Abstract
Background
To investigate whether melatonin supplementation can enhance cardiometabolic risk factors, reduce oxidative stress, and improve hormonal and pregnancy-related factors in patients with PCOS.
Methods
We conducted a systematic search of PubMed/Medline, Scopus, and the Cochrane Library for articles published in English from inception to March 2023. We included randomized controlled trials (RCTs) on the use of melatonin for patients with polycystic ovary syndrome (PCOS). We performed a meta-analysis using a random-effects model and calculated the standardized mean differences (SMDs) and 95% confidence intervals (CIs).
Results
Six studies met the inclusion criteria. The result of meta-analysis indicated that melatonin intake significantly increase TAC levels (SMD: 0.87, 95% CI: 0.46, 1.28, I2 = 00.00%) and has no effect on FBS, insulin, HOMA-IR, TC, TG, HDL, LDL, MDA, hs-CRP, mFG, SHBG, total testosterone, and pregnancy rate in patients with PCOS compare to controls. The included trials did not report any adverse events.
Conclusion
Melatonin is a potential antioxidant that may prevent damage from oxidative stress in patients with PCOS. However, the clear effect of melatonin supplementation on cardiometabolic risk factors, hormonal outcomes, and pregnancy-related outcomes needs to be evaluated further in large populations and long-term RCTs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-024-01450-z.
Keywords: Melatonin, PCOS, Cardiometabolic risk factors, Hormones, Pregnancy
Highlights
Melatonin supplementation increases total antioxidant capacity (TAC) levels in PCOS patients, suggesting its potential as an antioxidant therapy
Meta-analysis reveals no significant impact of melatonin on cardiometabolic risk factors, hormonal levels, or pregnancy rate in PCOS patients compared to controls
Melatonin intake shows no adverse events in PCOS patients, indicating its safety for supplementation
Further large-scale and long-term randomized controlled trials are needed to determine the full effects of melatonin supplementation in PCOS patients
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-024-01450-z.
Introduction
Polycystic ovarian syndrome (PCOS), a common endocrine disorder affecting a significant number of women before menopause, is mainly recognized by reproductive irregularities, increased androgen levels, and disrupted ovulation, impacting around 10% of premenopausal females [1]. Although the precise pathophysiology of PCOS is still not fully understood, evidences point to hyperandrogenism as having a major impact on this illness [2, 3]. The aberrant inflammatory reaction of ovarian theca cells to free oxygen radicals may lead to hyperandrogenism [4]. Furthermore, studies has indicated that PCOS is characterized by an ongoing condition of persistent mild inflammation and oxidative stress, which is strongly associated with further clinical and metabolic irregularities [5]. It is currently thought that PCOS is a condition characterized by oxidative stress and lower levels of antioxidants [6]. The presence of common insulin resistance and dyslipidemia can result in an overabundance of reactive oxygen species (ROS) due to endoplasmic reticulum stress and lipid peroxidation [7]. The activation of redox-sensitive transcription factors can be affected by oxidative stress, while the presence of lipid peroxidation might exacerbate the effects of PCOS and interfere with the regulation of glucose and lipid metabolism [8]. The development of PCOS in individuals experiencing oxidative stress might be associated with a lack of antioxidants, such as melatonin (N-acetyl-5-methoxytryptamine) [9].
The pineal gland is responsible for the primary release of melatonin [10]. Controlling sleep patterns and adjusting circadian rhythms are primary functions of the periodic release of melatonin into the bloodstream [11], and participation in the immunological response of the body [12]. Melatonin is a potent radical scavenger and an endogenous antioxidant [13]. dditionally, it plays a significant role in anti-inflammatory activities [14]. According to current studies, it appears that melatonin plays a role in different ovarian activities such as the growth of follicles, the functioning of the corpus luteum, the production of steroids, and the maturation of oocytes [15]. Melatonin appears to protect the oocyte from damage caused by ROS [16].
Moreover, the lack of melatonin appears to contribute to the underlying mechanisms of PCOS. Additionally, melatonin has demonstrated its ability to serve as a reliable indicator of oocyte quality, as a sufficient level of melatonin is positively linked to the appropriate quality of oocytes [17]. A randomized controlled trial revealed that the combined usage of melatonin and myo-inositol leads to a synergistic improvement in the quality of embryos and oocytes in women diagnosed with PCOS, who are undergoing in vitro fertilization (IVF), when compared to using myo-inositol alone [18]. Furthermore, it was found that the administration of melatonin over a duration of 12 weeks led to decreased concentrations of C-reactive protein (hs-CRP) and plasma malondialdehyde (MDA) among women diagnosed with PCOS. Simultaneously, melatonin supplementation increased total antioxidant capacity (TAC) levels and glutathione (GSH) levels. Additionally, melatonin was observed to reduce the expression of interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) genes [19]. However, there is no systematic review and meta-analysis that summarizes the effect of melatonin in patients with PCOS. Therefore, a systematic review was conducted to evaluate the effects of melatonin on cardiometabolic risk factors, oxidative stress, inflammatory factors, and hormonal profiles in women diagnosed with PCOS.
