Abstract
The discovery of a new lineage of helper T cells that selectively produces interleukin (IL) - 17 has provided exciting new insights into immunoregulation, host defense and the pathogenesis of autoimmune diseases. Although the factors that promote murine Th17 differentiation have been intensively examined, there has been much less information on the regulation of this cytokine in human T cells. IL-17 is readily produced by human memory T cells, which we now know express distinct patterns of chemokine receptor expression and may differentiate in response to selective pathogens. Recently it has been shown that IL-1, IL-6 and IL-23 are important in driving human Th17 differentiation. However, TGFβ−1 which is important for the differentiation of murine Th17 cells and inducible regulatory T cells (iTregs), is not required and even inhibits for human Th17 differentiation. In addition, human Th17 cells also produce other proinflammatory cytokines. Further characterization of the transcription regulation of human IL-17 expression, and the epigenetic regulation of human Il17 locus should improve our understanding the lineage commitment of human Th17 cells. Targeting the production and action of this cytokine is also likely to be beneficial therapeutically for autoinflammatory and autoimmune diseases.
1. Introduction
IL-17A is a proinflammatory cytokine initially identified in mouse cytotoxic T cells [1]. Later human IL-17 was cloned and the cDNA encodes a 155-amino acid polypeptide with an N-terminal hydrophobic signal sequence typical of cytokines [2]. Now we know that this family includes 6 family members: IL-17 (also denoted IL-17A), IL-17B, IL-17C, IL-17D, IL-17E and IL-17F [1–4] that share 16–50% amino acid identity but apparently have different tissue expression patterns. IL-17A and IL-17F appear to be important in host defense against extracellular bacteria and fungi [5–7]. Additionally, a number of autoimmune diseases are associated with overproduction of these cytokines and interference with IL-17 production or action attenuates autoimmune disease [8–14] .
Although it has been known for more than 10 years that IL-17 is a product of activated CD4+T cells, it was not until recently that it was recognized that a subset of helper T cells exists, Th17 cells, which preferentially produces IL-17 [15–17]. Conversely, it was also recognized that polarized Th1 and Th2 cells generally do not produce IL-17 and in fact, IFN-γ and IL-4 inhibit Th17 differentiation. Thus, it was proposed that IL-17-producing helper T cells or Th17 cells represent a new lineage of CD4+ T cell, although it should be borne in mind that cells producing both IFN-γ and IL-17 are readily detectable in physiologic and pathologic settings. During the past two years, cytokines and signaling pathways involved in regulating this new lineage differentiation, as well as its importance in the pathogenesis of autoimmune disease, have been intensively investigated in various mouse models. Given the pathogenic relevance of IL-17, it is obviously important to understand how this cytokine is controlled in human T cells and to define the conditions under which human naïve CD4+ T cells might become Th17 cells. Despite a great deal of information on the regulation of IL-17 in the mouse, until recently there has been a paucity of information of the control of IL-17 in human lymphocytes. Recent advances in our understanding of the similarities and differences in the regulation IL-17 in mice and humans will be discussed in this review.
2. Overview of murine Th17 regulation
Like other CD4+ T cell subsets, Th17 differentiation is largely controlled by products of dendritic cells (DCs) and other antigen presenting cells. Specifically, inflammatory cytokines like IL-6 in conjunction with TGFβ−1 are important initiators of the program that drives differentiation of naïve CD4+ T cells to become Th17 cells [18][19][20]. Accordingly, IL-6−/− mice have reduced but not absent Th17s [21]. Another product of DCs, IL-23, serves to expand and maintain Th17s in vivo. The current view is that IL-23 is not sufficient to drive Th17 differentiation of naïve cells principally because naïve CD4+ murine T cells lack receptors for this cytokine. Nonetheless, IL-23 is important for driving production of IL-17 in vivo [22–24][25]. Th17 cells preferentially produce IL-21, which has an important role as an autocrine positive regulator of Th17 differentiation and a negative regulator of Th1 differentiation [21, 26–29]. Thus, IL-21 acts in a manner analogous to IL-4 in that the lineage specific product promotes differentiation to the subset that produces this factor while simultaneously inhibiting other fates. Accordingly, both IL-21 and IL-21R-deficient mice also have significant impairments IL-17 production [26][21].
