Abstract
PURPOSE
Clinical efficiency is a key component of value-based health care. Our objective here was to identify workflow inefficiencies by using time-driven activity-based costing (TDABC) and evaluate the implementation of a new clinical workflow in high-volume outpatient radiation oncology clinics.
METHODS
Our quality improvement study was conducted with the Departments of GI, Genitourinary (GU), and Thoracic Radiation Oncology at a large academic cancer center and four community network sites. TDABC was used to create process maps and optimize workflow for outpatient consults. Patient encounter metrics were captured with a real-time status function in the electronic medical record. Time metrics were compared using Mann-Whitney U tests.
RESULTS
Individual patient encounter data for 1,328 consults before the intervention and 1,234 afterward across all sections were included. The median overall cycle time was reduced by 21% in GI (19 minutes), 18% in GU (16 minutes), and 12% at the community sites (9 minutes). The median financial savings per consult were $52 in US dollars (USD) for the GI, $33 USD for GU, $30 USD for thoracic, and $42 USD for the community sites. Patient satisfaction surveys (from 127 of 228 patients) showed that 99% of patients reported that their providers spent adequate time with them and 91% reported being seen by a care provider in a timely manner.
CONCLUSION
TDABC can effectively identify opportunities to improve clinical efficiency. Implementing workflow changes on the basis of our findings led to substantial reductions in overall encounter cycle times across several departments, as well as high patient satisfaction and significant financial savings.
INTRODUCTION
Health care systems are faced with the growing challenge of achieving the quadruple aim: improving population health, health care cost, patient experience, and staff well-being.1 These components of value in health care are especially relevant to the care of patients with cancer. The cost of cancer care in the United States continues to rise sharply and is associated with high personal costs for patients.2 Receipt of oncology care also represents a tremendous time burden as care often involves evaluation by multidisciplinary teams, requiring numerous subspecialty visits. Furthermore, appointments are associated with large opportunity costs as a single visit may account for up to 4.5 patient-hours, with a significant portion of that time being noncare time.3
Prolonged clinic wait times are a major source of dissatisfaction for patients and can have negative effects on patient experience.4–6 Patients commonly interact with several staff members in a single visit, including a medical assistant (MA), registered nurse (RN), advanced practice provider (APP), resident trainee, and attending physician (MD), some of whom have overlapping roles. Duplicated efforts can lead to clinic inefficiencies and increase staff workload, both of which lead to subjective time pressure and staff burnout.7 With burnout increasingly being recognized as requiring organization-level interventions, the need is increasing in parallel to support process improvement efforts to optimize clinic efficiency.8
Time-driven activity-based costing (TDABC) is a method of evaluating costs by assessing the time and resources required for various processes. TDABC is increasingly being used in health care institutions to guide quality improvements and increase operational efficiency.9 At our institution, we previously piloted TDABC in a high-volume, academic outpatient radiation oncology clinic and demonstrated a 21% reduction in patient cycle time.10 In this study, we report our experience in expanding that clinic efficiency initiative to additional clinics in academic departments, including clinics at community centers. Our primary outcome was to compare cycle times before versus after the initiative. We also estimated the associated financial savings and assessed patient satisfaction after implementation of the initiative.
METHODS
We conducted a quality improvement study of outpatient clinics in the Departments of GI Radiation Oncology, Genitourinary (GU) Radiation Oncology, and Thoracic Radiation Oncology (TRO) at a large academic cancer center and four additional community network sites. This retrospective study was approved by The University of Texas MD Anderson Cancer Center’s institutional review board, which also waived the requirement for patient consent for the use of anonymized data in retrospective studies.
The TDABC method was used to create a baseline process map outlining the entire care process (Fig 1, top), with the goal of identifying inefficiencies. Consultation visits were observed in-person and specifically identified all involved staff members, described the specific tasks performed at each component of the visit, and quantified the amount of time required at each step. Patient encounter metrics were also captured by using a real-time status function in the electronic medical record (EMR; Epic Systems, Verona, WI). At our institution, the EMR includes a digital patient status board that is accessible from any computer workstation and can be used by staff to track patients’ location and interactions with staff throughout the consultation process. Staff were educated on the status board functionality before the project was launched, and the EMR data were cross-referenced with the in-person observational data for validation.
FIG 1.
