Abstract
Although human immunodeficiency virus (HIV)-infected subjects without AIDS have a high frequency of HIV-specific CD8 T lymphocytes, cellular immunity is unable to control infection. Freshly isolated lymphocytes often do not lyse HIV-infected targets in 4-h cytotoxicity assays. A large fraction of circulating CD8 T cells from HIV-infected donors down-modulate CD3ζ, the signaling component of the T-cell receptor complex, which is reexpressed in vitro coincident with the return of cytotoxic function. To investigate further the link between CD3ζ down-modulation and possible CD8 T-cell functional defects, we used flow cytometry to characterize further the properties of the CD3ζ-down-modulated subset. HIV-specific CD8 T cells, identified by tetramer staining, are CD3ζ−. CD8 T cells with down-modulated CD3ζ also do not express the key costimulatory receptor CD28 and have the cell surface phenotype of activated or memory T cells (HLA-DR+ CD62L−). After T-cell activation, CD3ζ-down-modulated cells express the activation marker CD69 but not the high-affinity interleukin 2 (IL-2) receptor α-chain CD25 and produce gamma interferon but not IL-2. Therefore HIV-specific CD8 T cells have down-modulated key signaling molecules for T-cell activation and costimulation and require exogenous cytokine stimulation. The typical impairment of HIV-specific CD4 T helper cells, which would normally provide specific CD8 T-cell stimulation, means that in vivo CTL function in vivo is compromised in most HIV-infected individuals. In AIDS patients, the functional defect is more severe, since CD3ζ is not reexpressed even after IL-2 exposure.
During the asymptomatic phase of human immunodeficiency virus (HIV) infection there is a high frequency of circulating virus-specific CD8 T cells (17, 26). As many as 1 in 100 circulating CD8 T cells are specific for a single HIV peptide epitope in untreated patients (1, 37). The frequency of HIV-infected CD4 cells in either untreated patients or patients given highly active antiretroviral therapy (HAART) is generally many orders of magnitude lower. Even though the frequency of HIV-specific CD8 T cells falls as the viral load drops with treatment (40), there are still probably many more antiviral CD8 T cells than potential HIV-infected targets in patients who do not have AIDS (24). Nevertheless, effective immunosurveillance against HIV does not usually develop even after months of viral suppression with HAART; when antiviral drugs are withdrawn, HIV levels generally rebound (38). The following question then arises: why don't antiviral cytotoxic T lymphocytes (CTL) provide better protection? There are some recent clues to help answer this question. Many viruses have devised strategies to evade an immune response (42). During latent infection, viral proteins are not expressed and therefore latently infected cells are hidden from immune surveillance. Recent reports indicate that HIV Nef induces down-modulation of major histocompatibility complex class I molecules from the surface of the infected cell (8, 50). This is sufficient to inhibit recognition of HIV-infected cells by some, but not all, CTL (8, 51, 52, 59).
Although part of the reason for the lack of effective immune surveillance may stem from defects in antigen presentation of HIV-infected cells, we have also provided evidence that CD8 cytotoxic T-cell function is compromised in HIV infection. When freshly isolated blood mononuclear cells from HIV-infected donors who have not progressed to symptomatic disease are tested in short-term (4-h) cytotoxicity assays, they generally are unable to lyse HIV-infected targets at levels much above background (58). High levels of antiviral cytotoxicity develop in peripheral blood mononuclear cells (PBMC) from less-advanced patients in vitro; however, this occurs after overnight culture in an interleukin 2 (IL-2)-dependent manner. This suggests that circulating HIV-specific CD8 T cells may be partially anergic and may be unable to eliminate HIV-infected targets in vivo, especially in the setting of lacking or functionally impaired HIV-specific helper CD4 T cells (41, 46), which would normally provide IL-2 or other cytokines and possibly other forms of help that require cell-cell contact.
The molecular basis and etiology of the lack of cytotoxicity by freshly isolated cells are not certain (23, 55). A large fraction of circulating CD8 T cells in HIV-infected donors are already activated, as evidenced by high levels of expression of CD38, HLA-DR, and CD57 and the cytolytic serine esterase granzyme A (9, 14, 16, 45, 58, 60). Moreover, there is a good correlation in circulating CD8 T cells (but not in lymph node T cells [2]) between granzyme A and perforin expression (L. W. Kam and J. Lieberman, unpublished data). Therefore, it seems likely that they are armed for cytolysis.
In HIV infection, there is an increased proportion of activated/memory T cells that do not express CD28 (19, 28, 48, 62). HIV-specific cytotoxicity is mediated by the CD28− CD8 T-cell subpopulation (10, 62, 64). After activation by T-cell receptor (TCR) engagement, CD28− CD8 T cells in HIV-infected subjects produce gamma interferon (IFN-γ) but not IL-2 and have reduced proliferative capacity (5, 23, 28, 53). This suggests that CD28− CD8 T cells are terminally differentiated effector CTL.
Anomalies of T-cell signaling after activation in CD8 T cells from HIV-infected donors have been described (4, 23, 54, 58, 61). In fact, a large fraction of CD8 T cells in HIV-infected donors have down-modulated cell surface expression of CD3ζ, the key proximal signaling chain of the TCR (54, 58). CD3ζ expression decreases early in infection and correlates with declining CD4 counts and disease stage (13, 58). In less-advanced patients, CD8 T-cell CD3ζ expression increases in vitro after 6 to 8 h of culture in an IL-2-dependent manner, coincident with detection of HIV-specific cytotoxicity, which is also IL-2 dependent. A similar constellation of lack of antigen-specific cytotoxicity, signaling anomalies, and CD3ζ down-modulation has been described for tumor-infiltrating lymphocytes in mouse tumor models and human solid tumors, in circulating CD8 T cells in human lymphoma patients, in the blood of patients with systemic lupus erythematosis, and in the inflamed joints in rheumatoid arthritis (11, 27, 29, 35, 36). Signaling defects of lymphocytes from Hodgkin's disease and lymphoma patients have also been linked to abnormally low expression of CD3ζ (33, 43).
The aim of this study was to explore the phenotypic and functional properties of CD8 T cells with down-modulated CD3ζ. We found that HIV-specific CD8 T cells that stain with HIV epitope tetramers have down-modulated both CD3ζ and CD28. Upon activation, cells with decreased expression of CD3ζ and CD28 do not express CD25 and produce IFN-γ but not IL-2. We also found that the small proportion of CD8 T cells in healthy donors with down-modulated CD3ζ and CD28 behave similarly upon T-cell activation. Therefore, the down-modulation of these key T-cell activation receptors may be part of the normal regulation of CD8 T cells. However, whereas antigen-specific CD4 T cells that secrete IL-2 should be able to restore CD3ζ expression and cytotoxic function to antigen-specific CD8 T cells in vivo at the site of infection in healthy individuals, the paucity of HIV-specific CD4 T-cell help in HIV-infected individuals may be a barrier to effective cytotoxic function in vivo. Moreover, HIV-infected donors with symptomatic disease or AIDS have a more profound defect in CD3ζ expression. In advanced subjects, not only is the proportion of CD3ζ− CD8 T cells greater, but IL-2-induced up-regulation of CD3ζ is also impaired.
MATERIALS AND METHODS
Subjects.
Subjects were healthy volunteers and HIV type 1 (HIV-1)-seropositive patients at various disease stages (6) (Table 1). This study was approved by the Brigham and Women's Hospital and the Center for Blood Research institutional review boards, and informed consent was obtained from each subject. Samples were either freshly obtained or were cryopreserved using a programmed cell freezer (model 9000; Gordinier, Roseville, Mich.). Flow cytometry results obtained from thawed cells were comparable to those from freshly isolated cells in two samples studied.
TABLE 1.
