Abstract
Background
The clinical benefit of coronavirus disease 2019 (COVID-19) treatments against new circulating variants remains unclear. We sought to describe characteristics and clinical outcomes of highest risk patients with COVID-19 receiving early COVID-19 treatments in Scotland.
Methods
Retrospective cohort study of non-hospitalized patients diagnosed with COVID-19 from December 1, 2021–October 25, 2022, using Scottish administrative health data. We included adult patients who met ≥ 1 of the National Health Service highest risk criteria for early COVID-19 treatment and received outpatient treatment with sotrovimab, nirmatrelvir/ritonavir or molnupiravir, or no early COVID-19 treatment. Index date was defined as the earliest of COVID-19 diagnosis or early COVID-19 treatment. Baseline characteristics and acute clinical outcomes in the 28 days following index were reported. Values of ≤ 5 were suppressed.
Results
In total, 2548 patients were included (492: sotrovimab, 276: nirmatrelvir/ritonavir, 71: molnupiravir, and 1709: eligible highest risk untreated). Patients aged ≥ 75 years accounted for 6.9% (n = 34/492), 21.0% (n = 58/276), 16.9% (n = 12/71) and 13.2% (n = 225/1709) of the cohorts, respectively. Advanced renal disease was reported in 6.7% (n = 33/492) of sotrovimab-treated and 4.7% (n = 81/1709) of untreated patients, and ≤ 5 nirmatrelvir/ritonavir-treated and molnupiravir-treated patients. All-cause hospitalizations were experienced by 5.3% (n = 25/476) of sotrovimab-treated patients, 6.9% (n = 12/175) of nirmatrelvir/ritonavir-treated patients, ≤ 5 (suppressed number) molnupiravir-treated patients and 13.3% (n = 216/1622) of untreated patients. There were no deaths in the treated cohorts; mortality was 4.3% (n = 70/1622) among untreated patients.
Conclusions
Sotrovimab was often used by patients who were aged < 75 years. Among patients receiving early COVID-19 treatment, proportions of 28-day all-cause hospitalization and death were low.
Keywords: COVID-19, Sotrovimab, Molnupiravir, Nirmatrelvir/ritonavir, Omicron BA.1, Omicron BA.2, Omicron BA.5, Oral antivirals, Monoclonal antibody
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, as caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared by the World Health Organization in March 2020 [1]. Older individuals, immunocompromised patients or those with comorbidities such as cancer, diabetes, advanced renal disease or cardiovascular disease are at increased risk of developing severe COVID-19, which may result in hospitalization or death [2, 3].
In the UK, early COVID-19 treatment with either antivirals (e.g., nirmatrelvir/ritonavir, molnupiravir, remdesivir) or monoclonal antibodies (mAbs; e.g., casirivimab/imdevimab, sotrovimab) has been recommended for people with “highest risk” conditions. At the time of the study, examples of these conditions included solid cancer, advanced renal and liver disease, and human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) [4]. The emergence of the pandemic threatened to overwhelm healthcare systems in the UK and around the world. Strategies to minimize hospitalizations due to COVID-19 were key to the public health policy response, and hospitalization was an important outcome in the pivotal clinical trials of COVID-19 therapies [5–7].
Sotrovimab is a dual-action engineered human immunoglobulin G1κ mAb derived from the parental mAb S309, a potent neutralizing mAb directed against the spike protein of SARS-CoV-2 [8–11]. In the COVID-19 Monoclonal antibody Efficacy Trial-Intent to Care Early (COMET-ICE) randomized clinical trial (NCT04545060), sotrovimab (500 mg intravenous dose) was shown to significantly reduce the relative risk of all-cause > 24-h hospitalization or death by 79% compared with placebo in high-risk patients with mild-to-moderate COVID-19 [5]. In December 2021, sotrovimab received conditional marketing authorization in the UK for use in symptomatic adults and adolescents (aged ≥ 12 years and weighing ≥ 40 kg) with acute COVID-19 who did not require supplemental oxygen and were deemed at increased risk of progression to severe COVID-19 [12].
Two oral antivirals, molnupiravir and nirmatrelvir/ritonavir, have been shown to reduce the risk of progression to severe COVID-19 compared with placebo among high-risk patients with mild-to-moderate COVID-19 [6, 7, 13]. In the MOVe-OUT trial (NCT04575597), participants receiving molnupiravir had a lower risk of hospitalization or death through day 29 compared with the placebo group (6.8% vs 9.7%; difference, 3.0 percentage points; 95% CI, − 5.9 to − 0.1) [7]. In the EPIC-HR trial (NCT04960202), nirmatrelvir/ritonavir significantly reduced the incidence of hospitalization or death by day 28 compared with placebo (difference, 5.81 percentage points; 95% CI, –7.78 to –3.84; relative risk reduction, 88.9%) [6]. Molnupiravir and nirmatrelvir/ritonavir received conditional marketing authorization in November 2021 and December 2021, respectively, for use in patients with COVID-19 who were deemed at increased risk of disease progression [14, 15].
