Abstract
Forty-eight (48) drug products (DPs) containing amorphous solid dispersions (ASDs) have been approved by the U.S. Food and Drug Administration in the 12-year period between 2012 and 2023. These DPs comprise 36 unique amorphous drugs. Ten (10) therapeutic categories are represented, with most DPs containing antiviral and antineoplastic agents. The most common ASD polymers are copovidone (49%) and hypromellose acetate succinate (30%), while spray drying (54%) and hot melt extrusion (35%) are the most utilized manufacturing processes to prepare the ASD drug product intermediate (DPI). Tablet dosage forms are the most common, with several capsule products available. Line extensions of several DPs based on flexible oral solids and powders for oral suspension have been approved which provide patient-centric dosing to pediatric and other patient populations. The trends in the use of common excipients and film coating types are discussed. Eighteen (18) DPs are fixed-dose combinations, and some contain a mixture of amorphous and crystalline drugs. The DPs have dose/unit of amorphous drug ranging from <5 mg up to 300 mg, with the majority being ≤100 mg/unit. This review details several aspects of DPI and DP formulation and manufacturing of ASDs, as well as trends related to therapeutic category, dose, and patient-centricity.
Keywords: Amorphous solid dispersion, Drug product design, Therapeutic category, Dose, Patient centricity
Graphical abstract
1. Introduction
The increasing prevalence of poorly water-soluble compounds in pharmaceutical development pipelines has been acknowledged for the past few decades (Brouwers et al., 2009; Di et al., 2012; Lipinski, 2000; Shultz, 2019). Many solid form and formulation strategies to address the solubility limitation for oral drug products (DPs) have been investigated, including salts, cocrystals, cyclodextrins, lipid-based formulations (LBFs), nanocrystals, and amorphous solid dispersions (ASDs) (Bennett-Lenane et al., 2020; Jermain et al., 2018; Loftsson and Brewster, 2010; Saal and Becker, 2013; Williams et al., 2013; Wong et al., 2021). Of these, it is notable that the number of ASD formulations approved by the United States Food and Drug Administration (FDA) has been steadily increasing since their introduction (Jermain et al., 2018; Saha et al., 2023; Tan et al., 2020).
ASDs are a supersaturating drug delivery strategy where the amorphous drug and polymer carrier are formulated as a molecular-level dispersion (Williams et al., 2013). Enhanced bioavailability is achieved through the creation and maintenance of supersaturation due to the solubility advantage of the amorphous form, enabling improved absorption (Taylor and Zhang, 2016). For bioavailability enhancement to be realized, optimized formulation attributes such as drug loading and polymer selection may provide for enhanced dissolution rate, precipitation inhibition from solution, and physical stability upon storage (Hiew et al., 2022; Moseson and Taylor, 2023; Price et al., 2018; Saboo et al., 2020).
To translate the ASD formulation strategy into a DP, it must be manufactured as a drug product intermediate (DPI) and then formulated into a DP such as a tablet or capsule. This review sets out to analyze the ASD products approved by the FDA in the 12-year period between 2012 and 2023, detailing several aspects and trends of DPI and DP formulation and manufacturing, as well as aspects of therapeutic category, dose, and patient-centricity. The discussion presented here is pertinent for industrial pharmaceutical scientists seeking to understand competitor trends, academic research scientists seeking to understand industrially-relevant problem statements, as well as those new to ASD technology and DP development.
2. Analysis of approved drug products
2.1. Dataset
A summary of the ASD products approved by the FDA from 2012 to 2023 can be found in Table 1. The list was collated by first reviewing the monthly original new drug application approvals on Drugs@FDA in the years of interest for the list of DPs with proprietary names. Specifically, only DPs with submission classifications of Type 1 – New Molecular Entity, Type 2 – New Active Ingredient, Type 3 – New Dosage Form, Type 4 – New Combination, and Type 5 – New Formulation or New Manufacturer were shortlisted. The shortlisted DPs were further narrowed down by those containing polymers that may be used to formulate ASDs, as well as cross-referencing literature review papers highlighting ASD formulations (Bhujbal et al., 2021; Corrie et al., 2023; Jermain et al., 2018; Saha et al., 2023; Tan et al., 2020). The use of an ASD strategy was then confirmed by a review of publicly accessible documents such as prescribing information, patent families relating to the active ingredient, or other published works. For each ASD DP, the prescribing information was thoroughly reviewed for dosage forms and strengths, dosage and administration, and other information including molecular weight (MW), salt form, excipients used, packaging type, as well as storage and handling instructions. Pure amorphous DPs are not included in this review (i.e., those which do not include an ASD polymer). Additionally, Orilissa and Oriahnn, identified by other publications as ASDs, were excluded from this review, as the authors believe the formulation instead represents a melt granulation process of crystalline elagolix based on patent review (Qiu et al., 2019).
Table 1.
Summary of ASD products approved by the FDA between 2012 and 2023.
| Trade name | Drug name(s) | Dosage form | Dosage strength (mg) | Recommended dosage⁎ | Manufacturing technique | ASD polymer | Company& | Year^ | Therapeutic category# |
|---|---|---|---|---|---|---|---|---|---|
| Alvaiz | Eltrombopaga | Tablets | 9; 18; 36; 54 | 1 to 2 units QD | HME | PVPVA | Teva | 2023 | Blood Products and Modifiers |
| Astagraf XL | Tacrolimus | Capsules | 0.5; 1; 5 | Determined based on patient's weight | Solvent granulation | HPMC | Astellas | 2013 | Immunological Agents |
| Belsomra | Suvorexant | Tablets | 5; 10; 15; 20 | 1 to 2 units ON | HME | PVPVA | Merck | 2014 | Sleep Disorder Agents |
| Braftovi | Encorafenib | Capsules | 50b; 75 | 4 to 6 units QD | HME | PVPVA | Array | 2018 | Antineoplastics |
| Delstrigo | Doravirine/ Lamivudinec/ Tenofovir disoproxil fumaratec | Tablets | 100/300/300 | 1 unit QD | SD | HPMCAS | Merck | 2018 | Antivirals |
| Envarsus XR | Tacrolimus | Tablets | 0.75; 1; 4 | Determined based on patient's weight | Melt granulation | PEG | Veloxis | 2015 | Immunological Agents |
| Epclusa | Sofosbuvirc/ Velpatasvir | Tablets | 200/50d; 400/100 | 1 unit QD | SD | PVPVA | Gilead Sciences | 2016 | Antivirals |
| Epclusa | Sofosbuvirc/ Velpatasvir | Pellets | 150/37.5; 200/50 | 1 to 2 units QD | SD | PVPVA | Gilead Sciences | 2019 | Antivirals |
| Erleada | Apalutamide | Tablets | 60; 240e | 1 unit QD | SD | HPMCAS | Janssen | 2018 | Antineoplastics |
| Harvoni | Ledipasvir/ Sofosbuvirc | Tablets | 45/200f; 90/400 | 1 unit QD | SD | PVPVA | Gilead Sciences | 2014 | Antivirals |
| Harvoni | Ledipasvir/ Sofosbuvirc | Pellets | 33.75/150; 45/200 | 1 to 2 units QD | SD | PVPVA | Gilead Sciences | 2019 | Antivirals |
| Idhifa | Enasidenibg | Tablets | 50, 100 | 1 unit QD | SD | HPMCAS | Bristol Myers Squibb | 2017 | Antineoplastics |
| Jaypirca | Pirtobrutinib | Tablets | 50; 100 | 2 units QD | SD | HPMCAS | Loxo Oncology | 2023 | Antineoplastics |
| Jynarque | Tolvaptan | Tablets | 15; 30; 45; 60; 90 | 1 unit BID | SD | HPC | Otsuka | 2018 | Electrolytes/Minerals/Metals/Vitamins |
| Kalydeco | Ivacaftor | Tablets | 150 | 1 unit BID | SD | HPMCAS | Vertex | 2012 | Respiratory Tract/Pulmonary Agents |
| Kalydeco | Ivacaftor | Granules | 5.8h; 13.4h; 25i; 50; 75 | 1 unit BID | SD | HPMCAS | Vertex | 2015 | Respiratory Tract/Pulmonary Agents |
| Lynparza | Olaparib | Tablets | 100; 150 | 2 units BID | HME | PVPVA | AstraZeneca | 2017 | Antineoplastics |
| Mavyret | Glecaprevir/ Pibrentasvir | Tablets | 100/40 | 3 units QD | HME | PVPVA | AbbVie | 2017 | Antivirals |
| Mavyret | Glecaprevir/ Pibrentasvir | Pellets | 50/20 | 3 to 5 units QD | HME | PVPVA | AbbVie | 2021 | Antivirals |
| Norvir | Ritonavir | Powder for oral suspension | 100 | 1 to 6 units BD | HME | PVPVA | AbbVie | 2017 | Antivirals |
| Noxafil | Posaconazole | Tablets | 100 | 3 units QD or BID | HME | HPMCAS | Merck | 2013 | Antifungals |
| Noxafil | Posaconazole | PowderMix for oral suspension | 300 | 1 unit QD or BID | HME | HPMCAS | Merck | 2021 | Antifungals |
| Orkambi | Lumacaftorc/ Ivacaftor | Tablets | 100/125j; 200/125 | 2 units BID | SD | HPMCAS | Vertex | 2015 | Respiratory Tract/Pulmonary Agents |
| Orkambi | Lumacaftorc/ Ivacaftor | Granules | 75/94k; 100/125; 150/188 | 1 unit BID | SD | HPMCAS | Vertex | 2018 | Respiratory Tract/Pulmonary Agents |
| Paxlovidl | Nirmatrelvirc/ Ritonavir | Tablets | 150/100 | 3 units BID | HME | PVPVA | Pfizer | 2023 | Antivirals |
| Phyrago | Dasatinib | Tablets | 20; 50; 70; 80; 100; 140 | 1 unit QD | Electrospraying | Methacrylic acid-ethyl acrylate copolymer | Nanocopoeia | 2023 | Antineoplastics |
| Prograf | Tacrolimus | Granules for oral suspension | 0.2; 1 | Determined based on patient's weight | Solvent granulation | HPMC | Astellas | 2018 | Immunological Agents |
| Pifeltro | Doravirine | Tablets | 100 | 1 unit QD | SD | HPMCAS | Merck | 2018 | Antivirals |
| Qinlock | Ripretinib | Tablets | 50 | 3 units QD | SD | HPMCAS | Deciphera | 2020 | Antineoplastics |
| Qulipta | Atogepant | Tablets | 10; 30; 60 | 1 unit QD | HME | PVPVA | AbbVie | 2021 | Antimigraine Agents |
| Sotyktu | Deucravacitinib | Tablets | 6 | 1 unit QD | SD | HPMCAS | Bristol Myers Squibb | 2022 | Immunological Agents |
| Stivarga | Regorafenib | Tablets | 40 | 4 units QD | cPT | PVP | Bayer | 2012 | Antineoplastics |
| Sunlenca | Lenacapavirm | Tablets | 300 | 1 to 2 units QD | SD | PVPVA | Gilead Sciences | 2022 | Antivirals |
| Symdekol | Ivacaftor/ Tezacaftor and Ivacaftor | Tablets | 75/50 and 75n; 150/100 and 150 | 1 unit BID | SD | Ivacaftor – HPMCAS; Tezacaftor – HPMC | Vertex | 2018 | Respiratory Tract/Pulmonary Agents |
| Technivieb | Ombitasvir/ Paritaprevir/ Ritonavir | Tablets | 12.5/75/50 | 2 units QD | HME | PVPVA | AbbVie | 2015 | Antivirals |
| Tibsovo | Ivosidenib | Tablets | 250 | 2 units QD | SD | HPMCAS | Servier | 2018 | Antineoplastics |
| Tolsura | Itraconazole | Capsules | 65 | 2 units QD, BID, or TIDo | SD | HPMCP | Mayne Pharma | 2018 | Antifungals |
| Trikaftal | Elexacaftorc/ Ivacaftor/ Tezacaftor and Ivacaftor | Tablets | 50/37.5/25 and 75p; 100/75/50 and 150 | 2 units OM, 1 unit ON | SD | Ivacaftor – HPMCAS; Tezacaftor – HPMC | Vertex | 2019 | Respiratory Tract/Pulmonary Agents |
| Trikaftal | Elexacaftorc/ Ivacaftor/ Tezacaftor and Ivacaftor | Granules | 80/60/40 and 59.5; 100/75/50 and 75 | 1 unit BID | SD | Ivacaftor – HPMCAS; Tezacaftor – HPMC | Vertex | 2023 | Respiratory Tract/Pulmonary Agents |
| Tukysa | Tucatinib | Tablets | 50, 150 | 2 units BID | SD | PVPVA | Seagen | 2020 | Antineoplastics |
| Ubrelvy | Ubrogepant | Tablets | 50, 100 | 1 to 2 units QD | HME | PVPVA | AbbVie | 2019 | Antimigraine Agents |
| Venclexta | Venetoclax | Tablets | 10, 50, 100 | 1 to 6 units QD | HME | PVPVA | AbbVie | 2016 | Antineoplastics |
| Viekira PAKb, l | Dasabuvirc and Ombitasvir/ Paritaprevir/ Ritonavir | Tablets | 250 and 12.5/75/50 | 3 units OM, 1 unit ON | HME | PVPVA | AbbVie | 2014 | Antivirals |
| Viekira XRb | Dasabuvirc/ Ombitasvir/ Paritaprevir/ Ritonavir | Tablets | 200/8.33/50/33.33 | 3 units QD | HME | PVPVA | AbbVie | 2016 | Antivirals |
| Vosevi | Sofosbuvirc/ Velpatasvir/ Voxilaprevir | Tablets | 400/100/100 | 1 unit QD | SD | PVPVA | Gilead Sciences | 2017 | Antivirals |
| Welireg | Belzutifan | Tablets | 40 | 3 units QD | SD | HPMCAS | Merck | 2021 | Genetic, Enzyme, or Protein Disorder: Replacement, Modifiers, Treatment |
| Xtandi | Enzalutamide | Tablets | 40; 80 | 2 units QD | SD | HPMCAS | Astellas | 2020 | Antineoplastics |
| Zepatier | Elbasvir/ Grazoprevir | Tablets | 50/100 | 1 unit QD | SD | Elbasvir – HPMC; Grazoprevir – PVPVA | Merck | 2016 | Antivirals |
Recommended dosage does not take into account situations that require dosage adjustment.
U.S. New Drug Application holder.
Year of approval of ASD drug product.
Based on United States Pharmacopeia (USP) Drug Classification.
Drug is formulated as choline salt.
Product has been discontinued in the United States.
Drug is formulated in crystalline form.
Dosage strength was approved on March 19, 2020.
Dosage strength was approved on February 17, 2023.
Dosage strength was approved on August 28, 2019.
Drug is formulated as mesylate salt.
Dosage strength was approved on May 3, 2023.
Dosage strength was approved on April 29, 2019.
Dosage strength was approved on September 28, 2016.
Dosage strength was approved on September 2, 2022.
Co-packaged product.
Drug is formulated as sodium salt.
Dosage strength was approved on June 21, 2019.
TID dosing is used only as loading dose for life-threatening situations.
Dosage strength was approved on June 8, 2021.
