Abstract
Purpose
We report a patient with bilateral limbal stem cell deficiency (LSCD) like clinical manifestations and secondary corneal perforation presumably induced by durvalumab following its use for the treatment of non-small cell lung carcinoma.
Observations
A 65-year-old male diagnosed with non-small cell lung carcinoma was treated with monthly durvalumab infusions. Two months after starting durvalumab, the patient was found to have bilateral severe keratoconjunctivitis and LSCD-like clinical findings. Despite topical management and oral prednisone for presumed ocular cicatricial pemphigoid, the patient continued to worsen clinically. The patient was transferred to our institution about one year later with persistent inflammation. The patient eventually developed a corneal perforation of the left eye, which required the application of cyanoacrylic tissue adhesive. Due to the lack of response to oral prednisone, durvalumab was discontinued with the approval of the patient's oncologist. Several months following the discontinuation of durvalumab, the conjunctival inflammation subsided, and corneal epithelial breakdown and ulceration resolved.
Conclusions
We report an association between durvalumab and the development of bilateral LSCD-like clinical findings with subsequent corneal perforation. We hope this case reinforces the importance of routine ophthalmologic follow-up after starting any cancer treatment, especially in patients with symptoms and signs suggesting ocular surface disease or inflammation.
Keywords: Immune checkpoint inhibitors, Durvalumab, Limbal stem cell deficiency, Corneal perforation
1. Introduction
Immune checkpoint inhibitors (ICIs) have emerged as a promising therapy in the treatment of a variety of malignancies. These agents capitalize on immune response pathways that target proteins involved in modulating T-cell activity.1,2 Durvalumab is a selective, human IgG1 monoclonal antibody that blocks programmed death ligand 1 (PD-L1) binding to programmed death 1 (PD-1) to allow for T-cell recognition and attack against tumor cells.3 PD-L1 and PD-1 interaction plays an important inhibitory role by promoting the self-tolerance of T-cells through modulation and apoptosis. Their interaction normally downregulates the immune response. If the interaction between PD-L1 and PD-1 is blocked, the immune system is stimulated, enhancing the adaptive response.1,2
PD-L1 inhibitors like durvalumab permit the development of immune-mediated adverse effects that also target the eye. Ophthalmic adverse effects include uveitis, dry eye, and ocular myasthenia gravis, among other ophthalmic inflammatory conditions.4, 5, 6, 7 These events are rare; however, no specific report has previously described durvalumab as a contributing factor for limbal stem cell deficiency (LSCD)-like clinical findings and secondary corneal perforation. Here, we report a patient with bilateral LSCD-like clinical manifestations and secondary corneal perforation presumably induced by durvalumab following its use for the treatment of non-small cell lung carcinoma (NSCLC).
2. Case report
A 65-year-old male was diagnosed with non-small cell lung carcinoma in August of 2021. He was treated with induction chemotherapy—carboplatin and paclitaxel (discontinued due to an infusion reaction)—and radiation. Monthly infusions of durvalumab were started in December of 2021. The patient presented to an outside institution two months after starting durvalumab and was found to have severe bilateral conjunctivitis with negative cultures. His ocular history was notable for bilateral primary open-angle glaucoma controlled with dorzolamide-timolol (2 % and 0.5 %) twice daily in the left eye at the time of our evaluation. The patient was previously on brimonidine 0.2 % in both eyes, which was discontinued in March of 2022 following the onset of his new ocular symptoms. It was unclear why his latanoprost 0.005 % was discontinued. He also had a cataract in the right eye and pseudophakia in the left eye. He had no history of trauma, chemical exposure, or Steven-Johnson syndrome (SJS). The patient was treated for several months with aggressive lubrication and a variety of topical antibiotics and steroids. He was on erythromycin ointment and previously on moxifloxacin 0.5 %, trimethoprim/polymyxin, fluorometholone 0.1 %, and prednisolone 1 % drops for bilateral keratoconjunctivitis and limbal stem cell deficiency (LSCD) of unknown etiology. A Prokera® amniotic membrane corneal bandage was also trialed for the left eye alone in December of 2022. Approximately ten months after the onset of his symptoms, treatment with high-dose oral prednisone (60mg daily) was initiated for presumed ocular cicatricial pemphigoid (OCP). The patient was transferred to our institution about one year later while on maintenance durvalumab infusions.
