Abstract
Fluorescein is an agent that accumulates in areas of blood-brain barrier breakdown and is commonly used in neurosurgical oncology to assist with lesion localization and visualizing the extent of resection. It is considered to be cost-effective and has a favorable safety profile. Studies on the utilization of fluorescein demonstrate an improved extent of tumor resection and increased overall survival. Currently, fluorescein detection systems are all microscope based, leading to limitations such as decreased maneuverability, limited visualization of the entire operative field, and Significant cost associated with obtaining and maintaining a neurosurgical operating microscope. Three consecutive craniotomy patients for tumor resection were included, and surgery was carried out under loupe fluorescence guidance using the ReVeal 450 System, and also a surgical microscope for comparison. Loupe-mounted fluorescence system enabled excellent visualization of fluorescence in all three cases. In this manuscript, we describe our experience with a loupe-mounted fluorescein detection system in three patients with malignant gliomas. We found that the loupe-mounted system offered excellent ability to visualize fluorescein fluorescence. Although loupe-mounted systems are not an alternative to surgical microscopes, they could be a useful surgical adjunct for superficial lesions and in low-middle income counties.
Keywords: Fluorescein, Fluorescence, Neoplasms, Intraoperative monitoring
Fluorescent agents are commonly used in neurosurgery to assist with intraoperative visualization of both tumor and vascular pathology. Utilization of fluorescence in neurosurgery has been demonstrated to not only increase the rates of gross total resection, but also translated into improved outcomes.1 Most commonly used fluorescent agents in neurosurgery today include fluorescein, indocyanine-green (ICG), and 5-aminolevulinic acid (5-ALA). Fluorescein is an organic dye that has high absorptivity with maximum absorption at a wavelength of 494nm with a large fluorescence quantum yield at a peak emission of 521 nm.2 Fluorescein is a widely utilized fluorescent dye due to its lower-cost, ease of administration, and favorable safety profile. It is widely utilized in ophthalmology for retinal angiogaphy and has been used in dental medicine for plaque detection. Visualization of fluorescein requires a blue excitation light source and yellow filter. Fluorescein dye accumulates at areas of blood-brain barrier breakdown, such as in the cases of brain tumor. The most severe reported reaction to fluorescein administration is anaphylaxis. In ophthalmologic literature the rate of severe anaphylaxis is 1 in 15,000 cases.2–4
Use of fluorescein in clinical neurosurgery was first described in 1948 for the treatment of brain malignancy and since then has been widely studied in its use for both tumor and vascular pathologies.2, 5, 6 Numerous studies have demonstrated that the addition of fluorescein in brain tumor surgery resulted in decreased recurrence rates in metastatic disease6 and increased extent of resection and survival in patients with gliomas.1, 7–10 The requirement for a surgical microscope with optical filters for dye excitation and fluorescence detection can be a significant limiting factor for the wider adoption of fluorescein, especially in low to middle-income countries. For superficial lesions, many brain tumor surgeons primarily operate with surgical loupe magnification and thus are less likely to utilize fluorescein dye. Additionally, providers located in low- to middle income countries (LMIC) can be limited by microscope availability and may have to only rely on surgical loupe magnification for tumor surgery.11 Here we describe our experience with the ReVeal 450 system in 3 patients with malignant glioma. To our knowledge, this is the first description of a loupe-based fluorescein detection system in the treatment of human brain tumors. This is a proof-of-concept study on the feasibility of using a loupe-mounted fluorescein detection system in the surgical resection of brain tumors and it is not the intention of the authors to compare the effectiveness of loupe versus microscopic magnification in tumor surgery.
Study framework
Patient selection:
Three consecutive patients who under-went craniotomy for tumor resection by a single surgeon at our institution were included in this series (Table I). Off-label use of fluorescein for intraoperative tumor visualization has been approved by University of Pittsburgh Medical Center since 2012. Surgery was carried out under both white and blue light. Fluorescence pictures were taken with a cellphone camera (iPhone 11 Pro Max, Apple, Cupertino, CA, USA) covered by orange safety glass and through filtered telescopic lens to simulate surgeon’s view. Surgical microscope (OPMI Pentero, Zeiss, Oberkochen, Germany) was utilized in all cases to provide imaging comparison. Pre- and postoperative MRIs were obtained to evaluate extent of resection.
