Abstract
Objective:
This study aimed to propose a revised ypN (r-ypN) classification based on lymph node ratio (LNR) and to examine its prognostic value in postneoadjuvant esophageal cancer.
Background:
A new postneoadjuvant pathologic (ypTNM) staging classification has been introduced for esophageal cancer. However, the ypN classification currently defined by the number of positive lymph nodes is influenced by the extent of lymphadenectomy.
Methods:
Data on 7195 esophageal cancer patients receiving neoadjuvant chemoradiation were extracted from the National Cancer Database (NCDB). Four r-ypN stages were defined by 3 LNR thresholds (0%, 10%, and 20% using X-tile software). A revised ypTNM (r-ypTNM) classification was developed by solely changing N categories. Kaplan-Meier method and Cox proportional hazards models were used for survival analyses. Akaike information criterion (AIC) and Harrell’s concordance index (C-index) were used to compare the predictive performance of the current and the revised classification. External validation was performed using an independent cohort from the NEOCRTEC5010 clinical trial.
Results:
Both ypN (P < 0.001) and r-ypN (P < 0.001) were independent prognostic factors of overall survival (OS) for esophageal cancer patients. Kaplan-Meier curves demonstrated a better discrimination with r-ypN than ypN categories. Within each ypN category (except ypN3), OS was significantly different comparing r-ypN strata; however, there were no differences between ypN strata within each r-ypN category (except r-ypN3). r-ypN (AIC: 60752 vs 60782; C-index: 0.591 vs 0.587) and r-ypTNM (AIC: 60623 vs 60628; C-index: 0.613 vs 0.610) showed better predictive performance than the current staging system, with a lower AIC (better calibration) and higher C-index (improved discrimination). This advantage was also confirmed by external validation using the NEOCRTEC5010 cohort.
Conclusions:
LNR showed better performance than ypN in predicting OS of esophageal cancer patients after neoadjuvant chemoradiation and may be an improvement on the current staging system.
Keywords: esophageal neoplasms, neoadjuvant therapy, neoplasm staging, lymph node ratio, database
Esophageal cancer is one of the most aggressive malignancies worldwide; in 2018, it ranked seventh in terms of incidence (572,000 new cases) and sixth in mortality (509,000 deaths).1 In recent years, there has been increasing evidence that neoadjuvant therapy before surgery is associated with long-term survival benefits compared with surgery alone in patients with locoregionally advanced esophageal cancer. Neoadjuvant therapy has become the standard of care in these patients.2–4 With the widespread use of neoadjuvant therapy, both the extent of lymphadenectomy and the introduction of different staging systems have become topics of ongoing discussion.5–7 The eighth edition of the American Joint Committee on Cancer (AJCC-8) Tumor-Node-Metastasis (TNM) staging system launched in January 2017 and established a new postneoadjuvant pathologic (yp) staging groups to fulfill unmet needs in staging esophageal cancer patients given combined modality therapy.8–10 In this edition, the ypN stage is still defined according to the number of positive regional lymph nodes (LNs) (ie, ypN0 for no positive LNs; ypN1 for 1 or 2 positive LNs; ypN2 for 3–6 positive LNs, and ypN3 for 7 or more positive LNs). Although the optimal number of resected LNs remains controversial, a minimum resection of 15 LNs has been recommended to improve the accuracy of staging.11 However, the LN resection is influenced by the quality of the surgical procedure6; furthermore, neoadjuvant therapy has been associated with a decreased number of resected LNs and fewer metastatic LNs in esophageal cancer,5–7 which may result in stage migration. As such, there is a need to revise the current version of the ypTNM staging criteria.
Recently, the lymph node ratio (LNR), which is the ratio of pathologically metastatic to total harvested LNs, has been identified as a predictor of survival in several malignancies, including breast,12,13 gastric,14,15 and colorectal cancers.16 In esophageal cancer, some studies have shown better predictive value in using LNR instead of LN number for pN staging.17,18 However, few studies investigated the significance of LNR in esophageal cancer after neoadjuvant therapy and no relevant publications have compared LNR staging with AJCC-8 ypN staging.
In this study, we used the National Cancer Database (NCDB) to compare the prognostic value of LNR versus ypN as well as a modified staging system based on LNR versus the current AJCC-8 ypTNM staging system in predicting overall survival (OS) in patients undergoing esophagectomy after neoadjuvant chemoradiation. We aimed to propose a revised LN classification based on LNR for postneoadjuvant esophageal cancer patients.