CD3ζ expression on CD8 T cells from late-stage HIV-infected individuals remains suppressed even after overnight incubation with IL-2b
| Patient | Clinical stagea | CD4 count | % CD3ζ+ CD8 T cells (PHD, PA)c for:
|
||
|---|---|---|---|---|---|
| Fresh PBMC | Overnight incubation
|
||||
| −IL-2 | +IL-2 | ||||
| Healthy donors | |||||
| NS1 | Control | NDd | 90 | 82 | 85 |
| NS2 | Control | 625 | 93 | 78 | 87 |
| NS3 | Control | ND | 93 | 82 | 87 |
| Mean ± SD | 92 ± 1 | 80 ± 2 | 86 ± 1 | ||
| Primary infection | |||||
| 060569 | Primary infection | 1,390 | 55 | 78 | 98 |
| Stage A | |||||
| 150468 | A1 | 966 | 58 | 87 | 88 |
| 280875 | A1 | 892 | 31 | 77 | 92 |
| 605 | A1 | 840 | 80 | 82 | 82 |
| 604 | A1 | 675 | 71 | 81 | 94 |
| 503 | A2 | 493 | 58 | 74 | 84 |
| 502 | A2 | 470 | 68 | 58 | 85 |
| 603 | A2 | 410 | 66 | 67 | 86 |
| Mean ± SD | 62 ± 14 (<0.01) | 75 ± 9 (0.67) | 87 ± 4 (0.73) | ||
| Stage B | |||||
| 307 | B2 | 283 | 62 | 50 | 82 |
| BW001 | B3 | 411 | 64 | 61 | 56 |
| 228 | B3 | 194 | 21 | 32 | 71 |
| 355 | B3 | 121 | 20 | 16 | 28 |
| Mean ± SD | 42 ± 25 (<0.02, 0.13) | 40 ± 20 (<0.02, <0.003) | 59 ± 23 (0.11, <0.01) | ||
| Stage C | |||||
| 237 | C2 | 260 | 56 | 30 | 72 |
| 356 | C2 | 257 | 36 | 46 | 54 |
| BW006 | C3 | 258 | 27 | 31 | 46 |
| BW003 | C3 | 462 | 36 | 49 | 68 |
| BW002 | C3 | 149 | 60 | 56 | 58 |
| 354 | C3 | 6 | 48 | 37 | 53 |
| Mean ± SD | 44 ± 12 (<0.0004, <0.05) | 42 ± 10 (<0.0004, <0.0001) | 58 ± 9 (<0.002, <0.00002) | ||
Clinical stage assigned on the basis of lowest-known CD4 count using the classification of reference 6. Results for patient samples 502, 503, 604, and 605 were previously published (58).
Freshly isolated PBMC and PBMC incubated overnight with (+) or without (−) 600 IU of IL-2/ml were analyzed by flow cytometry for CD8 and CD3ζ.
PHD, P value compared to results for healthy donors; PA, P value compared to results for stage A donors.
ND, not determined.
Flow cytometry.
For external staining, freshly isolated PBMC (2 × 105 to 10 × 105/tube) in 50 μl of fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline with 2% fetal calf serum and 0.02% NaN3) were stained with 2 μl each of mixtures of the following antibodies: CD4-Cy-Chrome (monoclonal antibody [MAb] RPA-T4; Pharmingen, San Diego, Calif.), CD8-phycoerythrin (PE) (MAb B9.11; Immunotech, Westbrook, Maine), CD8-Cy5 (MAb B9.11; Immunotech), CD20-PE (MAb B9E9; Immunotech), CD3-Cy5 (MAb UCHT1; Immunotech), CD28-PE (MAb CD28.2; Immunotech), HLA-DR–PE (MAb 357; Immunotech), CD38-PE (MAb T16; Immunotech), CD57-PE (MAb NC1; Immunotech), and immunoglobulin G-fluorescein isothiocyanate (FITC), -PE, and -Cy5 isotype-matched controls (Immunotech). After incubation for 15 min at 4°C, washed cells were permeabilized using the Caltag Laboratories (Burlingame, Calif.) Fix and Perm kit according to the manufacturer's protocol and stained for 15 min at room temperature with 1 μl of CD3ζ-FITC (MAb 6B10.2; Santa Cruz Biotechnology, Santa Cruz, Calif.). Washed cells in FACS buffer plus 2% formaldehyde were analyzed on a tightly gated lymphocyte population using FACscalibur (Becton Dickinson). Gates for external markers were defined by requiring that <1% of the control antibody-stained cells be positive. Gates for internal markers were determined using CD20-staining cells as an internal negative control. Direct staining for CD3ζ was used to facilitate costaining for cell surface markers. The mean fluorescence intensity (MFI) for CD3ζ staining with FITC-conjugated antibody is significantly less than that obtained with indirect staining; however, it is possible, as previously described (58), to set the CD3ζ gates using CD20+ B cells as internal negative controls. When data were analyzed for median fluorescence intensity, results did not differ by more than 1 from values obtained for means.
Tetramer staining.
Bir A-modified HLA-A2 heavy chain and β2 microglobulin were synthesized and purified from plasmids (obtained from M. Davis and D. C. Wiley, respectively) and refolded with an A2-restricted HIV Gag epitope peptide (SLYNTVATL) (39) or an A2-restricted reverse transcriptase (RT) epitope (YTAFTIPSI) (51) to produce tetramers as described previously (1). For tetramer staining, 2 × 106 PBMC from A2-expressing seropositive subjects were resuspended in 500 μl of FACS buffer and stained with 0.5 μg of streptavidin-PE-conjugated tetramer/ml for 40 min at 4°C. Cells were then washed, stained, and analyzed for FITC-CD3ζ or FITC-CD28 and CD8-Cy5 as described above.
Analysis of apoptosis by TUNEL assay.
Cells were resuspended in 50 μl of Hanks balanced salt solution (HBSS), stained for Cy5-CD8, washed, and fixed and permeabilized and resuspended in 25 μl of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) label and 2.5 μl of TUNEL enzyme (Boehringer Mannheim, Philadelphia, Pa.). The negative control for nonspecific staining was cells incubated with TUNEL label without TUNEL enzyme. Positive controls for apoptosis included overnight stimulation with 1 ng of phorbol 12-myristate 13-acetate (PMA) and 250 ng of A23187 (Sigma, St. Louis, Mo.)/ml. Cells were incubated at 37°C in the dark for 1 h before being washed with 5 ml of HBSS and resuspended in 50 μl of FACS buffer with 1% formaldehyde. Samples were analyzed by flow cytometry on the FACscalibur (Becton Dickinson) within 24 h.
T-cell activation.
PBMC were stimulated with 1 ng of anti-CD3 MAb (12F6)/ml with and without anti-CD28 MAb L293 (0.5 μg/ml; Becton Dickinson) and cultured overnight at 2 × 106/ml in T-cell medium (25) before staining for CD8-Cy5 and CD3ζ-FITC with CD69-PE (MAb L78; Becton Dickinson) or CD25-PE (MAb 2A3; Becton Dickinson).
Cytokine production.
Freshly isolated or cryopreserved PBMC (3 × 106) enriched for CD28− T cells were activated with 10 ng of anti-CD3 and 1 ng of PMA (Sigma) in T-cell medium with 10 μM brefeldin A (Sigma) (12, 63). After overnight incubation, harvested cells were stained for Cy5-CD8 and, for some samples, FITC-CD28 or FITC-CD38. Samples were fixed and permeabilized as described above, stained with FITC-conjugated anti-CD3ζ (if not previously stained with anti-CD28 or anti-CD38) and 5 μl of PE–IFN-γ MAb (25723.11) or PE–IL-2 MAb (5334.2) (R&D Systems, Minneapolis, Minn.). Control samples were stained with PE-MslgG1 (R&D Systems).
Statistical analysis.
The statistical significance of correlations was evaluated by a two-sided Student t test; a P value less than 0.05 was considered significant.
RESULTS
CD3ζ is down-modulated in HIV-specific CD8 T cells.
