Abstract
Background
Hand, foot, and mouth disease (HFMD) is a common childhood infectious disease caused by a variety of enteroviruses (EVs). To explore the epidemiological characteristics and etiology of HFMD in Zhengzhou, China, we conducted a systematic analysis of HFMD surveillance data from Zhengzhou Center for Disease Control and Prevention from January 2009 to December 2021 (https://wjw.zhengzhou.gov.cn/).
Methods
Surveillance data were collected from Zhengzhou Center for Disease Control and Prevention from January 2009 to December 2021 (https://wjw.zhengzhou.gov.cn/). Cases were analyzed according to the time of onset, type of diagnosis, characteristics, viral serotype, and epidemiological trends.
Results
We found that the primary causative agent responsible for the HFMD outbreaks in Zhengzhou was Enterovirus A71 (EVA-71) (48.56%) before 2014. After 2015, other EVs gradually became the dominant strains (57.68%). The data revealed that the HFMD epidemics in Zhengzhou displayed marked seasonality, with major peaks occurring from April to June, followed by secondary peaks from October to November, except in 2020. Both the severity and case-fatality ratio of HFMD decreased following the COVID-19 pandemic (severity ‰: 13.46 vs. 0.17; case-fatality ‰: 0.21 vs. 0, respectively). Most severe cases were observed in patients aged 1 year and below, accounting for 45.81%.
Conclusions
Overall, the incidence rate of HFMD decreased in Zhengzhou following the introduction of the EVA-71 vaccine in 2016. However, it is crucial to acknowledge that HFMD prevalence continues to exhibit a distinct seasonal pattern and periodicity, and the occurrence of other EV infections poses a new challenge for children's health.
Keywords: HFMD, Epidemiology, Enterovirus serotypes, EVA-71, CVA-16
Graphical Abstract
Hand, foot, and mouth disease (HFMD) is a common childhood infectious disease caused by a variety of enteroviruses (EVs). To explore the epidemiological characteristics and etiology of HFMD in Zhengzhou, China, we conducted a systematic analysis of HFMD surveillance data from Zhengzhou Center for Disease Control and Prevention from January 2009 to December 2021.
1. Introduction
Hand, foot, and mouth disease (HFMD) is a highly transmissible ailment caused by multiple enteroviruses (EVs) exhibiting a high prevalence among the pediatric population. The first report of the characteristic coxsackievirus symptoms, including fever and a vesicular rash on the hands, feet, and oral mucosa, can be dated back to 1957 in Toronto [1]. In 1959, the term “hand, foot, and mouth disease” was initially used to name this disease [2]. The transmission routes of HFMD mainly include contact transmission, respiratory transmission, and fecal-oral transmission. On the basis of animal research, aerosol transmission via the respiratory tract has been demonstrated as a transmission route [3]. To validate the aerosol transmission pathway in the human population, further investigations are needed. Children under 5 years of age are the most susceptible population to HFMD [4]. Although the majority of HFMD infections tend to be mild and self-limiting, certain cases exhibit severe manifestations, such as meningitis, neurological complications, and pulmonary edema [5]. Data from large-scale surveillance reported approximately 13 million cases of HFMD in China during the period from 2008 to 2015. Of these, 12,000 were severe cases and more than 3300 deaths were documented [6]. Particular strains of EV type A, namely Enterovirus A71 (EVA-71), Coxsackievirus A16 (CVA-16), and Coxsackievirus A6 (CVA-6), serve as the main etiological agents of HFMD [7,8]. EVA-71-related HFMD was first detected in 1969 in the United States [9]. Large outbreaks or sporadic cases were also reported in Singapore, China, Australia, and several Asia-Pacific regions [10]. Recently, CVA-6 and CVA-10 have partially replaced EVA-71 and CVA-16 as the main pathogens responsible for HFMD outbreaks. Several outbreaks of HFMD due to CVA-6 and CVA-10 have been reported in Asia, the United States, and Europe since 2010 [1,[11], [12], [13]]. Despite an inactivated monovalent vaccine for EVA-71 being developed in 2016, the continued prevalence of HFMD remains a major public health issue in the Asia-Pacific region [14]. To explore the epidemiological characteristics and etiology of HFMD in Zhengzhou, seasonal patterns and disease incidence by age group and geographical distribution from 2009 to 2021 were detailed in this study.
2. Materials and methods
2.1. Data sources
Surveillance data were collected from Zhengzhou Center for Disease Control and Prevention from January 2009 to December 2021 (https://wjw.zhengzhou.gov.cn/). From 2008 onwards, patients clinically diagnosed with HFMD were required to be reported to the National Surveillance of Notifiable Infectious Disease Programme (NSNIDP). From June 2009 onwards, specimens were collected monthly from each county or district for the first five mild and all severe cases of HFMD. Viral RNA was extracted from specimens and tested by quantitative PCR. Only positive results were reported to the NSNIDP with virus categorization as EVA-71, CVA-16, or other EVs. HFMD was diagnosed if the patient had a skin papular or vesicular rash on the hands, feet, mouth, or buttocks, with or without fever, in accordance with the recommendations on the diagnosis and management of HFMD issued by the health administration [15]. Patients were classified as severe if neurological symptoms and severe cardiopulmonary dysfunction were present, otherwise, they were classified as mild [16]. Demographic data for Zhengzhou were obtained from the Zhengzhou Statistical Yearbook.