What these cytokines, IL-6, IL-21 and IL-23, all have in common is that they activate the transcription factor Stat3. Stat3 directly binds the Il17 and Il21 genes [30] [27]. Stat3 is also a positive regulator of another transcription factor, the orphan retinoid receptor, Rorγt, which is a critical regulator of Th17 differentiation [31]. Conversely, retroviral overexpression of constitutively active Stat3, results in increased production of IL-17 production [32]. Stat3 also regulates expression of IL-23R [26, 28, 33]. Suppressor of cytokine signaling (SOCS3) is a cytokine-inducible negative regulator of Stat3. Interestingly, deletion of Socs3 is associated with enhanced Stat3 phosphorylation and increased IL-17 production, and widespread inflammatory disease consistent with dysregulation of IL-17 production [30, 34].
In their original studies of murine Th17 differentiation, Stockinger’s group pointed out that LPS-stimulated DC cells were potent inducers of Th17 development[18]. In addition to IL-6 and TGFβ−1, they showed that inflammatory cytokines such as IL-1 and tumor necrosis factor (TNF) are contributors to murine Th17 differentiation. Although in vitro differentiation of Th17 cells can occur in the absence of IL-1, IL-1RI−/− mice have impaired Th17 cell differentiation and reduced incidence of EAE associated with failure to induce autoantigen-specific Th17 cells [35].
Given the intense inflammatory actions of IL-17, it is no surprise that there are also many negative regulators of IL-17 production. IL-2, IL-27, IFN-γ, and IL-4 all inhibit IL-17 production. Additionally, another DC product, retinoic acid (RA), inhibits IL-17 production [36–39] . The precise mechanism by RA inhibits IL-17 production is unknown. However, it is known that RA is selectively produced by CD103+ mucosal DCs and binds to retinoic acid receptors (RAR) to positively regulate Foxp3, and thereby inducing a Treg phenotype [38][40]. Whether there are endogenous ligands for Rorγ produced by immune cells, which positively regulate Th17 differentiation, is not known but is an intriguing possibility.
3. IL-17-producing human memory cells are readily detectable
IL-17 has been reported to be overproduced not only in mouse models of diseases, but also in several human autoimmune diseases including: multiple sclerosis, inflammatory bowel disease, psoriasis and rheumatoid arthritis [8][10][11][41]. Therefore, it is important to identify the cellular sources of IL-17 and the regulation of this cytokine in humans.
Although the conditions required to polarize naïve human CD4+ T cells to become selective IL-17 producers has been elusive, it has been readily apparent that human memory T cells are very effective producers of IL-17. This occurs by stimulation with anti-CD3 and anti-CD28 alone, although there is considerable individual variation in the propensity of IL-17 production[33][42]. Also of note is that IL-17- and IFN-γ-double producers are consistently detected in memory CD4+ T cells [33]. One marker of memory T cells that produce IL-17 is the IL-23 receptor [42]. Additionally though, both memory and effector IL-17-producing cells in peripheral blood and inflamed tissues express the chemokine receptor CCR6 cells [6][43]. However, it appears that T cell which express CCR6 and CCR4 only produce IL-17 whereas T cells that express CCR6 and CXCR3 and produce both IL-17 and IFN-γ [6].
By assessing memory T cell responses to various antigens, it has been possible to correlate responsiveness to certain pathogens with selective IL-17 production. That is, C. albicans – responsive T cells were noted to be selective IL-17 producers whereas M. tuberculosis– specific cells produced both IL-17 and IFN-γ. Interestingly, this pattern of cytokine production also correlated with specific chemokine receptor expression. That is, C. albicans – responsive T cells were primarily CCR6+CCR4+ (i.e. IL-17 producers) and M. tuberculosis–specific cells were mainly CCR6+CXCR3+ (IL-17, IFN-γ dual producers). As CCR6 and CCR4 are important for localization to mucosa and skin, the findings also suggest that different helper T cell subsets have distinct homing capacity. Whether murine Th17 cells have the similar pattern of chemokine receptor expression has not been reported; however, the majority of Rorγ-expressing cells in the mouse reside in the gut [31]. In humans, IL-17-producing cells have been detected in the gut in patients with Crohn’s disease; however, some of these IL-17-producing cells also produce IFNγ [43]. Notably, these IL-17 producing cells are CD45RO+ memory T cells.
As indicated, occupancy of the TCR alone is sufficient to induce large amounts of IL-17 in human memory T cells [33, 42]. TCR signaling activates a number of pathways and transcription factors, but one major pathway activated by the TCR, which appears to be very relevant to IL-17 regulation, is the production of intracellular calcium and the activation of the transcription nuclear factor of activated T cells (NFAT). Not surprisingly, the proximal promoter of the human IL17A gene contains two NFAT binding sites which appear to be important in regulation of IL-17 [44]. In addition, TCR occupancy in human memory T cells also upregulates RORγ expression although the regulation of expression of this transcription is not well understood [33].