Table I.—
Patient demographics.
| Patient # | Gender | Age | Location | Diagnosis | Prior surgery | Prior radiation |
|---|---|---|---|---|---|---|
|
| ||||||
| Patient 1 | Male | 37 | Parietal | GBM | Yes | Yes |
| Patient 2 | Female | 53 | Frontal | GBM | No | No |
| Patient 3 | Male | 57 | Temporal | GBM | Yes | No |
Fluorescein administration:
500 mg of 10% fluorescein (AK-Fluor, Akorn, Lake Forest, IL, USA) was administered after opening the dura mater and accessing the intradural space.
Equipment:
ReVeal 450 system was provided by Designs for Vision, Inc. (Bohemia, NY, USA). The system consists of a battery powered headlight with an excitation wavelength of 450 nm. The loupe itself has 2.5x magnification with long pass 495 nm filters which provides 91% light transmission while blocking everything below 495 nm. Carrier lenses also have custom 495nm filters. Orange safety glasses are provided to the assistants.
Results
The ReVeal 450 system includes a frame-mounted, battery-powered, headlight with an excitation wavelength of 450nm which can be switched off using either manual control on the battery pack or by flipping down the attached orange filter (Figure 1A, B). The ReVeal 450 is worn by the primary surgeon in usual surgical loupe fashion (Figure 1C). Orange safety glasses can be worn by the assistant and surgical technicians for visualization of fluorescence (Figure 1D).
Figure 1.—
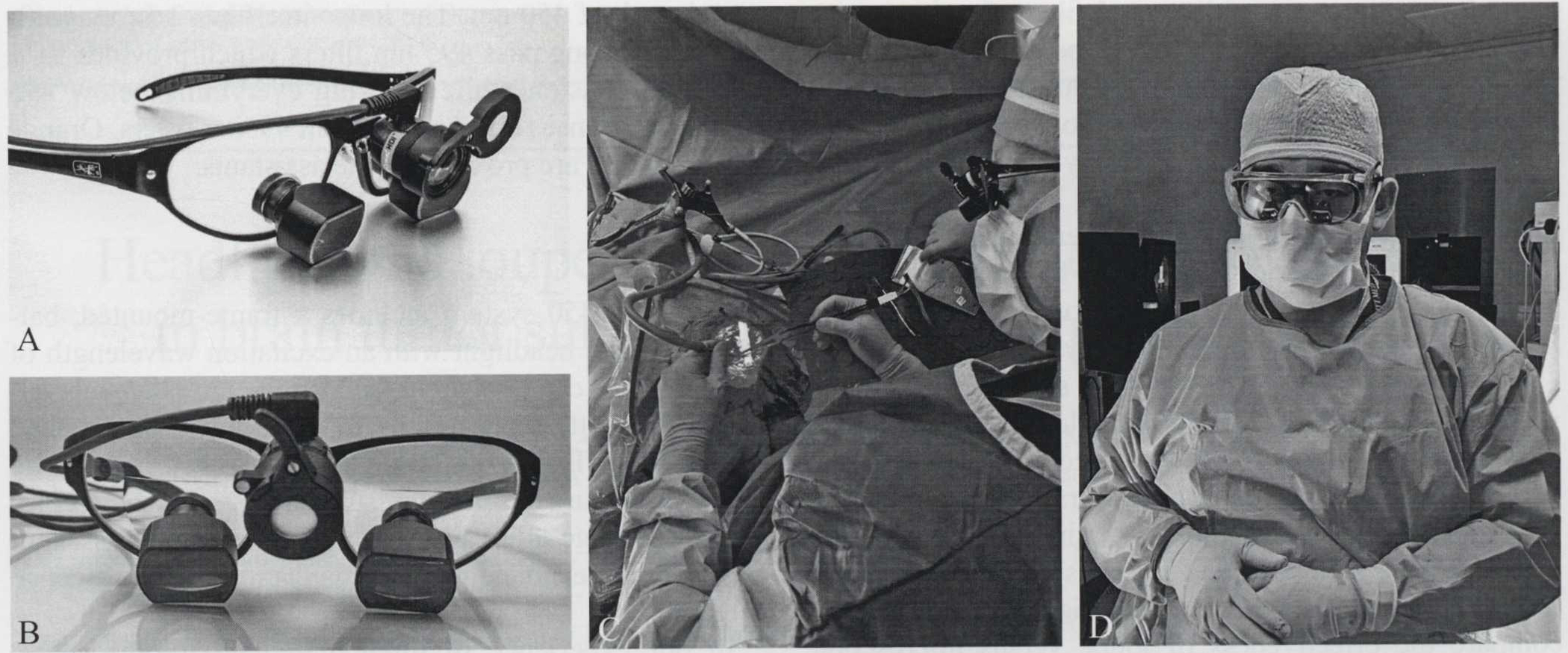
A, B) ReVeal 450 loupe and headlight system. Incorporating blue light emitting loupe-mounted headlight with 495-nm filters on carrier lens and 2.5x telescopes; C) blue light emission from ReVeal 450; D) orange safety glasses can be fitted over assistant’s unfiltered loupes.
