Abstract
Background
There is an imminent need to identify neural markers during preadolescence that are linked to developing depression during adolescence, especially among youth at elevated familial risk. However, longitudinal studies remain scarce and exhibit mixed findings. Here we aimed to elucidate functional connectivity (FC) patterns among preadolescents that interact with familial depression risk to predict depression two years later.
Methods
9–10 year-olds in the Adolescent Brain Cognitive Development (ABCD) Study were classified as healthy (i.e., no lifetime psychiatric diagnoses) at high familial risk for depression (HR; n=559) or at low familial risk for psychopathology (LR; n=1203). Whole-brain seed-to-voxel resting-state FC patterns with the amygdala, putamen, nucleus accumbens, and caudate were calculated. Multi-level, mixed-effects regression analyses were conducted to test whether FC at ages 9–10 interacted with familial risk to predict depression symptoms at ages 11–12.
Results
HR youth demonstrated stronger associations between preadolescent FC and adolescent depression symptoms (ps<0.001) as compared to LR youth (ps>0.001), primarily among amygdala/striatal FC with visual and sensory/somatomotor networks.
Conclusions
Preadolescent amygdala and striatal FC may be useful biomarkers of adolescent-onset depression, particularly for youth with family histories of depression. This research may point to neurobiologically-informed approaches to prevention and intervention for depression in adolescents.
Keywords: Depression, Familial risk for depression, ABCD study, Resting-state fMRI, Youth, Longitudinal study
1. Introduction
Depression is a leading cause of disability and suicide among adolescents (Disease et al., 2016). One consequence of the extensive neurobiological and social changes associated with adolescence is increased vulnerability to developing psychopathology (Lee, 2014). Psychiatric risk is further elevated by a familial history of illness; for example, youth who have parents experiencing depression are at three- to five-fold increased risk for developing depression themselves (Lieb, 2002, Williamson, 2004). These compounding risk factors highlight the pressing need to identify predictive neural markers for the development of depression prior to the onset of adolescence, especially among children who are already at high familial risk.
Extant research suggests that familial risk for depression may manifest through atypical development of neural circuits implicated in reward and emotion processing, with alterations observed in youth who are at high familial risk for depression, but who do not presently have depression (Gotlib, 2010, Frost Bellgowan, 2015, Chai, 2016, Chai, 2015, Fischer, 2022, Morgan, 2019, Morgan, 2022, Olino, 2014, Singh, 2018, Holt-Gosselin, 2023). Specifically, our recent study (Holt-Gosselin, 2023) identified alterations in striatal connectivity among youth at high familial risk for depression. While these studies provide important insight regarding potential neural markers of vulnerability to familial risk, there is scarce longitudinal research that identifies neural patterns that predict the future development of depression and how these patterns may differ depending on familial risk status among youth (Toenders, 2019). The small body of research that exists on this topic reveals that altered neural responses and functional connections within and between the amygdala, ventral and dorsal striatum, and frontal (e.g., anterior cingulate, dorsal prefrontal cortex) brain regions are associated with the subsequent onset of psychopathology (including depression) among youth at high familial risk for depression (Fischer, 2022, Pawlak et al., 2022, Hirshfeld-Becker, 2019, Shapero, 2019, Fischer, 2018). While there is relatively consistent evidence that reduced neural responses to reward in the ventral striatum and prefrontal cortex are associated with the future onset and course of depression in adolescence (Toenders, 2019), resting-state functional connectivity (FC) circuits have been less commonly investigated and current findings lack consistency (Toenders, 2019), warranting an imminent need for additional research.
Although existing studies revealing pre-existing neural markers of future depression among youth at high familial risk set a strong foundation, the identification of robust and consistent prognostic markers has been hindered by numerous study limitations. More specifically, prior work utilized small sample sizes (e.g., n<150), likely resulting in insufficient statistical power to detect complex interactions between brain function and risk factors in predicting future general and specific (e.g., depression) psychopathology. Additionally, the majority of previous studies do not include a low familial risk comparison group, which is essential for elucidating how familial risk specifically may contribute to future symptomology. Finally, prior research utilized samples spanning a wide range of developmental periods (e.g., 8–14, 8–17 years) and had inconsistent follow-up durations when assessing psychiatric symptoms (e.g., 3±2 years); these designs likely limited the studies’ ability to pinpoint neural markers specific to particular developmental periods and their timing with respect to the onset of psychopathology.
