Abstract
Objectives:
Head and neck cancer patients that require major reconstruction often have advanced-stage disease. Discharge disposition of patients can vary and impact time to adjuvant treatment. We sought to examine outcomes in patients discharged to skilled nursing facilities (SNF) compared to those discharged home, including the impact on adjuvant therapy initiation and treatment package time (TPT).
Methods:
Patients with head and neck squamous cell carcinoma treated with surgical resection and microvascular free flap reconstruction from 2019 to 2022 were included. Retrospective review was conducted to evaluate the impact of disposition on time to radiation (RT) and TPT.
Results:
230 patients were included, with 165 (71.7%) discharged to home and 65 (28.3%) discharged to SNF. 79.1% of patients were recommended adjuvant therapy. Average time to RT was 59 days for patients discharged to home compared to 70.1 days for patients discharged to SNF. Disposition was an independent risk factor for delays to starting RT (p = 0.03). TPT was 101.7 days for patients discharged to home versus 112.3 days for those who discharged to SNF. Patients discharged to SNF had higher rates of readmission (p < 0.005) compared to patients discharged home in an adjusted multivariate logistic regression.
Conclusions:
Patients discharged to an SNF had significantly delayed time to initiation of adjuvant treatment and higher rates of readmission. Timeliness to adjuvant treatment has recently been established as a quality measure, thus identifying delays to adjuvant treatment initiation should be a priority.
Level of Evidence:
3
Keywords: adjuvant therapy, disposition, head and neck, microvascular free flap, radiation therapy, readmission, reconstruction, skilled nursing facility, treatment package time
INTRODUCTION
Head and neck cancer patients who undergo oncologic resection and microvascular free flap reconstruction often require adjuvant therapy. Timely initiation of adjuvant therapy has been shown to play a significant role in treatment outcomes.1–4 Delays in receiving adjuvant treatment after surgery and longer treatment package time (TPT) have all been found to result in poorer survival and locoregional control.1–5 Studies report that initiating adjuvant therapy after 6 weeks is correlated with poorer survival with the risk of death progressively increasing after 7 weeks.2 Compliance with starting adjuvant within 6 weeks from surgery is low, with one study finding that over 50% of patients do not start their postoperative radiation treatment within this time.6
Multiple factors impact time to adjuvant treatment, but one of interest is discharge disposition.7 Postoperatively, patients are often discharged to skilled nursing facilities (SNF) to assist patients in recovering from surgery.8 However, in a recent study investigating patients with colorectal, pancreatic, bladder, or lung cancer, patients discharged to SNF were found to be less likely to receive timely adjuvant treatment and have poorer survival compared with patients who are discharged home.7
Generally, patients discharged to SNF tend to have worse functional status, are generally more frail, and have higher rates of postoperative mortality.7–9 Risk factors, including advanced age, longer hospital length of stay (LOS), higher number of comorbidities, and postoperative complications, have been associated with discharge to SNF.8–12 These patients often have pre-existing mobility issues and elevated frailty as measured by risk analysis index (RAI) and higher index of comorbidity measured by the Charlson Comorbidity Index (CCI).12,13
In addition, there are several steps before adjuvant therapy can begin, and often, SNF does not have the resources to coordinate numerous appointments as compared to a patient’s family/social support. Patients discharged to SNF often do not have caretakers available in the first place, which precludes their discharge back to home.
Currently, there is no literature on the impact of disposition on patients’ time to adjuvant treatment for head and neck malignancy. This study sought to investigate the effect of discharge disposition on patient outcomes. Specifically, we examined the time to adjuvant therapy and post-hospital complications in patients with head and neck squamous cell carcinoma (HNSCC) who underwent major oncologic resection and microvascular free flap reconstruction. We compared outcomes between patients who were discharged home with those discharged to SNF. We hypothesize that more frail patients are discharged to SNF, and such patients undergo adjuvant treatment in a delayed fashion compared with those who are discharged home. We also predict patients discharged to SNF have higher readmission rates and emergency department (ED) visits.7
MATERIALS AND METHODS
Four hundred forty-seven treatment-naïve patients with head and neck malignancy treated with surgical resection and microvascular free flap reconstruction from 2019 to 2022 within a large academic medical center were identified on initial screen (Fig. 1). Retrospective chart review of a database was performed to study the impact of disposition on time to radiation therapy (RT) and TPT. One hundred and ninety eight patients were excluded for the following reasons: diagnosis of non-squamous cell carcinoma malignancy, history of neoadjuvant therapy, double free flaps in initial reconstruction, loss to follow-up within 30 days from discharge, or if they declined recommended adjuvant treatment. Patients who underwent neoadjuvant therapy were excluded due to differences in their cancer treatment (i.e., immunotherapy or clinical trial). Double-free flaps were excluded due to our limited patient population (n = 2); the patients also had significantly prolonged operative times beyond our average. An additional 19 patients were excluded due to missing the recommended adjuvant treatment entirely. Reasons for missing adjuvant treatment included recurrence of malignancy before starting adjuvant therapy; alternative treatment preferred or offered (i.e., immunotherapy and clinical trial); radiation-mask intolerance (e.g., claustrophobia); declining further treatment after surgery, or death. Seven (36.8%) of these patients were discharged to SNF. Two hundred thirty patients were ultimately included in the final analysis. This study was approved by the institutional review board (IRB).