During the study period (December 2021–October 2022), National Health Service (NHS) guidelines recommended sotrovimab and nirmatrelvir/ritonavir as first-line treatment options, and molnupiravir as a third-line option [16]. It should be noted that nirmatrelvir/ritonavir may be contraindicated for certain highest-risk patients, including those with advanced renal disease or receiving ritonavir-containing medication for HIV/AIDS [17]. These guidelines apply to all parts of the UK, including Scotland [18].
Aim
Here, we describe real-world use of early COVID-19 treatments (including patient characteristics and clinical outcomes) for the management of non-hospitalized patients with COVID-19 at highest risk of developing severe disease in Scotland.
Methods
Study design and data source
This retrospective cohort study followed STROBE and RECORD reporting guidelines. We used data from administrative health datasets managed by Public Health Scotland and National Records of Scotland, linked and pseudonymized by the electronic Data Research and Innovation Service.
The study cohort was drawn from the Scottish general practitioner-registered population living within six health boards (i.e. Ayrshire & Arran, Dumfries & Galloway, Forth Valley, Greater Glasgow & Clyde, Lanarkshire and Lothian) that used the Hospital Electronic Prescribing and Medicines Administration (HEPMA) system for recording administration and prescription of COVID-19 therapies.
The index date (Day 1) was defined as the earliest date of a confirmed COVID-19 diagnosis (via reverse transcriptase polymerase chain reaction [RT-PCR] or lateral flow test [LFT]), or treatment date during the study period. Patients were followed up for 28 days (Day 28) from index (defined as the acute period), during which patient outcomes were evaluated. We included patients diagnosed with COVID-19 from December 1, 2021–October 25, 2022. The baseline period was defined as the 2 years prior to index for secondary care events and 1 year prior to index for general practitioner prescriptions.
Study population
Non-hospitalized patients (defined as having treatment for COVID-19 initiated in an outpatient or community setting) were eligible for inclusion if they were aged ≥ 18 years on the index date; had a COVID-19 diagnosis/positive SARS-CoV-2 RT-PCR or LFT; lived within 1 of the 6 geographical zones attached to a Scottish health board that used the HEPMA prescribing system; met ≥ 1 of the NHS highest-risk conditions criteria for receiving early treatment with sotrovimab, nirmatrelvir/ritonavir or molnupiravir (as defined by the presence of diagnosis codes) (Table 1); and received outpatient treatment with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir, or received no early COVID-19 treatment.
Table 1.
Highest and high-risk conditions criteria
| Highest risk conditions | High-risk conditions |
|---|---|
| Down syndrome | Age ≥ 70 years (without highest-risk conditions) |
| Solid cancer | Long-term respiratory conditions |
| Hematologic disease and stem-cell transplant recipients | Chronic heart diseasea |
| Advanced renal disease | Chronic kidney disease |
| Liver disease | Chronic liver disease |
| IMID | Chronic neurologic condition |
| Immune deficiencies | Diabetes |
| HIV/AIDS | Weakened immune system caused by medical condition or medicationa |
| Solid-organ transplant | Obesity (class III)a |
| Rare neurologic conditions | Pregnancy |
| Severe respiratory conditionsa | |
| Rare disease and inborn errors of metabolisma |
a No data available for this condition
AIDS acquired immune deficiency syndrome, HIV human immunodeficiency virus, IMID immune-mediated inflammatory disease
At the time of study, the NHS highest-risk criteria were Down’s syndrome, solid cancer, hematologic diseases (including cancers), advanced renal disease, advanced liver disease, immune-mediated inflammatory disease (IMID), immune deficiencies, HIV/AIDS, solid-organ and stem-cell transplant recipients, and rare neurologic conditions [4]. These highest-risk criteria were evaluated during the baseline period.
Patients were excluded if they received more than one COVID-19 treatment (sotrovimab, nirmatrelvir/ritonavir, molnupiravir, or remdesivir) in an outpatient setting during the acute period. Patients were also excluded if they received remdesivir as an early treatment in an outpatient setting, or initiated any COVID-19 treatment while in an inpatient setting (defined as overnight admission on the day of or prior to treatment, and discharge after the day of treatment). These latter two criteria were intended to ensure that only non-hospitalized patients with mild-to-moderate COVID-19 were included.
Study outcomes
The primary outcomes of this study were the proportions of patients with all-cause and COVID-19-related hospitalizations during the acute period (28 days following index). COVID-19-related hospitalizations were defined as any non-elective hospital visit for which COVID-19 was listed in the primary diagnosis field (among patients in whom the hospitalization episode was complete, i.e. discharge had occurred and clinical coding was complete).
Secondary outcomes included the number of all-cause and COVID-19-related inpatient hospitalization days, the proportion of patients with a critical care admission as part of hospitalization, the proportion of patients requiring non-invasive ventilation and mechanical ventilation, and the proportion of deaths during the acute period.
For all outcomes, patients were excluded if there was less than 45 days between the index diagnosis and data extraction censoring dates (regardless of whether they died in this interval) for the outcomes data (October 6, 2022). Forty-five days is the period recommended by Public Health Scotland to account for a reporting lag in the datasets used.