Beyond the lack of scarce research in this area, more studies are needed because this work is imperative for understanding screening for depression risk, delineating the neural mechanisms of risk for developing depression, and informing early intervention strategies. Specifically, it is useful to identify neurobiological markers of depression vulnerability in a sample of youth who are otherwise free of any psychiatric diagnosis (and thus may not behaviorally appear to be particularly vulnerable to depression) to inform evolving approaches to assessing depression risk. In the longer term, this research may contribute to the detection of psychopathology risk through the inclusion of the use of neuroimaging tools in conjunction with psychological, environmental, and behavioral assessments. Since it is well-established that depression is a result of environmental, behavioral, and biological factors (Ho, 2022), including additional modalities for risk assessment could potentially strengthen existing screening methods. Additionally, this research can advance understanding of the neural mechanisms underlying depression to inform the refinement of interventions and identify novel treatment targets.
Since adolescence marks a period of increased risk for psychopathology (Spear, 2004), as well as significant changes in brain maturation and in the environment (Cunningham et al., 2002, Giedd, 1999), preadolescence is a particularly useful window to identify pre-existing neural vulnerability markers that may put adolescents at even further risk for psychopathology during adolescence. Depression commonly emerges during a child’s transition to adolescence (Paus et al., 2008), which also coincides with pubertal changes that are associated with heightened risk for depression, particularly among girls (Lewis, 2018). Additionally, earlier-onset depression (e.g., during adolescence) is associated with a more severe illness course (Merikangas, 2010, Petito, 2020). Thus, identifying preadolescent neural vulnerability markers that are present prior to the onset of clinically significant depression symptoms during adolescence is valuable because this knowledge can lead to early identification of vulnerable youth. Since intervening early in illness course is associated with a less severe symptom course (Wittchen, 2011), early identification and treatment will ultimately improve mental health outcomes.
To address these gaps in knowledge, the current study leveraged a large sample from the ongoing Adolescent Brain Cognitive Development (ABCD) Study℠ (Casey, 2018) to test whether emotion- and reward-related FC patterns among healthy youth aged 9–10 interact with familial depression risk status to predict future depression symptoms at ages 11–12. Participants included healthy (i.e., no lifetime psychiatric diagnoses) youth who had at least one parent with a lifetime history of depression (HR, n=559) as well as healthy youth whose parents had no lifetime history of any psychiatric problems (LR, n=1203) at initial assessment (i.e., 9–10 years old). To our knowledge, this study utilized the largest sample to-date to elucidate prospective, preadolescent neural markers of adolescent-onset depression symptoms and how these associations differ based on familial history. The identification of prospective neural markers of depression, especially understanding how these may differ between youth at high versus low familial risk, is essential for prevention and early intervention strategies to mitigate depression severity, delay, and/or potentially prevent illness onset.
2. Methods
2.1. Study Design and Participants
Participants are from the ABCD Study® (Casey, 2018), which recruited 11,878 youth across 21 sites who are being followed over 10 years. Youth were 9 or 10 years old at the time of the baseline visit (between 2016 and 2018). Youth and their parents were recruited from public and private elementary schools within the catchment areas of the 21 sites. The study did not exclude twins, nor multiple siblings from the same family.
Inclusion criteria included: a) age 9–10 years at baseline visit, b) attending a public or private elementary school in the catchment area. Exclusion criteria included: a) not fluent in English, b) having a parent not fluent in English or Spanish, c) major medical or neurological conditions, d) gestational age <28 weeks or birthweight <1200 g, e) contraindications to MRI scanning, f) a history of traumatic brain injury, g) a current diagnosis of schizophrenia, moderate to severe autism spectrum disorder, intellectual disability, or alcohol/substance use disorder. Participants provided informed consent or assent (see (Clark, 2018) for ethics and oversight in the ABCD Study).
