Abstract
The gut serves as a vital immunological organ orchestrating immune responses and influencing distant mucosal sites, notably the respiratory mucosa. It is increasingly recognized as a central driver of critical illnesses, with intestinal hyperpermeability facilitating bacterial translocation, systemic inflammation, and organ damage. The “gut-lung” axis emerges as a pivotal pathway, where gut-derived injurious factors trigger acute lung injury (ALI) through the systemic circulation. Direct and indirect effects of gut microbiota significantly impact immune responses. Dysbiosis, particularly intestinal dysbiosis, termed as an imbalance of microbial species and a reduction in microbial diversity within certain bodily microbiomes, influences adaptive immune responses, including differentiating T regulatory cells (Tregs) and T helper 17 (Th17) cells, which are critical in various lung inflammatory conditions. Additionally, gut and bone marrow immune cells impact pulmonary immune activity, underscoring the complex gut-lung interplay. Moreover, lung microbiota alterations are implicated in diverse gut pathologies, affecting local and systemic immune landscapes. Notably, lung dysbiosis can reciprocally influence gut microbiota composition, indicating bidirectional gut-lung communication. In this review, we investigate the pathophysiology of ALI/acute respiratory distress syndrome (ARDS), elucidating the role of immune cells in the gut-lung axis based on recent experimental and clinical research. This exploration aims to enhance understanding of ALI/ARDS pathogenesis and to underscore the significance of gut-lung interactions in respiratory diseases.
Introduction
The intestine represents an expansive mucosal surface, positioning itself as the largest immunological organ, with immune cells distributed throughout both the lamina propria and epithelium of the intestinal mucosa. Playing the role of a major immune organ, it orchestrates immune responses upon confronting antigens and extends its influence to distant mucosal sites, particularly the respiratory mucosa [1].
Moreover, over the last decades, it has been postulated that the gastrointestinal (GI) tract serves as the central driver of critical illness, characterized by intestinal hyperpermeability, which facilitates bacterial translocation through the portal circulation and mesenteric lymphatics, culminating in subsequent systemic infection and damage to distant organs acting as the principal conduit for gut-derived injurious factors which potentially may trigger ARDS [2–4]. This substantiation lies in the lung’s initial encounter with the mesenteric lymph, where it is exposed to a high concentration of lymph contents before dilution by the systemic blood volume. Due to the fact that intestinal lymph enters the systemic circulation via the thoracic duct, which empties into the subclavian vein and subsequently reaches the right heart before being pumped into the pulmonary circulation, the pulmonary vascular bed is the first site to encounter mesenteric lymph [3]. Moreover, damage to the intestinal mucosa can result in the production of harmful substances such as endotoxins, microbial metabolites, and hormones or inflammatory mediators, disrupting the integrity of the gut barrier. Once these substances are absorbed into the mesenteric lymph duct, they enter the bloodstream and activate endothelial [5] and immune cells, ultimately leading to ALI [5, 6].
Various pathways of communication have been recognized within the gut-lung axis. These pathways include direct effects of gut microbiota, illustrated by the enhancement of the host’s immune response through the presence of peptidoglycan and lipopolysaccharide (LPS) [7]. Referring to the complex ecosystems of microorganisms inhabiting the intestinal tract, the term “gut microbiota” encompasses over 1000 types of microorganisms, spanning at least 4000 distinct species [8–11]. Additionally, indirect effects of gut microbiota are observed, such as unmetabolized short-chain fatty acids (SCFAs) influencing the development of immune cells upon entering the peripheral bloodstream [7]. Shaping adaptive immune responses, particularly the development and differentiation of CD4+ and CD8+ T cells, is another role of the gut microbiota. Dysbiosis triggers and activates Tregs, fosters the proliferation and differentiation of Tregs and Th17 cells, resulting in the production of interleukin (IL)-17 by intestinal Th17 cells, and prompts B cells to produce and release secretory immunoglobulin A (IgA) [12, 13]. Recent research indicates that immune cells, including Th17 and Treg cells, which primarily originate from progenitor cells in the bone marrow and among others from the gut, play a central role in various lung inflammatory pathologies such as chronic obstructive pulmonary disease (COPD), ARDS, sarcoidosis, asthma, and pulmonary infectious diseases [14–16]. Additionally, immune cells originating from the bone marrow trigger an immune response in the lungs, while intestinal immune cells migrate directly from the intestinal tract to the lungs via the bloodstream, impacting pulmonary immune activity [7].
Moreover, contrary to the long-standing notion of lung sterility, which has hindered systematic exploration of the lung microbiome and research progress, it is now firmly established that even healthy, asymptomatic individuals undergo colonization of alveoli by microbes [17]. Our knowledge about lung microbiota has been enhanced by improvements in both culture‐dependent and ‐independent techniques, aiding understanding not only of its presence in healthy lung tissue but also its significant impact on immune responses in both physiological and pathological states [18]. Although lung microbiota compositions in healthy individuals remain relatively stable, various clinical pathologies, including idiopathic pulmonary fibrosis (IPF), asthma, COPD, cystic fibrosis, cancer, and ALI/ARDS are associated with numerous patterns of lung dysbiosis [15, 19–25]. Furthermore, it is now well established that alterations in lung microbiota significantly affect both the local pulmonary and systemic landscape of immune cells [26], setting the stage for a feedback loop where local immune cells and microbiota engage in bidirectional communication [27]. Interestingly, alterations in lung microbiota may also influence the composition of gut microbiota [15, 26] in the context of dynamic crosstalk between gut and lung [27].
In the present review, we aim to further clarify the pathophysiology of ALI/ARDS by describing the various effects of immune cells within the gut-lung axis in patients with ARDS based on recent experimental and clinical data. While our focus is on elucidating shared pathophysiological mechanisms that critically influence the gut-lung axis, it is important to note that the variable effects of injury models (direct vs. indirect) and insult types (infectious vs. non-infectious) on disease outcomes and underlying mechanisms should be acknowledged.
The gut-lung axis during critical illness
Intensifying interest in biomedical research has emerged due to the recognition that gut microbiota functions as a vital superorganism and an autonomous organ, impacting various physiological processes in the host [28], including protection against enteric and systemic pathogens [29] by pleiotropic mechanisms as for example maintenance of intestinal epithelial integrity, nutritional competition and, immune system modulation [15, 30–33]. The term “gut microbiota” encompasses over 1000 types of microorganisms and at least 4000 distinct species, referring to the complex communities of commensal and pathogenic microorganisms residing in the intestinal tract [8–11] including bacteria, viruses, fungi, and parasites [34]. Despite normally engaging in a hormonally symbiotic relationship with the host under physiologic conditions, the mammalian gut microbiome undergoes a rapid decline in microbial density, membership composition, and comprehensive community structure and function within hours following a sudden physiological insult [32, 35] termed as “pathobiome” [36].