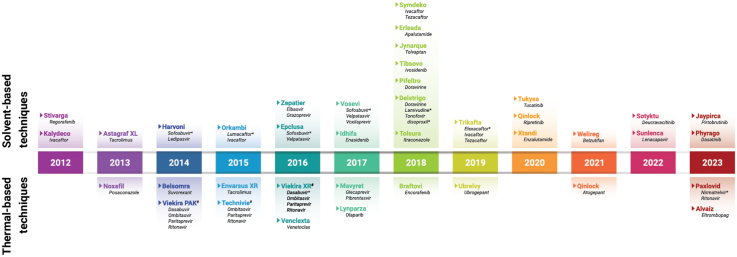
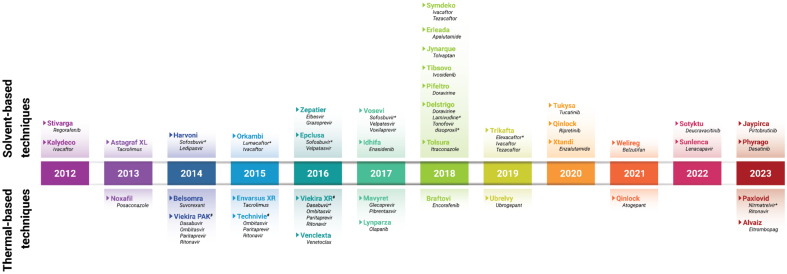
ASD DPs that received FDA approval are graphically displayed in the timeline found in Fig. 1. The timeline highlights the general trend of relatively consistent number of approvals per year, an average of four approvals per year (48 products over 12 years). The year 2018 had a record eight approvals.
Fig. 1.
Timeline of first FDA approval of ASD DPs between 2012 and 2023. For DPs with more than one DP type (e.g., tablets, granules, powder/granules for oral suspension), only the first approval is included in the figure.
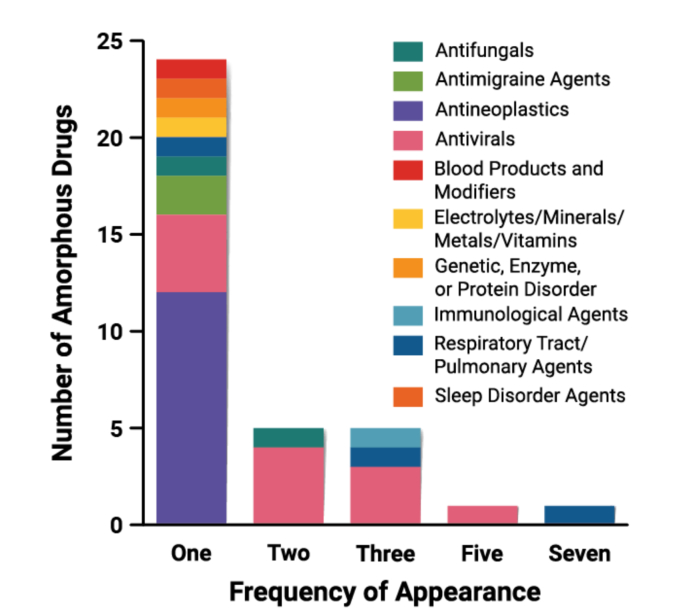
This dataset represents 36 unique amorphous drugs (Fig. 2). Three amorphous drugs, enasidenib, lenacapavir, and eltrombopag are formulated as mesylate, sodium, and choline salts, respectively. While the use of salt forms is less common for amorphous drugs, studies have shown that they can impact the physical stability and dissolution performance of ASDs (Hiew and Taylor, 2022; Mukesh et al., 2021). Therefore, the use of amorphous drug salts warrants careful consideration and evaluation. Several drugs also use hydrate crystalline solid forms as the in-going material (e.g., regorafenib, paritaprevir, tacrolimus), and the water is removed during the DPI manufacturing process. Deucravacitinib is the first novel deuterated FDA-approved drug molecule. Deuterium incorporation was first thought to increase metabolic stability of the compound, but it has since been shown that the pharmacokinetic improvements from deuteration may have a significant impact on drug efficacy and safety. Clinical relevance of deuterated drugs appears to be increasing, as there are at least 15 compounds under clinical investigation (Di Martino et al., 2023). In the case of deucravacitinib, the deuterium was incorporated to avoid the formation of a non-selective metabolite and preserve specificity of the parent drug for its target (Wrobleski et al., 2019).
Fig. 2.
Frequency of amorphous drugs appearing in one or more DPs (n = 36).
Several drugs are found in more than one approved DPs (Fig. 2). Tacrolimus appears in three (3) DPs (Astagraf XL, Envarsus XR, Prograf). Ivacaftor appears in seven (7) DPs, both as a monotherapy and fixed-dose combinations (FDCs). Technivie, Viekira PAK, and Viekira XR contain three amorphous drugs (ombitasvir, paritaprevir, ritonavir), and in two cases a crystalline drug dasabuvir. Ritonavir additionally appears within Norvir and Paxlovid. Epclusa includes sofosbuvir (a crystalline drug) and velpatasvir (an amorphous drug), while Vosevi adds a third drug in its amorphous form, voxilaprevir. FDC products are discussed further in Section 2.7. There are six (6) products (Epclusa, Harvoni, Kalydeco, Mavyret, Orkambi, Trikafta) that are available in both tablet and pellets/granules, and three (3) powders/granules for oral suspensions are new dosage forms of earlier product launches (Norvir, Noxafil, Prograf) (discussed further in Sections 2.5.1 and 2.5.5).
2.2. Therapeutic category
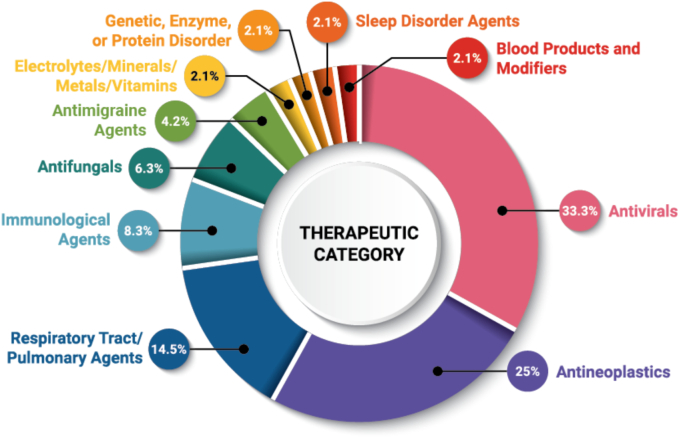
DPs comprising ASDs have been approved for drugs in a wide range of therapeutic categories, defined in Fig. 3 based on their United States Pharmacopeia (USP) Drug Classification. Antivirals (33.3%), antineoplastics (25%), and respiratory tract/pulmonary agents (14.5%) represent the majority of the ASD DPs. The prevalence of DPs within certain therapeutic categories is partly a result of reformulations and FDC products. For example, each DP that is pellet or granule type was originally launched in a tablet form. These dosage forms will be discussed in greater detail in Section 2.5.1. FDC products, where several drugs are combined into a single DP, will be discussed in detail in Section 2.7.
Fig. 3.
Frequency of therapeutic category served by ASD DPs (n = 48).
2.3. Drug physicochemical properties
The prevalence and beneficial patient-centric aspects of ASD technology being used for antiviral and antineoplastic agents were highlighted by McKelvey and Kesisoglou (2019) and Gala et al. (2020). The McKelvey and Kesisoglou review highlighted that molecules with similar structural motifs and requirements for target engagement may have similar physicochemical properties such as poor aqueous solubility (McKelvey and Kesisoglou, 2019). This can also be a reason that the use of ASD technology is limited to a select number of companies, based on the therapeutic areas in which they specialize. In the review by Gala et al., the limitations of alternate formulation approaches for poorly water-soluble antineoplastic agents were explored (e.g., low drug loading capacity, use of excipients with toxic side effects, altered drug distribution and clearance), ultimately highlighting the beneficial aspects of the ASD formulation approach which uses excipients that are generally recognized as safe (GRAS) to deliver these agents with improved pharmacokinetic and pharmacodynamic properties (Gala et al., 2020).
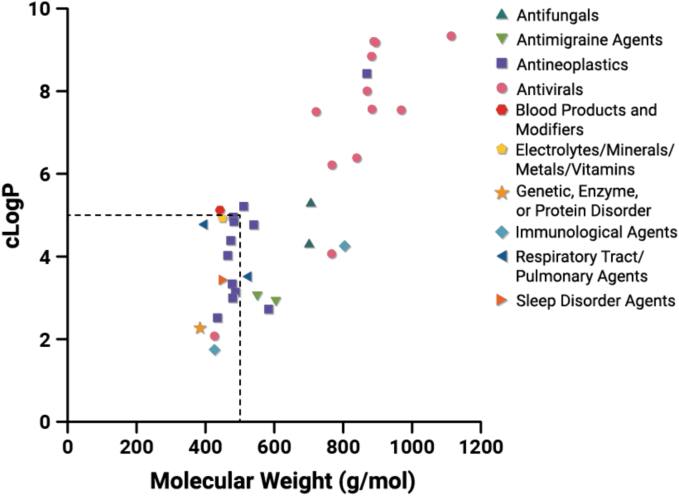
In 2000, Lipinski and coworkers introduced the rule of 5 (Ro5) regarding molecular descriptors of “drug-like” properties which can be used to estimate the likelihood of poor absorption based on permeation or solubility (Lipinski, 2000). The Ro5 criteria define four molecular descriptors: MW ≤ 500, logP ≤5, number of hydrogen bond donors (HBD) ≤ 5, number of hydrogen bond acceptors (HBA) ≤ 10. In the nearly 25 years since, drug molecules in industrial pipelines have continued to increase in MW and logP (Shultz, 2019). While not all Ro5 molecular descriptors have been found to correlate with permeability and solubility (Sutherland et al., 2012; Tinworth and Young, 2020; Winiwarter et al., 1998), they may offer value in understanding the development landscape, and enable translation into appropriate formulation strategies (Bergström et al., 2016; Stegemann et al., 2023; Yang et al., 2024). A recent review by Stegemann et al. (2023) found that between 1994 and 1997, 22% of oral products launched contained a drug with at least one descriptor beyond the rule of 5 (bRo5), while, in contrast, between 2013 and 2019, 40% of oral products contained a drug with at least one descriptor outside the Ro5 criteria. In our dataset containing amorphous drugs approved between 2012 and 2023 formulated into ASD DPs, 61% have at least one descriptor outside the Ro5 criteria (Fig. 4). MW is the most frequently violated rule (n = 21), followed by logP (n = 14), while few molecules violate the HBA (n = 1) and HBD (n = 0) rules. For all but one amorphous drug (eltrombopag), when the logP rule is violated, the MW rule is also violated. The trend is particularly notable for antiviral compounds. As physicochemical properties move toward higher MW and lipophilicity and into the bRo5 space, this has led to an increasing proportion of molecules with poor aqueous solubility. While solubility and bioavailability were identified as accounting for ∼40% of drug compound attrition in 1991 (Kola and Landis, 2004), technological advancements in strategies to address the solubility limitation, such as ASDs, have resulted in successful development of poorly water-soluble drugs for oral drug delivery.
Fig. 4.
Molecular descriptors of MW and logP for the amorphous drugs contained within the DPs approved between 2012 and 2023 (n = 36). The logP was calculated using the Molinspiration platform (www.molinspiration.com). MW and clogP properties of the free form of each amorphous drug are reported.
Other classification systems have been used to trend aqueous solubility and permeability or metabolism as predictors of drug absorption (Amidon et al., 1995; Butler and Dressman, 2010; Rosenberger et al., 2018; Wu and Benet, 2005). For example, using the Biopharmaceutics Drug Distribution Classification System (BDDCS), in a dataset of drugs approved by European Medicines Agency (EMA) and FDA between 2010 and 2017, approximately 80% of drugs formulated as solid dispersions were found to be in Class II or IV (indicating poor aqueous solubility), while approximately 40% of drugs formulated by conventional methods were found in Class II or IV (Bennett-Lenane et al., 2020). There are a few interesting observations to pull out of this comparative dataset, in particular on the importance of dose for formulation strategy (Bayliss et al., 2016; Charkoftaki et al., 2012). Since a large number of poorly water-soluble drugs are able to be formulated with conventional methods, the solubility value used with the BDDCS or other similar classification systems may not be a clinically significant threshold applicable to all drugs. Furthermore, given that 20% of drugs classified as Class I or III using the BDDCS are formulated as ASDs per the analysis of drugs approved by EMA and FDA between 2010 and 2017 by Bennett-Lenane et al. (2020), it implies that a high dose may be required to achieve the desired bioavailability, or that there may be other reasons beyond bioperformance that an ASD strategy was selected. First, the dose required of amorphous solid form may be less than that of a crystalline solid form to achieve therapeutic levels, which may result in cost savings or benefit to patients in the form of reduced pill burden or side effects. Second, where a polymer-stabilized amorphous form can be achieved, solid form challenges may be removed, such as an inability to develop a robust crystallization process, mechanical instability, or a complex polymorphism landscape (Chiang et al., 2023). Lastly, food effects or pH-dependent absorption limitations may be mitigated through the use of an ASD formulation (Larfors et al., 2023; Mudie et al., 2021; Wu et al., 2024). The approval of Phyrago as an ASD formulation of dasatinib in 2023 is an example of the need for formulation strategies to mitigate reduced exposures observed when the crystalline dasatinib formulation (Sprycel, approved in 2006) was co-medicated with proton pump inhibitors (Larfors et al., 2023; Wertz et al., 2022). Ultimately, the solubility classification from Biopharmaceutics Classification System (BCS) or other scheme may not be sufficient to indicate the need for mitigating formulation approaches such as ASD technology, nor is solubility the only driver to select such a formulation approach.
2.4. Drug product intermediate attributes
2.4.1. Manufacturing processes
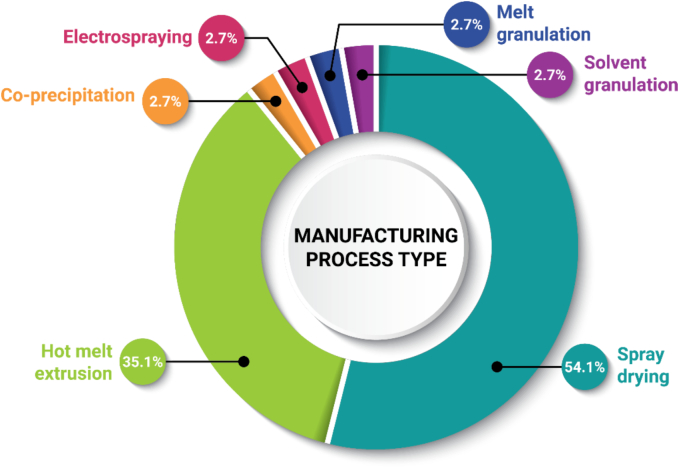
ASD DPI manufacturing processes fall into two general categories: solvent-based or thermal-based (Bhujbal et al., 2021; Huang and Williams, 2017). The manufacturing methods can be generalized into two main steps: amorphization is achieved by starting with a homogeneous distribution of drug and polymer (and possibly other components) and applying heat (thermal-based) or dissolving the materials in a suitable solvent system (solvent-based), followed by quenching through rapid cooling (thermal-based) or drying (solvent-based). Solvent-based methods used to prepare the approved DPs include spray drying (SD), co-precipitation (cPT), electrospraying, and solvent granulation, and thermal-based methods used to prepare the approved DPs include hot melt extrusion (HME) and melt granulation (Fig. 5). Other preparation methods such as electrospinning, microfluidics, fluid bed coating, supercritical fluid technology, milling, and KinetiSol, are explored in research works or clinical development.
Fig. 5.
Frequency of manufacturing process type used to manufacture ASD DPIs (n = 37). Each drug–polymer combination appears only once in the chart, even when used in multiple DPs. Tacrolimus was counted twice, since Astagraf XL and Prograf are both formulated with hypromellose (HPMC) via solvent granulation, while Envarsus XR is formulated with polyethylene glycol (PEG) via melt granulation.