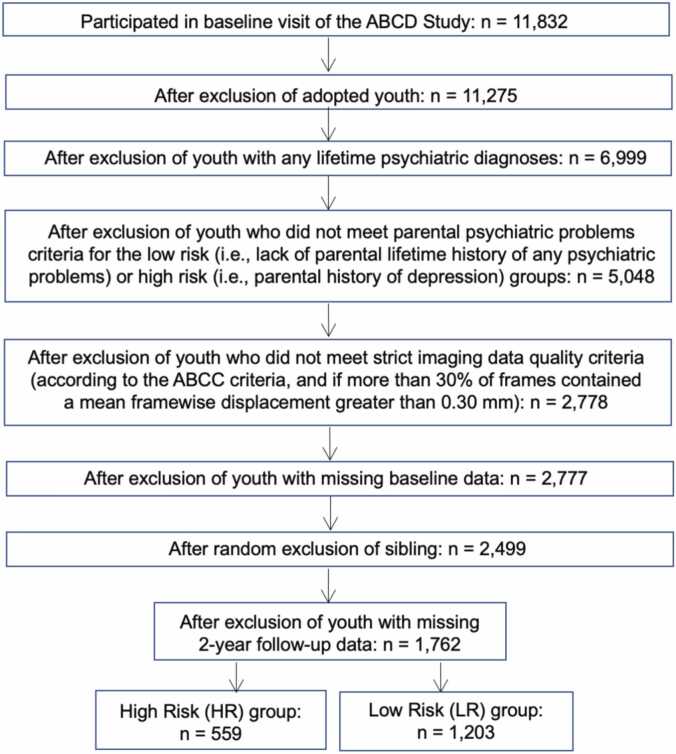
We used data from the 4.0 release (DOI: http://dx.doi.org/10.15154/1523041), which included baseline, 1- and 2-year data. A consort chart is reported in Fig. 1. The following exclusion criteria were used for our study: a) adopted youth, given that assessment of family history of psychiatric problems focuses on blood relatives; b) youth with any lifetime psychiatric diagnoses at baseline visit, reported by the parent about the youth (based on the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Youth for DSM-5 (K-SADS-5), see below); and c) resting-state fMRI data were recommended for exclusion by the ABCD-BIDS Community Collection (ABCC) team (Feczko et al., 2021). Youth were included in the high risk for depression (HR) group if there was a maternal and/or paternal history of depression (based on the Family History Assessment Module Screener (FHAM-S), see below). Youth were included in the low risk for psychiatric problems (LR) group if there was no parental lifetime history of any psychiatric problems. Youth with missing follow-up symptom data were excluded. For youth with siblings, one sibling was randomly selected and the other sibling(s) was excluded. Thus, the final sample includes 559 HR and 1203 LR youth.
Fig. 1.
Consort Chart of Youth in the Final High Risk and Low Risk Groups in 2-Year Follow-Up Longitudinal Sample. Exclusion criteria included: adopted youth, youth with any lifetime psychiatric diagnoses at baseline visit, and resting-state fMRI was recommended for exclusion by the ABCD-BIDS Community Collection (ABCC) team. Additionally, for the high risk group only, youth were excluded if there was a lack of parental history of depression. For the low risk group only, youth were excluded if there was a presence of parental lifetime history of any psychiatric problems. Youth were excluded if they lacked resting-state fMRI or diagnostic data, and siblings were excluded at random. Finally, youth with missing 2-year follow-up symptom data were excluded. Thus, this study includes 559 HR and 1203 LR youth.
2.2. Demographic Information
At baseline, parents reported the youth’s sex assigned at birth, age, and race/ethnicity, as well as parental education, marital status, and combined household income. At baseline, parents reported youth pubertal development using the Pubertal Development Scale (PDS) (Petersen, 1988).
