Abstract
Background and Objective
A significant number of individuals diagnosed with non-small cell lung cancer (NSCLC) have distant metastases, and the concept of oligometastatic NSCLC has shown promise in achieving a cure. Stereotactic body radiation therapy (SBRT) is currently considered a viable treatment option for a limited number of tumor metastases. It has also been demonstrated that third-generation tyrosine kinase inhibitors (TKIs) are effective in extending the survival of patients with epidermal growth factor receptor (EGFR)-mutated NSCLC. Hence, the combination of SBRT with third-generation TKIs holds the potential to enhance treatment efficacy in patients with oligometastatic EGFR-mutated NSCLC. This review aimed to assess the possibility of combining SBRT with TKIs as an optimum treatment option for patients with oligometastatic EGFR-mutated NSCLC.
Methods
We performed a narrative review by searching the PubMed, Web of Science, Elsevier and ClinicalTrials.gov databases for articles published in the English language from January 2009 to February 2024 and by reviewing the bibliographies of key references to identify important literature related to combining SBRT with third-generation TKIs in oligometastatic EGFR-mutated NSCLC.
Key Content and Findings
This review aimed to assess the viability of combining SBRT and EGFR-TKIs in oligometastatic EGFR-mutated NSCLC. Current clinical trials suggest that the combined therapies have better progression free survival (PFS) when using SBRT as either concurrent with EGFR-TKIs or consolidated with EGFR-TKIs. Furthermore, research with third-generation EGFR-TKIs and SBRT combinations has demonstrated tolerable toxicity levels without significant additional adverse effects as compared to prior therapies. However, further clinical trials are required to establish its effectiveness.
Conclusions
The combined approach of SBRT and TKIs can effectively impede the progression of oligometastatic NSCLC in patients harboring EGFR mutations and, most notably, can prolong progression-free survival rates. However, the feasibility of combining SBRT with third-generation TKIs in clinical trials remains unclear.
Keywords: Tyrosine kinase inhibitor (TKI), stereotactic radiation therapy, carcinoma, non-small cell lung cancer (NSCLC), oligometastasis
Introduction
Background
With 11.4% of new cancer cases and 18% of new cancer-related death being caused by lung cancer, it is now the second most prevalent type of cancer in the world, posing a serious threat to public health (1). The most prevalent form of lung cancer, non-small cell lung cancer (NSCLC), consists of adenocarcinoma, squamous cell carcinoma, and other pathological forms. As a result of the absence of the characteristic clinical symptoms of an early stage, between 35.4% and 38.8% of patients initially receive an advanced diagnosis, which has a 5-year survival rate lower than 6% (2).
Rationale and knowledge gap
In the traditional treatment paradigm of metastatic NSCLC, systemic therapy, such as chemotherapy, is frequently used to treat systemic tumor dissemination. However, drug resistance frequently arises shortly after treatment. In 1995, a concept was proposed, termed the oligometastatic state, that posited the existence of a state of limited metastatic disease burden wherein local treatment is still beneficial for patient survival (3). Local ablation has been shown to effectively remove a small number of distinguishable lesions in those with oligometastatic NSCLC, and this therapeutic strategy has been linked to better patient survival and prognosis (4). Stereotactic body radiation therapy (SBRT) is an effective form of local therapy that can target tumors with highly conformal radiation doses while minimizing harm to the surrounding healthy tissues and cells (5). Therefore, the accurate and efficient elimination of metastatic lesions with the use of SBRT in the treatment of oligometastatic NSCLC may better control overall disease course (6). The prevalence of epidermal growth factor receptor (EGFR) mutations ranges from between 5% and 10% in White patients in NSCLC and between 60% and 70% in never-smoking Asian patients with adenocarcinoma (7). Tyrosine kinase inhibitors (TKIs) have been demonstrated to be more successful than platinum-based chemotherapy in the treatment of patients with NSCLC and EGFR mutations. Use of TKIs as the first-line therapy in patients with EGFR mutations has greatly improved disease control and survival for these patients (8,9). Moreover, the ongoing development and enhancement of TKIs have led to the emergence of third-generation EGFR-TKIs, including osimertinib, almonertinib, vormetinib, which are more highly selective for EGFR-mutated cells and provide patients with advanced EGFR-mutated NSCLC improved efficacy and therapeutic window than older TKIs (8,10-12). As the origin and progression of tumors has come to be better understood, personalized tumor therapy tailored to individuals has steadily advanced and proliferated. The introduction of oligometastasis and the advancement of SBRT provide the opportunity for patients with locally advanced disease or distant metastasis to achieve long-term disease control and survival. Additionally, for patients with EGFR sensitizing mutations, third-generation TKIs provide significant increased progression free survival (PFS). Hence, in the case of patients with oligometastatic, limited tumor metastasis, or sensitizing EGFR mutations, the combination of SBRT and TKIs has the potential to yield enhanced treatment outcomes for these individuals. As the use of SBRT in the setting of oligometastatic EGFR-mutated NSCLC is becoming more common, we sought to conduct a review of the current evidence investigating SBRT and third-generation TKIs to better understand the therapeutic efficacy and toxicities of this treatment paradigm.
Objective
This review aimed to assess the possibility of combining SBRT with TKIs as an optimum treatment option for patients with oligometastatic EGFR-mutated NSCLC. We present this article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-414/rc).
Methods
We performed a search of the published literature in the PubMed, Web of Science, Elsevier, and ClinicalTrials.gov databases between October 1, 2023, and February 1, 2024 (Table 1). Search terms used included NSCLC, oligometastatic, advanced, EGFR, TKI, SBRT, stereotactic ablative radiotherapy (SABR), radiotherapy (RT), and local consolidative therapy. We included only English-language publications reporting original findings from randomized controlled trials, prospective or retrospective cohort studies, case reports or series, case-control studies, and translational preclinical studies, in addition to relevant review articles. Included articles were selected and assessed by X.Z. and S.Z. of this work.