Emerging experimental and epidemiological evidence has underscored a critical interplay between the intestinal microbiota and the lungs, recognized as the “gut-lung axis” [37]. This concept reflects the anatomical independence of the gut and lungs yet emphasizes the existence of complex interactions in both health and disease. These interactions involve not only the host-microbe relationship but also the crosstalk among different microbial communities, which influence local and systemic immune responses and airway homeostasis [37, 38]. The gut-lung axis functions through both local regulatory mechanisms and distant effects, impacting the progression of respiratory diseases by modulating immune and inflammatory responses compromising the integrity of the intestinal barrier which is associated with bacterial translocation, lung injury, sustained inflammation, and pulmonary fibrosis [37, 39–42].
Gut microbiome
Maintaining gut health heavily depends on the intestinal mucosal barrier, which balances the microbiota through antimicrobial peptides and the coordinated actions of the mucous layer and cell junctions [43]. During critical illnesses, the gut is notably vulnerable to injury, with approximately 50% of intensive care unit (ICU) patients experiencing enterocyte damage [44, 45]. Hypoperfusion and subsequent reperfusion of the intestinal wall, often induced by catecholamine administration to treat shock, sepsis, and robust non-infectious inflammation, can lead to reduced microvascular perfusion, severe mucosal inflammation, increased apoptosis, decreased proliferation of small bowel mucosal cells resulting to the compromise of the integrity of the intestinal mucus layer, decreased absorptive capacity, increased paracellular permeability and, alterations in systemic inflammation [46–52]. Moreover, intensive care settings commonly employ various clinical interventions, including enteral feeding, administration of proton-pump inhibitors, systemic catecholamines, and systemic antibiotics, all of which alter the environmental growth conditions for intestinal bacteria [53].
The mucus layer, an essential anatomical component of gut anatomy, acts as a physical barrier that hosts its own protective microbiota, separating the intestinal ecosystem from the host [53]. The resulting outcome of the aforementioned pathophysiologic alterations is an unstable and often decreased microbial diversity (as few as four species identified in some critically ill patients) [54, 55] with depletion of commensal microbes and proliferation of potentially pathogenic and inflammatory bacteria increasing susceptibility to hospital-acquired infections, sepsis, and multiple organ dysfunction syndrome (MODS) [56–58]. The upper GI tract, typically characterized by sparse microbial populations, becomes dominated by a limited number of species, such as Escherichia coli (E. coli), Enterococcus spp., and Pseudomonas aeruginosa (Ps. aeruginosa) [59, 60]. Moreover, during the first week in the ICU, critically ill patients showed a significantly different composition of intestinal microbiota compared to healthy subjects, with an increase in intestinal Enterobacteriales and Enterobacteriaceae associated with a 92% higher risk of 180-day adjusted mortality [61]. Freedberg and co-workers (2018) highlighted the predominance of pathogens such as E. coli, Pseudomonas species, Klebsiella species, and Clostridium difficile (Cl. difficile) in rectal swabs of 301 adult ICU patients. Additionally, an increased risk of death or all-cause infection was linked to the presence of Enterococcus as the predominant pathogen [62]. Furthermore, in a study of 81 patients with severe systemic inflammatory response syndrome (SIRS), the analysis of gut flora revealed that the complication of enteritis was primarily associated with a reduction in total obligate anaerobes and an increase in Staphylococcus and Enterococcus [63]. Finally, in an experimental study, fecal microbiota from patients with ARDS caused by community-acquired pneumonia (CAP) was transferred to antibiotics-treated recipient male mice, revealing that the intestinal flora of ARDS/CAP patients exhibited higher abundances of Gram-negative bacteria compared to normal controls. Additionally, mice that received fecal transplants from ARDS/CAP patients exhibited increased systemic LPS, systemic inflammation, and intestinal permeability. Interestingly, the altered gut microbiota from ARDS/CAP patients resulted in neuroinflammation and behavioral disturbances in mice [64].
Lung microbiome
Under normal conditions, the alveolar space, unlike the GI tract, provides an environment that is generally hostile to most bacteria, resulting in minimal bacterial growth [53], mainly including Prevotella, Veillonella, Streptococcus, and Fussobacterium species [65–67]. However, critical illness leads to lung dysbiosis by altering local physiochemical and metabolic characteristics within the alveoli, such as pH levels, oxygen concentration, free radical occurrence, and nutrient availability [68–70]. Indeed, in pathological conditions such as ARDS, the previously empty alveolar airspaces are filled with protein-rich fluid, providing a favorable energy milieu for proliferating microorganisms [69]. Moreover, the development of anaerobic zones due to alveolar oedema or collapse, and consequent atelectasis in injured lungs, creates a more favorable environment for the proliferation of potential pathogens [68], exhibiting characteristics more similar to the gut environment than to healthy lung tissue [53]. Indeed, in patients with ARDS, Panzer and colleagues (2018) found an enrichment of the lung microbiome with gut-associated microbes, such as Bacteroidetes and Enterobacteriaceae. Additionally, the study revealed that in patients with severe blunt trauma undergoing mechanical ventilation, early alterations of the lung microbiome correlate with elevated markers of inflammation (IL-6, IL-8), making these patients susceptible to the development of ARDS [71]. These findings are in line with the findings of Dickson et al., who investigated the bacterial composition of lung samples obtained via bronchoalveolar lavage (BAL) from both ARDS and non-ARDS patients. Streptococcaceae, Veillonellaceae, Prevotellaceae, Verrucomicrobiaceae, and Flavobacteriaceae were identified as the dominant species in patients without ARDS. In contrast, BAL specimens from ARDS patients revealed the presence of Pasteurellaceae and Enterobacteriaceae [25].
As previously noted, the development of ARDS has been strongly correlated with gut-associated bacteria in the lungs. Yet, limited data exists on the consistency of microbiota changes in both the gut and lungs. Conducting 16S ribosomal RNA (rRNA) sequencing analysis on 26 patients with acute pancreatitis-induced ARDS, a study found that alterations in gut microbiota mirror those observed in lung microbiota from earlier studies, suggesting that the translocation of gut microbiota may result in changes in lung microbiota [72]. The transfer of gut microbiota to the lungs can occur through several mechanisms. Impaired intestinal permeability, as suggested by recent studies, allows gut microbes like Bacteroidetes and Enterobacteriaceae to translocate into the lungs of ARDS patients [71]. However, it is crucial to understand that gut-derived critical illness extends beyond bacterial translocation, as evidenced by experimental studies of trauma or haemorrhagic shock where post-shock mesenteric lymph shows an absence of bacterial 16 s rRNA genetic material but an increase in damage-associated molecular pattern material (DAMPs), indicating the transport of pro-inflammatory mediators to distant organs, especially the lungs [73]. The invasion of native gut bacteria through the intestinal mucosa into normally sterile tissues, potentially causing disease, is referred to as "bacterial translocation," a process that also includes the infiltration and movement of inflammatory molecules produced at the intestinal wall or toxic products from the intestine, potentially resulting in systemic damage [74]. Indeed, local activation of the mucosal immune system (MIS) is triggered by bacterial translocation, leading to the production of inflammatory mediators known as DAMPs, which travel through the mesenteric lymphatics to the lungs and systemic circulation. Innate immune cells recognize these molecules, which further stimulate pro-inflammatory pathways that hasten organ damage and the development of MODS [30].
