Abstract
OBJECTIVE
To examine the association of area-level socioeconomic status, rural-urban residence, and type of insurance with overall and cancer-specific mortality among patients with muscle-invasive bladder cancer.
METHODS
Using the Pennsylvania Cancer Registry, which collects demographic, insurance, and clinical information on every patient with cancer within the state, we identified all patients diagnosed with non-metastatic muscle-invasive bladder cancer between 2010 and 2016 based on clinical and pathologic staging. We used the Area Deprivation Index (ADI) as a surrogate for socioeconomic status and Rural-Urban Commuting Area codes to classify urban, large town, and rural communities. ADI was reported in quartiles, with 4 representing the lowest socioeconomic status. We fit multivariable logistic regression and Cox models to assess the relationship of these social determinants with overall and cancer-specific survival adjusting for age, sex, race, stage, treatment, rural-urban classification, insurance and ADI.
RESULTS
We identified 2597 patients with non-metastatic muscle-invasive bladder cancer. On multivariable analysis, Medicare (hazards ratio [HR] 1.15), Medicaid (HR 1.38), ADI 3 (HR 1.16) and ADI 4 (HR 1.21) were independent predictors of greater overall mortality (all P < 0.05). Female sex and receipt of non-standard treatment were associated with increased overall mortality and bladder cancer-specific mortality. There was no significant difference in both overall and cancer-specific survival between patients who were non-Hispanic White compared to non-White or between those from urban areas, large towns, or rural locations.
CONCLUSION
Lower socioeconomic status and Medicare and Medicaid insurance were associated with a greater risk of overall mortality while rural residence was not a significant factor. Implementation of public health programs may help reduce the gap in mortality for low SES at-risk populations.
Muscle-invasive bladder cancer (MIBC) is a serious condition that leads to high rates of mortality. A third of patients will die within 5 years.1 Despite advances in the management and treatment of MIBC, patient mortality has not significantly improved over the last 30 years.2 This is fairly unique to muscle-invasive bladder cancer, as during the same time period mortality from all cancers has decreased by more than 25%.3
The reasons for high mortality rates associated with MIBC are multifactorial, but likely include social determinants of health. Overall there is growing recognition of the importance of social determinants of health on cancer outcomes, but limited evidence specific to MIBC.4,5 Lower socioeconomic status, female sex, non-private health insurance, and being a racial minority are associated with increased bladder cancer-specific mortality.6–9 A potential reason for these differences in mortality stems from the fact that social determinants can negatively impact outcomes on many levels ranging from the individual level to the societal level. They are also impacted by multiple domains including the biological, behavioral, physical and sociocultural environment, and health care system, as presented in the National Institute on Minority Health and Health Disparities framework.9–13 However, only a few prior studies have specifically examined the effect of social determinants of health on outcomes for patients with muscle-invasive disease.8,9,14 Further, as treatment for MIBC becomes more concentrated in centers of excellence, traditionally underserved patient groups are less likely to receive care at these centers.15 Thus, a better understanding of the effects of social determinants on patient outcomes is important in the development of health policies and initiatives to decrease disparities in bladder cancer care.
For these reasons, we sought to better understand the role of social determinants and type of treatment received on outcomes for patients with MIBC. We utilized the Pennsylvania Cancer Registry, a large state-wide database, to examine the association of select proxies for social determinants with overall and cancer-specific mortality for patients with MIBC.