Fig. 1.
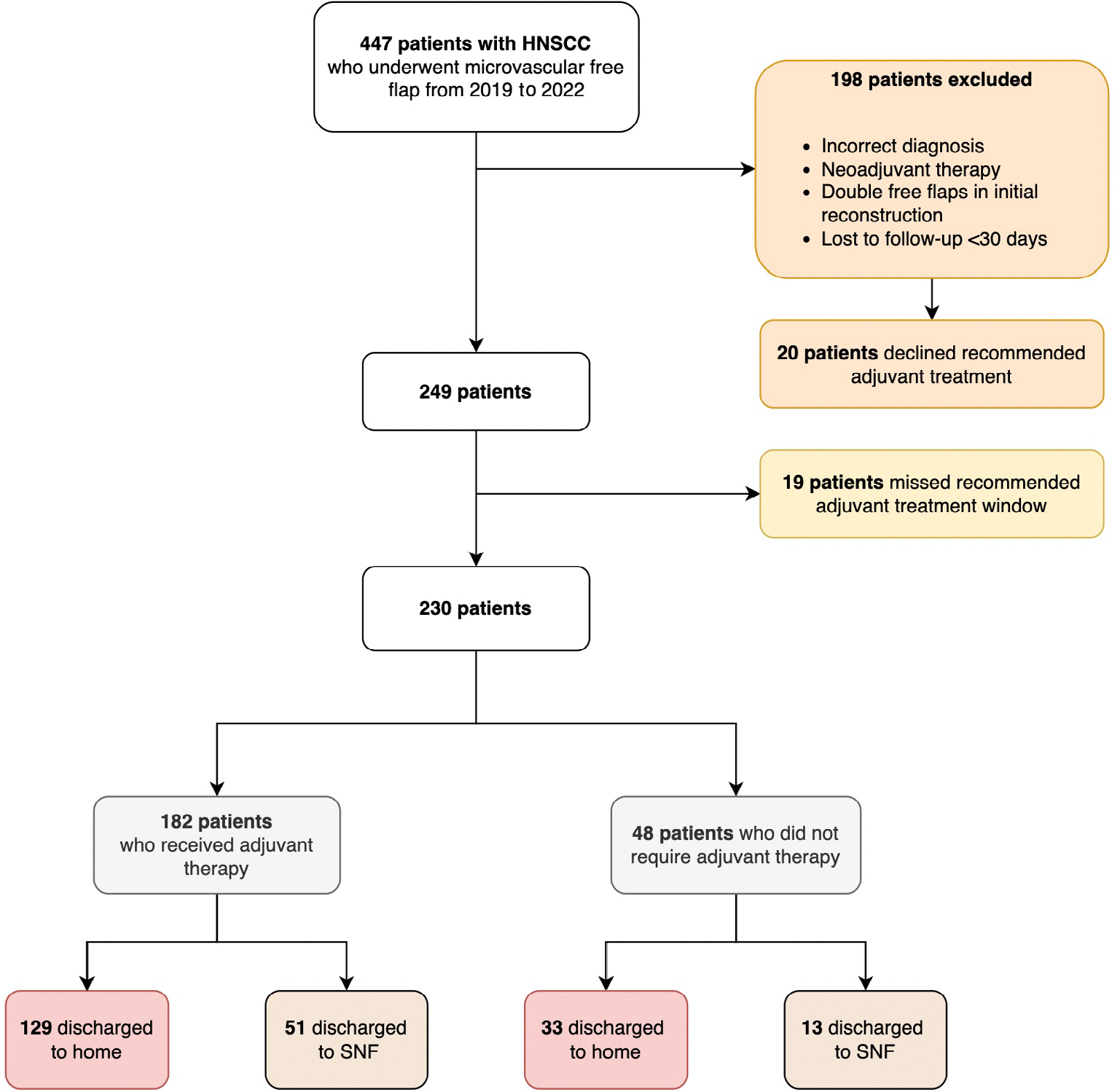
Patient selection. HNSCC = head and neck squamous cell carcinoma; SNF = skilled nursing facility. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Study Design, Cohort, and Outcomes
Data including age, primary surgical site, cancer stage, reconstruction type, risk analysis index (RAI), CCI, readmission rates, ED visits, hospital LOS, presence of a surgical airway in the form of a tracheostomy or laryngectomy, presence of enteral feeds in the form of a nasogastric tube (NGT) or gastric tube (GT), and disposition location to home or SNF were collected. Data on adjuvant therapy recommendation (chemotherapy and radiation [CRT] or RT alone), time to RT and calculated TPT were also collected.