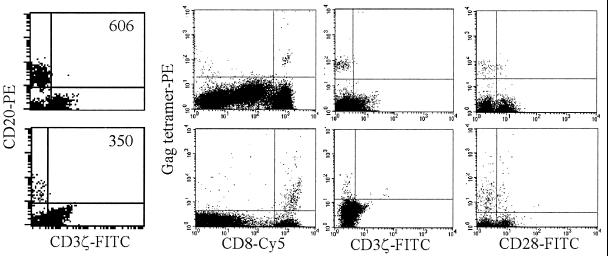
To determine whether CD3ζ down-modulation is a feature of HIV-specific CD8 T cells, PBMC from five HLA A2.1-expressing HIV-seropositive donors were stained for CD8 and for binding to the streptavidin-PE-conjugated HIV Gag peptide (SLVNTVATL) or RT peptide (YTAFTIPSI) A2.1 tetramer (1, 39, 51) and then fixed and permeabilized for staining with FITC-conjugated CD3ζ. (Tetramer binding is not expected to interfere with the binding of antibody to CD3ζ, since the tetramer binds to the outermost surface of the TCR, while the CD3ζ antibody recognizes an intracytoplasmic determinant of CD3ζ.) Representative flow cytometry analyses for two HIV-infected subjects are shown in Fig. 1 and Table 2. Most (88% ± 8%) of the tetramer+ CD8 T cells do not express any CD3ζ above background. Because HIV-infected patients have an expansion of CD28− CD8 T cells, we also costained samples from these subjects for CD28 and A2.1-restricted HIV peptide tetramers. The proportion of CD28− CD8 T cells was comparable to the proportion of CD3ζ− CD8 T cells; 85% ± 6% of tetramer+ CD8 T cells were also CD28−. Therefore, the majority of antigen-specific CD8 T cells have down-modulated both the principal transducing protein for the TCR and the principal costimulatory signaling molecule.
FIG. 1.
HIV-specific CD8 T cells, stained with tetramers, mostly have down-modulated CD3ζ and CD28. Shown are representative flow cytometry dot plots of PBMC from stage A HIV-infected donors 606 (top) and 350 (bottom) costained with HIV A2.1 Gag tetramers and CD8, CD3ζ, or CD28. The dot plots in the two left columns are tightly gated on the total lymphocyte population; in the two right columns, plots represent data for CD8bright lymphocytes. CD3ζ staining of CD20+ B cells was used to set the gate for CD3ζ.
TABLE 2.
Summary of quantitative flow cytometry results for five donors
| Subject | Tetramer | % Tetramer+ cells that are:
|
|
|---|---|---|---|
| CD3ζ− | CD28− | ||
| 219 | Gag | 79 | 81 |
| 350 | Gag | 93 | 88 |
| 606 | Gag | 99 | 89 |
| 701 | Gag | 83 | 91 |
| 703 | RT | 84 | 78 |
| Mean ± SD | 88 ± 8 | 85 ± 6 | |
Although the proportion of all CD8 T cells in these subjects that are CD3ζ− CD28− is high, the proportion of CD3ζ− CD28− cells in the HIV-specific tetramer+ subset is even higher (data not shown). For example, the proportion of all CD8 T cells in these five patients that are CD28− is 68% ± 11% (range, 53 to 83%), compared with 85% ± 6% of the tetramer+ subset. CD28− CD8 T cells probably include other HIV-specific cells that recognize other epitopes, as well as previously activated CD8 T cells that recognize other pathogens.
CD3ζ is down-modulated in activated and memory T cells.
To determine if CD3ζ down-modulation occurs only in certain phenotypically defined subsets of CD8 T cells in HIV infection, we stained PBMC from healthy and HIV-seropositive donors for CD3ζ, CD8, and another T-cell marker using three-color flow cytometry. In 13 HIV-seropositive donors, CD3ζ expression was disproportionately reduced in CD28−, HLA-DR+, and CD62L− CD8 T cells (Fig. 2; Table 3). CD28+ CD8 T cells stained with CD3ζ at an MFI of 13 ± 3, whereas the MFI of CD28− CD8 T cells was 9 ± 2 (P < 0.0000003). Similar differences were seen for CD3ζ expression on CD62L high-, medium-, and low-expression populations (CD62L high, 14 ± 3; CD62L medium, 12 ± 2; CD62L low, 11 ± 2; P < 0.008 and P < 0.0002). On the other hand, CD8 T cells expressing the activation marker HLA-DR had a CD3ζ MFI of 9 ± 2 whereas HLA-DR− CD8 T cells had an MFI of 11 ± 2 (P < 0.0008). This suggests that activated and/or memory CD8 T cells have reduced CD3ζ expression. Differences of CD3ζ expression were found to be not significant when cells were analyzed by CD45RA expression. This may reflect the fact that CD45RA stains both naive T cells and previously activated effector CTL (15, 44).
FIG. 2.
CD3ζ staining of gated CD8 T cells from a representative HIV-infected donor, costained for CD28, HLA-DR, and CD62L. Shaded histograms, CD3ζ staining profiles of cells expressing markers; open histograms, CD3ζ staining of cells that do not express markers. CD3ζ is down-modulated in CD28−, HLA-DR+, and CD62L− CD8 T cells.
TABLE 3.
CD3ζ MFI is reduced in activated and memory CD8 T-cell subsetsa
| Phenotypic marker | Healthy donor
|
HIV-seropositive donor
|
P (sero+ vs sero−)b | ||
|---|---|---|---|---|---|
| n | CD3ζ MFI (P) | n | CD3ζ MFI (P) | ||
| HLA-DR+ | 7 | 12 ± 2 | 13 | 10 ± 2 | 0.01 |
| HLA-DR− | 16 ± 1 (<0.00001) | 12 ± 2 (<0.00008) | 0.00005 | ||
| CD28+ | 7 | 17 ± 2 | 13 | 14 ± 2 | 0.01 |
| CD28− | 11 ± 3 (<0.000004) | 10 ± 2 (0.00001) | 0.3 | ||
| CD62L high | 7 | 18 ± 3 | 13 | 14 ± 3 | 0.03 |
| CD62L medium | 15 ± 3 (0.00002) | 12 ± 2 (0.008) | 0.01 | ||
| CD62L low | 15 ± 3 (<0.00002) | 11 ± 2 (0.00002) | 0.002 | ||
| CD45RA+ | 3 | 18 ± 2 | 6 | 13 ± 2 | 0.02 |
| CD45RA− | 14 ± 2 (0.05) | 12 ± 1 (0.08) | 0.09 | ||
T cells from both HIV-seropositive and seronegative donors that correspond to memory (CD62 medium and low, CD45RA−) and activated (CD28− HLA-DR+) phenotypes have significantly decreased CD3ζ MFI. MFI values were obtained by direct staining with FITC-CD3ζ, Cy5-CD8, and PE-conjugated antibodies to CD62L, HLA-DR, CD28, and CD45RA. The P values in parentheses represent the statistical analysis of the differences in CD3ζ MFI when cells expressing the activation marker in each group (healthy donor or HIV-seropositive donor) are compared to cells that do not. P values are calculated by a paired two-sided t test.
Sero+, seropositive; sero−, seronegative.
Activated CD8 T cells from healthy donors also have down-modulated CD3ζ.
Surprisingly, similar differences were found when CD3ζ expression was correlated with activation phenotype in seven healthy-donor samples (Table 3). Although the proportion of circulating activated and memory CD8 T cells is much lower in HIV-seronegative healthy donors than in HIV-seropositive donors, HLA DR+ CD28− CD62L− CD8 T cells from healthy-donor PBMC had significantly lower CD3ζ expression than CD8 T cells with a naive-cell phenotype. If anything the differences in CD3ζ expression were more striking in healthy-donor samples. This probably reflects the fact that cells that do not express any one activation marker are truly naive in healthy donors, whereas in HIV-seropositive donors there is considerable diversity in phenotypic subsets (44). For example, cells that are HLA-DR− in healthy donors are likely to be CD28+ CD62L+ CD45RA+ as well, whereas in HIV-infected donors a substantial fraction of HLA-DR− CD8 T cells are not naive and express some of the activation markers.