MATERIAL AND METHODS
Data Source and Study Population
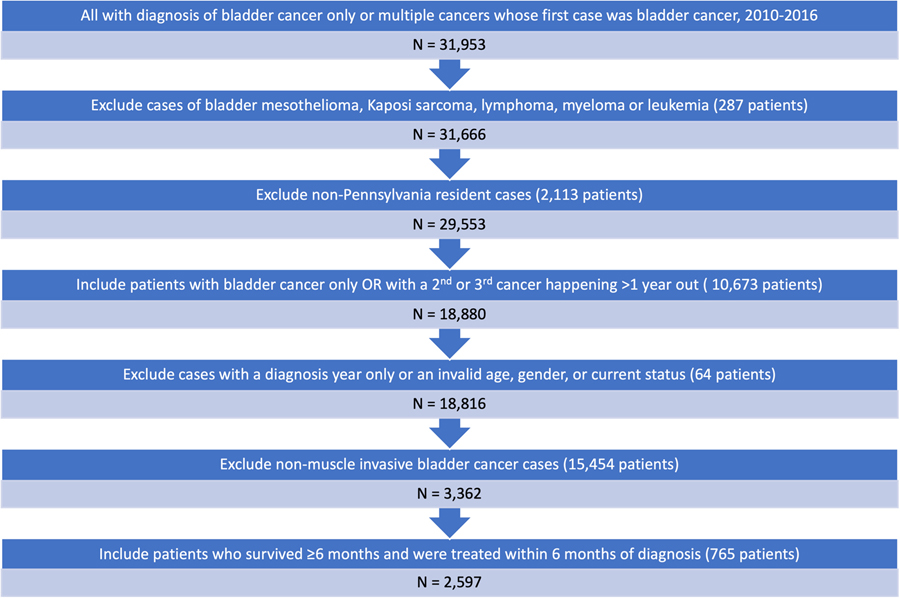
Using the Pennsylvania Cancer Registry, we identified patients diagnosed with nonmetastatic MIBC between 2010 and 2016. We excluded patients with pre-existing malignancies and patients diagnosed at autopsy or death. Additionally, we excluded patients who died within 6 months of diagnosis. Using these criteria, our study consisted of 2597 patients (Fig. 1).
Figure 1.
Patients’ inclusion and exclusion criteria for the final analytic sample. (Color version available online.)
Outcomes
The primary outcomes were overall and bladder cancer-specific survival. Overall survival was the occurrence of death from any cause. Bladder cancer-specific survival was determined based on the cause of death indicated in the Pennsylvania Cancer Registry. Since patients were excluded if they died within 6 months of diagnosis, duration of survival was defined as 6 months after diagnosis until death or censor date of December 31, 2016 (Fig. 1).
Independent Variables
The primary exposures included demographics, measures of select social determinants of health (race/ethnicity, sex, geographic location, insurance type, and Area Deprivation Index (ADI)), as well as type of treatment received. We used summary information on the first course of treatment abstracted by the registry. Treatments received included radical cystectomy, trimodal therapy (i.e., transurethral resection of the bladder tumor, chemotherapy, and radiation), and non-standard treatments (i.e., patients underwent only one or two of the trimodal treatment modalities).
Geographic residence was defined based on the United States Department of Agriculture Rural-Urban Commuting Area (RUCA) codes. The RUCA codes assign a value of 1–10 to census tracts, and we merged this information with patient census tract of residence codes available in the registry data. The RUCA value reflects population density and travel/commuting distance to urban centers. We defined urban as primary RUCA codes 1–3, large towns as RUCA codes 4–6, and rural areas as RUCA codes 7–10 as previously described.16 The ADI was used as a surrogate for socioeconomic status and was assigned to each patient based on census tract of residence. The ADI uses 17 census measures including poverty, income, education, employment, and housing status to provide a composite measure of neighborhood socioeconomic status.17 It is reported in quartiles as ADI 1–4 with 4 representing the highest deprivation or lowest area socioeconomic status.
Statistical Analysis
First, we delineated the overall number and frequency of demographic factors for patients with MIBC overall and stratified by treatment received (radical cystectomy, trimodal therapy, and non-standard treatments). Second, we fit a multivariable cox regression model for overall mortality, and a Fine and Gray proportional subdistribution hazards model for cancer-specific mortality treating any non-cancer mortality as a competing event. For both survival analysis models, we adjusted for age, sex, race, tumor stage, nodal stage, treatment type, insurance type, geographic location, and ADI. For overall mortality, death from any cause was the end point. For cancer-specific mortality, we used a Fine and Gray model in which any non-cancer-specific death was considered a competing event. Finally, we performed a survival analysis to generate Kaplan-Meier curves illustrating trends in survival by geographic location, ADI, and insurance type. Analyses were performed in SAS, version 9.4. All tests were 2-sided with the probability of a type I error set at 0.05. The Institutional Review Board deemed the study exempt from review (IRB STUDY19020149).