One common feature of many studies of human T cell differentiation, is their increased flexibility or plasticity compared to mouse T cells [45]. This feature is also observed in human Th17 cells. Although clearly Th17 differentiation is inhibited by Th1 and Th2 polarizing conditions, after one week culture, restimulation with anti-CD3/CD28 nonetheless resulted in IL-17 production in these cells suggesting more plasticity than mouse cells [33]. Additional evidence of plasticity is the coexistence of dual IL-17- and IFN-γ-producing memory T cells (see above) [6][33, 42][43]. Whether IL-17-producers and IL-17+IFN-γ+ double producers represent distinct two subsets or are developmentally related is not clear. Functional study of these two subsets showed similar cytotoxic capability, suppression by Treg cells, and ability to help B cells, Furthermore, both subsets reportedly express similar levels of RORγt and T-bet [43].
Thus, it is clear that like the mouse, there are circumstances in which human memory cells can arise that preferentially produce IL-17. This appears to be relevant to the pathogenesis of autoimmune disease and presumably in normal host defense. This begs the question, how do human Th17 cells arise?
4. Human Th17 differentiation – lessons learned
As discussed above, IL-6, IL-21, and IL-23, cytokines that activate Stat3, are now recognized to be a key regulators of IL-17 production. Additionally, in murine studies inflammatory cytokines such as IL-1 were also important in driving IL-17 production. Therefore, it was logical to explore whether combinations of such cytokines are capable of promoting human Th17 differentiation. In fact, it appears that these cytokines do promote Th17 differentiation of naïve human CD4+ T cells; however, not all groups agree on all of the details.
In the recent studies by Acosta-Rodriguez et al and Wilson et al, the authors report that IL-6 alone fails to induce either the expression of Rorγt or IL-17 [42][46]. Furthermore, IL-1 alone is not sufficient to induce sustained IL-17 expression and only transiently induces expression of RORγ. Sallusto’s group has found that IL-1 synergizes with IL-6 to induce human Th17 development with sustained Rorγ expression [42, 46]. Of note, is that this cytokine combination induces substantial numbers of cells producing both IL-17 and IFN-γ [46]. Studies from Sallusto’s group also examined the ability of different types of antigen presenting cells (APCs) to drive Th17 differentiation. They showed that LPS-activated DCs produce large amounts of IL-12. Unsurprisingly, such cells promoted Th1 cell differentiation rather than Th17 differentiation. By using various ligands of toll-like receptors with different APCs, these investigators found that activation of monocytes with peptidoglycan (a ligand for TLR2) resulted in high level IL-6 production with little IL-12 production, thereby providing a particularly effective stimulus for generating cells that selectively produce IL-17 [46].
As discussed previously, IL-23 was initially thought to be the driver of Th17 differentiation in murine cells; however, it is now thought that this cytokine does not act on naïve murine T cells. This is consistent with the idea that naïve murine T cells express low levels of IL-23R [33, 42]. However the role of IL-23 in regulating human Th17 differentiation is a bit more controversial. Both Wilson et al and Chen et al showed that IL-23 alone is quite effective in driving human Th17 differentiation. In addition, IL-23 is a potent inducer of another inflammatory cytokine-IL-22 [42][33]. In contrast, Sallusto and colleagues reported that IL-23 alone was a poor inducer of IL-17. It should be noted that temporally, IL-23 has small effects on IL-17 induction at early stages of Th17 differentiation, which can be explained by minimal IL-23R expression on naive T cells [33]. However IL-23R expression can be upregulated by TCR stimulation, IL-6, IL-21 and even IL-23 itself [26, 33, 47]. It is therefore not surprising that IL-23-dependent IL-17 production was apparent at later stages of Th17 differentiation [33][42]. Both Sallusto’s and de Waal Malefyt’s groups did show that the combination of IL-1 and IL-23 generates more IL-17–producers than does IL-1 alone. Cells stimulated with IL-23 alone or in combination with IL-1 generated a mixed population of IL-17 single producers and IL-17–IFN-γ ‘double-producing’ cells. This contrasts sharply with mouse data in which IL-23 is not an effective inducer of Th17 differentiation in naïve CD4+ T cells.
One explanation for the difference between mouse and human T cell responsiveness could simply be the regulation of expression of IL-23R. It is possible that naïve human T cells have higher levels of expression of IL-23R or that it is more easily inducible. An alternative explanation is that it is harder to be certain that human T cells are completely “naïve” compared to mouse cells in which this can be more carefully ascertained. While this distinction between converting naïve into Th17 cells vs. maintaining and expanding Th17s is an interesting conceptual distinction, with respect to targeting this pathway therapeutically it may not be an important practical distinction. Moreover, the recent finding of the relationship between IL-23R polymorphisms and the prevalence of inflammatory bowel disease and other autoimmune diseases clearly argues for relevance of this cytokine in human autoimmune disease [48, 49].