CD8 T cells with down-modulated CD3ζ expression are almost exclusively CD28−.
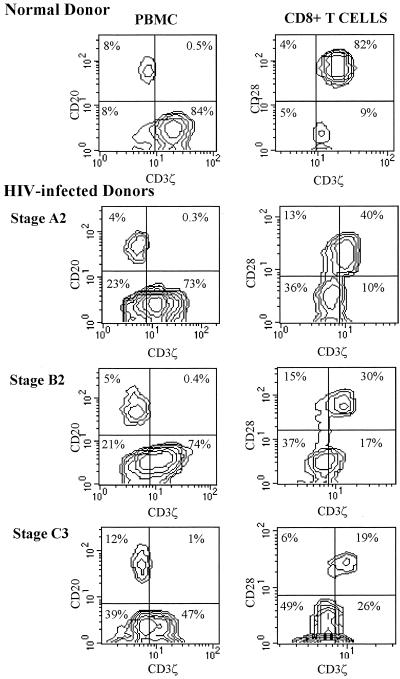
CD28 expression appears to correlate most closely with CD3ζ. CD8 T cells that are CD28−, whether from healthy or HIV-infected donors, uniformly have reduced expression of CD3ζ (Fig. 3). However, most CD28− T cells from healthy donors have reduced but not absent ζ-chain expression, while in the CD28− CD8 T-cell population from HIV-infected donors there is a mixture of CD3ζdim cells and truly CD3ζ− cells, in which staining is comparable to that of CD3ζ− B cells. Although both HIV-seropositive and seronegative donors have some CD28− CD8 T cells, this population is greatly expanded in HIV-seropositive subjects. In nine healthy donors, 22% ± 8% of CD8 T cells were CD28− (range, 11 to 39%) compared with a mean of 57% ± 14% (range, 32 to 83%) in 24 HIV-seropositive samples (P < 0.0000001). Although the expansion of CD28− CD8 T cells tends to increase with disease progression, the correlation with CD4 count or disease stage was not statistically significant for this sample.
FIG. 3.
Representative flow cytometry contour plots of PBMC (left column) or gated CD8 T cells (right column) from a healthy donor and three HIV-seropositive donors at indicated disease stages. Quadrant markers were set using CD20+ B cells (y axis) as internal negative controls for background CD3ζ staining (x axis) as shown in the left plot for each subject. CD28 and CD3ζ staining of CD8 T cells from HIV-seropositive and healthy donors demonstrates the coincident down-modulation of CD3ζ and CD28 and the increase in doubly down-modulated CD8 T cells with HIV infection and disease progression. CD3ζ is dimly expressed, but present above background, in the CD28− subset in the healthy donor.
Expression of CD28 and that of CD3ζ are not directly linked.
The strong correlation between CD28 and CD3ζ expression led us to ask whether expression of these two proteins is directly linked. CD3ζ expression by CD8 T cells from asymptomatic HIV-infected individuals normalizes after 8 to 10 h of incubation at 37°C in high concentrations of IL-2 (58). However, in five samples studied, there was no change in the proportion of CD28-expressing CD8 T cells after overnight incubation in the presence or absence of IL-2 even though CD3ζ levels increased significantly (data not shown). In one representative subject (subject 228), 17% of freshly isolated CD8 T cells were positive for CD28. After overnight incubation in medium alone, 21% of CD8 T cells were CD28+ while in medium containing 600 IU of IL-2/ml 18% were CD28+. However, the percentage of CD8 T cells expressing CD3ζ increased from 21% in freshly isolated T cells to 32% after overnight incubation in medium to 71% in the presence of 600 IU of IL-2/ml. This finding suggests that different pathways may be involved in controlling CD28 and CD3ζ expression.
Apparent CD3ζ reexpression after overnight incubation with IL-2 might have been due to preferential apoptosis of the CD3ζ-down-modulated T cells. To verify that this was not the case, we analyzed PBMC from two HIV-infected individuals (subjects 356 and 237) for apoptosis using TUNEL staining. PBMC were incubated overnight in medium alone or in medium supplemented with 600 IU of IL-2/ml. Cells incubated with PMA plus the Ca+2 ionophore A23187, which stimulates activation-induced apoptosis, were stained as a positive control. Although the proportion of CD3ζ-expressing cells increased as expected in the presence of IL-2, there was no significant difference in the proportion of TUNEL+ apoptotic CD8 T cells when IL-2 was added (data not shown). Therefore, it is not likely that preferential apoptosis of CD3ζ− cells can explain the up-regulation of CD3ζ staining after IL-2 exposure.
CD8 T cells from late-stage HIV-infected individuals do not up-regulate CD3ζ expression after incubation with IL-2.
To determine whether up-regulation of CD3ζ expression might be impaired in advanced disease, we compared in vitro changes in CD8 T-cell CD3ζ expression in PBMC samples from 3 healthy donors and from 18 HIV-infected individuals at various stages of disease (Table 1). CD8 T cells from healthy donors showed a slight decrease in T-cell CD3ζ expression after overnight incubation in medium alone, which could be reversed by adding exogenous IL-2. PBMC from seven of eight donors experiencing primary HIV infection or with asymptomatic stage A disease had partially restored CD3ζ expression after overnight incubation in the absence of exogenous IL-2. We previously showed that this is due primarily to endogenous production of IL-2, since it can be blocked by adding IL-2 antibody (58). Restoration of CD3ζ expression to a level comparable to that of healthy-donor PBMC occurred after overnight incubation with 600 IU of IL-2/ml in all eight subjects. T cells from stage B patients had little change in the percentages of CD3ζ+ CD8+ T cells after overnight incubation in medium alone. This was a statistically significant change compared with the results for stage A donors (P < 0.003). In the presence of IL-2, CD8 T cells from two of four subjects tested were able to restore CD3ζ expression to near normal levels. However, for six individuals with stage C disease, overnight incubation in medium had little effect on CD3ζ levels and IL-2 only partially restored CD8 T-cell ζ-chain expression (Table 1). The differences between stage A and C patients as to baseline CD3ζ expression and reexpression in the absence or presence of IL-2 were all highly significant. Therefore, loss of CD3ζ expression becomes irreversible even with exogenous cytokines around the time of progression to AIDS.
CD3ζ-down-modulated T cells do not express the high-affinity IL-2 receptor upon TCR-mediated activation.