2.3. Family History of Psychiatric Problems
The FHAM-S (Rice, 1995) is a brief interview that was used to assess family history of psychiatric problems in all first- and second-degree biological relatives of the child (i.e., full and half-siblings, parents, grandparents, aunts, uncles), as reported by a parent of the youth at the baseline visit. The FHAM-S assesses the presence/absence of symptoms associated with alcohol and substance use disorder, depression, anxiety, mania, psychosis, and antisocial personality disorder in all blood relatives. Our study examined parental history in order to facilitate comparison across prior studies (most of which focused on parental history, e.g., (Fischer, 2022, Pawlak et al., 2022, Hirshfeld-Becker, 2019, Shapero, 2019, Fischer, 2018)), limit heterogeneity, and due to stronger relations between parental history and youth psychopathology compared to sibling and second-degree relative histories (Gotlib, 2010, Fischer, 2018, Fischer, 2019, Luking, 2016). All youth in the HR group have at least one biological parent with a history of depression, whereas all youth in the LR group have both parents with no lifetime history of any psychiatric problems.
2.4. Youth Psychiatric Diagnoses
At the baseline visit, current and lifetime psychiatric diagnoses for youth were assessed using the parent-report responses to the computerized K-SADS-5 (Geller, 2001).
2.5. Youth Psychiatric Symptoms
Youth psychiatric symptoms were assessed using the parent-report Child Behavior Checklist (CBCL) (Achenbach, 2009) at baseline (9–10 years) and 2-year follow-up (11–12 years), which includes questions regarding symptoms of depression, anxiety, somatic, attention-deficit/hyperactivity, oppositional defiant, and conduct disorder. The current study used the depression symptoms, internalizing symptoms, externalizing symptoms, and total problems subscales.
2.6. Imaging Acquisition and Preprocessing
ABCD Study imaging procedures have been described in detail in Casey et al. (Casey, 2018). Youth completed four 5-minute resting-state fMRI scans at the baseline visit, whereby youth were instructed to fixate on a cross hair. Resting-state images were acquired in the axial plane using an echo-planar imaging (EPI) sequence. Other resting-state imaging parameters varied by 3 T scanner and have been previously described in Casey et al. (Casey, 2018). Data were pre-processed by the ABCD Consortium’s data analytic core (Hagler and Jr, 2019). Human Connectome Project (HCP) minimal preprocessing steps were implemented (Glasser, 2013). We used the resting-state fMRI data that were preprocessed by the ABCD-BIDS Community Collection (ABCC) team (Feczko et al., 2021).
Additional preprocessing of resting-state BOLD data was conducted by the Developmental Cognition and Neuroimaging lab, which consisted of three steps. First, fMRI data were de-meaned and de-trended with respect to time, where the central tendency was estimated based on low head-movement data, excluding frames with a framewise displacement (FD) threshold of 0.3 mm. Next, a first-level, multiple regression model was used to remove confounding nuisance signals from the processed fMRI data. Regressors included mean time series for white matter, cerebrospinal fluid, and the global signal, and translational (X, Y, Z) and rotational (roll, pitch, and yaw) motion parameters, which were estimated on non-censored head movement data (i.e., frames with FD<0.3 mm) and applied to the non-censored (i.e., low head movement) dataset. After denoising, the time series were band-pass filtered between 0.008 and 0.09 Hz using a second-order Butterworth filter applied in the forward and backward direction to avoid the introduction of lags in-phase. To avoid the introduction of head-movement artifacts when applying the band-pass filter, data coming from frames with a FD>0.3 mm were replaced with interpolated data from the remaining frames. Since this FD threshold (FD>0.3 mm) is higher than the FD threshold used later for motion censoring, the interpolated data were not used for the FC analyses. Timepoints were further censored with outlier detection. Participants’ data were excluded from subsequent analyses if greater than 30% of their frames were censored based on the mean FD>0.3 mm threshold. CIFTI dense time series for cortex were converted to voxel-level volumes by hemisphere using Connectome Workbench’s cifti-separate and metric-to-volume-mapping. For subcortex volumes, only cifti-separate was required. Finally, hemispheric cortical and subcortical volumes were combined to form a single map of voxel-level time series. Volumetric data were used here to conform to previously-reported analyses (Fischer, 2022, Pawlak et al., 2022, Hirshfeld-Becker, 2019, Shapero, 2019, Fischer, 2018), facilitating comparison of results.