IL-21 has recently emerged to be another key regulator of Th17 cells, analogous to the role of IL-4 in Th2 cells. However, relatively little work has been done on IL-21 and human Th17 cells. Whether this cytokine has similar effect on naive and memory helper T cells has not been addressed. How is this cytokine involved in human Th17 differentiation has not been systematically studied.
5. Species-specific differences in the regulation of Th17 differentiation
TGFβ−1 has pleiotropic functions in immune responses and in many respects, TGFβ−1 is viewed as a critical immunosuppressive cytokine. However, all studies in murine cells agree that it is an important in vitro and in vivo regulator of Th17s [18][19][20]. Nonetheless, despite the critical role of TGFβ−1 in murine Th17 differentiation, TGFβ−1 has not only been reported to be dispensable for human Th17 differentiation; in fact, it inhibits IL-17 production in human cells in a dose-dependent manner [33, 42, 46].
This is especially perplexing and may simply point to species-specific differences in regulation of the Il17 gene. While this may certainly be the case, it is not an especially appealing explanation at this point. The explanation is particularly unappealing because TGFβ−1 is also a critical regulator of another subset of helper T cells-Treg cells – principally for their in vitro differentiation [50]. For murine T cells, functional Tregs, with high level expression of the transcription factor Foxp3, can be readily generated from naïve CD4+ T cells by the combination of IL-2 and TGFβ−1. The effects of TGFβ−1 in the context of a different cytokine milieu (i.e IL-6) have been an argument for the potential relatedness of Th17 and Treg cells. More recent data directly document the ability of Foxp3+ positive cells to convert to Th17s. Foxp3+ cells obtained from mice can be changed to IL-17-producing cells by in vitro culture with IL-6 and LPS- activated bone marrow derived DC [51]. This results in loss of Foxp3 expression and induction of IL-17 expression. The possibility that Tregs and Th17s might interconvert is a fascinating possibility, which could have immense implications with respect to immunoregulation; in such scenarios, TGFβ−1 would be a major player for both of these fates.
So why does TGFβ−1 have such seemingly strange effects on human Th17 differentiation? It is worth bearing in mind in this context that Foxp3 expression is less tightly controlled in human T cells compared to mouse T cells. Foxp3 is widely expressed in activated human T cells and its pattern of expression is not necessarily indicative of a population of cells with suppressive activity [52]. However, this does not explain the seemingly strange effects of TGFβ−1 vis-à-vis Th17 difference – indeed, it adds to the puzzle. Curiously, by studying the expression of IL-17 and Foxp3 in human T cells, we observed the coexsistance of IL17+Foxp3+ cells and IL17+Foxp3- cells in response to TGFβ−1 and IL-6 stimulation [33]. Therefore, exactly what the functional significance of such Foxp3+IL-17+ cells remains to be determined.
TGFβ−1 inhibits both Th1/Th2 differentiation, downregulating both T-bet and Gata3, so it is possible that indirect actions are important aspects of the action of this cytokine in murine T cells [53, 54]. Thus, it is possible that Il17 or Rorg transcription per se may not be directly regulated by TGFβ−1 signaling – TGFβ−1 may primarily acting to inhibit other fates. Another possibility – also somewhat unappealing - is that “naïve” human T cells obtained from peripheral blood may not be the appropriate counterparts of naïve murine CD4+ T cells generally obtained from primary or secondary lymphoid organs.
6. Negative regulation of human Th17 cells
As discussed above, given the highly inflammatory effects of IL-17, it comes as no surprise that Th17 cell differentiation is tightly regulated; there is no shortage of cytokines that negatively regulate murine Th17. Indeed, IFN-γ and IL-4, the signature cytokines of Th1 or Th2 cells, inhibited both human Th17 differentiation, but the repression of IL-17 production is incomplete [16, 17, 29, 55].