METHODS
Patient Population
The original cohort consisted of primary esophageal cancer patients given neoadjuvant chemoradiotherapy in the NCDB (diagnosed between 2004 and 2015). The NCDB collected data from over 1500 hospital-based tumor registries in the United States.19
For each patient, the study retrieved: (i) demographic characteristics: age, sex, and year of diagnosis; (ii) treatment information: neoadjuvant chemoradiation and surgery; (iii) pathologic data: histology, number of resected LNs, number of positive LNs, and pT, pN, and pM stages according to AJCC-6 or AJCC-7 staging; and, (iv) follow-up information: current status, and survival (in months). After data extraction, AJCC-6 or AJCC-7 pT, pN, and pM stages were converted into AJCC-8 stages, and the ypTNM stage for each patient was determined. OS was measured from the date of diagnosis of esophageal cancer until death or last follow-up. Patients alive at last follow-up were considered as censored observations. Patients with no LNs dissected, missing information on pT, pN, pM stage, or inadequate follow-up were excluded. These criteria left 7195 patients in the original cohort (NCDB) for analysis (Fig. 1).
FIGURE 1.
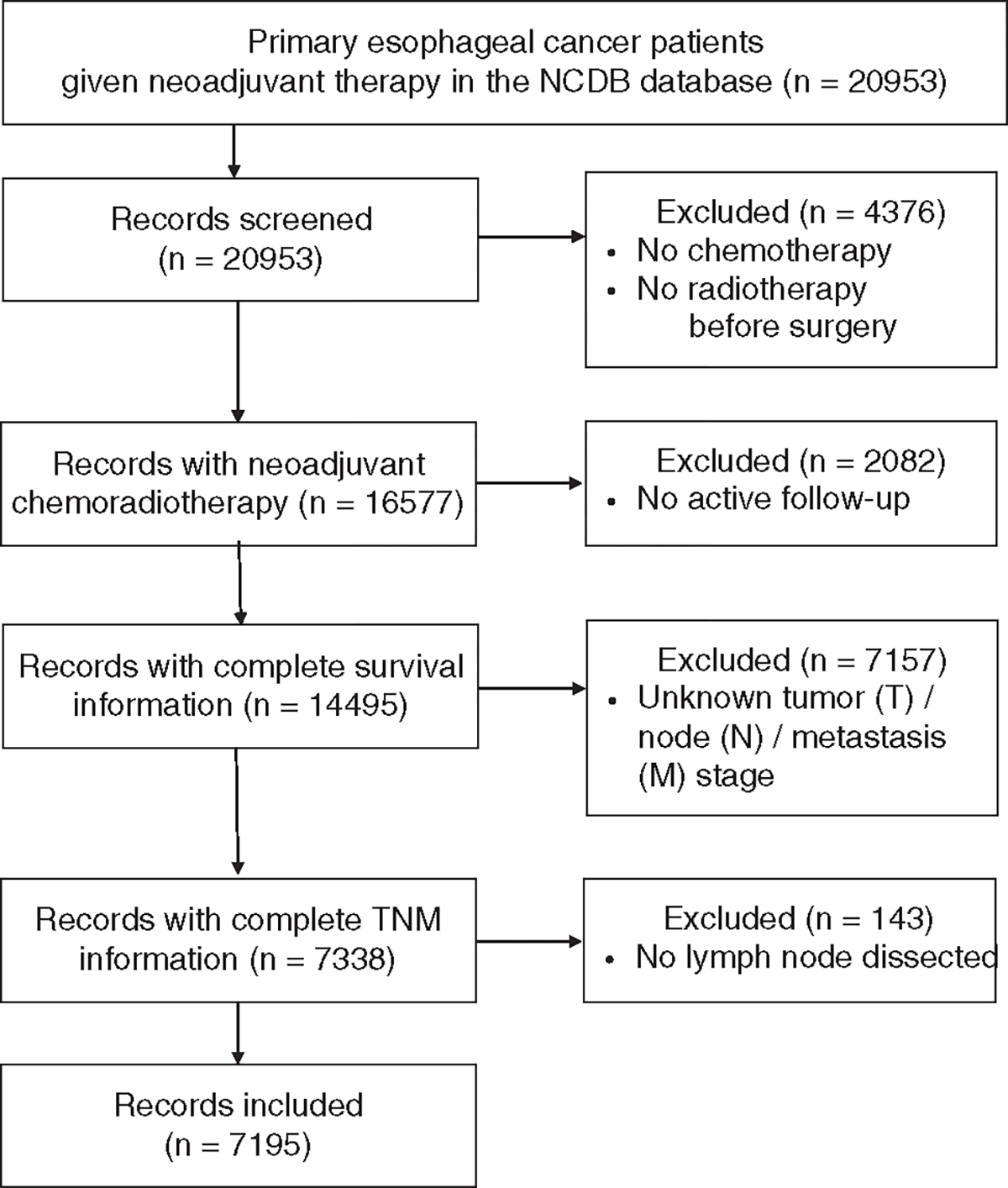
Flow diagram of patient selection.
An independent cohort from the NEOCRTEC5010 trial, a multicenter open-label randomized phase 3 clinical trial conducted at 8 centers in China,4 was used for external validation. Locally advanced esophageal squamous cell carcinoma patients (diagnosed between 2007 and 2014) in the CRT group (neoadjuvant chemoradiation followed by surgery) with at least 1 LN dissected and complete information on clinical and pathologic characteristics were included (n = 182).