In our experience of three malignant glioma cases, we were able to use the ReVeal 450 system to visualize fluorescence in all the cases. Fluorescence intensity was comparable between ReVeal 450 and the operative microscope. The system’s ability to distinguish fluorescence from tumor tissue and background fluorescence is also comparable to that of an operative microscope (Figure 2B, C). In Figure 3B, we provide an example of non-specific staining of areas of gliosis by fluorescein in a case of redo craniotomy. In all three cases we were able to achieve gross total resection using fluorescein-enabled loupes (Figure 2, 3). Fluorescent tissues were sent to pathology and was confirmed to be high-grade glioma. We found that when compared to microscopic magnification, loupe-based magnification offered the benefit of maneuverability and allowed us to visualize the whole surgical field. A significant limitation we encountered is the poor illumination offered by 450 nm blue light. We propose that the assistant should wear a white light headlight to allow for rapid switching between blue and white light conditions. Future models should allow emission of both white and blue light and have a mechanism that allows for easy switching between light modes. Another expected drawback with the loupe-based system was our difficulty with visualizing deep-seated lesions, where the intraoperative microscope provided a large improvement in visualization. In all three cases, intraoperative neuro-navigation and ultrasonography were also utilized to assist with tumor resection.
Figure 2.—
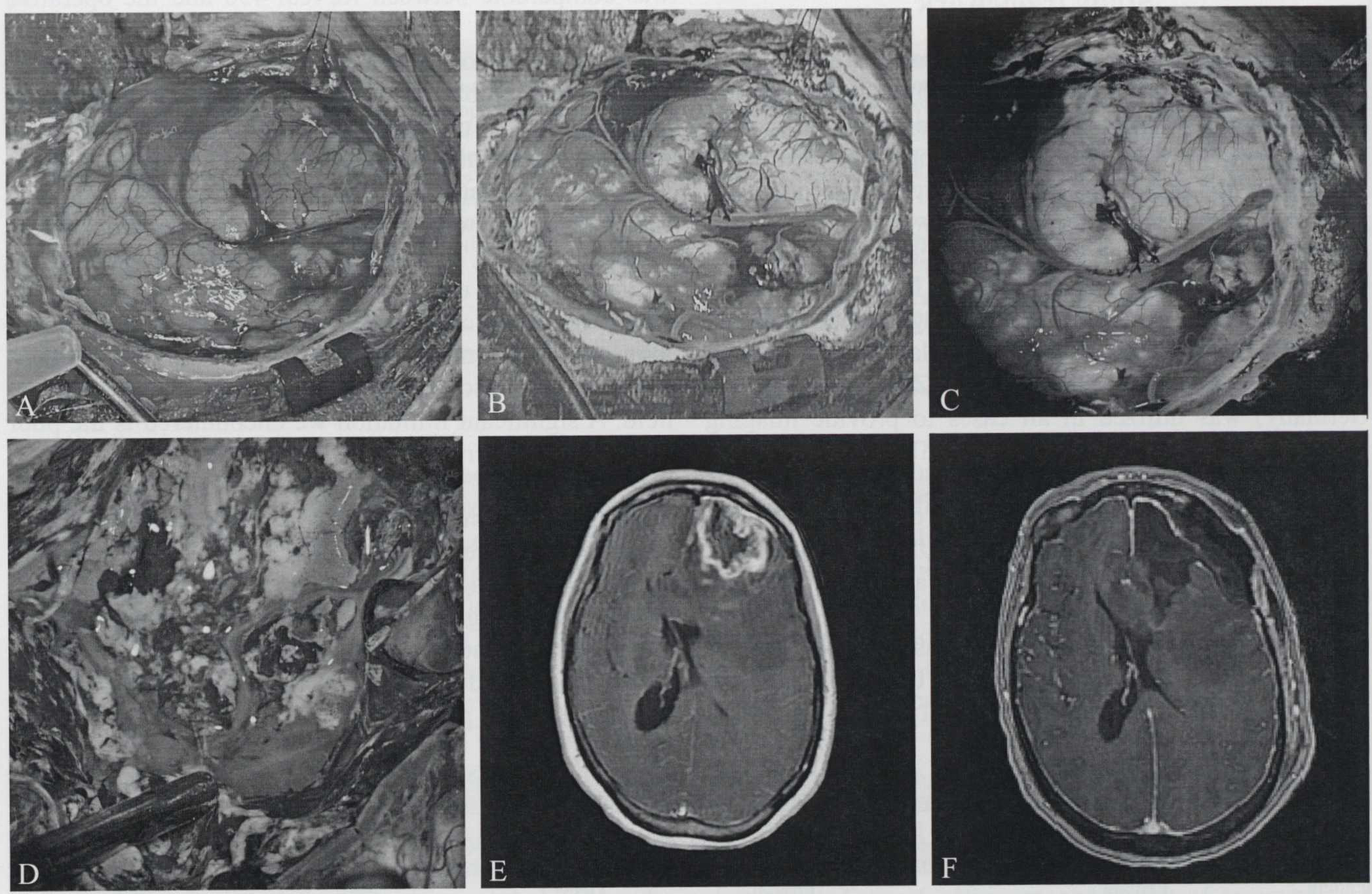
A-C) Side to side comparison of white-light, ReVeal 450, and operative microscope; D) intraoperative image demonstrating increased background fluorescence; E, F) pre- and postoperative MRI demonstrating gross-total resection.
Figure 3.—
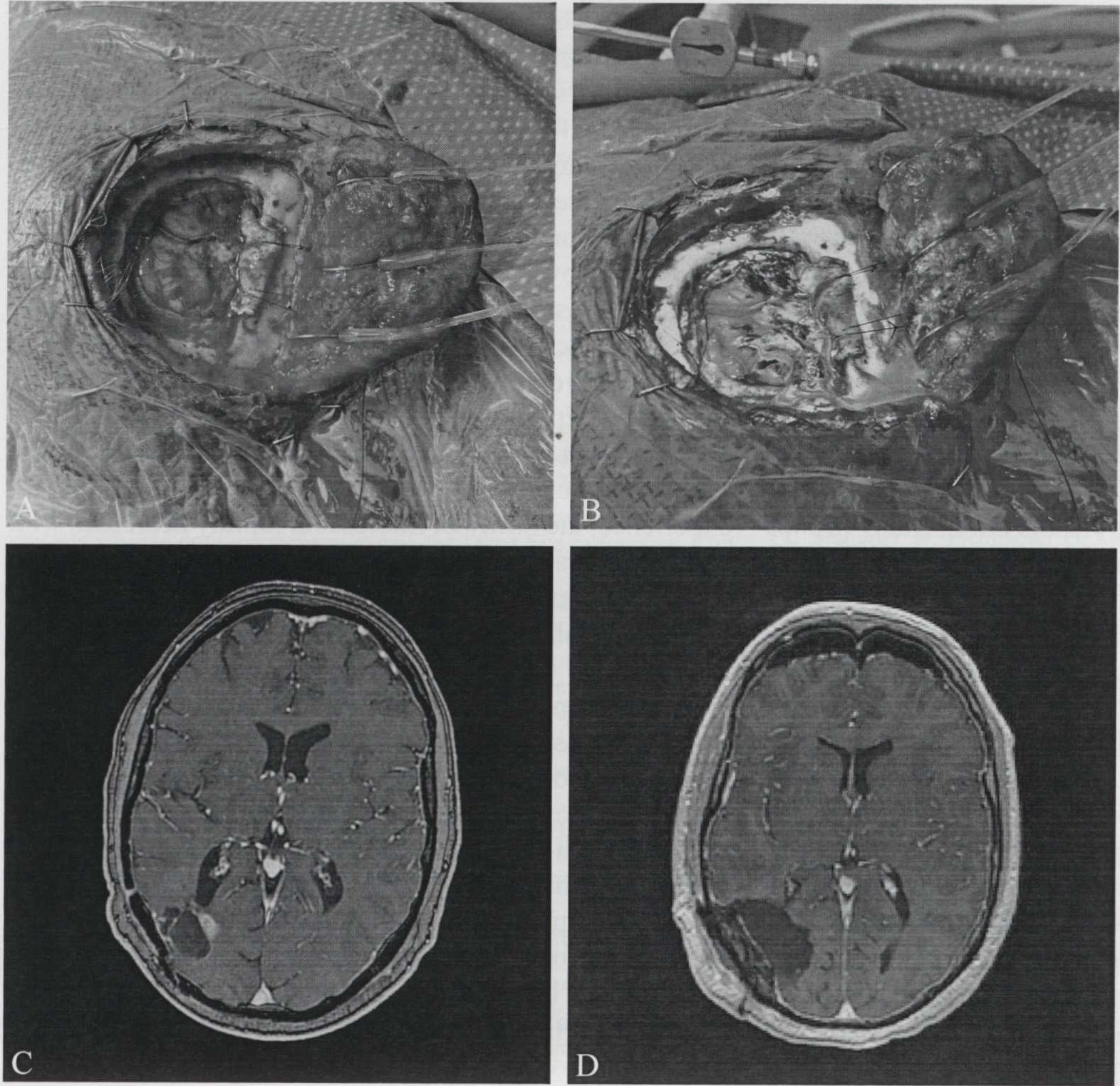
A, B) Side to side comparison of white-light and ReVeal 450 in a patient with deep recurrence of tumor. Note non-specific staining in the areas gliosis and scarring from prior surgery; C, D) pre- and postoperative MRIs demonstrating gross-total resection.
Discussion