2.2. Data analysis
2.2.1. Statistical methods
HFMD cases were analyzed according to the time of onset, type of diagnosis, characteristics, viral serotype, and epidemiological trends from 2009 to 2021. We used Chi-square test or Fisher's exact test to compare differences in proportions as appropriate. All statistical analyses were performed using SPSS version 26.0, and P < 0.05 was considered statistically significant. Figures were graphed in Excel tables (Excel 2016, Microsoft, Washington, DC, USA), GraphPad Prism 9.0.0 (GraphPad 9.0.0 Software, San Diego, CA, USA), and Adobe Illustrator 2020 (Adobe Systems Incorporated, USA).
2.2.2. Wavelet analysis and monthly incidence prediction
The methods of wavelet analysis and monthly incidence prediction were described in the Supplementary materials.
3. Results
3.1. General epidemiological characteristics of HFMD in Zhengzhou, China
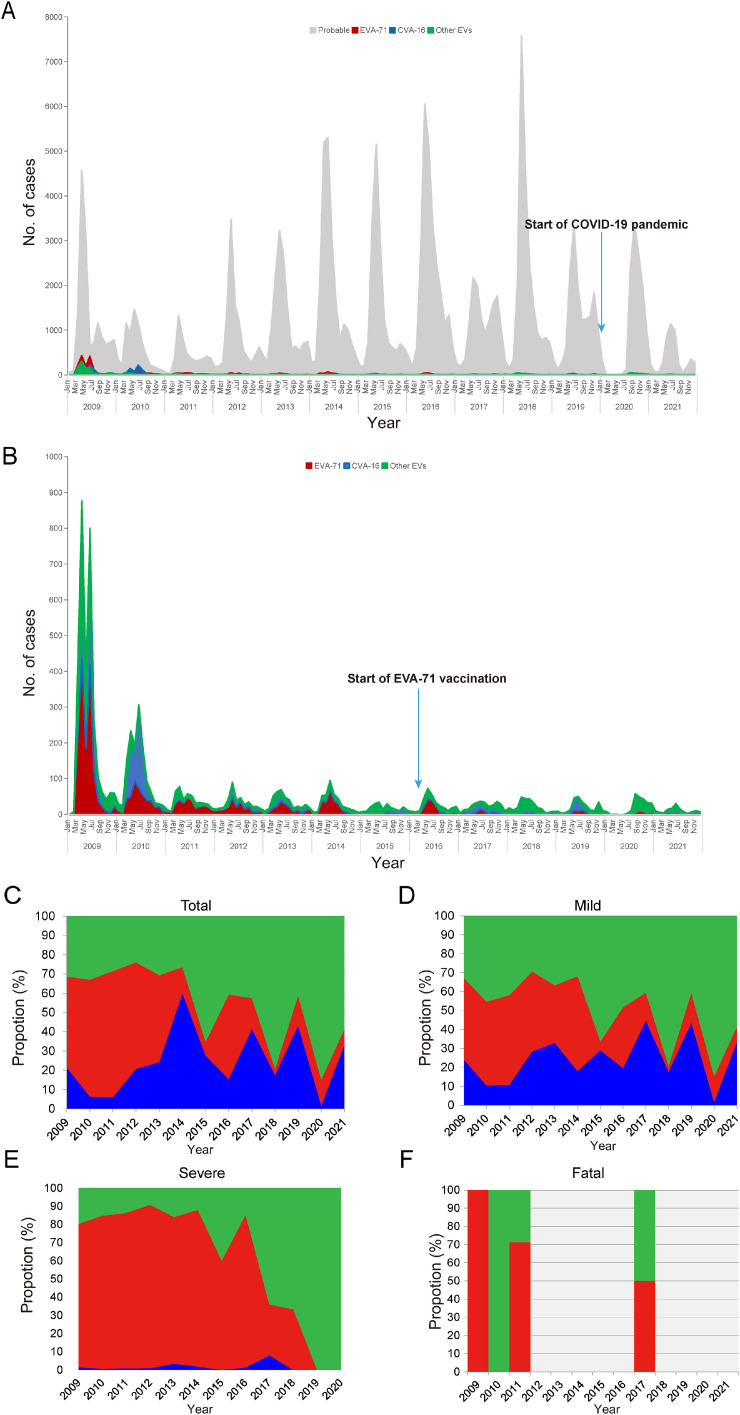
In Zhengzhou, a total of 192,494 HFMD cases were reported to the NSNIDP from 2009 to 2021, among which 2362 and 37 were diagnosed as severe and fatal cases, respectively (Table 1, Fig. 1). There was a notable decline in the number of HFMD cases from 2011 to 2021 (Fig. 1A). This study examined the distribution among sexes, with 115,360 cases in male (59.95%) and 77,096 cases in female individuals (40.05%). During the study period, the incidence and severity rate of HFMD were found to be significantly higher in male individuals (χ2 = 6966.88, P < 0.001; χ2 = 6.628, P < 0.001) (Supplementary Table 1). Out of 2362 severe cases, 1135 were confirmed through laboratory testing, while the remaining cases were diagnosed based on clinical symptoms. Among the reported cases, patients were from various backgrounds, including 135,183 (70.26%) scattered children (infants and toddlers who are raised at home from birth to seven years instead of being sent to a nursery, daycare center, or kindergarten), 4367 (2.27%) students, 52,197 (27.12%) preschool children, and 673 (0.35%) individuals from other age groups. Additionally, among 7266 laboratory-confirmed HFMD cases, 3161 cases were EVA-71 positive (43.50%), 1423 were CVA-16 positive (19.58%), and the remaining 2682 cases tested positive for other EVs (36.91%) that could not be typed (Table 1).
Table 1.