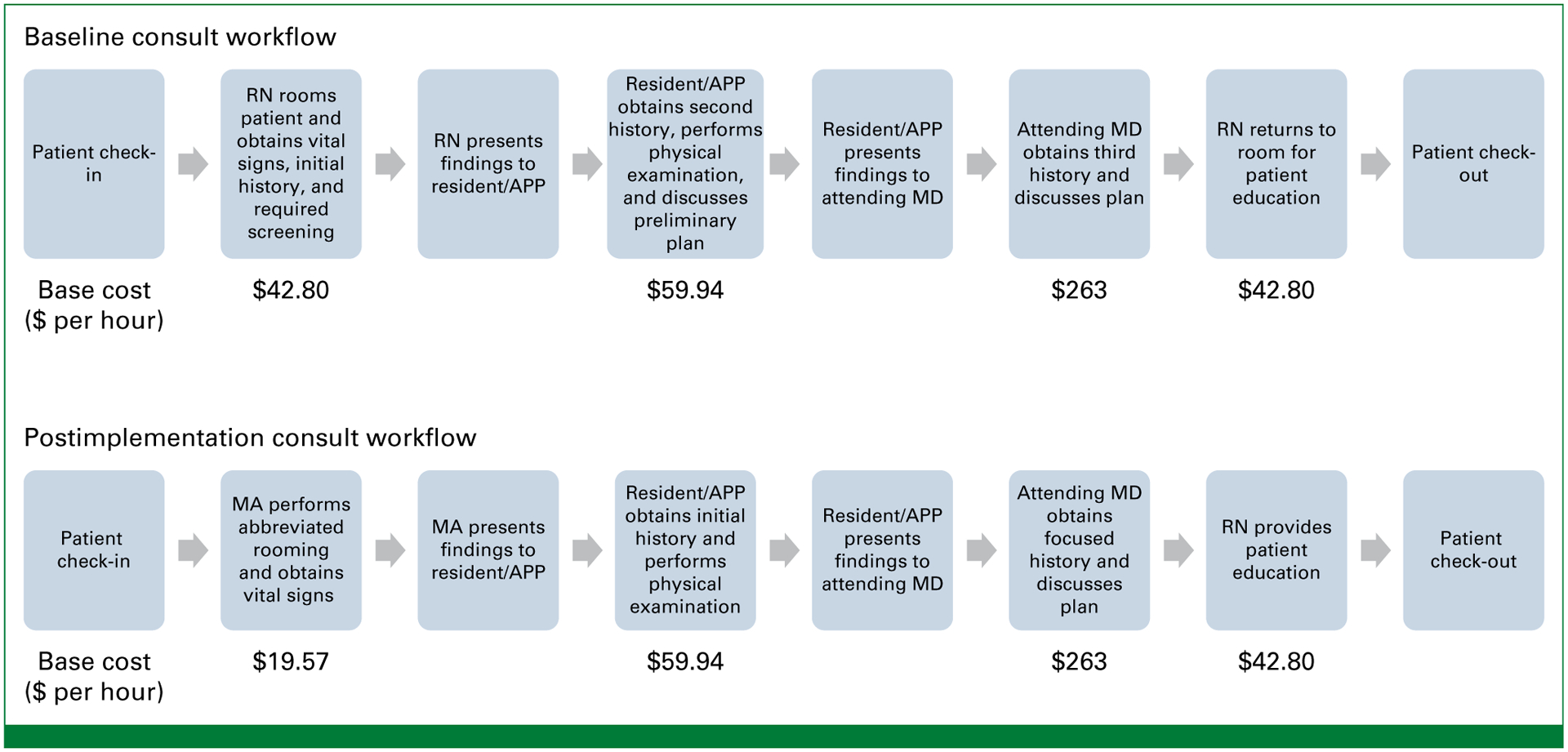
Clinic workflows before and after implementation of the process improvement team’s recommendations. APP, advanced practice provider; MA, medical assistant; MD, attending physician; RN, registered nurse; $, US dollars.