Stratification of LNR and the Revised ypTNM (r-ypTNM) Staging
Using X-tile software (http://www.tissuearray.org/rimmlab), the optimal cutoff values for positive LNR (LNR > 0%) were calculated as 10.26% and 20.69% by selecting the highest log-rank χ2 among all possible partitions (Supplemental Digital Content Fig. 1, http://links.lww.com/SLA/D863).20 For consistency with the current TNM staging system, 4 LNR categories were considered and defined as “revised ypN (r-ypN) stages”: r-ypN0 (LNR = 0%), r-ypN1 (0% < LNR ≤ 10%), r-ypN2 (10% < LNR ≤ 20%), and r-ypN3 (20% < LNR ≤ 100%). In keeping with AJCC-8, we developed an r-ypTNM classification (r-I, r-II, r-IIIA, r-IIIB, r-IVA, r-IVB) by simply converting the current ypN categories into r-ypN categories stratified by LNR.
Statistical Analysis
Univariate survival analyses were first carried out using the Kaplan-Meier method. This analysis provided estimates of 5-year OS rates (with their standard errors, SEs) as well as the corresponding median survival times. Survival curves were compared using the log-rank test.
Two multivariable Cox proportional hazard models were developed comparing ypN and r-ypN, respectively, to evaluate their prognostic values after adjustment. All the other categories (age, sex, histology type, number of resected LNs, ypT, and ypM) were included in each model as covariates. A P value for each variable was given by the likelihood ratio test between the complete model and the model excluding that variable. Trend tests were additionally conducted by assigning 0, 1, 2, and 3 to the increasing stages as quantitative variables.
The Spearman correlation analyses were conducted to detect correlations between the number of resected LNs and the number of positive LNs, or the LNR.
To evaluate the predictive performance of each variable, the Akaike information criterion (AIC) and Harrell’s concordance index (C-index) were calculated in the univariate Cox regression models of both the original NCDB cohort and the external validation cohort. The AIC was defined as (2×the number of parameters in the model)−(2×the log maximum likelihood). A lower AIC implies an improved goodness-of-fit model calibration.21 Harrell’s C-index corresponds to the proportion of pairs of patients whose predicted survival times are correctly ordered among all pairs that can actually be ordered. A higher Harrell’s C-index is associated with a great discriminant ability of the model.22,23
All statistical analyses were performed using R (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria). A P value ≤0.05 was considered statistically significant.
RESULTS
Clinicopathologic Characteristics of Study Patients
The clinical and pathologic characteristics of all 7195 esophageal cancer patients given neoadjuvant chemoradiation are shown in Table 1. At last follow-up, 3513 patients were still alive and 3682 had died. The median follow-up was 41.8 months. Median survival was 36.8 months (95% confidence interval: 35.5–38.5 months). The 1-, 3-, and 5-year OS rates were 82.7% (SE: 4.5%), 50.6% (SE: 6.4%), and 37.5% (SE: 7.7%), respectively.
TABLE 1.
Clinicopathologic Characteristics and Univariate Analyses of OS in Esophageal Cancer Patients Given Neoadjuvant Chemoradiotherapy (N= 7195)
| Variables | n (%) | OS |
||
|---|---|---|---|---|
| 5-y OS (SE) (%) | Median Survival (mo) | Log-rank P | ||
|
| ||||
| Age (y) | <0.001 | |||
| ≤60 | 2888 (40.1) | 40.3 (1.21) | 41.3 | |
| >60 | 4307 (59.9) | 35.6 (1.00) | 34.2 | |