ASD DPIs used to prepare the approved DPs are most commonly manufactured by SD (54.1%) or HME (35.1%), as shown in Fig. 5, based on review of patent literature and other published works. Considerations for manufacturing process selection include drug and polymer physicochemical properties, ease of screening in early development, availability of manufacturing processing equipment, and company preference. For example, all ASD products in our dataset by AbbVie are produced by HME (n = 9) and all by Vertex are produced by SD (n = 7). The primary limitation for selection of a thermal method is thermal stability of the drug and other components and ensuring complete amorphous transformation during processing (Kyeremateng et al., 2022; Moseson and Taylor, 2023). The primary limitation for selection of a solvent-based method is having adequate solubility in the solvent system of choice to enable sufficient throughput at commercial scale (Singh and Van den Mooter, 2016).
2.4.2. Polymer selection
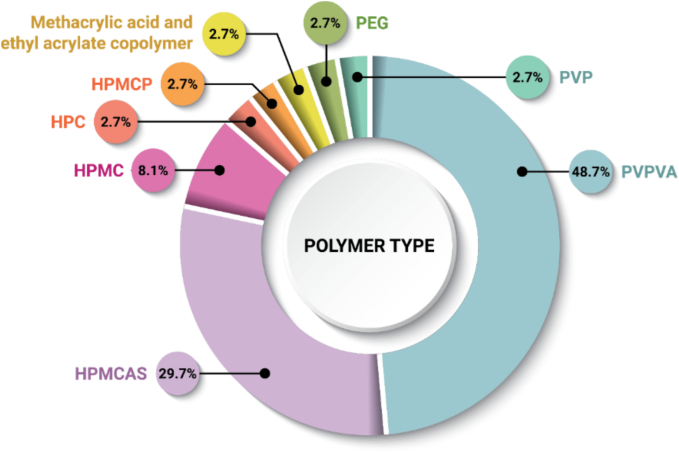
To date, the ASDs approved by the FDA have been commonly formulated with a range of different polymers (Fig. 6). These polymers fall into several categories, including amorphous neutral polymers (copovidone; PVPVA, HPMC, hydroxypropyl cellulose; HPC, povidone; PVP), amorphous ionic polymers (hypromellose acetate succinate; HPMCAS, hypromellose phthalate; HPMCP, polymethacrylates), and crystalline polymers (PEG) (Duong and Van den Mooter, 2016a; Duong and Van den Mooter, 2016b). Polymer selection is multifaceted, as the polymer is used for multiple functions, such as to enhance physical stability through miscibility, drug–polymer interactions, and anti-plasticization, as a dissolution rate enhancer, to inhibit crystallization from supersaturated solutions, and as a processing aid. Beyond these formulation and manufacturing process reasons, security of supply (i.e., single source excipients) and precedence of use are key drivers for polymer selection (McKelvey and Kesisoglou, 2019). PVPVA is found in the majority of ASD DPIs (n = 18), while HPMCAS is the second most common (n = 11). One (1) product, Alvaiz, is a multi-component ASD formulation containing several polymer and plasticizer materials (PVPVA, PVP, PEG 4000, and poloxamer 188) (Choudhari et al., 2022), but will be treated as a single polymer formulation containing only PVPVA for the subsequent analysis.
Fig. 6.
Frequency of polymers used to formulate ASD DPIs (n = 37). Each drug–polymer combination is counted once in the dataset, even when used in multiple DPs.
The authors can speculate reasons for the selection of the polymers within this dataset. The selection of HPMC (n = 3) may be limited due to its relative insolubility in many organic solvents (Maskova et al., 2020; Mugheirbi et al., 2017). It is possible that HPMCP (n = 1) and polymethacrylates such as methacrylic acid and ethyl acrylate copolymer (n = 1), also known as Eudragit L100–55, may see greater application in the future as alternative ionic polymer formulation options to HPMCAS, as precedence in commercial products was just recently established (Tolsura, approved in 2018; Phyrago, approved in 2023). HPMCP is most commonly supplied by Shin-Etsu and is available in two grades, HP-50 and HP-55. HP-50 and HP-55 are soluble above pH 5.0 and 5.5, respectively. Polymethacrylates are a family of enteric polymers which include methacrylic acid and ethyl acrylate copolymer (e.g., Eudragit L100–55), soluble above pH 5.5, or methacrylic acid and methyl methacrylate copolymer (e.g., Eudragit L100), soluble above pH 6. It is possible that PVP (n = 1) may be used less often in the future due to high hygroscopicity in comparison to the chemically similar PVPVA. To date, HPC (n = 1) has not been shown to provide for significant miscibility with model compounds, and therefore its use may not expand in the future (Luebbert et al., 2021). PEG (n = 1) is a crystalline polymer and is therefore outside the norm for stabilizing amorphous drugs. Within Envarsus XR, tacrolimus is dissolved within molten PEG then sprayed onto a powder bed (a melt granulation process) (Holm et al., 2013). Due to its low dose and physicochemical properties, tacrolimus may also not require a polymer for stabilization (Trasi et al., 2017).
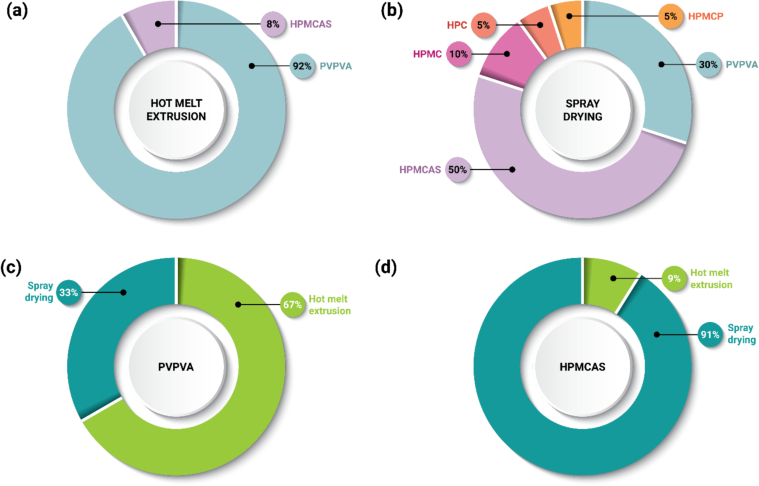
Selection of PVPVA may be attributed, in part, to its ease of processability by HME. Twelve (12) out of 13 DPIs made by HME include the use of this polymer (Fig. 7a). PVPVA has excellent thermal stability upon exposure to the high temperature of the extrusion process and has excellent viscoelastic properties enabling a wide processing temperature design space (Gupta et al., 2014; Moseson et al., 2020; Moseson and Taylor, 2018). PVPVA is a neutral (non-ionic) polymer with moderate hygroscopicity, is soluble in many organic solvents, and is also an excellent crystallization inhibitor for many compounds (Cheng et al., 2019; Jackson et al., 2014; Patel and Serajuddin, 2022; Raina et al., 2015; Trasi et al., 2015). PVPVA forms a strong gel upon water exposure, which may be a challenge for DP formulation where amorphous–amorphous phase separation or crystallization in the gel layer might adversely affect release performance upon hydration (Deac et al., 2023; Moseson et al., 2023). Additionally, PVPVA is available from multiple suppliers.
Fig. 7.
(a) Frequency of polymer selection for ASD DPIs prepared by HME (n = 13). (b) Frequency of polymer selection for ASD DPIs prepared by SD (n = 20). (c) Frequency of manufacturing process selection for ASDs formulated with PVPVA (n = 18). (d) Frequency of manufacturing process selection for ASDs formulated with HPMCAS (n = 11). Each drug appears only once in the chart, even if it appears in multiple DPs.
In this dataset, HPMCAS is the most common polymer used for spray dried ASDs, appearing in 10 out of 20 DPIs (Fig. 7b). HPMCAS may be selected for reasons such as its solubility in many organic solvents, low hygroscopicity, pH-sensitive release above pH 5.5–6.5, and excellent crystallization inhibition properties (Butreddy, 2022; Friesen et al., 2008; Patel et al., 2022; Song et al., 2016; Zhang et al., 2021). HPMCAS is available in three grades (L, M, H) which differ in their succinoyl and acetyl substitutions, resulting in difference in their pH solubility profiles (Butreddy, 2022). HPMCAS L and M grades are mostly commonly found in research studies because they are soluble at relatively lower pH conditions compared to HPMCAS H. HPMCAS is more challenging to process than PVPVA by HME due to its higher viscosity and greater reactivity during thermal processing which may result in drug and/or polymer degradation (Alvarenga Jr et al., 2022; Corum et al., 2023; Meena et al., 2014; Sarode et al., 2014). Due to its ionic properties, HPMCAS may form hydrophobic ion-pairs with some drugs based on their pKa, yielding poor release performance of formulated ASDs (Bapat et al., 2024; Hiew et al., 2022), or formulations may have pH-sensitive dissolution performance (Nguyen et al., 2023a; Nguyen et al., 2023b). Shin-Etsu is the most common supplier of HPMCAS.
Itraconazole forms an interesting case study regarding polymer selection. Tolsura, approved in 2018, is the second DP approved comprising an itraconazole ASD. The first DP is Sporanox, approved in 1992, which uses HPMC as a polymer to prepare amorphous drug-layered beads (Gilis et al., 1997). Unlike Sporanox, which is available in 100 mg capsules, Tolsura contains 65 mg of itraconazole formulated with HPMCP. While different in dose, Sporanox and Tolsura were shown to be bioequivalent when administered under fed conditions (Thompson et al., 2020). However, the same cannot be said under fasted conditions (Borbás et al., 2019). Sporanox was reported to be susceptible to food and acid-suppressive effects, where a low stomach pH is required to achieve therapeutic blood levels. By comparison, Tolsura is less affected by the prandial state, with a moderate increase in bioavailability reported under fasted conditions. Moreover, while bioequivalence between these products have been demonstrated, they are dosed differently. For example, for the treatment of aspergillosis, Sporanox is dosed BID but QD dosing with Tolsura may be sufficient to achieve effective serum concentrations. In addition, while Sporanox is approved for the treatment of blastomycosis, histoplasmosis, aspergillosis, and onychomycosis, Tolsura is only approved for the treatment of blastomycosis, histoplasmosis, and aspergillosis but not for onychomycosis. Therefore, these products are not considered interchangeable or substitutable per the Tolsura prescribing information. The difference observed between Sporanox and Tolsura can be rationalized based on the polymer used to formulate the ASD DPIs. With HPMC (Sporanox), a neutral polymer with pH-independent solubility, drug release can commence in any environment, leading to possible variation in drug release depending on the pH environment, given that itraconazole has greater solubility at low pH. With HPMCP (Tolsura), an enteric polymer that is insoluble at gastric pH but is soluble at duodenal pH, when paired with a weakly basic drug such as itraconazole, the majority of drug dissolution would only commence in the intestine, leading to consistent drug release in both fed and fasted conditions. However, in the gastric environment, itraconazole is ionized and may leach from the formulation, leading to risks of crystallization, desupersaturation, or incomplete drug release in the intestinal environment. This pattern of drug release was demonstrated with several weakly basic drugs (Elkhabaz et al., 2019; Monschke et al., 2021; Nguyen et al., 2023b; Nunes et al., 2022; Wang et al., 2022).
2.4.3. Intersection of polymer selection and manufacturing process
As ASD polymer selection and DPI manufacturing process type are not completely independent of one another for ASD formulations, our dataset enables further investigation of manufacturing trends. The selection of commercial manufacturing technique may be biased by formulation design during early clinical phases, where limited quantities of drug are available for formulation and process development, and speed to clinic and formulation properties (e.g., solubility and solid form control), take higher priority over manufacturability (Anane-Adjei et al., 2022; Hu et al., 2013; Mosquera-Giraldo et al., 2021). Improved approaches for in silico formulation screening such as PC-SAFT should enable greater choice of polymer selection, where additional considerations such as manufacturability can be factored into early development (Deac et al., 2023; Dohrn et al., 2021; Kyeremateng et al., 2022; Lehmkemper et al., 2017; Pavlis et al., 2023).
Based on the analysis of approved DPs herein, PVPVA is versatile within the two dominant manufacturing platforms, HME and SD (Fig. 7c). As interest grows within the pharmaceutical industry to transition to “greener” manufacturing platforms (Solomos et al., 2023; Trenkenschuh et al., 2024), HME technology is positioned to see greater utilization, particularly in later development phases as greater amounts of drug are available for pharmaceutical development studies. For formulations initially developed with a solvent-based process, those with PVPVA may be best positioned to transition to HME, as PVPVA has a flexible temperature processing window.
On the other hand, few approved DPs in this dataset prepared by HME use polymers beyond PVPVA, which may suggest greater difficulty in process development with polymers such as HPMCAS, HPMCP, or HPMC due to thermal instability or viscoelastic properties of polymers (Meena et al., 2014; Moseson et al., 2020). New HPMC-based (Affinisol) and vinylpyrrolidone-based (Soluplus) polymers have been developed to address this limitation and may see greater utilization in the future (Gupta et al., 2016; Huang et al., 2016). This results in HPMCAS-based DPIs being almost exclusively manufactured by SD (n = 10 out of 11) (Fig. 7d), due to characteristics described in Section 2.4.2.
Co-precipitation (cPT), or microprecipitated bulk powder processing, is also expected to see greater utilization, as the processing step to prepare the cPT DPI can take the place of a crystallization step, improving speed to the clinic, flexibility, and reducing costs (Shah et al., 2012; Strotman and Schenck, 2021). While utilization of this technique was found only with Stivarga to prepare a regorafenib/PVP DPI, it was also used to prepare vemurafenib/HPMCAS DPI (Zelboraf, approved in 2011). Solvent selection for the cPT process is also broader than SD due to differences in the manufacturing processes, since rapid evaporation of the solvent is not required and can instead be removed through filtration. Current research work has used a wide range of polymers to prepare ASDs by cPT, including PVPVA, HPMCAS, polymethacrylates, cellulose acetate phthalate, and HPMCP (Hiew et al., 2023; Mann et al., 2018; Solomos et al., 2023).
Several new technologies are utilized within clinical development or in recent commercial approvals. Electrospraying is a new manufacturing technology used to prepare ASD products, appearing in 2023 with the approval of Phyrago. Atomization of the feed solution is generated by electrical forces, and particles are formed through rapid evaporation of the solvent, similar to spray drying (Nguyen et al., 2016; Smeets et al., 2018). Due to method differences with respect to particle atomization, temperature, and pressure, solvent selection for electrospraying may be broader than that of spray drying (Bhujbal et al., 2021). Supercritical fluid (SCF) particle engineering technologies are also currently available for clinical development (Tran and Park, 2021). In the supercritical anti-solvent (SAS) process, the drug–polymer solution is injected into a supercritical fluid which acts as an anti-solvent, leading to the formation of particles (Liu et al., 2020). In the rapid expansion of supercritical solution (RESS) process, the solute is solubilized in the supercritical fluid, then rapidly expanded by sudden decompression to generate particles (Riekes et al., 2015). This method requires the drug and polymer to be soluble in the supercritical fluid but is solvent-free. Notably, a reformulation of dasatinib as a PVPVA-based ASD manufactured by SCF technology is anticipated to receive approval in 2024 (Larfors et al., 2023). KinetiSol technology is a solvent-free, thermal/mechanical-based processing method to prepare ASDs. While not found in any currently marketed DPs, this technology has greater flexibility with respect to drug physicochemical properties and polymer selection (Ellenberger et al., 2018), and does not require the polymer to have a matching organic solvent solubility profile to that of the drug. Thus, KinetiSol is expected to see greater utilization to replace solvent-based processes or to process molecules with challenging physicochemical properties.