Immune cells within the gut-lung axis: impact of gut-derived immune cells in the pathophysiology of ALI/ARDS
As mentioned above, the gut microbiota contributes significantly to maintaining immune homeostasis by modulating the balance between pro-inflammatory Th17 and anti-inflammatory Treg cells in the GI tract [75]. At the same time, an imbalanced microbiome has been linked to an elevation in CD4+ -IL-17 cells within the gut [76]. These immune cell-mediated regulatory properties of the microbiota are not limited to the local intestinal level but extend to peripheral organs and systems such as the brain [77–79], lungs [25, 80, 81], and liver [82] through T cell migration [83, 84] underscoring a direct link within the gut-lung axis.
Th17/Tregs
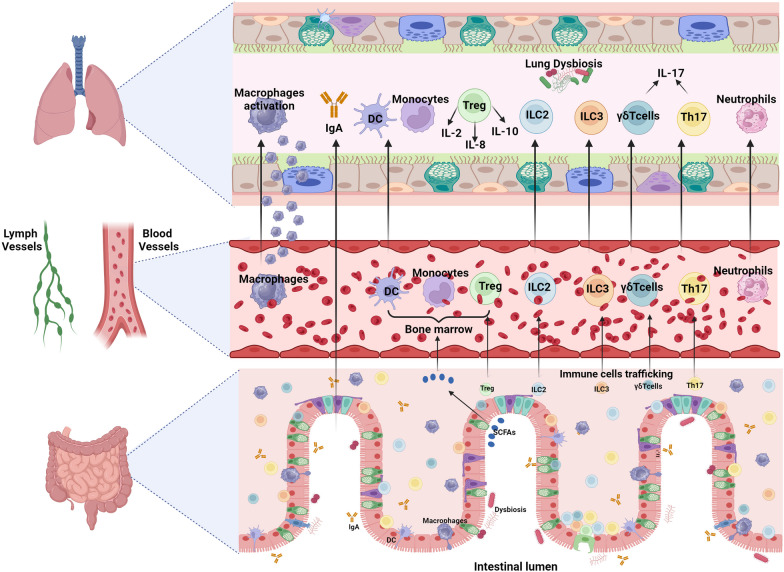
ALI manifests as hypoxaemia and enhanced alveolo-capillary permeability, leading to acute respiratory failure with a notable mortality rate. The immune system, particularly Tregs, plays a strategic role in the transition from injury to resolution in survivors of acute ALI/ARDS, with increasing evidence indicating that Tregs contribute to the suppression of lung inflammation [85]. Tregs have the capacity to mitigate inflammation-induced tissue damage through various indirect mechanisms, including their anti-inflammatory properties through suppression of macrophage anti‐inflammatory cytokine secretion [38, 39] and antiapoptotic capabilities [86], which contribute to establishing a conducive immune microenvironment for tissue repair and regeneration [87]. Treg cells exert anti-inflammatory effects through direct cell–cell interactions or by releasing cytokines like IL-10 or transforming growth factor (TGF)-β, with their differentiation dependent on the transcription factor FoxP3 [88, 89]. Moreover, acting as a “cytokine sink,” Tregs have the ability to capture specific effector cytokines [90]. Recent studies investigating the pathophysiology of ARDS have validated that the direct instillation of human umbilical cord mesenchymal stem cells into the lungs results in an increase of the alveolar Tregs, which was associated with the regulation of pro- and anti-inflammatory factors, including tumor necrosis factor (TNF)-α [91]. Moreover, it has been shown that the Tregs/CD4+ ratio in the bloodstream of ARDS patients is significantly higher compared to non-ARDS patients, while at the alveolar level, the Tregs/CD4+ ratio is reduced [92, 93]. Equivalent research has revealed a notable decline in CD4+, CD8+, and B lymphocyte counts, activation of Th1 and Th2 pathways, cytotoxicity, apoptosis, and endothelial dysfunction in ARDS patients. Interestingly, changes in the intestinal microbiome composition correlate with fluctuations in lymphocyte counts [93–95]. In addition, indirect evidence proposes that Tregs may influence IL-2 [96] and IL-8 [97] (Fig. 1). Moreover, Wang et al. (2012) investigated the chemotactic receptor for leukotriene B4 (LTB4) in the recruitment of Tregs to bronchoalveolar lavage fluid (BALF) in LPS-induced ALI. They examined BLT1 expression in both mouse and human Tregs and assessed its role in Treg migration both in vitro and in vivo. The research demonstrated that BLT1 plays a novel anti-inflammatory role in resolving ALI by mediating the alveolar recruitment of Tregs, suggesting that therapies disrupting the LTB4-BLT1 pathway after ALI onset could hinder recovery [98]. Additionally, an experimental study using a mouse model of LPS-induced ALI demonstrated that Tregs play a critical role during lung injury resolution by altering innate immune responses, indicating possible therapeutic targets for ALI treatment [99]. Indeed, Treg cells, crucial regulators of immune responses through cell-to-cell contact and secretion of inhibitory cytokines such as IL-10 and TGF-β1, infiltrate the lungs and may contribute to the pathogenesis of ARDS. They accumulate in the BALF of mice and patients with ALI, promoting the resolution of ALI by inducing TGF-β1 secretion and neutrophil apoptosis [99].
Fig. 1.
The migration of cells from the gut to the lungs has the potential to impact respiratory immunity, as illustrated by the movement of Tregs, Th17 cells, ILC2s, ILC3s, and γδ T cells from the gut to the lungs. Moreover, SCFAs derived from dietary fibers enter the peripheral circulation and bone marrow, playing a role in immune cell development, including Treg cells, which contribute to lung immune responses. Elevated Th17 levels in both alveolar and circulating compartments correlate with enhanced proportions of alveolar neutrophils, increased alveolar permeability, and organ dysfunction in ARDS. Finally, IgA derived from the gut might play a role in regulating immune responses beyond the mucosal tissues of the lung. ARDS acute respiratory distress syndrome, IgA immunoglobulin A, ILC innate lymphoid cells, SCFAs short-chain fatty acids, Th17 T helper 17, Treg T regulatory cells
Further research using a murine model of lung injury has demonstrated that Tregs play a significant role in resolving ALI fibroproliferation by decreasing fibrocyte recruitment through the chemokine (C-X-C motif) ligand 12 (CXCL12)–CXCR4 axis [100].
On the other hand, clinical and experimental studies highlight the essential involvement of pro-inflammatory Th17 cells in initiating pulmonary inflammation and the cardinal role of the imbalance of Th17/Treg in the progression of the disease [101]. Th17 cells, a subset of CD4+ T lymphocytes, contribute to both the pathogenicity and immunoprotective mechanisms observed in diverse autoimmune diseases and play a crucial role in generating an inflammatory milieu during infections [102–104] by producing mainly cytokine IL-17 and, to a lesser degree, other inflammatory mediators like IL-6, TNF-α, and granulocyte–macrophage colony-stimulating factor [88]. Accumulating data demonstrate that Th17 represents a “double-edged sword” paradigm where Th17 has a fundamental function in eliminating pathogen eradication but concurrently contributes to lung damage via neutrophil recruitment [105]. In a nematode infection model, the central role of IL-17A in eliminating parasites was related to lung damage via neutrophil recruitment [106]. Moreover, in murine models of lung injury induced by Influenza A (H1N1) infection, the involvement of Th17 was associated with an early and appropriate immune response to pathogens while simultaneously fostering pathological inflammation in the lung [107, 108].