Table 1. Summary of the search strategy.
| Item | Specification |
|---|---|
| Date of search | October 1, 2023 to February 1, 2024 |
| Databases and other sources searched | PubMed, Web of Science, Elsevier, and ClinicalTrials.gov |
| Search terms used | NSCLC, oligometastatic, advanced, EGFR, TKI, SBRT, SABR, RT, and local consolidative therapy |
| Timeframe | From January 2009 to February 2024 |
| Inclusion and exclusion criteria | Inclusion criteria: English-language articles; randomized controlled trials, prospective or retrospective cohort studies, case reports or series, case-control studies and translational preclinical studies |
| Exclusion criteria: non-English language articles; editorial comments, abstracts, conference materials, review articles, guidelines, consensus statements, and study protocols | |
| Selection process | Study selection and full-text articles were assessed by X.Z. and S.Z. |
NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; SBRT, stereotactic body radiation therapy; SABR, stereotactic ablative radiotherapy; RT, radiotherapy.
SBRT for oligometastatic NSCLC
Definition of oligometastatic NSCLC
Studies have indicated that tumor cells exhibit organ specificity and must acquire specific features and abilities in order to disseminate throughout the body. Consequently, there exists an intermediate stage of tumor development known as oligometastasis, characterized by a relatively indolent state with several metastatic sites of disease, prior to the occurrence of disseminated, or poly-metastases (3). This state can be considered a transitional phase between locally advanced disease and subsequent widespread metastasis. In this stage, the tumor cells lack the capacity for extensive dissemination, and the emergence of a limited number of metastases is attributable to the presence of micrometastatic tumor cells that have infiltrated the blood arteries and subsequently implanted into specific target organs (2).
A universally accepted and dependable definition for oligometastatic NSCLC remains lacking. The majority of papers discussing suitable stage IV NSCLC commonly indicate a range of 1 to 8 for defining the maximum number of metastatic lesions. Among these articles, a prevailing choice is to define the maximum number of metastatic lesions as 5. However, the bulk of research in this field does not provide a clear definition regarding the specific quantity of metastatic sites inside the afflicted organs, and only 27.8% of reported cases include mediastinal lymph node involvement as the location of metastasis (13). After thorough deliberation, the European Consensus Conference on Thoracic Tumours ultimately reached a consensus for the criteria of Synchronous oligometastatic NSCLC (sOM NSCLC), which included the presence of up to five metastatic lesions, with no more than three lesions in each organ. It was also agreed upon that mediastinal lymphadenopathy does not meet the definition of oligometastasis. Additionally, cases with diffuse serosal metastasis or bone marrow involvement were excluded from the defined category of sOM NSCLC (14). Guckenberger et al. further classified oligometastatic disease state based on the count of tumor metastases at the initial diagnosis and the recurrence status following therapy and outlined the following types: synchronous oligometastatic disease, metachronous oligorecurrence, metachronous oligoprogression, repeat oligorecurrence, repeat oligopersistence, repeat oligoprogression, induced oligorecurrence, induced oligopersistence, and induced oligoprogression (15). If multimetastasis is not observed throughout the diagnostic and treatment process, the features of oligometastatic tumors may be considered present, indicating a low degree of tumor metastasis.
The treatment of SBRT
SBRT is often used in the treatment of patients with NSCLC diagnosed with oligometastasis or oligoprogression due to its notable benefits, including accurate tumor targeting, enhanced tumor ablation capabilities, and favorable lung tolerance. Several prospective clinical trials have demonstrated improved PFS and overall survival (OS) when incorporating metastasis-directed therapy (MDT), such as SBRT or surgery.
The findings from the Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastases (SABR-COMET) clinical trial demonstrated that the application of SBRT as an initial treatment in conjunction with standard therapy yielded superior PFS and OS outcomes compared to standard therapy alone (16). Furthermore, the incorporation of SBRT resulted in a reduced incidence of adverse reactions of grade 3 or higher while not significantly impinging upon the patients’ quality of life. Additionally, a clinical study has attested to the significant benefits associated with SBRT as a local consolidation therapy in oligometastatic NSCLC, especially for extending PFS (17). SBRT has also shown the potential to substantially delay tumor progression when used as a local treatment prior to chemotherapy. Furthermore, in the aforementioned clinical studies, SBRT did not result in significant toxicity. Moreover, the randomized controlled study by Tsai et al. confirmed that SBRT is also suitable for oligoprogressive NSCLC, leading to more than a four-times increase in PFS compared with standard care (18). In this study, only 16% of patients in the SBRT group had grade 2 or worse toxicities related to SBRT. Hence, the use of SBRT as a means of achieving local control presents a favorable alternative for individuals diagnosed with both oligometastatic and oligoprogressive NSCLC. However, it is worth noting that these studies did not investigate the efficacy of combining TKIs and SBRT in patients with EGFR mutations.
While there may be variability in the inclusion criteria and dose fractionation protocols of SBRT for NSCLC across various trial designs, it has been generally observed that SBRT yields favorable outcomes in terms of local control (19,20). The National Comprehensive Cancer Network (NCCN) guidelines of the United States have provided a set of recommendations for the segmentation design of thoracic SBRT (21). The treatment protocols for small tumors (<2 cm) situated in the periphery and tumors within 1 cm to the chest wall are 25–34 Gy/1 fraction, 45–60 Gy/3 fractions, 48–50 Gy/4 fractions, and 50–55 Gy/5 fractions. Meanwhile, the treatment protocols for cancers situated in central regions are 48–50 Gy/4 fractions, 50–55 Gy/5 fractions, and 60–70 Gy/4–8 fractions. Furthermore, the Radiation Therapy Oncology (RTOG) 0813 protocol offers a robust framework for the administration of SBRT to tumors situated in central locations (22). This is achieved by the implementation of a five-point dose escalation strategy, starting at 10 Gy/fraction and increasing by 0.5 Gy/fraction every second to third day to 12 Gy/fraction, which is afterward assessed to be safe and effective. Through systematic review, Mayinger et al. recently recognized SBRT for lung oligometastases to be an effective definitive treatment (23). It is evident that SBRT is a strongly recommended local therapeutic modality for patients diagnosed with pulmonary oligometastatic disease and an indication for definitive local therapy. Furthermore, the biologically equivalent dose (BED) was calculated using the assumption that tumour and normal tissue alpha/beta ratios were 10 Gy (BED10) and 3 Gy (BED3), respectively. A research has shown that when utilizing appropriate fractionation schedules in which the BED10 ≥100 Gy and BED3 ≤210 Gy, local control exceeded 85% and the risk of treatment-related mortality was less than 1% (24). However, for metastases with an ultracentral location, the doses may need to be reduced, particularly when targets are overlapping with the esophagus and/or proximal bronchial tree (25).