2.7. Functional Connectivity (FC) Analysis
We conducted seed-to-voxel FC analyses as the final step in first-level fMRI preprocessing. A whole-brain FC map was generated for each participant and each spatially-independent seed: whole amygdala, putamen, nucleus accumbens, and caudate, split by hemisphere and defined by the Brainnetome Atlas (Fan, 2016). FC was calculated using AFNI’s (Cox, 1996) 3dNetCorr, where the partial correlation between mean residual time courses from the 8 regions of interest (ROIs) and all other voxels was calculated. Analyses were restricted to only gray matter voxels (defined by AFNI’s standard template). All correlation coefficients were converted to Z-scores using the Fisher transformation. Finally, FC outliers were censored from second-level analyses using a threshold of ±3 standard deviations from the global mean.
2.8. Primary Analysis: Preadolescent Functional Connectivity (FC) Patterns Interact with Familial Risk Status to Predict Depression Symptoms at 2-Year Follow-Up
We conducted multi-level, mixed-effects regression analyses to test for preadolescent (ages 9–10) whole-brain FC patterns with the amygdala, putamen, nucleus accumbens, and/or caudate (i.e., emotion and reward-related circuits) that interact with familial risk status to predict depression symptoms two years later (ages 11–12). Z-transformed correlation coefficients were used in regression models implemented with AFNI’s 3dLMEr (Chen, 2013) for each ROI, with depression symptoms at 2-year follow-up as the dependent variable. The interaction between familial risk status (i.e., HR versus LR group membership) and preadolescent FC patterns, as well as their main effects, were included as independent variables. Fixed-effect covariates included baseline age, sex assigned at birth, and baseline depression symptoms. A random intercept controlling for differences based on study site was also included. We conducted post-hoc simple slopes analyses to probe all significant interactions. For all main effects and interactions, voxel-level thresholding was set to p<0.001. Cluster-level multiple comparison corrections were conducted with AFNI’s 3dClustSim (with the updated -acf option), and a cluster was significant at a threshold of α (i.e., corrected cluster p) <0.05. The parameters applied in 3dClustSim were 2-sided thresholding (i.e., positive and negative Gaussian noise-only values are above the threshold and then clustered together) and a clustering option of nearest neighbors 3 (i.e., two voxels are part of the same cluster if their faces, edges, or corners converge). The acf parameters were estimated from the residual time series from the DCAN lab nuisance regression. No additional corrections were applied across the 8 ROIs, as we had little a priori reason to believe that the 8 ROIs would function similarly with respect to future depression symptoms.
To enhance interpretability of our results, we conducted an empirical search to determine which network each of the significant clusters belonged to according to the Power et al. atlas (Power, 2011). Specifically, we extracted the peak voxel from each of the significant clusters. Then, we used a nearest neighbor approximation with the Power et al. network assignments to identify the Power et al. ROI that was nearest to the peak voxel that was identified. Finally, we assigned the peak voxel to the network that Power et al. assigned it.
2.9. Sensitivity Analyses: Inclusion of Puberal Status as a Covariate
Given that prior work has demonstrated differential links between brain alterations and depression symptoms (Toenders, 2019) depending on pubertal status, we re-ran analyses after covarying for baseline pubertal status.
2.10. Sensitivity Analyses: Examination of Other Youth Psychiatric Symptoms (Besides Depression)
To determine whether findings observed were specific to the development of youth depression symptoms (versus generalizing to other types of psychiatric symptoms), we ran two additional analyses. First, we re-ran analyses with baseline total problems and follow-up total problems as covariates when predicting follow-up depression symptoms. To further probe whether findings were specific to depression symptoms (or internalizing symptoms more broadly), we re-ran analyses replacing our dependent variable (i.e., follow-up depression symptoms) with follow-up internalizing symptoms, covarying for baseline internalizing symptoms and follow-up externalizing symptoms.