Previous research illustrated a substantial activation of Th17 cells during the initial phase of ARDS and an alleviation of the severity of ARDS by mitigating the Th17 response [109]. Moreover, it is well established that elevated levels of CD4+ T cell activation and proliferation, coupled with the presence of Th17 cells, mediate inflammatory cascades in the lungs, contributing to the pathophysiology of ARDS [110, 111]. Furthermore, enhanced levels of Th17 in alveolar and circulating compartments are associated with increased proportions of alveolar neutrophils, enhanced alveolar permeability, and organ dysfunction in ARDS [105, 112]. Moreover, a recent experimental study using a mouse model of LPS-induced ARDS has highlighted that IL-33 production increases the Th17/Treg ratio. At the same time, IL-33 deficiency eliminates the differentiation of T cells into Th17 cells, thereby restoring Th17/Treg balance. As a result, IL-33 deficiency notably suppresses inflammation, whereas treatment with recombinant IL-33 exacerbates lung inflammation [101]. These findings are in line with previous experimental research underscoring the diverse role of IL-33 in shaping the development and maintenance of the Th17 immune response by influencing proinflammatory Th17 cells in the small intestine to transition into a regulatory phenotype with immunosuppressive properties [113]. Interestingly, IL-33 has been demonstrated in experimental studies to directly suppress the expression of tight junction-related proteins both in vitro and in vivo, while also significantly increasing intestinal permeability in vivo, promoting bacterial translocation and exacerbating systemic and colonic inflammation [114, 115]. Finally, probiotic intervention demonstrates preventive effects against experimental particulate matter (PM)2.5-induced lung injury. Indeed, analysis of 16 S rRNA sequences revealed that probiotic treatment in a rat lung damage model induced by PM2.5 exposure could diminish microbiota abundance and diversity, enhance the presence of potentially beneficial bacteria, and diminish the levels of bacteria linked to inflammation. This effect was attributed to the suppression of inflammatory responses, modulation of Th17/Treg balance, and preservation of intestinal internal environment stability, indicating the pivotal contribution of intestinal Th17/Treg in the pathophysiology of ALI [116].
The generation, proliferation, and survival of Tregs are significantly influenced by host-derived nutrients and hormones [117]. Certainly, dietary components such as probiotics and prebiotics have the ability to impact health by altering the composition and function of the mucosal immune system and gut microbiota. Through competition or inhibition of adherence, they can increase pathogen exclusion and enhance intestinal epithelial integrity. Furthermore, both the gut and systemic immune systems can be influenced by these dietary factors, particularly by stimulating various components of the innate and adaptive immune responses. This involves the activation of regulatory T and B cells (Bregs), Th1, Th2, and Th17 responses, alongside the humoral response [118, 119]. Moreover, metabolites derived from commensal microbiota, including SCFAs, play a crucial role in regulating Treg homeostasis and function within the gut-associated lymphoid tissue (GALT) (Fig. 1) [117]. SCFAs stand out as potentially significant. Originating from the processing of dietary fiber by gut microbiota, SCFAs emerge as the primary intestinal metabolic elements by regulating immune reactions, including the differentiation, activation, and recruitment of immune cells (Fig. 1). Additionally, SCFAs demonstrate immunomodulatory properties that extend beyond exerting effects solely within the gut. Recent research findings suggest SCFAs as potential therapeutic strategies to reduce Th17 cell differentiation while simultaneously boosting Treg cells and enhancing their suppressive potential [120]. Indeed, SCFAs trigger the release of TGF-β1 by hindering histone deacetylase-mediated activating protein-1 activation within intestinal epithelial cells (IECs). Consequently, this process promotes the generation of Tregs and elicits anti-inflammatory, immune effects [121–123].
Given the restricted therapeutic strategies in the management of patients with ALI/ARDS, enhancing our understanding of the functions of Tregs and Th17 in the pathophysiology of ALI/ARDS holds the potential to prepare the way for the development of innovative treatments for affected individuals.
γδ T cells
Γδ T cells represent a distinctive subpopulation of Th17 lymphocytes located at epithelial surfaces, including the intestine and the lung, playing a strategic role in the modulation of immune responses against microbial pathogens [124, 125]. Despite γδ T cells being most frequent in the lamina propria/smooth muscle of the airways and less so in the alveolar epithelium, their localization in the subepithelium of alveolar regions within the lung [126] implies a role in influencing the immune response against bacterial infections [127], among others, through their ability to regulate macrophage homeostasis and activation [128]. This is supported by experimental studies showing that in individuals deficient in γδ T cells, total lung inflammation and alveolar-capillary leak were enhanced and that γδ T cells protected against LPS-induced lung inflammation and alveolar-capillary dysfunction [99, 129, 130].
Furthermore, it is highlighted that modifications in mucosal barrier function associated with enhanced permeability have been linked to bacterial translocation and immune dysfunction. This alteration has a causal connection with several pathological conditions, including inflammatory bowel disease and ischaemic stroke. Additionally, increased intestinal permeability triggers intestinal inflammatory responses and may, in part, contribute to the transition from protective γδ T cells to pathogenic γδ T cells [83, 131–134]. Very recently, an experimental study in a murine model of stroke demonstrated that γδ T cells, rather than CD4+ Th17 cells, serve as a principal source of IL-17A in the injured brain and lung following stroke. Importantly, it is noteworthy that the migration of γδ T cells from the small intestine to the brain and lung plays a pivotal role in exacerbating stroke and lung injury. Additionally, the subdiaphragmatic vagus nerve actively participates in mediating the migration of small intestine-derived γδ T cells into the brain and lung after a stroke [131]. Moreover, a current experimental study of lung infection with Ps. aeruginosa found that prophylactic nutritional intervention with inulin supplementation triggers a higher proportion of γδ T cells in the blood, accompanied by a higher infiltration of IL-17-producing γδ T cells within the lungs [135]. Finally, the crucial role of γδ T cells in the gut-lung crosstalk is indicated by studies underscoring the elevated susceptibility to chronic inflammatory lung disease observed in individuals with inflammatory bowel disease [136]. Exploring the foundational role of γδ T cells in the communication between the gut and the lung would provide insights into whether adjusting the migratory behavior of γδ T cells could offer an additional therapeutic avenue to mitigate the onset or advancement of ALI resulting from the disruption of intestinal barrier function.