2.5. Drug product attributes
2.5.1. Drug product type
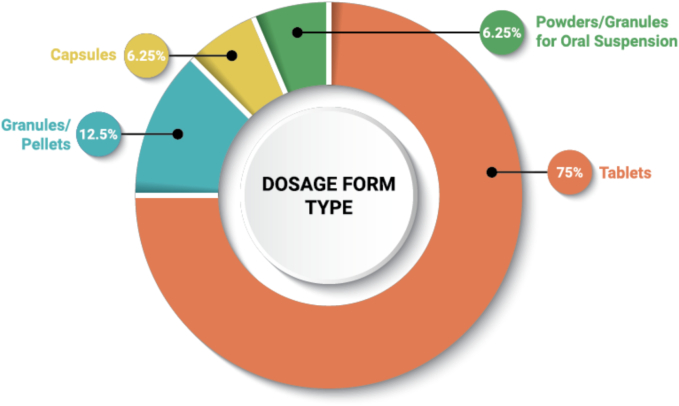
Common DP types for ASD oral DPs include tablets, capsules, pellets/granules, and powders/granules for oral suspension. Tablets are the most common dosage form type used for ASD DPs (75%) (Fig. 8). There are only three (3) DPs formulated as capsules, so there are no clear trends to be observed with respect to the choice of tablet or capsule. The pellets/granules and powders/granules for oral suspension dosage forms each represent line extensions intended to provide dosing options and flexibility for the pediatric and other patient populations.
Fig. 8.
Frequency of DP types used for ASD DPs (n = 48).
Three (3) powders/granules for oral suspension have been approved within this dataset as a line extension of an earlier tablet or capsule approval. All are considered substitutable for the tablet or capsule DP and contain pediatric dosing instructions. Norvir oral powder is recommended to be mixed with soft food (e.g., apple sauce, pudding) or with a liquid (e.g., water, milk, infant formula), and used within two hours of preparation. Noxafil PowderMix is a kit based on a powder and mixing liquid. Upon combining the two components, the reconstituted suspension should be used within one hour. Prograf granules are to be combined with water and given immediately after preparation.
Several other products have multiple DP types (Kalydeco, Harvoni, Orkambi, Mavyret, Epclusa, Trikafta), all of which are either antivirals or respiratory tract/pulmonary agents. Pellet- or granule-based DPs may include powders, multi-particulates, or minitablets. Each of the pellet- or granule-based DP appears to have been created for the purpose of expanding dosing into specific patient populations, such as pediatrics (Meruva et al., 2024). Each of these dosage form presentations can be considered flexible oral solids, which enables greater dose flexibility, ease of administrations, and better acceptance of drug formulations in children (Ivanovska et al., 2014; Virtanen et al., 2024). In some cases, the formulation used for pellet- or granule-based DP may be different than that of the original DP. This is exemplified with Kalydeco granules, which substitute mannitol and sucralose for microcrystalline cellulose (MCC), likely to increase palatability. These alternative pellet- or granule-based DPs are typically available 3–5 years later following FDA approval of the original DP, reflecting the time required for development studies, clinical trials, and regulatory approval of the new dosage form. Of note, these DPs fall under priority review or orphan drug designations, which expedite review and regulatory pathways, so this timeframe is likely faster than for pediatric approvals that do not fall under priority designations (Hudgins et al., 2018).
Several products are specifically designated as delayed- or extended-release. Per the prescribing information, Noxafil tablets and PowderMix are considered delayed-release products due to the formulation of the ASD DPI with HPMCAS. There are 13 DPIs formulated with enteric polymers HPMCAS, HPMCP, and polymethacrylates, so this classification of Noxafil as a delayed-release product is an outlier for ASD DPs. Envarsus XR and Astagraf XL are extended-release versions of tacrolimus, initially launched as an immediate-release product (Prograf) prior to 2012. Viekira XR is an extended-release DP, where the crystalline drug dasabuvir is formulated as part of a bilayer tablet formulation to provide a longer release profile to reduce the daily dosing from BID to QD. From this dataset, it can be inferred that extended-release formulations of amorphous drugs are difficult to achieve, only being successful in the case of low dose drugs which are highly stable against crystallization (Maincent and Williams, 2018; Tran et al., 2011).
In this dataset, several ASD DPs were first approved as LBFs. Lynparza was first approved as a 50 mg LBF capsule in 2014, then approved as an ASD tablet (100 mg and 150 mg dosage strengths) in 2017; the LBF has now been discontinued. Xtandi was first approved as a 40 mg LBF capsule in 2012, then launched as an ASD tablet (40 mg and 80 mg dosage strengths) in 2020. Several additional examples of drugs formulated using both LBF and ASD technology include ritonavir and lopinavir (Bennett-Lenane et al., 2020). Notably, ritonavir was reformulated from a semisolid capsule formulation into an ASD formulation, due to the late appearance of a new polymorph (Chemburkar et al., 2000). This change also impacted lopinavir, originally produced in combination with ritonavir in the LBF, which was then reformulated into the ASD formulation. For Lynparza and Xtandi, higher dosage strengths became available with the approval of the ASD tablets, which may suggest an additional rationale for the reformulation.
Another interesting trend is the late appearance of additional dosage strengths. In most cases, the secondary approval provides for lower dosage strengths. This is exemplified by Kalydeco granules, where the additional dosage strengths open up dosing to younger patient populations. In the case of Erleada, the initial approval of the 60 mg tablet required patients to take four tablets QD. A new strength of 240 mg was approved in 2023, reducing the pill burden to one tablet QD.
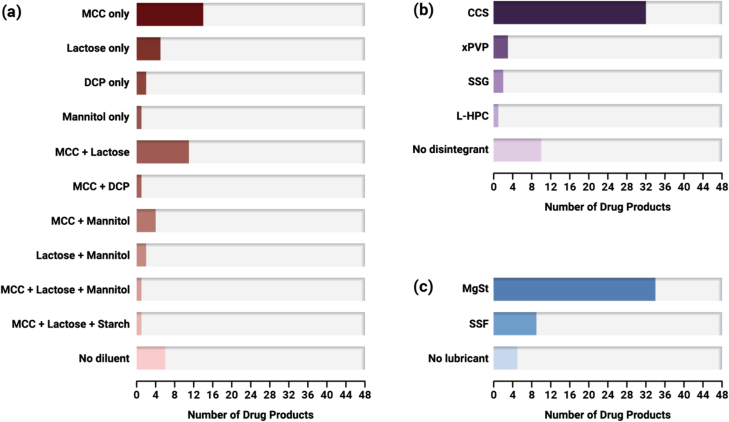
2.5.2. Excipients
The excipients used to formulate ASD DPs from the three main categories of diluent, disintegrant, and lubricant are detailed in Fig. 9. In order to formulate and manufacture tablets and capsules at commercial scale, diluent excipients are added to impart mechanical strength, flowability characteristics, and increase the bulk of the tablet (Yu and Hoag, 2024). Disintegrants are included to promote de-aggregation of the solid dosage form upon contact with an aqueous environment (Berardi et al., 2022). Lubricants are an essential formulation component for reducing friction of the dosage form components with processing equipment during preparation of tablets and capsules (Paul and Sun, 2018). Other excipient types such as flow aids (e.g., silicon dioxide) or pH modifying agents are occasionally included in the ASD DP formulation, but will not be discussed in detail, as their use is less frequent. Excipients that serve as surfactants/plasticizers such as d-α-tocopherol polyethylene glycol 1000 succinate, sodium lauryl sulfate, or poloxamer are also found in many DPs. In most cases, these may be formulated within the DPI to improve wetting, dissolution rate, or serve as processing aids.
Fig. 9.
Frequency of excipients used in ASD DP formulations: (a) diluents, (b) disintegrants, and (c) lubricants (n = 48).
Several diluent excipients are found in our dataset of ASD DPs (Fig. 9a). The majority of ASD tablets contain MCC (66.7%), as its high compressibility provides for high tablet tensile strength of ASD tablet formulations (Dinunzio et al., 2012; Yu and Hoag, 2024). Diluents may also be needed to prevent gelation of ASD tablets (Zhang et al., 2021). Lactose is the second most common diluent (41.7%). Most DPs are formulated with only MCC or lactose, but not both (n = 26 of 48), with those containing MCC only being more common. In many cases, multiple diluents are selected to optimize mechanical and disintegration properties. Thirteen (13) DPs contain both MCC and lactose, and five (5) DPs contain both MCC and mannitol. Nine (9) ASD DPs do not contain either lactose or MCC (Lynparza, Norvir oral powder, Noxafil PowderMix, Paxlovid (ASD tablet), Technivie, Tolsura, Venclexta, Viekira PAK (ASD tablet), Viekira XR). The diluent used in Lynparza is mannitol, while the diluent in Paxlovid (ASD tablet) and Venclexta is dibasic calcium phosphate (DCP). Norvir oral powder, Noxafil PowderMix, Technivie, Tolsura, Viekira PAK (ASD tablet), and Viekira XR do not contain diluent.
Disintegrants are found in the majority of ASD DPs in our dataset (79.2%) (Fig. 9b). Croscarmellose sodium (CCS) is the most commonly used disintegrant and is found in 32 DPs. This is followed by crospovidone (xPVP) found in three (3) DPs, sodium starch glycolate (SSG) found in two (2) DPs, and low-substituted hydroxypropyl cellulose (L-HPC) found in one (1) DP. Use of salts such as sodium chloride as part of the disintegrant system is found with several DPs (e.g., Qulipta, Tukysa, Ubrelvy, Zepatier) formulated with neutral polymers. This disintegrant system strategy is used to reduce disintegration time of ASDs formulated with neutral polymers by disrupting gelation (Xi et al., 2020). There are several tablet DPs that do not contain a disintegrant. For Astagraf XL, Envarsus XR, and Viekira XR, the absence of a disintegrant can be rationalized based on their matrix tablet design for extended-release, where the tablets are designed to release the drug over an extended period of time through gradual erosion of the tablet matrix. DPs such as Lynparza, Technivie, and Venclexta are also designed to release the drug through an erosion mechanism, albeit over an immediate-release timeframe.
Lubricants found in our dataset of ASD DPs are either magnesium stearate (MgSt) (70.8%) or sodium stearyl fumarate (SSF) (18.8%) (Fig. 9c). For Viekira XR, only the ER layer, which contains the crystalline drug dasabuvir, has a lubricant added (MgSt), but the IR ASD layer does not contain a lubricant. The other DPs that do not contain a lubricant are Orkambi granules, as well as the three (3) powders/granules for oral suspension (Norvir, Noxafil, Prograf).
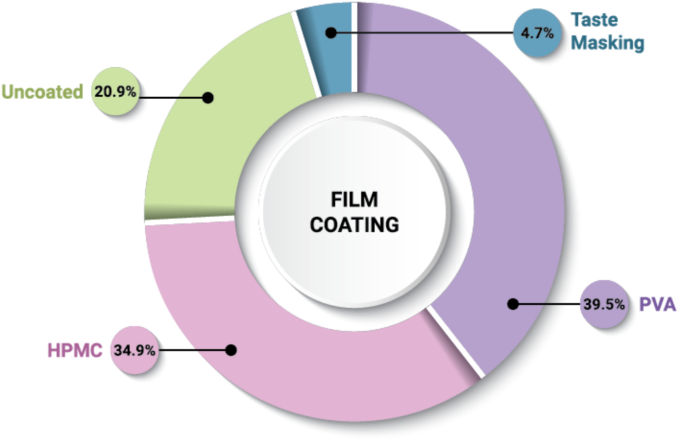
2.5.3. Film coating
A majority of ASD DPs in our dataset are film coated (79.1%) as shown in Fig. 10. Film coatings are widely used to impart aesthetics or functionality such as taste masking, controlled release, or improved mechanical integrity (Felton and Porter, 2013). The prevalence of coating types is split between HPMC-based (n = 15) and polyvinyl alcohol (PVA)-based (n = 17). PVA-based film coatings are known to impart improved moisture barrier properties (Yang et al., 2019), suggesting a possible reason for their prevalence within ASD DPs. Coating components may impart additional crystallization inhibition properties to the supersaturated solution (Sakai et al., 2018), but may also induce risk of crystallization due to migration of plasticizers that are in direct contact with ASD particles or hygroscopicity (Punia et al., 2023). Additionally, the coating process should be carefully designed to protect the ASD from phase separation or crystallization that may be induced by solvent/water exposure (Boel and Van den Mooter, 2023).
Fig. 10.
Frequency of film coating and type used with ASD tablets and pellet- or granule-based DPs (n = 43). The three (3) powders/granules for oral suspension (Norvir, Noxafil, Prograf) and three (3) capsules (Astagraf XL, Braftovi, Tolsura) are excluded from the dataset. Alvaiz is included twice, as its different dosage strengths are formulated with two types of film coating.
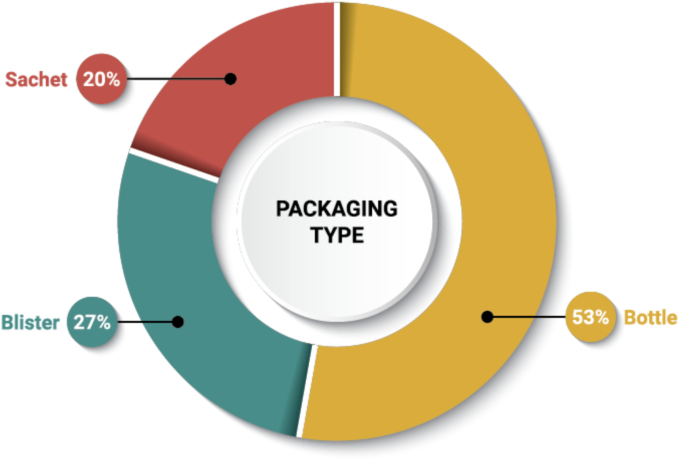
2.5.4. Packaging and storage recommendations
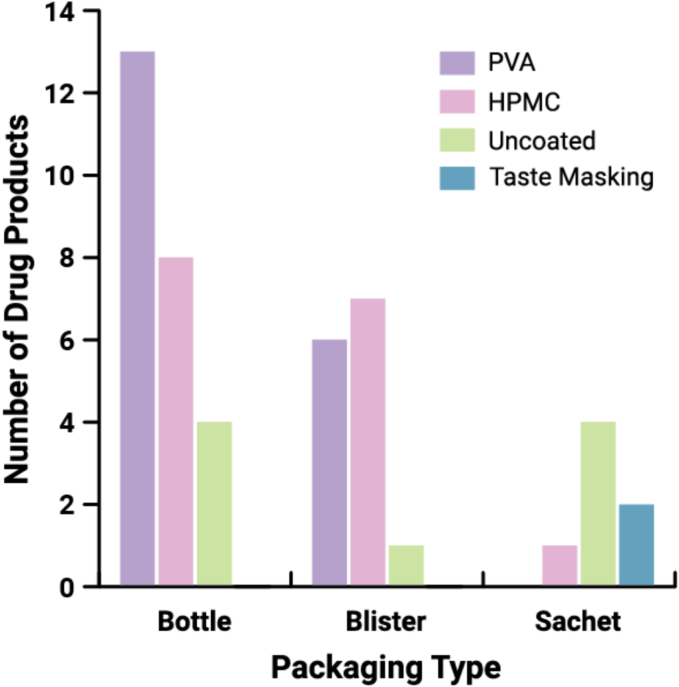
As shown in Fig. 11, high-density polyethylene bottles are the most widely used primary packaging for ASD DPs in our dataset (53%). This preference may stem from the ease of ensuring child-resistant packaging regulations are met for U.S. products. Blister packaging is also quite common for ASD DPs (27%) and may be used to confer numerous advantages, such as unit-dose packaging, tamper-evidence, and protection from oxygen, light, and/or moisture (Chen, 2017). Co-packaged DPs in our dataset are packaged in blisters, for ease of patient compliance. All pellets/granules and powder/granules for oral suspension DPs in our dataset are packaged in unit-dose sachets. Kalydeco tablets, Mavyret tablets, and Venclexta tablets are provided in blisters and bottles.
Fig. 11.
Frequency of packaging type used with ASD DPs (n = 51). Forty eight (48) DPs were analyzed, with three (3) DPs available in both bottle and blister packaging (Kalydeco tablets, Mavyret tablets, Venclexta tablets).
