in a rabbit reticulocyte lysate translation system generated a product that was comparable in size, based on migration in a denaturing gel, to the human αv subunit (Fig. 1a). In contrast, in the rabbit reticulocyte lysate system, the bovine β3 subunit migrated faster, to an apparent lower molecular weight than the human subunit did (Fig. 1a). Since differences in glycosylation may account for differences in migration on SDS-PAGE, the transcription-translation reactions were repeated utilizing a wheat germ extract. In contrast to the result seen in the rabbit reticulocyte lysate, the bovine and human β3 subunits synthesized in this system were comparable in size, as were the αv subunits (Fig. 1b). While O-linked glycosylation occurs in rabbit reticulocyte lysates in the absence of microsomal membranes, this posttranslational modification cannot take place in wheat germ extracts in the absence of membranes. In contrast, N-linked glycosylation occurs only in the presence of microsomal membranes in either extract (38, 58). Since we did not add microsomal membranes to either lysate, differences seen between the bovine and human β3 subunits in the rabbit reticulocyte lysate may have been due to differences in glycosylation. Examination of potential O-linked glycosylation sites in the β3 subunits revealed differences between human and bovine β3 subunits which may account for the variation in apparent molecular weight seen in the rabbit reticulocyte lysate system (results not shown).
FIG. 1.
Transcription-translation of cloned bovine integrin subunits. Plasmids containing sequences representing bovine αv (pBovαvZEO), bovine β3 (pBovβ3ZEO), human αv (pHumαvZEO), or human β3 (pHumβ3ZEO) integrin sequences were analyzed for protein expression by coupled in vitro transcription-translation using either a rabbitreticulocyte lysate (a) or wheat germ extract system (b), as described in Materials and Methods. Translation products were analyzed by SDS-PAGE (7.5% polyacrylamide). Bovine and human subunits are indicated by b and h, respectively, above the lanes.
Sequence comparisons of bovine and human integrin subunits.
The cloned bovine subunit cDNAs were sequenced using automated sequencing, as described in Materials and Methods, and compared with the reported human αv and β3 subunit sequences (24, 60). Analysis of the two bovine subunit constructs revealed a high degree of sequence similarity to their counterpart human homolog subunits. Alignment of DNA sequence using the Martinez/Needlemen-Wunsch alignment method, with a gap penalty of 1.10 and a gap length penalty of 0.33, showed that the sequence similarities between the bovine and human αv and β3 extracellular ectodomains were 93.6 and 90.1%, respectively (Table 1). When the deduced amino acid sequences within this region were compared by Lipman-Pearson alignment (gap penalty of 4 and gap length penalty of 12), the sequence similarity was about 96% for both subunits (Table 1). The transmembrane and cytoplasmic domains of both bovine subunits displayed the highest level of amino acid similarity to their human homologs: the αv subunits were identical in this domain, and the β3 subunits had a 99.9% similarity even though the nucleotide sequence similarity was rather low at 88% (Table 1). We also compared sequences which lie within the putative ligand-binding domains of these subunits. The ligand-binding domains of the human αv and β3 subunits have been determined using a number of methods, most prominently photoaffinity cross-linking and generation of chimeric receptors with closely related αv and β3 subunits. These studies have estimated that the ligand-binding regions for the αv and β3 subunits lie between amino acid residues 1 and 340 (41, 57) and between residues 85 and 207 (13, 19, 39, 56, 61, 62), respectively (amino acid residue 1 is the first amino acid after cleavage of the signal sequence). Within this domain, the amino acid sequence similarity between bovine and human sequences was quite high for the αv subunit (98.8%) but was only 93.5% for the β3 subunit.
TABLE 1.
Nucleotide and encoded amino acid sequence similarities between the human and bovine αv and β3 integrin subunits
| Subunit | Nucleotide similarity/amino acid similaritya in:
|
||
|---|---|---|---|
| Ectodomain | Transmembrane and cytoplasmic domains | Ligand-binding domainb | |
| αv | 93.6%/95.6% | 94.5%/100% | 98.1%/98.8% |
| β3 | 90.1%/95.5% | 88%/99.9% | 91.6%/93.5% |
The first number of each pair represents the sequence similarity of nucleotides aligned using the Martinez/Needleman-Wunsch alignment method and determined using a gap penalty of 1.10 and a gap length penalty of 0.33. The second number represents the sequence similarity of amino acids aligned using the Lipman-Pearson alignment method and determined using a gap penalty of 4 and a gap length penalty of 12.
Transient expression of integrin subunits in COS-1 cells.