Patient characteristics were also recorded, including age, sex, COVID-19 vaccination status, and comorbidity history. Cohorts were described in relation to “highest-risk” conditions that made patients eligible for early treatment with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir, as mentioned above. Additionally, the cohorts were described in relation to other “high-risk” conditions that may predispose patients to severe COVID-19 outcomes (Table 1).
Outcomes were reported for the following cohorts: Cohort 1, patients receiving early treatment with sotrovimab; Cohort 2, patients receiving early treatment with nirmatrelvir/ritonavir; Cohort 3, patients receiving early treatment with molnupiravir; and Cohort 4, patients at highest risk who received no early COVID-19 treatment.
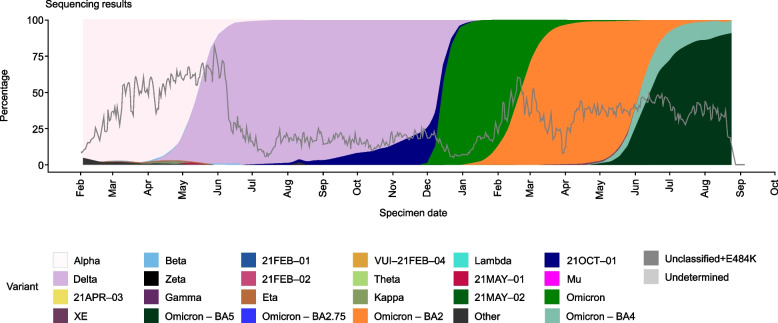
A subgroup analysis was also conducted. We described 28-day COVID-19-related hospitalization among sotrovimab-treated patients (Cohort 1) and those without any early COVID-19 treatments (Cohort 4) during the periods of Omicron BA.1 (December 1, 2021–February 28, 2022), Omicron BA.2 (March 1–May 31, 2022) and Omicron BA.5 (June 1–September 30, 2022) subvariant predominance in the UK (Fig. 1) [19]. These analyses were not performed for patients treated with nirmatrelvir/ritonavir or molnupiravir due to small sample sizes. Due to low sequencing rates, periods of most prevalent circulating variants were used as a proxy for the infecting variant.
Fig. 1.
SARS-CoV-2 variant predominance from February 2021 to September 2022. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 45. 2022. Reprinted from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf [19]. Contains public sector information licensed under the Open Government Licence v3.0: https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/. Accessed 17 Aug 2023. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Data analysis
Continuous variables (e.g. age) were summarized using mean, standard deviation, median, interquartile range, and range. Categorical variables (e.g. sex) were described using frequencies and percentages. Due to the study’s information governance and data suppression rules, counts of between 0 and 5 were suppressed and are reported as n = ≤ 5 throughout (unless they were structural zeros caused by inclusion/exclusion criteria). This suppression did not apply to mortality data.
Results
Patient demographics and baseline characteristics
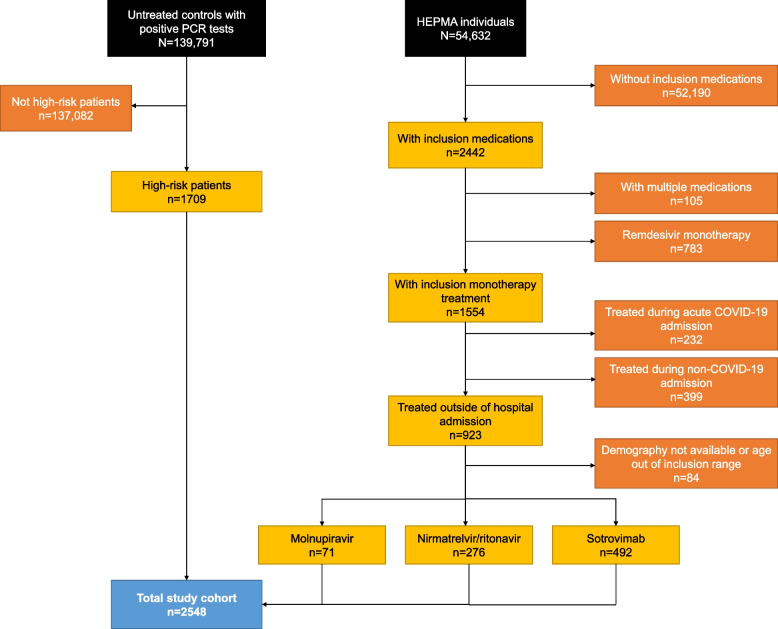
Following application of the eligibility criteria, demographics and baseline characteristics were available for 2548 patients, including 492 patients treated with sotrovimab, 276 patients treated with nirmatrelvir/ritonavir, 71 patients treated with molnupiravir, and 1709 eligible highest-risk untreated patients (Fig. 2). Baseline characteristics are reported in Table 2.
Fig. 2.
Flow diagram of patient cohort inclusion/exclusion criteria. COVID-19, coronavirus disease 2019; HEPMA, Hospital Electronic Prescribing and Medicines Administration; PCR, polymerase chain reaction
Table 2.