| Sex | <0.001 | |||
| Male | 6054 (84.1) | 36.1 (0.83) | 35.5 | |
| Female | 1141 (15.9) | 45.3 (2.03) | 51.5 | |
| Histology type | <0.001 | |||
| EAC | 5449 (75.7) | 37.0 (0.89) | 36.6 | |
| ESCC | 1170 (16.3) | 43.6 (1.86) | 45.5 | |
| Adenosquamous carcinoma | 58 (0.8) | 39.8 (7.32) | 31.3 | |
| Other types | 518 (72.0) | 28.6 (2.77) | 27.1 | |
| Resected LNs | <0.001 | |||
| <15 | 3775 (52.5) | 35.5 (1.02) | 33.2 | |
| ≥15 | 3420 (47.5) | 39.7 (1.16) | 40.6 | |
| ypT stage (AJCC-8) | <0.001 | |||
| ypT0 | 1752 (24.4) | 51.8 (1.62) | 62.6 | |
| ypTis/T1 | 1388 (19.3) | 45.4 (1.82) | 50.1 | |
| ypT2 | 1298 (18.0) | 38.8 (1.80) | 37.1 | |
| ypT3 | 2691 (37.4) | 25.2 (1.11) | 25.5 | |
| ypT4 | 66 (0.9) | 8.18 (4.62) | 14.9 | |
| ypN stage (AJCC-8) | <0.001 | |||
| ypN0 | 4358 (60.6) | 47.7 (1.01) | 53.9 | |
| ypN1 | 1707 (23.7) | 27.3 (1.46) | 28.6 | |
| ypN2 | 798 (11.1) | 15.7 (1.81) | 21.8 | |
| ypN3 | 332 (4.6) | 13.4 (2.86) | 16.4 | |
| ypM stage (AJCC-8) | <0.001 | |||
| ypM0 | 6960 (96.7) | 38.6 (0.79) | 38.2 | |
| ypM1 | 235 (3.3) | 12.1 (2.23) | 14.8 | |
| ypTNM (AJCC-8) | <0.001 | |||
| I | 3188 (44.3) | 52.1 (1.19) | 66.1 | |
| II | 1081 (15.0) | 37.9 (1.95) | 36.9 | |
| IIIA | 857 (11.9) | 33.0 (2.29) | 37.1 | |
| IIIB | 1488 (20.7) | 20.2 (1.41) | 23.1 | |
| IVA | 346 (4.8) | 13.9 (2.73) | 16.6 | |
| IVB | 235 (3.3) | 12.1 (2.23) | 14.8 | |
| r-ypN stage | < 0.001 | |||
| r-ypN0 (LNR = 0%) | 4358 (60.6) | 47.7 (1.01) | 53.9 | |
| r-ypN1 (0%<LNR≤10%) | 963 (13.4) | 31.5 (2.03) | 34.0 | |
| r-ypN2 (10%<LNR≤20%) | 776 (10.8) | 20.8 (2.07) | 25.2 | |
| r-ypN3 (20%<LNR≤100%) | 1098 (15.3) | 15.5 (1.51) | 19.8 | |
| r-ypTNM | <0.001 | |||
| r-I | 3188 (44.3) | 52.1 (1.19) | 66.1 | |
| r-II | 1081 (15.0) | 37.9 (1.95) | 36.9 | |
| r-IIIA | 509 (7.1) | 36.9 (2.98) | 40.3 | |
| r-IIIB | 1159 (16.1) | 23.2 (1.73) | 26.5 | |
| r-IVA | 1023 (14.2) | 17.1 (1.63) | 20.2 | |
| r-IVB | 235 (3.3) | 12.1 (2.23) | 14.8 | |
EAC indicates, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; SE, standard error.
Univariate and Multivariable Survival Analyses
The results of univariate Kaplan-Meier analyses and log-rank tests for OS are shown in Table 1. Younger age (60 y and below), female sex, squamous cell carcinoma, more LNs resected ( ≥15), lower ypT, lower ypN, lower ypM, lower ypTNM, lower r-ypN (based on LNR), and lower r-ypTNM (based on r-ypN) were all significantly associated with better prognoses in terms of OS (P < 0.001 for all factors).
Supplemental Digital Content Table 1 (http://links.lww.com/SLA/D863) shows the hazard ratios (with corresponding 95% confidence intervals) for OS for the multivariable models comparing the current and revised nodal classification systems. After adjusting for age ( ≤ 60 vs > 60), sex, histology, number of resected LNs (< 15 vs ≥ 15), and ypT and ypM stage, both ypN (P < 0.001) and r-ypN (P < 0.001) were still independent predictors of OS. Trend tests for both ypN and r-ypN were significant (P < 0.001 for both factors) suggesting more advanced nodal stages were associated with a worse prognosis.
Comparison Between Number of Positive LNs and LNR
Kaplan-Meier curves and risk tables demonstrated an imbalanced distribution of ypN stage groups (Fig. 2A), while r-ypN staging led to better discrimination and well-balanced distribution of the number at risk (Fig. 2B). The 5-year OS rates for patients with ypN0–3 and r-ypN0–3 stage tumors reclassified by LNR were listed respectively in Table 1.
FIGURE 2.