Cells were cotransfected with each integrin subunit using FuGENE 6 as described in Materials and Methods. The cells were grown for 48 h, fixed, and immunostained using the anti-αvβ3 MAb LM609. Figure 2 shows that cells expressing either bovine or human αvβ3 were stained equally well with this MAb, which reacts only with the heterodimeric αvβ3 integrin (17). This confirms previously reported results showing that LM609, which was generated against the human integrin, also reacts with the complete bovine integrin (12, 28, 55). Furthermore, these results indicate that both integrins were expressed to approximately comparable levels in the COS-1 cells. We also examined the expression of the αvβ3 integrin in cells transfected with mixed bovine-human subunits. The results in Fig. 2 show that these cultures also expressed the integrin to levels comparable to those seen in either complete bovine or complete human integrin expression. Because integrins can be composed of different combinations of α and β subunits (35), transfections were also done with the individual subunits and cells were analyzed by IHC. In all cases, no staining above the level seen in the control cells was observed (data not shown), indicating that the staining obtained in cells transfected with both subunits was not due to the formation of heterodimers between the transfected subunits and endogenous monkey integrin subunits.
FIG. 2.
Analysis of integrin expression in transfected COS-1 cells. Cells were transfected with integrin subunit-encoding plasmids, as shown above each panel, and integrin expression was analyzed by IHC with MAb LM609 48 h after transfection, as described in Materials and Methods.
In the experiments reporting FMDV replication in integrin subunit-transfected cells shown below, parallel cultures were always examined for integrin expression by IHC and only experiments where the integrin expression in all cultures was qualitatively equivalent are reported. In addition, in some experiments, cells were analyzed for integrin expression using fluorescence-activated cell sorting (FACS), and these results were comparable to those seen using IHC analysis (data not shown).
Replication of FMDV in integrin subunit-transfected cells.
COS-1 cells were transfected with complete bovine or human integrin subunit cDNAs or with mixed bovine-human subunits and infected with FMDV as outlined in Materials and Methods. We used three different viruses for these studies: our tissue culture-adapted type A12, an A12 variant containing sequences isolated from the tongue of an infected bovine (vRM-SSP) (51), and a highly cattle-virulent variant of type O1Campos (vCRM8) (54). These viruses utilize only αvβ3 as a receptor to infect cultured cells (47). Transfected-infected cells were labeled overnight with [35S]methionine, and lysates were analyzed by RIP and SDS-PAGE as described in Materials and Methods. In all of these assays, equal numbers of TCA-precipitable cpm and equal amounts of protein were immunoprecipitated within each experiment. This normalized the various conditions and allowed us to make semiquantitative comparisons of the levels of viral protein synthesis. The results in Fig. 3 show the presence of viral proteins only in infected cells expressing either bovine or human αvβ3, confirming our previous findings obtained in experiments with human integrin-transfected cells (47).
FIG. 3.
Analysis of viral protein synthesis in COS-1 cells transfected with integrin subunit cDNAs. Cells were transfected with plasmids encoding human or bovine integrin subunits as shown. Transfected cells were infected with FMDV type A12, vRM-SSP, or vCRM8 at an MOI of 10 PFU/cell. Cells were labeled with [35S]methionine, RIP was performed on cell lysates, and the products were analyzed by SDS-PAGE (10% polyacrylamide). Locations of viral structural proteins are denoted on the left. con, nontransfected cells; M, marker viral proteins from FMDV-infected BHK-21 cells also labeled with [35S]methionine; bov, bovine integrin subunits; hum, human integrin subunits.
A comparison of the level of viral protein synthesis in cultures expressing the different integrins, however, showed that viral protein synthesis was greater in cultures transfected with the bovine integrin (Fig. 3), even though the bovine and human integrins were expressed to the same level, as determined by IHC. We also examined the level of viral protein synthesis in cells transfected with mixed bovine-human integrin subunits. The results in Fig. 3 show that with all three viruses used, the level of viral protein synthesis was always greater when the bovine β3 was expressed, regardless of which αv subunit was transfected. In fact, expression of the bovine β3 subunit along with the human αv subunit resulted in a level of viral protein synthesis comparable to that seen with the complete bovine integrin. The differences in the level of viral protein synthesis appeared to be more pronounced when the cells were infected with the laboratory strain A12 virus than when they were infected with either of the other two animal-derived viruses. However, it is clear in all cases that viral replication, as measured in this assay, was greater when the bovine αvβ3 integrin was used as a receptor. In nontransfected COS-1 cells, there was a very low level of viral protein synthesis in cells infected with the vCRM8 virus (Fig. 3). We are not sure why this occurred; however, it may be the result of a low level of virus which utilizes cell surface heparan sulfate as a receptor (47, 54), either present in the original seed or generated during the overnight incubation.