Epidemiological characteristics of hand, foot and mouth disease cases in Zhengzhou, China from 2009 to 2021.
| Category | Mild | Severe | Fatal | Total |
|---|---|---|---|---|
| Disease severity | ||||
| Number of cases | 190058 | 2362 | 37 | 192494 |
| Male | 113883 | 1477 | 24 | 115360 |
| Female | 76175 | 885 | 13 | 77096 |
| Case category | ||||
| Laboratory-confirmed cases | 5931 | 1335 | 14 | 7266 |
| Clinically-diagnosed cases | 184127 | 1027 | 23 | 185154 |
| Crowd | ||||
| Preschool children | 51881 | 316 | 7 | 52197 |
| Students | 4356 | 11 | 0 | 4367 |
| Scattered children | 133148 | 2035 | 30 | 135183 |
| Others | 673 | 0 | 0 | 673 |
| Serotype | ||||
| EVA-71 | 2072 | 1089 | 8 | 3161 |
| CVA-16 | 1404 | 19 | 0 | 1423 |
| Other EVs | 2455 | 227 | 6 | 2682 |
Fig. 1.
Number of HFMD cases with different Enterovirus serotypes in Zhengzhou, China. (A) Monthly notifications of probable and laboratory-confirmed HFMD cases. (B) Monthly notifications of laboratory-confirmed HFMD cases. (C) Pathogen spectrum of all laboratory-confirmed HFMD patients. (D) Pathogen spectrum of mild HFMD patients. (E) Pathogen spectrum of severe HFMD patients. (F) Pathogen spectrum of fatal HFMD patients.
3.2. Enterovirus serotypes of HFMD cases in Zhengzhou, China
As shown in Fig. 1B, EVA-71 was the dominant serotype among HFMD cases from 2009 to 2014, and in 2016. Of note, EVA-71 infection accounted for over 60% of laboratory-confirmed infections in 2014, and other serotypes increased progressively between 2015 and 2021. Because only the first five mild cases of HFMD were tested and reported to the NSNIDP, we were not able to distinguish the serotype for the other cases, and therefore the prevalence of different viruses may be underestimated. Hence, further studies in virologic diagnosis for all HFMD cases are needed to better quantify the HFMD incidence. In 2020, 84.90% of laboratory-confirmed cases were infected with other EVs (Fig. 1C). Between 2009 and 2017, more than 80% of laboratory-confirmed severe HFMD cases were EVA-71 infections, with the proportion of severe cases caused by EVA-71 reaching 89.90% in 2012 (Fig. 1E). Compared with CVA-16 and other EVs, EVA-71 accounted for 57.14% of laboratory-confirmed fatal cases between 2009 and 2021 (Fig. 1F). In Zhengzhou and other places, EVA-71 associated HFMD cases significantly declined between 2009 and 2021. We propose two possible explanations for this decline. First, since 2014, the pathogen spectrum of HFMD has altered, with CVA-16 and other EVs emerging as alternatively co-circulating pathogens. Second, a vaccination campaign targeting children aged 0.5–5 years with EVA-71 vaccine was implemented in Zhengzhou from September 2016, resulting in a notable decline in EVA-71 infections by 2017. This vaccine has been shown to be extremely effective in children [17]. EVA-71 was one of the major pathogenic strains causing severe and fatal cases, but since 2018, it has not been detected among severe cases and the percentage of CVA-6- and CVA-10-positive cases has gradually increased. CVA-6-associated HFMD presents a more severe and extensive rash, and is also characterized by a higher incidence in adults [18]. A notable increase in patients infected with CVA-10 has been found in Xiamen over recent years [19].
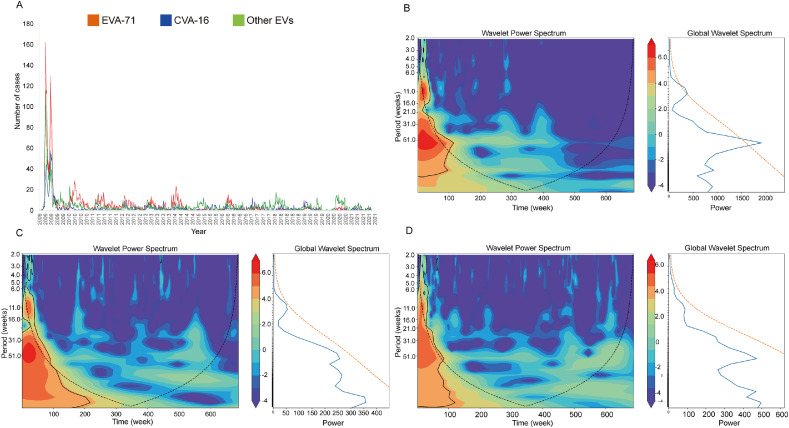
3.3. Periodicity of HFMD in Zhengzhou, China
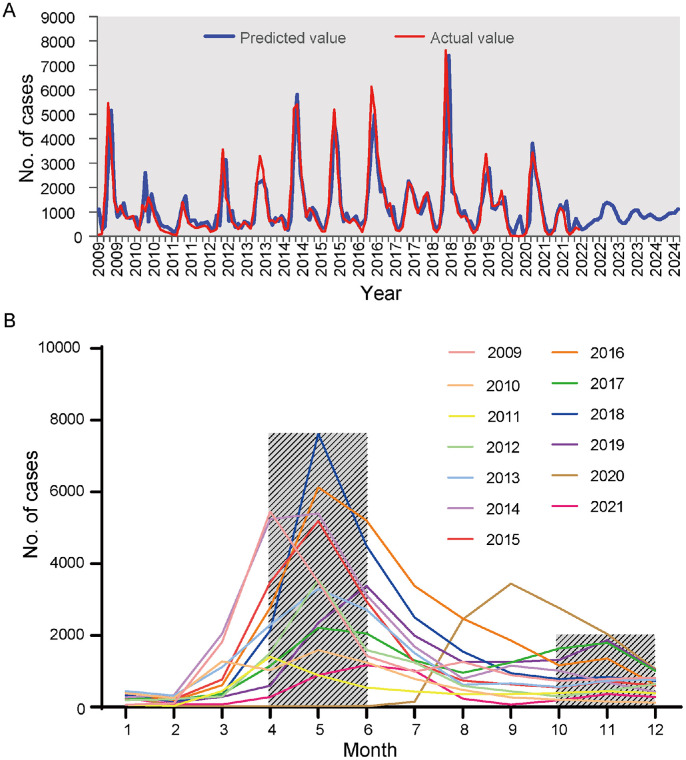
Wavelet analysis showed that HFMD had an annual periodicity during the study period (Fig. 2A). Studies have shown that EVA-71, CVA-16, and other EVs have a 51-week periodicity (Fig. 2B–D), suggesting that HFMD cases attributed to all serotypes showed an annual periodicity. According to the global power spectrum, the 51-week periodicity of EVA-71 is stronger than that of other viruses. Fig. 3A shows that the simulation and prediction trends of HFMD using the model were relatively consistent with the actual situation, validating the reliability of the SARIMA model in assessing the epidemiological trends of HFMD. Fig. 3B shows a clear seasonal trend in the incidence of HFMD, with primary peaks from April to June and secondary peaks from October to November. Most cases were reported in May. Supplementary Figure 1 shows the ACF and PACF after square root conversion of the data. On the basis of the comparative results of the various goodness-of-fit tests, our study identified the optimal SARIMA (2,0,1)(2,0,0)12 model. The Ljung-Box test demonstrated that the residuals were white noise (Ljung-Box, P = 0.512), verifying that the fitted data were completely summarized. Supplementary Table 2 displays the parameter estimation for the SARIMA (2,0,1)(2,0,0)12 model, which was found to be statistically significant.