Methods
Search strategy
This systematic review and meta-analysis was undertaken based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Cochrane handbook for systematic reviews of interventions [20]. Two authors independently searched various electronic databases, including MEDLINE, Scopus, and the Cochrane Library, from inception to April 2023. The following search terms were used in this systematic review: “Melatonin” OR “Pineal hormone” and terms related to PCOS (including MeSH search using “Polycystic Ovary Syndrome”, “PCOS” and terms related to study design (such as “Randomized controlled trial”, “Controlled Clinical Trial”, “Randomized”, “Randomly”, “Placebo”, “Trial”). The searches conducted were restricted to humans only, but not restricted based on the language. Additionally, the references of original published articles and reviews, as well as input from experts, were also explored during the search process. The complete search strategy, key terms, and syntaxes for searching each individual database are presented in Supplementary File 1.
Inclusion and exclusion criteria inclusion criteria
The studies met the inclusion criteria if they had the following characteristics: (I) The research was conducted as a crossover or parallel randomized controlled trial (RCT). (II) The participants in the research were females with polycystic ovary syndrome (PCOS) of any age. (III) The intervention group was given melatonin alone or in combination with other treatments, while the control group received no treatment or only other treatments. (IV) The primary outcome of the article consists of at least one of the following factors: anthropometric indices such as weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), and glycemic parameters such as fasting blood sugar (FBS), insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and lipid profile parameters including total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), as well as oxidative stress and inflammatory indicators such as total antioxidant capacity (TAC), malondialdehyde (MDA), and high-sensitivity C-reactive protein (hs-CRP), and finally hormonal and pregnancy-related factors such as total testosterone, sex hormone-binding globulin (SHBG), Modified Ferriman–Gallwey Score (mFG), endometrial thickness, and pregnancy rate. The process involved excluding literature reviews, observational studies, case reports, and molecular and animal studies. Following this, duplicate studies were removed, and two reviewers individually assessed the remaining studies for inclusion based on their titles, abstracts, and full texts if necessary.
Data extraction and quality appraisal
Two reviewers extracted data separately, including study details and primary results. In case of any inconsistencies, they resolved them by discussing with a third author. Additionally, information such as the country of origin, dose and duration of melatonin intake, as well as patient-related details like age and the number of patients, were also gathered. The collected data included the means, the standard deviation of those means, and the number of participants in each group. If precise data was not provided, such as with graphs or bar charts, we requested unpublished data from the author. If we could not obtain this information, we used a digital ruler to estimate the data from the graphs or charts. The included studies were appraised and graded independently by two reviewers according to the Cochrane risk of bias evaluation tool [21].
Meta-analytic methods
We performed meta-analyses utilizing Stata software version 17.0 (Stata Corp, College Station, TX, USA). We assessed the influence of melatonin intervention using the standardized mean difference (SMD) of the variables of interest. The results for continuous variables were presented as effect sizes along with confidence intervals (CIs). A random-effects model was used to calculate the effect sizes of the variables of interest and a meta-analysis was conducted. We considered data from intention to-treat analyses. The heterogeneity of the study was investigated using the Chi-square test of homogeneity (p < 0.05) in addition to the I2 statistic. A level of I2 ≥ 50% was considered indicative of a high level of heterogeneity.
Results
Study selection
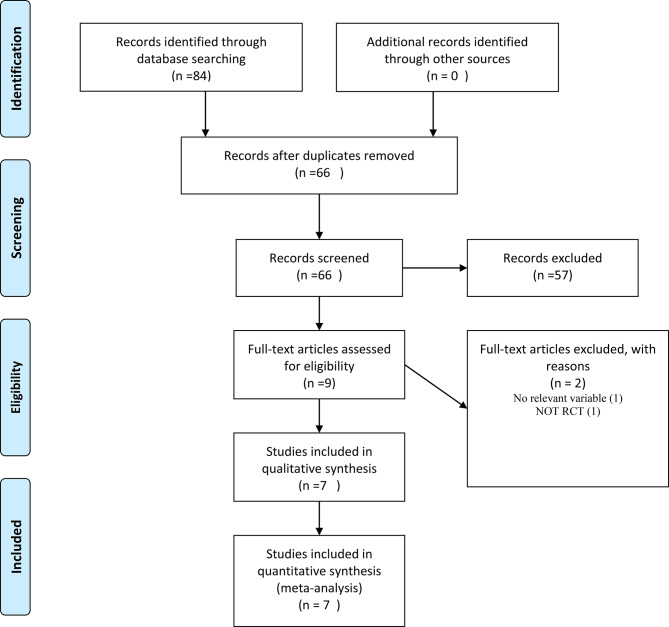
The primary search identified 84 articles from searched databases. After duplicate removal, there were 66 records for screening of title and abstract. Nine full texts were obtained for final screening, and out of those nine, three were excluded because they did not evaluate relevant variables (n = 2) and were not RCTs (n = 1). Finally, six trials met the inclusion criteria for this systematic review and meta-analysis [5, 18, 19, 22–24]. The PRISMA flow diagram of the studies selection is presented in Fig. 1.