Initial examination revealed best-corrected visual acuity (BCVA) of 20/400 in the right eye and hand motion (HM) in the left eye. The intraocular pressure was normal in each eye. Further examination revealed good eyelid closure and eyelid apposition with no significant ectropion, entropion, fornix loss, or symblepharon. Marked conjunctival inflammation and thickening were present bilaterally, worse in the left eye. Slit lamp examination revealed findings concerning for a severe LSCD in each eye with 360 degrees of peripheral superficial corneal neovascularization, a diffuse verticillate keratopathy and diffuse subepithelial and stromal scarring bilaterally. In addition, examination of the left eye revealed an epithelial defect in the inferior peripheral cornea with significant stromal thinning but no infiltrate, descemetocele, or perforation. The anterior chamber was deep in each eye. A nuclear sclerotic cataract was present in the right eye, and a posterior chamber intraocular lens was present in the left eye. Dilated fundus examination was unremarkable in the right eye. B-scan revealed no vitreous opacities, retinal, or choroidal abnormalities in the left eye. The patient continued treatment with oral prednisone 40mg once daily, and moxifloxacin drops four times daily in the left eye. The patient's glaucoma drops were tapered to minimize any toxicity, and aggressive lubrication was also advised.
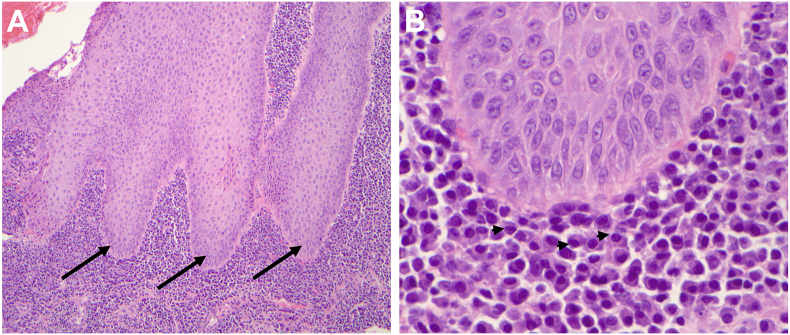
A conjunctival biopsy was performed in the left eye to rule out carcinoma and OCP. The conjunctival biopsy revealed pseudoepitheliomatous hyperplasia and severe inflammation in the substantia propria. There were no pathological or immunohistochemical features of carcinoma or OCP (Fig. 1). His vitamin A level was normal, and an inflammatory workup revealed an elevated anti-nuclear antibody (ANA) titer (greater than 1:1280), a normal angiotensin-converting enzyme (ACE), anti-double stranded DNA (anti-dsDNA), and rheumatoid factor (RF).
Fig. 1.
Photomicrographs of the left conjunctiva taken prior to discontinuation of durvalumab revealing A, conjunctival pseudoepitheliomatous hyperplasia (arrows) with chronic inflammation in the substantia propria; hematoxylin-eosin (H&E) stain, x10 B, the inflammatory cells are mainly plasma cells (arrowheads), indicating a predominant B-cell response against the epithelium; H&E stain, x60.
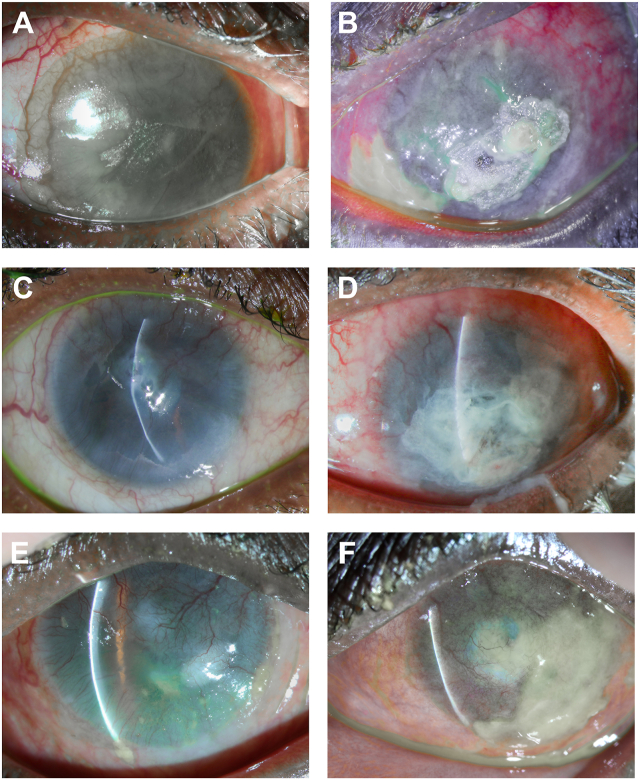
The conjunctival inflammation persisted in each eye, and the patient eventually developed a corneal perforation of the left eye, which required the application of cyanoacrylic tissue adhesive (Fig. 2A and B). Due to the lack of response to oral prednisone, the durvalumab was discontinued with the approval of the patient's oncologist. Several months following the discontinuation of durvalumab, the conjunctival inflammation subsided, and corneal epithelial breakdown and ulceration resolved (Fig. 2C and D). The oral prednisone was tapered and discontinued over the course of a year. At his most recent examination, his BCVA is HM in the right eye and light perception in the left eye with persistent LSCD-like clinical findings, including corneal scarring, vascularization, and thinning but with an intact ocular surface and no epithelial defect or ulceration (Fig. 2E and F). He is a poor surgical candidate due to his underlying medical comorbidities.