Fig. 2.
Wavelet analyses for time series of serotype-specific notifications of HFMD in Zhengzhou, China. (A) Weekly numbers of laboratory-confirmed cases by serotype. B–D: Local wavelet power spectrum and global power spectrum for EVA-71 (B), CVA-16 (C) and other EVs (D). Color is the power spectrum, power increases from blue to red with red denoting stronger periodicities. Solid circles indicate boundary of statistical significance; dashed lines are “core-of-influence” estimates.
Fig. 3.
Seasonal distribution and prediction diagram of HFMD in Zhengzhou, China. (A) Actual value and prediction value of SARIMA (2,0,1)(2,0,0)12 model. (B) Seasonal distribution of HFMD from 2009 to 2021.
3.4. Incidence, severity, and case-fatality ratio of HFMD in Zhengzhou, China
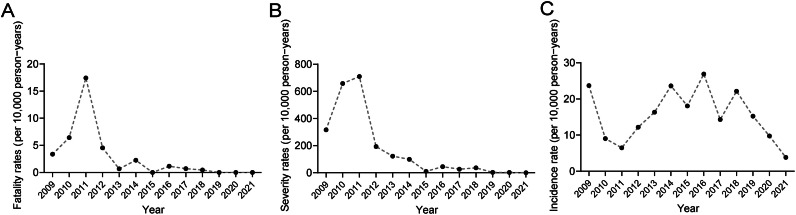
During the study period, the mean annual incidence rate of HFMD was about 15.51 per 10,000 persons, while the maximum annual incidence rate was approximately 26.88 per 10,000 persons in 2016 (χ2 = 41306.985, P < 0.001) (Supplementary Table 3, Fig. 4A). After 2018, there was a gradual decline in the incidence of HFMD (Fig. 4A). The severity rate of HFMD peaked in 2011 (7.10%) and then decreased over subsequent years (Supplementary Table 3, Fig. 4B). In 2011, the case-fatality ratio was recorded at 17.43 per 10,000 persons, surpassing those observed in other years (χ2 = 54.144, P < 0.001, Fig. 4C). Meanwhile, the data presented in Supplementary Table 4 reveal the significant decrease in the severity and case-fatality ratio of HFMD following implementation of the EVA-71 vaccine (P < 0.001). EVA-71 is the most frequent cause of severe HFMD. Vaccination has significantly decreased the EVA-71 epidemic, which in turn has reduced the severity and case fatality rate. Similar patterns in severity rates were also observed in the aftermath of the COVID-19 pandemic (Supplementary Table 5).
Fig. 4.
Annual incidence, severity, and case-fatality rate of HFMD in Zhengzhou, China. (A) Annual incidence rate of HFMD in Zhengzhou, China. (B) Annual severity rate of HFMD in Zhengzhou, China. (C) Annual case-fatality rate of HFMD in Zhengzhou, China.
Notably, when comparing different age groups, EVA-71 infections exhibited significantly higher severity and case-fatality ratios compared with CVA-16 infections or other EV infections. The severity rate of HFMD showed broad age-specific variations (χ2 = 343.044, P < 0.001, Table 2). Most severe cases were observed in patients aged 1 year and below, accounting for 45.81%. As shown in Fig. 5A, the severity rate peaked at 1 year of age (44.21%) for cases infected with EVA-71, while cases infected with CVA-16 and other EVs peaked in the age group below 1 year at 2.15% and 14.22%, respectively. These differences were found to be statistically significant (χ2 = 343.044, P < 0.001). The highest severity rate for HFMD was observed at 1 year of age (Table 2). Furthermore, among laboratory-confirmed cases, the case-fatality ratio caused by EVA-71 and other EVs peaked at 3 years of age at 0.50% and 0.38%, respectively. After peaking, both the severity and case-fatality ratio decreased with increasing age. Additionally, the severity-fatality rate peaked at the age of 3 years for both EVA-71 and other EVs, at 2.04% and 7.14%, respectively (Fig. 5C). Following this peak, a decline in the case-fatality ratio was observed with increasing age. This is because young children have low immunity levels and poor hygiene awareness and habits, making them more susceptible to EVs, potentially resulting in severe infections and fatalities [16].