Fig. 1.
PRISMA flow diagram of study selection
Study characteristics
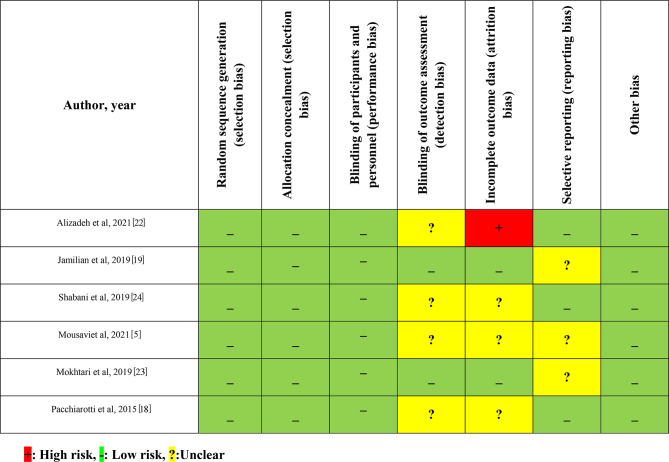
The included studies characteristics and findings are shown in Table 1. All six studies, which were included in this systematic review, were double-blind RCTs published between 2015 and 2021. These studies were conducted in Iran and Italy. The trials that were included had 1006 participants who were administered melatonin orally, with doses ranging from 3 to 10 mg/day. The age of participants varied between 25 and 31 years, and their BMI ranged from 22 to 29 kg/m2, while the duration of the intervention lasted between 3 and 12 weeks. All the included trials were evaluated as being of good quality, and the report of the quality appraisal for these trials is presented in Fig. 2. Included studies did not provide any information on adverse events.
Table 1.
Main characteristics of included studies
| Study (ref) | Country | Sample size | Melatonin dosage (mg/day) | Duration (week) |
Age (years) | BMI (kg/m2) | Main outcome§ | ||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Mean ± SD |
Placebo Mean ± SD |
Intervention Mean ± SD |
Placebo Mean ± SD |
||||||
| Alizadeh et al., 2021 [22] | Iran | 84 | 6 | 8 | 25.57 ± 4.99 | 26.20 ± 5.72 | 28.40 ± 3.86 | 26.94 ± 3.83 | ↑HDL, ↔BMI, ↔WC, ↔FBS, ↔Ins, ↔HOMA-IR, ↔HOMA-B, ↔TC, ↔TG, ↔LDL, ↔Total Testosterone, ↔SHBG, ↔mFG |
| Jamilian et al., 2019 [19] | Iran | 56 | 10 | 12 | 28.7 ± 2.1 | 28.3 ± 2.3 | 29.1 ± 4.6 | 29.2 ± 3.5 |
↓mFG, ↓Total Testosterone, ↓hs-CRP, ↓MDA, ↑TAC, ↑GSH |
| Shabani et al., 2019 [24] | Iran | 58 | 10 | 12 | 26.5 ± 3.5 | 26.0 ± 3.3 | 27.1 ± 4.6 | 27.8 ± 4.7 | ↓Ins, ↓HOMA-IR, ↓TC, ↓LDL,↑QUICKI, ↔FBS, ↔TG, ↔VLDL |
| Mousaviet al, 2021 [5] | Iran | 84 | 6 | 8 | 25.57 ± 4.99 | 26.20 ± 5.70 | 28.40 ± 3.86 | 26.94 ± 3.83 | ↑TAC, ↔mFG, ↔hs-CRP, ↔MDA |
| Mokhtari et al., 2019 [23] | Iran | 198 | 3 | 3 | 28.4 ± 5.5 | 29.3 ± 5.6 | 27.6 ± 4.0 | 28.1 ± 3.7 | ↑Chemical pregnancy, ↑Endometrial thickness |
| Pacchiarotti et al., 2015 [18] | Italy | 526 | 3 | 3 | 31.2 ± 2.1 | 31.5 ± 2.8 | 22.8 ± 1.3 | 23.1 ± 1.7 | ↑oocyte and embryo quality, |
symbol is a sign of decreasing variables in the intervention group
↑This symbol is a sign of increasing variables in the intervention group
↔This sign indicates that there is no difference between the two groups. NR: not reported
§INS: Insulin, HOMA-IR: homeostatic model assessment-insulin resistance, HOMA-B: homeostatic model assessment-Beta cell function, QUICKI: quantitative insulin sensitivity check index, TG: triglycerides, VLDL: Very Low Density Lipoproteins, HDL: High Density Lipoproteins, LDL: Low Density Lipoproteins, TC: Total Cholesterol, FBS: Fasting blood Sugar, hs-CRP: high-sensitivity C-reactive protein, MDA: Malondialdehyde, TAC: Total Antioxidant Capacity, GSH: glutathione, NO: nitric oxide, SHBG: sex hormone-binding globulin, FSH: Follicle-Stimulating Hormone, LH: Luteinizing Hormone
Fig. 2.