The benefit of fluorescence in brain tumor surgery has been widely studied, with 5-ALA and fluorescein as the most promising agents. Currently, the only approved indication for the use of fluorescein in the USA is for retinal angiography.12 The use of fluorescein for intraoperative visualization of brain tumor is on an off-label basis. There is preponderance of safety data on fluorescein use in patients. Due to its favorable safety profile, fluorescein has been approved for intraoperative visualization of brain tumors at our institution since 2012. Although intravenous fluorescein administration has been associated with anaphylactic reactions, there has been no published report of anaphylaxis or other life-threatening side-effects from fluorescein use in neuro-oncology.13 Given its off-label nature, it is important to explicitly discuss the use of fluorescein with the patient and other members of the care team to both ensure adequate understanding and proper monitoring of side effects. A significant barrier to more widespread intraoperative use of fluorescein in brain tumor surgery is the requirement of an operative microscope. This represents a technical barrier to surgeons who prefer to resect tumors under surgical loupe magnification as well as a financial barrier to surgeons in LMIC where fluorescein-capable microscopes are limited in availability.6 Prior attempts at overcoming the above-mentioned limitations included use of 3D printed microscope attachments.14 Additionally, for superficial and less vascularized lesions, many tumor surgeons prefer to operate using loupe magnification over microscopy due to the ability to visualize a larger area of the operative field without constant adjustment of the microscope. The ReVeal 450 system represents the first commercial loupe-based system for intraoperative visualization of fluorescein. In our experience, The ReVeal 450 system provided excellent infraoperative visualization of fluorescein labelled tissue. The use of ReVeal 450 integrated well with the operating room workflow without the need for additional accommodations. It is important to note that the timing our fluorescein administration is different from those reported in the literature. We administered fluorescein immediately following incision and opening of the dura. It is reported in literature that to minimize non-specific extravasation, fluorescein should be administered between 2–4 hours prior to the need for visualization. Many groups have found that the optimal timing for administration is at the time of anesthesia induction.15