IL-2 is a well-known T cell growth factor in vitro, but the deficiency of IL-2 is severe multi-organ autoimmune disease characterized by overproduction of IL-17. These observations led to the finding that IL-2 inhibits Th17 differentiation while promoting Treg differentiation [56, 57]. Whether the effect of IL-2 is due solely to induction of Foxp3 is unknown. Nonetheless, IL-2 does appear to have similar effects on human Th17 differentiation, although there are also clear temporal considerations. Sallusto and colleagues assessed at this issue systematically in their study and showed that early on, high dose of IL-2 inhibits early Th17 differentiation of human CD4+ T cells [46]. However, they also show that IL-2 will ultimately led to the population expansion of Th17 cells. Similarly, the study by de Waal Malefyt’s group shows that cells treated with IL-2 produce more but not less IL-17, at least in some donors. They concluded that this is another example of species-specific regulation [42]; however, in view of the data from Sallusto’s group, it seems likely that this represents an effect on differentiated cells. The effects of IL-2 are also consistent with another study showing that IL-17-producing memory T cell populations are expanded by IL-2 in both species [55]. IL-27 is another potent negative regulator of mouse Th17 differentiation, but whether this cytokine also has directly inhibitory effects on human IL-17 production is less clear [55].
7. Unanswered questions in human IL-17 regulation
As should be evident, there is still much to be learned about IL-17 regulation in humans. Although, much emphasis has been placed on the production of IL-17 by CD4+ T cells, they are clearly not the only cell that produces IL-17. Certainly invariant NK T and γδ T cells can rapidly produce IL-17 [58][59]. Clearly, it will be important to carefully assess the production of IL-17 in immunologically relevant cells in health and disease in humans, assessing the capacity of both adaptive immune and innate immune cells. Recent evidence points to distinct chemokine receptor expression of IL-17-producing cells. It will be important to understand how different pathogens elicit such cells and how these cells traffic. The identification of IL-17- and IFN-γ-dual producing cells with distinct chemokine receptor expression, which are apparently induced by different microorganisms is also fascinating. What the relationship (if any) between these two populations of IL-17-producing cells will be important to dissect.
Obviously, a practical aspect of understanding what cells make IL-17 and what factors regulate its production is the possibility of targeting such factors in autoimmune disease. In the mouse, considerable effort is devoted to understanding lineage commitment and there are some apparent puzzling differences in the control human Th17 differentiation. However, these subtleties (e.g. does IL-23 act on naïve or memory human T cells) may be less important in considering target IL-17; targeting molecules is likely to be beneficial regardless of the details of how such a cytokine might be acting. While there may be benefit of targeting IL-17 in immune-mediated pathogenesis, it will also be important to keep a close eye on the role of IL-17 in normal host defense against extracellular bacteria in considering the risk/benefit ratio of targeting IL-17.
Th17 cells are also reported to be high producers of another important proinflammatory cytokine, IL-22. In human CD4+ T cells, IL-23 is an also potent inducer of not only IL-17 and IL-22, but also IFN-γ, TNF and IL-26 - cytokines that all participate in inflammation and autoimmune diseases [33, 42, 46, 60–62]. Additionally, IL-22 may also be produced by cells other than Th17 cells. While this seemingly caucophonous pattern of cytokine production may not fit neatly with the lineage commitment model of mouse helper T cell differentiation, it nonetheless offers opportunities for therapeutic intervention. That is, the concept that a major driver of autoimmune diseases can be explained solely by a discrete IL23/IL-17 connection is likely may be an oversimplification in humans, but that does not take away for the potential therapeutic benefit of targeting IL-23.
The negative regulation of IL-17 production, is clearly another important area of investigation, particularly with respect to the development of new therapeutic strategies. A series of recent studies on mouse model show that RA, the active metabolite of vitamin A produced by mucosal DC reciprocally regulates Th17 and Treg differentiation [36–39] [40, 63]. That is, RA downregulates expression of RORγt, and enhances the expression of Foxp3, arguing that the balance of Treg vs. Th17 differentiation might be influenced by engagement of RAR and RORγt. Despite this intriguing possibility, the role of RA in human cells has not been studied. We also do not know whether RA can act on cells that are already polarized to be IL-17-producers and downregulate production of this cytokine in memory T cells – this, of course, has important therapeutic implications. Currently it is not clear whether and how RA-regulated expression of RORγt and Foxp3 might influence Th17 and Treg function in human cells, but this will surely be an important area for future investigation. Indeed, further investigation of the apparent relationship of Th17 cells and Tregs is clearly of great importance. Considering the immense effort devoted to bringing Treg-based cellular therapy to the clinic, it is more than a little disturbing that thymic derived-Tregs can apparently convert to Th17s cells [51]. Adding to this consternation are the observations that human CD4+ T cells seem to be much more plastic than their mouse counterparts. Moreover, Foxp3 expression is a less reliable marker of cells with immunosuppressive capability in human than in mice. Clearly, these issues will need to be carefully considered in human Treg trials.