Risk of bias assessment of included trials [5, 18, 19, 22–24]
Meta-analysis
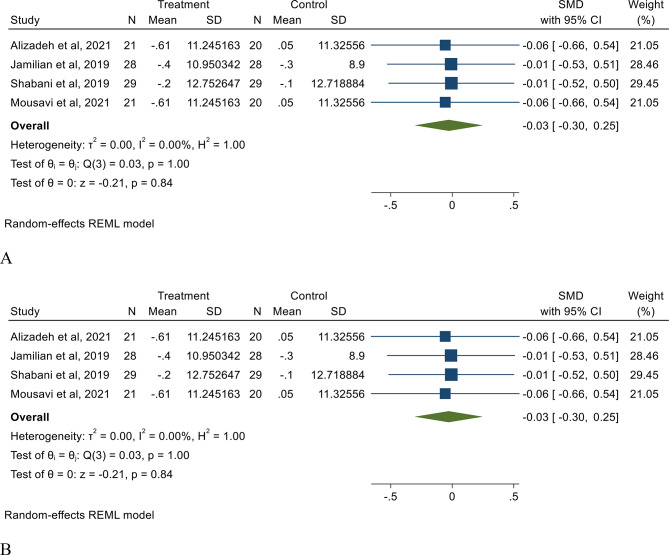
The results of random-effect meta-analysis indicated that melatonin intake has no effect on weight (SMD: −0.03, 95% CI: −0.30, 0.25, I2 = 00.00%) and BMI (SMD: −0.03, 95% CI: −0.30, 0.25, I2 = 00.00%) in patients with PCOS (Fig. 3.). The results of subgroup and sensitivity analysis showed no significant difference. The result of meta-analysis also indicated that melatonin intake has no effect on FBS, insulin, HOMA-IR, TC, TG, HDL, LDL, MDA, and hs-CRP. Our meta-analysis indicated that melatonin intake significantly increase TAC levels (SMD: 0.87, 95% CI: 0.46, 1.28, I2 = 00.00%) compare to placebo (Table 2). Additionally, while the impact of melatonin intake on mFG, SHBG, total testosterone, endometrial thickness, and pregnancy rate was not statistically significant, a marginal decrease in total testosterone and a slight increase in endometrial thickness were detected (Table 2). We investigate the source of heterogeneity in our meta-analysis by performing a sensitivity analysis. The results of the sensitivity analysis are presented in Supplementary File 2.
Fig. 3.
The effect of melatonin intake on weight (A), and BMI (B) in PCOS patients compare to placebo
Table 2.
Meta-analysis of effect of melatonin in PCOS patients
| Variable | effect size SMD |
95% CI | I2 (%) | P for heterogeneity | |
|---|---|---|---|---|---|
| Glycemic parameters | FBS | -0.15 | -0.54, 0.24 | 00.00 | 0.450 |
| Insulin | -0.35 | -0.99, 0.28 | 60.99 | 0.280 | |
| HOMA-IR | -0.33 | -0.93, 0.27 | 56.29 | 0.280 | |
| Lipid Profile | TC | -0.04 | -0.60, 0.52 | 50.66 | 0.890 |
| TG | -0.01 | -0.38, 0.39 | 00.00 | 0.980 | |
| HDL | 0.06 | -0.33, 0.45 | 00.00 | 0.770 | |
| LDL | -0.07 | -0.53, 0.39 | 27.45 | 0.760 | |
| Oxidative Stress and Inflammation | TAC | 0.87 | 0.46, 1.28* | 00.00 | 0.000 |
| MDA | 0.08 | -0.91, 1.08 | 83.62 | 0.870 | |
| hs-CRP | -0.22 | -0.61, 0.17 | 00.00 | 0.270 | |
| Hormonal and pregnancy outcomes | mFG | 0.15 | -0.54, 0.24 | 00.00 | 0.460 |
| SHGB | 0.10 | -0.29, 0.49 | 00.00 | 0.620 | |
| Total Testosterone | -0.25 | 0.65, 0.14 | 00.00 | 0.210 | |
| Endometrial thickness | 0.23 | 0.53, 0.99 | 95.01 | 0.550 | |
| RR | 95% CI | I 2 (%) | P for heterogeneity | ||
| Pregnancy Rate | 0.30 | -0.16, 0.77 | 56.60 | 0.200 |
SMD: Standard mean difference, CI: confidence interval, FBS: Fasting Blood Sugar, HOMA-IR: homeostatic model assessment-insulin resistance, TC: Total Cholesterol, TG: triglycerides, HDL: High Density Lipoproteins, LDL: Low Density Lipoproteins, hs-CRP: high-sensitivity C-reactive protein, MDA: Malondialdehyde, TAC: Total Antioxidant Capacity, SHBG: sex hormone-binding globulin, mFG: Modified Ferriman–Gallwey Score
*statistically significant
Discussion
Our current systematic review and meta-analysis entails compiling data from six RCTs that investigate the effects of melatonin supplementation on cardiometabolic risk factors, including anthropometric indices, glycemic parameters, lipid profile, oxidative stress, and inflammatory factors, as well as hormonal and pregnancy outcomes, in patients with PCOS. The primary outcome of the present research indicated that melatonin supplementation led to a significant rise in TAC levels in comparison to the placebo group. Nevertheless, there was a slight overall decrease in hs-CRP and total testosterone levels. To the best of our knowledge, this is the first comprehensive evaluation and meta-analysis that examines the effects of melatonin on individuals diagnosed with PCOS. However, there is evidence in the literature from systematic reviews that shows melatonin concentration is reduced in patients with PCOS [25]. It has also been demonstrated that melatonin supplementation can be effective in increasing pregnancy rates in infertile women who undergo ART techniques [26].