An interesting correlation exists for the selection of film coating type and packaging type (Fig. 12). For ASD film-coated tablets packaged in bottles, the majority are coated with a PVA-based film coating. For ASD tablets packaged in blisters, the proportion of those film coated are split relatively evenly between HPMC-based and PVA-based coating. This suggests that the moisture barrier properties imparted by PVA-based coatings are desirable when bottle packaging is used. Blisters may be selected when moisture barrier properties are desired, but also may have been selected for ease of use for patients.
Fig. 12.
Analysis of packaging type and film coating type for ASD tablets and pellet- or granule-based DPs (n = 46). Forty two (42) DPs were analyzed, with four (4) tablet DPs counted twice (Kalydeco, Mavyret, Venclexta, Alvaiz) as three (3) are available in both bottle and blister packaging (Kalydeco, Mavyret, Venclexta), while different dosage strengths of Alvaiz tablets are coated with either HPMC or PVA. The three (3) powders/granules for oral suspension (Norvir, Noxafil, Prograf) and three (3) capsules (Astagraf XL, Braftovi, Tolsura) are excluded from the dataset.
All ASD DPs in our dataset suggest room storage conditions. USP controlled room temperature is defined as “Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).” None require cold storage.
2.5.5. Patient-centric formulations
Traditional tablet and capsule dosage forms may not always meet the needs of certain patient populations, such as pediatrics, geriatrics, or those with dysphagia or using feeding tubes (Page et al., 2022). For this reason, there has been a shift toward more patient-centric products, as seen with the nine (9) powders/granules for oral suspension and pellet- or granule-based DPs in this dataset. In the absence of such a dosage form, patients may be instructed to split or crush the tablet, or open the capsule to empty its contents. However, this may not be suitable for ASDs, as liquid vehicles may alter the integrity of the formulation or compromise oral bioavailability (Uttaro et al., 2021). If the ASD dosage form is crushed, the lack of predictable absorption may put patients at risk of toxicity from rapid absorption or reduced total bioavailability that could result in subtherapeutic serum drug levels (Cornish, 2005).
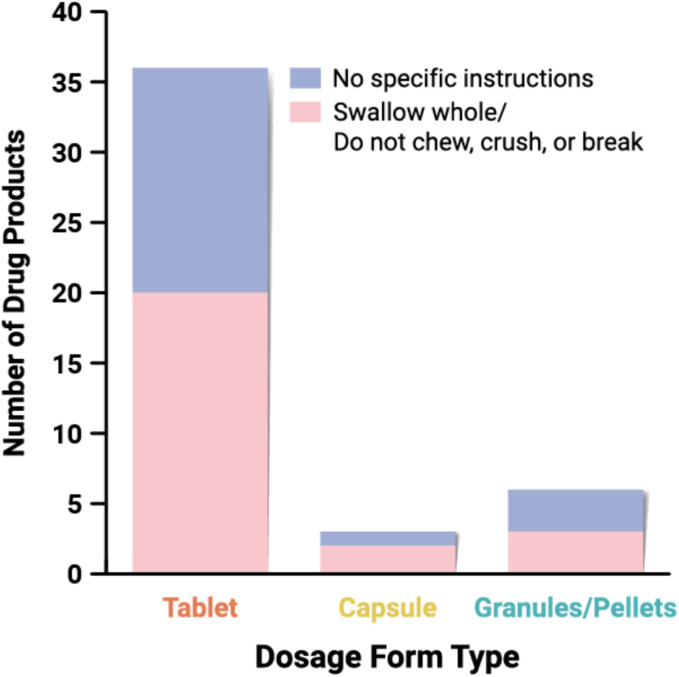
Prescribing information for the DPs in our dataset identifies 25 formulations which instruct patients to either swallow the dosage form whole or to not chew, crush, or break the dosage form, while 20 formulations provide no specific instructions (Fig. 13). Specifically, three (3) pellets/granules-based DPs (Epclusa, Harvoni, Mavyret) state that the DP should not be crushed or chewed to avoid a bitter aftertaste. For capsule products, no specific instructions on DP administration are provided for Braftovi, while Astagraf XL and Tolsura capsules should not be crushed or chewed. Some formulations which instruct patients not to crush the tablets provided alternate administration instructions. For example, the prescribing instructions for Erleada provide instructions for dispersing the tablets in applesauce and consumed within one hour of preparation, as well as instructions for administering through a feeding tube after dispersal in water.
Fig. 13.
Frequency of DPs which have prescribing instructions around chewing/crushing (n = 45). The three (3) powders/granules for oral suspension are excluded from this dataset.
Noxafil forms an interesting case study as an ASD DP with “do not divide, crush, or chew” prescribing instructions for the tablet formulation. Several dosage forms are available, including an oral suspension (approved in 2006, discontinued in 2023), oral tablet (approved in 2013), intravenous solution (approved in 2014), and PowderMix for oral suspension (approved in 2021). Prior to the introduction of the PowderMix for oral suspension DP, additional need for non-tablet-based dosage forms was evident. The oral tablet formulation is favored over the oral suspension formulation due to QD dosing and improved absorption (Mason et al., 2019), and the oral suspension is not considered substitutable for the tablet formulation. Several small studies have been published where the ASD DP was crushed, and subtherapeutic serum concentrations were achieved in some of the patients. This was addressed by increasing the dose provided or switching to BID dosing (Bio et al., 2024; Dieringer et al., 2022; Mason et al., 2019; Stevens et al., 2023). The subtherapeutic serum concentrations achieved are rationalized by the pH-sensitive release profile of the drug and polymer in the ASD formulation (Pas et al., 2020), and how this may be altered based on dosing the intact or crushed tablet. With the introduction of the Noxafil PowderMix for oral suspension in 2021, a substitutable formulation is now available for the oral tablet formulation, demonstrating that innovative formulation strategies can meet the needs of various patient populations.
2.6. Dose
The DPs in this dataset have dose/unit of amorphous drug ranging from <5 mg up to 300 mg. In Fig. 14, the maximum dose/unit of amorphous drug is presented to provide a baseline assessment of the amount of drug as an ASD that can be loaded into a dosage form. For example, Trikafta contains 125 mg of amorphous drug, comprising 75 mg of ivacaftor and 50 mg of tezacaftor. Crystalline drugs, when present, are not counted. The loading of ASD within a tablet as a percentage is more difficult to determine, without knowing the weight of each dosage form and the drug loading within the ASD DPI.
Fig. 14.
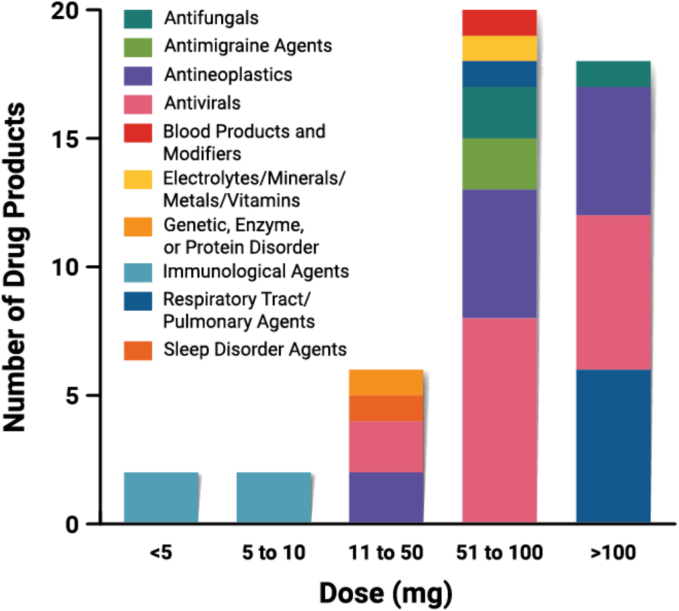
Total amount of amorphous drug in each unit dose (n = 48). For FDC products, this represents the sum of all amorphous drugs in the dosage form. The highest dose available is used.
The majority of DPs have doses ≤100 mg/unit (Fig. 14). The maximum dose/unit is 300 mg in Sunlenca and Noxafil PowderMix for oral suspension. FDC products also commonly have total amorphous drug dose/tablet >100 mg/unit. Doses on the low end of the range (<10 mg) are specific to the DPs delivering immunological agents (e.g., tacrolimus, deucravacitinib) or developed for pediatric purposes (e.g., Kalydeco granules). Beyond these purposes, low doses are not commonly expected for ASD products, as a main goal is to provide sufficient bioavailability beyond what a crystalline drug can deliver (Brouwers et al., 2009; Williams et al., 2013). In essence, if a reasonable quantity of crystalline drug could meet the therapeutic need, the increased complexity and cost of an amorphous drug delivery strategy is not likely to be justified.
Many ASD products require taking more than one unit at each dosing interval, highlighting that ASD formulation design limits the maximum amount of amorphous drug that unit doses can accommodate. This is for several reasons. First, the DPI contains both amorphous drug and polymer. Second, many ASD DPs are designed with immediate-release disintegration characteristics, which limits the amount of ASD that can be contained within the dosage form (Zhang et al., 2021). Additionally, spray dried ASDs typically require a dry granulation step to have sufficient powder flowability characteristics to be processed on a high-speed tablet press (Ekdahl et al., 2019; Singh and Van den Mooter, 2016), which adds to the excipient burden and limits the maximum dose/unit (Frank et al., 2023).
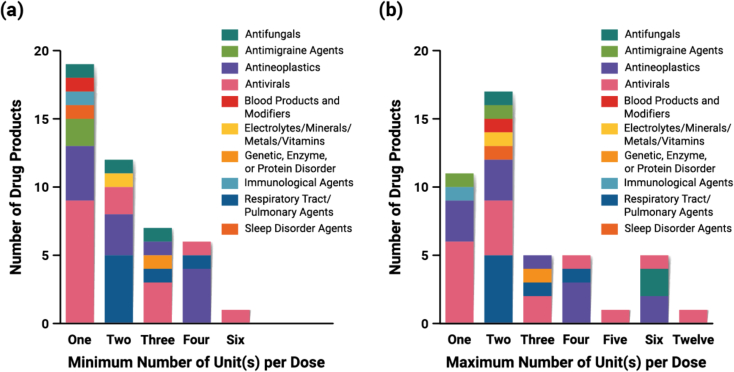
The pill burden for each DP is highly varied in terms of the minimum and maximum number of dosage units that may be prescribed per day (Fig. 15). It is notable that more than half of the DPs require multiple units per day even at the lowest recommended dosage (Fig. 15a). Several DPs may prescribe up to 6–12 units per day (Braftovi, Norvir, Noxafil, Paxlovid, Tolsura, Venclexta). The therapeutic classes where dosing regimen is more varied are the antineoplastics, antivirals, and antifungals, so the difference between minimum and maximum number of units to be taken per day is larger. For example, the recommended dosage of Venclexta, which contains an antineoplastic agent, ranges from one to six tablets per day. The use of pellets/granules and powders/granules for oral suspension provide for greater flexibility of the dose provided in a single dosage form, as the size constraints related to total dose and excipients and overall dosage form size are eliminated.
Fig. 15.
(a) Minimum and (b) maximum number of units of each DP taken per day (n = 45). The three tacrolimus products (Astagraf XL, Envarsus XR, Prograf) are excluded from this dataset because the dosage of tacrolimus is determined based on the patient's weight.
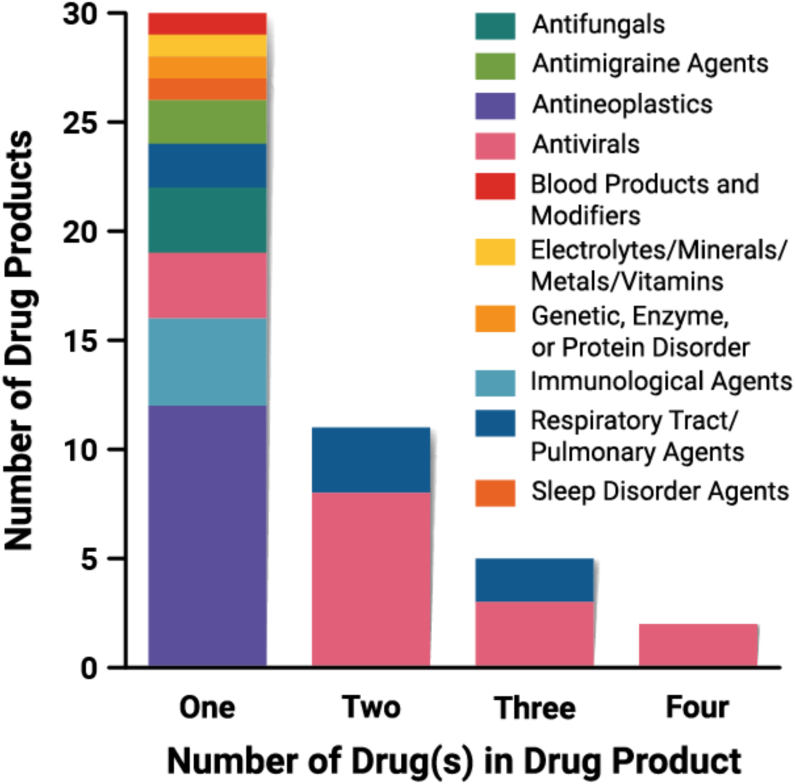
2.7. Fixed-dose combination products
Fixed-dose combination (FDC) products comprise two or more separate drug components in a single dosage form. A co-packaged product consists of two or more separate DPs in their final dosage forms packaged together to support combination use. Eighteen (18) of the 48 DPs in this dataset comprise multiple drugs (Fig. 16). Three (3) DPs are prepared as bilayer tablets (Delstrigo, Mavyret, Viekira XR). Five (5) DPs are co-packaged (Paxlovid, Symdeko, Trikafta tablets, Trikafta granules, Viekira PAK) to enhance therapeutic efficacy through synergistic effects. For example, the Symdeko dosing regimen consists of a tezacaftor/ivacaftor FDC tablet in the morning and an ivacaftor-only tablet in the evening. The blister packaging includes appropriate labeling to support the combination use through incorporation of colors, symbols, and text.
Fig. 16.
Frequency of DPs containing one or more drugs (n = 48).
The success of FDC therapies over monotherapies in several therapeutic categories is represented in this dataset. Each of these FDC DPs are found in just two therapeutic categories: antivirals or respiratory tract/pulmonary agents, as shown in Fig. 16. FDCs facilitate improved patient compliance with their treatment regimen, by minimizing the pill burden (number of tablets taken), reducing dosing frequency, and providing simpler dosing instructions (Page et al., 2022). This, in turn, enhances medication adherence and patient compliance. On the other hand, FDC unit dosage forms may need to be quite large in order to accommodate the total amount of drug included within, which may lead to swallowability issues.
The majority of the DPs in our dataset contain only one drug (n = 30), while there are 18 FDC DPs (Fig. 16). All the DPs that contain more than one drug are either antivirals or respiratory tract/pulmonary agents, with FDCs more common for antivirals (n = 13). Of the FDC DPs, several include combinations of amorphous and crystalline drugs, and several include combinations of only amorphous drugs. There are nine (9) FDC products that contain at least one crystalline drug (Delstrigo, Epclusa, Harvoni, Orkambi, Paxlovid, Trikafta, Viekira XR, Viekira PAK, Vosevi). Epclusa, Harvoni, Orkambi, and Paxlovid comprise one crystalline drug and one amorphous drug. Destrigo comprises three drugs: two crystalline drugs and one amorphous drug. Trikafta and Vosevi contain three drugs, one crystalline drug and two amorphous drugs. Viekira PAK and Viekira XR include four drugs, one as a crystalline drug (dasabuvir), and three amorphous drugs (ombitasvir, paritaprevir, ritonavir). The other four (4) FDC products include only combinations of amorphous drugs (Mavyret, Symdeko, Technivie, Zepatier). Five (5) of the FDC DP brands are available as both tablets and pellets/granules (Epclusa, Harvoni, Mavyret, Orkambi, Trikafta).