The FLUOGLIO trial evaluated fluorescein-guided resection in high-grade gliomas. In the prospective phase II report, Acerbi et al. enrolled 46 patients with HGGs and was able to achieve greater than 98% resection in 93.4% of the cases.16 Furthermore, they performed a total of 50 biopsies at tumor margins and found that fluorescein had a sensitivity and specificity of 80.8% and 79.1% respectively.16 A prospective study by Koc et al. compared rates of gross total resection between patients who received intraoperative fluorescein and those who did not. They found that use of fluorescein increases the rate of gross total resection from 55% to 83%.9 Okuda et al. evaluated the effectiveness of fluorescein in 36 patients with metastatic brain lesions. Gross total resection was achieved in 31 out of 36 patients. They found that they had substantially lower rates of local recurrence (20%) when compared to published historical controls suggesting that fluorescein leads to improved resection of brain metastases and improved local confrol.6
Significant controversy surrounds the use of fluorescein in brain tumor surgery and in particular when compared with 5-ALA. This controversy stems from the mechanism of fluorescein accumulation within areas of blood-brain barrier breakdown, whereas 5-ALA is metabolized and accumulates intracellularly in the form of fluorescent porphyrins. While fluorescein can accumulate within tumors, it is also found in areas of edematous brain, and areas of brain that have undergone mechanical manipulation.17 5-ALA in contrast is very specific to tumor cells.17 In a series of studies where both fluorescein and 5-ALA were co-administered to patients with malignant glioma, the authors found that fluorescein tends to accumulate in areas of disrupted blood-brain barrier and performed poorly in the margins of tumor when compared to 5-ALA.18, 19 In a retrospective single-institutional comparison of fluorescein with 5-ALA for malignant gliomas, the authors found no statistically significant differences in residual tumor volume, or percentage of patients with residual tumors. Of concern, the authors also noted increased progression -free survival and overall survival in the fluorescein group which raises the prospect of patient selection bias.20 We certainly share the concern over lack of specificity with fluorescein. However, given its ability to stain lower-grade tumors, its cost-effectiveness, and low side-effect