Antibody-secreting cells
The regulation of humoral immunity involves a special population of B cells with the capacity to produce and release substantial quantities of antibodies, known as antibody-secreting cells (ASC) [137]. Within the gastrointestinal milieu, a combination of foreign diet antigens, commensal microbiota, and intermittent harmful pathogens results in the consistent differentiation of B cells into ASC. This sustained immune response establishes the gut as the residence for over 80% of mammalian ASC predominantly expressing IgA [138, 139]. Playing a central role in local immunity, IgA serves as the primary antibody within the mucosal immune system. It is essential for resisting the invasion of external pathogenic microorganisms, neutralizing bacterial toxins, and contributing to the establishment of the immune barrier and immune clearance while mediating immune responses, which can influence the progression of autoimmune diseases [140, 141]. Current research indicates that ASC derived from the gut plays a role in regulating immune responses beyond mucosal tissues, including in the blood, central nervous system (CNS), and the kidney [139]. To our knowledge, the data regarding the role of IgA in the pathophysiology of ALI/ARDS are scarce. This scarcity can be attributed to the fact that secretory IgA, primarily produced by mucosa-associated lymphoid tissue, is the main immunoglobulin in the upper airways. In contrast, IgG is the principal immunoglobulin in the alveolar spaces, mainly entering through passive diffusion from the systemic circulation. At the bronchial surfaces, IgM, also derived from mucosa-associated lymphoid tissue, is dominant and enhances pathogen opsonization by activating the complement system [142, 143]. However, research investigating the pathophysiology of other inflammatory lung diseases characterized by chronic neutrophil infiltration, such as COPD, indicates an impaired lung IgA immune function and a correlation between the decrease in IgA levels and the severity of the disease [144–146]. Moreover, recent data in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection proposes a dual nature of IgA’s impact on the disease progression. In the early stages, IgA-induced Neutrophil Extracellular Traps (NET) release is posited to be beneficial, acting as a defense mechanism against SARS-CoV-2 entry into the mucosal region. However, as the disease advances, NET release may become detrimental, potentially exacerbating tissue damage [140].
Innate lymphoid cells
The innate lymphoid cells (ILCs) are categorized into three main groups: ILC1, ILC2, and ILC3, each comprising multiple sub-populations [147]. Recent evidence highlights that the intestinal microbiota may influence lung immune responses in newborn mice, particularly in combating pneumonia, by triggering the production of IL-22 derived from ILC3 in the lungs of animals (Fig. 1) [148]. In addition, ILCs are found to play crucial roles in lung injury, lung allergies, fibrosis, and pulmonary infections [149–152]. Accumulating evidence suggests that ILC2s present in the lung may derive from the intestine through circulation (Fig. 1). Huang and co-workers (2018) reported that ILC2s, originating from a resting state in the lamina propria of the intestine, enter the lymphatics and recruited into the lung through circulation as a result of sphingosine 1-phosphate (S1P)-mediated chemotaxis [153]. Moreover, using integrated microbiota dysbiosis approaches, Pu and co-authors reveal that the gut microbiota plays a role in guiding the migration of ILC2s from the gut to the lung, establishing a gut-lung axis. Specifically, they identified Proteobacteria as the pivotal species within the gut microbiome that supports the natural migration of ILC2s, with elevated Proteobacteria levels leading to increased IL-33 production [154]. Nevertheless, as the pathophysiology of migration of ILC2s within the lung during inflammatory conditions remains mainly unclear and there is limited knowledge about the signals that could potentially regulate this process, further studies are needed to investigate the plausible participation of ILCs of intestinal origin in the pathogenesis of ALI/ARDS.
Areas of further research
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) aims to restore the functions of an altered gut microbiota by introducing fecal material from a healthy donor. Interest in this method, which has well-established benefits for treating conditions such as food poisoning and dysentery over centuries, surged in 2013 after the publication of a randomized control trial that demonstrated FMT's substantial superiority over standard care for treating recurrent Cl. difficile infections (CDI) [155, 156]. Notably, in critically ill patients with severe and complicated CDI, rescue FMT emerged as a promising alternative to surgical treatment, achieving a primary cure rate of nearly 80%, thereby enabling almost 90% of patients to avoid colectomy [157]. However, there is limited evidence regarding the use of FMT in managing critically ill patients with antibiotic-associated diarrhea (AAD) caused by pathogens other than Cl. difficile or those of unknown origin, which account for about two-thirds of AAD cases [158–161]. Yet, in a case series studying the use of FMT in critically ill patients with AAD, Dai, and coworkers (2019) described good clinical outcomes without infectious complications [161]. Moreover, experimental research in recent years has suggested that by maintaining the balance of pulmonary flora and altering the structure and diversity of both pulmonary and intestinal microbiota, FMT might effectively prevent and treat chronic respiratory diseases through the regulation of the pulmonary and the intestinal flora [162]. Furthermore, the effectiveness of FMT, as highlighted by clinical and experimental studies in CDI, might arise from a combination of direct microbiological actions against Cl. difficile and indirect mechanisms, such as the production of microbiota-derived metabolites like secondary bile acids and SCFAs. FMT appears to modulate the intense inflammatory response triggered by Cl. difficile by involving Tregs, which play a crucial role in reducing various cells and soluble inflammatory mediators [163], making it a significant treatment modality for ARDS patients. However, considering potential complications such as sepsis and exacerbation of inflammatory processes, further research is necessary to ensure safety and efficacy in critically ill ARDS patients [164].
The aforementioned findings hold promise for the development of successful therapies to manage critically ill patients, including those with ARDS. However, caution is warranted, as not all experimental results from animal models translate directly to humans. Further research is needed to explore the relevance of these findings in the management of critically ill patients.
Conclusions
In summary, the pleiotropic relationship between the gut microbiota and immune cells plays a crucial role in maintaining immune balance by impacting systemic responses, including those seen in ALI/ARDS. Treg cells offer protection by suppressing lung inflammation, while Th17 cells can worsen lung damage. SCFAs from the gut microbiota, probiotics, and FMT emerge as potential therapies, influencing immune cell function, gut dysbiosis, and intestinal barrier integrity. Additionally, γδ T cells, ASCs, and ILCs play roles in lung immune responses, with implications for ALI/ARDS. Understanding these interactions offers hope for innovative treatments to address the urgent need for effective ALI/ARDS therapies.
Acknowledgements
Figure 1 created with BioRender.com.
Author contributions
The study was designed by MZ and AE. MZ searched the articles and drafted the manuscript, to which AE contributed and revised. Both authors read and approved the final manuscript.
Funding
Publication costs for this article were funded by the authors' institutions.