Formulation and manufacturing process development to prepare FDC products is complex, due to the critical quality attributes, stability, and performance required of each drug or ASD DPI and the overall DP (Frantz, 2006). For example, the drugs may chemically react toward one another or alter the in vitro/in vivo dissolution performance and absorption profile (Desai et al., 2013). To minimize the extent of process development required, the risk of reactivity between drugs, and control of disintegration/dissolution properties, the manufacturing process for DPIs is typically separate for each amorphous drug. This is readily apparent for some FDC products where the two amorphous drugs are formulated with different polymers. Symdeko contains ivacaftor, which is formulated with HPMCAS, and tezacaftor, which is formulated with HPMC. Zepatier contains elbasvir, which is formulated with HPMC, and grazoprevir, which is formulated with PVPVA. Some products contain only one polymer type, e.g., PVPVA is used for all amorphous drugs contained within Mavyret, Technivie, and Vosevi. The use of individual DPI manufacturing processes for each drug results from the different processing design space needed for each amorphous drug–polymer combination. Additionally, during the development process, each drug is initially developed individually, and only later placed into a FDC product. This could be done by combining DPIs or additional crystalline drugs into a common blend, or through innovative formulation design (e.g., bilayer tablet). The re-designing of a manufacturing process to incorporate multiple drugs would be a significant technical challenge and may be cost-prohibitive. There is one example from an ASD-based DP not included in this dataset (Kaletra), where patent literature suggests that a common HME process may be used to prepare a single ASD DPI containing two drugs, ritonavir and lopinavir (Rosenberg et al., 2014). This may be possible since both drugs have reasonably low melting points: 126 °C and 94 °C, respectively (Alvarenga Jr et al., 2022; Li and Taylor, 2019).
Combining drugs into a DP may also have impact on drug solubility, supersaturation, and subsequent absorption. Many drugs have specific dosing instructions to take with or without food, depending on the needs of the drug to facilitate absorption and bioavailability, so any combination therapy must take this into account. As some drugs experience pH-dependent solubility, concomitant dosing with acid reducing agents is a further consideration. A specific concern with combination drugs from supersaturating drug delivery systems is the likelihood that supersaturation and absorption are impacted. In a study of ritonavir and lopinavir supersaturated solutions, the presence of the second drug reduced the maximum achievable supersaturation and membrane transport rates of the first drug, and vice versa (Trasi and Taylor, 2015). This type of effect may impact the pharmacokinetics of combination therapies, whether co-dosed or co-formulated.
The DPs which use a bilayer tablet strategy form interesting case studies for the application of FDCs. The Mavyret DP is an immediate-release bilayer tablet, where the glecaprevir and pibrentasvir DPIs are in separate layers even though both are amorphous DPIs formulated with the same polymer. Based on the relevant patent, it can be inferred that the rationale for this strategy may have been to optimize in vitro/in vivo performance compared to separately formulated tablets (Sever et al., 2022). Viekira XR contains an extended-release layer with crystalline dasabuvir and ASD immediate-release layer (Miller et al., 2018). This formulation design reduces the dosing frequency of this therapeutic regimen to QD, in comparison to Viekira PAK. In the case of Delstrigo, a bilayer tablet design was selected to address a number of issues related to tablet disintegration, dissolution, processability, and chemical compatibility (Panmai et al., 2020). The tablet design utilizes a layer consisting of doravirine ASD and a layer consisting of separately dry granulated lamivudine and tenofovir disoproxil fumarate crystalline drugs. The separate ASD layer was necessary to avoid poor dissolution of doravirine observed when all components were combined into a monolithic tablet, as well as to address the chemical stability considerations of the crystalline tenofovir disoproxil fumarate. Significant process optimization of the roller compaction, compression, and film coating steps was necessary to address poor tablet tensile strength issues encountered due to the overall high loading of ASD (500 mg total), and two crystalline drugs (600 mg) in a 1600 mg tablet.
3. Conclusion and future perspective
In the last several decades, ASD formulations have been demonstrated to be a clinically successful strategy to deliver poorly water-soluble drugs. Early approvals of ASD products such as Sporanox (itraconazole) and Prograf (tacrolimus) in the 1990s, prepared by the traditional pharmaceutical manufacturing techniques of fluid bed bead layering and solvent granulation, demonstrated that amorphous materials could be physically and chemically stable. Manufacturing techniques of SD and HME were adapted from other industries, and opened up the ASD manufacturing landscape with approvals such as Intelence (etravirine) and Kaletra (ritonavir/lopinavir). These foundations have led to the contemporary state-of-the-art of ASD DPs for oral delivery of small molecules as discussed in this review.
Herein, examining the dataset of 48 ASD DPs approved by the FDA between 2012 and 2023 has allowed many trends to be observed regarding formulation strategies by therapeutic category, formulation and processing strategies of ASD DPIs and DPs, and aspects of patient-centricity (e.g., dosage form type, pill burden). It is apparent to the authors that formulating a drug as an ASD is not as simple as selecting a polymer and a manufacturing process. Despite a significant majority of new chemical entities having poor aqueous solubility characteristics, the number of formulations commercialized using an ASD strategy are yet relatively few in number, averaging four per year.
In the coming years, the authors expect expanded range of formulation and manufacturing strategies to be utilized for ASD DPI and DP formulations. Additional product launches with patient-centric delivery strategies are expected, in particular for the pediatric patient population. Similarly, as new drugs in certain therapeutic categories (e.g., antivirals) continue to be developed, new FDC products are likely to be launched. While PVPVA and HPMCAS are the most commonly used, diversification of polymer type used for ASD formulations is expected to continue in the future. This is exemplified by polymers such as polymethacrylates and HPMCP, each appearing in one recent ASD product approval. New polymers such as Soluplus may see their first commercial approval. While HPMC was among the first identified polymers useful for formulation of ASDs, poor processability characteristics limited its application. A new class of HPMC polymers (Affinisol) has been developed with improved HME processability characteristics and organic solvent solubility, potentially opening up formulation and processing strategies with this polymer chemistry. As the pharmaceutical industry pursues sustainability, greater efficiencies in SD manufacturing are expected (e.g., solvent recycling), as well as growth in utilization of techniques such as HME, KinetiSol, electrospraying, SCF, and cPT.
CRediT authorship contribution statement
Dana E. Moseson: Conceptualization, Data curation, Formal analysis, Project administration, Visualization, Writing – original draft. Trong Bien Tran: Data curation, Writing – review & editing. Bharathi Karunakaran: Data curation, Writing – review & editing. Rohan Ambardekar: Data curation, Writing – review & editing. Tze Ning Hiew: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dana E. Moseson and Rohan Ambardekar report a relationship with Pfizer Inc. that includes employment and stock ownership. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Jeremy Bartlett for the insightful discussion on pediatric drug delivery strategies. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data will be made available on request.
References
- Alvarenga B.R., Jr., Moseson D.E., Carneiro R.L., Taylor L.S. Impact of polymer type on thermal degradation of amorphous solid dispersions containing ritonavir. Mol. Pharm. 2022;19:332–344. doi: 10.1021/acs.molpharmaceut.1c00823. [DOI] [PubMed] [Google Scholar]
- Amidon G.L., Lennernäs H., Shah V.P., Crison J.R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- Anane-Adjei A.B., Jacobs E., Nash S.C., Askin S., Soundararajan R., Kyobula M., Booth J., Campbell A. Amorphous solid dispersions: utilization and challenges in preclinical drug development within AstraZeneca. Int. J. Pharm. 2022;614 doi: 10.1016/j.ijpharm.2021.121387. [DOI] [PubMed] [Google Scholar]
- Bapat P., Paul S., Tseng Y.C., Taylor L.S. Interplay of drug–polymer interactions and release performance for HPMCAS-based amorphous solid dispersions. Mol. Pharm. 2024;21:1466–1478. doi: 10.1021/acs.molpharmaceut.3c01106. [DOI] [PubMed] [Google Scholar]
- Bayliss M.K., Butler J., Feldman P.L., Green D.V.S., Leeson P.D., Palovich M.R., Taylor A.J. Quality guidelines for oral drug candidates: dose, solubility and lipophilicity. Drug Discov. Today. 2016;21:1719–1727. doi: 10.1016/j.drudis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Bennett-Lenane H., O’Shea J.P., O’Driscoll C.M., Griffin B.T. A retrospective biopharmaceutical analysis of >800 approved oral drug products: are drug properties of solid dispersions and lipid-based formulations distinctive? J. Pharm. Sci. 2020;109:3248–3261. doi: 10.1016/j.xphs.2020.08.008. [DOI] [PubMed] [Google Scholar]
- Berardi A., Janssen P.H.M., Dickhoff B.H.J. Technical insight into potential functional-related characteristics (FRCs) of sodium starch glycolate, croscarmellose sodium and crospovidone. J. Drug Deliv. Sci. Technol. 2022;70 doi: 10.1016/j.jddst.2022.103261. [DOI] [Google Scholar]
- Bergström C.A.S., Charman W.N., Porter C.J.H. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv. Drug Deliv. Rev. 2016;101:6–21. doi: 10.1016/j.addr.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Bhujbal S.V., Mitra B., Jain U., Gong Y., Agrawal A., Karki S., Taylor L.S., Kumar S., Zhou Q. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm. Sin. B. 2021;11:2505–2536. doi: 10.1016/j.apsb.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio L.L., Hiroshima L., Schwenk H.T., Green S. Successful enteral administration of crushed posaconazole delayed-release tablets in children. Pediatr. Blood Cancer. 2024;71 doi: 10.1002/pbc.30782. [DOI] [PubMed] [Google Scholar]
- Boel E., Van den Mooter G. The impact of applying an additional polymer coating on high drug-loaded amorphous solid dispersions layered onto pellets. Int. J. Pharm. 2023;630 doi: 10.1016/j.ijpharm.2022.122455. [DOI] [PubMed] [Google Scholar]
- Borbás E., Kádár S., Tsinman K., Tsinman O., Csicsák D., Takács-Novák K., Völgyi G., Sinkó B., Pataki H. Prediction of bioequivalence and food effect using flux- and solubility-based methods. Mol. Pharm. 2019;16:4121–4130. doi: 10.1021/acs.molpharmaceut.9b00406. [DOI] [PubMed] [Google Scholar]
- Brouwers J., Brewster M.E., Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009;98:2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- Butler J.M., Dressman J.B. The developability classification system: Application of biopharmaceutics concepts to formulation development. J. Pharm. Sci. 2010;99:4940–4954. doi: 10.1002/jps.22217. [DOI] [PubMed] [Google Scholar]
- Butreddy A. Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: a review from bench to clinic. Eur. J. Pharm. Biopharm. 2022;177:289–307. doi: 10.1016/j.ejpb.2022.07.010. [DOI] [PubMed] [Google Scholar]
- Charkoftaki G., Dokoumetzidis A., Valsami G., Macheras P. Elucidating the role of dose in the biopharmaceutics classification of drugs: the concepts of critical dose, effective in vivo solubility, and dose-dependent BCS. Pharm. Res. 2012;29:3188–3198. doi: 10.1007/s11095-012-0815-4. [DOI] [PubMed] [Google Scholar]
- Chemburkar S.R., Bauer J., Deming K., Spiwek H., Patel K., Morris J., Henry R., Spanton S., Dziki W., Porter W., Quick J., Bauer P., Donaubauer J., Narayanan B.A., Soldani M., Riley D., McFarland K. Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development. Org. Process. Res. Dev. 2000;4:413–417. doi: 10.1021/op000023y. [DOI] [Google Scholar]
- Chen Y. In: Developing Solid Oral Dosage Forms: Pharmaceutical Theory & Practice. Qiu Y., Chen Y., Zhang G., Yu L., Mantri R.V., editors. Elsevier Academic Press; 2017. Packaging selection for solid oral dosage forms; pp. 637–651. [Google Scholar]
- Cheng H., Mao L., Zhang S., Lv H. Impacts of polymeric additives on nucleation and crystal growth of indomethacin from supersaturated solutions. AAPS PharmSciTech. 2019;20 doi: 10.1208/s12249-019-1387-y. [DOI] [PubMed] [Google Scholar]
- Chiang C.W., Lubach J.W., Chen T., Chin S., Ly J., Zhang W., Hou H.H., Nagapudi K. Development of an amorphous solid dispersion formulation for mitigating mechanical instability of crystalline form and improving bioavailability for early phase clinical studies. Mol. Pharm. 2023;20:2452–2464. doi: 10.1021/acs.molpharmaceut.2c01056. [DOI] [PubMed] [Google Scholar]
- Choudhari A., Gopi A.K.T., Pattanayek S., Shah P., Joshi M., inventors; Actavis Laboratories, assignee. 2022 March 17. Eltrombopag Choline Dosage Forms. US 2022/0079883 A1. [Google Scholar]
- Cornish P. "Avoid the crush": Hazards of medication administration in patients with dysphagia or a feeding tube. CMAJ. 2005;172:871–872. doi: 10.1503/cmaj.050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrie L., Ajjarapu S., Banda S., Parvathaneni M., Bolla P.K., Kommineni N. HPMCAS-based amorphous solid dispersions in clinic: a review on manufacturing techniques (hot melt extrusion and spray drying), marketed products and patents. Materials. 2023;16 doi: 10.3390/ma16206616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corum I., Spangenberg A., Miller K., Kucera S., Miller D. Minimization of acid-catalyzed degradation in KinetiSol processing through HPMCAS neutralization. Mol. Pharm. 2023;20:1599–1612. doi: 10.1021/acs.molpharmaceut.2c00791. [DOI] [PubMed] [Google Scholar]
- Deac A., Qi Q., Indulkar A.S., Purohit H.S., Gao Y., Zhang G.G.Z., Taylor L.S. Dissolution mechanisms of amorphous solid dispersions: Role of drug load and molecular interactions. Mol. Pharm. 2023;20:722–737. doi: 10.1021/acs.molpharmaceut.2c00892. [DOI] [PubMed] [Google Scholar]
- Desai D., Wang J., Wen H., Li X., Timmins P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm. Dev. Technol. 2013;18:1265–1276. doi: 10.3109/10837450.2012.660699. [DOI] [PubMed] [Google Scholar]