profile, we make the argument that there is a role for fluorescein in brain tumor surgery, especially in low-middle income countries. In our experience, the utility of fluorescein rapidly decreases with increased duration of surgery and frequent tissue manipulation. Often times, the degree of fluorescence, rather than its presence or absence, is the true indicator of tumor versus normal brain tissue. This can be misleading to inexperienced surgeons who are perhaps to a lesser degree able to adequately distinguish background fluorescence of normal brain and more avid tumor fluorescence. Intraoperative pathological examination of fluorescent tissue was consistent with high-grade glioma. With increased comfort with fluorescein, the surgeon can distinguish “true” tumor fluorescence from fluorescence from edema or tissue injury. Accounting for this, we would like to propose that fluorescein-enabled loupes allow for increased maneuverability and speed, while allowing the user a greater view of the operative field and improve the surgeon’s ability to distinguish between disease tissue and background fluorescence.
In future work, we aim to collect a larger case series incorporating both low and high-grade gliomas to fully determine the utility of a loupe-mounted fluorescein detection system. As loupe-based technologies improve, we anticipate that this will become increasingly integrated into augmented reality solutions and become an integral part in neurosurgical oncology. It is important to note that fluorescence is only a single tool in the surgeon’s armamentarium which should also include other adjunct technologies such as neuro-navigation and intraoperative ultrasound, which is another low-cost solution that could increase surgical resection rates.21–23
Conclusions
Fluorescein-enabled headlight and loupe system offers excellent intraoperative visualization of glioblastoma. The system offers additional benefit of maneuverability and wider field of view. We believe this system can be of interest to neurosurgeons who prefer performing brain tumor surgery under loupe magnification and to those neurosurgeons who are located in low-middle income countries where an operating microscope is not readily available.
Footnotes
Conflicts of interest.—Pascal O. Zinn received ReVeal 450 headlight and loupe system from Design for Vision. all other authors have no conflicts of interest.