A comprehensive examination of 757 cases of oligometastatic NSCLC treated with SBRT revealed that many parameters were significantly associated with OS, including performance status prior to treatment, maximum diameter of metastatic lesions, histology of the main tumor, number of metastases, diagnosis of the primary tumor and the timing of using SBRT. Therefore, there are many aspects that should be considered for better efficacy of SBRT therapy when clinical trials are being devised (26).
Third-generation TKIs for NSCLC with EGFR sensitizing mutations
Although the first and second generations of TKIs have demonstrated favorable overall response rates (ORRs) and PFS in patients with NSCLC and EGFR sensitizing mutations, it is common for these patients to experience acquired resistance (AR) within a period of around 9–12 months after the initiation of therapy. Drug resistance may arise via many processes, including as a result of additional EGFR mutations, the activation of alternative signaling pathways, and histopathological change (8,27). The development of the third generation of TKIs was driven by an improved understanding of the resistance mechanism of EGFR-TKIs. These TKIs demonstrate notable efficacy against mutations in exon 19, exon 21, and the T790M mutation. Specifically, they target the cysteine 797 residue within the adenosine triphosphate (ATP)-binding site, forming a covalent bond and establishing irreversible binding to the EGFR tyrosine kinase domain in a specialized manner, thus exerting a heightened and prolonged impact on the locations of EGFR mutations.
The T790M mutation is widely recognized as the primary driver of resistance to EGFR-TKI therapy. In response to this challenge, novel third-generation TKIs, including osimertinib, almonertinib, vormetinib, have been developed to specifically target the T790M mutation. Consequently, these third-generation TKIs have demonstrated remarkable efficacy in the treatment of patients with NSCLC and EGFR sensitizing mutations (28-30). When third-generation TKIs are administered as first therapy, they effectively suppress the activity of the EGFR mutation and mitigate the development of drug resistance associated with the T790M mutation. For example, the phase I AURA study and phase III FLAURA trial investigated osimertinib as a first-line medication. The final findings indicated that osimertinib treatment yielded superior ORR and PFS outcomes compared to gefitinib or afatinib when used as a first-line drug. Specifically, osimertinib yielded an ORR ranging from 67% to 87% and PFS durations ranging from 22.1 to 19.3 months, whereas gefitinib and afatinib yielded an ORR of 80% to 76% and PFS durations of 17.2 to 8.5 months. Furthermore, the most frequently observed adverse events associated with osimertinib administration included rash, diarrhea, nausea, and loss of appetite. It is worth noting that the overall toxicity profile of osimertinib was not deemed to be severe (8). The AENEAS phase III trial aimed to compare the efficacy of almonertinib with that of first-generation TKIs. In this trial, almonertinib demonstrated superior outcomes compared to first-generation TKIs in terms of both median PFS (19.3 vs. 9.9 months) and median duration of response (18.1 vs. 8.3 months). Additionally, almonertinib was associated with lower incidence rates of rash and diarrhea when compared to first-generation TKIs (11). The findings of phase III FURLONG trial demonstrated that vormetinib had a superior PFS (20.8 months) compared to first-generation TKIs (11.1 months). Furthermore, the study did not observe significant adverse effects associated with vormetinib administration. In addition, clinical trials such as AURA3 and APOLLO have further confirmed that the administration of third-generation TKIs as second-line treatment can yield superior ORR and PFS outcomes compared to conventional chemotherapy. This is particularly evident in patients with NSCLC who exhibit secondary EGFR mutations, notably the T790M mutation, which is known to confer resistance to first- and second-generation TKIs (31,32).
The occurrence of brain metastases (BMs) in patients with NSCLC following therapy ranges from 25% to 30%. This incidence is particularly high in patients with EGFR sensitizing mutations, with the mutation rate of BMs reaching as high as 64.7% (33). In comparison to the first and subsequent iterations of TKIs, the third-generation TKIs exhibit an enhanced ability to traverse the blood–brain barrier and to confer therapeutic efficacy in the context of central nervous system metastases (34-36). Hence, the introduction of third-generation TKIs presents a more favorable option for individuals afflicted with BMs.
SBRT combined with TKIs for oligometastatic NSCLC
Rationale for combination therapy
Based on the aforementioned information, it has been shown that SBRT may be used for the localized irradiation of oligometastatic NSCLC, which in turn can facilitate the optimal eradication of tumor metastases thus prolonging the life of patients. Additionally, for the treatment of NSCLC with an EGFR sensitizing mutation, the use of third-generation TKIs as the first therapeutic option might successfully mitigate the development of drug resistance associated with the T790M mutation, improving response rates and the PFS outcomes for this patient group. While third-generation TKIs efficacy is high, durability of response is variable, and resistance eventually occurs in most patients. Therefore, the integration of SBRT with third-generation TKIs as the first-line therapeutic approach for those with oligometastatic and EGFR sensitizing mutations has the potential to optimize tumor cell eradication and effectively prolong disease control.
A preclinical study has demonstrated that the combination of RT and TKIs can effectively suppress cell proliferation, induce cell cycle redistribution, promote apoptosis, and impair DNA damage response in Hela S3 cells. And the effect is attributed to the attenuation of expression and function of homologous recombination (HR) protein RAD51 by EGFR-TKIs (37). One the one hand, the administration of EGFR-TKIs and RT leads to the buildup of tumor cells in the G1 and G2-M phases, respectively. As a consequence, there is a decrease in the number of tumor cells in the S phase, ultimately impeding the progression of tumor development. In contrast, the combination of TKIs with RT has also demonstrated the ability to not only facilitate a greater reduction in the S phase fraction but to also trigger cellular apoptosis, suppress EGFR autophosphorylation, and downregulate the expression of RAD51 recombinase subsequent to radiation exposure, resulting in enhanced radiosensitivity (38). However, in comparison to concurrent chemoradiotherapy, patients treated with combination TKIs and SBRT have been reported to exhibit a higher incidence of grade 2 or worse radiation pneumonitis (39). This underscores the crucial need for physicians to meticulously consider the timing of radiotherapy. Consequently, as research continues to progress, the integration of TKIs and RT will garner wider recognition in the management of NSCLC, particularly in patients with oligometastatic NSCLC with visceral metastasis and BM.