Table 2.
Severity rates and case-fatality rates with ages.
| Rate | < 1 | 1 | 2 | 3 | 4 | 5 | 6–14 | ≥15 | χ2 | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Severity | 254 (1.51) | 1082 (1.66) | 569 (1.42) | 303 (0.8) | 104 (0.58) | 35 (0.49) | 15 (0.22) | 0 (0) | 343.044 | <0.001 |
| Case-fatality | 2 (0.12) | 15 (0.23) | 10 (0.25) | 8 (0.21) | 0 (0) | 2 (0.28) | 0 (0) | 0 (0) | 7.281* | 0.314 |
Note: Severity rate: Per 100-people. Case-fatality rate: Per 1000-people.
Fisher Exact Probability test.
Fig. 5.
Severity, fatality, and severity-fatality rate of HFMD in different ages in Zhengzhou, China. (A) The severity rate of notified HFMD cases in different ages by serotype in Zhengzhou, China from 2009 to 2021. (B) The case-fatality rate of notified HFMD cases in different ages by serotype in Zhengzhou, China from 2009 to 2021. (C) The severity-fatality rate of notified HFMD cases in different ages by serotype in Zhengzhou, China from 2009 to 2021.
Among the several districts in Zhengzhou, Huiji District had the highest incidence, with an average yearly incidence of 28.98 per 10,000 persons (Supplementary Table 6; χ2 = 70,466.04, P < 0.001). Gongyi County had the highest severity rate (502.79 per 10,000 persons, χ2 = 1,253.896, P < 0.001), and Dengfeng City had the highest case-fatality ratio (7.61 per 10,000 persons), but this difference was not statistically significant (χ2 = 19.773, P = 0.081).
4. Discussion
HFMD is a public health threat of global concern. Our findings align with previous investigations [20], confirming the higher prevalence of infection and the greater proportion of severe cases among male compared with female individuals. This discrepancy may be attributed to the increased physical activity and willingness of boys to engage in outdoor pursuits, thereby increasing their likelihood of exposure and subsequent infection [21]. Notably, scattered children demonstrated the highest rates of severity and fatality, consistent with previous findings in Beijing [22]. Consequently, it is imperative to enhance the promotion of HFMD prevention measures within childcare facilities and communities.
In contrast to previous investigations conducted in Xi'an [23,24], the etiology of HFMD was predominantly attributed to the primary pathogen EVA-71, which was found to be responsible for severe cases of HFMD in our study. EVA-71 demonstrated the highest prevalence of severe infections and mortality across different age cohorts, surpassing other viral agents. Conversely, CVA-16 accounted for the lowest number of cases and exhibited less severe manifestations, with no reported fatalities linked to CVA-16 throughout the duration of the study. With a decrease in the frequency of EVA-71 and CVA-16 infections and an increase in other EVs, the etiological agents causing HFMD infections in Zhengzhou have become more diverse in recent years. We propose two possible explanations for this decline in EVA-71 and CVA-16 infections. First, since 2014, the pathogen spectrum of HFMD has altered, with CVA-16 and other EVs emerging as alternatively co-circulating pathogens. Second, a vaccination campaign targeting children aged 0.5–5 years with EVA-71 vaccine was implemented in Zhengzhou from September 2016, resulting in a notable decline in EVA-71 infections by 2017. This vaccine has been shown to be extremely effective at protecting children from infection [17]. After 2017, the number of severe cases linked to other EVs surpassed those associated with EVA-71 and CVA-16. Given the potential threat from pathogenetic diversification, comprehensive virological surveillance of other EVs and the development of multivalent vaccines may be critical in the early response to HFMD outbreaks. The results from wavelet analysis suggested that the number of cases of EVA-71, CVA-16, or other EVs showed close to 1-year periodicity, which was consistent with other studies [25,26]. Our findings on the epidemiological cycle of HFMD in Zhengzhou are not in agreement with the general view of a 2-year cycle for all serotypes reported elsewhere [4]. Hence, health promotion and prevention activities may be conducted far in advance of the epidemic period to limit the virus's rapid spread and minimize the number of infections. HFMD exhibits year-round occurrence but displays distinct seasonal patterns and an overall bimodal distribution. Consistent with other areas of China [16,27], with the exception of the year 2020, the highest frequency of HFMD outbreaks in Zhengzhou was predominantly observed during April and June, followed by secondary peaks in October and November. In Xi'an, the months of April through July experienced the highest incidence rates, followed by October and November [23]. Additionally, in Guangdong Province, May to June and October to November observed the highest seasonal incidences [28]. The annual peak of HFMD cases manifested periodically. The peak incidence was reached in May in most years, including 2010, 2012, 2015, 2014, 2016, 2017, and 2018. The seasonal incidence trend was predicted by the SARIMA model. Notably, a potential increased incidence was anticipated in July and November of 2023, as well as from April to August of 2024. Taking into account the relaxation of control measures pertaining to COVID-19 in 2023, it is plausible that the number of HFMD cases in both 2023 and 2024 may surpass initial predictions. This is confounded by the altered distribution of EVs that is associated with changes in environmental factors such as season, climate, and humidity [29], [30], [31], [32]. The rise in temperature and humidity during the summer months enhances the proliferation and propagation of EVs, thereby facilitating the transmission of HFMD [29,33]. Notably, there has been a significant decline in August each year, which could potentially be attributed to the summer vacations and reduced gatherings in school. However, the seasonal distribution of HFMD in 2020 exhibited a distinctive “single-peak” trend, characterized by a solitary outbreak in November. It is crucial to prioritize early prevention and control measures before the annual peak epidemic to reduce the occurrence of severe cases and improve the protection of children.