Readmission rates and ED visits were measured if they occurred from the day of discharge up to 60 days from the day of surgery, or prior to initiation of adjuvant therapy, whichever date occurred first. ED visits that did not result in an admission were collected as a separate variable.
30-day and 90-day mortality as well as recurrence were collected; however, during the time of the study, most patients were within 3 months to 3 years from surgery, and thus, longer disease-free survival or overall survival was not analyzed.
Primary surgical sites included all head and neck subsites: oral cavity, oropharyngeal, larynx/hypopharynx, sinonasal cavity, salivary gland, cutaneous face/neck, and unknown primary. For the purpose of our study, larynx and hypopharynx were categorized as one anatomical site because all these patients underwent total laryngectomy or total laryngopharyngectomy. Cancer staging followed the American Joint Committee on Cancer (AJCC) 8th edition, which was the most updated guideline at the time of our study. Reconstruction types included all microvascular free flaps (Table I). The RAI is measured prior to surgery. CCI is calculated using several comorbid conditions, including age, cardiovascular disease, peripheral vascular disease, cerebrovascular accident, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome.
TABLE I.
Demographics of Study Sample Combined.
| Patients (N = 230) | Median (or n) | Interquartile distance (or %) |
|---|---|---|
|
| ||
| Age (years) | 63* | 56–72 |
| CCI score | 3* | 2–5 |
| RAI score | 23* | 20–28 |
| Cancer site | ||
| Oral cavity | 161 | 70% |
| Oropharynx | 5 | 2.2% |
| Larynx/hypopharynx | 30 | 13% |
| Sinonasal cavity | 2 | 0.9% |
| Cutaneous face/neck | 27 | 11.7% |
| Salivary gland | 5 | 2.2% |
| Cancer stage | ||
| 1 | 11 | 4.7% |
| 2 | 30 | 13% |
| 3 | 42 | 18.3% |
| 4a | 94 | 40.9% |
| 4b | 53 | 23% |
| Reconstruction type | ||
| ALT | 116 | 50.4% |
| Fibula | 52 | 22.6% |
| Scapula | 18 | 7.8% |
| Radial forearm | 43 | 18.7% |
| Other | 1 | 0.4% |
| Surgical airway | 118 | 51.3% |
| NGT or GT feeds | 152 | 66.1% |
| LOS | 9* | 7–13 |
| Dispo | ||
| Home | 165 | 71.7% |
| SNF | 65 | 28.3% |
| Completion of adjuvant therapy | 182 | 79.1% |
| Readmissions | 68 | 29.6% |
| ED visits | 40 | 17.4% |
| 30-day mortality | 1 | 0.4% |
| 90-day mortality | 3 | 1.3% |
Categorical variables are otherwise reported as frequency (percentage). Surgical airway is defined as tracheostomy or laryngectomy.
Continuous variables are reported as median (interquartile distance).
ALT = anterolateral thigh; CCI = Charlson Comorbidity Index; ED = emergency department; Gt = gastric tube; LOS = length of stay; NGT = nasogastric tube; RAI = risk analysis index; SNF = skilled nursing facility.
Long-term acute care (LTAC) and in-patient rehabilitation (IPR) facilities were few in our cohort and were therefore categorized in the SNF group. RAI was a marker for frailty, and CCI was a marker for comorbidity with higher scores meaning worse frailty or comorbidity, respectively. Time to RT (in days) was analyzed as a continuous variable from the date of surgery to the date of adjuvant treatment initiation. TPT was measured as a continuous variable from the date of surgery to the date of adjuvant treatment completion.