The results of the current systematic review and meta-analysis indicate that melatonin supplementation has no effect on glycemic parameters, including FBS, insulin, and HOMA-IR, in patients with PCOS. These findings are not in accordance with previous systematic reviews and meta-analyses regarding the effect of melatonin on glycemic control and diabetes. Previous studies have shown that melatonin can decrease FBS levels [27, 28]. The limited number of trials included in the current systematic review and the differing pathophysiology of PCOS and diabetes may have prevented us from finding a significant effect of melatonin on FBS. However, previous systematic reviews have also supported the notion that melatonin intake does not have an effect on HOMA-IR and insulin levels [27, 28]. The results of the current systematic review and meta-analysis indicate that melatonin intake has no effect on lipid profiles, including TC, TG, HDL, and LDL, in patients with PCOS. Previous systematic review and meta-analyses on the effects of melatonin on lipid profiles have indicated that melatonin can decrease TC and TG levels, but has no effect on HDL and LDL [29]. The results of other systematic reviews in the field indicate that intake of melatonin can decrease levels of LDL and TG, while having no effect on TC and HDL levels [30]. It appears that the controversial outcomes of prior studies and the current systematic review highlight the necessity to assess the impact of melatonin on lipid profile in patients with PCOS through larger and longer-term RCTs.
The results of our systematic review indicate that melatonin supplementation significantly increases TAC levels and has no effect on MDA and hs-CRP in patients with PCOS. These findings are consistent with previous reviews regarding the effect of melatonin on oxidative stress parameters [31]. Based on the available data, it appears that melatonin possesses antioxidant protective properties through both its direct free radical scavenging ability and its indirect antioxidant activity [32, 33]. It has been demonstrated that Melatonin effectively interacts with different reactive oxygen and reactive nitrogen species [34]. Moreover, it upregulates antioxidant enzymes while downregulating pro-oxidant enzymes [35]. It has also been demonstrated that melatonin decreases the generation of ROS through its effect on TNF-α gene expression [36, 37]. TNF-α can increase the expression of NOX by activating NF-κB signaling, thereby inducing the generation of ROS [38, 39]. The production and removal of ROS are primarily influenced by the equilibrium between oxidase and antioxidase. Major sources of ROS include NADPH oxidative enzymes, such as NOX1 and NOX2 [40, 41]. The expression of NADPH oxidative enzymes is upregulated by the presence of inflammatory cytokines, leading to increased proliferation of ROS [42]. The presence of TNF-α led to a significant elevation in the expression of both NOX1 and NOX2 [43]. Recent findings have revealed that melatonin can significantly reduce the production of ROS, which was previously increased due to TNF-α induction [44]. It has also been demonstrated that melatonin can lead to the downregulation of NOX1 and NOX2 expression, as well as the upregulation of primary antioxidant enzymes such as SOD1 and CAT expression [45, 46]. It has also been reported that melatonin can decrease ROS production by suppressing the expression of the NF-kB gene [47].