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ardain A, et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature. 2019;570(7762):528–532. doi: 10.1038/s41586-019-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019;7:17. doi: 10.1186/s40560-019-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207(Suppl 1):E103–E111. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore EE, Claude H. Organ, Jr. memorial lecture: Splanchnic hypoperfusion provokes acute lung injury via a 5-lipoxygenase-dependent mechanism. Am J Surg. 2010;200(6):681–9. doi: 10.1016/j.amjsurg.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y, et al. The gut-lung axis in systemic inflammation. Role of mesenteric lymph as a conduit. Am J Respir Cell Mol Biol. 2021;64(1):19–28. doi: 10.1165/rcmb.2020-0196TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauche D, et al. IL-23 and IL-2 activation of STAT5 is required for optimal IL-22 production in ILC3s during colitis. Sci Immunol. 2020;5(46):66. doi: 10.1126/sciimmunol.aav1080. [DOI] [PubMed] [Google Scholar]
- 7.Ma PJ, Wang MM, Wang Y. Gut microbiota: a new insight into lung diseases. Biomed Pharmacother. 2022;155:113810. doi: 10.1016/j.biopha.2022.113810. [DOI] [PubMed] [Google Scholar]
- 8.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Atarashi K, Honda K. Microbiota in autoimmunity and tolerance. Curr Opin Immunol. 2011;23(6):761–768. doi: 10.1016/j.coi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Chervonsky AV. Intestinal commensals: influence on immune system and tolerance to pathogens. Curr Opin Immunol. 2012;24(3):255–260. doi: 10.1016/j.coi.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Paun A, Danska JS. Immuno-ecology: how the microbiome regulates tolerance and autoimmunity. Curr Opin Immunol. 2015;37:34–39. doi: 10.1016/j.coi.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Chhor V, et al. Role of microglia in a mouse model of paediatric traumatic brain injury. Brain Behav Immun. 2017;63:197–209. doi: 10.1016/j.bbi.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, et al. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother. 2023;164:114985. doi: 10.1016/j.biopha.2023.114985. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R, Qiao S, Yang X. Th17/Treg imbalance: Implications in lung inflammatory diseases. Int. J. Mol. Sci. 2023;24(5):66. doi: 10.3390/ijms24054865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziaka M, Exadaktylos A. Pathophysiology of acute lung injury in patients with acute brain injury: the triple-hit hypothesis. Crit Care. 2024;28(1):71. doi: 10.1186/s13054-024-04855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, et al. Immunosuppression and neuroinflammation in stroke pathobiology. Exp Neurobiol. 2021;30(2):101–112. doi: 10.5607/en20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huffnagle GB, Dickson RP. The bacterial microbiota in inflammatory lung diseases. Clin Immunol. 2015;159(2):177–182. doi: 10.1016/j.clim.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson RP, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12(6):821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Dwyer DN, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(9):1127–1138. doi: 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathieu E, et al. Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front Physiol. 2018;9:1168. doi: 10.3389/fphys.2018.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, et al. Dysregulated lung commensal bacteria drive Interleukin-17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity. 2019;50(3):692e7–706e7. doi: 10.1016/j.immuni.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Coburn B, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep. 2015;5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin C, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell. 2019;176(5):998e16–1013e16. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson RP, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1(10):16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson RP, et al. The microbiome and the respiratory tract. Annu Rev Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, et al. The impact of lung microbiota dysbiosis on inflammation. Immunology. 2020;159(2):156–166. doi: 10.1111/imm.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 29.Haak BW, Levi M, Wiersinga WJ. Microbiota-targeted therapies on the intensive care unit. Curr Opin Crit Care. 2017;23(2):167–174. doi: 10.1097/MCC.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 30.Ziaka M, Exadaktylos A. Exploring the lung-gut direction of the gut-lung axis in patients with ARDS. Crit Care. 2024;28(1):179. doi: 10.1186/s13054-024-04966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev. 2017;279(1):90–105. doi: 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45(2):337–347. doi: 10.1097/CCM.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Ursell LK, et al. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38–S44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oami T, Chihade DB, Coopersmith CM. The microbiome and nutrition in critical illness. Curr Opin Crit Care. 2019;25(2):145–149. doi: 10.1097/MCC.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 38.Enaud R, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, et al. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J Neuroinflamm. 2022;19(1):76. doi: 10.1186/s12974-022-02435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley CP, et al. Segmented filamentous bacteria provoke lung autoimmunity by inducing gut-lung axis Th17 cells expressing dual TCRs. Cell Host Microbe. 2017;22(5):697–704. doi: 10.1016/j.chom.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, et al. Gut-lung dysbiosis accompanied by diabetes mellitus leads to pulmonary fibrotic change through the NF-kappaB signaling pathway. Am J Pathol. 2021;191(5):838–856. doi: 10.1016/j.ajpath.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Boesch M, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403. doi: 10.1080/2162402X.2021.1988403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albert-Bayo M, et al. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells. 2019;8(2):66. doi: 10.3390/cells8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reintam A, et al. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53(3):318–324. doi: 10.1111/j.1399-6576.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 45.Piton G, et al. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41(9):2169–2176. doi: 10.1097/CCM.0b013e31828c26b5. [DOI] [PubMed] [Google Scholar]
- 46.Sertaridou E, et al. Gut failure in critical care: old school versus new school. Ann Gastroenterol. 2015;28(3):309–322. [PMC free article] [PubMed] [Google Scholar]
- 47.Sautner T, et al. Early effects of catecholamine therapy on mucosal integrity, intestinal blood flow, and oxygen metabolism in porcine endotoxin shock. Ann Surg. 1998;228(2):239–248. doi: 10.1097/00000658-199808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung DH, et al. Burn-induced transcriptional regulation of small intestinal ornithine decarboxylase. Am J Surg. 1992;163(1):157–62. doi: 10.1016/0002-9610(92)90269-W. [DOI] [PubMed] [Google Scholar]
- 49.Lu Q, et al. The anatomic sites of disruption of the mucus layer directly correlate with areas of trauma/hemorrhagic shock-induced gut injury. J Trauma. 2011;70(3):630–635. doi: 10.1097/TA.0b013e3181e1221b. [DOI] [PubMed] [Google Scholar]
- 50.Rupani B, et al. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141(4):481–489. doi: 10.1016/j.surg.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Coopersmith CM, et al. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30(1):195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Dominguez JA, et al. Epidermal growth factor improves survival and prevents intestinal injury in a murine model of pseudomonas aeruginosa pneumonia. Shock. 2011;36(4):381–389. doi: 10.1097/SHK.0b013e31822793c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaborin A, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. mBio. 2014;5(5):e01361-14. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald D, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1(4):66. doi: 10.1128/mSphere.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–466. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 57.Latorre M, Krishnareddy S, Freedberg DE. Microbiome as mediator: Do systemic infections start in the gut? World J Gastroenterol. 2015;21(37):10487–10492. doi: 10.3748/wjg.v21.i37.10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. 2016;32(2):203–212. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marshall JC, et al. The microbiology of multiple organ failure. The proximal gastrointestinal tract as an occult reservoir of pathogens. Arch Surg. 1988;123(3):309–15. doi: 10.1001/archsurg.1988.01400270043006. [DOI] [PubMed] [Google Scholar]