RESULTS
We identified 1923 men (74%) and 674 women (26%) diagnosed with non-metastatic MIBC between 2010 and 2016 (Table 1). The median follow up for our cohort was 11.5 months and there were 1226 total deaths (Supplementary Table 1). Compared with patients undergoing radical cystectomy, patients undergoing non-standard treatments had higher hazard of overall mortality (hazard ratio [HR] 2.20, 95% confidence interval [CI] 1.95–2.49) (Table 2). Female sex was associated with higher overall mortality (HR 1.26, 95% CI 1.14–1.40). Patients with Medicare (HR 1.15, 95% CI 1.01–1.31) and Medicaid (HR 1.38, 95% CI 1.11–1.72) insurance had worse overall survival than patients with private insurance. Overall mortality increased as ADI quartile increased, and differences relative to Q1 were significant for both Q3 (HR 1.16, 95% CI 1.02–1.33) and Q4 (HR 1.21, 95% CI 1.05–1.4). Patients receiving non-standard treatments also had worse bladder cancer-specific survival (HR 1.94, 95% CI 1.67–2.25) compared with those who had cystectomy or trimodal therapy. Additionally, female sex was associated with worse bladder cancer-specific survival (HR 1.32, 95% CI 1.17–1.5).
Table 1.
Patients’ baseline characteristics by treatments received.
| N | Total 2597 | Radical cystectomy 1321 | Trimodal therapy 323 | Non-standard treatments 953 |
|---|---|---|---|---|
| Age, years | ||||
| < 60 | 507 (19.5) | 335 (25.4) | 27 (8.4) | 145 (15.2) |
| 60–69 | 697 (26.8) | 423 (32.0) | 61 (18.9) | 213 (22.4) |
| 70–79 | 725 (27.9) | 412 (31.2) | 101 (31.3) | 212 (22.2) |
| > 80 | 668 (25.7) | 151 (11.4) | 134 (41.5) | 383 (40.2) |
| Race/ethnicity | ||||
| Non-Hispanic White | 2391 (92.1) | 1228 (93.0) | 291 (90.1) | 872 (91.5) |
| Non-White | 206 (7.9) | 93 (7.0) | 32 (9.9) | 81 (8.5) |
| Sex | ||||
| Male | 1923 (74.0) | 1016 (76.9) | 223 (69.0) | 684 (71.8) |
| Female | 674 (26.0) | 305 (23.1) | 100 (31.0) | 269 (28.2) |
| Tumor stage | ||||
| < =T2 | 1658 (63.8) | 582 (44.1) | 271 (83.9) | 805 (84.5) |
| T3 | 628 (24.2) | 544 (41.2) | 30 (9.3) | 54 (5.7) |
| T4 | 311 (12.0) | 195 (14.8) | 22 (6.8) | 94 (9.9) |
| Nodal stage | ||||
| N0/Nx | 2140 (82.4) | 982 (74.3) | 294 (91.0) | 864 (90.7) |
| > =N1 | 457 (17.6) | 339 (25.7) | 29 (9.0) | 89 (9.3) |
| Health insurance type | ||||
| Private | 758 (29.2) | 483 (36.6) | 46 (14.2) | 229 (24.0) |
| Medicare | 1500 (57.8) | 654 (49.5) | 246 (76.2) | 600 (63.0) |
| Medicaid | 156 (6.0) | 99 (7.5) | 13 (4.0) | 44 (4.6) |
| Other/Uninsured/Unknown | 183 (7.0) | 85 (6.4) | 18 (5.6) | 80 (8.4) |
| Rurality of residence | ||||
| Urban | 2205 (84.9) | 1117 (84.6) | 279 (86.4) | 809 (84.9) |
| Large Town | 252 (9.7) | 135 (10.2) | 24 (7.4) | 93 (9.8) |
| Rural | 140 (5.4) | 69 (5.2) | 20 (6.2) | 51 (5.4) |
| Area Deprivation Index | ||||
| Q1 | 650 (25.0) | 337 (25.5) | 79 (24.5) | 234 (24.6) |
| Q2 | 642 (24.7) | 326 (24.7) | 83 (25.7) | 233 (24.4) |
| Q3 | 652 (25.1) | 335 (25.4) | 76 (23.5) | 241 (25.3) |
| Q4 | 653 (25.1) | 323 (24.5) | 85 (26.3) | 245 (25.7) |
NOTE: frequency with column percents are presented. Trimodal therapy includes transurethral resection of the bladder tumor, chemotherapy, and radiation. Non-standard treatments describe cases in which patients underwent only one or two of the trimodal treatment modalities.