Statistical Analysis
Of the 230 patients that met inclusion criteria, 182 patients were recommended for adjuvant therapy. Demographics and preoperative health indices were analyzed with descriptive statistics (Table I) overall and separated into cohorts based on disposition (Tables II and III). Dependent outcome variables of interest included readmissions and ED visits for the entire study cohort. Dependent outcome variables of interest for the adjuvant population were time to RT and TPT. Univariate analyses were performed with independent sample t-test and χ2 to assess for significant associationis as predictors for these outcome variables individually. Logistic regression analyses were then performed to analyze independent variables as predictors of readmissions and ED visits. Linear regression analyses were performed to analyze predictors of time to RT and TPT.
TABLE II.
Univariate Analysis of Total Study Population by Disposition (N = 230).
| Home (n = 165, 71.7%) |
SNF (n = 65, 28.3%) |
p-Value | |||
|---|---|---|---|---|---|
| n | Mean | n | Mean | ||
|
| |||||
| Age | 165 | 61.5 years | 65 | 67.1 years | 0.001* |
| CCI score | 165 | 3.3 | 65 | 4.3 | 0.002* |
| RAI score | 165 | 22.8 | 65 | 27.3 | <0.001* |
| Cancer stage | 0.1 | ||||
| 1 | 10 | 6.1% | 0 | 0% | |
| 2 | 24 | 14.6% | 6 | 9% | |
| 3 | 33 | 20% | 9 | 14% | |
| 4a | 64 | 38.8% | 30 | 46% | |
| 4b | 33 | 20% | 20 | 31% | |
| Surgical airway | 73 | 44.2% | 45 | 69% | <0.001* |
| NGT or GT feeds | 98 | 59.4% | 54 | 83% | <0.001* |
| LOS | 165 | 9.2 days | 65 | 13.9 days | <0.001* |
| Readmissions | 36 | 21.8% | 32 | 49% | <0.001* |
| ED visits | 23 | 13.9% | 17 | 26% | 0.03* |
| 30-day mortality | 0 | 0% | 1 | 2% | - |
| 90-day mortality | 2 | 1.2% | 1 | 2% | - |
Surgical airway is defined as tracheostomy or laryngectomy.
Statistical significance.
CCI = Charlson Comorbidity Index; ED = emergency department; GT = gastric tube; LOS = length of stay; NGT = nasogastric tube; RAI = risk analysis index; SNF = skilled nursing facility.
TABLE III.
Univariate Analysis of Patients Recommended for Adjuvant Therapy (N = 182).
| Home (n = 129, 70.9%) |
SNF (n = 53, 29.1%) |
p-Value | |||
|---|---|---|---|---|---|
| n | Mean (Range, SD) | n | Mean (Range, SD) | ||
|
| |||||
| Age (years) | 129 | 60.2 | 65.6 | 0.01* | |
| CCI score | 129 | 3.2 | 4.1 | 0.04* | |
| RAI score | 129 | 22.3 | 27.1 | <0.001* | |
| Cancer stage | 0.15 | ||||
| 1 | 4 | 3.1% | 0 | 0% | |
| 2 | 3 | 2.3% | 2 | 4% | |
| 3 | 28 | 21.7% | 6 | 11% | |
| 4a | 62 | 48.1% | 25 | 47% | |
| 4b | 32 | 24.8% | 20 | 38% | |
| Surgical airway | 64 | 49.6% | 40 | 75% | <0.001* |
| Enteral feeds | 85 | 65.9% | 45 | 85% | 0.01* |
| LOS (days) | 9.4 | 13.9 | <0.001* | ||
| Time to RT (days) | 106 | 59 (15–110, 13.8) | 40 | 70.1 (41–147, 22.0) | 0.007* |
| TPT (days) | 112 | 101.7 (78–147, 13.5) | 42 | 112.3 (44–203, 29.4) | 0.005* |
| Readmissions | 34 | 26.4% | 26 | 49.1% | 0.003* |
| ED visits | 20 | 15.5% | 15 | 28.3% | 0.05* |
| 30-day mortality | 0 | 0% | 1 | 1.9% | - |
| 90-day mortality | 2 | 1.6% | 1 | 1.9% | - |
Surgical airway is defined as tracheostomy or laryngectomy.
Statistical significance.
CCI = Charlson Comorbidity Index; ED = emergency department; GT = gastric tube; LOS = length of stay; NGT = nasogastric tube; RAI = risk analysis index; SD = standard deviation; SNF = skilled nursing facility.
Statistical significance is defined as p < 0.05. Statistical analysis was done using IBM SPSS Statistics Version 28.0.1.0.