Patient characteristics
| Characteristic | Sotrovimab (n = 492) | Nirmatrelvir/ritonavir (n = 276) | Molnupiravir (n = 71) | Untreated (n = 1709) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 54.6 (15.0) | 60.9 (17.3) | 59.6 (14.0) | 55.1 (17.9) |
| Median (IQR) | 55 (44–65) | 62 (50–73) | 60 (49.5–69.5) | 57 (41–69) |
| Age group (years), n (%) | ||||
| 18–54 | 242 (49.2) | 95 (34.4) | 22 (31.0) | 783 (45.8) |
| 55–64 | 121 (24.6) | 61 (22.1) | 20 (28.2) | 369 (21.6) |
| 65–74 | 95 (19.3) | 62 (22.5) | 17 (23.9) | 332 (19.4) |
| ≥ 75 | 34 (6.9) | 58 (21.0) | 12 (16.9) | 225 (13.2) |
| Female sex, n (%) | 286 (58.1) | 164 (59.4) | 42 (59.2) | 949 (55.5) |
| Number of vaccinations, n (%) | ||||
| 0–2 | 21 (4.3) | 19 (6.9) | ≤ 5 | 315 (18.4) |
| 3 | 281 (57.1) | 126 (45.7) | 41 (57.7) | 1036 (60.6) |
| 4 | 160 (32.5) | 88 (31.9) | 23 (32.4) | 283 (16.6) |
| ≥ 5 | 30 (6.1) | 43 (15.6) | ≤ 5 | 75 (4.4) |
| Time since last vaccination (days), n (%) | ||||
| 1–60 | 164 (33.3) | 37 (13.4) | 17 (23.9) | 369 (21.6) |
| 61–120 | 204 (41.5) | 56 (20.3) | 28 (39.4) | 542 (31.7) |
| 121–180 | 74 (15.0) | 88 (31.9) | 10 (14.1) | 371 (21.7) |
| ≥ 181 | 50 (10.2) | 95 (34.4) | 16 (22.5) | 427 (25.0) |
| Highest risk conditions, n (%)a | ||||
| Solid cancer | 45 (9.1) | 16 (5.8) | ≤ 5 | 501 (29.3) |
| Hematologic disease, sickle cell disease and stem-cell transplant recipients | 28 (5.7) | 15 (5.4) | ≤ 5 | 89 (5.2) |
| Advanced renal disease | 33 (6.7) | ≤ 5 | ≤ 5 | 81 (4.7) |
| Liver disease | ≤ 5 | ≤ 5 | ≤ 5 | 23 (1.3) |
| IMID | 31 (6.3) | 12 (4.3) | ≤ 5 | 964 (56.4) |
| Immune deficiencies | ≤ 5 | ≤ 5 | ≤ 5 | 7 (0.4) |
| HIV/AIDS | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Solid-organ transplant | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Rare neurologic conditions | 9 (1.8) | ≤ 5 | ≤ 5 | 72 (4.2) |
| Patients with no highest risk diagnoses for developing COVID-19 complications, n (%) | 353 (71.7) | 235 (85.1) | 61 (85.9) | 0 |
| High-risk conditions, n (%)b | ||||
| Age ≥ 70 years (and no highest risk conditions) | 56 (11.4) | 84 (30.4) | 17 (23.9) | 0 |
| Chronic respiratory conditions | 15 (3.0) | 8 (2.9) | ≤ 5 | 49 (2.9) |
| Other chronic kidney disease | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Other chronic liver disease | ≤ 5 | ≤ 5 | ≤ 5 | 8 (0.5) |
Due to information governance and data suppression rules used in the study, counts of between 0 and 5 were suppressed and are reported as n = ≤ 5 throughout, excluding structural zeros (due to inclusion/exclusion criteria)
aNo cases of Down syndrome were identified from the available data; b No cases of pregnancy, chronic, moderate-risk neurologic conditions, or diabetes were identified from the available data
AIDS acquired immune deficiency syndrome, COVID-19 coronavirus disease 2019; HIV, human immunodeficiency virus, IMID immune-mediated inflammatory disease, IQR interquartile range, SD standard deviation
Patients aged ≥ 75 years accounted for 6.9% (n = 34/492) of the sotrovimab-treated cohort, 21.0% (n = 58/276) of the nirmatrelvir/ritonavir-treated cohort, 16.9% (n = 12/71) of the molnupiravir-treated cohort, and 13.2% (n = 225/1709) of untreated patients.
A high proportion of patients receiving an early COVID-19 treatment did not have an identifiable highest-risk condition in the database (71.7% for sotrovimab [n = 353/492], 85.1% for nirmatrelvir/ritonavir [n = 235/276], 85.9% for molnupiravir [n = 61/71], and 0% for untreated patients [resulting from the cohort’s inclusion criteria]). A high percentage of untreated patients had solid cancer (29.3%, n = 501/1709) and IMID (56.4%, n = 964/1709), while comparatively lower percentages were reported for other highest-risk comorbidities among this cohort (Table 2). In the treated cohorts, most patients did not have data available on highest-risk comorbidities, and low proportions of such conditions were therefore reported. Among sotrovimab-treated patients, 9.1% (n = 45/492) had solid cancer and 6.3% (n = 31/492) had IMID. Solid cancer was the most frequently reported highest-risk condition for nirmatrelvir/ritonavir-treated patients (5.8%, n = 16/276), and each of the highest-risk comorbidities were reported in five or fewer molnupiravir-treated patients. Advanced renal disease was reported for 6.7% (n = 33/492) of sotrovimab-treated patients and 4.7% (n = 81/1709) of untreated patients. Five or fewer patients treated with nirmatrelvir/ritonavir (n = ≤ 5/276) and molnupiravir (n = ≤ 5/71) had advanced renal disease. Due to the high level of missing data, the proportions of patients with highest-risk comorbidities could not be compared between cohorts.