FMDV infection in cells expressing chimeric bovine-human β3 subunit receptors.
Since the results presented in the previous section indicated that the bovine β3 subunit was necessary for the higher level of viral protein synthesis seen in transfected-infected cells, we generated chimeric bovine-human β3 subunits to delineate which portions of the subunit were responsible for this phenomenon. To do this, we took advantage of two unique restriction sites that are conserved in both the bovine and human β3 cDNAs. A schematic diagram for the chimeric β3 subunits is shown in Fig. 4a. The first two were created using a KpnI restriction site, which facilitated a reciprocal swap within the ligand-binding domain at amino acid residue 136. These swaps generated the proteins hkbβ3, which contains human sequences from the N terminus to codon 136 and bovine sequences for the rest of the subunit, and bkhβ3, which contains bovine sequences from the N terminus to codon 136 and human sequences for the rest of the subunit. The second set of chimeric β3 subunits were created using a SmaI restriction site, which allowed a reciprocal swap within the C-terminal one-third of the ectodomain at amino acid residue 488, far outside the ligand-binding domain. These swaps generated the proteins hsbβ3, which contains human sequences from the N terminus to codon 488 and bovine sequences for the rest of the subunit, and bshβ3, which contains bovine sequences from the N terminus to codon 488 and human sequences for the rest of the subunit.
FIG. 4.
Analysis of viral protein synthesis in COS-1 cells transfected with bovine-human chimeric β3 subunits. (a) Schematic diagram of chimeric β3 subunits, showing the locations of the ligand-binding domain (LBD), transmembrane domain (TMD), and cytoplasmic domain (CD), as well as the locations of the KpnI (codon 136) and SmaI (codon 488) sites used to generate them (see Materials and Methods). The white background with the black stipples represents human sequences, and the black background with the white stipples represents bovine sequences. (b) FMDV type A12-infected-radiolabeled cell lysates prepared from cells cotransfected with a bovine αv subunit and the β3 subunits shown in panel a were analyzed as described in the legend to Fig. 3 and Materials and Methods.
These chimeras and the wild-type β3 subunit were cotransfected into COS-1 cells, along with the human or bovine αv subunit. The resulting cultures were checked for αvβ3 expression level by IHC, infected with FMDV type A12, labeled overnight, and analyzed by RIP and SDS-PAGE. The results of transfections with the intact bovine and human β3 subunits confirmed the importance of the bovine β3 subunit in increased receptor utilization (Fig. 4b). The results of transfections with the chimeric β3 subunits showed that the hkbβ3 or hsbβ3 chimeras supported replication to the same level as the intact bovine β3 did. Interestingly, these results suggest that the presence of bovine or human sequences from the N terminus of the β3 subunit to amino acid residue 488, including the ligand-binding domain, did not influence the level of viral protein synthesis observed. In contrast, the higher levels of viral protein synthesis were observed only when the β3 subunit contained bovine sequences downstream from codon 488 (hkbβ3 and hsbβ3). To rule out any influence of the bovine αv subunit, we repeated the experiment using the human αv subunit and obtained similar results to those seen in Fig. 4b (data not shown).
Sequence comparison within the C-terminal region of the β3 subunit ectodomain.
The β3 subunit has a high cysteine content, as do all integrin β subunits (15). The bovine subunit contains 54 cysteine residues, 30 of which are located within a region of four tandem amino acid repeats near the C terminus of the ectodomain. This is within the region downstream from amino acid residue 488, which appears to be responsible for the observed increase in viral protein synthesis. A comparison of the amino acid sequences of the bovine and human β3 subunits in this region is shown in Fig. 5. The vertical arrow at amino acid residue 488 represents the location of the SmaI restriction endonuclease site, and the horizontal arrows show the four tandem repeats. It can be seen that downstream from residue 488 there are only seven amino acid residues which differ between the bovine and human subunits. However, all of the cysteines are conserved, as they are in the entire subunit, with the exception of one, at residue 503, within the second repeat region, which is an arginine in the bovine integrin. The significance of changes within this region on the ability of the subunit to function as a viral receptor is examined in Discussion.
FIG. 5.