Initial baseline findings were discussed with key stakeholders (nurses, physicians, department leadership, and administrative staff) to identify opportunities for improvement and optimize clinic workflow. The recommended workflow changes included several concurrent interventions. First, staff roles were clearly defined to minimize duplicated effort, thereby facilitating the ability of providers to work to the fullest extent of their license. This intervention was created to address the observation that patients were frequently asked the same questions about their medical history. For example, an RN would obtain an initial history, which would then be presented to the APP or resident, who would repeat the same questions and present the results to the MD. The physician would then confirm the patient history for a third time before discussing the plan for treatment. The new workflow specifically incorporated a role for an MA to perform basic rooming tasks, including obtaining vital signs. The APP/resident would then obtain a history of the present illness and perform a physical examination. The MD was encouraged to minimize duplicative history taking and focus on discussing the treatment plan with the patient. If needed on the basis of the plan, the RN would then wrap up the visit by providing further education for patients who had been assigned to receive radiation therapy, including instructions for treatment simulation, parking, and other logistical considerations. These changes allowed the RNs to tailor patient education to the final plan after the patient had been evaluated by the physician. This change also minimized the number of times the nurses were required to enter and exit the patient room. Additional changes to the workflow included improving communication among staff members regarding patient status during the visit. Because several patients are typically roomed simultaneously at the clinics (ie, several patients could be waiting, in different examination rooms, at the same time), the clinical team was often unsure of which patients had been evaluated and would need to wait for clarification. The intervention consisted of educating all team members on the digital patient status function of the EMR and encouraging that it be used at every step of the process. This digital monitoring capability allowed any staff member, at any workstation, to quickly check on which patients were ready to be seen and to review any previous notes on that patient. This helped to reduce idle time among the clinical team waiting to be updated about the next patient.
The new workflow (Fig 1, bottom) had been piloted in the GI clinic in May 2019-September 2020, and findings from the initial time savings analysis were published previously.10 This workflow initiative was subsequently extended to the GU and thoracic clinics in September 2020-April 2021 and to the community network sites in June-December 2021. Metrics of clinical efficiency were to be captured with the EMR and included total cycle time for the consult visit, which began with rooming time, and included patient wait times and time spent with each staff member (Appendix Fig A1, online only). Waiting room time was also recorded as time elapsed from patient check in to being roomed. Because this interval can be highly variable depending on patient arrival time, we did not include waiting room time in the overall consult cycle time or cost analysis. Patients who arrived later than their scheduled appointment time were included in the analysis, but their consult time metrics were calculated on the basis of when they were roomed as opposed to their scheduled appointment time. Metrics missed by the digital EMR were omitted from the analysis, and all available metrics were included in the patient encounter. All metrics were compared before and after implementation of the new workflow.
Cost per consult visit was estimated from the median time metrics for each step of the patient encounter multiplied by the hourly wages for each staff member. Hourly wages for MA, RN, and APP were based on the 2022 US Bureau of Labor Statistics11 (Table 1) national averages. Radiation oncology physician salary was estimated using the 2023 Doximity Physician Compensation Report,12 which includes 31,000 physician compensation surveys conducted in 2022 for full-time, US-based physicians. The hourly wages was calculated using the national average annual compensation divided by 52 weeks and 40 hours per week. The difference in cost from before to after implementation of the new workflow for each step of the patient encounter was totaled to determine the cost savings per consult. As part of the new workflow, an MA performed the rooming portion of the patient encounter instead of a RN, and therefore, the MA salary was used for the rooming cost after implementation. No nursing positions or schedules were changed in this initiative. Anonymous patient satisfaction telephone surveys were also given to patients seen at the community network sites after their visit. These phone calls were made by a third independent party (NRC Health, Lincoln, NE). Statistical analyses were done with SPSS (version 25.0, IBM, Armonk, NY) and GraphPad (version 9.2, GraphPad Software, San Diego, CA). Mann-Whitney U tests were used to compare nonparametric data, which were reported as medians and IQRs (25th–75th percentiles). Chi-squared analysis was used for proportional comparisons.
TABLE 1.