Further investigation of the basic biology of human Th17 cells is also warranted. Epigenetic regulation of the Il17 and other cytokine loci is clearly a critical area for future research. One would imagine that many aspects of T cell memory will ultimately be explained by epigenetic modifications of cytokine loci. One might further speculate that some of the vexing differences between mouse and human cells might be explained by differences in these modifications. The Il17a gene is linked to the Il17f gene on mouse chromosome 1 and human chromosome 6 in a tail-to-tail configuration. Most data point to coordinate expression of these two cytokines; although IL-17F regulation has not been intensively studied in humans [33]. In mouse Il17 locus, the promoter regions of both the Il17a and Il17f genes undergo histone H3 acetylation and K4 tri-methylation in response to TGFβ−1 and IL-6, implying increased accessibility of the locus [64]. In addition, there are eight conserved noncoding sequences in this locus, four of which reside in the intergenic region. These sites also appear to undergo preferential histone acetylation. Human memory T cells clearly maintain the capacity to promptly generate IL-17 indicating that this locus remains accessible in memory CD4+ T cells – even under the circumstances which favor IFN-γ and IL-4 production [33]. However, there have been no studies of the epigenetic regulation of the Il17a/f loci in human cells. Remodeling of the IL-17 locus in memory cells might allow for efficient production of this cytokine, and may also explain some aspects of the plasticity of human CD4+ T cells, but this area has not been explored.
Aside from Stat3, which is known to bind the Il17a gene, we know essentially nothing about the details of the regulation of the Il17a/f locus. Whether and how RORγt and Stat3 might contribute to the chromatin remodeling of Il17a/Il17 locus have not been assessed, but based on what we know about the regulation of the Th2 and Ifng loci it is likely that this is a strong possibility. Since there are interchromosomal interactions between the Ifng and Th2 loci, one wonders whether interactions between Il17a/f and Foxp3 loci also exist and how interchromosomal and intrachromosomal interactions might change with activation and differentiation of T cells [65]. Brustle and colleagues have recently reported that interferon regulatory factor (IRF)-4 is a positive regulator in Th17 differentiation [66]; however, the expression of this transcription in human Th17 has not been explored. IRF4 is known to interact with NFAT and this may be one mechanism through which it could regulate IL-17 production [67, 68].
8. Conclusions
Unquestionably, our sophisticated understanding of mouse immunology and the tools provided by genetic manipulation have provided enormous insights into immunologic mechanisms. However, the gap between the knowledge obtained from the mouse its applicability to human immunology is disturbingly vast. One of the more striking discrepancies recognized in recent studies relates to the function TGFβ−1 in mouse vs. human cells. In the mouse, this is clearly a critical cytokine that links Treg and Th17 differentiation. Its function in human T cell differentiation is clearly more enigmatic. A second lesson is that despite the elegant work on mouse T helper T cell lineage commitment, human CD4+ T cells appear to be much more plastic and much less “terminally differentiated”. The molecular mechanisms underlying these fascinating differences is unclear, but one can speculate that epigenetic modifications are likely to be at the heart of these differences. Clearly this will be important to dissect. Despite these puzzles, the advances in understanding IL-17 regulation offer many opportunities for increasing our sophistication in dissecting immunological mechanisms in autoimmune disease. Furthermore, these advances should offer many new therapeutic opportunities.
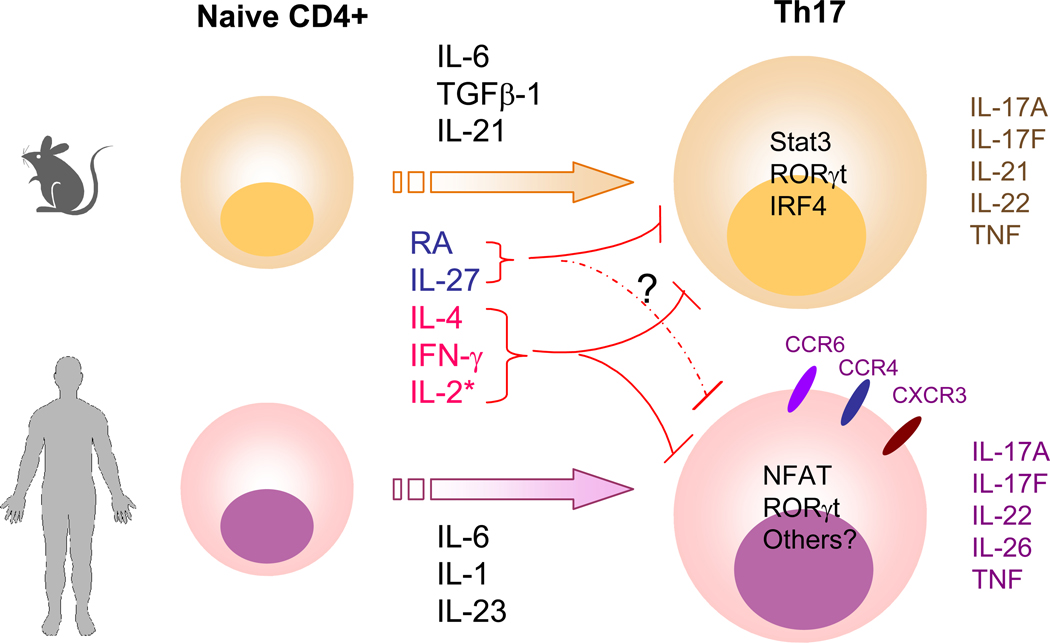
Divergence and similarity of mouse and human Th17 differentiation.