Comparison of amino acid sequences within the cysteine-rich repeat region between bovine and human β3 subunits. Deduced amino acid sequences of the bovine and human β3 subunits from amino acid residue 394 to the last residue in the subunit ectodomain (residue 692) are shown. The conserved sequences (con) are shown in lowercase type, with the exception of the cysteine residues, which are capitalized. Sequences which differ between bovine (bov) and human (hum) subunits are shown in capital letters. The horizontal arrows indicate the location of the four cysteine-rich repeats, and the vertical arrow indicates the location of the SmaI site. The dotted line represents the putative disulfide bond assignment for cysteine 503 in the human β3 subunit (15).
DISCUSSION
Previous results have implicated integrin αvβ3 as a receptor for FMDV by demonstrating inhibition of infection using integrin-specific antibodies (11) and by demonstrating that cells transfected with cloned human integrin subunit cDNAs became susceptible to infection by FMDV (47). Since these studies were performed with cDNAs encoding integrin subunits from a host that is not susceptible to foot-and-mouth disease (see Introduction), we have repeated these studies with molecularly cloned bovine αv and β3 cDNAs. The results of these studies indicate that FMDV was able to utilize the bovine integrin more efficiently than it utilized the human homolog, and this increased efficiency appears to correlate with the presence of the bovine β3 subunit.
We have utilized transient expression of αv and β3 subunits of bovine and human origin in COS-1 cells to examine receptor utilization by FMDV. Using this system, we have examined the replication of our laboratory strain of FMDV (type A12), its bovine tongue-derived variant, vRM-SSP (51), and the highly cattle-virulent type O1Campos variant, vCRM8 (54), which all utilize only the integrin αvβ3 as receptor (47). These experiments showed that for these three viruses, the cotransfection of cells with a bovine β3 subunit and either a bovine or human αv subunit resulted in the expression of a more efficiently utilized receptor than did the cotransfection of cells with both human subunits or bovine αv and human β3 subunits.
To determine the regions of the bovine β3 subunit that might be responsible for the increased efficiency of use as an FMDV receptor, we generated chimeric bovine-human β3 subunit cDNAs and tested their efficiency as receptors for type A12 FMDV. The results of these studies indicated that bovine sequences downstream from codon 488, outside of the putative ligand-binding domain, appeared to be responsible for the increased efficiency of the bovine receptor (see Results) (Fig. 4).
Comparison of the amino acid sequences of the bovine and human β3 subunit between codon 488 and the C terminus of the subunit reveals virtually identical transmembrane and cytoplasmic domains, with only seven amino acid changes in the ectodomain (Fig. 5). This region of the subunit is rich in cysteines which contribute to the overall structure of the integrin through disulfide bonding (15). In the mature bovine β3 subunit, 7% of the amino acids are cysteines (a total of 54 cysteine residues). Thirty of these cysteine residues are within a region of four tandem repeats within the integrin stalk region, known as the cysteine-rich repeats (15, 30, 33, 35). A cysteine residue just upstream of the first cysteine-rich repeat region, at codon 435, forms a disulfide bond with a cysteine near the N terminus, at codon 5, and probably contributes to the formation of the β3 globular head that interacts with a similar structure on the α subunit to form the complete ligand-binding region (16, 49). The SmaI site at codon 488 occurs at the beginning of the second repeat (Fig. 5). Within the cysteine-rich repeat region of all the β subunits, the positions of the cysteines are highly conserved, with seven residues in the first repeat and eight residues in the second through fourth repeats. Similar cysteine-rich repeat regions are also found in laminin B chains and epidermal growth factor (10). Examination of the amino acid sequence comparisons between the bovine and human subunits within this region reveals that the cysteine found at residue 503 in the human subunit has been changed to an arginine in the bovine subunit. Thus, the region of the bovine β3 subunit that confers the higher efficiency of utilization of the subunit as a receptor for FMDV is missing one cysteine residue.
There are six other amino acid changes within the β3 region that confers increased receptor efficiency (Fig. 5), and we have not yet determined which of these changes may play a role in receptor efficiency. The loss of the cysteine at residue 503, however, is an intriguing change. The overall structure of the β subunits, based on primary sequence, is conserved among many species, and much of that structure appears to be dependent on the disulfide bonding between cysteine residues (30). Therefore, reports that cysteine 503 in the human β3 subunit forms a disulfide bond with cysteine 536 in the third repeat region (15) makes the absence of a cysteine at this position particularly interesting. The cysteine-rich repeat region has been implicated in the modulation of integrin activation and ligand-binding activity. A naturally occurring mutation within this region in the human β3 subunit is responsible for the activation of both αvβ3 and αIIbβ3 (37), and a MAb which binds to the cysteine-rich repeat region of the β1 subunit increases the affinity of the α5β1 integrin for its natural ligand, fibronectin (21). In addition, binding of β3 integrins to their ligands induces conformational changes within the subunit, exposing new epitopes, defined by their ability to bind certain MAbs. These new epitopes are known as ligand-induced binding sites (LIBS). The conformational changes that expose the LIBS can increase the affinity of the β3 receptor for its ligand (20). Mapping of a number of LIBS has shown that some of them reside within the cysteine-rich repeat region (32).