RESULTS
Patient Characteristics
Two hundred thirty patients with stage 1–4b HNSCC were included in the study (Fig. 1). Two hundred two patients were recommended for adjuvant therapy in the form of radiation or chemoradiation. One hundred eighty-two (90.1%) of these patients received adjuvant therapy. Demographics of our entire study sample are listed in Table I. The median age was 63 years. The most common cancer site was the oral cavity (n = 161, 70%), and the most common cancer stage was stage 4a (n = 94, 40.9%), followed by stage 4b (n = 53, 23%) and stage 3 (n = 42, 18.3%). The most common reconstruction type was the anterolateral thigh (ALT) free flap (n = 116, 50.4%), followed by the fibula osteocutaneous free flap (n = 52, 22.6%).
Collectively, the rate of discharge to SNF was 28.3% for the entire study group (Table I): SNF discharge rate was 50%, 24.4%, and 17.6% in 2019, 2020, and 2021, respectively. Discharge data in 2022 were not analyzed due to data collection concluding by June 2022 and did not include the entire 2022 population. Of note, our institution was affected by the coronavirus disease 2019 (COVID-19), which had a significant impact on hospital operations starting around March 2019.
Total Study Population by Disposition
A univariate analysis (Table II) comparing all patients discharged home compared to SNF revealed significant differences in average patient age (61.5 vs. 67.1 years, p = 0.001), CCI score (3.3 vs. 4.3, p = 0.002), and RAI score (22.8 vs. 27.3, p < 0.001). In addition, when compared to patients discharged to home, patients discharged to SNF had a higher incidence of a surgical airway (44.2% vs. 69%, p < 0.001) and enteral access (59.4% vs. 83%, p < 0.001). LOS was higher for patients discharged to SNF (9.2 vs. 13.9 days, p < 0.001). Patients discharged to SNF also had significantly higher rates of readmissions (21.8% vs. 49%, p < 0.001) and ED visits (13.9% vs. 26%, p = 0.03). Reasons for readmissions range from recipient site infections to donor site infections, GT issues, tracheostomy or laryngectomy site issues, and less commonly, pneumonia, respiratory failure, and bleeding.
Adjuvant Study Population by Disposition
Of the 182 patients who underwent adjuvant therapy, the rate of SNF disposition was 29.1% (Table III). A univariate analysis comparing patients undergoing adjuvant therapy who went home compared to those who went to an SNF showed significant differences in average patient age (60.2 vs. 65.6 years), CCI score (3.2 vs. 4.1), and RAI score (22.3 vs. 27.1) (Table III). Patients discharged to SNF had a higher frequency of a surgical airway (75% vs. 49.6%, p < 0.001), a higher frequency of enteral access (85% vs. 65.9%, p = 0.01), and a higher LOS (13.9 vs. 9.4 days, p < 0.001). They also had higher rates of readmissions (49.1% vs. 26.4%, p = 0.003) and ED visits (28.3 vs. 15.5%, p = 0.05). When evaluating expediency to adjuvant therapy initiation, patients discharged to the SNF had a longer time to start adjuvant therapy (70.1 days vs. 59 days, p = 0.007) and a longer TPT (112.3 days vs. 101.7 days, p = 0.005) (Table III).
Readmissions and ED Visits
A univariate analysis was performed on the entire study cohort to determine significant associations with readmission; disposition to SNF, advanced cancer stage diagnosis, presence of NGT or GT, presence of a surgical airway, and longer LOS were significant factors. However, after adjusting for these factors, logistic regression analysis demonstrated that discharge to SNF (OR = 2.80, CI 1.37–5.72) and presence of a surgical airway (OR = 2.84, CI 1.30–6.17) were found to be significantly associated with higher readmission rates.
Similarly, univariate analysis showed disposition to SNF and advanced cancer stage diagnosis were significant associations with ED visits. However, adjusting for these factors in a logistic regression analysis to determine predictors of ED visits found no significant associations (Table IVb).
TABLE IV.
Multivariate Logistic Regression to Predict (a) Readmissions and (b) ED Visits (N = 230).
| Factor | OR | CI | p-Value |
|---|---|---|---|
|
| |||
| (a) | |||
| Discharge to SNF | 2.80 | 1.37, 5.72 | 0.005* |
| Advanced cancer stage at diagnosis | - | - | 0.56 |
| Presence of NGT or GT feeds | 0.99 | 0.42, 2.31 | 0.97 |
| Presence of surgical airway | 2.84 | 1.30, 6.17 | 0.009* |
| Longer LOS | 0.99 | 0.94, 1.06 | 0.98 |
| (b) | |||
| Discharge to SNF | 1.87 | 0.91, 3.84 | 0.09 |
| Advanced cancer stage at diagnosis | - | - | 0.67 |
Surgical airway is defined as tracheostomy or laryngectomy.