When compared with nationwide data for China, Zhengzhou has a high incidence rate. The national incidence of HFMD in China during 2010–2015 was reported to be 12.7 per 10,000 person-years [23]. Our study observed that the incidence rate of HFMD infections in Zhengzhou was around 15.51 per 10,000 person-years during 2009–2021. However, a declining pattern in the incidence of HFMD has been observed annually from 2018 to 2021. This finding aligns with prior investigations [16,20], indicating that the prevention and control measures in place for HFMD have achieved some success. EVA-71 vaccination was initiated in Zhengzhou in September 2016, mainly targeting children under 5 years of age. According to previous studies, vaccination plays a crucial role in decreasing the incidence, severity, and fatality rates of HFMD [34,35]. To date, monovalent vaccines have been utilized for the prevention HFMD, while polyvalent vaccines are currently in the phase of clinical trials. Three bivalent vaccines against EVA-71 and CVA-16 infection have been approved for clinical trials in China, including those developed by AIM Vaccine, Beijing Sinovac Vaccine, and the Institute of Medical Biology Chinese Academy of Medical Sciences (IMBCAMS, https://www.cde.org.cn/). The most accessible inactivated whole virus vaccines for EVA-71 have been developed by Sinovac, Sinopharmc, and IMBCAMS. According to the phase 3 randomized experiment conducted in China, a 97.4% efficacy rate was shown for the inactivated EVA-71 vaccine [36]. Additionally, a clinical trial of the bivalent vaccine was approved and showed protection against both CVA-16 and EVA-71 infections. Following the introduction of vaccination, the surveillance of HFMD etiology revealed a gradual reduction in the proportion of cases attributed to EVA-71, accompanied by a declining trend in both severity and fatality rates. This decline in severe infections and mortality may be associated with the decreased prevalence of EVA-71 following vaccination. Notably, the EVA-71 vaccine is presently not part of the complimentary and standardized vaccination program for children. Consequently, immunization is advised for younger children because of their increased vulnerability to infection and relatively elevated risk of experiencing severe symptoms. The pathogenic spectrum of HFMD has changed, with EVA-71 and CVA-16 gradually being replaced by other serotypes such as CVA-6 and CVA-10 as the primary pathogens. Notably, the EVA-71 vaccine does not provide cross-protection against other EVs, leaving children susceptible to infection by other serotypes of HFMD even after vaccination [17,37,38]. Therefore, it is crucial to acknowledge and address this risk. In response to the changing epidemic strains of HFMD, the development of multivalent vaccines that include EVA-71 and other major serotypes has become an accepted strategy for preventing HFMD [24]. Since the initiation of the COVID-19 pandemic, the government has implemented comprehensive interventions, including imposing strict restrictions on individuals’ movement and temporarily closing kindergartens, with the aim of interrupting the transmission of COVID-19. During the COVID-19 epidemic in 2020, the incidence of HFMD significantly decreased, as determined by our assessment of COVID-19 Non-Pharmaceutical Interventions (NPIs) impact on EV spread. This finding supports a previous study that observed the nationwide impact of NPIs on HFMD incidence in China [39]. These findings provide evidence that effective measures can be implemented to intervene in the incidence of HFMD.
The severity rates associated with all three serotypes exhibited a declining trend as age increased beyond 1 year, which is consistent with previous serological evidence [40]. A significant factor contributing to the disparity observed across various age groups is that levels of EV antibodies increase with age [16,41]. Simultaneously, young children are more vulnerable to EVs due to their relatively weaker immune response and limited awareness of hygiene practices [16]. To address this, it is imperative to implement a holistic strategy that encompasses heightened preventive and treatment measures for HFMD, particularly in educational institutions and childcare facilities. Additionally, efforts should be made to augment public awareness and education among high-risk populations, while ensuring the provision of standardized vaccination services.
Among the different districts of Zhengzhou, Huiji District exhibited the highest incidence rate, potentially attributable to factors such as the high population density, the mobility of the population, and the relatively advanced economy [21]. Gongyi County and Dengfeng City had the highest severity rate and case-fatality ratio, which might be associated with factors such as medical care and economic level [21]. Wang et al. [42] found that high population density, population mobility, and well-developed transportation facilitated the spread of HFMD in metropolitan areas, whereas in less developed areas, limited medical care made it difficult to control HFMD. Attention and surveillance should be strengthened to reduce the incidence of HFMD in schools in less developed areas.
This study had several limitations. First, only 3.6% of reported cases are tested for EVs associated with HFMD; therefore, the prevalence of different viruses may be underestimated. Future efforts should focus on improving the detection of various viruses to better understand the epidemiology of infections. Second, with the limited laboratory data on other EVs, we are unable to evaluate the impact of specific serotypes other than EVA-71 and CVA-16, especially CVA-6, which have increased dramatically in recent years [43], [44], [45]. Third, surveillance data on a self-limited illness are used in this research where cases may have been underreported, especially regarding asymptomatic infections. This may be due to the fact that the symptoms of infection are mild and therefore patients do not attend hospital. Fourth, our study was based on a descriptive analysis of surveillance data.