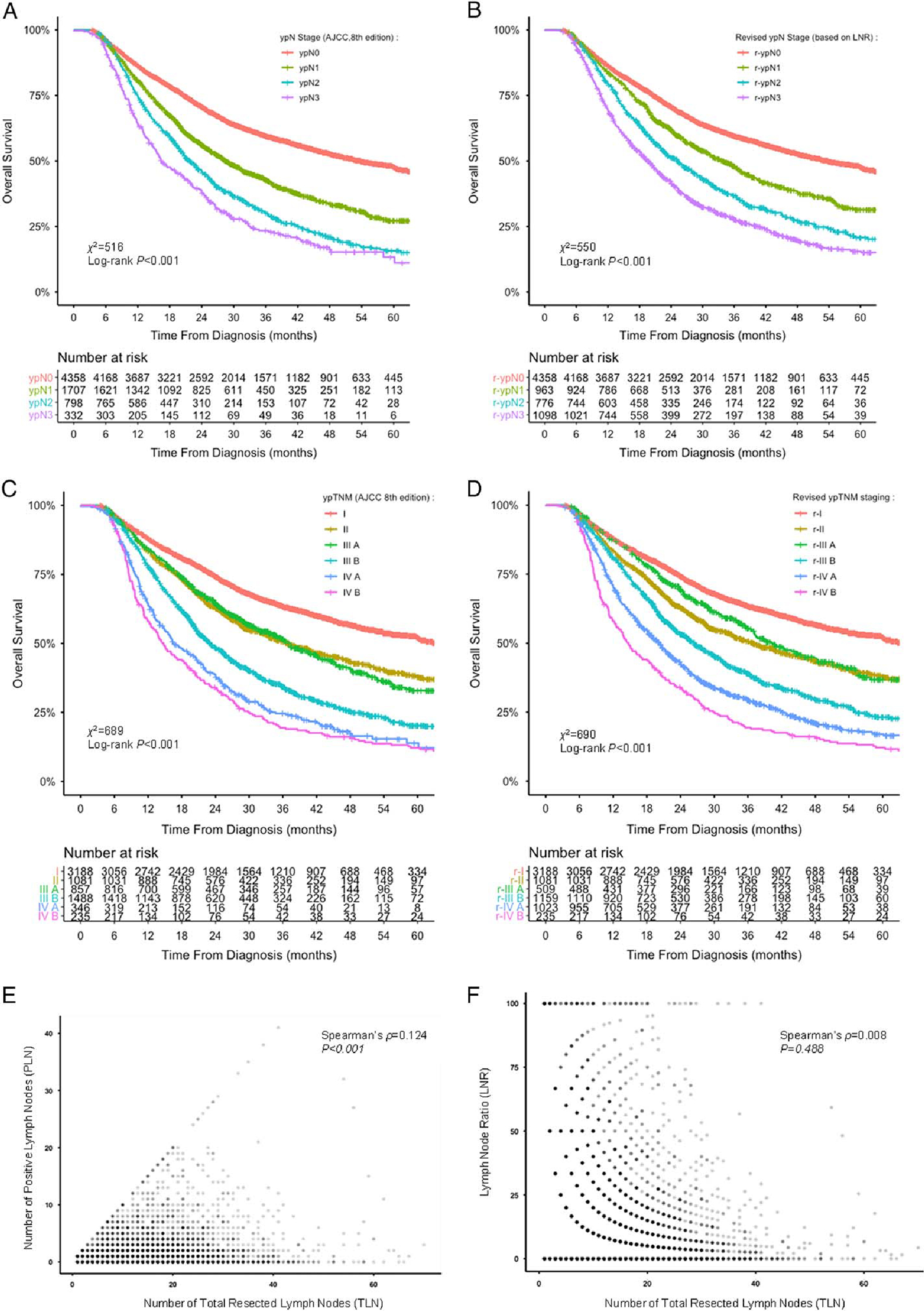
Comparison between the number of positive LNs and the LNR. Kaplan-Meier survival curves of OS in the entire cohort stratified according to the AJCC-8 ypN stage (A), r-ypN stage based on the LNR (B), ypTNM staging (C), and r-ypTNM staging based on the LNR (D); Spearman correlation analyses showing a significant correlation between the number of TLNs and the number of PLNs (E) but not between the number of TLNs and the LNR (F).
Regarding the significant prognostic value of the number of resected LNs (P < 0.001), Spearman ρ was calculated to detect rank correlation between this number and positive LNs or LNR. The number of positive LNs (a determining factor for the current ypN stage) was found to correlate with the number of resected LNs (ρ = 0.124, P < 0.001; Fig. 2E), whereas no such correlation was found between LNR and the number of resected LNs (ρ = 0.008, P = 0.488; Fig. 2F).
The 3-dimensional bar plot shows the reclassification of pathologic nodal stages and their prognostic contribution to OS (Fig. 3A). OS was significantly different between r-ypN strata within the ypN1 (P < 0.001; Fig. 3B) and ypN2 (P = 0.021; Fig. 3C) groups, but not within the ypN3 group (P = 0.360; Fig. 3D). However, there were no statistical differences in OS between ypN strata within each r-ypN group except r-ypN3 (P = 0.910 for r-ypN1; P = 0.590 for r-ypN2; P = 0.016 for r-ypN3; Figs. 3E–G).
FIGURE 3.