All percentages may not add up to 100 due to rounding.
Table 2.
Adjusted risks of overall and cancer-specific mortality among the study population.
| Overall Mortality HR (95% CI) | Cancer-Specific Mortality HR (95% CI) | |
|---|---|---|
| Age, years | ||
| < 60 | Reference | Reference |
| 60–69 | 0.95 (0.8–1.13) | 0.96 (0.8–1.15) |
| 70–79 | 1.30 (1.09–1.55) | 1.10 (0.90–1.35) |
| > 80 | 1.83 (1.54–2.19) | 1.40 (1.15–1.72) |
| Race/Ethnicity | ||
| Non-Hispanic White | Reference | Reference |
| Non-White | 0.99 (0.83–1.17) | 1.13 (0.90–1.40) |
| Sex | ||
| Male | Reference | Reference |
| Female | 1.26 (1.14–1.40) | 1.39 (1.17–1.50) |
| Tumor stage | ||
| < =T2 | Reference | Reference |
| T3 | 1.42 (1.24–1.62) | 1.58 (1.34–1.85) |
| T4 | 1.90 (1.67–2.16) | 1.88 (1.59–2.23) |
| Nodal stage | ||
| N0/Nx | Reference | Reference |
| > =N1 | 1.57 (1.39–1.78) | 1.87 (1.61–2.16) |
| Primary treatment for MIBC | ||
| Cystectomy | Reference | Reference |
| Trimodal | 1.07 (0.90–1.28) | 1.19 (0.96–1.46) |
| Non-standard | 2.20 (1.95–2.49) | 1.94 (1.67–2.25) |
| Health insurance type | ||
| Private | Reference | Reference |
| Medicare | 1.15 (1.01–1.31) | 1.03 (0.88–1.20) |
| Medicaid | 1.38 (1.11–1.72) | 1.16 (0.9–1.49) |
| Other/Uninsured/Unknown | 1.10 (0.89–1.36) | 0.89 (0.67–1.16) |
| Rurality of residence | ||
| Urban | Reference | Reference |
| Large Town | 0.89 (0.76–1.05) | 0.83 (0.67–1.04) |
| Rural | 0.93 (0.75–1.15) | 1.09 (0.85–1.40) |
| Area Deprivation Index | ||
| Q1 | Reference | Reference |
| Q2 | 1.00 (0.88–1.15) | 1.03 (0.88–1.22) |
| Q3 | 1.16 (1.02–1.33) | 1.10 (0.92–1.30) |
| Q4 | 1.21 (1.05–1.40) | 1.06 (0.88–1.27) |
CI, Confidence Interval; HR, Hazard Ratio; MIBC, Muscle-Invasive Bladder Cancer
NOTE: for overall mortality, we used a multivariable cox regression model; for cancer-specific mortality, we used a Fine and Gray model. For both of these regressions, we adjusted for age, sex, race, tumor stage, nodal stage, type of primary treatment for MIBC, health insurance type, rurality of residence, and ADI. Bold values are statistically significant (P value < 0.05).