Denotes statistical significance.
ED = emergency department; GT = gastric tube; LOS = length of stay; NGT = nasogastric tube; SNF = skilled nursing facility.
Time to RT Initiation
Univariate analysis showed disposition to SNF, readmissions, increased LOS, and older age were significantly associated with longer time to RT. Adjusting for these variables in a linear regression analysis demonstrated that discharge to SNF (p = 0.03) was significantly associated with increasing time to RT, while readmissions, LOS, and age were not (Table V).
TABLE V.
Multivariate Linear Regression of Time to Radiation Therapy Initiation.
| Factor | B | CI | p-Value |
|---|---|---|---|
|
| |||
| (Constant) | 41.8 | 26.1, 57.5 | <0.001* |
| Discharge to SNF | 7.23 | 0.75, 13.7 | 0.03* |
| Readmission | 5.49 | −0.52, 11.5 | 0.07 |
| LOS (days) | 0.53 | −0.06, 1.13 | 0.12 |
| Age (years) | 0.19 | −0.05, 0.42 | 0.08 |
Statistical significance.
B = unstandardized beta (value represents slope of the line between predictor and dependent variable); CI = 95% confidence interval; LOS = length of stay; SNF = skilled nursing facility.
Treatment Package Time
Disposition to SNF, readmissions, and increased LOS were significantly associated with longer TPT on univariate analysis. When adjusting for these factors, linear regression analysis evaluating predictors affecting TPT demonstrated that readmission (p = 0.03) and increased LOS (p = 0.04) were significantly associated with increasing TPT, but discharge to SNF (p = 0.056) was not (Table VI).
TABLE VI.
Multivariate Linear Regression of Treatment Package Time.
| Factor | B | CI | P-Value |
|---|---|---|---|
|
| |||
| (Constant) | 93.4 | 86.3, 100.6 | <0.001* |
| Discharge to SNF | 6.88 | −0.17, 13.9 | 0.056 |
| Readmission | 7.67 | 0.84, 14.5 | 0.03* |
| LOS (days) | 0.72 | 0.05, 1.38 | 0.04* |
Statistical significance.
B = unstandardized beta (value represents slope of the line between predictor and dependent variable); CI = 95% confidence interval; LOS = length of stay; SNF = skilled nursing facility.
DISCUSSION
We sought to understand the impact of discharge disposition on patients after head and neck free flap reconstruction, including time to adjuvant therapy initiation and TPT as well as readmission rates and ED visits.
It has been thoroughly studied that the timing of adjuvant therapy after surgery for patients with head and neck cancer is correlated with survival.5 A study of 47,000 patients in the National Cancer Database (NCDB) found that 56% of patients do not initiate RT within 6 weeks.6 Factors associated with delayed care include sociodemographic factors, increased comorbidities, LOS, 30-day readmission rates, treatment at an academic medical center, and fragmentation of care.
In our cohort, 28.3% of patients required SNF at discharge. These patients were older, had higher frailty and comorbidity indices, had longer LOS, and were more likely to have a surgical airway or require enteral feeds when compared to those who went home. Similarly, Lepse et al. found that having tracheostomy on discharge and increasing age were significantly associated with the need for post-acute care.14 In a recent multi-institutional study, Sweeny et al. reported risk factors associated with higher likelihood of SNF disposition, including older age, a higher comorbid burden specifically relating to cardiopulmonary or vascular disease, and a higher incidence of postoperative complications leading to SNF disposition. Interestingly, their findings revealed no differences between post-discharge surgical complications or 30-day readmission rates between different discharge destinations.15 Previous studies have also shown that patient education, postoperative sequelae, coordination of care, fragmentation across care organizations, a lack of family support, and socioeconomic status are all factors that have been shown to impact a patient’s ability to receive the treatment they need on time.5,6,14,16,17 Our patients discharged to SNF initiated adjuvant therapy later than those discharged home (70.1 vs. 59 days, respectively). In multivariate analysis, adjusting for confounding variables, we found that discharge to an SNF was an independent risk factor for delayed time to RT. Similarly, TPT was noted to be significantly longer in the SNF cohort on univariate analysis (112.3 vs. 101.7 days). When adjusting for confounding variables, disposition was no longer a statistically significant variable but only by a narrow margin (p = 0.056, 95% CI [−0.17, 13.9]). Despite this, we suspect disposition remains clinically relevant in affecting TPT. Readmissions and increased LOS were significantly associated with increased TPT.