2.11. Follow-Up Analyses
While both 1- and 2-year follow-up symptom data are available, we chose to examine 2-year follow-up because we were most interested in capturing the transition to adolescence specifically (i.e., ages 11–12), which marks a period of increased risk for psychopathology (Lee, 2014), and hypothesized that findings would have greater predictive validity if effects were detected with more time elapsed. However, given the availability of the 1-year symptom data, we also examined whether FC patterns interact with familial risk status to predict depression symptoms at 1-year follow-up (i.e., ages 10–11; see Supplement).
3. Results
3.1. Demographic and Clinical Characteristics
Demographic and clinical characteristics are reported in Table 1. HR and LR groups did not differ in age, sex at birth, pubertal status, or parental education, but differed in race and ethnicity (p<0.001), baseline and 2-year follow-up psychiatric symptoms (all ps<0.001), parental marital status (p<0.001), and household income (p=0.001). HR and LR groups did not differ in mean framewise displacement (p=0.923).
Table 1.
Demographic and Clinical Characteristics in High Risk versus Low Risk Groups in 2-Year Follow-Up Longitudinal Sample. Means and standard deviations (continuous variables), and frequencies and percentages (categorical variables) of demographic and clinical characteristics displayed for high and low risk groups. One-way ANOVAs (continuous variables) and Chi-Square tests (categorical variables) were conducted (as appropriate) for all variables of interest.
| Characteristic |
High Risk for Depression (HR) n=559 |
Low Risk for Psychiatric Problems (LR) n=1203 |
Statistical Value | P Value |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| Youth Age | 9.95 (0.64) | 10.00 (0.61) | t(1261.1)=1.04 | 0.299 |
| Youth Sex at Birth | n (%) | n (%) | ||
| Male | 254 (45.44 %) | 586 (48.71 %) | X2(1)=0.64 | 0.423 |
| Female | 305 (54.56 %) | 617 (51.29 %) | ||
| Youth Race and Ethnicity | n (%) | n (%) | ||
| Asian | 4 (0.72 %) | 35 (2.91 %)a | X2(4)=48.41 | <0.001b |
| Black | 40 (7.16 %) | 127 (10.56 %)a | ||
| Hispanic | 74 (13.24 %) | 246 (20.45 %)a | ||
| Native Hawaiian, Pacific Islander, Alaskan Native, American Indian, or Multiracial |
58 (10.38 %) | 89 (7.40 %) | ||
| White | 383 (68.52 %)a | 706 (58.69 %) | ||
| Youth Psychiatric Symptomsc | M (SD) | M (SD) | ||
| Depression Symptoms | 0.86 (1.31)a | 0.50 (1.01) | t(1088.5)=-4.96 | <0.001b |
| Depression Symptoms (2-Year) | 1.40 (2.07)a | 0.69 (1.29) | t(765.97)=-7.42 | <0.001b |
| Internalizing Symptoms | 4.21 (4.25)a | 2.72 (3.07) | t(1002.7)=-7.22 | <0.001b |
| Internalizing Symptoms (2-Year) | 4.85 (5.26)a | 2.82 (3.40) | t(782.67)=-8.35 | <0.001b |
| Externalizing Symptoms | 2.86 (3.62)a | 1.86 (2.60) | t(1015.3)=-6.21 | <0.001b |
| Externalizing Symptoms (2-Year) | 2.78 (3.72)a | 1.80 (2.87) | t(876.63)=-5.39 | <0.001b |
| Total Problems | 13.00 (11.32)a | 8.87 (8.39) | t(1039.1)=-7.65 | <0.001b |
| Total Problems (2-Year) | 13.27 (12.78)a | 8.53 (9.07) | t(828.54)=-7.90 | <0.001b |
| Youth Pubertal Statusd | n (%) | n (%) | ||
| Pre Puberty | 298 (54.48 %) | 612 (52.31 %) | X2(4)=1.94 | 0.746 |