- Di L., Fish P.V., Mano T. Bridging solubility between drug discovery and development. Drug Discov. Today. 2012;17:486–495. doi: 10.1016/j.drudis.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Di Martino R.M.C., Maxwell B.D., Pirali T. Deuterium in drug discovery: Progress, opportunities and challenges. Nat. Rev. Drug Discov. 2023;22:562–584. doi: 10.1038/s41573-023-00703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieringer T.D., Schaenman J.M., Davis M.R. Enteral feeding tube administration with therapeutic drug monitoring of crushed posaconazole tablets and opened isavuconazonium sulfate capsules. J. Antimicrob. Chemother. 2022;77:1417–1423. doi: 10.1093/jac/dkac035. [DOI] [PubMed] [Google Scholar]
- Dinunzio J.C., Schilling S.U., Coney A.W., Hughey J.R., Kaneko N., McGinity J.W. Use of highly compressible Ceolus microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev. Ind. Pharm. 2012;38:180–189. doi: 10.3109/03639045.2011.595415. [DOI] [PubMed] [Google Scholar]
- Dohrn S., Rawal P., Luebbert C., Lehmkemper K., Kyeremateng S.O., Degenhardt M., Sadowski G. Predicting process design spaces for spray drying amorphous solid dispersions. Int. J. Pharm. X. 2021;3 doi: 10.1016/j.ijpx.2021.100072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong T.V., Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part I: crystalline and semi-crystalline carriers. Expert Opin. Drug Deliv. 2016;13:1583–1594. doi: 10.1080/17425247.2016.1198768. [DOI] [PubMed] [Google Scholar]
- Duong T.V., Van den Mooter G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: amorphous carriers. Expert Opin. Drug Deliv. 2016;13:1681–1694. doi: 10.1080/17425247.2016.1198769. [DOI] [PubMed] [Google Scholar]
- Ekdahl A., Mudie D., Malewski D., Amidon G., Goodwin A. Effect of spray-dried particle morphology on mechanical and flow properties of felodipine in PVP VA amorphous solid dispersions. J. Pharm. Sci. 2019;108:3657–3666. doi: 10.1016/j.xphs.2019.08.008. [DOI] [PubMed] [Google Scholar]
- Elkhabaz A., Sarkar S., Simpson G.J., Taylor L.S. Characterization of phase transformations for amorphous solid dispersions of a weakly basic drug upon dissolution in biorelevant media. Pharm. Res. 2019;36:174. doi: 10.1007/s11095-019-2718-0. [DOI] [PubMed] [Google Scholar]
- Ellenberger D.J., Miller D.A., Williams R.O. Expanding the application and formulation space of amorphous solid dispersions with KinetiSol®: a review. AAPS PharmSciTech. 2018;19:1933–1956. doi: 10.1208/s12249-018-1007-2. [DOI] [PubMed] [Google Scholar]
- Felton L.A., Porter S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013;10:421–435. doi: 10.1517/17425247.2013.763792. [DOI] [PubMed] [Google Scholar]
- Frank D.S., Nie H., Chandra A., Coelho A., Dalton C., Dvorak H., Elkhabaz A., Fahy M., Ormes J., Parker A., Punia A., Rowe J., Schenck L., Smith D., Strotman N.A., Wang M., Wareham L. High bulk-density amorphous dispersions to enable direct compression of reduced tablet size amorphous dosage units. J. Pharm. Sci. 2023;112:2037–2045. doi: 10.1016/j.xphs.2022.09.007. [DOI] [PubMed] [Google Scholar]
- Frantz S. The trouble with making combination drugs. Nat. Rev. Drug Discov. 2006;5:881–882. doi: 10.1038/nrd2188. [DOI] [PubMed] [Google Scholar]
- Friesen D.T., Shanker R., Crew M., Smithey D.T., Curatolo W.J., Nightingale J.A.S. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview. Mol. Pharm. 2008;5:1003–1019. doi: 10.1021/mp8000793. [DOI] [PubMed] [Google Scholar]
- Gala U.H., Miller D.A., Williams R.O. Harnessing the therapeutic potential of anticancer drugs through amorphous solid dispersions. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188319. doi: 10.1016/j.bbcan.2019.188319. [DOI] [PubMed] [Google Scholar]
- Gilis P.M.V., De Condé V.F.V., Vandecruys R.P.G., inventors, Janssen Pharmaceutica NV, Assignee . 1997 May 27. Beads Having a Core Coated with an Antifungal and a Polymer. US Patent 5,633,015. [Google Scholar]
- Gupta S.S., Meena A.K., Parikh T., Serajuddin A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion - I: Polyvinylpyrrolidone and related polymers. J. Excipients Food Chem. 2014;5:32–45. [Google Scholar]
- Gupta S.S., Solanki N., Serajuddin A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, IV: Affinisol™ HPMC HME polymers. AAPS PharmSciTech. 2016;17:148–157. doi: 10.1208/s12249-015-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiew T.N., Taylor L.S. Combining drug salt formation with amorphous solid dispersions – a double edged sword. J. Control. Release. 2022;352:47–60. doi: 10.1016/j.jconrel.2022.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiew T.N., Zemlyanov D.Y., Taylor L.S. Balancing solid-state stability and dissolution performance of lumefantrine amorphous solid dispersions: the role of polymer choice and drug−polymer interactions. Mol. Pharm. 2022;19:392–413. doi: 10.1021/acs.molpharmaceut.1c00481. [DOI] [PubMed] [Google Scholar]
- Hiew T.N., Saboo S., Zemlyanov D.Y., Punia A., Wang M., Smith D., Lowinger M., Solomos M.A., Schenck L., Taylor L.S. Improving dissolution performance and drug loading of amorphous dispersions through a hierarchical particle approach. J. Pharm. Sci. 2023;112:2057–2068. doi: 10.1016/j.xphs.2022.12.019. [DOI] [PubMed] [Google Scholar]
- Holm P., Norling T., inventors, Veloxis Pharmaceuticals, Assignee . 2013 November 26. Modified Release Compositions Comprising Tacrolimus. US patent 8,591,946 B2. [Google Scholar]
- Hu Q., Choi D.S., Chokshi H., Shah N., Sandhu H. Highly efficient miniaturized coprecipitation screening (MiCoS) for amorphous solid dispersion formulation development. Int. J. Pharm. 2013;450:53–62. doi: 10.1016/j.ijpharm.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Huang S., Williams R.O. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech. 2017;19:1971–1984. doi: 10.1208/s12249-017-0861-7. [DOI] [PubMed] [Google Scholar]
- Huang S., O’Donnell K.P., Keen J.M., Rickard M.A., McGinity J.W., Williams R.O. A new extrudable form of hypromellose: AFFINISOLTM HPMC HME. AAPS PharmSciTech. 2016;17:106–119. doi: 10.1208/s12249-015-0395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins J.D., Bacho M.A., Olsen K.L., Bourgeois F.T. Pediatric drug information available at the time of new drug approvals: a cross-sectional analysis. Pharmacoepidemiol. Drug Saf. 2018;27:161–167. doi: 10.1002/pds.4351. [DOI] [PubMed] [Google Scholar]
- Ivanovska V., Rademaker C.M., van Dijk L., Mantel-Teeuwisse A.K. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- Jackson M.J., Toth S.J., Kestur U.S., Huang J., Qian F., Hussain M.A., Simpson G.J., Taylor L.S. Impact of polymers on the precipitation behavior of highly supersaturated aqueous danazol solutions. Mol. Pharm. 2014;11:3027–3038. doi: 10.1021/mp500201s. [DOI] [PubMed] [Google Scholar]
- Jermain S.V., Brough C., Williams R.O. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery – an update. Int. J. Pharm. 2018;535:379–392. doi: 10.1016/j.ijpharm.2017.10.051. [DOI] [PubMed] [Google Scholar]
- Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Kyeremateng S.O., Voges K., Dohrn S., Sobich E., Lander U., Weber S., Gessner D., Evans R.C., Degenhardt M. A hot-melt extrusion risk assessment classification system for amorphous solid dispersion formulation development. Pharmaceutics. 2022;14:1044. doi: 10.3390/pharmaceutics14051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larfors G., Andersson P., Jesson G., Liljebris C., Brisander M., Lennernäs H., Stenke L. Despite warnings, co-medication with proton pump inhibitors and dasatinib is common in chronic myeloid leukemia, but XS004, a novel oral dasatinib formulation, provides reduced pH-dependence, minimizing undesirable drug–drug interactions. Eur. J. Haematol. 2023;111:644–654. doi: 10.1111/ejh.14059. [DOI] [PubMed] [Google Scholar]
- Lehmkemper K., Kyeremateng S.O., Heinzerling O., Degenhardt M., Sadowski G. Impact of polymer type and relative humidity on the long-term physical stability of amorphous solid dispersions. Mol. Pharm. 2017;14:4374–4386. doi: 10.1021/acs.molpharmaceut.7b00492. [DOI] [PubMed] [Google Scholar]
- Li N., Taylor L.S. Microstructure formation for improved dissolution performance of lopinavir amorphous solid dispersions. Mol. Pharm. 2019;16:1751–1765. doi: 10.1021/acs.molpharmaceut.9b00117. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Liu G., Gong L., Zhang J., Wu Z., Deng H., Deng S. Development of nimesulide amorphous solid dispersions via supercritical anti-solvent process for dissolution enhancement. Eur. J. Pharm. Sci. 2020;152 doi: 10.1016/j.ejps.2020.105457. [DOI] [PubMed] [Google Scholar]
- Loftsson T., Brewster M.E. Pharmaceutical applications of cyclodextrins: basic science and product development. J. Pharm. Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- Luebbert C., Stoyanov E., Sadowski G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X. 2021;3 doi: 10.1016/j.ijpx.2020.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maincent J., Williams R.O. Sustained-release amorphous solid dispersions. Drug Deliv. Transl. Res. 2018;8:1714–1725. doi: 10.1007/s13346-018-0494-8. [DOI] [PubMed] [Google Scholar]
- Mann A.K.P., Schenck L., Koynov A., Rumondor A.C.F., Jin X., Marota M., Dalton C. Producing amorphous solid dispersions via co-precipitation and spray drying: Impact to physicochemical and biopharmaceutical properties. J. Pharm. Sci. 2018;107:183–191. doi: 10.1016/j.xphs.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Maskova E., Kubova K., Raimi-Abraham B.T., Vllasaliu D., Vohlidalova E., Turanek J., Masek J. Hypromellose - a traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release. 2020;324:695–727. doi: 10.1016/j.jconrel.2020.05.045. [DOI] [PubMed] [Google Scholar]
- Mason M.J., McDaneld P.M., Musick W.L., Kontoyiannis D.P. Serum levels of crushed posaconazole delayed-release tablets. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.02688-18. e02688–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey C.A., Kesisoglou F. Enabling an HCV treatment revolution and the frontiers of solid solution formulation. J. Pharm. Sci. 2019;108:50–57. doi: 10.1016/j.xphs.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Meena A., Parikh T., Gupta S.S., Serajuddin A.T.M. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion - II: Cellulosic polymers. J. Excipients Food Chem. 2014;5:46–55. [Google Scholar]
- Meruva S., Singaraju A.B., Vinjamuri B.P., Ternik R., Stagner W.C. Current state of minitablet product design: a review. J. Pharm. Sci. 2024;113:1123–1154. doi: 10.1016/j.xphs.2024.02.016. [DOI] [PubMed] [Google Scholar]
- Miller J.M., Morris J.B., Sever N.E., Schmitt E.A., Gao P.X., Shi Y., Gao Y., Liepold B., Moosmann A., Pauli M., Durak F., Kessler T., Hoelig P., Rosenblatt K., Kostelac D., Gokhale R., Costello M., Knable C., George S., inventors, AbbVie, Assignee . 2018 October 23. Solid Antiviral Dosage Forms. US patent 10,105,365 B2. [Google Scholar]
- Monschke M., Kayser K., Wagner K.G. Influence of particle size and drug load on amorphous solid dispersions containing pH-dependent soluble polymers and the weak base ketoconazole. AAPS PharmSciTech. 2021;22:44. doi: 10.1208/s12249-020-01914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseson D.E., Taylor L.S. The application of temperature-composition phase diagrams for hot melt extrusion processing of amorphous solid dispersions to prevent residual crystallinity. Int. J. Pharm. 2018;553:454–466. doi: 10.1016/j.ijpharm.2018.10.055. [DOI] [PubMed] [Google Scholar]
- Moseson D.E., Taylor L.S. Crystallinity: a complex critical quality attribute of amorphous solid dispersions. Mol. Pharm. 2023;20:4802–4825. doi: 10.1021/acs.molpharmaceut.3c00526. [DOI] [PubMed] [Google Scholar]
- Moseson D.E., Jordan M.A., Shah D.D.C., Corum I.D., Alvarenga B.R., Jr., Taylor L.S. Application and limitations of thermogravimetric analysis to delineate the hot melt extrusion chemical stability processing window. Int. J. Pharm. 2020;590 doi: 10.1016/j.ijpharm.2020.119916. [DOI] [PubMed] [Google Scholar]
- Moseson D.E., Hiew T.N., Su Y., Taylor L.S. Formulation and processing strategies which underpin susceptibility to matrix crystallization in amorphous solid dispersions. J. Pharm. Sci. 2023;112:108–122. doi: 10.1016/j.xphs.2022.03.020. [DOI] [PubMed] [Google Scholar]
- Mosquera-Giraldo L.I., Donoso M., Stefanski K., Foster K., Gesenberg C., Abraham P., Ren Y., Rose A., Freeden C., Ranasinghe A. Solvent-casted films to assist polymer selection for amorphous solid dispersions during preclinical studies: In-vitro and in-vivo exploration. Pharm. Res. 2021;38:901–914. doi: 10.1007/s11095-021-03040-w. [DOI] [PubMed] [Google Scholar]
- Mudie D.M., Stewart A.M., Rosales J.A., Biswas N., Adam M.S., Smith A., Craig C.D., Morgen M.M., Vodak D.T. Amorphous solid dispersion tablets overcome acalabrutinib pH effect in dogs. Pharmaceutics. 2021;13:557. doi: 10.3390/pharmaceutics13040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugheirbi N.A., Marsac P.J., Taylor L.S. Insights into water-induced phase separation in itraconazole-hydroxypropylmethyl cellulose spin coated and spray dried dispersions. Mol. Pharm. 2017;14:4387–4402. doi: 10.1021/acs.molpharmaceut.7b00499. [DOI] [PubMed] [Google Scholar]
- Mukesh S., Joshi P., Bansal A.K., Kashyap M.C., Mandal S.K., Sathe V., Sangamwar A.T. Amorphous salts solid dispersions of celecoxib: Enhanced biopharmaceutical performance and physical stability. Mol. Pharm. 2021;18:2334–2348. doi: 10.1021/acs.molpharmaceut.1c00144. [DOI] [PubMed] [Google Scholar]
- Nguyen D.N., Clasen C., Van den Mooter G. Pharmaceutical applications of electrospraying. J. Pharm. Sci. 2016;105:2601–2620. doi: 10.1016/j.xphs.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Nguyen H.T., Van Duong T., Jaw-Tsai S., Bruning-Barry R., Pande P., Taneja R., Taylor L.S. Fed- and fasted-state performance of pretomanid amorphous solid dispersions formulated with an enteric polymer. Mol. Pharm. 2023;20:3170–3186. doi: 10.1021/acs.molpharmaceut.3c00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.T., Van Duong T., Taylor L.S. Impact of gastric pH variations on the release of amorphous solid dispersion formulations containing a weakly basic drug and enteric polymers. Mol. Pharm. 2023;20:1681–1695. doi: 10.1021/acs.molpharmaceut.2c00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P.D., Pinto J.F., Henriques J., Paiva A.M. Insights into the release mechanisms of ITZ:HPMCAS amorphous solid dispersions: the role of drug-rich colloids. Mol. Pharm. 2022;19:51–66. doi: 10.1021/acs.molpharmaceut.1c00578. [DOI] [PubMed] [Google Scholar]
- Page S., Khan T., Kuhl P., Schwach G., Storch K., Chokshi H. Patient centricity driving formulation innovation: Improvements in patient care facilitated by novel therapeutics and drug delivery technologies. Annu. Rev. Pharmacol. Toxicol. 2022;62:341–363. doi: 10.1146/annurev-pharmtox-052120-093517. [DOI] [PubMed] [Google Scholar]