Clinical trials on combination therapy
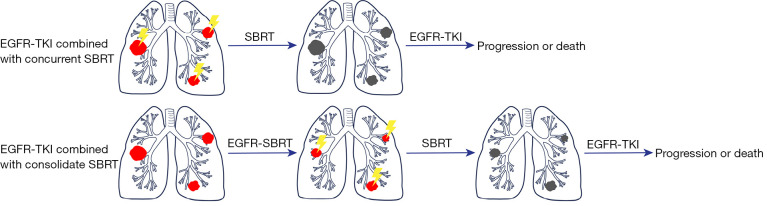
A number of studies have been conducted to investigate the potential enhancement of survival rates via combination of radiation and targeted treatments in patients diagnosed with oligometastatic NSCLC (Table 2). Currently, the combination of SBRT and TKIs therapy can be classified into two distinct categories based on the timing of irradiation (Figure 1). The first approach involves the combination of EGFR-TKIs and concurrent SBRT, which utilizes radiation to ablate the limited number of metastases prior to the administration TKI targeted therapy. The second method involves the combination of EGFR-TKIs and consolidation SBRT. This approach entails initiating systemic therapy as the primary treatment and then applying SBRT to stable lesions or those in remission to consolidate the treatment. Systemic therapy is then continued to conclude the treatment process.
Table 2. Summary of trials on EGFR-TKIs combined with SBRT.
| Therapy | Study design | Case | Conditions | Treatment | Efficacy | AEs | Reference |
|---|---|---|---|---|---|---|---|
| EGFR-TKIs combined with concurrent SBRT | Phase III | 133 | Synchronous oligometastatic EGFR-mutated NSCLC | Control group: EGFR-TKI (gefitinib, erlotinib, icotinib) alone. Experimental group: EGFR-TKI + RT (25–40 Gy in 5 fractions), upfront RT before first-line EGFR-TKI | mPFS 12.5 vs. 20.2 months (P<0.001); mOS 17.4 vs. 25.5 months (P<0.001) | Grade 3–4 pneumonitis 6% | (40) |
| Phase II | 10 | Stage IV NSCLC harboring EGFR activating mutations | EGFR-TKI (erlotinib 150 mg or gefitinib 250 mg per day), thoracic radiotherapy (54–60 Gy/27–30 fractions/5.5–6 weeks) | 1-year PFS rate 57.1%, mPFS 13 months, median time to progression of irradiated lesion 20.5 months, ORR 50%, DCR 100% | Radiation pneumonitis (20%), rash (10%) | (41) | |
| Systemic therapy combined with consolidative SBRT | Phase II | 62 | Stage IV NSCLC with no more than 5 metastatic foci | Control group: first-generation EGFR-TKIs alone. Study group: SBRT (30–50 Gy in 5 fractions) after EGFR-TKI administration in patients who had achieved SD or PR | mPFS 9.0 vs. 17.6 months (HR =0.52; 95% CI: 0.31–0.89; P=0.02); mOS 23.2 vs. 33.6 months (HR =0.53; 95% CI: 0.30–0.95; P=0.03) | Grade 2 AEs: control group 45.2%, study group 50%; no grade 3 AEs | (42) |
| Phase II | 49 | Oligometastatic NSCLC without progression after first-line systemic therapy with ≤3 metastatic lesions | MT/O: maintenance treatment alone. LCT: radiotherapy or resection; chemotherapy 84%, TKI 16% | mPFS 3.9 months (95% CI: 2.3–6.6) vs. 11.9 months (95% CI: 5.7–20.9) (HR =0.35; 95% CI: 0.18–0.66; P=0.0054) | Control group: fatigue (n=1), anemia (n=1); experimental group: esophagitis (n=2), anemia (n=1), pneumothorax (n=1), abdominal pain (n=1) | (19) | |
| Phase II | 49 | Oligometastatic NSCLC without progression after first-line systemic therapy with ≤3 metastatic lesions | MT/O: maintenance treatment alone. LCT: radiotherapy or resection; chemotherapy 84%, TKI 16% | mPFS 4.4 months (95% CI: 2.2–8.3) vs. 14.2 months (95% CI: 7.4–23.1), P=0.02. mOS 17.0 months (95% CI: 10.1–39.8) vs. 41.2 months (95% CI: 18.9–not reached), P=0.02 | No additional grade 3 or greater toxicities | ||
| Phase II | 47 | Oligometastatic NSCLC without progression after first-line systemic therapy with ≤5 metastatic lesions | Single-arm: SABR (45–60 Gy in 3–5 fractions). Consolidative therapy after front-line systemic therapy (chemotherapy 61.7%, TKI 38.3%) | mPFS 34.3 months (95% CI: 31.1–38.8) | Pneumonitis: grade 1, 79.5%; grade 2, 12.8%; grade 3, 7.7% | (43) | |
| Retrospective | 122 | Older adult patients with oligometastatic EGFR-mutated NSCLC | TKI alone group: TKI monotherapy. TKIs + LCRT group: TKI combined with local consolidative radiation therapy | mPFS 12 months (95% CI: 11.05–12.95) vs. 17 months (95% CI: 15.37–18.63), P<0.001. mOS 29 months (95% CI: 26.86–31.14) vs. 38 months (95% CI: 35.61–40.39), P<0.001 | TKI + LCRT group: grade 1–2 pneumonia, 26%; grade 3, pneumonia 6% | (44) | |
| Retrospective | 194 | Synchronous oligometastatic NSCLC with ≤3 metastatic lesions | LCT: radiotherapy, surgical therapy, or other local ablative therapy | Comprehensive LCT (MST 29.2 months, 95% CI: 25.2–42.3; 1-year OS: 85.0%; 3-year OS: 42.7%; 5-year OS: 32.0%). All other patients (MST 22.9 months: 95% CI: 16.2–35.1; 1-year OS: 72.2%; 3-year OS: 34.6%; 5-year OSL: 18.9%) | NA | (45) | |
| Retrospective | 145 | Synchronous oligometastatic EGFR-mutated NSCLC | Consolidative LAT: all-LAT, part-LAT, non-LAT (surgery, radiotherapy) | mPFS in the all-LAT, part-LAT, and non-LAT groups of 20.6, 15.6, and 13.9 months, respectively (P<0.001). mOS in the all-LAT, part-LAT, and non-LAT groups of 40.9, 34.1, and 30.8 months, respectively (P<0.001) | Grade ≥3 pneumonitis (7.7%), esophagitis (16.9%) | (46) | |