Upon activation by antigen-presenting cells, in the presence of IL-6, TGFβ−1 and IL-21, mouse naïve CD4+ T cells differentiate to Th17 cells, which produce IL-17A, IL-17F, IL-21, IL-22 and TNFα. Transcription factors including RORγt, Stat3, and IRF4 are involved in regulating this process. In human naïve CD4+ T cells, cytokines IL-6, IL-1 and IL-23 promote Th17 differentiation but surprisingly, TGFβ−1 is not involved. The transcription factors NFAT (from TCR stimulation), RORγt have been implicated in regulating human IL-17 expression, although it is also likely that Stat3 is also important. In addition to IL-17A, IL-17F, IL-22 and TNF, human Th17 cells also produce IL-26. Cytokines such as IL-4 and IFN-γ negatively regulate both mouse and human IL-17 production. IL-2 signaling through Stat5 inhibits murine Th17 differentiation but it appears to have distinct temporal effects, especially in regulating human Th17 development (denoted by *). That is, at early points in differentiation, IL-17 production is inhibited. Later on IL-2 appears to expand IL-17 producing effector/memory cells. Retinoic acid (RA) is another negative regulator for IL-17 expression in murine T cells but this effect has not been confirmed in human T cells. Although IL-27 negatively regulates murine Th17 differentiation, it is uncertain if this cytokine directly inhibits IL-17 production in human T cells. Human Th17 cells express chemokine receptors CCR6 and CCR4 whereas cells that produce IL-17 and IFN-γ express CCR6 and CXCR3. Similar chemokine receptor expression pattern has not been characterized in mouse Th17 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference:
- [1].Rouvier E, Luciani MF, Mattei MG, Denizot F and Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 1993; 150: 5445–56. [PubMed] [Google Scholar]
- [2].Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol 1995; 155: 5483–6. [PubMed] [Google Scholar]
- [3].Moseley TA, Haudenschild DR, Rose L and Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 2003; 14: 155–74. [DOI] [PubMed] [Google Scholar]
- [4].Gaffen SL, Kramer JM, Yu JJ and Shen F. The IL-17 cytokine family. Vitam Horm 2006; 74: 255–82. [DOI] [PubMed] [Google Scholar]
- [5].Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 2005; 202: 761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8: 639–46. [DOI] [PubMed] [Google Scholar]
- [7].LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 2007; 8: 630–8. [DOI] [PubMed] [Google Scholar]
- [8].Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002; 8: 500–8. [DOI] [PubMed] [Google Scholar]
- [9].Vaknin-Dembinsky A, Balashov K and Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol 2006; 176: 7768–74. [DOI] [PubMed] [Google Scholar]
- [10].Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003; 52: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 1999; 103: 1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koenders MI, Joosten LA and van den Berg WB. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Ann Rheum Dis 2006; 65 Suppl 3: iii29-iii33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA and Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 1998; 111: 645–9. [DOI] [PubMed] [Google Scholar]
- [14].Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2006; [DOI] [PubMed] [Google Scholar]
- [15].Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6: 1123–32. [DOI] [PubMed] [Google Scholar]
- [17].Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6: 1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM and Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24: 179–89. [DOI] [PubMed] [Google Scholar]
- [19].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441: 235–8. [DOI] [PubMed] [Google Scholar]
- [20].Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006; 441: 231–4. [DOI] [PubMed] [Google Scholar]
- [21].Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007; 448: 484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest 2006; 116: 1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med 2006; 203: 2473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med 2006; 203: 2485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006; 116: 1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007; 448: 480–3. [DOI] [PubMed] [Google Scholar]
- [27].Wei L, Laurence A, Elias K and O’Shea J. IL-21 is produced by Th17 cells and drives IL-17 production in a Stat3-dependent manner. J Biol Chem 2007; submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007; [DOI] [PubMed] [Google Scholar]
- [29].Hoeve MA, Savage ND, de Boer T, Langenberg DM, de Waal Malefyt R, Ottenhoff TH, et al. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol 2006; 36: 661–70. [DOI] [PubMed] [Google Scholar]
- [30].Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 2006; 103: 8137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The Orphan Nuclear Receptor RORgammat Directs the Differentiation Program of Proinflammatory IL-17(+) T Helper Cells. Cell 2006; 126: 1121–33. [DOI] [PubMed] [Google Scholar]