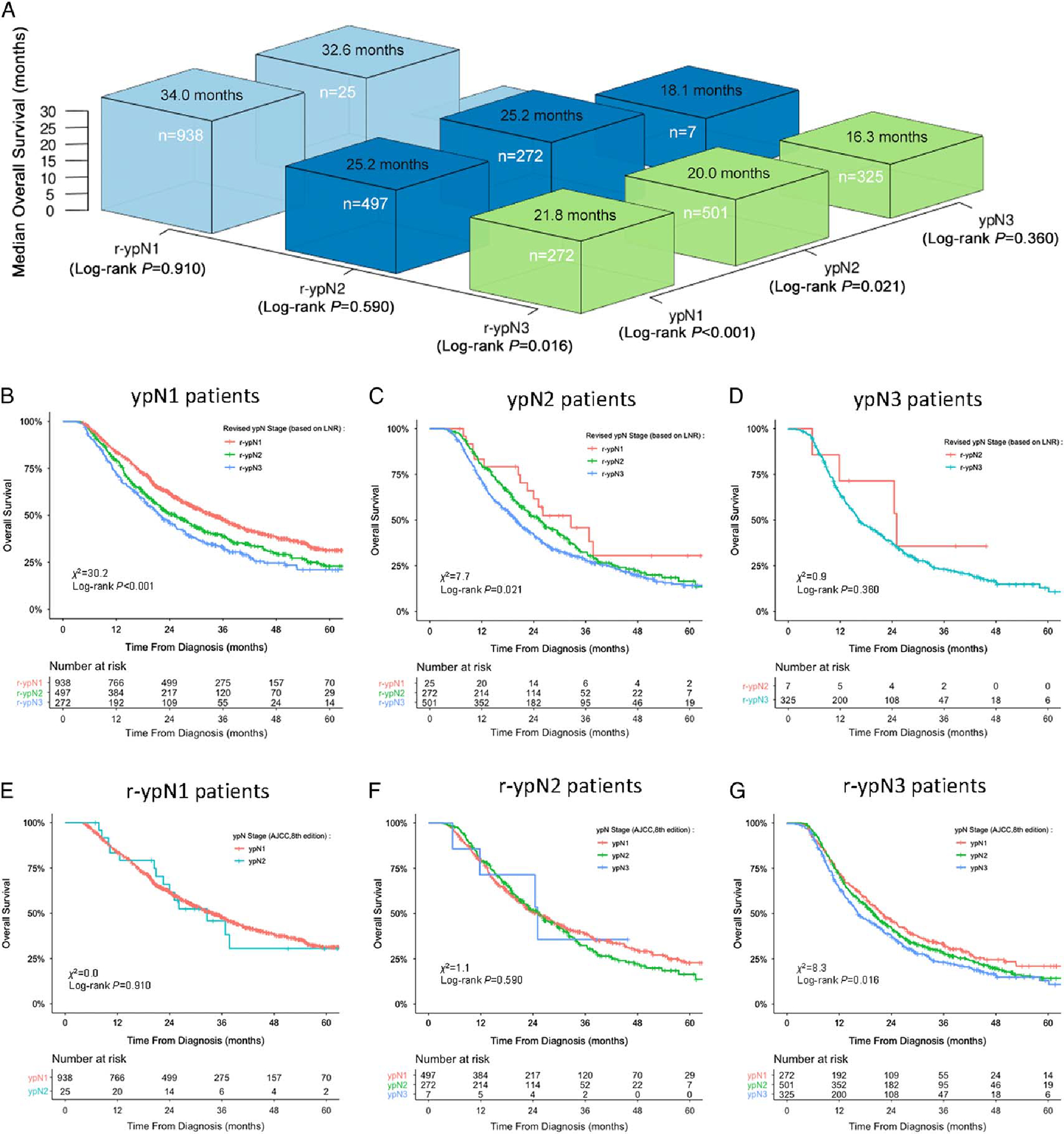
Reclassification of the pathologic nodal stage for prediction of OS. A, Three-dimensional bar plot of median survival times stratified by current and revised pN categories. B–D, Kaplan-Meier curves of OS of different subgroups stratified by r-ypN categories within each ypN group. E–G, Same curves in subgroups stratified by ypN stage within each r-ypN group.
Predictive Performance of the Current and the Revised Classification
The AIC and Harrell’s C-index (Table 2) were used to compare the predictive performance between the current and revised classification in unadjusted Cox proportional hazards models. The r-ypN classification showed a better-penalized goodness-of-fit (AIC = 60752 with r-ypN vs 60782 with ypN) and a better discriminant ability (Harrell’s C-index = 0.591 with r-ypN vs 0.587 with ypN) than the current ypN classification in predicting OS. In comparison with the AJCC-8 ypTNM staging, the revised staging showed better calibration (AIC = 60623 with r-ypTNM vs 60628 with ypTNM) and discrimination (Harrell’s C-index = 0.613 with r-ypTNM vs 0.610 with ypTNM) in predicting OS.
TABLE 2.
Comparison and Validation of Predictive Performance of the Current Versus the Revised Classification in Unadjusted Cox Regression Models for OS
| Subgroup and Unadjusted Cox Model | OS |
|
|---|---|---|
| AIC* | Harrell’s C-index† | |
|
| ||
| Entire cohort (n=7195) | ||
| ypN | 60782 | 0.587 |
| r-ypN | 60752 | 0.591 |
| ypTNM | 60628 | 0.610 |
| r-ypTNM | 60623 | 0.613 |
| External validation, NEOCRTEC5010 (n=182) | ||
| ypN | 665.20 | 0.682 |
| r-ypN | 651.24 | 0.701 |
| ypTNM | 669.91 | 0.688 |
| r-ypTNM | 663.45 | 0.699 |
The lower the AIC value is, the better the calibration.