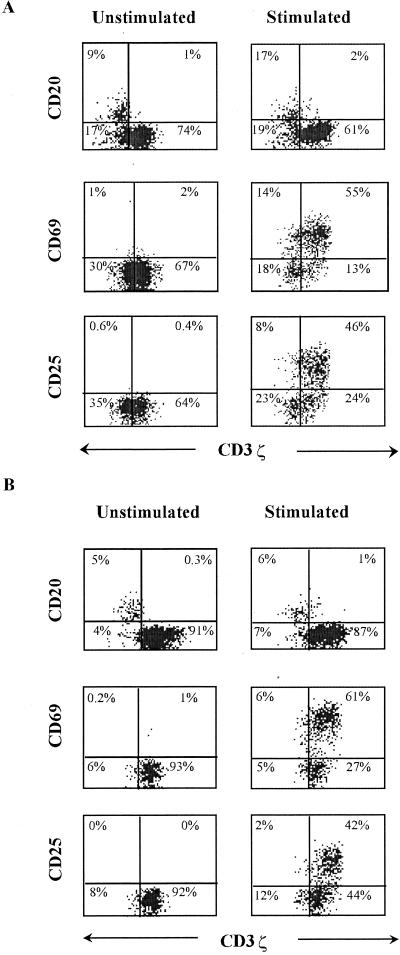
Since phosphorylation of the ζ-chain is the most important proximal event in TCR-mediated signal transduction (65), the down-modulation of CD3ζ should alter activation of these cells via the TCR, perhaps as a way to regulate excessive cell-mediated lysis. This presumed decrease in TCR signaling should be exacerbated by the lack of CD28 expression on the CD3ζ-down-modulated T cells, since CD28 engagement is an important costimulatory signal. In fact, Stefanova and colleagues have shown aberrant signaling in T cells in HIV infection (54). To test whether CD3ζ down-modulation interferes with activation through the TCR, we stimulated PBMC from five healthy donors and five HIV-infected donors with a suboptimal concentration (1 ng/ml) of anti-CD3ɛ antibody 12F6 and 1 μg of anti-CD28/ml and measured the induction of activation markers CD69 and CD25 the next day. (CD3ɛ and the other components of the TCR-CD3 complex are not down-modulated when CD3ζ is [58].) Data from a representative healthy donor and an HIV-infected donor are shown in Fig. 4. In both types of donors, CD69 expression was induced in cells with both normal and down-modulated CD3ζ expression, although the level of CD69 cell surface staining was somewhat reduced in the down-modulated T cells. However, CD25, the α-chain of IL-2R required for high-affinity binding, was induced only on CD3ζbright cells. Moreover, the CD3ζ MFI of cells that were activated to express CD69 or CD25 was significantly higher than that for CD8 T cells that were not activated for both healthy and HIV-infected donors. CD8 T cells from five HIV-seropositive donors with induced CD69 expression stained for CD3ζ with an MFI of 14 ± 1, while CD8 T cells that were not induced had a CD3ζ MFI of 12 ± 2 (background MFI of B cells was 6 ± 1). This difference was statistically significant (P < 0.005). CD25 expression also developed only in T cells that had increased levels of CD3ζ expression (ζ-chain MFI of CD25+ CD8 T cells was 14 ± 2 versus the CD25− CD8 T cell MFI of 11 ± 1; P < 0.006).
FIG. 4.
CD8 T cells with down-modulated CD3ζ from an HIV-infected donor (patient 605) (A) or a healthy donor (B) do not express cell surface CD25 after CD3/CD28 cross-linking. PBMC were enriched for CD28− T cells by partial immunomagnetic depletion of CD28+ T cells and incubated overnight with or without activating antibodies before staining for CD69, CD25, and CD3ζ. CD3ζ staining of CD3ζ− CD20+ B cells was used to set the gate for CD3ζ. The CD20 dot plots were gated on the total lymphocyte population by forward and side scattering; the CD69 and CD25 dot plots were obtained after gating on CD8bright lymphocytes.
Similar results were found for activation of healthy-donor PBMC. After TCR activation, five healthy donors also had significantly higher CD3ζ expression in CD69+ CD8 T cells (MFI, 15 ± 2) than in CD69− CD8 T cells (MFI, 12 ± 2; P < 0.0001) and higher expression in CD25+ CD8 T cells (MFI, 16 ± 2) than in CD25− CD8 T cells (MFI, 12 ± 2; P < 0.006). The B-cell background CD3ζ MFI was 7 ± 1 for the healthy-donor samples. Levels of induction of CD69 and CD25 expression were similar when cells were activated with 1 ng of anti-CD3/ml without anti-CD28 (data not shown). These results show that at least some of the signaling changes in CD3ζ-down-modulated CD8 T cells are not specific to HIV infection but rather occur as part of a normal immune response.
Because there is a greater proportion of CD3ζ− CD28− T cells in more-advanced patients, we also examined the proportion of CD8 T cells, which fail to express CD25 when activated, in HIV-infected patients and healthy controls (Table 4). About 70% of healthy-donor CD8 T cells are activated by 1 ng of anti-CD3/ml and 0.5 μg of anti-CD28/ml to express CD69 the next day. About two-thirds of these (44% ± 2%) coexpress CD25. In five stage A donor samples, the proportion of CD69+ CD8 T cells activated to express CD69 was somewhat higher (77%; not significant) and the proportion activated to express CD25 was significantly higher (61% ± 8%; P < 0.02). In two patients with advanced disease, however, although a comparable number of CD8 T cells expressed CD69 (59%), the proportion that expressed CD25 was only 18% ± 6% (P < 0.006 compared with healthy donors). This substantial reduction in CD25 expression supports the hypothesis that there is more-pronounced CD8 T-cell anergy in more-advanced patients.
TABLE 4.
CD8 T-cell activation after stimulation with anti-CD3 and anti-CD28a
| HIV status and donor | % of CD8 T cells expressing:
|
|
|---|---|---|
| CD69 | CD25 | |
| HIV seronegative | ||
| SM | 67.0 | 44.0 |
| RSF | 66.1 | 41.5 |
| MEP | 76.1 | 45.4 |
| Mean ± SD | 69.7 ± 5.5 | 43.6 ± 2.0 |
| Stage A | ||
| 9013 | 73.5 | 52.0 |
| 9010 | 86.4 | 54.2 |
| 6080 | 80.7 | 58.4 |
| 431 | 79.7 | 68.2 |
| 605 | 64.3 | 71.1 |
| Mean ± SD | 76.9 ± 8.4 | 60.8 ± 8.5 |
| Stage B or C | ||
| BW005 | 54.5 | 13.2 |
| BW004 | 62.6 | 22.2 |
| Mean ± SD | 58.6 ± 5.7 | 17.7 ± 6.4b |
The proportion of activated CD8 T cells that express CD25 is markedly reduced in late-stage HIV-infected subjects compared to healthy uninfected donors or stage A HIV-infected donors.
Significantly different compared to results for healthy donors (P < 0.01) and stage A donors (P < 0.002).
CD3ζ-down-modulated T cells produce IFN-γ but not IL-2 or IL-4.
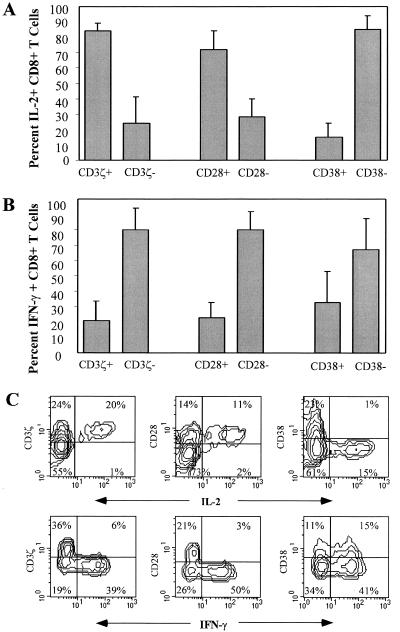
We next looked at cytokine production on a single-cell basis with flow cytometry after maximal overnight stimulation of PBMC from nine HIV-infected donors with PMA and anti-CD3ɛ in the presence of brefeldin A (Fig. 5). Cells were costained with CD8 and either CD3ζ, CD28, or CD38 in addition to IL-2, IFN-γ, and IL-4. Flow cytometry analysis for gated CD8 T cells from a representative HIV-infected donor sample is shown in Fig. 5C. There was no significant IL-4 production by CD8 T cells in any of the samples (data not shown). IL-2 was produced almost exclusively by cells with a naive phenotype (CD3ζ+ CD28+ CD38−). In samples from nine donors, 84% ± 5% of the IL-2-producing CD8 T cells were CD3ζ+ and 72% ± 12% were CD28+. In contrast, the CD3ζ− CD28− population was primarily responsible for IFN-γ production; 80% ± 14% of the IFN-γ-producing CD8 T cells were CD3ζ− and 80% ± 12% were CD28−. There was no clear association of CD38 expression with IFN-γ production. Although fewer cells were activated when samples were stimulated with anti-CD3ɛ and anti-CD28 in place of PMA and anti-CD3ɛ, the same pattern of cytokine production was seen (data not shown).
FIG. 5.