The experiments reported here are indirect measures of receptor utilization and have not addressed whether the bovine receptor has a higher affinity for the virus or whether the C-terminal region of the ectodomain may be mediating an event subsequent to adsorption, either penetration or eclipse. However, an examination of virus binding in relation to the level of viral protein synthesis observed in transfected-infected cells indicated that cells transfected with bovine subunit cDNA adsorbed higher levels of type A12 virus than did cells transfected with human integrin cDNAs (data not shown). Furthermore, analysis of infected cells by IHC using a virus-specific MAb showed that increased numbers of infected cells were present in cultures transfected with the bovine β3 subunit (not shown).
Finally, it is interesting to speculate, from the standpoint of receptor utilization, why foot-and-mouth disease is limited to cloven-hoofed animals. Results from this and previous studies have shown that FMDV can utilize human and simian αvβ3 as a receptor in cell culture (11, 47). However, it is quite clear that the virus replicates to a greater extent in cells expressing the bovine integrin, specifically the cysteine-rich repeat region of the β3 subunit of that integrin, which has a high degree of structural conservation among all β subunits (30). Thus, it is possible that FMDV evolved into a disease of cloven-hoofed livestock because the structure of their αvβ3 receptors resulted in a more advantageous “fit” with the viral surface that would, in turn, lead to much greater viral replication and disease within these species. However, since this integrin probably performs similar functions in a wide variety of species, the structural differences cannot be radically different, as evidenced by the high degree of sequence similarity between the bovine and human integrins. It is also important to note that receptors alone may not necessarily determine FMDV species tropism. Recent results have shown that a type O virus, isolated from an outbreak which occurred only in swine in Taiwan in 1997, contained a deletion in nonstructural protein 3A, which led to restricted growth in bovine cells and attenuation in cattle (9). In the case of poliovirus, which causes disease only in primates, a murine homolog of the poliovirus receptor has been found and is unable to bind the virus (46). This inactivity of the murine homolog has been mapped to a few amino acid differences with the human homolog in the first immunoglobulin domain of the receptor (2, 31). We have transfected COS-1 cells with the murine β3 subunit (a kind gift from Erich Mackow and Eric Brown) and have found that viral protein synthesis was comparable to that seen with the human receptor (data not shown). We are currently cloning the porcine αv and β3 subunits, and it will be interesting to see whether the changes within the β3 cysteine-rich region are similar those seen in the bovine integrin.
ACKNOWLEDGMENTS
We thank Michael LaRocca for excellent technical assistance and William Golde for performing the FACS analysis.
REFERENCES
- 1.Acharya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature. 1989;337:709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- 2.Aoki J, Koike S, Ise I, Sato-Yoshida Y, Nomoto A. Amino acid residues on human poliovirus receptor involved in interaction with poliovirus. J Biol Chem. 1994;269:8431–8438. [PubMed] [Google Scholar]
- 3.Baranowski E, Ruiz-Jarabo C M, Sevilla N, Andreu D, Beck E, Domingo E. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J Virol. 2000;74:1641–1647. doi: 10.1128/jvi.74.4.1641-1647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barteling S J, Vreeswijk J. Development in foot-and-mouth disease vaccines. Vaccine. 1991;9:75–88. doi: 10.1016/0264-410x(91)90261-4. [DOI] [PubMed] [Google Scholar]
- 5.Baxt B. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 1987;7:257–271. doi: 10.1016/0168-1702(87)90032-3. [DOI] [PubMed] [Google Scholar]
- 6.Baxt B, Becker Y. The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes. 1990;4:73–83. doi: 10.1007/BF00308567. [DOI] [PubMed] [Google Scholar]
- 7.Baxt B, Mason P W. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology. 1995;207:503–509. doi: 10.1006/viro.1995.1110. [DOI] [PubMed] [Google Scholar]