The higher the Harrell’s C-index is, the better the discrimination.
r-ypN indicates revised N stage defined by lymph node ratio; r-ypTNM, revised ypTNM by replacing pN stage with r-pN; ypN, pathologic N stage (AJCC-8); ypTNM, postneoadjuvant therapy staging group (AJCC-8).
External Validation
Using the independent cohort from the NEOCRTEC5010 trial for external validation, the AIC value of the revised system was lower than that of the current system (651.24 with r-ypN vs 665.20 with ypN; 663.45 with r-ypTNM vs 669.91 with ypTNM) when predicting OS. The Harrell’s C-index of the revised classification was higher than the current system (0.701 with r-ypN vs 0.682 with ypN; 0.699 with r-ypTNM vs 0.688 with ypTNM). These results were consistent with our findings from the original NCDB cohort.
DISCUSSION
In the AJCC-8 Cancer Staging Manual established by the Worldwide Esophageal Cancer Collaboration (WECC) on data from 22,654 patients spanning 6 continents, the postneoadjuvant pathologic stage (ypTNM) was first separated from the pathologic stage after esophagectomy alone (pTNM) in cancer of the esophagus and the esophagogastric junction.8 In this new staging system, N remains the most important prognostic factor because survival decreases markedly with the number of positive LNs in postneoadjuvant esophageal cancer9; thus, threshold values of 0, 1, 3, and 7 positive LNs are still used for N categorization. However, neoadjuvant therapy has been shown to reduce the number of harvested LNs because of stromal atrophy, fibrosis, and shrinkage of LNs during esophageal and rectal surgeries.6,24 In the post hoc analysis from a phase III randomized controlled trial comparing neoadjuvant chemoradiation and surgery with surgery alone, Robb et al6 indicated neoadjuvant therapy was associated with a 27% reduction in the mean number of LNs harvested and a similar decrease in the number of metastatic LNs. They found that neoadjuvant therapy was an independent predictor of fewer LNs harvested during esophagectomy and concluded that staging systems must evolve to accurately reflect nodal downstaging after neoadjuvant therapy.
Recent studies have confirmed the value of adequate lymphadenectomy in patients given neoadjuvant therapy for esophageal cancer. Using the NCDB, Lutfi et al25 found that among esophageal cancer patients with a pathologic complete response after neoadjuvant therapy and esophagectomy, those who had ≥15 nodes sampled had improved 5-year OS versus patients who had <15 nodes sampled (56.1% vs 50.0%; P = 0.011). Using WECC data (AJCC-8), Raja et al26 found that lymphadenectomy during esophagectomy was a valuable adjunct to neoadjuvant therapy and that survival was maximized when a stage-dependent optimal number of nodes was resected. The present study also found that adequate lymphadenectomy resulted in better OS. This is probably because inadequate lymphadenectomy leads to understaging, resulting in an underestimation of disease severity.
Thus, the LNR, a new prognostic factor accounting for the number of metastatic LNs as well as that of resected LNs, has been proposed as a simple, convenient, and reproducible indicator to better identify subgroups of cancer patients with various tumors.12–14,16–18 The present results confirm that the number of positive LNs was found to be correlated with the number of resected LNs, whereas no such correlation was found between LNR and the number of resected LNs.
Here, the LNR was used to create 4 categories (0%, 0%–10%, 11%–20%, and 21%–100%) and these categories were used to develop a r-ypN. Using this new staging system showed survival differences between the categories; in fact, the ability of the r-ypN staging system to distinguish OS in patients staged r-ypN1 and r-ypN2 was better than the ability of the ypN staging system in patients staged ypN1 and ypN2. In addition, OS of ypN1 or ypN2 patients differed between r-ypN strata, whereas survival of r-ypN1 or r-ypN2 patients did not significantly differ between ypN strata. This suggested that the r-ypN classification based on LNR categories might be more homogeneous in predicting OS than ypN categories in postneoadjuvant esophageal cancer patients. Furthermore, the AIC and C-index showed that the predictive performance of r-ypN was better than that of ypN and that the performance of r-ypTNM was better than that of ypTNM.
ypTNM groupings according to the AJCC-8 are identical in squamous cell carcinoma and adenocarcinoma. However, in the NCDB used for this study, adenocarcinoma accounts for the vast majority, up to 75.7%. Therefore, we used another independent cohort from the NEOCRTEC5010 trial for external validation, which was a prospective study of neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced esophageal cancer from China, and all research subjects had squamous cell carcinoma. Patients who underwent neoadjuvant chemoradiation followed by surgery were analyzed as a validation cohort (n = 182). The results showed that the revised classification was superior to the current classification in distinguishing OS in squamous cell carcinoma, which was consistent with our findings in the original cohort from the NCDB. These results indicated that, in both esophageal adenocarcinoma and squamous cell carcinoma, LNR showed better performance than ypN in predicting OS in patients who underwent esophagectomy after neoadjuvant chemoradiation.