| Retrospective | 231 | Oligometastatic EGFR-mutated lung adenocarcinoma | Monotherapy group: EGFR-TKI alone. Combination group: EGFR-TKI + LCT (surgery, radiotherapy) | mPFS 10 months (95% CI: 8.936–11.064) vs. 15 months (95% CI: 13.611–16.389) (HR =0.610; 95% CI: 0.461–0.807; P<0.001); mOS 21 months (95% CI: 18.445–23.555) vs. 34 months (95% CI: 27.889–40.111) (HR =0.593; 95% CI: 0.430–0.817; P=0.001) | NA | (47) | |
| Retrospective | 40 | Oligometastatic EGFR-mutated NSCLC | SBRT with a median BED10 value of 102.7 Gy (94.5–113.5 Gy) | mOS 40 months (95% CI: 32.562–47.438); 1-, 2-, and 3-year OS rates of 100.0%, 72.5%, and 62.5%, respectively; 1-, 2-, and 3-year PFS rates of 65.0%, 10.0%, and 0%, respectively | Grade 1–2: 36 (90.0%), Grade 3: 2 (5.0%), CTCAE v. 5.0 | (48) |
SBRT, stereotactic radiation therapy; SABR, stereotactic ablative radiotherapy; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; LCT, local consolidative therapy; LCRT, local consolidative radiation therapy; LAT, local ablative therapy; RT, radiation therapy; MT/O, maintenance therapy or observation; mPFS, median progression-free survival; mOS, median overall survival; ORR, objective response rate; CI, confidence interval; HR, hazard ratio; MST, median survival time; CTCAE, Common Terminology Criteria for Adverse Events; DCR, disease control rate; SD, stable disease; PR, partial response; AEs, adverse events.
Figure 1.
The therapy of EGFR-TKIs combined with SBRT. SBRT, stereotactic radiation therapy; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
The combination of EGFR-TKIs and concurrent SBRT has been shown to effectively eliminate tumor lesions to the greatest degree possible in a timely manner. This approach aligns with the treatment plan for low metastatic tumor states, hence mitigating the risk of tumor recurrence in the initial lesion. Wang et al. conducted a phase III clinical trial that examined the efficacy of combining first-generation EGFR-TKIs with RT in the treatment of oligometastatic EGFR-mutated NSCLC in 113 patients (40). In the RT group, patients received a radiation regimen of 25–40 Gy/5 fractions prior to TKI treatment. The median PFS in the group receiving TKI monotherapy was 12.5 months, but in the group receiving a combination of TKIs and RT, the median PFS was 20.2 months. Similarly, the median OS in the TKI monotherapy group was 17.4 months, while it was 25.5 months in the TKI-RT group. No grade 5 adverse events (AEs) were observed in the TKI-RT group, and the incidence of symptomatic grade 3–4 pneumonia was 6%. In a phase II single-arm study conducted by Zheng et al., the efficacy of combining EGFR-TKIs with chest radiation was evaluated in the treatment of 10 patients diagnosed with EGFR-mutated NSCLC (41). The study revealed that the number of metastatic lesions detected did not exceed 10. After the administration of EGFR-TKIs, the patients underwent thoracic RT at a dosage range of 54–60 Gy that was administered with 27–30 fractions over a period of 5.5–6 weeks. Ultimately, the study observed a 1-year PFS rate of 57.1%, a median PFS of 13 months, a median time to progression of the irradiated lesion of 20.5 months, an ORR of 50%, and a disease control rate (DCR) of 100%. In addition, among the grade ≥3 AEs, radiation pneumonitis had the highest incidence at 20%, followed by rash at 10%.
Furthermore, considering the risk of toxicity, it is still important to carefully evaluate whether TKIs usage should be interrupted during radiotherapy. Previous research has demonstrated that the timing of TKIs usage does not result in the incidence of pneumonitis during radiotherapy (49). According to the review of previous clinical studies, the incidence of grade ≥3 pneumonitis was 20% in the Zheng et al.’s trial with TKIs maintenance and 6% in the Wang et al.’s trial with TKIs interruption (40,41). Consequently, more prospective experiments with larger sample sizes are required to confirm the association between the incidence of pneumonitis and the time of TKIs usage.
EGFR-TKIs combined with consolidation SBRT also appear relatively safe. Peng et al.’s phase II randomized controlled trial of SBRT examined 62 patients with oligometastatic EGFR-mutated NSCLC without progression after EGFR-TKI treatment (42). The control group was treated with EGFR-TKIs alone, while the study group was additionally treated with SBRT after three months. The median PFS of the control group and the study group were 9.0 and 17.6 months [hazard ratio =0.52; 95% confidence interval (CI): 0.31–0.89, P=0.02], respectively, and the median OS was 23.2 and 33.6 months (hazard ratio =0.53, 95% CI: 0.30–0.95; P=0.03), respectively. In addition, no grade ≥3 AEs occurred in either group, and the incidence of grade 2 AEs was 45.2% and 50% in the control group and study group, respectively.