- 8.Baxt B, Morgan D O, Robertson B H, Timpone C H. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J Virol. 1984;51:298–305. doi: 10.1128/jvi.51.2.298-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard C W, Mason P W. Genetic determinants of altered virulence of Taiwanese foot-and-mouth disease virus. J Virol. 2000;74:987–991. doi: 10.1128/jvi.74.2.987-991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg R W, Leung E, Gough S, Morris C, Yao W-P, Wang S-X, Ni J, Krissansen W. Cloning and characterization of a novel β integrin-related cDNA coding for the protein TIED (“ten β integrin EGF-like repeat domains”) that maps to chromosome band 13q33: a divergent stand-alone integrin stalk structure. Genomics. 1999;56:169–178. doi: 10.1006/geno.1998.5707. [DOI] [PubMed] [Google Scholar]
- 11.Berinstein A, Roivainen M, Hovi T, Mason P W, Baxt B. Antibodies to the vitronectin receptor (integrin αvβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69:2664–2666. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya S, Chenzhong F, Bhattacharya J, Greenberg S. Soluble ligands of the αvβ3 integrin mediate enhanced tyrosine phosphorylation of multiple proteins in adherent bovine pulmonary artery endothelial cells. J Biol Chem. 1995;270:16781–16787. doi: 10.1074/jbc.270.28.16781. [DOI] [PubMed] [Google Scholar]
- 13.Bitan G, Scheibler L, Greenberg Z, Rosenblatt M, Chorev M. Mapping the integrin αvβ3-ligand interface by photoaffinity cross-linking. Biochemistry. 1999;38:3414–3420. doi: 10.1021/bi981946c. [DOI] [PubMed] [Google Scholar]
- 14.Blystone S D, Lindberg F P, LaFlamme S E, Brown E J. Integrin β3 cytoplasmic tail is necessary and sufficient for regulation of α5β1 phagocytosis by αvβ3 and integrin-associated protein. J Cell Biol. 1995;130:745–754. doi: 10.1083/jcb.130.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvete J J, Henschen A, González-Rodríguez J. Assignment of disulphide bonds in human platelet GPIIIa. A disulphide pattern for the β-subunits of the integrin family. Biochem J. 1991;274:63–71. doi: 10.1042/bj2740063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrell N A, Fitzgerald L A, Steiner B, Erickson H P, Phillips D R. Structure of human platelet membrane glycoproteins IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985;260:1743–1749. [PubMed] [Google Scholar]
- 17.Cheresh D. Human endothelial cells synthesize and express an arg-gly-asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cieutat A M, Ross J P, Letourneur F, Poncz M, Rifat S. A comparative analysis of cDNA-derived sequences for rat and mouse β3 integrins (GPIIIa) with their human counterpart. Biochem Biophys Res Commun. 1993;193:771–778. doi: 10.1006/bbrc.1993.1692. [DOI] [PubMed] [Google Scholar]
- 19.D'Souza S E, Haas T A, Piotrowicz R S, Byers-Ward V, McGrath D E, Soule H R, Cierniewski C, Plow E F, Smith J W. Ligand and cation binding are dual functions of a discrete segment of the integrin β3 subunit: cation displacement is involved in ligand binding. Cell. 1994;79:659–667. doi: 10.1016/0092-8674(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 20.Du X, Gu M, Weise J W, Nagaswami C, Bennett J S, Bowditch R, Ginsberg M H. Long range propagation of conformational changes in integrin αIIbβ3. J Biol Chem. 1993;268:23087–23092. [PubMed] [Google Scholar]
- 21.Faull R J, Wang J, Leavesley D I, Puzon W, Russ G R, Vestweber D, Takada Y. A novel activating anti-β1 integrin monoclonal antibody binds to the cysteine-rich repeats in the β1 chain. J Biol Chem. 1996;271:25099–25106. doi: 10.1074/jbc.271.41.25099. [DOI] [PubMed] [Google Scholar]
- 22.Fernández C, Clark K, Burrows L, Schofield N R, Humphries M J. Regulation of the extracellular ligand binding activity of integrins. Front Biosci. 1998;3:684–700. doi: 10.2741/a313. [DOI] [PubMed] [Google Scholar]
- 23.Fox G, Parry N R, Barnett P V, McGinn B, Rowlands D, Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid) J Gen Virol. 1989;70:625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- 24.Frachet P, Uzan G, Thevenon D, Denarier E, Prandini M H, Marguerie G. GPIIb and GPIIIa amino acid sequences deduced from human megakaryocyte cDNAs. Mol Biol Rep. 1990;14:27–33. doi: 10.1007/BF00422712. [DOI] [PubMed] [Google Scholar]