There are a number of limitations in this study that need to be addressed. This is a retrospective study, and although the NCDB offers excellent follow-up records, surgical and pathologic bias between hospitals may have affected the number of resected LNs. A second limitation is the lack of information regarding the location of the metastatic LNs in the NCDB. The location of the positive LNs has been shown in previous studies to impact prognosis in patients with esophageal cancer,27 but we could not account for that using this database. Another limitation is the potential for overfitting the data by choosing the optimal cutoff points for LNRs based on the entire cohort (using X-tile software). Finally, further external validation is necessary in different cohorts and populations to confirm these results.
In conclusion, LNR is an independent prognostic factor in esophageal cancer patients undergoing esophagectomy after neoadjuvant therapy and performs better and compensates for some of the potential shortcomings of the AJCC-8 ypN staging classification related to decreased LNs obtained after chemoradiation. This modified nodal staging system may provide more accurate staging for patients receiving neoadjuvant chemoradiation, thus resulting in more precise adjuvant treatment for these patients.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jean Iwaz (Service de Biostatistique-Bioinformatique, Hospices Civils de Lyon) for comments and revisions that have greatly improved the manuscript.
Supported by the National Key Research and Development Program of China (2021YFC2500900) National Natural Science Foundation of China (81871882, 82072557, 81902951), and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20172005) and Outstanding Academic Leader of Shanghai (20XD1402300).
Footnotes
J.L. is a robotic proctor for Intuitive Surgical Inc. The remaining authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36:2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan C, Shi Y, Jiang W, et al. How many lymph nodes should be dissected in esophagectomy with or without neoadjuvant therapy to get accurate staging? Dis Esophagus. 2020;33:doz009. [DOI] [PubMed] [Google Scholar]
- 6.Robb WB, Dahan L, Mornex F, et al. Impact of neoadjuvant chemoradiation on lymph node status in esophageal cancer: post hoc analysis of a randomized controlled trial. Ann Surg. 2015;261:902–908. [DOI] [PubMed] [Google Scholar]
- 7.Robb WB, Maillard E, Mariette C. Lymph node status after neoadjuvant chemoradiotherapy for esophageal cancer: implications for the extent of lymphadenectomy. Ann Surg. 2017;266:e53–e54. [DOI] [PubMed] [Google Scholar]
- 8.Rice TW, Ishwaran H, Kelsen DP, et al. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice TW, Lerut TEMR, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–883. [DOI] [PubMed] [Google Scholar]
- 12.Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. [DOI] [PubMed] [Google Scholar]
- 13.Soran A, Ozmen T, Salamat A, et al. Lymph node ratio (LNR): predicting prognosis after neoadjuvant chemotherapy (NAC) in breast cancer patients. Eur J Breast Health. 2019;15:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C-M, Lin B-J, Lu H-S, et al. Prognostic impact of metastatic lymph node ratio in advanced gastric cancer from cardia and fundus. World J Gastroenterol. 2008;14:4383–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262:991–998. [DOI] [PubMed] [Google Scholar]
- 16.Tong L, Gao P, Wang Z, et al. Can lymph node ratio take the place of pN categories in the UICC/AJCC TNM classification system for colorectal cancer? Ann Surg Oncol. 2011;18:2453–2460. [DOI] [PubMed] [Google Scholar]
- 17.Shao Y, Geng Y, Gu W, et al. Assessment of lymph node ratio to replace the pN categories system of classification of the TNM system in esophageal squamous cell carcinoma. J Thorac Oncol. 2016;11:1774–1784. [DOI] [PubMed] [Google Scholar]
- 18.Tan Z, Ma G, Yang H, et al. Can lymph node ratio replace pN categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol. 2014;9:1214–1221. [DOI] [PubMed] [Google Scholar]
- 19.American College of Surgeons. About the National Cancer Database. Accessed August 16, 2021. Available at: http://www.facs.org/quality-programs/cancer/ncdb/about.
- 20.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. [DOI] [PubMed] [Google Scholar]
- 21.Akaike H Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of Hirotugu Akaike. New York, NY: Springer. 199–213. [Google Scholar]
- 22.Harrell FE, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 23.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 24.Gao P, Song Y, Yang Y, et al. What is the minimum number of examined lymph nodes after neoadjuvant therapy in rectal cancer? J Gastrointest Surg. 2018;22:1068–1076. [DOI] [PubMed] [Google Scholar]
- 25.Lutfi W, Martinez-Meehan D, Dhupar R, et al. Higher lymph node harvest in patients with a pathologic complete response after neoadjuvant therapy for esophageal cancer is associated with improved survival. J Surg Oncol. 2020;121:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja S, Rice TW, Murthy SC, et al. Value of lymphadenectomy in patients receiving neoadjuvant therapy for esophageal adenocarcinoma. Ann Surg. 2021;274:e320–e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata H, Sugimura K, Yamasaki M, et al. Clinical impact of the location of lymph node metastases after neoadjuvant chemotherapy for middle and lower thoracic esophageal cancer. Ann Surg Oncol. 2019;26:200–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