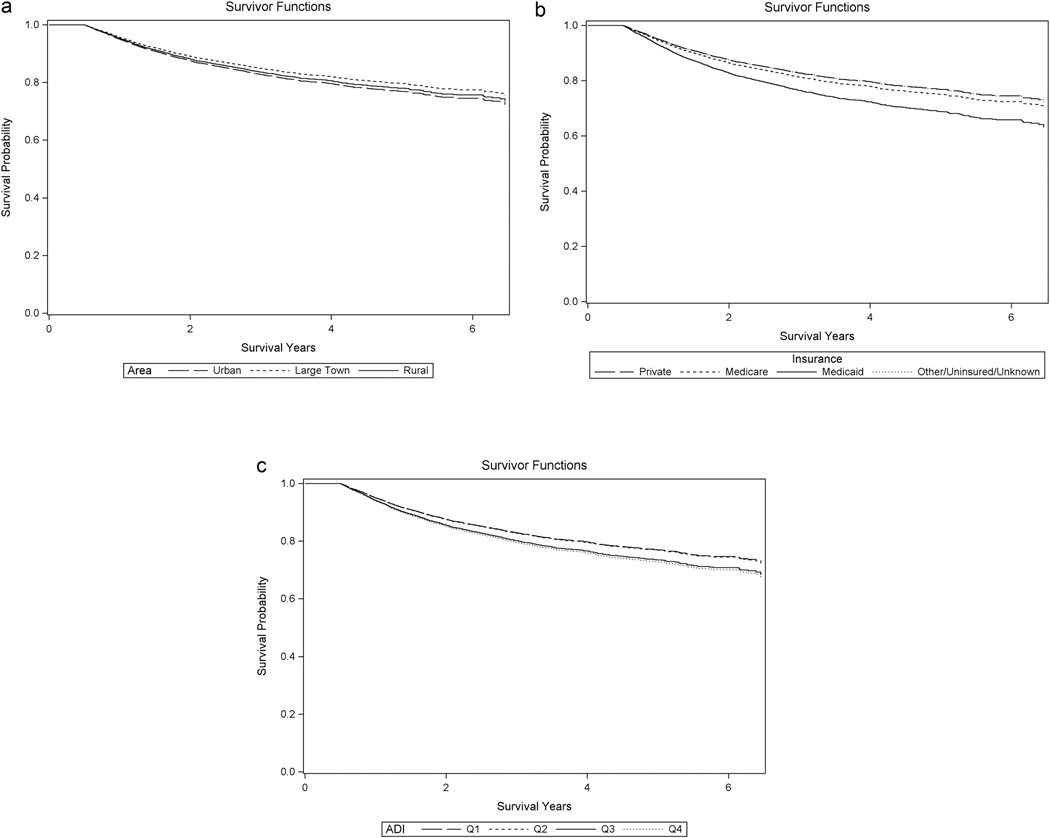
Kaplan-Meier curves indicate cumulative survival stratified by rurality of residence, health insurance type, and ADI quartiles (Fig. 2). There was no significant difference in overall survival between patients from urban areas, large towns, or rural locations. Patients living in more deprived areas (quartiles 3 [P = 0.03] and 4 [P < 0.01]) as well as those with Medicaid (P < 0.01) or Medicare (P = 0.04) insurance had decreased overall survival.
Figure 2.
Kaplan-Meier overall survival curves stratified by social determinants of health. Overall survival by geographic region (A) there was no significant difference was seen between patients from urban (black dashed line), large town (black dotted line), and rural areas (black solid line). Overall survival by insurance type (B) patients with Medicare (black dotted line) (P = 0.04) and Medicaid (black solid line) (P < 0.01) insurance compared with private insurance (black dashed line) had decreased survival. There was no significant overall mortality difference for other/unknown/uninsured (gray dotted line tracking closely with the black dashed line) compared with private insurance (black dashed line). Overall survival by ADI quartile (C): there were no significant differences between Q1 (black dashed line) and Q2 (black dotted line tracks closely with black dashed line), but Q3 (black solid line) (P = 0.03) and Q4 (P < 0.01) (gray dotted line) where both associated with worse survival outcomes.