- 60.de la Cal MA, et al. Selective digestive decontamination and bacterial resistance. Lancet Infect Dis. 2013;13(9):738. doi: 10.1016/S1473-3099(13)70217-2. [DOI] [PubMed] [Google Scholar]
- 61.Xu R, et al. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit Care. 2019;23(1):195. doi: 10.1186/s13054-019-2488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freedberg DE, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018;44(8):1203–1211. doi: 10.1007/s00134-018-5268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu K, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56(4):1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng H, et al. Gastrointestinal microbiome of ARDS patients induces neuroinflammation and cognitive impairment in mice. J Neuroinflamm. 2023;20(1):166. doi: 10.1186/s12974-023-02825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly BJ, et al. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:7. doi: 10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shukla SD, et al. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunol. 2017;6(3):e133. doi: 10.1038/cti.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yagi K, et al. The lung microbiome during health and disease. Int J Mol Sci. 2021;22(19):16. doi: 10.3390/ijms221910872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunther A, et al. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153(1):176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 70.Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med. 2013;7(3):245–257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panzer AR, et al. Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. Am J Respir Crit Care Med. 2018;197(5):621–631. doi: 10.1164/rccm.201702-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu X, et al. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Front Cell Infect Microbiol. 2023;13:1127369. doi: 10.3389/fcimb.2023.1127369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi J, et al. A "Clean Case" of systemic injury: mesenteric lymph after hemorrhagic shock elicits a sterile inflammatory response. Shock. 2015;44(4):336–340. doi: 10.1097/SHK.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab. 2017;71(Suppl 1):11–16. doi: 10.1159/000479918. [DOI] [PubMed] [Google Scholar]
- 75.Delgado Jimenez R, Benakis C. The gut ecosystem: a critical player in stroke. Neuromol Med. 2021;23(2):236–241. doi: 10.1007/s12017-020-08633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh V, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, et al. RhANP attenuates endotoxin-derived cognitive dysfunction through subdiaphragmatic vagus nerve-mediated gut microbiota-brain axis. J Neuroinflamm. 2021;18(1):300. doi: 10.1186/s12974-021-02356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hattori N, Yamashiro Y. The gut-brain axis. Ann Nutr Metab. 2021;77(Suppl 2):1–3. doi: 10.1159/000512226. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, et al. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10(1):186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dessein R, et al. Antibiotic-related gut dysbiosis induces lung immunodepression and worsens lung infection in mice. Crit Care. 2020;24(1):611. doi: 10.1186/s13054-020-03320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, et al. Emerging trends and hotspot in gut-lung axis research from 2011 to 2021: a bibliometrics analysis. Biomed Eng Online. 2022;21(1):27. doi: 10.1186/s12938-022-00987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tripathi A, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benakis C, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni S, et al. Gut microbiota regulate migration of lymphocytes from gut to lung. Microb Pathog. 2023;183:106311. doi: 10.1016/j.micpath.2023.106311. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, et al. Regulatory T cells in inflammation and resolution of acute lung injury. Clin Respir J. 2022;16(9):587–595. doi: 10.1111/crj.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arpaia N, et al. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162(5):1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei H, et al. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front Pharmacol. 2015;6:184. doi: 10.3389/fphar.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 90.Lin S, et al. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol. 2018;9:1545. doi: 10.3389/fimmu.2018.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun J, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+ CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27(5):587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 92.Halter S, et al. T regulatory cells activation and distribution are modified in critically ill patients with acute respiratory distress syndrome: a prospective single-centre observational study. Anaesth Crit Care Pain Med. 2020;39(1):35–44. doi: 10.1016/j.accpm.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 93.Zhang DW, et al. Gut microbiota and its metabolic products in acute respiratory distress syndrome. Front Immunol. 2024;15:1330021. doi: 10.3389/fimmu.2024.1330021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouadma L, et al. Immune alterations in a patient with SARS-CoV-2-related acute respiratory distress syndrome. J Clin Immunol. 2020;40(8):1082–1092. doi: 10.1007/s10875-020-00839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y, et al. Gut microbiota dysbiosis correlates with abnormal immune response in moderate COVID-19 patients with fever. J Inflamm Res. 2021;14:2619–2631. doi: 10.2147/JIR.S311518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Willems MG, et al. Systemic interleukin-2 administration improves lung function and modulates chorioamnionitis-induced pulmonary inflammation in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2016;310(1):L1–7. doi: 10.1152/ajplung.00289.2015. [DOI] [PubMed] [Google Scholar]
- 97.Bai J, et al. Erythromycin enhances CD4+ Foxp3+ regulatory T-cell responses in a rat model of smoke-induced lung inflammation. Mediat Inflamm. 2012;2012:410232. doi: 10.1155/2012/410232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, et al. BLT1-dependent alveolar recruitment of CD4(+)CD25(+) Foxp3(+) regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med. 2012;186(10):989–998. doi: 10.1164/rccm.201202-0261OC. [DOI] [PubMed] [Google Scholar]
- 99.D'Alessio FR, et al. CD4+ CD25+ Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10):2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garibaldi BT, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013;48(1):35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng L, et al. IL-33 deficiency attenuates lung inflammation by inducing Th17 response and impacting the Th17/Treg balance in LPS-induced ARDS mice via dendritic cells. J Immunol Res. 2022;2022:9543083. doi: 10.1155/2022/9543083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer A, Parmar PJ, Shahrara S. Significance of IL-7 and IL-7R in RA and autoimmunity. Autoimmun Rev. 2022;21(7):103120. doi: 10.1016/j.autrev.2022.103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robert M, Miossec P, Hot A. The Th17 pathway in vascular inflammation: culprit or consort? Front Immunol. 2022;13:888763. doi: 10.3389/fimmu.2022.888763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paiva IA, et al. Th17 cells in viral infections-friend or foe? Cells. 2021;10(5):66. doi: 10.3390/cells10051159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mikacenic C, et al. Interleukin-17A is associated with alveolar inflammation and poor outcomes in acute respiratory distress syndrome. Crit Care Med. 2016;44(3):496–502. doi: 10.1097/CCM.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sutherland TE, et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15(12):1116–1125. doi: 10.1038/ni.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li C, et al. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22(3):528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crowe CR, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M, et al. Protein kinase C theta inhibition attenuates lipopolysaccharide-induced acute lung injury through notch signaling pathway via suppressing Th17 cell response in mice. Inflammation. 2019;42(6):1980–1989. doi: 10.1007/s10753-019-01058-2. [DOI] [PubMed] [Google Scholar]
- 110.Risso K, et al. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur J Clin Microbiol Infect Dis. 2015;34(6):1111–1118. doi: 10.1007/s10096-015-2333-x. [DOI] [PubMed] [Google Scholar]
- 111.Yu ZX, et al. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19(1):82. doi: 10.1186/s13054-015-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai H, et al. Treatment with a neutralising anti-rat interleukin-17 antibody after multiple-trauma reduces lung inflammation. Injury. 2015;46(8):1465–1470. doi: 10.1016/j.injury.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 113.Pascual-Reguant A, et al. T(H)17 cells express ST2 and are controlled by the alarmin IL-33 in the small intestine. Mucosal Immunol. 2017;10(6):1431–1442. doi: 10.1038/mi.2017.5. [DOI] [PubMed] [Google Scholar]