Clinic Time and Cost Metrics
| Metrics by Clinic | Cycle Time | In Room to With MD | Waiting Room Time | Rooming Time | Wait for APP | Time With APP | Wait for MD | Time With MD | Wait for RN Wrap Up | Time for RN Wrap Up |
|---|---|---|---|---|---|---|---|---|---|---|
| GI clinic | ||||||||||
| Baseline (n = 485), minutes | 91 (71–114) | 54 (39–72) | 14 (8–26) | 13 (9–18) | 11 (5–20) | 22 (12–32) | 20 (11–13) | 33 (25–48) | – | – |
| After intervention (n = 71), minutes | 72 (52–82) | 47 (33–60) | 5 (3–14) | 12 (9–14) | 5 (3–8) | 19 (22–26) | 6 (3–15) | 23 (15–31) | – | – |
| Time reduction, minutes | 19 | 7 | 9 | 1 | 6 | 3 | 14 | 10 | ||
| Baseline cost, $ | 176 | 9 | 22 | 145 | ||||||
| After intervention cost, $ | 124 | 4 | 19 | 101 | ||||||
| Cost savings, $ | 52 | 5 | 3 | 44 | ||||||
| Genitourinary clinic | ||||||||||
| Baseline (n = 247), minutes | 89 (71–116) | 57 (38–77) | 0 (0–3) | 26 (18–33) | 9 (4–24) | 22 (16–33) | 16 (12–34) | 25 (12–47) | – | – |
| After intervention (n = 205), minutes | 73 (56–95) | 48 (27–60) | 0 (0–9) | 11 (7–16) | 7 (3–14) | 21 (13–31) | 7 (3–16) | 21 (13–31) | 1 (0–4) | 5 (3–13) |
| Time reduction, minutes | 16 | 9 | 0 | 15 | 2 | 1 | 9 | 4 | ||
| Baseline cost, $ | 150 | 19 | 22 | 109 | ||||||
| After intervention cost, $ | 117 | 4 | 21 | 92 | ||||||
| Cost savings, $ | 33 | 15 | 1 | 17 | ||||||
| Thoracic clinic | ||||||||||
| Baseline (n = 345), minutes | 72 (55–93) | 48 (35–63) | 0 (0–2) | 16 (12–24) | 8 (4–14) | 14 (9–19) | 10 (3–18) | 21 (13–33) | 8 (3–13) | 11 (7–17) |
| After intervention (n = 233), minutes | 69 (57–83) | 38 (27–51) | 0 (0–3) | 5 (3–7) | 8 (4–15) | 15 (11–22) | 7 (2–16) | 16 (10–26) | 2 (1–6) | 11 (6–17) |
| Time reduction, minutes | 3 | 10 | 0 | 11 | 0 | −1 | 3 | 5 | 6 | 0 |
| Baseline cost, $ | 125 | 11 | 14 | 92 | 8 | |||||
| After intervention cost, $ | 95 | 2 | 15 | 70 | 8 | |||||
| Cost savings, $ | 30 | 9 | −1 | 22 | 0 | |||||
| Community clinics | ||||||||||
| Baseline (n = 251), minutes | 75 (56–98) | 38 (26–54) | 11 (6–18) | 18 (13–29) | 8 (5–14) | 16 (13–22) | 12 (5–20) | 31 (17–45) | – | – |
| After intervention (n = 725), minutes | 66 (51–82) | 34 (21–48) | 9 (6–16) | 5 (3–9) | 7 (3–15) | 16 (12–26) | 7 (3–15) | 24 (14–35) | 4 (2–8) | 11 (6–17) |
| Time reduction, minutes | 9 | 4 | 2 | 13 | 1 | 0 | 5 | 7 | ||
| Baseline cost, $ | 165 | 13 | 16 | 136 | ||||||
| After intervention cost, $ | 123 | 2 | 16 | 105 | ||||||
| Cost savings, $ | 42 | 11 | 0 | 31 |
NOTE. Numbers are medians (25th to 75th IQRs). Cycle time = from entering examination room until check-out. In room to with MD = time from the patient entering examination room until MD arrival. Base Costs per hour: MA $19.57, RN $42.80, APP $59.94, MD $263.
Abbreviations: APP, advanced practice provider; MA, medical assistant; MD, attending physician; RN, registered nurse; $, US dollars.
RESULTS
Patient encounter data were extracted for 1,328 consults before the workflow improvement and 1,234 consults afterward across all departments. Encounters included 556 GI consults (485 before, 71 after), 452 GU consults (247 before, 205 after), 578 TRO consults (345 before, 233 after), and 976 community site consults (251 before, 725 after; Table 1). The nature of the intervention was the same at all clinic sites; however, the performance improvement team met with the members of the thoracic, GU, and community clinics more frequently to encourage use of the EMR status board function and capture time points more consistently. The numbers of MDs in each clinic were eight in GI, seven in GU, 11 in TRO, and 12 in total at the four community sites; the average numbers of consult visits per month for each clinic were 51 in GI, 72 in thoracic, 57 in GU, and 163 in total in community clinics.
