Abstract
Shi et al. (2021) in “Short-term Western diet intake promotes IL-23-mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice” show that Western diet (WD) exacerbates an IL-23 minicircle–mediated model of psoriasis and psoriatic arthritis, with an expansion of IL-17A–expressing γδ T cells and shifts to the gut microbial community. WD-associated inflammation is mitigated by diet manipulation or antibiotic administration. These results suggest that dietary manipulation may be useful in the treatment of IL-23–mediated disease, possibly through the modulation of the gut microbiota.
Hippocrates noted, “Let medicine be thy food and let food by thy medicine.” With the timeless focus on diet and more recent discoveries implicating the gut microbiota in mediating health and disease (Singh et al., 2017), many patients with autoimmune diseases, such as psoriasis and psoriatic arthritis (PsA), inquire whether diet manipulation may alleviate inflammatory disease through the modulation of the gut microbiota. Anecdotally, a subset of patients find dietary interventions (e.g., reduction of sugar or processed food intake) that seemingly alleviate aspects of their disease. A recent systematic review of the literature (Ford et al., 2018) suggests that weight reduction with a hypocaloric diet in individuals who are overweight and obese is beneficial as an adjunctive intervention to pharmacologic therapy. Despite these findings, the mechanisms by which dietary interventions modulate psoriatic disease severity remain unclear. In a new study, Shi et al. (2021), using a murine model of psoriasis and PsA, find that Western diet (WD) leads to an expansion of IL-17A–expressing γδ T cells and to shifts in gut microbiota composition, raising the possibility that diet may influence host disease through the modulation of host immunity and the gut microbiota.
Shi et al. (2021) treated C57BL/6 mice with either a conventional diet (CD) or a WD for 6 weeks, followed by injection with IL-23 minicircle DNA; mice received each diet for four more weeks. They found that IL-23 cytokine–mediated skin and joint disease were exacerbated in the mice that received a WD relative to those that received a CD (Figure 1a-e). Skin and joint immune cell populations, including neutrophils and γδ T cells, and IL-17A cytokine production were elevated in the mice that received a WD relative to those that received a CD. These diet-induced exacerbations and inflammation were reproduced in a second autoimmune-prone mouse model. Together, these findings demonstrate that WD exacerbates IL-23–mediated inflammatory skin and joint disease relative to the CD by altering host immunity, as has been reported by the authors in a different model of psoriatic dermatitis (Shi et al., 2020) and is in this study extended to IL-23–mediated inflammatory arthritis.