SBRT is also being used in several clinical trials as consolidation treatment following systemic therapy, which also includes targeted therapy and chemotherapy. These clinical studies have also illustrated the promise of systemic treatment in conjunction with local therapy for oligometastatic NSCLC. Gomez et al. completed a phase II randomized controlled trial that examined the efficacy of local consolidation treatment for 49 patients with oligometastatic NSCLC who did not experience disease progression after initial systemic therapy (19). EGFR mutations were present in 12% of these patients. The local consolidation therapy group adhered to a treatment approach that included the continuation of systemic therapy with further surgical procedures or radiation for suitable lesions. In contrast, the maintenance therapy group only received first-line systemic therapy until disease progression. The median PFS in the maintenance and consolidation groups was 4.4 months (95% CI: 2.2–8.3) and 14.2 months (95% CI: 7.4–23.1), respectively (P=0.02); meanwhile, the median OS was 17.0 months (95% CI: 10.1–39.8) and 41.2 months (95% CI: 18.9–not achieved) respectively (P=0.02). Overall survival was shown to be considerably improved in the consolidation therapy group, with no observed occurrence of grade 3 or above toxicity. The study by Blake-Cerda et al. investigated the impact of SABR on a cohort of 47 patients diagnosed with oligometastatic NSCLC who showed no signs of disease progression after first systemic treatment (43). And in the first systemic treatment, 38.3% of the patients were treated with TKIs. The administered dosage of radiation ranged from 45 to 60 Gy, which was delivered over a course of 3–5 fractions. The median PFS was 34.3 months (95% CI: 31.1–38.8), and the frequency of AEs associated with radiation treatment, including pneumonia, was 79.5% for grade 1 AEs, 12.8% for grade 2 AEs, and 7.7% for grade 3 AEs. Several retrospective trials have also examined the impact of RT as consolidation therapy in individuals diagnosed with oligometastatic NSCLC (44-48). The findings indicate that compared to that of maintenance therapy alone, the efficacy of SBRT as consolidation therapy is significantly enhanced, while the incidence of radiotherapy-related adverse reactions is not significantly increased.
Ongoing clinical trials of third-generation TKIs combined with SBRT
Only a limited number of clinical trials and cases have been reported related to the use of SBRT in combination with third-generation TKIs as a first-line treatment. In contrast, numerous retrospective experimental studies and clinical trials have investigated the efficacy of third-generation TKIs as second-line drugs for managing drug-resistant NSCLC or their potential application in treating NSCLC with brain metastases (50). In the context of assessing the feasibility of integrating RT with osimertinib treatment, empirical investigations have revealed the potential occurrence of grade 3 or higher radiation pneumonitis and radiation dermatitis, which can generally be managed, with patients exhibiting a relatively favorable capacity to tolerate RT in conjunction with osimertinib treatment (51). Furthermore, a recent comparative trial comparing the effects of thoracic irradiation with various generation EGFR-TKIs revealed that the first-, second-, and third-generation EGFR-TKIs groups had an overall incidence of clinical radiation pneumonitis of 29%, 48%, and 28% (P=0.043), respectively. Pneumonitis was less common when third-generation TKIs and thoracic radiation were used together (52). Additionally, Chen et al.’s prospective single-arm study examined the safety of consolidation SBRT at the maximal osimertinib response through the assessment of five patients (53). They reported no grade 3/4 acute or late radiation toxicity, thus supporting the high tolerability of osimertinib and SBRT used in combination.
The current body of experimental evidence does not provide a definitive conclusion regarding the efficacy of combining SBRT with third-generation TKIs for the treatment of patients with oligometastatic NSCLC and EGFR sensitizing mutations. However, ongoing clinical trials are being conducted to investigate this treatment approach, and the final results of these studies hold promise for further insights into its effectiveness (Table 3).
Table 3. Summary of the clinical trials on third-generation EGFR-TKIs combined with SBRT.
| Clinical trial | Setting | Cases | Condition | Treatment | Measures | Study completion |
|---|---|---|---|---|---|---|
| NCT04908956 | Phase II | 60 | Oligo-metastatic EGFR-mutant NSCLC | Risk-adapted SBRT × up to 5 fractions, osimertinib 80 mg once daily | Pneumonia grade ≥2, PFS, RECIST v. 1.1 | 2023/3/26 |
| NCT05167851 | Phase II | 68 | Oligometastatic EGFR-mutant NSCLC | Lazertinib 240 mg once daily oral (PO); SBRT to oligometastatic sites | PFS, OS, distant PFS, ORR, DOR, CTCAE | 2023/12/25 |
| NCT04764214 | Phase II | 56 | Oligometastatic EGFR-mutant NSCLC | Osimertinib 80 mg once daily; consolidative SBRT to all residual disease | PFS, OS, CTCAE v. 5.0 | 2023/12/31 |
| NCT05583409 | Not applicable | 72 | EGFR-mutant NSCLC with fewer than 5 metastatic focus | Received SBRT after 3 months of osimertinib treatment; osimertinib 80 mg once daily | PFS, OS | 2025/12/30 |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; SBRT, stereotactic radiation therapy; NSCLC, non-small cell lung cancer; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors; PO, per os; OS, overall survival; ORR, objective response rate; DOR, duration of response; CTCAE, Common Terminology Criteria for Adverse Events.
Currently, no research has examined how combination therapy works to treat oligometastases at various sites, particularly brain metastases. Therefore, more research is required to determine whether or not SBRT in conjunction with third-generation TKIs is a more effective management approach for brain metastases. Furthermore, a number of studies did not implement high-dose ablative RT for oligometastatic NSCLC patients, but still achieved good local control rates (19,40). Therefore, additional investigation is required to ascertain if the efficiency and toxicity of the combination treatment are substantially impacted by the SBRT dosage. Similarly, more research is required to determine whether interrupting TKIs during SBRT may reduce the risk of pneumonia. In the future, it will be necessary to investigate if the first-line usage of third-generation TKIs in conjunction with SBRT could affect second-line management. Furthermore, additional research on fourth-generation EGFR-TKIs may offer promising therapeutic strategies to overcome mutant EGFR-based TKI resistance (54,55). A clinical trial is investigating the efficacy of fourth-generation TKIs in patients with advanced EGFR-mutated NSCLC (56).