DISCUSSION
In this population-based analysis using data from the Pennsylvania Cancer Registry, we found an association between select indicators of key social determinants of health and mortality. These proxies for social determinants of health that we found to be associated with higher overal mortality include living in a more deprived area as well as having Medicaid or Medicare insurance. However, higher area deprivation and Medicaid or Medicare insurance were not associated with increased bladder cancer-specific mortality. Lastly, female patients and those receiving non-standard treatment had worse overall mortality and bladder cancer-specific mortality.
Unlike genotypes, which are not modifiable risk factors, there are modifiable factors related to social determinants of health that were associated with increased overall mortality. Our results suggest that, after accounting for differences in treatment received, patients in more socioeconomically deprived areas continued to experience outcomes worse than their peers from less socioeconomically deprived areas. There are multiple potential contributors to this finding, such as differences in employment status, which intersects with low education level and limited access to care, and also, lack of healthcare infrastructure in these deprived neighborhoods.10 Additionally, this may be due, in part, to patients from more deprived areas not having the means to get to the hospital that originally provided their treatment. Thus, they may end up in lower volume centers closer to their home for follow-up care, which could impact outcomes.18 The role of each of these factors is difficult to assess and outside the scope of our data, but our results indicate that area deprivation is an important correlate of mortailty among patients with muscle-invasive bladder cancer.
The observed higher overall mortality in patients with MIBC from more deprived areas is likely impacted by worse health behaviors and higher rates of comorbid conditions. Individuals from a lower socioeconomic positions are twice as likely to have an unhealthy diet, have higher prevelance of tobacco usage, and lower rates of physical activity compared with peers from higher positions.18 Furthermore, rates of medical conditions such as cardiovascular disease, cardiovascular disease-specific mortality, and diabetes are increased in deprived areas.19–21 Finally, patients who developed a second malignancy greater than 1 year after the diagnosis of bladder cancer were included in our survival analysis. It is known that being from a more deprived area and having non-private insurance has a negative effect on survival for patients with other malignancies such as breast, uterine, cervical, head and neck, lung, colorectal, and prostate cancer.4,5 The impact of these malignancies on survival is beyond the scope of our study.
Another potentially modifiable factor is insurance status. Patients with non-private insurance had worse overall mortality, which is consistent with findings from other studies.7,9 This previously has been attributed to higher rates of surveillance for patients with Medicare insurance.22 Other factors accounting for this finding may include delays in treatment greater than 90 days for this group of patients.7 Additionally, patients with non-private insurance are less likely to receive their care at high-volume centers and are less likely to receive neoadjuvant chemotherapy.7 Although it is inherently difficult to asses the direct effect of insurance type as the population of patients covered by each differs signficantly, our multivariate results suggest that there may be important differences across insurance groups in access to treatment.
Additionally, only 63% of patients in our sample received treatment considered standard of care (i.e., cystectomy or trimodal therapy). Those receiving non-standard treatments had significantly higher mortality. The failure of patients to receive appropriate treatment for MIBC is a known issue, with recent studies showing that only around half of patients with MIBC receive treatment with curative intent.12,14,22 The reasons for this include far travel distance to centers offering definitive treatments, racial disparities, patients declining radical cystectomy due to its high morbidity, and even confusion among urologists, radiation oncologists, and medical oncologists on optimal chemotherapy and radiotherapy regimens.11,12,23
In addition to receiving non-standard treatment, female sex was associated with both worse overall and bladder cancer-specific mortality, even after adjusting for cancer stage at time of diagnosis. This is in agreement with other studies that have shown an association between female sex and mortality.8,24,25 Possible explanations for this difference in outcomes include delays in diagnosis of bladder cancer for women due to the degree of similarity between urinary tract infection symptoms and bladder cancer symptoms.8,26,27
The lack of association between social determinants such as race, type of health insurance, and ADI with worse bladder-cancer-specific outcomes was unexpected given the results of previous studies.4–7,9 However, recent evidence has found that receipt of complex cancer surgery at a high-quality hospital was associated with no significant differences in mortality between individuals living in the most deprived neighborhoods compared to least deprived.28 Therefore, a possible explanation for our findings may be that patients receiving care at high-quality hospitals, such as a National Cancer Institute or Commission on Cancer designated center, in our cohort had similar outcomes despite the presence of social determinants that have been associated with negative outcomes. However, our dataset is limited in that it does not contain the details of the hospital at which each patient was treated. This represents an area of research where further work is needed to validate these findings.