CD3ζ− CD28− CD8+ T cells produce IFN-γ but not IL-2. PBMC from six to nine HIV-seropositive subjects at various disease stages were activated overnight with anti-CD3 and PMA in the presence of brefeldin A and analyzed for IL-2 (A) or IFN-γ (B) production and CD3ζ, CD28, and CD38 staining. (C) Contour plots for representative subject BW004.
DISCUSSION
We recently found that freshly isolated circulating CD8 T cells from HIV-infected donors have limited ability to lyse HIV-infected target cells but become highly cytolytic after overnight culture in the presence of IL-2 (58). In looking for a molecular basis for the lack of cytotoxic function, we found that a large fraction of CD8 T cells in HIV-infected donors have down-modulated the principal signaling chain of the TCR-CD3 complex, as has been reported for tumor-infiltrating lymphocytes in mice and humans (11, 29, 33, 36). We also found that CD3ζ became up-regulated in parallel with the return to cytotoxicity. Here we show that HIV-specific CD8 T cells that stain with HIV peptide epitope tetramers have almost uniformly down-modulated not only CD3ζ but also the principal costimulatory receptor CD28. We previously found that the circulating tetramer+ cells are likely armed to kill since they stain for granzyme A (52). Therefore the lack of HIV-specific cytotoxicity is probably due to aberrant signaling.
CD3ζ down-modulation is a feature of cells that have been previously activated, as evidenced by HLA-DR expression and lack of CD28 and CD62L expression. Because the CD3ζ− CD28− phenotype typically represents 20 to 80% of circulating CD8 T cells in HIV-infected patients (Table 1), the CD3ζ− CD28− population probably includes not only HIV-specific T cells but also cells specific for other pathogens.
CD3ζ down-modulation correlates closely with CD28 down-modulation in CD8 T cells. Previous studies have shown, and their results are confirmed here by tetramer staining, that the expanded CD28− CD8 T-cell subpopulation in HIV infection contains most of the HIV-specific CTL (10, 62). However, the CD8 T-cell suppressor factor CAF is produced by CD28+ T cells and is therefore not a product of antigen-specific cells (3). Previous studies have also shown that activation of CD28− T cells induces IFN-γ production but not that of IL-2 and that these cells have reduced proliferative capacity (5, 23, 28, 53). Consistent with this is our finding that cells that produce IFN-γ upon TCR activation are predominantly CD3ζ− CD28−, while cells that produce IL-2 are predominantly CD28+ CD3ζ+. The reduced proliferative capacity of these cells may be linked to their failure to produce IL-2 and to express the high-affinity IL-2 receptor upon activation.
What is the functional significance of dual down-modulation of CD3ζ and CD28? Although CD3ζ is not detectable above background staining in a large proportion of CD8 (but not CD4) T cells in HIV infection, there is probably some CD3ζ present since antibodies to CD3ɛ stimulated CD69 expression and IFN-γ production by CD3ζ− cells. Estimates of the numbers of TCR molecules that need to be engaged for CD8 T-cell activation go down to as few as one molecule per cell, well below the sensitivity of flow cytometry detection (56). Since the CD3ζ− cells require IL-2 exposure to up-regulate CD3ζ before they are cytotoxic (58), the dually down-modulated cells are partially anergic; they produce IFN-γ but are not cytotoxic and do not produce IL-2. Therefore, the threshold for triggering cytotoxicity may be higher than that needed to activate CD69 expression or to trigger IFN-γ production. That the parallel down-regulation of CD3ζ and cytotoxicity is a major factor in the lack of immune surveillance in cancer and HIV seems likely but is a matter of conjecture.
CD3ζ down-modulation, which increases with disease progression and which interferes with T-cell activation, is corrected within 6 to 10 h of in vitro culture in an IL-2-dependent manner (58). However, development of cytotoxic function in vitro requires a longer incubation time (16 to 24 h) than that required for reexpression of CD3ζ (data not shown). Therefore the lack of cytolytic function is not simply due to CD3ζ down-modulation. Reversal of modifications of the key TCR-associated tyrosine kinases Lck, Fyn, and ZAP70, which have been described in HIV infection (56), may be required before cytotoxicity can be triggered.
We do not know what induces CD3ζ and CD28 down-modulation in CD8 T cells in HIV infection, cancer, and autoimmunity. However, in this study we found that in healthy donors approximately 5 to 10% of CD8 T cells have down-modulated both CD3ζ and CD28. Moreover, the down-modulated cells behave after TCR stimulation like their counterparts in HIV infection. This suggests that CD3ζ and CD28 down-modulation may be a normal consequence of CD8 T-cell activation and not an aberration secondary to chronic immune stimulation or HIV infection. In support of this, we recently found a massive expansion of CD3ζ− CD28− CD8 T cells in three patients undergoing acute viral infections of diverse etiologies (57). These cells, like the comparable subset in HIV infection, were effector CTL that produced IFN-γ but not IL-2 and were not cytotoxic until cultured in high concentrations of IL-2. Like the down-modulated cells in HIV-seropositive donors, upon TCR activation they failed to express CD25. Moreover, corroborative results were found in a mouse green fluorescent protein model recently developed to study CD8 T-cell differentiation (32). In the mice, CD3ζ mRNA is decreased in effector CD8 CTL. The mouse effector cells also had limited proliferative capacity, produced IFN-γ but not IL-2, and, in fact, mostly underwent apoptosis upon TCR activation unless they were provided with IL-2.
These findings taken together suggest that CD3ζ and CD28 down-modulation is a normal correlate of CD8 T-cell activation. We suspect that it serves two purposes: (i) to prevent immunopathology due to cytolysis of bystander cells that may express self peptide-HLA pairs with low affinity for the TCR and (ii) to insure that CD8 T cells are dependent on T-cell help for their function and proliferation. Lack of IL-2 production and high-affinity IL-2R expression means that the activated CTL are likely dependent on exogenous cytokines not only for survival but also for cytolytic function. This finding is consistent with a recent study of murine lymphocytic choriomeningitis virus infection in which functional antigen-specific CD8 T cells did not provide antiviral protection in mice depleted of CD4 T cells (66). In HIV infection, the paucity of HIV-specific CD4 helper T-cell function is also likely to be an important contributor to HIV-specific CTL dysfunction (18, 58).
Our finding of partial anergy in the antiviral CD8 T-cell compartment may also be true of the HIV response of CD4 T cells. A CD4 proliferative response to HIV antigens is generally not detectable above background except in some long-term nonprogressors or in some instances after treatment (30, 31, 46, 47, 49). This finding led investigators to assume that HIV-specific CD4 T cells are absent in most HIV-infected individuals. However, a recent study showed that in most HIV-infected patients CD4 memory cells that produce IFN-γ in response to stimulation with the HIV Gag protein are readily detectable by flow cytometry (41). This suggests that the HIV-specific CD4 T-cell response is not absent but partially anergized. It may be that the absence of a proliferative response to HIV antigens seen in most patients reflects a lack of IL-2 secretion, similar to what we find for CD8 T cells. IL-2 production is likely required for CD4 T-cell proliferation in response to an antigen and may be needed for effective help for CD8 CTL function.
Based on this study and results with acute viral infection (57) and with the mouse green fluorescent protein model (32), we propose a working model for CD8 T-effector-cell differentiation outlined in Fig. 6. After TCR activation, CD8 T cells, unlike their CD4 counterparts, down-modulate expression of CD3ζ and CD28. The dually down-modulated cells secrete IFN-γ upon repeat exposure to antigens but are not cytotoxic unless they encounter antigens in proximity to IL-2-secreting antigen-specific CD4 helper cells. In that case they upregulate CD3ζ expression, become cytotoxic, and proliferate. If they reencounter antigens in the absence of specific helper cells, the CD8 T cells, although they have the molecular machinery for cytolysis, are not cytolytic and do not proliferate.
FIG. 6.