Fig. 2.
Serial slit lamp images while on durvalumab (April of 2023) (A,B) and off durvalumab at 1 (C,D) and 7 (E,F) months. A, right eye, 360 pannus with vascularization, central epithelial defect, and bandage-contact lens B, left eye, severely inflamed and thickened conjunctiva with intact cyanoacrylic tissue adhesive following corneal perforation. C, right eye, healed epithelial defect with severe stromal thinning, central pseudocysts but no perforation D, left eye, marked resolution of conjunctival inflammation and thickening, intact cyanoacrylic tissue adhesive, and a formed chamber. E, right eye, and F left eye, improved with persistent limbal stem cell deficiency (LSCD) like clinical manifestations, including corneal scarring, vascularization, thinning, and thick mucoid discharge (as demonstrated inferiorly in 2F) without epithelial defect or ulceration.
3. Discussion
ICIs upregulate the immune response, risking the development of autoimmune-like side effects. Ocular side effects following use of ICIs are rare. They are reported to occur in approximately 1 % of patients following its use.6 The most common ocular side effects include uveitis, dry eye, and ocular myasthenia gravis.4, 5, 6 If divided among target ligands, they are even less frequent in PD-L1 inhibitors—likely related to the fewer number of patients exposed to the drug class.4, 5, 6, 7
Durvalumab, a PD-1 inhibitor, has been associated with the development of keratitis and uveitis.3,6 We report an association between durvalumab and the development of bilateral LSCD-like clinical findings with subsequent corneal perforation. The differential diagnosis for a non-healing epithelial defect is broad and includes dry eye syndrome, exposure, mechanical trauma, toxicity, neurotrophic keratopathy, or LSCD. We speculate that durvalumab might be the cause of his bilateral LSCD-like clinical findings with subsequent chronic corneal epithelial instability. The cornea constitutively expresses high levels of PD-L1, contributing to its immune privilege. Under normal conditions, PD-L1 downregulates antigen-presenting cells (APCs) located in the peripheral cornea, limiting lymphocytic infiltration. Inhibition of PD-1/PD-L1 enhances the immune response leading to an upregulation of APCs and the recruitment of lymphocytes into the cornea; therefore, ICIs like durvalumab can be a cause of autoimmune corneal inflammation.8, 9, 10
The patient's symptoms started about 2 months after the initiation of durvalumab, for which he was continued on for a year prior to our evaluation. Our patient's corneal pathology was consistent with severe LSCD-like clinical manifestations with subsequent epithelial breakdown and ulceration. He had a normal vitamin A level and intact corneal sensation. His ocular adnexa appeared normal with no ectropion, entropion, or lagophthalmos. He had no fornix loss or symblepharon. He had no history of trauma, contact lens wear, chemical exposure, or inflammatory disease. His autoimmune workup did reveal elevated ANA titers; however, we believed that the elevated ANA titers were related to a non-specific, pro-inflammatory state instead of an autoimmune disease. The patient had no previous history of or current evidence of autoimmune disease. Furthermore, LSCD is generally not associated with ANA-type autoimmune disease.
The patient presented with severe bilateral chronic conjunctivitis and underwent a biopsy to exclude malignant masqueraders and OCP. Although the biopsy was negative for carcinoma and OCP, it did reveal pseudoepitheliomatous hyperplasia and severe inflammation in the substantia propria with plasma cells signifying a predominant B-cell response against the conjunctival epithelium. Even though PD-1/PD-L1 signaling has been extensively studied in T-cell tumor immunosuppression, recent advances report that B-cells also play an important role in tumorigenesis and immunotherapy. Some studies have shown that B-cell abundance is positively associated with favorable outcomes, while other studies suggest B-cells are “tumor-promoting”.11 These findings suggest that B-cell function is vast and complex, helping to explain the B-cell response identified in his histopathology.