The results of the current meta-analysis indicate no effect of melatonin supplementation on hormonal and pregnancy outcomes in patients with PCOS. However, there was a marginal trend towards decreasing androgens and increasing endometrial thickness after melatonin intake in women with PCOS. Melatonin is an important hormone that plays a crucial role in regulating the development of follicles in the ovaries [48]. The presence of receptors MT1 and MT2 in the ovarian follicle has been shown by multiple authors, which supports the idea that melatonin has a role in the functioning of the ovaries [49]. It has been demonstrated that the administration of melatonin can increase the synthesis of insulin-like growth factor I (IGF-I), a significant growth factor in granulosa cells that promotes follicular development [50]. Recent research has shown that melatonin at a concentration of 0.1 mM can affect the expression of IGF-I receptors and may induce the development of primary and antral follicles [51]. Recent findings have discovered that melatonin is present in the follicular fluid of preovulatory follicles in humans, and its levels in this fluid are higher than those in serum [52]. The elevated concentrations of melatonin in follicular fluid are believed to be crucial for the growth and appropriate maturation of ovarian follicles, whereas low levels may result in anovulation and inferior oocyte quality in women with PCOS [53].
The role of melatonin is crucial as it serves as an antioxidant, antiapoptotic, and anti-inflammatory factor [31, 54]. It has the potential to protect the oocyte and surrounding cells from oxidative stress damage, thereby preventing follicular atresia [17, 55]. Research findings suggest that oxidative stress can harm granulosa cells, leading to an increased rate of apoptosis and damaging oocyte DNA [56]. Studies also indicate that melatonin treatment resulted in a higher number of antral and primary follicles compared to the control group [57, 58]. Furthermore, administering melatonin to animals before induction of permanent estrus state showed a higher number of ovarian follicles [59].
This study, which is the initial extensive meta-analysis, investigates the impact of melatonin supplementation on factors related to cardiometabolic health, oxidative stress, and hormones in individuals diagnosed with PCOS. Nevertheless, there were certain constraints associated with this research. Primarily, a majority of the trials examined in this meta-analysis consisted of a limited number of participants, and the overall count of studies incorporated was relatively small. Theoretically, this limitation could result in uncertain evaluations of treatment outcomes. Secondly, the included trials were limited to only two specific countries of origin, which limits the generalizability of our findings. Finally, wide heterogeneity was detected between the included trials, and due to the limited number of included studies, we are unable to perform subgroup analysis to deal with this high heterogeneity.
Conclusion
The current meta-analysis demonstrated that the addition of melatonin as a supplement led to a substantial elevation in TAC levels among individuals diagnosed with PCOS. However, it had no significant effects on other cardiometabolic and hormonal factors, as well as pregnancy-related factors in these patients. It seems that large population and long-duration RCTs are still needed to draw a clear conclusion about the effect of melatonin supplementation in patients with PCOS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- SMD
Standard Mean Difference
- CI
Confidence Interval
- FBS
Fasting Blood Sugar
- HOMA-IR
Homeostatic Model Assessment-insulin Resistance
- TC
Total Cholesterol
- TG
Triglycerides
- HDL
High Density Lipoproteins
- LDL
Low Density Lipoproteins
- hs-CRP
High-sensitivity C-reactive Protein
- MDA
Malondialdehyde
- TAC
Total Antioxidant Capacity
- SHBG
Sex Hormone-binding Globulin
- mFG
Modified Ferriman–Gallwey Score
Author contributions
SZ and JH designed the study. MH, ED, and MM collected the data. MH and JH analyzed data. MM and SZ wrote the manuscript. KK contributed to the revision by performing additional and supplementary analysis, and by editing and preparing the entire revision. All authors revised and finalized the report.
Funding
Not applicable.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publish this article in this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baptiste CG, Battista M-C, Trottier A, Baillargeon J-P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2010;122(1–3):42–52. doi: 10.1016/j.jsbmb.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–57. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 3.Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol. 1997;47(1):93–9. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 4.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metabolism. 2006;91(1):336–40. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 5.Mousavi R, Alizadeh M, Asghari Jafarabadi M, Heidari L, Nikbakht R, Babaahmadi Rezaei H, et al. Effects of melatonin and/or magnesium supplementation on biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2022;200(3):1010–9. doi: 10.1007/s12011-021-02725-y. [DOI] [PubMed] [Google Scholar]