Working model for regulation of CD8 T-cell-effector function. Following the initial TCR activation of a naive CD8 T cell, CD3ζ and CD28 are down-modulated and cytotoxic granule proteins are expressed as part of normal CD8 T-cell differentiation. If the CD8 T cell encounters antigens on infected cells in the absence of specific helper cells, the effector CD8 T cell can produce IFN-γ but is not cytotoxic and does not proliferate. In the presence of antigen-specific CD4 T cells, the CD8 T cell up-regulates CD3ζ but not CD28, becomes cytolytically competent, and proliferates.
We previously found that the defect in CD3ζ expression is more pronounced in HIV-infected patients with more advanced immunodeficiency (58). This is corroborated by reference 13 and the study of additional patients reported here. We now find that IL-2 does not restore in vitro CD3ζ expression on CD8 T cells in advanced patients and that the defect in expression of the high-affinity IL-2 receptor after T-cell activation is more profound in advanced patients. This suggests that antigen excess, prolonged chronic antigenic stimulation, or particular HIV gene products (such as Tat) (6, 55) might result in additional alterations in the signaling pathway or transcription factors that are important in normal CD8 CTL development. This finding may also be a contributing factor to the lack of response to IL-2 therapy in more-advanced patients (20). Global defects in CD3ζ expression on T and NK cells and profound signaling defects have been found in tumor-infiltrating cells in mice and humans with advanced cancers (21, 34). A recent study of tetramer-staining melanoma-specific CD8 T cells in patients with metastatic melanoma found that melanoma-specific CD8 T cells in these patients were profoundly anergic (22). Although they were perforin+, they were not cytotoxic against their specific targets. In addition, specific CD8 T cells from these patients did not express CD69 or produce cytokines after specific antigenic exposure. In more recent work (P. Shankar, M. Russo, B. Harnisch, M. Patterson, P. R. Skolnik, and J. Lieberman, submitted for publication), we also found that IFN-γ production in response to HIV-infected primary T cells or by HIV tetramer+ cells is compromised in more-advanced HIV-infected donors. In some donors, the IFN-γ production defect, like the defect in cytotoxicity, can be reversed by supplying IL-2 in vitro. Our results suggest that a similarly profound anergy might be found in HIV-infected patients with AIDS and might reflect derangements of immune regulation associated with chronic antigenic excess. The functional properties of HIV-specific T cells in more-advanced patients require further study.
ACKNOWLEDGMENTS
We thank Chiron Oncology for recombinant human IL-2, M. Davis and D. Wiley for plasmids used to express A2.1 and β2 microglobulin to produce tetramers, Rachel Friedman, Melissa Russo, and Zhan Xu for expert technical assistance, and N. Manjunath for useful discussions.
J.L. was supported by National Institutes of Health grants 42519 and 45406 from the National Institute for Allergy and Infectious Diseases.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Andersson J, Behbahani H, Lieberman J, Connick E, Landay A, Patterson B, Sonnerborg A, Lore K, Uccini S, Fehniger T E. Perforin is not co-expressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. AIDS. 1999;13:1295–1303. doi: 10.1097/00002030-199907300-00005. [DOI] [PubMed] [Google Scholar]
- 3.Barker E, Bossart K N, Fujimura S H, Levy J A. CD28 costimulation increases CD8+ cell suppression of HIV replication. J Immunol. 1997;159:5123–5131. [PubMed] [Google Scholar]
- 4.Borthwick N J, Bofill M, Gombert W M, Akbar A N, Medina E, Sagawa K, Lipman M C, Johnson M A, Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. Acta Chem Scand. 1994;8:431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Caruso A, Licenziati S, Canaris A D, Cantalamessa A, Corulli M, Benzoni B, Peroni L, Balsari A, Turano A. Characterization of T cell subsets involved in the production of IFN-gamma in asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses. 1996;12:135–141. doi: 10.1089/aid.1996.12.135. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1993;41:1–19. [PubMed] [Google Scholar]
- 7.Chirmule N, Than S, Khan S A, Pahwa S. Human immunodeficiency virus Tat induces functional unresponsiveness in T cells. J Virol. 1995;69:492–498. doi: 10.1128/jvi.69.1.492-498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 9.Cossarizza A, Ortolani C, Mussini C, Borghi V, Guaraldi G, Mongiardo N, Bellesia E, Franceschini M G, De Rienzo B, Franceschi C. Massive activation of immune cells with an intact T cell repertoire in acute human immunodeficiency virus syndrome. J Infect Dis. 1995;172:105–112. doi: 10.1093/infdis/172.1.105. [DOI] [PubMed] [Google Scholar]
- 10.Dalod M, Fiorentino S, Delamare C, Rouzioux C, Sicard D, Guillet J G, Gomard E. Delayed virus-specific CD8+ cytotoxic T lymphocyte activity in an HIV-infected individual with high CD4+ cell counts: correlations with various parameters of disease progression. AIDS Res Hum Retroviruses. 1996;12:497–506. doi: 10.1089/aid.1996.12.497. [DOI] [PubMed] [Google Scholar]
- 11.Finke J H, Zea A H, Stanley J, Longo D L, Mizoguchi H, Tubbs R R, Wiltrout R H, O'Shea J J, Kudoh S, Klein E, et al. Loss of T-cell receptor zeta chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 12.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 13.Geertsma M F, van Wengen-Stevenhagen A, van Dam E M, Risberg K, Kroon F P, Groeneveld P H P, Nibbering P H. Decreased expression of ζ molecules by T lymphocytes is correlated with disease progression in human immunodeficiency virus-infected persons. J Infect Dis. 1999;180:649–658. doi: 10.1086/314941. [DOI] [PubMed] [Google Scholar]
- 14.Gougeon M L, Laurent-Crawford A G, Hovanessian A G, Montagnier L. Direct and indirect mechanisms mediating apoptosis during HIV infection: contribution to in vivo CD4 T cell depletion. Semin Immunol. 1993;5:187–194. doi: 10.1006/smim.1993.1022. [DOI] [PubMed] [Google Scholar]
- 15.Hamann D, Baars P A, Rep M H, Hooibrink B, Kerkhof-Garde S R, Klein M R, van Lier R A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho H N, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C C, O'Rourke S, Taylor J M, Giorgi J V. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 17.Hoffenbach A, Langlade-Demoyen P, Dadaglio G, Vilmer E, Michel F, Mayaud C, Autran B, Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–462. [PubMed] [Google Scholar]
- 18.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammerer R, Iten A, Frei P C, Burgisser P. Expansion of T cells negative for CD28 expression in HIV infection. Relation to activation markers and cell adhesion molecules, and correlation with prognostic markers. Med Microbiol Immunol. 1996;185:19–25. doi: 10.1007/s004300050010. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs J A, Baseler M, Dewar R J, Vogel S, Davey R T, Jr, Falloon J, Polis M A, Walker R E, Stevens R, Salzman N P, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N Engl J Med. 1995;332:567–575. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 21.Lai P, Rabinowich H, Crowley-Nowick P A, Bell M C, Mantovani G, Whiteside T L. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 22.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 23.Lewis D E, Tang D S, Adu-Oppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 24.Lieberman J. Cytotoxic T lymphocyte adoptive immunotherapy for HIV infection. In: Sitkovsky M V, Henkart P A, editors. Cytotoxic cells: basic mechanisms and medical applications. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. p. 441. [Google Scholar]
- 25.Lieberman J, Fabry J A, Kuo M C, Earl P, Moss B, Skolnik P R. Cytotoxic T lymphocytes from HIV-1 seropositive individuals recognize immunodominant epitopes in Gp160 and reverse transcriptase. J Immunol. 1992;148:2738–2747. [PubMed] [Google Scholar]