Conclusions
For individuals with oligometastatic EGFR-mutated NSCLC, one suggested therapeutic approach involves the combination of local therapy and systemic therapy that takes into consideration the features of the tumor oligometastatic state and the commonly used treatment techniques for metastatic NSCLC. The combination of SBRT and third-generation TKIs can effectively impede the progression of oligometastatic NSCLC in patients harboring EGFR mutations and may also mitigate the emergence of subsequent drug resistance mutations, ultimately leading to prolonged PFS and OS rates in affected individuals. The confirmation of the feasibility of combining SBRT with third-generation TKIs in clinical trials is currently pending, and we eagerly anticipate the publication of their outcomes in the near future. In addition, further research is still required to determine if combination therapy may effectively treat oligometastases in various locations, if the dosage of SBRT and the timing of TKIs usage could affect the toxicity and effectiveness of combination therapy and what is the optimal configuration for second-line therapy following third-generation TKIs resistance.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the Sichuan Science and Technology Program (No. 2022YFS0212), the Chengdu Science and Technology Innovation Research and Development Project (No. 2021-YF05-02143-SN), and the Clinical Research and Transformation Fund of Sichuan Provincial People’s Hospital (No. 2021LY25).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-414/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-414/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-414/coif). B.H.K. receives research support from the NIH and the Botha-Chan Low Grade Glioma Consortium, and receives honoraria from DayOne Biopharmaceuticals and ASTRO. The other authors have no conflicts of interest to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. 10.6004/jnccn.2019.0059 [DOI] [PubMed] [Google Scholar]
- 3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 4.Bansal P, Rusthoven C, Boumber Y, et al. The role of local ablative therapy in oligometastatic non-small-cell lung cancer: hype or hope. Future Oncol 2016;12:2713-27. 10.2217/fon-2016-0219 [DOI] [PubMed] [Google Scholar]
- 5.Andruska N, Stowe HB, Crockett C, et al. Stereotactic Radiation for Lung Cancer: A Practical Approach to Challenging Scenarios. J Thorac Oncol 2021;16:1075-85. 10.1016/j.jtho.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Jasper K, Stiles B, McDonald F, et al. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:635-41. 10.1200/JCO.21.01719 [DOI] [PubMed] [Google Scholar]
- 7.Tan DS, Mok TS, Rebbeck TR. Cancer Genomics: Diversity and Disparity Across Ethnicity and Geography. J Clin Oncol 2016;34:91-101. 10.1200/JCO.2015.62.0096 [DOI] [PubMed] [Google Scholar]
- 8.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 11.Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase III Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol 2022;40:3162-71. 10.1200/JCO.21.02641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med 2022;10:1019-28. 10.1016/S2213-2600(22)00168-0 [DOI] [PubMed] [Google Scholar]
- 13.Giaj-Levra N, Giaj-Levra M, Durieux V, et al. Defining Synchronous Oligometastatic Non-Small Cell Lung Cancer: A Systematic Review. J Thorac Oncol 2019;14:2053-61. 10.1016/j.jtho.2019.05.037 [DOI] [PubMed] [Google Scholar]
- 14.Dingemans AC, Hendriks LEL, Berghmans T, et al. Definition of Synchronous Oligometastatic Non-Small Cell Lung Cancer-A Consensus Report. J Thorac Oncol 2019;14:2109-19. 10.1016/j.jtho.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 15.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. 10.1016/S1470-2045(19)30718-1 [DOI] [PubMed] [Google Scholar]
- 16.Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capone L, Antonia Allegretta S, Bianciardi F, et al. The impact of a mono-institutional experience in lung metastases treated with stereotactic body radiation therapy (SBRT): a retrospective analysis. Ther Radiol Oncol 2023;7:13. [Google Scholar]
- 18.Tsai CJ, Yang JT, Shaverdian N, et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet 2024;403:171-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daly ME, Perks JR, Chen AM. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J Thorac Oncol 2013;8:202-7. 10.1097/JTO.0b013e318279155f [DOI] [PubMed] [Google Scholar]
- 22.Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol 2019;37:1316-25. 10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayinger M, Kotecha R, Sahgal A, et al. Stereotactic Body Radiotherapy for Lung Oligo-metastases: Systematic Review and International Stereotactic Radiosurgery Society Practice Guidelines. Lung Cancer 2023;182:107284. 10.1016/j.lungcan.2023.107284 [DOI] [PubMed] [Google Scholar]
- 24.Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276-82. 10.1016/j.radonc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 25.Meng MB, Wang HH, Zaorsky NG, et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci 2019;110:3553-64. 10.1111/cas.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. 10.1016/j.cllc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Yoneda K, Imanishi N, Ichiki Y, et al. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J UOEH 2019;41:153-63. 10.7888/juoeh.41.153 [DOI] [PubMed] [Google Scholar]
- 28.Yang JC, Camidge DR, Yang CT, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial. J Thorac Oncol 2020;15:1907-18. 10.1016/j.jtho.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 29.Ahn MJ, Han JY, Lee KH, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol 2019;20:1681-90. 10.1016/S1470-2045(19)30504-2 [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Wu L, Hu P, et al. A Novel Third-generation EGFR Tyrosine Kinase Inhibitor Abivertinib for EGFR T790M-mutant Non-Small Cell Lung Cancer: a Multicenter Phase I/II Study. Clin Cancer Res 2022;28:1127-35. 10.1158/1078-0432.CCR-21-2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadimitrakopoulou VA. AURA3 trial: does Tagrisso (osimertinib) have the potential to become the new standard of care for second-line treatment of patients with EGFR T790M mutation-positive locally advanced or metastatic NSCLC. Lung Cancer Manag 2016;5:159-62. 10.2217/lmt-2017-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S, Wang Q, Zhang G, et al. Efficacy of Aumolertinib (HS-10296) in Patients With Advanced EGFR T790M+ NSCLC: Updated Post-National Medical Products Administration Approval Results From the APOLLO Registrational Trial. J Thorac Oncol 2022;17:411-22. 10.1016/j.jtho.2021.10.024 [DOI] [PubMed] [Google Scholar]