In addition, the SNF population also had higher rates of readmissions when compared to home disposition. Having a surgical airway was also noted to be an independent risk factor for higher rates of readmission, regardless of disposition. The most common reasons for readmissions were related to infections (e.g., neck infection, flap infection, wound dehiscence, orocutaneous fistula, salivary leak, hardware infection), tracheostomy or laryngectomy issues (obstruction, bleeding, dehiscence), and enteral tube feed obstruction/dislodgement. ED visits that did not result in admission were not found to be significantly associated with any variables of interest in a multivariate analysis.
From our experience and in current literature, patients who are discharged to an SNF often require additional rehabilitation or wound care that require assistance that they are unable to access at home due to limited family/social support.14 However, discharge to SNF is also challenging, as searching for an available facility to accept head and neck patients is difficult; this stems from a lack of resources and staff in caring for patients with tracheostomy or laryngectomy, enteral feeds, and complex surgical wounds. These facilities are often understaffed with a nurse-to-patient ratio close to 1:15 and an even higher medical provider-to-patient ratio. Many (79.1%) of our patients underwent adjuvant therapy. These patients require coordination of care with radiation and/or medical oncologists in a timely manner and frequent appointments. Patients discharged to SNF or other facilities also often have limited resources related to transportation to and from adjuvant treatment centers. Furthermore, literature shows discordant understanding of patients’ diagnoses and care between SNF providers and primary medical providers.
In addition, the cost of starting adjuvant therapy while at SNF would have to be absorbed by these facilities, as Singh et al. reported, as SNF is covered by Medicare Part A, while adjuvant therapy is considered an outpatient service and therefore is only covered by Medicare Part B.7 Though we did not specifically collect patients’ insurance status/coverage, our patients also do not initiate treatment until they are discharged from the facility. This leads to further delays in initiating RT within the recommended 6 weeks and can negatively impact overall survival.6
Our findings in this study should encourage providers to consider a variety of patient social factors, baseline demographics, and functional status/health when deciding discharge disposition, especially for those who require adjuvant therapy. Though we did not perform multivariate analysis regarding risk factors leading to SNF disposition, prior studies have identified that older patients are at higher risk of being discharged to SNF in the head and neck free flap population. Notably, a proportionate increase in age was associated with a 5% increased chance for SNF disposition.8
Major limitations of this retrospective study include the inability to ascertain specific reasons for patient discharge to SNF, reasons for delays in discharge to SNF, as well as the quality of care and any coordination difficulties at these post-acute care facilities. Twenty-three of our patients discharged to SNF were documented as “pending SNF placement,” indicating a delay in discharge, but we were unable to determine the specific reasons for delay or quantify impact on patients’ LOS. We postulate that these placement delays may be associated with insurance-related causes. We did not investigate patients’ insurance type but Lepse et al. reported that having government insurance was associated with higher discharge to post-acute facility (i.e., SNF or rehabilitation center) compared to private insurance or non-insurance.14 This knowledge may aid in earlier preparation in obtaining insurance authorization and approval for patients to be discharged to these facilities. Acute care facilities are often not associated with large medical centers, and obtaining access to these records is challenging. Thus, only delays that required medical/surgical intervention in the form of ED visits, readmission rates, or clinic visits could be reviewed in our study. Furthermore, we do not have information on how long patients stayed at acute care facilities. Another limitation is lack of documentation on the reasons for those who declined adjuvant treatment or missed adjuvant treatment entirely. As our data is less than 5 years old, we are unable to assess overall survival or disease-free survival. However, it would be an important interest for future investigation. Finally, our investigation occurred in the midst of the COVID-19 pandemic, which may have played a role in the significant decrease in discharge to SNF (50%, 24.4%, and 17.6% in 2019, 2020, and 2021, respectively), as there were more stringent testing criteria as well as limited resources and staffing at facilities during the height of the pandemic.
CONCLUSION
Our study shows that discharge to an SNF led to significant delays in starting adjuvant therapy in patients with head and neck cancer who underwent microvascular free flap reconstruction. These patients had greater frailty and higher incidences of readmissions as well. This may suggest a difference in quality of or access to care in SNF compared to care at home. Future directions include better understanding of factors that lead to SNF disposition, including socioeconomic factors, patient ease of access to adjuvant treatment centers, 5-year overall survival and disease-free survival, and including non-free flap head and neck cancer patients in our analysis. Future investigation also includes further understanding of care coordination needed to improve initiation of adjuvant therapy.
Footnotes
The authors have no funding, financial relationships, or conflicts of interest to disclose.
The Triological Society 2023 Combined Sections Meeting at Coronado, California, USA on January 28, 2023.