Acute period outcomes
In total, 8.5% (n = 216/2548) of patients did not have sufficient observation time (45 days, including after death, from index to data extraction date) to assess acute period outcomes (sotrovimab: n = 16/492 [3.3%]; nirmatrelvir/ritonavir: n = 101/276 [36.6%]; molnupiravir: n = 12/71 [16.9%]; untreated: n = 87/1709 [5.1%]). Fewer than five patients with insufficient observation time died.
The percentage of patients who experienced an all-cause hospitalization was 5.3% (n = 25/476) for sotrovimab-treated patients and 6.9% (n = 12/175) for nirmatrelvir/ritonavir-treated patients (Table 3). Five or fewer molnupiravir-treated patients (n = ≤ 5/59) experienced an all-cause hospitalization. For untreated patients, the percentage of all-cause hospitalizations was 13.3% (n = 216/1622). The median (interquartile range) number of days in hospital due to any cause during the acute period was 5.0 (2–9) for sotrovimab-treated patients, 3.5 (2–9.5) for patients treated with nirmatrelvir/ritonavir, and 6.0 (3–14) for untreated patients. Median length of stay could not be reported for molnupiravir due to low patient numbers. Five or fewer patients in all cohorts required non-invasive ventilation or mechanical ventilation. Critical care admissions were experienced by five or fewer patients in each of the treated cohorts and 10 (0.6%) untreated patients.
Table 3.
Acute period all-cause and COVID-19-related outcomes
| Outcome | Sotrovimab (n = 476) | Nirmatrelvir/ritonavir (n = 175) | Molnupiravir (n = 59) | Untreated (n = 1622) |
|---|---|---|---|---|
| All-cause | ||||
| Inpatient (overnight) hospitalization, n (%) | 25 (5.3) | 12 (6.9) | ≤ 5 | 216 (13.3) |
| Inpatient hospitalization days | ||||
| Mean (SD) | 7.0 (6.0) | 8.8 (11.1) | NR | 10.8 (14.0) |
| Median (IQR) | 5 (2–9) | 3.5 (2–9.5) | 6 (3–14) | |
| Critical care admission, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | 10 (0.6) |
| Non-invasive ventilation, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Mechanical ventilation, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Inpatient (overnight) hospitalization with COVID-19 recorded in any diagnosis field, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | 66 (4.1) |
| Death, n (%) | 0 | 0 | 0 | 70 (4.3) |
| COVID-19-related | ||||
| Inpatient (overnight) hospitalization (primary diagnosis), n (%) | ≤ 5 | ≤ 5 | ≤ 5 | 48 (3.0) |
| Inpatient hospitalization days | ||||
| Mean (SD) | NR | NR | NR | 8.4 (7.4) |
| Median (IQR) | 6 (3–12) | |||
| Critical care admission, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Non-invasive ventilation, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Mechanical ventilation, n (%) | ≤ 5 | ≤ 5 | ≤ 5 | ≤ 5 |
| Death, n (%) | 0 | 0 | 0 | 13 (0.8) |
The hospitalizations dataset ran from December 1, 2019 to October 11, 2022, the intensive care unit dataset ran from December 1, 2021 to October 23, 2022, and the deaths dataset ran from December 2, 2021 to October 6, 2022. These three outcomes datasets were censored to align with the earliest dataset (to October 6, 2022) for consistency
Due to information governance and data suppression rules used in the study, counts of between 0 and 5 were suppressed and are reported as n = ≤ 5 throughout, except for mortality data, which does not require suppression
COVID-19 coronavirus disease 2019, IQR interquartile range, NR not reported, SD standard deviation
Five or fewer patients treated with each of sotrovimab, nirmatrelvir/ritonavir, and molnupiravir experienced COVID-19-related hospitalizations during the acute period (Table 3). For untreated patients, the percentage of COVID-19-related hospitalizations was 3.0% (n = 48/1622). Five or fewer patients in all cohorts required non-invasive ventilation or mechanical ventilation, or experienced critical care admission.
There were no deaths within 28 days of index for patients treated with sotrovimab, nirmatrelvir/ritonavir or molnupiravir. Mortality was 4.3% (n = 70/1622) in untreated patients, of whom 13 patients (18.6%) had COVID-19 as the primary cause (Table 3).
COVID-19-related hospitalizations during BA.1, BA.2, and BA.5 predominance
During BA.1 predominance, 283 patients were treated with sotrovimab and 721 received no treatment (Table 4). Similar to the overall analysis, five or fewer sotrovimab-treated patients (n = ≤ 5/283) experienced a COVID-19-related hospitalization. The percentage for untreated patients was 3.1% (n = 22/721).
Table 4.