While we cannot be certain that previous chemotherapy or other factors did not have a role in this patient's severe ocular surface disease, he was followed regularly for glaucoma, and his clinical appearance of inflammation and LSCD did not appear until after initiation of durvalumab. Many mild to moderate adverse ocular effects of checkpoint inhibitors have been managed with topical or periocular steroids; more severe cases have been managed with systemic corticosteroids or cessation of therapy.6 Our patient progressively worsened despite the use of high-dose systemic corticosteroids. It was not until durvalumab was discontinued that the patient's bilateral conjunctival inflammation subsided, and his chronic corneal epithelial defects and ulcerations resolved. Unfortunately, the patient is left with severe bilateral LSCD-like clinical findings including marked corneal scarring and is not a good candidate for surgery or ocular surface restoration procedures.
4. Conclusions
There are case reports of ulceration following the use of other immune checkpoint inhibitors, including ipilimumab, nivolumab, and pemebrolizumab.12, 13, 14 While it is impossible to know whether earlier discontinuation of the drug would have led to different outcomes, we hope this case reinforces the importance of routine ophthalmologic follow-up after starting any new cancer treatment, especially in patients with symptoms and signs suggesting ocular surface disease or inflammation. We also suggest that a careful history of medication use be obtained in all patients with new onset ocular surface disease or LSCD-like clinical findings in efforts to prevent vision loss.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Nahrain Putris Schumaier: Writing – review & editing, Writing – original draft, Conceptualization. David G. Heidemann: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Chirag Gupta: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727. pmc/articles/PMC7136921/ [PMC free article] [PubMed] [Google Scholar]
- 2.Alsaab H.O., Sau S., Alzhrani R., et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8(AUG) doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AstraZeneca Pharmaceuticals L.P. AstraZeneca Pharmaceuticals LP; Wilmington, DE: 2017. Imfinzi (Durvalumab) Injection, for Intravenous Use [Prescribing Information]www.fda.gov/medwatch [Google Scholar]
- 4.Fang T., Maberley D.A., Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319–322. doi: 10.1016/j.joco.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young L., Finnigan S., Streicher H., et al. Ocular adverse events in PD-1 and PD-L1 inhibitors. J Immunother Cancer. 2021;9(7) doi: 10.1136/jitc-2020-002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalvin L.A., Shields C.L., Orloff M., Sato T., Shields J.A. vol. 38. 2018. http://journals.lww.com/retinajournal (Review CHECKPOINT INHIBITOR IMMUNE THERAPY Systemic Indications and Ophthalmic Side Effects). [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.J., Lee J.S., Lee J., et al. Factors associated with ocular adverse event after immune checkpoint inhibitor treatment. Cancer Immunol Immunother. 2020;69(12):2441–2452. doi: 10.1007/s00262-020-02635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moshirfar M., Basharat N.F., Seitz T.S., Ply B.K., Ronquillo Y.C., Hoopes P.C. Corneal transplant rejections in patients receiving immune checkpoint inhibitors. J Clin Med. 2022;11(19):5647. doi: 10.3390/JCM11195647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L., Jin Y., Freeman G.J., Sharpe A.H., Dana M.R. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J Immunol. 2007;179(6):3672–3679. doi: 10.4049/JIMMUNOL.179.6.3672. [DOI] [PubMed] [Google Scholar]
- 10.Yang W., Li H., Chen P.W., et al. PD-L1 expression on human ocular cells and its possible role in regulating immune-mediated ocular inflammation. Invest Ophthalmol Vis Sci. 2009;50(1):273–280. doi: 10.1167/IOVS.08-2397. [DOI] [PubMed] [Google Scholar]
- 11.Zhang E., Ding C., Li S., et al. Roles and mechanisms of tumour-infiltrating B cells in human cancer: a new force in immunotherapy. Biomark Res. 2023;11(1):1–14. doi: 10.1186/S40364-023-00460-1. 2023 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramaekers A., Aspeslagh S., De Brucker N., et al. Bilateral corneal perforation in a patient under anti-PD1 therapy. Cornea. 2021;40(2):245–247. doi: 10.1097/ICO.0000000000002490. [DOI] [PubMed] [Google Scholar]
- 13.Aschauer J., Donner R., Lammer J., Schmidinger G. Bilateral corneal perforation in Ipilimumab/Nivolumab - associated peripheral ulcerative keratitis. Am J Ophthalmol Case Rep. 2022;28 doi: 10.1016/J.AJOC.2022.101686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen A.T., Elia M., Materin M.A., Sznol M., Chow J. Cyclosporine for dry eye associated with nivolumab: a case progressing to corneal perforation. Cornea. 2016;35(3):399–401. doi: 10.1097/ICO.0000000000000724. [DOI] [PubMed] [Google Scholar]