- 114.Sedhom MA, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2013;62(12):1714–1723. doi: 10.1136/gutjnl-2011-301785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palmieri V, et al. Interleukin-33 signaling exacerbates experimental infectious colitis by enhancing gut permeability and inhibiting protective Th17 immunity. Mucosal Immunol. 2021;14(4):923–936. doi: 10.1038/s41385-021-00386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Y, et al. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2.5 induced lung injury. Ecotoxicol Environ Saf. 2022;244:114060. doi: 10.1016/j.ecoenv.2022.114060. [DOI] [PubMed] [Google Scholar]
- 117.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36(1):3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pujari R, Banerjee G. Impact of prebiotics on immune response: from the bench to the clinic. Immunol Cell Biol. 2021;99(3):255–273. doi: 10.1111/imcb.12409. [DOI] [PubMed] [Google Scholar]
- 119.Bermudez-Brito M, et al. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 120.Haase S, et al. Propionic acid rescues high-fat diet enhanced immunopathology in autoimmunity via effects on Th17 responses. Front Immunol. 2021;12:701626. doi: 10.3389/fimmu.2021.701626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin-Gallausiaux C, et al. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci Rep. 2018;8(1):9742. doi: 10.1038/s41598-018-28048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 123.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur J Immunol. 2013;43(8):1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 125.Born WK, et al. gammadelta T lymphocytes-selectable cells within the innate system? J Clin Immunol. 2007;27(2):133–144. doi: 10.1007/s10875-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 126.Wands JM, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol. 2005;78(5):1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 127.Nanno M, et al. gammadelta T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 128.Tramonti D, et al. Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis. Eur J Immunol. 2006;36(7):1729–1738. doi: 10.1002/eji.200635959. [DOI] [PubMed] [Google Scholar]
- 129.Wehrmann F, et al. gammadelta T cells protect against LPS-induced lung injury. J Leukoc Biol. 2016;99(2):373–386. doi: 10.1189/jlb.4A0115-017RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakajima T, et al. T cell pathways involving CTLA4 contribute to a model of acute lung injury. J Immunol. 2010;184(10):5835–5841. doi: 10.4049/jimmunol.0903238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie B, et al. Reversal of the detrimental effects of social isolation on ischemic cerebral injury and stroke-associated pneumonia by inhibiting small intestinal gammadelta T-cell migration into the brain and lung. J Cereb Blood Flow Metab. 2023;43(8):1267–1284. doi: 10.1177/0271678X231167946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stanley D, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22(11):1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- 133.Sanchez de Medina F, et al. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis. 2014;20(12):2394–404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 134.Wu P, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boucher E, et al. Inulin prebiotic protects against lethal pseudomonas aeruginosa acute infection via gammadelta T cell activation. Nutrients. 2023;15(13):66. doi: 10.3390/nu15133037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raftery AL, et al. Development of severe colitis is associated with lung inflammation and pathology. Front Immunol. 2023;14:1125260. doi: 10.3389/fimmu.2023.1125260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nutt SL, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 138.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12(12):821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 139.Keppler SJ, Goess MC, Heinze JM. The wanderings of gut-derived IgA plasma cells: impact on systemic immune responses. Front Immunol. 2021;12:670290. doi: 10.3389/fimmu.2021.670290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang X, et al. SIgA in various pulmonary diseases. Eur J Med Res. 2023;28(1):299. doi: 10.1186/s40001-023-01282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rees F, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 142.Baumann U, et al. The lung in primary immunodeficiencies: new concepts in infection and inflammation. Front Immunol. 2018;9:1837. doi: 10.3389/fimmu.2018.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burnett D. Immunoglobulins in the lung. Thorax. 1986;41(5):337–344. doi: 10.1136/thx.41.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Fays C, et al. Secretory immunoglobulin a immunity in chronic obstructive respiratory diseases. Cells. 2022;11(8):66. doi: 10.3390/cells11081324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bohlander F. A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases. Front Immunol. 2023;14:1127339. doi: 10.3389/fimmu.2023.1127339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gohy ST, et al. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am J Respir Crit Care Med. 2014;190(5):509–21. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 147.Elemam NM, Hannawi S, Maghazachi AA. Innate lymphoid cells (ILCs) as mediators of inflammation, release of cytokines and lytic molecules. Toxins. 2017;9(12):66. doi: 10.3390/toxins9120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gray J, et al. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. 2017;9(376):66. doi: 10.1126/scitranslmed.aaf9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Globinska A, Kowalski ML. Innate lymphoid cells: the role in respiratory infections and lung tissue damage. Expert Rev Clin Immunol. 2017;13(10):991–999. doi: 10.1080/1744666X.2017.1366314. [DOI] [PubMed] [Google Scholar]
- 150.Pan L, et al. Innate lymphoid cells exhibited IL-17-expressing phenotype in active tuberculosis disease. BMC Pulm Med. 2021;21(1):318. doi: 10.1186/s12890-021-01678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pasha MA, et al. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc. 2019;40(3):138–145. doi: 10.2500/aap.2019.40.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ardain A, et al. Type 3 ILCs in lung disease. Front Immunol. 2019;10:92. doi: 10.3389/fimmu.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang Y, et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359(6371):114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pu Q, et al. Gut microbiota regulate gut-lung axis inflammatory responses by mediating ILC2 compartmental migration. J Immunol. 2021;207(1):257–267. doi: 10.4049/jimmunol.2001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Nood E, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 156.Gaines S, Alverdy JC. Fecal micobiota transplantation to treat sepsis of unclear etiology. Crit Care Med. 2017;45(6):1106–1107. doi: 10.1097/CCM.0000000000002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alukal J, et al. Safety and efficacy of fecal microbiota transplant in 9 critically ill patients with severe and complicated Clostridium difficile infection with impending colectomy. J Dig Dis. 2019;20(6):301–307. doi: 10.1111/1751-2980.12750. [DOI] [PubMed] [Google Scholar]
- 158.Wurm P, et al. Antibiotic-associated apoptotic enterocolitis in the absence of a defined pathogen: the role of intestinal microbiota depletion. Crit Care Med. 2017;45(6):e600–e606. doi: 10.1097/CCM.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Q, et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care. 2015;19(1):37. doi: 10.1186/s13054-015-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wei Y, et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care. 2016;20(1):332. doi: 10.1186/s13054-016-1491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dai M, et al. Rescue fecal microbiota transplantation for antibiotic-associated diarrhea in critically ill patients. Crit Care. 2019;23(1):324. doi: 10.1186/s13054-019-2604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu T, et al. 16S rDNA analysis of the effect of fecal microbiota transplantation on pulmonary and intestinal flora. 3 Biotech. 2017;7(6):370. doi: 10.1007/s13205-017-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Soveral LF, et al. Immunological mechanisms of fecal microbiota transplantation in recurrent Clostridioides difficile infection. World J Gastroenterol. 2022;28(33):4762–4772. doi: 10.3748/wjg.v28.i33.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park SY, Seo GS. Fecal microbiota transplantation: Is it safe? Clin Endosc. 2021;54(2):157–160. doi: 10.5946/ce.2021.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.