The median overall cycle time (ie, the time at which the patient was brought to the examination room [roomed] until check-out) was reduced by 21% in GI (91 v 72 minutes; P < .001), 18% in GU (89 v 73 minutes; P < .001), and 12% in the community sites (75 v 66 minutes; P < .001). No statistically significant decrease in median overall cycle time was noted in the thoracic clinic (72 v 69 minutes; P 5 .10). The median interval between rooming and being seen by the MD decreased by 13% in GI (7 minutes; P < .001), 16% in GU (9 minutes; P < .001), 21% in thoracic (10 minutes; P < .001), and 10% at the community sites (4 minutes; P < .005). Patient wait time for the APP or resident decreased by 55% in GI (6 minutes; P < .001) and 22% in GU (2 minutes; P < .001) but did not change in the thoracic (3 minutes; P = 5 .57) or community (1 minutes; P = 5 .34) clinics. The patient wait time for MD decreased by 70% in GI (14 minutes; P < .001), 57% in GU (9 minutes; P < .001) and 38% in the community sites (5 minutes; P = 5 .002). No change in wait time for the MD was noted in the thoracic clinic (3 minutes; P = 5 .10). The percentage of patients who were >15 minutes late to their appointment was similar before and after the intervention (GI 12% before v 14% after; GU 8% v 5%; thoracic 11% v 9%; community sites 8% v 9%).
A financial savings was noted for each consult as well: The median savings per consult were $52 USD for GI, $33 USD for GU, $30 USD for thoracic, and $42 USD for the community sites. Applying these savings to the study cohort of 2,562 USD patients in all clinics would represent a total savings of $102,000 USD. An average savings of $39 USD per consult and an average 12,000 consults across our institution per month would result in savings of $5,616,000 USD per year.
The response rate to the patient satisfaction telephone survey was 56% (127 of 228; Table 2). In that survey, 99% of patients reported that their providers spent adequate time with them, 97% reported spending enough time to discuss what mattered most to them, 86% reported their appointment beginning on time, and 93% reported being seen by a care provider in a timely manner.
TABLE 2.
Patient Satisfaction Survey Responses
| All Community Location | Participation Rate, % | No. of Responses | No. of Surveys Sent | Percentage, % |
|---|---|---|---|---|
| Question | 55.7 | 127 | 228 | |
| Did this provider spend enough time with you? | ||||
| Yes, definitely | 99.2 | |||
| Did we spend enough time to discuss what matters most to you? | ||||
| Yes, definitely | 96.8 | |||
| Did your appointment begin on time? | ||||
| Yes, definitely | 86.4 | |||
| Yes, somewhat | 12.8 | |||
| Were you seen by a care provider in a timely manner? | ||||
| Yes, definitely | 92.7 | |||
| Yes, somewhat | 7.3 |
DISCUSSION
In this study, we demonstrated that TDABC is an effective tool to assess and optimize clinic workflow efficiency for outpatient consults in both academic and community settings. We found that simple changes to more clearly distinguish the responsibilities of each team member and enhance communication through the EMR patient status board (Fig 1) led to significant reductions in overall consult cycle time, wait time, and time to being seen by the MD. The benefit of workflow optimization was greatest for teams that had had the longest cycle times and wait times at baseline. Similarly, the financial savings from the improved workflow were the greatest for clinics with longer consult cycle times at baseline.
Our results are comparable with previous reports of clinic flow optimization initiatives at other institutions. A study that involved similar Lean Six Sigma strategies in an outpatient ophthalmology clinic demonstrated a reduction of median patient cycle times by 18% and a decrease in the IQR by 32%.13 These time savings were accomplished through simple, low-cost changes to clinic processes and were sustained despite a 9% increase in patient volume per clinic.13 Another study conducted in a primary care clinic used a similar strategy of engaging key stakeholders in the clinic and implementing continuous process improvement14; interventions included incorporating a MA to room patients during the busiest clinic times, improving nurse and physician communication regarding scheduled patients, and incorporation of automated EMR time stamp metrics.14 Over the 1-year study period, cycle times decreased by 12% from 71 to 65 minutes,14 a magnitude similar to that in our experience. Patient satisfaction scores in that study were also high after implementation of the improved workflow and did not seem to be adversely affected by decreased cycle times,14 as was the case in our study as well.