These findings have important implications for policies and guidelines to support effective and equitable cancer care delivery. First, the undertreatment of MIBC impacts patient outcomes such as mortality. The American Urological Association Office of Education has sought to address this with state-of-the-art courses, plenary sessions, and clear guidelines.29 Despite this, undertreatment persists. Second, policies could be developed with the aim of targeting people with non-private insurance or who live in deprived areas to ensure they get the care they need. The treatment of MIBC continues to migrate towards centers of excellence, with up to 80% of patients getting treated at such locations.12 This can function as a barrier to care as the rate of treatment with radical cystectomy dropped by 40% if patients lived over 50 miles from a center of excellence.22 Potential solutions include ensuring that all patients are able to access care at these centers by streamlining care between these centers and community providers, helping patients with transportation, and initiating a prompt transfer from community hospitals to these centers should a complication occur.
Our study should be evaluated in the context of several limitations. First, we rely on registry data without accompanying medical record or claims information, thus we cannot account for details not present in the registry, including comorbidities or office visit information. Second, our patient population is limited in that it is from a single state, potentially limiting generalizability. However, Pennsylvania is a large state with substantial populations of patients across all demographic groups, that closely parallel the United States as a whole.30 Despite these limitations, this study is important because it highlights the association of social determinants of health, some of which are modifiable, that negatively impact mortality, and have important implications for policy and practice. Our study is unique in that it joins only a few prior studies reporting on the association of social determinants of health and mortality outcomes specifically for patients with MIBC.6,8
Supplementary Material
Footnotes
Financial Disclosure:
Valentina Grajales was supported in part by the 2020 Urology Care Foundation Residency Research Award Program and The Kahlert Foundation. Bruce L. Jacobs is supported in part by the Shadyside Hospital Foundation. The remaining authors declare no conflict of interest.
APPENDIX A. SUPPORTING INFORMATION
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.urology.2023.02.045.
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. JCO. 2001;19:666–675.(Feb 1). [DOI] [PubMed] [Google Scholar]
- 2.Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–225.(Jun). [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.(Jan). [DOI] [PubMed] [Google Scholar]
- 4.Boyd C, Zhang-Salomons JY, Groome PA, Mackillop WJ. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999;17:2244–2255.(Jul). [DOI] [PubMed] [Google Scholar]
- 5.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2:403–411. (Jun). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gild P, Wankowicz SA, Sood A, et al. Racial disparity in quality of care and overall survival among black vs. white patients with muscle-invasive bladder cancer treated with radical cystectomy: a national cancer database analysis. Urol Oncol. 2018;36:469.e1–469.e11. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher SA, Cole AP, Lu C, et al. The impact of underinsurance on bladder cancer diagnosis, survival, and care delivery for individuals under the age of 65 years. Cancer. 2020;126:496–505.(01). [DOI] [PubMed] [Google Scholar]
- 8.Grajales V, Bandari J, Hale NE, et al. Associations between female sex and treatment patterns and outcomes for muscle-invasive bladder cancer. Urology. 2020;151:169–175.(Jul) (S0090429520308232). [DOI] [PubMed] [Google Scholar]
- 9.Smith ND, Prasad SM, Patel AR, et al. Bladder cancer mortality in the United States: a geographic and temporal analysis of socioeconomic and environmental factors. J Urol. 2016;195:290–296. (Feb). [DOI] [PubMed] [Google Scholar]
- 10.National Institute on Minority Health and Health Disparities [Internet]. NIMHD Research Framework. [cited 2021 Jan 18]. Available from: 〈https://www.nimhd.nih.gov/about/overview/research-framework/nimhd-framework.html〉.