Acute period COVID-19-related hospitalizations during periods of Omicron BA.1, BA.2, and BA.5 predominance
| Sotrovimab | Untreated | |
|---|---|---|
| BA.1 (December 1, 2021 to February 28, 2022) | ≤ 5/283 | 22/721 (3.1%) |
| BA.2 (March 1, 2022 to May 31, 2022) | ≤ 5/144 | 14/625 (2.2%) |
| BA.5 (June 1, 2022 to September 30, 2022) | ≤ 5/49 | 12/276 (4.3%) |
Due to information governance and data suppression rules used in the study, counts of between 0 and 5 were suppressed and are reported as n = ≤ 5 throughout
COVID-19 coronavirus disease 2019
During BA.2 predominance, 144 patients were treated with sotrovimab and 625 received no treatment (Table 4). Five or fewer sotrovimab-treated patients (n = ≤ 5/144) and 2.2% (n = 14/625) of untreated patients had COVID-19-related hospitalizations.
During BA.5 predominance, 49 patients were treated with sotrovimab and 276 received no treatment (Table 4). Five or fewer sotrovimab-treated patients (n = ≤ 5/49) and 4.3% (n = 12/276) of untreated patients had COVID-19-related hospitalizations.
Discussion
This study described the characteristics and severe clinical outcomes of non-hospitalized patients who received early COVID-19 treatment in Scotland from December 1, 2021–October 25, 2022, or those who were likely eligible but did not receive treatment. Our findings indicate that sotrovimab was used among patients who were slightly younger (6.9% were aged ≥ 75 years vs 21.0% for nirmatrelvir/ritonavir and 16.9% for molnupiravir). We also found that patients who received early COVID-19 treatment with sotrovimab or antivirals experienced low levels of 28-day hospitalization. Low levels of all-cause death were also observed.
The observed characteristics of sotrovimab-treated patients differed to those described in a similar real-world study conducted in another part of the UK [20]. In a retrospective cohort study of non-hospitalized patients who received early treatment for, or were diagnosed with, COVID-19 in Northwest London from December 1, 2021–May 31, 2022, Patel et al. reported descriptive results for patients treated with sotrovimab, nirmatrelvir/ritonavir, or molnupiravir. Sotrovimab was found to be used most often among older patients with multiple comorbidities that increased their risk of severe COVID-19, such as advanced renal disease [20]. In the present study, we found that sotrovimab-treated patients were younger than the other treated cohort. Of note, we observed a particularly high number of untreated patients with IMID (56.4%). This suggests that the effective eligibility criteria may have been narrower than the definitions used herein, with certain IMIDs associated with higher risk and real-world treatment allocation reflecting this. Alternatively, it may indicate genuine clinical differences between the treated and untreated cohorts.
We found that the proportion of all-cause hospitalizations was low for patients treated with sotrovimab and antivirals. These results are supported by other real-world studies. Patel et al. reported low all-cause hospitalizations among patients treated with sotrovimab, nirmatrelvir/ritonavir, and molnupiravir, with low hospitalization rates for sotrovimab being consistent among patients with advanced renal disease, those aged 18–64 years and ≥ 65 years, and across periods of Omicron BA.1, BA.2, and BA.5 predominance (through July 2022) [20]. Our results for the overall cohort are also similar to those of a recent retrospective cohort study of patients presumed to be treated with sotrovimab in England, where 4.6% of patients experienced an all-cause hospitalization [21].
A recent publication investigated outcomes among patients on kidney replacement therapy in Scotland and England using OpenSAFELY and UK Renal Registry data. Among this population, 1.1% (n = 21/1852) of sotrovimab-treated patients experienced 28-day COVID-19-related hospitalization or death compared with 3.3% (n = 17/515) of molnupiravir-treated patients [22]. In an analysis of sotrovimab vs molnupiravir in England (conducted from December 2021 to February 2022), 0.1% (n = 32/3331) of sotrovimab-treated patients and 2.0% (n = 55/2689) of molnupiravir-treated patients experienced 28-day COVID-19-related hospitalization or death [23]. In a US study conducted during Delta and early Omicron variant predominance, 2.67% (n = 418/15,633) of sotrovimab-treated patients experienced 30-day all-cause hospitalization and 0.08% (n = 13/15,633) died, compared with 5.57% (n = 84,307/1,514,868) and 0.54% (n = 8167/1,514,868), respectively, of patients who did not receive mAb treatment [24]. In a further study conducted from February to October 2022, very similar proportions of patients treated with sotrovimab and nirmatrelvir/ritonavir experienced 28-day COVID-19-related hospitalization or death [25]. In another recent study, the OpenSAFELY platform was used to emulate target trials to estimate the effectiveness of sotrovimab vs no treatment during BA.1 and BA.2 predominance. Estimated hazard ratios for 28-day COVID-19-related hospitalization or death were 0.76 (95% confidence interval [CI]: 0.66–0.89) during BA.1 and 0.92 (95% CI: 0.79–1.06) during BA.2 for sotrovimab vs no treatment [26].
We previously assessed the uptake of mAbs and antiviral therapies in Scotland, and discussed whether these treatments were used as recommended [27]. We reported that only around half of eligible patients received a mAb/antiviral for COVID-19, but the vast majority of patients who received treatment did so within the recommended timeframe. In this study, we also found that some eligible patients (based on COVID-19 diagnosis and high-risk comorbidities) were untreated; the reasons for this were not ascertained as part of this study but are of interest for future research.