References
- 1.Francaviglia N, Iacopino DG, Costantino G, Villa A, Impallaria P, Meli F, et al. Fluorescein for resection of high-grade gliomas: A safety study control in a single center and review of the literature. Surg Neurol Int 2017;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewelt C, Nemes A, Senner V, Wölfer J, Brokinkel B, Stummer W, et al. Fluorescence in neurosurgery: its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B 2015;148:302–9. [DOI] [PubMed] [Google Scholar]
- 3.Kwan AS, Barry C, McAllister IL, Constable I. Fluorescein angiography and adverse drug reactions revisited: the Lions Eye experience. Clin Exp Ophthalmol 2006;34:33–8. [DOI] [PubMed] [Google Scholar]
- 4.Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, et al. Fluorescein angiography complication survey. Ophthalmology 1986;93:611–7. [DOI] [PubMed] [Google Scholar]
- 5.Moore GE, Peyton WT. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg 1948;5:392–8. [DOI] [PubMed] [Google Scholar]
- 6.Okuda T, Kataoka K, Yabuuchi T, Yugami H, Kato A. Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J Clin Neurosci 2010;17:118–21. [DOI] [PubMed] [Google Scholar]
- 7.Acerbi F, Broggi M, Eoli M, Anghileri E, Cuppini L, Polio B, et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien) 2013;155:1277–86. [DOI] [PubMed] [Google Scholar]
- 8.Höhne J, Schebesch KM, de Laurentis C, Akçakaya MO, Pedersen CB, Brawanski A, et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg 2019;125:e158–64. [DOI] [PubMed] [Google Scholar]
- 9.Koc K, Anik I, Cabuk B, Ceylan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg 2008;22:99–103. [DOI] [PubMed] [Google Scholar]
- 10.Schebesch KM, Proescholdt M, Höhne J, Hohenberger C, Hansen E, Riemenschneider MJ, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir (Wien) 2013;155:693–9. [DOI] [PubMed] [Google Scholar]
- 11.Mansur A, Oswari S, Perdana Wahjoepramono PO, Kusdiansah M, Bernstein M. Awake Craniotomy in a Low- to Middle-Income Country: A Sustainability Analysis. World Neurosurg 2018;118:332–41. [DOI] [PubMed] [Google Scholar]
- 12.Tsang SH, Sharma T. Fluorescein Angiography. Adv Exp Med Biol 2018;1085:7–10. [DOI] [PubMed] [Google Scholar]
- 13.Ha SO, Kim DY, Sohn CH, Lim KS. Anaphylaxis caused by intravenous fluorescein: clinical characteristics and review of literature. Intern Emerg Med 2014;9:325–30. [DOI] [PubMed] [Google Scholar]
- 14.Lovato RM, Vitorino Araujo JL, Esteves Veiga JC. Low-Cost Device for Fluorescein-Guided Surgery in Malignant Brain Tumor. World Neurosurg 2017;104:61–7. [DOI] [PubMed] [Google Scholar]
- 15.Belykh E, Shaffer KV, Lin C, Byvaltsev VA, Preul MC, Chen L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front Oncol 2020;10:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acerbi F, Broggi M, Schebesch KM, Höhne J, Cavallo C, De Laurentis C, et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin Cancer Res 2018;24:52–61. [DOI] [PubMed] [Google Scholar]
- 17.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401. [DOI] [PubMed] [Google Scholar]
- 18.Schwake M, Stummer W, Suero Molina EJ, Wölfer J. Simultaneous fluorescein sodium and 5-ALA in fluorescence-guided glioma surgery. Acta Neurochir (Wien) 2015;157:877–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano H, Nakayama N, Ohe N, Miwa K, Shinoda J, Iwama T. Pathological analysis of the surgical margins of resected glioblastomas excised using photodynamic visualization with both 5-aminolevulinic acid and fluorescein sodium. J Neurooncol 2017;133:389–97. [DOI] [PubMed] [Google Scholar]
- 20.Rasmus WH, Christian BP, Bo H, Anders RK, Mette KS, Bjame WK, et al. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: a single-center retrospective study. Journal of Neurosurgery 2020;133:1324–31. [DOI] [PubMed] [Google Scholar]
- 21.Orringer DA, Golby A, Jolesz F. Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 2012;9:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pino MA, Imperato A, Musca I, Maugeri R, Giammalva GR, Costantino G, et al. New Hope in Brain Glioma Surgery: The Role of Intraoperative Ultrasound. A Review. Brain Sci 2018;8:E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W 3rd, et al. Applications of Ultrasound in the Resection of Brain Tumors. J Neuroimaging 2017;27:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