- 6.Macut D, Bjekić-Macut J, Savić-Radojević A. Dyslipidemia and oxidative stress in PCOS. Polycystic Ovary Syndrome. 2013;40:51–63. doi: 10.1159/000341683. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10(1):86. doi: 10.4103/ijpvm.IJPVM_576_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göçer H, Akıncıoğlu A, Öztaşkın N, Göksu S, Gülçin İ. Synthesis, antioxidant, and antiacetylcholinesterase activities of sulfonamide derivatives of Dopamine-R elated compounds. Arch Pharm. 2013;346(11):783–92. doi: 10.1002/ardp.201300228. [DOI] [PubMed] [Google Scholar]
- 10.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42(10):1634–50. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15(4):432–7. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 12.Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity: circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986;13(1):19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- 13.Tan D-X, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20(10):18886–906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 2010;8(3):228–42. doi: 10.2174/157015910792246155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanavde V, Maitra A. In vitro modulation of steroidogenesis and gene expression by melatonin: a study with porcine antral follicles. Endocr Res. 2003;29(4):399–410. doi: 10.1081/ERC-120026946. [DOI] [PubMed] [Google Scholar]
- 16.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan D-X, Sugino N, et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92(1):328–43. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Pacchiarotti A, Carlomagno G, Antonini G, Pacchiarotti A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol. 2016;32(1):69–73. doi: 10.3109/09513590.2015.1101444. [DOI] [PubMed] [Google Scholar]
- 19.Jamilian M, Foroozanfard F, Mirhosseini N, Kavossian E, Aghadavod E, Bahmani F, et al. Effects of melatonin supplementation on hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. Front Endocrinol. 2019;10:273. doi: 10.3389/fendo.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group* t. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343. [DOI] [PMC free article] [PubMed]
- 22.Alizadeh M, Karandish M, Asghari Jafarabadi M, Heidari L, Nikbakht R, Babaahmadi Rezaei H, et al. Metabolic and hormonal effects of melatonin and/or magnesium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Nutr Metabolism. 2021;18(1):1–11. doi: 10.1186/s12986-021-00586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokhtari F, Asbagh FA, Azmoodeh O, Bakhtiyari M, Almasi-Hashiani A. Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: a randomized clinical trial. Int J Fertility Steril. 2019;13(3):225. doi: 10.22074/ijfs.2019.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shabani A, Foroozanfard F, Kavossian E, Aghadavod E, Ostadmohammadi V, Reiter RJ, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250:51–6. doi: 10.1016/j.jad.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Liu M, Zhang C. Women with polycystic ovary syndrome (PCOS) have reduced melatonin concentrations in their follicles and have mild sleep disturbances. BMC Womens Health. 2022;22(1):79. doi: 10.1186/s12905-022-01661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu K-L, Ye X, Wang S, Zhang D. Melatonin application in assisted reproductive technology: a systematic review and meta-analysis of randomized trials. Front Endocrinol. 2020;11:160. doi: 10.3389/fendo.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doosti-Irani A, Ostadmohammadi V, Mirhosseini N, Mansournia MA, Reiter RJ, Kashanian M, et al. The effects of melatonin supplementation on glycemic control: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2018;50(11):783–90. doi: 10.1055/a-0752-8462. [DOI] [PubMed] [Google Scholar]
- 28.Delpino FM, Figueiredo LM, Nunes BP. Effects of melatonin supplementation on diabetes: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr. 2021;40(7):4595–605. doi: 10.1016/j.clnu.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37(6):1943–54. doi: 10.1016/j.clnu.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Loloei S, Sepidarkish M, Heydarian A, Tahvilian N, Khazdouz M, Heshmati J, et al. The effect of melatonin supplementation on lipid profile and anthropometric indices: a systematic review and meta-analysis of clinical trials. Diabetes Metabolic Syndrome: Clin Res Reviews. 2019;13(3):1901–10. doi: 10.1016/j.dsx.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Morvaridzadeh M, Sadeghi E, Agah S, Nachvak SM, Fazelian S, Moradi F, et al. Effect of melatonin supplementation on oxidative stress parameters: a systematic review and meta-analysis. Pharmacol Res. 2020;161:105210. doi: 10.1016/j.phrs.2020.105210. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro‐oxidant actions. J Pineal Res. 2014;57(2):131–46. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 33.Sevilla A, Chéret J, Slominski RM, Slominski AT, Paus R. Revisiting the role of melatonin in human melanocyte physiology: a skin context perspective. J Pineal Res. 2022;72(3):e12790. doi: 10.1111/jpi.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu X, Wang X, Qiu J, Zhu Y, Liang T, Gao B et al. Melatonin rescued reactive oxygen species-impaired osteogenesis of human bone marrow mesenchymal stem cells in the presence of tumor necrosis factor-alpha. Stem cells international. 2019;2019. [DOI] [PMC free article] [PubMed]
- 35.Ghosh P, Dey T, Majumder R, Datta M, Chattopadhyay A, Bandyopadhyay D. Insights into the antioxidative mechanisms of melatonin in ameliorating chromium-induced oxidative stress-mediated hepatic and renal tissue injuries in male Wistar rats. Food Chem Toxicol. 2023;173:113630. doi: 10.1016/j.fct.2023.113630. [DOI] [PubMed] [Google Scholar]