We also found that five or fewer sotrovimab-treated patients experienced COVID-19-related hospitalizations across periods of BA.1, BA.2 and BA.5 predominance. Due to data suppression rules and small patient numbers (particularly with decreasing sotrovimab use during BA.5), interpretation of these results is challenging, particularly during BA.2 and BA.5 predominance. The risk of ecologic bias should be noted, as we did not have access to sequencing data to confirm each patient’s variant.
It should be noted that these descriptive results require confirmation with formal statistical testing adjusting for confounding factors. In the current study, formal comparison was not considered appropriate for several reasons. Due to the relatively small sample size for some groups, and low numbers of events observed (resulting in suppressed numbers), it was highly unlikely that there would be sufficient power to show statistical significance. More importantly, despite it being an inclusion criterion, for a high number of patients in the treatment groups (> 70%) their highest-risk conditions could not be identified in the database; this meant it was impossible to adequately adjust for potential confounders.
This study has several limitations. Firstly, we present descriptive analyses with no adjustment for differences in patient characteristics between cohorts, meaning that results may be subject to bias and confounding. Further, the limited sample size (particularly for molnupiravir) led to frequent suppression of small values. We did not collect data on COVID-19 severity and symptoms at disease onset, and therefore cannot confirm the cohorts were comparable in this regard. It is possible that the sotrovimab-treated cohort had mild-to-moderate COVID-19, while some untreated patients may have been asymptomatic, mildly symptomatic, or improving. In addition, 85% of the treated cohort had no identified RT-PCR or (registered) LFT in the 40 days prior to treatment, whereas the untreated cohort were required to have a positive test recorded. These “untested” treated patients were likely to have tested at home and not reported their results. There were limited sequencing data available for included patients, and dominance period for Omicron subvariant was used as a surrogate; hence, there is potential for misclassification of the infecting variant. Also, comorbidities were identified using inpatient admissions and procedures data; conditions treated in specialist departments (including maternity and renal wards) or in primary care could not, therefore, be determined, which may partially explain the level of missing data observed. Finally, there was an imbalance across study cohorts in the proportion of patients from each group who could not be included for analysis due to insufficient observation time (< 45 days); in particular, more than one-third of those in the nirmatrelvir/ritonavir group had an insufficient observation period, which may have impacted our findings. Patients with observation time < 45 days were excluded from the main analysis even if they died; this accounted for less than five patients, and their inclusion in a sensitivity analysis had no impact on study results (data not shown).
Conclusion
Our findings indicate that among patients who received early COVID-19 treatment with sotrovimab or antivirals in Scotland, low proportions experienced all-cause hospitalizations and death within 28 days of treatment. Sotrovimab was observed to be frequently utilized in patients aged below 75 years old in Scotland. Most treated patients had missing data for their high/highest-risk status and conditions, which reduced the feasibility of conducting a comparative effectiveness analysis to assess the impact of sotrovimab in preventing severe COVID-19 among this population.
Acknowledgements
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables, grammatical editing and referencing) was provided by Kathryn Wardle of Apollo, OPEN Health Communications, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022), and was funded by GSK and Vir Biotechnology, Inc.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- CI
Confidence interval
- COMET-ICE
COVID-19 Monoclonal antibody Efficacy Trial-Intent to Care Early
- COVID-19
Coronavirus disease 2019
- HEPMA
Hospital Electronic Prescribing and Medicines Administration
- HIV
Human immunodeficiency virus
- IMID
Immune-mediated inflammatory disease
- IQR
Interquartile range
- LFT
Lateral flow test
- mAb
Monoclonal antibody
- NHS
National Health Service
- NR
Not reported
- PCR
Polymerase chain reaction
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SD
Standard deviation
Authors’ contributions
MD, HT, VP, EJL, WK, HJB, and AS were involved in the study design, conception, and execution. HT, DCG, and CM took part in the acquisition and analysis of data. All authors were involved in data interpretation; drafting, revising, or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript.
Funding
This study was funded by GSK in collaboration with Vir Biotechnology, Inc (study number: 219082).
Availability of data and materials
Data for this study (study ID: 2223–0033) are held by the National Services Scotland electronic Data Research and Innovation Service in the National Safe Haven. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data would be made available from a reasonable request to myriam.g.drysdale@gsk.com.
Declarations
Ethics approval and consent to participate
This study received data access and processing approval from the Public Benefit and Privacy Panel for Health and Social Care, NHS Scotland on November 9, 2022. We complied with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred as part of this study; therefore, informed consent, ethics committee, or IRB approval were not required, as determined by the NHS Health Research Authority Research Ethics Committee decision tool (https://www.hra-decisiontools.org.uk/ethics/). Study results were reported in tabular form and aggregate analyses that omitted subject identification. Publications, reports or any other research outputs do not include subject identifiers and low patient numbers were suppressed.
Consent for publication
Not applicable.