Developing multivalent EV vaccines based on monitoring the spectrum of HFMD pathogens, comprehensive virological surveillance on specific virus serotypes, and encouraging EV prevention through personal protective measures, especially before a pandemic, will be crucial in reducing the adverse impacts of HFMD on human health.
5. Conclusion
Since the implementation of the EVA-71 vaccination program, there has been a notable reduction in the incidence, severity rate, and case-fatality ratio of HFMD in Zhengzhou, China. However, it is important to note that the prevalence of HFMD still exhibits a discernible seasonal pattern and periodicity, and the emergence of outbreaks related to other EV infections presents a new obstacle to the effective management of HFMD. Consequently, it is imperative to undertake surveillance of additional EV serotypes, pursue the development of multiple vaccines, and prioritize health education as essential measures for the future control of HFMD.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82273695, No. 82002147, and No. 82073618) and the Open Research Fund of the National Health Commission Key Laboratory of Birth Defects Prevention & Henan Key Laboratory of Population Defects Prevention (ZD202301). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Author contributions
B.W.D., G.W.L., and Y.F.J.: Conceptualization; B.W.D.: Methodology, Data curation, Writing—original draf; Y.F.J.: Software, Supervision, Writing—review and editing; Y.F.J.: Resources, Project administration; Y.F.J., H.Y.Y., and G.C.D.: Funding acquisition; Y.C., S.J.H., S.H.C., F.W., H.F.F., X.L.Z., and W.L.L.: Validation; G.C.D.: Formal analysis; H.Y.Y.: Investigation; S.Y.C.: visualization. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
None.
Declaration of competing interest
The authors have declared that no competing interests exist.
Data available statement
All relevant data are within the manuscript and its Supporting Information files.
Ethics statement
Not applicable.
Informed consent
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imj.2024.100114.
Contributor Information
Guowei Li, Email: lgw@xxmu.edu.cn.
Yuefei Jin, Email: jyf201907@zzu.edu.cn.
Appendix. Supplementary materials
References
- 1.Sinclair C., Gaunt E., Simmonds P., et al. Atypical hand, foot, and mouth disease associated with coxsackievirus A6 infection, Edinburgh, United Kingdom, January to February 2014. Euro Surveill. 2014;19(12):20745. doi: 10.2807/1560-7917.es2014.19.12.20745. [DOI] [PubMed] [Google Scholar]
- 2.Alsop J., Flewett T.H., Foster J.R. "Hand-foot-and-mouth disease” in Birmingham in 1959. BMJ. 1960;2(5214):1708–1711. doi: 10.1136/bmj.2.5214.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoorn B., Tyrrell D.A. On the growth of certain “newer” respiratory viruses in organ cultures. Br. J. Exp. Pathol. 1965;46(2):109–118. [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon T., Lewthwaite P., Perera D., et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10(11):778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Hu T., Sun D., et al. Epidemiological characteristics of hand, foot, and mouth disease in Shandong, China, 2009–2016. Sci. Rep. 2017;7(1):8900. doi: 10.1038/s41598-017-09196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J., Liao Q., Ooi M.H., et al. Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg. Infect. Dis. 2018;24(3):432–442. doi: 10.3201/eid2403.171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y., Yeo A., Phoon M.C., et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 2010;14(12):e1076–e1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Ooi M.H., Wong S.C., Lewthwaite P., et al. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9(11):1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt N.J., Lennette E.H., Ho H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974;129(3):304–309. doi: 10.1093/infdis/129.3.304. [DOI] [PubMed] [Google Scholar]
- 10.Puenpa J., Wanlapakorn N., Vongpunsawad S., et al. The history of enterovirus A71 outbreaks and molecular epidemiology in the asia-pacific region. J. Biomed. Sci. 2019;26(1):75. doi: 10.1186/s12929-019-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca M.C., Sarmiento L., Resik S., et al. Coxsackievirus A6 and enterovirus 71 causing hand, foot and mouth disease in Cuba, 2011–2013. Arch. Virol. 2014;159(9):2451–2455. doi: 10.1007/s00705-014-2071-x. [DOI] [PubMed] [Google Scholar]
- 12.Hayman R., Shepherd M., Tarring C., et al. Outbreak of variant hand-foot-and-mouth disease caused by coxsackievirus A6 in Auckland, New Zealand. J. Paediatr. Child Health. 2014;50(10):751–755. doi: 10.1111/jpc.12708. [DOI] [PubMed] [Google Scholar]
- 13.Puenpa J., Mauleekoonphairoj J., Linsuwanon P., et al. Prevalence and characterization of enterovirus infections among pediatric patients with hand foot mouth disease, herpangina and influenza like illness in Thailand, 2012. PLoS ONE. 2014;9(6):e98888. doi: 10.1371/journal.pone.0098888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Yin H., An Z., et al. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine. 2015;33(9):1107–1112. doi: 10.1016/j.vaccine.2014.10.081. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Feng Z., Yang Y., et al. Hand, foot, and mouth disease in China: patterns of spread and transmissibility. Epidemiology. 2011;22(6):781–792. doi: 10.1097/EDE.0b013e318231d67a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing W., Liao Q., Viboud C., et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect. Dis. 2014;14(4):308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., An Z., Huo D., et al. Enterovirus A71 vaccine effectiveness in preventing enterovirus A71 infection among medically-attended hand, foot, and mouth disease cases, Beijing, China. Hum. Vaccin. Immunother. 2019;15(5):1183–1190. doi: 10.1080/21645515.2019.1581539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian L., Wang Y., Yao X., et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev. Anti Infect. Ther. 2015;13(9):1061–1071. doi: 10.1586/14787210.2015.1058156. [DOI] [PubMed] [Google Scholar]