- [32].Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley J T, et al. Stat3 and Stat4 Direct Development of IL-17-Secreting Th Cells. J Immunol 2007; 178: 4901–7. [DOI] [PubMed] [Google Scholar]
- [33].Chen Z, Tato C, Muul L, Laurence A and O’Shea JJ. Distinct Regulation of IL-17 in Human Helper T Lymphocytes. Arthritis Rheum 2007; 56: 2936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest 2006; 116: 1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sutton C, Brereton C, Keogh B, Mills KH and Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006; 203: 1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal Th-17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science 2007; [DOI] [PubMed] [Google Scholar]
- [37].Benson MJ, Pino-Lagos K, Rosemblatt M and Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 2007; 204: 1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta} and retinoic acid dependent mechanism. J Exp Med 2007; 204: 1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007; 204: 1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Elias K, Laurence A, Davidson T, Stephens G, Y. K, EM. S, et al. Retinoic Acid Inhibits Th17 Polarization and Enhances FoxP3 Expression through a Stat-3/Stat-5 Independent Signaling Pathway. Blood 2007; submitted manuscript: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Albanesi C, Scarponi C, Cavani A, Federici M, Nasorri F and Girolomoni G. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000; 115: 81–7. [DOI] [PubMed] [Google Scholar]
- [42].Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007; [DOI] [PubMed] [Google Scholar]
- [43].Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204: 1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu XK, Lin X and Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J Biol Chem 2004; 279: 52762–71. [DOI] [PubMed] [Google Scholar]
- [45].Sundrud MS, Grill SM, Ni D, Nagata K, Alkan SS, Subramaniam A, et al. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J Immunol 2003; 171: 3542–9. [DOI] [PubMed] [Google Scholar]
- [46].Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A and Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007; [DOI] [PubMed] [Google Scholar]
- [47].Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282: 9358–63. [DOI] [PubMed] [Google Scholar]
- [48].Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science 2006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Consortium WTCC. Association scan of 14,500 nsSNPs in four common diseases identifies variants involved in autoimmunity. Nat Genetics 2007; in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li MO, Wan YY and Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007; 26: 579–91. [DOI] [PubMed] [Google Scholar]
- [51].Xu L, Kitani A, Fuss I and Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol 2007; 178: 6725–9. [DOI] [PubMed] [Google Scholar]
- [52].Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 2006; 25: 195–201. [DOI] [PubMed] [Google Scholar]
- [53].Gorelik L, Fields PE and Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 2000; 165: 4773–7. [DOI] [PubMed] [Google Scholar]
- [54].Gorelik L, Constant S and Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 2002; 195: 1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 2007; 13: 711–8. [DOI] [PubMed] [Google Scholar]
- [56].Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007; 26: 371–81. [DOI] [PubMed] [Google Scholar]
- [57].Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 2007; 178: 6730–3. [DOI] [PubMed] [Google Scholar]
- [58].Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med 2007; 204: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS and Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005; 22: 285–94. [DOI] [PubMed] [Google Scholar]
- [60].Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203: 2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K and Sabat R. IL-22 increases the innate immunity of tissues. Immunity 2004; 21: 241–54. [DOI] [PubMed] [Google Scholar]
- [62].Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 2006; 36: 1309–23. [DOI] [PubMed] [Google Scholar]
- [63].Schambach F, Schupp M, Lazar MA and Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol 2007; [DOI] [PubMed] [Google Scholar]
- [64].Akimzhanov AM, Yang XO and Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem 2007; 282: 5969–72. [DOI] [PubMed] [Google Scholar]
- [65].Lee GR, Kim ST, Spilianakis CG, Fields PE and Flavell RA. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity 2006; 24: 369–79. [DOI] [PubMed] [Google Scholar]
- [66].Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol 2007; [DOI] [PubMed] [Google Scholar]
- [67].Hu CM, Jang SY, Fanzo JC and Pernis AB. Modulation of T cell cytokine production by interferon regulatory factor-4. J Biol Chem 2002; 277: 49238–46. [DOI] [PubMed] [Google Scholar]
- [68].Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H and Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med 2002; 195: 1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]