- Panmai S., Tatavarti A., Farrington A.M., Biyyala V., Allain L.R., Nefliu M., Klinzing G.R., Ren J., Lamm M., inventors; Merck Sharp and Dohme, assignee. 2020 March 31. Pharmaceutical Compositions Containing Doravirine, Tenofovir Disoproxil Fumarate and Lamivudine. US patent 10,603,282 B2. [Google Scholar]
- Pas T., Verbert S., Appeltans B., Van den Mooter G. The influence of crushing amorphous solid dispersion dosage forms on the in-vitro dissolution kinetics. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118884. [DOI] [PubMed] [Google Scholar]
- Patel N.G., Serajuddin A.T.M. Moisture sorption by polymeric excipients commonly used in amorphous solid dispersion and its effect on glass transition temperature: I. Polyvinylpyrrolidone and related copolymers. Int. J. Pharm. 2022;616 doi: 10.1016/j.ijpharm.2022.121532. [DOI] [PubMed] [Google Scholar]
- Patel N.G., Banella S., Serajuddin A.T.M. Moisture sorption by polymeric excipients commonly used in amorphous solid dispersions and its effect on glass transition temperature: II. Cellulosic polymers. J. Pharm. Sci. 2022;111:3114–3129. doi: 10.1016/j.xphs.2022.07.020. [DOI] [PubMed] [Google Scholar]
- Paul S., Sun C.C. Systematic evaluation of common lubricants for optimal use in tablet formulation. Eur. J. Pharm. Sci. 2018;117:118–127. doi: 10.1016/j.ejps.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Pavlis J., Mathers A., Fulem M., Klajmon M. Can pure predictions of activity coefficients from PC-SAFT assist drug–polymer compatibility screening? Mol. Pharm. 2023;20:3960–3974. doi: 10.1021/acs.molpharmaceut.3c00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D.J., Ditzinger F., Koehl N.J., Jankovic S., Tsakiridou G., Nair A., Holm R., Kuentz M., Dressman J.B., Saal C. Approaches to increase mechanistic understanding and aid in the selection of precipitation inhibitors for supersaturating formulations – a PEARRL review. J. Pharm. Pharmacol. 2018;71:483–509. doi: 10.1111/jphp.12927. [DOI] [PubMed] [Google Scholar]
- Punia A., Biyyala V., Faassen F., Ash J., Lamm M.S. Detrimental effect of the film coat chemistry and thickness on the physical stability of amorphous solid dispersions in tablet formulations. J. Pharm. Sci. 2023;112:708–717. doi: 10.1016/j.xphs.2022.09.013. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Gong Y., Ruggles A., Baird J.A., Chwalisz K., Owens C.D., Thomas J.W., Castelli-Haley J., Gordon K., Snabes M.C., Soliman A.M., Flores O.A., Jain R., Ng J.W.-K., North J.D., Palac H., Peloso P.M., Williams L.A., Zu H., Hu Y., inventors, Neurocrine Biosciences, Assignee . 2019 February 21. Solid Pharmaceutical Formulations for Treating Endometriosis, Uterine Fibroids, Polycystic Ovary Syndrome or Adenomyosis. US Patent Application Publication 2019/0054027 A1. [Google Scholar]
- Raina S.A., Van Eerdenbrugh B., Alonzo D.E., Mo H., Zhang G.G.Z., Gao Y., Taylor L.S. Trends in the precipitation and crystallization behavior of supersaturated aqueous solutions of poorly water-soluble drugs assessed using synchrotron radiation. J. Pharm. Sci. 2015;104:1981–1992. doi: 10.1002/jps.24423. [DOI] [PubMed] [Google Scholar]
- Riekes M.K., Caon T., da Silva J., Sordi R., Kuminek G., Bernardi L.S., Rambo C.R., de Campos C.E.M., Fernandes D., Stulzer H.K. Enhanced hypotensive effect of nimodipine solid dispersions produced by supercritical CO2 drying. Powder Technol. 2015;278:204–210. doi: 10.1016/j.powtec.2015.03.029. [DOI] [Google Scholar]
- Rosenberg J., Reinhold U., Liepold B., Berndl G., Breitenbach J., Alani L., Ghosh S., inventors, AbbVie, Assignee . 2014 Apr 8. Solid Pharmaceutical Dosage Form. US Patent 8,691,878 B2. [Google Scholar]
- Rosenberger J., Butler J., Dressman J. A refined developability classification system. J. Pharm. Sci. 2018;107:2020–2032. doi: 10.1016/j.xphs.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Saal C., Becker A. Pharmaceutical salts: a summary on doses of salt formers from the Orange Book. Eur. J. Pharm. Sci. 2013;49:614–623. doi: 10.1016/j.ejps.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Saboo S., Moseson D.E., Szeto R., Kestur U.S., Taylor L.S. Patterns of drug release as a function of drug loading from amorphous solid dispersions: a comparison of five different polymers. Eur. J. Pharm. Sci. 2020;155 doi: 10.1016/j.ejps.2020.105514. [DOI] [PubMed] [Google Scholar]
- Saha S.K., Joshi A., Singh R., Dubey K. Review of industrially recognized polymers and manufacturing processes for amorphous solid dispersion based formulations. Pharm. Dev. Technol. 2023;28:678–696. doi: 10.1080/10837450.2023.2233595. [DOI] [PubMed] [Google Scholar]
- Sakai T., Hirai D., Kimura S.I., Iwao Y., Itai S. Effects of tablet formulation and subsequent film coating on the supersaturated dissolution behavior of amorphous solid dispersions. Int. J. Pharm. 2018;540:171–177. doi: 10.1016/j.ijpharm.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Sarode A.L., Obara S., Tanno F.K., Sandhu H., Iyer R., Shah N. Stability assessment of hypromellose acetate succinate (HPMCAS) NF for application in hot melt extrusion (HME) Carbohydr. Polym. 2014;101:146–153. doi: 10.1016/j.carbpol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Sever N.E., Westedt U., Lander U., Schneider K., Steitz B., Mueller T., Reul R., Obermiller C., Jayasankar A., Simon M., Gao Y., Hach H., Kyeremateng S., Asmus K., Tong P., Zhu D., Naris M., Garrett C., inventors, AbbVie, Assignee . 2022 February 15. Solid Pharmaceutical Compositions for Treating HCV. US Patent 11,246,866 B2. [Google Scholar]
- Shah N., Sandhu H., Phuapradit W., Pinal R., Iyer R., Albano A., Chatterji A., Anand S., Choi D.S., Tang K., Tian H., Chokshi H., Singhal D., Malick W. Development of novel microprecipitated bulk powder (MBP) technology for manufacturing stable amorphous formulations of poorly soluble drugs. Int. J. Pharm. 2012;438:53–60. doi: 10.1016/j.ijpharm.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Shultz M.D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J. Med. Chem. 2019;62:1701–1714. doi: 10.1021/acs.jmedchem.8b00686. [DOI] [PubMed] [Google Scholar]
- Singh A., Van den Mooter G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016;100:27–50. doi: 10.1016/j.addr.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Smeets A., Koekoekx R., Clasen C., Van den Mooter G. Amorphous solid dispersions of darunavir: Comparison between spray drying and electrospraying. Eur. J. Pharm. Biopharm. 2018;130:96–107. doi: 10.1016/j.ejpb.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Solomos M.A., Punia A., Saboo S., John C., Boyce C.W., Chin A., Taggart R.V., Smith D., Lamm M.S., Schenck L. Evaluating spray drying and co-precipitation as manufacturing processes for amorphous solid dispersions of a low Tg API. J. Pharm. Sci. 2023;112:2087–2096. doi: 10.1016/j.xphs.2023.02.011. [DOI] [PubMed] [Google Scholar]
- Song Y., Zemlyanov D., Chen X., Su Z., Nie H., Lubach J.W., Smith D., Byrn S., Pinal R. Acid-base interactions in amorphous solid dispersions of lumefantrine prepared by spray-drying and hot-melt extrusion using X-ray photoelectron spectroscopy. Int. J. Pharm. 2016;514:456–464. doi: 10.1016/j.ijpharm.2016.06.126. [DOI] [PubMed] [Google Scholar]
- Stegemann S., Moreton C., Svanback S., Box K., Motte G., Paudel A. Trends in oral small-molecule drug discovery and product development based on product launches before and after the rule of five. Drug Discov. Today. 2023;28 doi: 10.1016/j.drudis.2022.103344. [DOI] [PubMed] [Google Scholar]
- Stevens R.W., O’Connell C., Huang A., Epps K.L., Ilges D. Therapeutic drug monitoring following crushed administration of delayed-release posaconazole tablets via enteral feeding tubes. J. Antimicrob. Chemother. 2023;78:553–555. doi: 10.1093/jac/dkac420. [DOI] [PubMed] [Google Scholar]
- Strotman N.A., Schenck L. Coprecipitated amorphous dispersions as drug substance: Opportunities and challenges. Org. Process. Res. Dev. 2021;26:10–13. doi: 10.1021/acs.oprd.1c00380. [DOI] [Google Scholar]
- Sutherland J.J., Raymond J.W., Stevens J.L., Baker T.K., Watson D.E. Relating molecular properties and in vitro assay results to in vivo drug disposition and toxicity outcomes. J. Med. Chem. 2012;55:6455–6466. doi: 10.1021/jm300684u. [DOI] [PubMed] [Google Scholar]
- Tan D.K., Davis D.A., Miller D.A., Williams R.O., Nokhodchi A. Innovations in thermal processing: Hot-melt extrusion and KinetiSol® dispersing. AAPS PharmSciTech. 2020;21:312. doi: 10.1208/s12249-020-01854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L.S., Zhang G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016;101:122–142. doi: 10.1016/j.addr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson G.R., Lewis P., Mudge S., Patterson T.F., Burnett B.P. Open-label crossover oral bioequivalence pharmacokinetics comparison for a 3-day loading dose regimen and 15-day steady-state administration of SUBA-itraconazole and conventional itraconazole capsules in healthy adults. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/aac.00400-20. e00400–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinworth C.P., Young R.J. Facts, patterns, and principles in drug discovery: Appraising the rule of 5 with measured physicochemical data. J. Med. Chem. 2020;63:10091–10108. doi: 10.1021/acs.jmedchem.9b01596. [DOI] [PubMed] [Google Scholar]
- Tran P., Park J.S. Application of supercritical fluid technology for solid dispersion to enhance solubility and bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121247. [DOI] [PubMed] [Google Scholar]
- Tran P.H., Tran T.T., Park J.B., Lee B.J. Controlled release systems containing solid dispersions: strategies and mechanisms. Pharm. Res. 2011;28:2353–2378. doi: 10.1007/s11095-011-0449-y. [DOI] [PubMed] [Google Scholar]
- Trasi N.S., Taylor L.S. Thermodynamics of highly supersaturated aqueous solutions of poorly water-soluble drugs—impact of a second drug on the solution phase behavior and implications for combination products. J. Pharm. Sci. 2015;104:2583–2593. doi: 10.1002/jps.24528. [DOI] [PubMed] [Google Scholar]
- Trasi N.S., Abbou Oucherif K., Litster J.D., Taylor L.S. Evaluating the influence of polymers on nucleation and growth in supersaturated solutions of acetaminophen. CrystEngComm. 2015;17:1242–1248. doi: 10.1039/c4ce02179g. [DOI] [Google Scholar]
- Trasi N.S., Purohit H.S., Taylor L.S. Evaluation of the crystallization tendency of commercially available amorphous tacrolimus formulations exposed to different stress conditions. Pharm. Res. 2017;2142–2155 doi: 10.1007/s11095-017-2221-4. [DOI] [PubMed] [Google Scholar]
- Trenkenschuh E., Blattner S.M., Hirsh D., Hoffmann R., Luebbert C., Schaefer K. Development of ternary amorphous solid dispersions manufactured by hot-melt extrusion and spray-drying horizontal line comparison of in vitro and in vivo performance. Mol. Pharm. 2024;21:1309–1320. doi: 10.1021/acs.molpharmaceut.3c00696. [DOI] [PubMed] [Google Scholar]
- Uttaro E., Pudipeddi M., Schweighardt A., Zhao F. To crush or not to crush: a brief review of novel tablets and capsules prepared from nanocrystal and amorphous solid dispersion technologies. Am. J. Health Syst. Pharm. 2021;78:389–394. doi: 10.1093/ajhp/zxaa412. [DOI] [PubMed] [Google Scholar]
- Virtanen A.A., Myslinska M., Healy A.M., Power E., Madi A., Siven M. The challenge of downstream processing of spray dried amorphous solid dispersions into minitablets designed for the paediatric population – a sustainable product development approach. Eur. J. Pharm. Sci. 2024;196 doi: 10.1016/j.ejps.2024.106752. [DOI] [PubMed] [Google Scholar]
- Wang B., Nethercott M.J., Narula A., Hanrahan M., Kuang S., Wenslow R.M., Li N. pH-dependent supersaturation from amorphous solid dispersions of weakly basic drugs. Pharm. Res. 2022;39:2919–2936. doi: 10.1007/s11095-021-03147-0. [DOI] [PubMed] [Google Scholar]
- Wertz C.F., Chen T., inventors, Nanocopoeia, LLC, assignee . 2022 May 10. Amorphous solid dispersions of dasatinib and uses thereof. US patent 11,324,745 B2. [Google Scholar]
- Williams H.D., Trevaskis N.L., Charman S.A., Shanker R.M., Charman W.N., Pouton C.W., Porter C.J.H. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013;65:315–499. doi: 10.1124/pr.112.005660. [DOI] [PubMed] [Google Scholar]
- Winiwarter S., Bonham N.M., Ax F., Hallberg A., Lennernäs H., Karlén A. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 1998;41:4939–4949. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- Wong S.N., Chen Y.C.S., Xuan B., Sun C.C., Chow S.F. Cocrystal engineering of pharmaceutical solids: therapeutic potential and challenges. CrystEngComm. 2021;23:7005–7038. doi: 10.1039/d1ce00825k. [DOI] [Google Scholar]
- Wrobleski S.T., Moslin R., Lin S., Zhang Y., Spergel S., Kempson J., Tokarski J.S., Strnad J., Zupa-Fernandez A., Cheng L., Shuster D., Gillooly K., Yang X., Heimrich E., McIntyre K.W., Chaudhry C., Khan J., Ruzanov M., Tredup J., Mulligan D., Xie D., Sun H., Huang C., D'Arienzo C., Aranibar N., Chiney M., Chimalakonda A., Pitts W.J., Lombardo L., Carter P.H., Burke J.R., Weinstein D.S. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J. Med. Chem. 2019;62:8973–8995. doi: 10.1021/acs.jmedchem.9b00444. [DOI] [PubMed] [Google Scholar]
- Wu C.Y., Benet L.Z. Predicting drug disposition via application of BCS: Transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- Wu D., Liu J., Paragas E.M., Yadav J., Aliwarga T., Heimbach T., Escotet-Espinoza M.S. Assessing and mitigating pH-mediated DDI risks in drug development – formulation approaches and clinical considerations. Drug Metab. Rev. 2024;1-20 doi: 10.1080/03602532.2024.2345632. [DOI] [PubMed] [Google Scholar]
- Xi H., Ren J., Novak J.M., Kemp E., Johnson G., Klinzing G., Johnson M.A., Xu W. The effect of inorganic salt on disintegration of tablets with high loading of amorphous solid dispersion containing copovidone. Pharm. Res. 2020;37:70. doi: 10.1007/s11095-020-2772-7. [DOI] [PubMed] [Google Scholar]
- Yang Q., Yuan F., Xu L., Yan Q., Yang Y., Wu D., Guo F., Yang G. An update of moisture barrier coating for drug delivery. Pharmaceutics. 2019;11:436. doi: 10.3390/pharmaceutics11090436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Saboo S., Zhou L., Askin S., Bak A. Early evaluation of opportunities in oral delivery of PROTACs to overcome their molecular challenges. Drug Discov. Today. 2024;29 doi: 10.1016/j.drudis.2023.103865. [DOI] [PubMed] [Google Scholar]
- Yu D., Hoag S.W. The impact of diluents on the compaction, dissolution, and physical stability of amorphous solid dispersion tablets. Int. J. Pharm. 2024;654 doi: 10.1016/j.ijpharm.2024.123924. [DOI] [PubMed] [Google Scholar]
- Zhang W., Noland R., Chin S., Petkovic M., Zuniga R., Santarra B., Conklin B., Hou H.H., Nagapudi K., Gruenhagen J.A., Yehl P., Chen T. Impact of polymer type, ASD loading and polymer-drug ratio on ASD tablet disintegration and drug release. Int. J. Pharm. 2021;592 doi: 10.1016/j.ijpharm.2020.120087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.