| Early Puberty | 129 (23.58 %) | 291 (24.87 %) | ||
| Mid Puberty | 113 (20.66 %) | 255 (21.79 %) | ||
| Late Puberty | 7 (1.28 %) | 11 (0.94 %) | ||
| Post Puberty | 0 (0.00 %) | 1 (0.09 %) | ||
| Parental Educatione | n (%) | n (%) | ||
| High School or Less | 221 (39.53 %) | 466 (38.73 %) | X2(2)=2.46 | 0.292 |
| Bachelor’s Degree | 191 (34.17 %) | 378 (31.42 %) | ||
| Graduate Degree | 147 (26.30 %) | 359 (29.84 %) | ||
| Parental Marital Status | n (%) | n (%) | ||
| Divorced | 59 (10.55 %)a | 61 (5.07 %)a | X2(6)=52.59 | <0.001b |
| Living with a Partner | 31 (5.55 %)a | 36 (2.99 %) | ||
| Married | 403 (72.09 %) | 997 (82.88 %) | ||
| Never Married | 38 (6.80 %) | 75 (6.23 %) | ||
| Separated | 21 (3.76 %) | 20 (1.66 %) | ||
| Widowed | 5 (0.89 %) | 6 (0.50 %) | ||
| Refused to Answer | 2 (0.36 %) | 8 (0.67 %) | ||
| Household Incomef | n (%) | n (%) | ||
| Less than $50,000/year | 110 (19.68 %) | 218 (18.12 %) | X2(3)=16.99 | 0.001b |
| $50,000-$100,000/year | 188 (33.63 %)a | 313 (26.02 %) | ||
| Greater than $100,000/year | 231 (41.32 %) | 569 (47.30 %) | ||
| Don’t know/Refused to Answer | 30 (5.37 %) | 103 (8.56 %) |
Notes: All characteristics were assessed at the baseline visit unless otherwise noted.
Indicates the group n or mean was significantly higher than the other group.
Indicates significant difference between groups (p<0.05).
Youth psychiatric symptoms were measured using the Child Behavior Checklist (CBCL).
Youth pubertal status was measured using the parent-report of the Pubertal Development Scale (PDS).
Based on which parent was reporting; 1532 (86.95 %) biological mothers, 230 (13.05 %) biological fathers.
Household income was measured as total household income before taxes and deductions during the last 12 months.
On the CBCL depression subscale, a cut-off score of 3.5 or higher indicates clinical levels of depression (Jiang, 2023). For HR youth, 4.5 % (n=25/559) of youth at baseline and 11 % (n=64/559) of youth at 2-year follow-up had a score of 3.5 or higher. For LR youth, 2.4 % (n=29/1203) of youth at baseline and 3.9 % (n=47/1203) of youth at 2-year follow-up had a score of 3.5 or higher. While participants in both the HR and LR groups exhibited low depression symptoms on average at baseline and 2-year follow-up, depression symptoms significantly increased over the two years within both the HR and LR groups (t(558)=-6.65, p<0.001; t(1202)=-5.47, p<0.001, respectively).
3.2. Primary Analysis: Preadolescent FC Patterns Interact with Familial Risk Status to Predict Depression Symptoms at 2-Year Follow-Up
In general, HR youth demonstrated stronger associations between preadolescent FC and depression symptoms at 2-year follow-up as compared to LR youth. FC with the amygdala, caudate, and nucleus accumbens (but not the putamen) interacted with familial risk status to predict future depression symptoms. See Table S1 for cluster-level statistics, sizes, and peak/center-of-mass coordinates of significant clusters. Table S1 additionally includes the networks to which each significant cluster belongs according to prior work by Power et al. (Power, 2011). See Supplemental Results for findings with the 1-year follow-up depression symptoms.