Competing interests
MD, DCG, EJL, WK, and HJB and are employees of, and/or shareholders in, GSK. VP was an employee of GSK at the time of the study and is now an employee of KVM Analytics. HT, CM, and AS are employees of the University of Edinburgh, which received funding from GSK and Vir Biotechnology, Inc. to conduct the study. AS has also served on a number of Scottish Government and UK Government COVID-19 advisory groups, all of which have been unremunerated.
Footnotes
Vishal Patel at the time of study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/europe/emergencies/situations/covid-19. Accessed 17 Aug 2023.
- 2.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hippisley-Cox J, Khunti K, Sheikh A, Nguyen-Van-Tam JS, Coupland CAC. QCovid 4 - predicting risk of death or hospitalisation from COVID-19 in adults testing positive for SARS-CoV-2 infection during the Omicron wave in England. medRxiv. 2022; 10.1101/2022.08.13.22278733.
- 4.Department of Health & Social Care. Defining the highest-risk clinical subgroups upon community infection with SARS-CoV-2 when considering the use of neutralising monoclonal antibodies (nMABs) and antiviral drugs: independent advisory group report. https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report/defining-the-highest-risk-clinical-subgroups-upon-community-infection-with-sars-cov-2-when-considering-the-use-of-neutralising-monoclonal-antibodies. Accessed 17 Aug 2023.
- 5.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Rodrigues Falci D, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15:e1002493. doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 11.Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid M, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2022; 10.1101/2021.03.09.434607.
- 12.Medicines & Healthcare products Regulatory Agency. Summary of product characteristics for Xevudy. https://www.gov.uk/government/publications/regulatory-approval-of-xevudy-sotrovimab/summary-of-product-characteristics-for-xevudy. Accessed 17 Aug 2023.
- 13.Hung YP, Lee JC, Chiu CW, Lee CC, Tsai PJ, Hsu IL, et al. Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark? Antibiotics (Basel) 2022;11:220. doi: 10.3390/antibiotics11020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medicines & Healthcare products Regulatory Agency. Summary of product characteristics for Paxlovid. https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid/summary-of-product-characteristics-for-paxlovid. Accessed 17 Aug 2023.
- 15.Medicines & Healthcare products Regulatory Agency. Last updated 19/10/22 - summary of product characteristics for Lagevrio. https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir/summary-of-product-characteristics-for-lagevrio. Accessed 17 Aug 2023.
- 16.Department of Health & Social Care. Interim clinical commissioning policy: antivirals or neutralising monoclonal antibodies for non-hospitalised patients with COVID-19 (Version 6). https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=104044. Accessed 17 Aug 2023.
- 17.National Health Service. Who can and cannot take Paxlovid. https://www.nhs.uk/medicines/paxlovid/who-can-and-cannot-take-paxlovid/#:~:text=severe%20liver%20disease,have%20a%20weakened%20immune%20system. Accessed 17 Aug 2023.
- 18.Scottish Medicines Consortium. Sotrovimab (Xevudy). https://www.scottishmedicines.org.uk/medicines-advice/sotrovimab-xevudy-full-smc2555/. Accessed 17 Aug 2023.
- 19.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 45. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf. Accessed 17 Aug 2023.
- 20.Patel V, Yarwood MJ, Levick B, Gibbons DC, Drysdale M, Kerr W, et al. Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England. medRxiv. 2022; 10.1101/2022.11.28.22282808.
- 21.Patel V, Levick B, Boult S, Gibbons DC, Drysdale M, Lloyd EJ, et al. Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in NHS hospitals in England. medRxiv. 2023; 10.1101/2023.02.08.23285654. [DOI] [PMC free article] [PubMed]
- 22.The OpenSAFELY Collaborative, Zheng B, Campbell J, Carr EJ, Tazare J, Nab L, et al. Comparative effectiveness of sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the OpenSAFELY-UKRR linked platform and SRR database. medRxiv. 2022; 10.1101/2022.12.02.22283049.
- 23.Zheng B, Green ACA, Tazare J, Curtis HJ, Fisher L, Nab L, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ. 2022;379:e071932. doi: 10.1136/bmj-2022-071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng MM, Reyes C, Satram S, Birch H, Gibbons DC, Drysdale M, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States. medRxiv. 2022; 10.1101/2022.09.07.22279497. [DOI] [PMC free article] [PubMed]
- 25.Zheng B, Tazare J, Nab L, Mehrkar A, MacKenna B, Goldacre B, et al. Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform. medRxiv. 2023; 10.1101/2023.01.20.23284849.
- 26.The OpenSAFELY collaborative, Tazare J, Nab L, Zheng B, Hulme WJ, Green ACA, et al. Effectiveness of sotrovimab and molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform. medRxiv. 2023; 10.1101/2023.05.12.23289914.
- 27.Tibble H, Mueller T, Proud E, Hall E, Kurdi A, Robertson C, et al. Uptake of monoclonal antibodies and antiviral therapies for COVID-19 in Scotland. Lancet. 2023;401:101–102. doi: 10.1016/S0140-6736(22)02398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study (study ID: 2223–0033) are held by the National Services Scotland electronic Data Research and Innovation Service in the National Safe Haven. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data would be made available from a reasonable request to myriam.g.drysdale@gsk.com.