- 25.Fry E E, Lea S M, Jackson T, Newman J W I, Ellard F M, Blakemore W E, Abu-Ghazaleh R, Samuel A, King A M Q, Stuart D I. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrilovskaya I N, Brown E J, Ginsberg M H, Mackow E R. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J Virol. 1999;73:3951–3959. doi: 10.1128/jvi.73.5.3951-3959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrilovskaya I N, Shepley M, Shaw R, Ginsberg M H, Mackow E R. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci USA. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson M A, Leavesley D I, Ashman L K. Microfibril-associated glycoprotein-2 specifically interacts with a range of bovine and human cell types via the αvβ3 integrin. J Biol Chem. 1999;274:13060–13065. doi: 10.1074/jbc.274.19.13060. [DOI] [PubMed] [Google Scholar]
- 29.González-Amaro R, Sánchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol. 1999;19:389–429. [PubMed] [Google Scholar]
- 30.Green L, Mould A P, Humphries M J. The integrin beta subunit. Int J Biochem Cell Biol. 1998;30:179–184. doi: 10.1016/s1357-2725(97)00107-6. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Bowman V D, Mueller S, Bator C M, Bella J, Peng X, Baker T S, Wimmer E, Kuhn R J, Rossmann M G. Interaction of the poliovirus receptor with poliovirus. Proc Natl Acad Sci USA. 2000;97:79–84. doi: 10.1073/pnas.97.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda S, Tomiyama Y, Pelletier A J, Annis D, Honda Y, Orcheskowski R, Ruggeri Z, Kunicki T J. Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin β3 subunit. J Biol Chem. 1995;270:11947–11954. doi: 10.1074/jbc.270.20.11947. [DOI] [PubMed] [Google Scholar]
- 33.Horton M A. The αvβ3 integrin “vitronectin receptor.”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 34.Humphries M J. Towards a structural model of an integrin. Biochem Soc Symp. 1999;65:63–78. [PubMed] [Google Scholar]
- 35.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 36.Jackson T, Ellard F M, Abu-Ghazaleh R, Brooks S M, Blakemore W E, Corteyn A H, Stuart D I, Newman J W I, King A M Q. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J Virol. 1996;70:5282–5287. doi: 10.1128/jvi.70.8.5282-5287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashiwagi H, Tomiyama Y, Tadokoro S, Honda S, Shiraga M, Mizutani H, Handa M, Kurata Y, Matsuzawa Y, Shattil S J. A mutation in the extracellular cysteine-rich repeat region of the β3 subunit activates integrins αIIbβ3 and αvβ3. Blood. 1999;93:2559–2568. [PubMed] [Google Scholar]
- 38.Kottler M L, Counis R, Degrelle H. Sex steroid-binding protein: identification and comparison of the primary product following cell-free translation of human monkey (Macaca fascicularis) liver RNA. J Steroid Biochem. 1989;33:201–207. doi: 10.1016/0022-4731(89)90295-1. [DOI] [PubMed] [Google Scholar]
- 39.Lin E C K, Ratnikov B I, Tsai P M, Carron C P, Meyers D M, Barbas III C F, Smith J W. Identification of a region in the integrin β3 subunit that confers ligand binding specificity. J Biol Chem. 1997;272:23912–23920. doi: 10.1074/jbc.272.38.23912. [DOI] [PubMed] [Google Scholar]
- 40.Lindberg F P, Gresham H D, Schwarz E, Brown E J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loftus J C, Halloran C E, Ginsberg M H, Feigen L P, Zablock J A, Smith J W. The amino-terminal one-third of αIIb defines the ligand recognition specificity of integrin αIIbβ3. J Biol Chem. 1996;271:2033–2039. doi: 10.1074/jbc.271.4.2033. [DOI] [PubMed] [Google Scholar]
- 42.Logan D, Abu-Ghazaleh R, Blakemore W, Curry S, Jackson T, King A, Lea S, Lewis R, Newman J, Parry N, Rowlands D, Stuart D, Fry E. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature. 1993;362:566–568. doi: 10.1038/362566a0. [DOI] [PubMed] [Google Scholar]
- 43.Martínez M, Verdaguer N, Mateu M G, Domingo E. Evolution subverting essentiality: dispensability of the cell attachment arg-gly-asp motif in multiply passaged foot-and-mouth disease virus. Proc Natl Acad Sci USA. 1997;94:6798–6802. doi: 10.1073/pnas.94.13.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason P W, Baxt B, Brown F, Harber J, Murdin A, Wimmer E. Antibody-complexed foot-and-mouth disease, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology. 1993;192:568–577. doi: 10.1006/viro.1993.1073. [DOI] [PubMed] [Google Scholar]