Improving clinic efficiency by reducing waiting time and redundant tasks would be expected to improve patient satisfaction. Another study addressing patient satisfaction examined the effects of improved clinic efficiency in a multidisciplinary transplantation clinic.15 The investigators identified suboptimal communication between staff as a key bottleneck in clinic workflow. By implementing workflow changes that included using EMR patient status indicators and adding a patient status monitor to the clinic, staff members were easily able to identify patients who were ready to be seen and were able to communicate delays to patients. In that study, these changes resulted in a sustained improvement in patient satisfaction scores over 5 years as measured by Press Ganey surveys.13 Given the high number of subspecialty appointments that patients with cancer often require, reducing unnecessary wait time in clinics may well reduce patients’ overall time burden associated with receiving cancer treatment.
We recognize that improvements in clinical efficiency should not sacrifice quality of time spent with patients. Because patients value the time the spent with their physicians, we did not aim to reduce the time spent with the radiation oncologist. Although a significant proportion of the cost savings was due to the physician portion of the patient encounter, this was driven by the much higher physician salary rather than reduced time spent with physicians. Moreover, patient satisfaction was high in domains such as adequate time for discussion and timely care with the new workflow, which we took to indicate that patients felt they had sufficient time with their physicians. Our focus was to reduce cycle times by minimizing wait time between steps in the visit and minimizing repeated questioning. Overall, by improving clinical efficiency without compromising quality or time with the physician, busy clinics may be able to achieve high patient satisfaction while being more resilient to clinic delays or staff shortages.
For health care staff, rates of burnout have increased substantially since the start of the COVID-19 pandemic. One physician survey demonstrated an increase in physicians having at least one symptom of burnout from 38% in 2020 to 63% in 2022.16 This drastic rise in the prevalence of burnout likely results from many factors associated with the COVID-19 pandemic and is likely exacerbated by staffing shortages that increase workload. In this context, inefficiencies in clinic workflow may worsen burnout by increasing time pressure and interfering with staff work-life balance.7 Although our study did not directly assess the effects of our workflow changes on rates of employee burnout, improving clinic efficiency and having clear definitions of staff roles can reduce the workload for individual employees and may alleviate subjective workload. Burnout is a topic of great concern and is being assessed at the institutional level at many tertiary cancer care centers.
Our study had several limitations. First, it was conducted solely in radiation oncology consultation clinics, which may limit the generalizability of our results to other medical specialties. However, our study was conducted at both a large academic center and several community network locations. Second, cost estimates were based on average salaries derived from information provided by the US Bureau of Labor and Statistics and thus represent estimates. Third, data in this study were largely collected through the EMR patient status function, which relies on staff members to update information on patient status in real time; EMR status data were not always comprehensive for every patient. To mitigate these effects, we omitted missing time intervals from our analysis and manually reviewed the automated EMR status data for validity. The benefit of using the EMR status function was that it facilitated collection data from a much larger number of patients than would be possible with in-person observation, which could also be burdensome on staff. We also acknowledge that greater appreciation of long patient cycle time and increased focus on patient flow by the clinic staff may have influenced our results. Finally, we obtained patient satisfaction data via Press Ganey surveys from a limited subset of patients after implementation of the new workflow and did not have baseline data for comparison. These telephone surveys were also limited by the phrasing of the questions asked, specifically use of the term provider, which could possibly be interpreted as referring to the APP, resident, or MD. However, use of these standardized Press Ganey surveys has since been expanded, and the question is meant to assess patient satisfaction with physicians; in general, we expect that patients associate this term with the physician. Nevertheless, it is reassuring that patient satisfaction was high with the new workflow, and we plan to collect baseline patient satisfaction data in future initiatives across the entire range of radiation oncology clinics.
In summary, TDABC can be a useful strategy to improve outpatient clinic workflow. By implementing automated EMR time metrics tracking, the downstream effects of changes can be monitored, and feedback can be provided regularly to staff members. Simple changes in workflow to minimize duplication of effort and improve communication can lead to substantial reductions in visit cycle time and reduce patient wait time. These reductions might be expected to lead to improved patient satisfaction and reduce time pressure and burnout for staff. On an institutional level, the decrease in consult cycle times may also result in financial savings and increase clinic capacity, which could lead to improved access for patients. Future efforts are underway to assess the effects of similar workflow changes on patient satisfaction and to expand this initiative to other departments at our institution.
CONTEXT.