Contributor Information
Sophia Dang, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Terral Patel, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Isabella Lao, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, U.S.A..
Shaum S. Sridharan, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.; Department of Plastic and Reconstructive Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.
Mario G. Solari, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.; Department of Plastic and Reconstructive Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.
Seungwon Kim, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Umamaheswar Duvvuri, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Robert Ferris, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A..
Mark Kubik, Department of Otolaryngology – Head and Neck Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.; Department of Plastic and Reconstructive Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, U.S.A.
BIBLIOGRAPHY
- 1.Rosenthal DI, Liu L, Lee JH, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24(2):115–126. 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 2.Graboyes EM, Garrett-Mayer E, Ellis MA, et al. Effect of time to initiation of postoperative radiation therapy on survival in surgically managed head and neck cancer. Cancer. 2017;123(24):4841–4850. 10.1002/cncr.30939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghanem AI, Schymick M, Bachiri S, et al. The effect of treatment package time in head and neck cancer patients treated with adjuvant radiotherapy and concurrent systemic therapy. World J Otorhinolaryngology Head Neck Surg. 2019;5(3):160–167. 10.1016/j.wjorl.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langendijk JA, de Jong MA, Leemans CR, et al. Postoperative radiotherapy in squamous cell carcinoma of the oral cavity: the importance of the overall treatment time. Int J Radiat Oncol Biol Phys. 2003;57(3):693–700. 10.1016/s0360-3016(03)00624-2. [DOI] [PubMed] [Google Scholar]
- 5.Tam M, Wu SP, Gerber NK, et al. The impact of adjuvant chemoradiotherapy timing on survival of head and neck cancers. Laryngoscope. 2018;128(10):2326–2332. 10.1002/lary.27152. [DOI] [PubMed] [Google Scholar]
- 6.Graboyes EM, Garrett-Mayer E, Sharma AK, Lentsch EJ, Day TA. Adherence to National Comprehensive Cancer Network guidelines for time to initiation of postoperative radiation therapy for patients with head and neck cancer. Cancer. 2017;123(14):2651–2660. 10.1002/cncr.30651. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Eguchi M, Min SJ, Fischer S. Outcomes of patients with cancer discharged to a skilled nursing facility after acute care hospitalization. J Natl Compr Canc Netw. 2020;18(7):856–865. 10.6004/jnccn.2020.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatcher JL, Bell EB, Browne JD, Waltonen JD. Disposition of elderly patients after head and neck reconstruction. JAMA Otolaryngology Head Neck Surg. 2013;139(11):1236–1241. 10.1001/jamaoto.2013.5054. [DOI] [PubMed] [Google Scholar]
- 9.Freeman MH, Shinn JR, Fernando SJ, et al. Impact of preoperative risk factors on inpatient stay and facility discharge after free flap reconstruction. Otolaryngol Head Neck Surg. 2022;166(3):454–460. 10.1177/01945998211037541. [DOI] [PubMed] [Google Scholar]
- 10.Parhar HS, Chang BA, Durham JS, Anderson DW, Hayden RE, Prisman E. Post-acute care use after major head and neck oncologic surgery with microvascular reconstruction. Laryngoscope. 2018;128(11):2532–2538. 10.1002/lary.27190. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Kim S, Albergotti WG, et al. Selection of ideal candidates for surgical salvage of head and neck squamous cell carcinoma: effect of the Charlson-age comorbidity index and oncologic characteristics on 1-year survival and hospital course. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107(11 Pt 1):1469–1475. 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175–182. 10.1001/jamasurg.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepse J, Sykes KJ, Kakarala K. Risk factors for post-acute care following free flap reconstruction of the oral cavity. Otolaryngol Head Neck Surg. 2021;164(4):767–773. 10.1177/0194599820955175. [DOI] [PubMed] [Google Scholar]
- 15.Sweeny L, Slijepcevic A, Curry JM, et al. Factors impacting discharge destination following head and neck microvascular reconstruction. Laryngoscope. 2023;133(1):95–104. 10.1002/lary.30149. [DOI] [PubMed] [Google Scholar]
- 16.Graboyes EM, Halbert CH, Li H, et al. Barriers to the delivery of timely, guideline-adherent adjuvant therapy among patients with head and neck cancer. JCO Oncol Pract. 2020;16(12):e1417–e1432. 10.1200/OP.20.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noyes EA, Burks CA, Larson AR, Deschler DG. An equity-based narrative review of barriers to timely postoperative radiation therapy for patients with head and neck squamous cell carcinoma. Laryngosc Investig Otolaryngol. 2021;6(6):1358–1366. 10.1002/lio2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]