- 45.Mason P W, Rieder E, Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement mechanism. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison M E, Racaniello V R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neff S, Carvalho S, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelsen-Salz B, Eggers H J, Zimmermann H. Integrin αvβ3 (vitronectin receptor) is a candidate receptor for the virulent echovirus 9 strain Barty. J Gen Virol. 1999;80:2311–2313. doi: 10.1099/0022-1317-80-9-2311. [DOI] [PubMed] [Google Scholar]
- 49.Nermut M V, Green N M, Eason P, Yamada S S, Yamada K M. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7:4093–4099. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulli T, Koivunen E, Hyypiä T. Cell-surface interactions of echovirus 22. J Biol Chem. 1997;272:21176–21180. doi: 10.1074/jbc.272.34.21176. [DOI] [PubMed] [Google Scholar]
- 51.Rieder E, Baxt B, Mason P W. Animal-derived antigenic variants of foot-and-mouth disease virus type A12 have low affinity for cells in culture. J Virol. 1994;68:5296–5299. doi: 10.1128/jvi.68.8.5296-5299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rieder E, Bunch T, Brown F, Mason P W. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J Virol. 1993;67:5139–5145. doi: 10.1128/jvi.67.9.5139-5145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roivainen M, Piiraninen L, Hovi T, Virtanen I, Rikonen T, Heino J, Hyypiä T. Entry of coxsackievirus A9 into host cells: specific interaction with αvβ3 integrin, the vitronectin receptor. Virology. 1994;203:357–365. doi: 10.1006/viro.1994.1494. [DOI] [PubMed] [Google Scholar]
- 54.Sá-Carvalho D, Rieder E, Baxt B, Rodarte R, Tanuri A, Mason P W. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol. 1997;71:5115–5123. doi: 10.1128/jvi.71.7.5115-5123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider G B, Whitson S W, Cooper L F. Restricted and coordinated expression of β3-integrin and bone sialoprotein during cultured osteoblast differentiation. Bone. 1999;24:321–327. doi: 10.1016/s8756-3282(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 56.Smith J W, Cherish D A. The arg-gly-asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61–203 of the β subunit. J Biol Chem. 1988;263:18726–18731. [PubMed] [Google Scholar]
- 57.Smith J W, Cheresh D A. Integrin (αvβ3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990;265:2168–2172. [PubMed] [Google Scholar]
- 58.Starr C M, Hanover J A. Glycosylation of nuclear pore protein p62. Reticulocyte lysate catalyzes O-linked N-acetylglucosamine addition in vitro. J Biol Chem. 1990;265:6868–6873. [PubMed] [Google Scholar]
- 59.Stave J W, Card J L, Morgan D O, Vakharia V N. Neutralization sites of type O1 foot-and-mouth disease virus defined by monoclonal antibodies and neutralization-escape virus variants. Virology. 1988;161:21–29. doi: 10.1016/0042-6822(88)90390-x. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki S, Argraves W S, Arai H, Languino L R, Pierschbacher M D, Ruoslahti E. Amino acid sequence of the vitronectin receptor α subunit and comparative expression of the adhesion receptor mRNAs. J Biol Chem. 1987;262:14080–14085. [PubMed] [Google Scholar]
- 61.Takagi J, Kamata T, Meredith J, Puzon-McLaughlin W, Takada Y. Changing ligand specificities of αvβ1 and αvβ3 integrins by swapping a short diverse sequence of the β subunit. J Biol Chem. 1997;272:19794–19800. doi: 10.1074/jbc.272.32.19794. [DOI] [PubMed] [Google Scholar]
- 62.Tozer E C, Liddington R C, Sutcliffe M J, Smeeton A H, Loftus J C. Ligand binding to integrin αIIbβ3 is dependent on a MIDAS-like domain in the β3 subunit. J Biol Chem. 1996;271:21978–21984. doi: 10.1074/jbc.271.36.21978. [DOI] [PubMed] [Google Scholar]
- 63.Wada J, Kumar A, Liu Z, Ruoslahti E, Reichardt L, Marvaldi J, Kanwar Y S. Cloning of mouse integrin αv cDNA and role of the αv related matrix receptors in metanephric development. J Cell Biol. 1996;132:1161–1176. doi: 10.1083/jcb.132.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 65.Zimrin A B, Eisman R, Vilaire G, Schwartz E, Bennett J S, Poncz M. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors. J Clin Investig. 1988;81:470–475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]