- 36.Haddadi GH, Fardid R. Oral administration of melatonin modulates the expression of tumor necrosis factor-α (TNF-α) gene in irradiated rat cervical spinal cord. Rep Practical Oncol Radiotherapy. 2015;20(2):123–7. doi: 10.1016/j.rpor.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tocharus J, Khonthun C, Chongthammakun S, Govitrapong P. Melatonin attenuates methamphetamine-induced overexpression of pro‐inflammatory cytokines in microglial cell lines. J Pineal Res. 2010;48(4):347–52. doi: 10.1111/j.1600-079X.2010.00761.x. [DOI] [PubMed] [Google Scholar]
- 38.Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249–61. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-α. Antioxid Redox Signal. 2009;11(6):1249–63. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metabolism. 2014;25(9):452–63. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, et al. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56(3):490–7. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 42.Taylor JP, Hubert MT. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021;48:102159. doi: 10.1016/j.redox.2021.102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandoval R, Lazcano P, Ferrari F, Pinto-Pardo N, González-Billault C, Utreras E. TNF-α increases production of reactive oxygen species through Cdk5 activation in nociceptive neurons. Front Physiol. 2018;9:65. doi: 10.3389/fphys.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebaid H, Bashandy SA, Abdel-Mageed AM, Al-Tamimi J, Hassan I, Alhazza IM. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr Metabolism. 2020;17:1–14. doi: 10.1186/s12986-019-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Najafi M, Shirazi A, Motevaseli E, Geraily G, Amini P, Shabeeb D, et al. Evaluating the expression of NOX2 and NOX4 signaling pathways in rats’ lung tissues following local chest irradiation; modulatory effect of melatonin. Int J Mol Cell Med. 2018;7(4):220. doi: 10.22088/IJMCM.BUMS.7.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8‐hydroxy‐2’‐deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54(3):303–12. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 47.Colombo J, Jardim-Perassi BV, Ferreira JP, Braga CZ, Sonehara NM, Júnior RP et al. Melatonin differentially modulates NF-кB expression in breast and liver cancer cells. Anti-cancer agents in Medicinal Chemistry (formerly current Medicinal Chemistry-Anti-cancer agents). 2018;18(12):1688–94. [DOI] [PubMed]
- 48.Mojaverrostami S, Asghari N, Khamisabadi M, Khoei HH. The role of melatonin in polycystic ovary syndrome: a review. Int J Reproductive Biomed. 2019;17(12):865. doi: 10.18502/ijrm.v17i12.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo YM, Sun TC, Wang HP, Chen X. Research progress of melatonin (MT) in improving ovarian function: a review of the current status. Aging. 2021;13(13):17930. doi: 10.18632/aging.203231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seymen CM, Sağlam ASY, Elmazoğlu Z, Arık GN, Kaplanoğlu GT. Involvement of endometrial IGF-1R/IGF-1/Bcl-2 pathways in experimental polycystic ovary syndrome: identification of the regulatory effect of melatonin. Tissue Cell. 2021;73:101585. doi: 10.1016/j.tice.2021.101585. [DOI] [PubMed] [Google Scholar]
- 51.Lombardi LA, Mattos LSd, Simões RS, Florencio-Silva R, Sasso GRS, Carbonel AAF, et al. Melatonin may prevent or reverse polycystic ovary syndrome in rats. Revista Da Associação Médica Brasileira. 2019;65:1008–14. doi: 10.1590/1806-9282.65.7.1008. [DOI] [PubMed] [Google Scholar]
- 52.Khan HL, Bhatti S, Khan YL, Abbas S, Munir Z, Sherwani IARK, et al. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J Gynecol Obstet Hum Reprod. 2020;49(1):101624. doi: 10.1016/j.jogoh.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Minguini IP, Luquetti CM, Baracat MCP, Maganhin CC, Nunes CO, Simões RS, et al. Melatonin effects on ovarian follicular cells: a systematic review. Revista Da Associação Médica Brasileira. 2019;65:1122–7. doi: 10.1590/1806-9282.65.8.1122. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz-Jurado A, Escribano BM, Caballero-Villarraso J, Galván A, Agüera E, Santamaría A, et al. Melatonin and multiple sclerosis: antioxidant, anti-inflammatory and immunomodulator mechanism of action. Inflammopharmacology. 2022;30(5):1569–96. doi: 10.1007/s10787-022-01011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Deng H, Jiang Z, Li Q, Shi M, Chen H, et al. Effects of melatonin on follicular atresia and granulosa cell apoptosis in the porcine. Mol Reprod Dev. 2016;83(8):692–700. doi: 10.1002/mrd.22676. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, Shen M, Chen Y, Wei Y, Tao J, Liu H. Melatonin represses mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules. 2021;11(7):968. doi: 10.3390/biom11070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, et al. Melatonin prevents cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO 3a pathway activation in the mouse ovary. J Pineal Res. 2016;60(3):336–47. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 58.Kandemir YB, Aydin C, Gorgisen G. The effects of melatonin on oxidative stress and prevention of primordial follicle loss via activation of mTOR pathway in the rat ovary. Cell Mol Biol. 2017;63(2):100–6. doi: 10.14715/cmb/2017.63.2.16. [DOI] [PubMed] [Google Scholar]
- 59.Mohammadghasemi F, Jahromi SK, Hajizadeh H, Homafar MA, Saadat N. The protective effects of exogenous melatonin on nicotine-induced changes in mouse ovarian follicles. J Reprod Infertility. 2012;13(3):143. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.