Key Objective
Our objective was to optimize clinical workflow to improve clinical operations for high-volume outpatient radiation oncology clinics using time-driven activity-based costing (TDABC).
Knowledge Generated
We identified common opportunities for improvement and implemented workflow changes including reduction of patient wait times, defining staff roles to work at the top of their license, and improving clinical team communication though an electronic medical record patient status dashboard. We demonstrated a significant reduction in overall consult cycle times, potential financial savings by integrating a medical assistant, and high patient satisfaction across multiple clinics in both academic and community settings.
Relevance
Our findings suggest TDABC can be an effective strategy to identify simple interventions that enhance clinic workflow and decrease cycle times. Reducing inefficiency in the clinical encounter may improve patient experience, reduce staff burnout, and increase clinic capacity.
ACKNOWLEDGMENT
We thank Christine Wogan, MS, ELS, of MD Anderson’s Division of Radiation Oncology, for her review of the manuscript and editorial assistance.
SUPPORT
Supported in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center (PI: PW Pisters). This funding body had no role in the design of the study or the collection, analysis, and interpretation of data or in writing the manuscript.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Optimizing Outpatient Radiation Oncology Consult Workflow by Using Time-Driven Activity-Based Costing: Efficiency and Financial Impacts
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = 5 Immediate Family Member, Inst = 5 My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Julius Weng
Employment: MD Anderson Cancer Center
Research Funding: AstraZeneca (Inst)
Shane Mesko
Employment: Scripps Health
Consulting or Advisory Role: Oscar Health
Percy Lee
Employment: City of Hope National Medical Center
Honoraria: OncLive/MJH Life Sciences, ViewRay, AstraZeneca, Genentech, Varian Medical Systems, Roche
Consulting or Advisory Role: Johnson & Johnson/Janssen, ViewRay, AstraZeneca
Research Funding: AstraZeneca
Prajnan Das
Employment: MD Anderson Cancer Center
Honoraria: Bayer
Consulting or Advisory Role: American Society for Radiation Oncology
Albert C. Koong
Stock and Other Ownership Interests: Aravive
Katy French
Research Funding: Ambu (Inst)
Thomas Aloia
Employment: BioIntellisense
Elizabeth Bloom
Employment: Carl D. Bloom, DMD, PA
Consulting or Advisory Role: MD Anderson Physician Network
Zhongxing Liao
Honoraria: Varian Medical Systems
Consulting or Advisory Role: ReHeva Biosciences, AIQ Global
Speakers’ Bureau: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
No other potential conflicts of interest were reported.
APPENDIX
FIG A1.
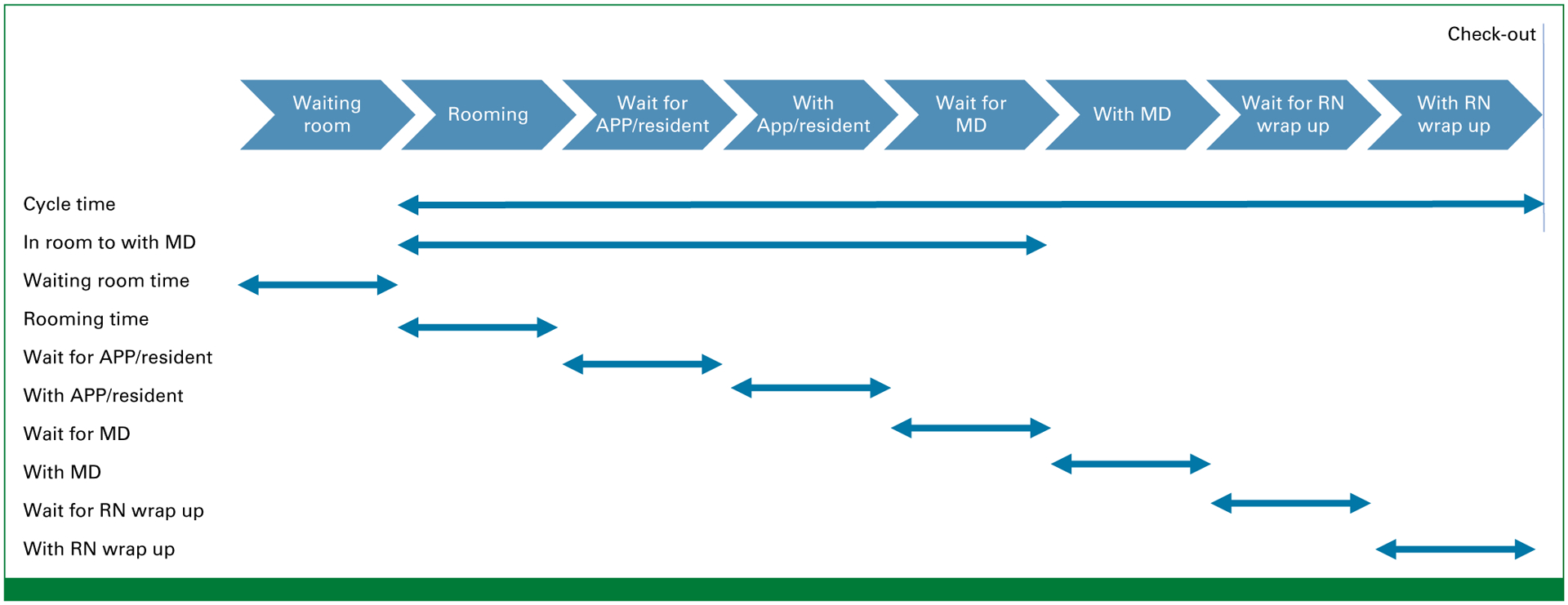
Measured time metrics. APP, advanced practice provider; MD, attending physician; RN, registered nurse.
Footnotes
PRIOR PRESENTATION
Presented in part at ASCO Quality 2022, Chicago, IL, September 30, 2022 - October 1, 2022; American Radium Society 2022, Scottsdale, AZ, May 19–22, 2022; and the Institute of Healthcare Improvement 2021, Virtual Conference, December 5–8, 2021.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI https://doi.org/10.1200/OP.23.00037.