- 19.He S.Z., Chen M.Y., Xu X.R., et al. Epidemics and aetiology of hand, foot and mouth disease in Xiamen, China, from 2008 to 2015. Epidemiol. Infect. 2017;145(9):1865–1874. doi: 10.1017/S0950268817000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J., Zeng H., Zheng H., et al. Hand, foot and mouth disease in Guangdong, China, in 2013: new trends in the continuing epidemic. Clin. Microbiol. Infect. 2014;20(7):O442–O445. doi: 10.1111/1469-0691.12468. [DOI] [PubMed] [Google Scholar]
- 21.Pan Q., Liu F., Zhang J., et al. Regional-level risk factors for severe hand-foot-and-mouth disease: an ecological study from mainland China. Environ. Health Prev. Med. 2021;26(1):4. doi: 10.1186/s12199-020-00927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Sun Y., Du Y., et al. Characterization of coxsackievirus A6- and enterovirus 71-associated hand foot and mouth disease in Beijing, China, from 2013 to 2015. Front. Microbiol. 2016;7:391. doi: 10.3389/fmicb.2016.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Xiang X., Pu Z., et al. Epidemic pattern of hand-foot-and-mouth disease in Xi'an, China from 2008 through 2015. BMC Infect. Dis. 2019;19(1):19. doi: 10.1186/s12879-018-3624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M.S., Chang L.Y. Development of enterovirus 71 vaccines. Expert Rev. Vaccines. 2010;9(2):149–156. doi: 10.1586/erv.09.152. [DOI] [PubMed] [Google Scholar]
- 25.Yang B., Liu F., Liao Q., et al. Epidemiology of hand, foot and mouth disease in China, 2008 to 2015 prior to the introduction of EV-A71 vaccine. Euro Surveill. 2017;22(50):16–00824. doi: 10.2807/1560-7917.ES.2017.22.50.16-00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian C.W., Wang H., Luo X.M. Time-series modelling and forecasting of hand, foot and mouth disease cases in China from 2008 to 2018. Epidemiol. Infect. 2019;147:e82. doi: 10.1017/S095026881800362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma E., Chan K.C., Cheng P., et al. The enterovirus 71 epidemic in 2008—public health implications for Hong Kong. Int. J. Infect. Dis. 2010;14(9):e775–e780. doi: 10.1016/j.ijid.2010.02.2265. [DOI] [PubMed] [Google Scholar]
- 28.Deng T., Huang Y., Yu S., et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS One. 2013;8(2):e56943. doi: 10.1371/journal.pone.0056943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P., Goggins W.B., Chan E.Y.Y. Hand, foot and mouth disease in Hong Kong: a time-series analysis on its relationship with weather. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo C., Ma Y., Liu Y., et al. The burden of childhood hand-foot-mouth disease morbidity attributable to relative humidity: a multicity study in the Sichuan Basin. China, Sci. Rep. 2020;10(1):19394. doi: 10.1038/s41598-020-76421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., Rosenberg M.W., Chen H., et al. Epidemiological characteristics and spatiotemporal patterns of hand, foot, and mouth disease in Hubei, China from 2009 to 2019. PLoS One. 2023;18(6) doi: 10.1371/journal.pone.0287539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Huang R., Wang H., et al. Spatial-temporal heterogeneity and meteorological factors of hand-foot-and-mouth disease in Xinjiang, China from 2008 to 2016. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Zhang S. Epidemiological characteristics and trends of hand-foot-mouth disease in Shanghai, China from 2011 to 2021. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1162209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swain S.K., Gadnayak A., Mohanty J.N., et al. Does enterovirus 71 urge for effective vaccine control strategies? Challenges and current opinion. Rev. Med. Virol. 2022;32(4):e2322. doi: 10.1002/rmv.2322. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X., Zhang Y., Li H., et al. Hand-foot-and-mouth disease-associated enterovirus and the development of multivalent HFMD vaccines. Int. J. Mol. Sci. 2022;24(1):169. doi: 10.3390/ijms24010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R., Liu L., Mo Z., et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014;370(9):829–837. doi: 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Zhou Y., Cheng Y., et al. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017-18: a test-negative case-control study. Lancet Child Adolesc. Health. 2019;3(10):697–704. doi: 10.1016/S2352-4642(19)30185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F.C., Meng F.Y., Li J.X., et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381(9882):2024–2032. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z., Zheng C., Qi H., et al. Impact of the coronavirus disease 2019 interventions on the incidence of hand, foot, and mouth disease in mainland China. Lancet Reg. Health West. Pac. 2022;20 doi: 10.1016/j.lanwpc.2021.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B., Wu P., Wu J.T., et al. Seroprevalence of enterovirus 71 antibody among children in China: a systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2015;34(12):1399–1406. doi: 10.1097/INF.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu F.C., Liang Z.L., Meng F.Y., et al. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS One. 2012;7(5):e37206. doi: 10.1371/journal.pone.0037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C., Li X., Zhang Y., et al. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the county level in mainland China, 2008–2012. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buttery V.W., Kenyon C., Grunewald S., et al. Atypical presentations of hand, foot, and mouth disease caused by coxsackievirus A6: Minnesota, 2014. MMWR Morb. Mortal. Wkly Rep. 2015;64(29):805. doi: 10.15585/mmwr.mm6429a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu H., Ma C., Yang Q., et al. Hand, foot and mouth disease caused by coxsackievirus A6, Beijing, 2013. Pediatr. Infect. Dis. J. 2014;33(12):1302–1303. doi: 10.1097/INF.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 45.Zeng H., Lu J., Zheng H., et al. The epidemiological study of coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guangdong, China. Sci. Rep. 2015;5:10550. doi: 10.1038/srep10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.