- 26.Lieberman J, Fabry J A, Shankar P, Beckett L, Skolnik P R. Ex vivo expansion of HIV type 1-specific cytolytic T cells from HIV type 1-seropositive subjects. AIDS Res Hum Retroviruses. 1995;11:257–271. doi: 10.1089/aid.1995.11.257. [DOI] [PubMed] [Google Scholar]
- 27.Liossis S N, Ding X Z, Dennis G J, Tsokos G C. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosis. J Clin Investig. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd T E, Yang L, Tang D N, Bennett T, Schober W, Lewis D E. Regulation of CD28 costimulation in human CD8+ T cells. J Immunol. 1997;158:1551–1558. [PubMed] [Google Scholar]
- 29.Loeffler C M, Smyth M J, Longo D L, Kopp W C, Harvey L K, Tribble H R, Tase J E, Urba W J, Leonard A S, Young H A, et al. Immunoregulation in cancer-bearing hosts. Down-regulation of gene expression and cytotoxic function in CD8+ T cells. J Immunol. 1992;149:949–956. [PubMed] [Google Scholar]
- 30.Lori F, Jessen H, Lieberman J, Finzi D, Rosenberg E, Tinelli C, Siliciano R F, Walker B, Lisziewicz J. Treatment of human immunodeficiency virus infection with hydroxyurea, didanosine, and a protease inhibitor before seroconversion is associated with normalized immune parameters and limited viral reservoir. J Infect Dis. 1999;180:1827–1832. doi: 10.1086/315113. [DOI] [PubMed] [Google Scholar]
- 31.Lori F, Rosenberg E, Lieberman J, Foli A, Maserati R, Seminari E, Alberici F, Walker B, Lisziewicz J. Hydroxyurea and didanosine long-term treatment prevents HIV breakthrough and normalizes immune parameters. AIDS Res Hum Retroviruses. 1999;15:1333–1338. doi: 10.1089/088922299310034. [DOI] [PubMed] [Google Scholar]
- 32.Manjunath N, Shankar P, Stockton B, Dubey P D, Lieberman J, von Andrian U H. A transgenic mouse model to analyze CD8+ effector T cell differentiation in vivo. Proc Natl Acad Sci USA. 1999;96:13932–13937. doi: 10.1073/pnas.96.24.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massaia M, Attisano C, Beggiato E, Bianchi A, Pileri A. Correlation between disease activity and T-cell CD3 zeta chain expression in a B-cell lymphoma. Br J Haematol. 1994;88:886–888. doi: 10.1111/j.1365-2141.1994.tb05133.x. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda M, Petersson M, Lenkei R, Taupin J-L, Magnusson I, Mellstedt H, Anderson P, Kiessling R. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995;61:765–772. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- 35.Maurice M M, Lankester A C, Bezemer A C, Geertsma M F, Tak P-P, Breedveld F C, van Lier R A W, Verweij C L. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. J Immunol. 1997;159:2973–2978. [PubMed] [Google Scholar]
- 36.Mizoguchi H, O'Shea J J, Longo D L, Loeffler C M, McVicar D W, Ochoa A C. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 37.Moss P A, Rowland-Jones S L, Frodsham P M, McAdam S, Giangrande P, McMichael A J, Bell J I. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann A U, Tubiana R, Calvez V, Robert C, Li T S, Agut H, Autran B, Katlama C. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. AIDS. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 39.Nixon D F, Townsend A R, Elvin J G, Rizza C R, Gallwey J, McMichael A J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 40.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 42.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 43.Renner C, Ohnesorge S, Held G, Bauer S, Jung W, Pfitzenmeier J P, Pfreundschuh M. T cells from patients with Hodgkin's disease have a defective T-cell receptor zeta chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- 44.Roederer M, De Rosa S C, Watanabe N, Herzenberg L A. Dynamics of fine T-cell subsets during HIV disease and after thymic ablation by mediastinal irradiation. Semin Immunol. 1997;9:389–396. doi: 10.1006/smim.1997.0097. [DOI] [PubMed] [Google Scholar]
- 45.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Investig. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg E S, LaRosa L, Flynn T, Robbins G, Walker B D. Characterization of HIV-1-specific T-helper cells in acute and chronic infection. Immunol Lett. 1999;66:89–93. doi: 10.1016/s0165-2478(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 48.Saukkonen J J, Kornfeld H, Berman J S. Expansion of a CD8+CD28− cell population in the blood and lung of HIV-positive patients. J Acquir Immune Defic Syndr. 1993;6:1194–1204. [PubMed] [Google Scholar]
- 49.Schwartz D, Sharma U, Busch M, Weinhold K, Matthews T, Lieberman J, Birx D, Farzedagen H, Margolick J, Quinn T, et al. Absence of recoverable infectious virus and unique immune responses in an asymptomatic HIV+ long-term survivor. AIDS Res Hum Retroviruses. 1994;10:1703–1711. doi: 10.1089/aid.1994.10.1703. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility class I molecules is induced by the HIV-Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 51.Shankar P, Sprang H, Lieberman J. Effective lysis of HIV-1-infected primary CD4+ T cells by a cytotoxic T-lymphocyte clone directed against a novel A2-restricted reverse-transcriptase epitope. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:111–120. doi: 10.1097/00042560-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 52.Shankar P, Xu Z, Lieberman J. Viral-specific cytotoxic T lymphocytes lyse HIV-infected primary T lymphocytes by the granule exocytosis pathway. Blood. 1999;94:3084–3093. [PubMed] [Google Scholar]
- 53.Sousa A, Victorino R. Single-cell analysis of lymphokine imbalance in asymptomatic HIV-1 infection: evidence for a major alteration within the CD8+ T cell subset. Clin Exp Immunol. 1998;112:294–302. doi: 10.1046/j.1365-2249.1998.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanova I, Saville M W, Peters C, Cleghorn F R, Schwartz D, Venzon D J, Weinhold K J, Jack N, Bartholomew C, Blattner W A, Yarchoan R, Bolen J B, Horak I D. HIV infection-induced posttranslational modification of T cell signaling molecules associated with disease progression. J Clin Investig. 1996;98:1290–1297. doi: 10.1172/JCI118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanyam M, Gutheil W G, Bachovchin W W, Huber B T. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993;150:2544–2553. [PubMed] [Google Scholar]
- 56.Sykulev Y, Joo M, Vturina I, Tsomides T J, Eisen H N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 57.Trimble, L. A., L. W. Kam, R. S. Friedman, Z. Xu, and J. Lieberman. CD3ζ and CD28 down-modulation on CD8 T cells during viral infection. Blood, in press. [PubMed]
- 58.Trimble L A, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3ζ, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–594. [PubMed] [Google Scholar]
- 59.Tsomides T J, Aldovini A, Johnson R P, Walker B D, Young R A, Eisen H N. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanham G, Kestens L, Gigase P, Colebunders R, Vandenbruaene M, Brijs L, Ceuppens J L. Evidence for circulating activated cytotoxic T cells in HIV-infected subjects before the onset of opportunistic infections. Clin Exp Immunol. 1990;82:3–9. doi: 10.1111/j.1365-2249.1990.tb05395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vingerhoets J, Kestens L, Penne G, De Vuyst H, Vandenbruaene M, Pelgrom Y, Bosmans E, de Boer M, Kasran A, Azuma M, Colebunders R, Ceuppens J L, Vanham G. CD8+ cells and not CD4+ T cells are hyporesponsive to CD28- and CD40L-mediated activation in HIV-infected subjects. Clin Exp Immunol. 1997;107:440–447. doi: 10.1046/j.1365-2249.1997.d01-964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vingerhoets J H, Vanham G L, Kestens L L, Penne G G, Colebunders R L, Vandenbruaene M J, Goeman J, Gigase P L, De Boer M, Ceuppens J L. Increased cytolytic T lymphocyte activity and decreased B7 responsiveness are associated with CD28 down-regulation on CD8+ T cells from HIV-infected subjects. Clin Exp Immunol. 1995;100:425–433. doi: 10.1111/j.1365-2249.1995.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Investig. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weekes M P, Carmichael A J, Wills M R, Mynard K, Sissons J G. Human CD28−CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J Immunol. 1999;162:7569–7577. [PubMed] [Google Scholar]
- 65.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 66.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]