REFERENCES
- 1.Bodenheimer T, Sinsky C: From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med 12:573–576, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laviana AA, Luckenbaugh AN, Resnick MJ: Trends in the cost of cancer care: Beyond drugs. J Clin Oncol 38:316–322, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bange EM, Doucette A, Gabriel PE, et al. : Opportunity costs of receiving palliative chemotherapy for metastatic pancreatic ductal adenocarcinoma. JCO Oncol Pract 16:e678–e687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feddock CA, Hoellein AR, Griffith CH III, et al. : Can physicians improve patient satisfaction with long waiting times? Eval Health Prof 28:40–52, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Lis CG, Rodeghier M, Gupta D: Distribution and determinants of patient satisfaction in oncology: A review of the literature. Patient Prefer Adherence 3:287–304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandoval GA, Brown AD, Sullivan T, et al. : Factors that influence cancer patients’ overall perceptions of the quality of care. Int J Qual Health Care 18:266–274, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kleiner S, Wallace JE: Oncologist burnout and compassion fatigue: Investigating time pressure at work as a predictor and the mediating role of work-family conflict. BMC Health Serv Res 17:639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeChant PF, Acs A, Rhee KB, et al. : Effect of organization-directed workplace interventions on physician burnout: A systematic review. Mayo Clinic Proc Innov Qual Outcomes 3:384–408, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keel G, Savage C, Rafiq M, et al. : Time-driven activity-based costing in health care: A systematic review of the literature. Health Policy 121:755–763, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Mesko S, Weng J, Das P, et al. : Using patient flow analysis with real-time patient tracking to optimize radiation oncology consultation visits. BMC Health Serv Res 22:1517, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Bureau of Labor Statistics. https://www.bls.gov/
- 12.Doximity physician compensation report 2023. https://press.doximity.com/research
- 13.Kam AW, Collins S, Park T, et al. : Using Lean Six Sigma techniques to improve efficiency in outpatient ophthalmology clinics. BMC Health Serv Res 21:38, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson J, Porter M, Montalvo Y, et al. : Losing the wait: Improving patient cycle time in primary care. BMJ Open Qual 9:e000910, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pashankar DS, Brown T, Votto P, et al. : Sustained improvement in patient experience by optimizing patient flow in ambulatory settings. J Patient Exp 9:23743735221092610, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanafelt TD, West CP, Dyrbye LN, et al. : Changes in burnout and satisfaction with work-life integration in physicians during the first 2 years of the COVID-19 pandemic. Mayo Clin Proc 97: 2248–2258, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]