- 11.Washington SL, Neuhaus J, Meng MV, Porten SP. Social determinants of appropriate treatment for muscle-invasive bladder cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1339–1344. (Aug). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the national cancer data base. Eur Urol. 2013;63:823–829.(May). [DOI] [PubMed] [Google Scholar]
- 13.Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–78.(Jan). [DOI] [PubMed] [Google Scholar]
- 14.Shah AA, Sun Z, Eom KY, et al. Treatment disparities in muscle-invasive bladder cancer: evidence from a large statewide cancer registry. Urol Oncol Semin Orig Investig. 2022;40:164.e17–164.e23. (Jan) (S1078143921005524). [DOI] [PubMed] [Google Scholar]
- 15.Mossanen M, Izard J, Wright JL, et al. Identification of underserved areas for urologic cancer care: Underserved Areas for Urologic Cancer Care. Cancer. 2014;120:1565–1571.(May 15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maganty A, Sabik LM, Sun Z, et al. Under treatment of prostate cancer in rural locations. J Urol. 2020;203:108–114.(Jan). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93:1137–1143. (Jul 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinh VQ, Trinh QD, Tian Z, et al. In-hospital mortality and failure-to-rescue rates after radical cystectomy. BJU Int. 2013;112:E20–E27.(Jul 1). [DOI] [PubMed] [Google Scholar]
- 19.Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Can J Cardiol. 2010;26(Suppl C):S8C–S13C.(Sep). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly V Diabetes prevalence and socioeconomic status: a population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J Epidemiol Commun Health. 2000;54:173–177.(Mar 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. Int J Epidemiol. 2002;31:600–613.(Jun). [DOI] [PubMed] [Google Scholar]
- 22.Gore JL, Litwin MS, Lai J, et al. Use of radical cystectomy for patients with invasive bladder cancer. JNCI J Natl Cancer Inst. 2010;102:802–811.(Jun 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120–137. (Jul). [DOI] [PubMed] [Google Scholar]
- 24.Kluth LA, Rieken M, Xylinas E, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. Eur Urol. 2014;66:913–919.(Nov 1). [DOI] [PubMed] [Google Scholar]
- 25.Messer JC, Shariat SF, Dinney CP, et al. Female gender is associated with a worse survival after radical cystectomy for urothelial carcinoma of the bladder: a competing risk analysis. Urology. 2014;83:863–868.(Apr 1). [DOI] [PubMed] [Google Scholar]
- 26.Marks P, Soave A, Shariat SF, Fajkovic H, Fisch M, Rink M Female with bladder cancer: what and why is there a difference? Translational Andrology and Urology; vol 5, No 5 (October 2016): Translational Andrology and Urology (Urothelial Carcinoma) [Internet]. 2016. [cited 2016 Jan 1]; Available from: 〈http://tau.amegroups.com/article/view/9763〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69:300–310.(Feb 1). [DOI] [PubMed] [Google Scholar]
- 28.Bonner S, Ibrahim AM, Kunnath N, Dimick JB, Nathan H. Neighborhood deprivation, hospital quality and mortality after cancer surgery. Ann Surg. 2022;277:73–78..Sep 19 [cited 2022 Dec 2]; Publish Ahead of Print. Available from. 〈https://journals.lww.com/10.1097/SLA.0000000000005712〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitti VW. Role of the urologist in managing bladder cancer. AUA News. 2018:21–22.(Oct). [Google Scholar]
- 30.DATA USA: Pennsylvania [Internet]. DATA USA. [cited 2020 Dec 3]. Available from: 〈https://datausa.io/profile/geo/pennsylvania#demographics〉. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.