- 33.Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. 10.1097/JTO.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 34.Colclough N, Chen K, Johnström P, et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res 2021;27:189-201. 10.1158/1078-0432.CCR-19-1871 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhang Y, Niu W, et al. Experimental Study of Almonertinib Crossing the Blood-Brain Barrier in EGFR-Mutant NSCLC Brain Metastasis and Spinal Cord Metastasis Models. Front Pharmacol 2021;12:750031. 10.3389/fphar.2021.750031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Chen G, Wang X, et al. Central Nervous System Efficacy of Furmonertinib (AST2818) Versus Gefitinib as First-Line Treatment for EGFR-Mutated NSCLC: Results From the FURLONG Study. J Thorac Oncol 2022;17:1297-305. 10.1016/j.jtho.2022.07.1143 [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Gu Y, Liu H, et al. Icotinib hydrochloride enhances chemo- and radiosensitivity by inhibiting EGFR signaling and attenuating RAD51 expression and function in Hela S3 cells. Onco Targets Ther 2018;11:1245-58. 10.2147/OTT.S152613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai YC, Ho PY, Tzen KY, et al. Synergistic Blockade of EGFR and HER2 by New-Generation EGFR Tyrosine Kinase Inhibitor Enhances Radiation Effect in Bladder Cancer Cells. Mol Cancer Ther 2015;14:810-20. 10.1158/1535-7163.MCT-13-0951 [DOI] [PubMed] [Google Scholar]
- 39.Jia W, Guo H, Jing W, et al. An especially high rate of radiation pneumonitis observed in patients treated with thoracic radiotherapy and simultaneous osimertinib. Radiother Oncol 2020;152:96-100. 10.1016/j.radonc.2020.07.051 [DOI] [PubMed] [Google Scholar]
- 40.Wang XS, Bai YF, Verma V, et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer. J Natl Cancer Inst 2023;115:742-8. 10.1093/jnci/djac015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng L, Wang Y, Xu Z, et al. Concurrent EGFR-TKI and Thoracic Radiotherapy as First-Line Treatment for Stage IV Non-Small Cell Lung Cancer Harboring EGFR Active Mutations. Oncologist 2019;24:1031-e612. 10.1634/theoncologist.2019-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng P, Gong J, Zhang Y, et al. EGFR-TKIs plus stereotactic body radiation therapy (SBRT) for stage IV Non-small cell lung cancer (NSCLC): A prospective, multicenter, randomized, controlled phase II study. Radiother Oncol 2023;184:109681. 10.1016/j.radonc.2023.109681 [DOI] [PubMed] [Google Scholar]
- 43.Blake-Cerda M, Lozano-Ruíz F, Maldonado-Magos F, et al. Consolidative stereotactic ablative radiotherapy (SABR) to intrapulmonary lesions is associated with prolonged progression-free survival and overall survival in oligometastatic NSCLC patients: A prospective phase 2 study. Lung Cancer 2021;152:119-26. 10.1016/j.lungcan.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 44.Hu X, Li H, Kang X, et al. First-Line Tyrosine Kinase Inhibitors Combined With Local Consolidative Radiation Therapy for Elderly Patients With Oligometastatic Non-Small Cell Lung Cancer Harboring EGFR Activating Mutations. Front Oncol 2022;12:766066. 10.3389/fonc.2022.766066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell KG, Farooqi A, Ludmir EB, et al. Improved Overall Survival With Comprehensive Local Consolidative Therapy in Synchronous Oligometastatic Non-Small-Cell Lung Cancer. Clin Lung Cancer 2020;21:37-46.e7. 10.1016/j.cllc.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Zhou F, Liu H, et al. Consolidative Local Ablative Therapy Improves the Survival of Patients With Synchronous Oligometastatic NSCLC Harboring EGFR Activating Mutation Treated With First-Line EGFR-TKIs. J Thorac Oncol 2018;13:1383-92. 10.1016/j.jtho.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 47.Hu F, Xu J, Zhang B, et al. Efficacy of Local Consolidative Therapy for Oligometastatic Lung Adenocarcinoma Patients Harboring Epidermal Growth Factor Receptor Mutations. Clin Lung Cancer 2019;20:e81-90. 10.1016/j.cllc.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 48.Hu X, Li H, Liu H, et al. Assessing efficacy and safety of stereotactic body radiation therapy for oligometastatic non-small cell lung cancer with epidermal growth factor receptor (EGFR) wild type. Transl Cancer Res 2021;10:184-94. 10.21037/tcr-20-2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Zhang Z, Rinsurongkawong W, et al. Toxicity and Local Control of Lung Stereotactic Body Radiation Therapy With or Without Concurrent Molecularly Targeted Therapies. Int J Radiat Oncol Biol Phys 2021;111:e446. [Google Scholar]
- 50.Zhai X, Li W, Li J, et al. Therapeutic effect of osimertinib plus cranial radiotherapy compared to osimertinib alone in NSCLC patients with EGFR-activating mutations and brain metastases: a retrospective study. Radiat Oncol 2021;16:233. 10.1186/s13014-021-01955-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banla LI, Tzeng A, Baillieul JP, et al. Pneumonitis in Patients Receiving Thoracic Radiotherapy and Osimertinib: A Multi-Institutional Study. JTO Clin Res Rep 2023;4:100559. 10.1016/j.jtocrr.2023.100559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mu F, Fan B, Li B, et al. Comparison of the Incidence Rate of Radiation Pneumonitis Observed in Patients with Advanced Lung Adenocarcinoma Treated with Simultaneous Thoracic Radiotherapy and 1G/2G/3G EGFR-TKIs. Cancer Manag Res 2023;15:351-62. 10.2147/CMAR.S404874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C, Wang Y, Liao S, et al. Safety and efficacy of stereotactic body radiotherapy (SBRT) to oligoresidual sites combine with osimertinib in stage IV EGFR mutant NSCLC patients: A prospective pilot study. J Clin Oncol 2023;41:e21069. [Google Scholar]
- 54.Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. 10.1038/nature17960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashima K, Kawauchi H, Tanimura H, et al. CH7233163 Overcomes Osimertinib-Resistant EGFR-Del19/T790M/C797S Mutation. Mol Cancer Ther 2020;19:2288-97. 10.1158/1535-7163.MCT-20-0229 [DOI] [PubMed] [Google Scholar]
- 56.Elamin YY, Nagasaka M, Shum E, et al. BLU-945 Monotherapy and Combination With Osimertinib in Previously Treated Patients With Advanced EGFR-Mutant (EGFRm) NSCLC in Phase 1/2 SYMPHONY Study. J Clin Oncol 2023;41:9011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as