Figure 1. Western diet-induced exacerbation of IL-23 mediated skin and joint disease can be mitigated with antibiotics or diet manipulation.
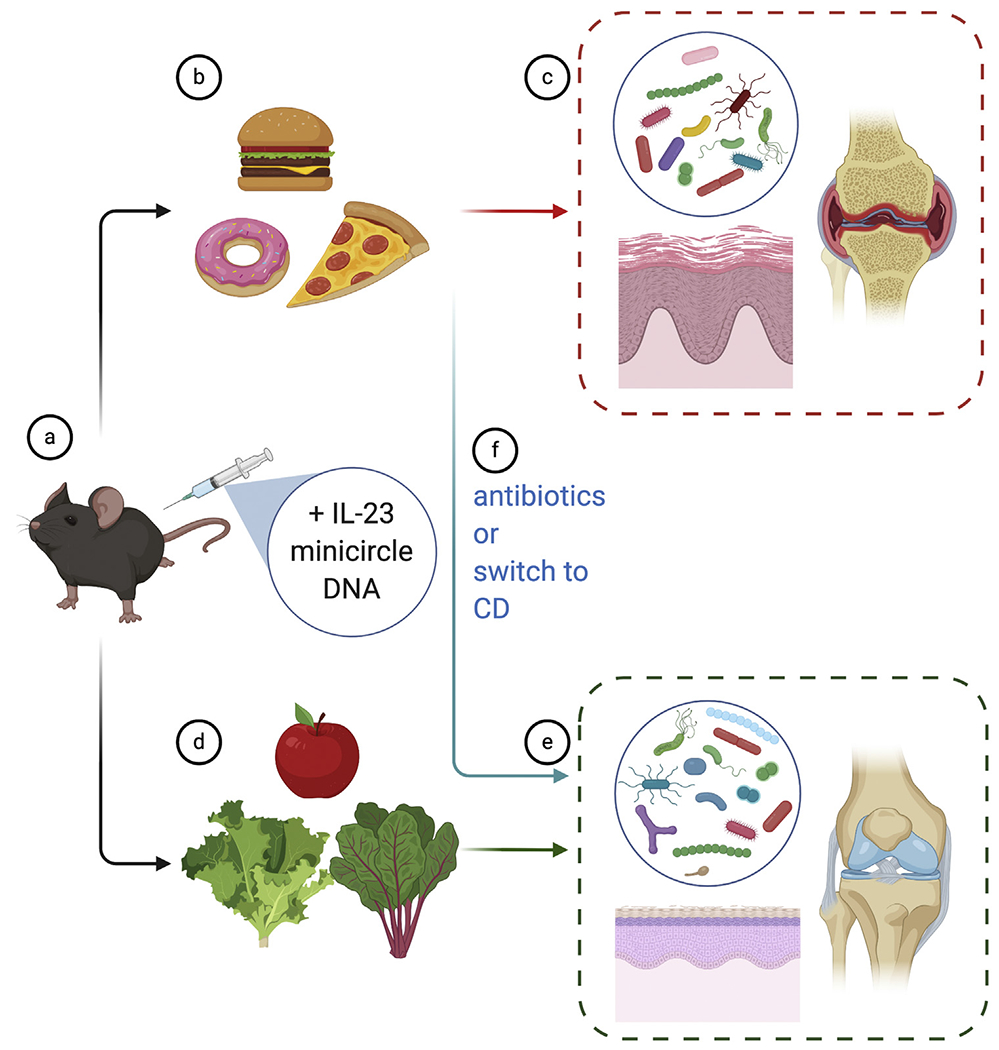
Mice treated with (a) IL-23 minicircle DNA and placed on a (b) WD developed (c) worse psoriatic-like skin and joint disease accompanied by changes to the gut microbiota. In contrast, those fed a (d) CD exhibited (e) less severe skin and joint disease. Treatment of WD-fed mice with either (f) antibiotics or a switch to a CD alleviated disease. CD, conventional diet; WD, western diet.
To investigate whether diet alters the gut microbial community, Shi et al. (2021) undertook 16S ribosomal RNA gene amplicon sequencing of fecal samples and found alterations in community composition as well as in differential abundance of several taxa in mice on a WD relative to those on a CD, consistent with findings from multiple studies in both humans (Singh et al., 2017) and murine models (Bisanz et al., 2019). In addition to diet, IL-23 overexpression shifted community composition more so in mice fed a WD than in those fed a CD, suggesting a synergistic effect of diet and cytokine activation in modulating microbiota composition. They then treated WD-fed IL-23–expressing mice with broad-spectrum antibiotics and found improvement in skin disease. Interestingly, this was also true for mice on a CD. Switching diets from WD to CD led to improvements in skin disease as well as shifts in community composition (Figure 1f). Fecal microbiota transplant (FMT) experiments from WD-fed donors into mice pretreated with broad-spectrum antibiotics revealed that WD-exposed microbiota induced γδ T-cell infiltration into the dermis without a concomitant worsening of disease severity. These findings suggest that WD-induced changes to community composition may contribute to altered immune-activating properties of the microbiota.
Overall, these findings support the hypothesis that a high-fat, high-sugar diet can exacerbate IL-23–mediated autoimmune disease. Dietary components may act on both host immunity and the microbiota. These findings begin to bridge the gap in our understanding of the mechanisms by which diet–host–microbiota interactions modulate IL-23–mediated diseases.
As with all intriguing studies, this study raises additional questions. Are the effects of diet different in skin versus joint disease? Skin disease seemed to be more responsive to dietary shifts or antibiotic treatment in this IL-23 minicircle–mediated model of disease. Does the microbiota contribute to disease even in the absence of a WD? Mice fed a CD derived benefit from antibiotics similar to those fed a WD. Alternatively, are the beneficial effects of antibiotics secondary to the direct effects of antibiotics on the host? Studies in germ-free animals have demonstrated that although antibiotics were developed to target microbes, they can also influence host programs (Yang et al., 2017). Thus, the germ-free equivalents of the studies undertaken by Shi et al. (2021) would be useful in understanding the contribution of the microbiota in mediating disease severity. Relatedly, the transfer of inflammatory phenotype but not disease severity in the FMT studies undertaken by Shi et al. (2021) may suggest that diet affects both the host and the microbiota to promote disease. Alternatively, the FMT studies may have featured incomplete engraftment of disease-exacerbating microbial communities such that transplanted microbial strains could not outcompete resident strains.
Many open questions remain before these findings can be translated into clinical practice. Although WD exacerbates many diseases (Manzel et al., 2014) and advising patients to avoid a high-fat, high-sugar diet is likely to be prudent, we lack the ability to guide patients regarding what they should eat. There is much to be learned about the specific dietary components that lead to inflammation, host factors that mediate the effects of diet on the immune system, and microbial effectors that may be responsible for diet-induced inflammation (Alexander and Turnbaugh, 2020). Such mechanistic understanding is necessary to help us counsel patients on effective strategies to treat disease.
In summary, the findings of Shi et al. (2021) add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and PsA (Herbert et al., 2018; Nakamizo et al., 2017) and raise the possibility that the microbiome may contribute to disease severity. Expansion of γδ T cells and IL-17A pathways implicate a role for the microbiota, and identification of the relevant microbes or causal microbial genes remains to be elucidated. The recapitulation of these findings in patients with psoriasis or PsA remains to be investigated, with the caveat that the tremendous genetic, dietary, and microbial variation in humans may make identification of such patterns challenging. The findings of Shi et al. (2021) bring us a step closer to understanding and targeting diet-dependent host and microbial pathways that can be modulated to treat autoimmunity.
Clinical Implications.
Western diet (WD) exacerbates IL-23–mediated skin and joint disease.
WD expands IL-17A–producing γδ T cells and alters the gut microbiota. Antibiotics mitigate these effects.
Diet and microbiota manipulation may be useful in treating IL-23–mediated disease.
Footnotes
CONFLICT OF INTEREST
The author states no conflict of interest.
REFERENCES
- Alexander M, Turnbaugh PJ. Deconstructing mechanisms of diet-microbiome-immune interactions. Immunity 2020;53:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe 2019;26:265–72.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AR, Siegel M, Bagel J, Cordoro KM, Garg A, Gottlieb A, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the Medical Board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol 2018;154:934–50. [DOI] [PubMed] [Google Scholar]
- Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, et al. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J Invest Dermatol 2018;138:1999–2009. [DOI] [PubMed] [Google Scholar]
- Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 2014;14:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamizo S, Honda T, Adachi A, Nagatake T, Kunisawa J, Kitoh A, et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci Rep 2017;7:14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Wu X, Rocha CS, Rolston M, Garcia Melcor E, Huynh M, et al. Short-term Western diet intake promotes IL-23-mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol 2021;141:1780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Wu X, Yu S, Huynh M, Jena PK, Nguyen M, et al. Short-term exposure to a western diet induces psoriasiform dermatitis by promoting accumulation of IL-17A-producing γδ T cells. J Invest Dermatol 2020;140:1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Bhargava P, McCloskey D, Mao N, Palsson BO, Collins JJ. Antibiotic-induced changes to the host metabolic environment inhibit drug efficacy and alter immune function. Cell Host Microbe 2017;22:757–65.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