3.3. Amygdala FC
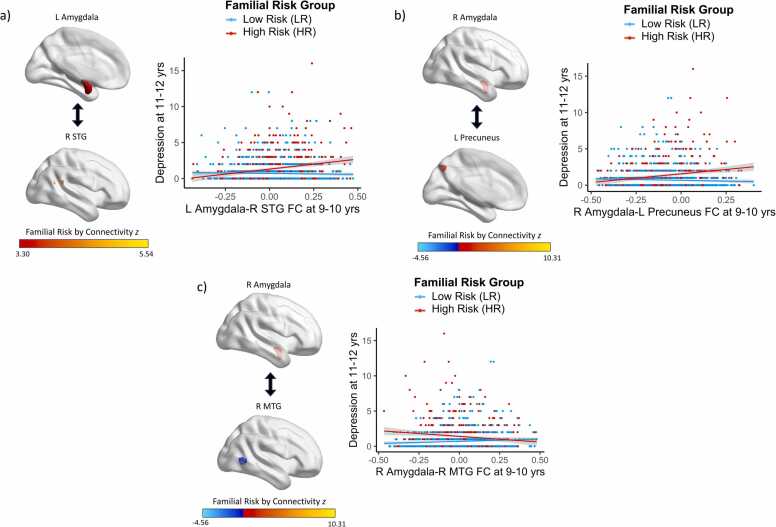
Preadolescent amygdala FC interacted with familial risk status to predict depression symptoms two years later (Fig. 2, Table S2, Table S3). More specifically, there were positive associations between amygdala FC with the superior temporal gyrus (STG; β=2.377, t=5.78, p<0.001) and precuneus (β=2.044, t=5.06, p<0.001) and future depression symptoms for HR youth; these relations were not significant for LR youth (β=−0.401, t=−1.39, p=0.163; β=-0.512, t=-1.77, p=0.077, respectively). Additionally, there was a negative association between amygdala FC with the middle temporal gyrus (MTG) and future depression symptoms for HR youth (β=-1.607, t=-3.95, p<0.001), whereas this relation was positive for LR youth (β=.623, t=2.29, p=0.022).
Fig. 2.
: Amygdala FC Patterns at Baseline Interact with Familial Risk Status to Predict Depression Symptoms at 2-Year Follow-Up. There were positive associations between amygdala FC with the a) superior temporal gyrus and b) precuneus and subsequent depression symptoms for HR youth, whereas these relations were not significant for LR youth. There was a negative association between amygdala FC with the c) middle temporal gyrus and subsequent depression symptoms for HR youth, whereas this relation was positive for LR youth.
3.4. Caudate FC
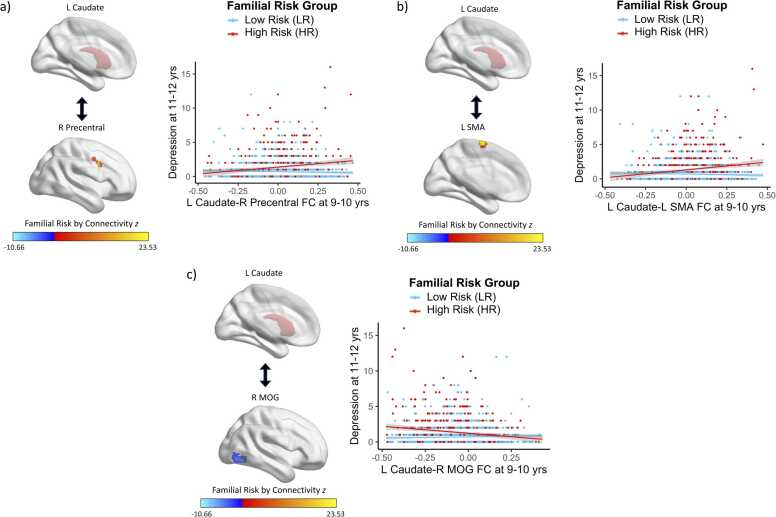
Preadolescent caudate FC interacted with familial risk status to predict depression symptoms two years later (Fig. 3, Table S2, Table S3). Specifically, there were positive associations between caudate FC with the precentral gyrus (β=2.288, t=5.54, p<0.001) and supplementary motor area (SMA; β=2.267, t=5.47, p<0.001) and future depression symptoms for HR youth; these relations were not significant for LR youth (β=-0.341, t=-1.26, p=0.210; β=-0.270, t=-0.96, p=0.335, respectively). Furthermore, there was a negative association between caudate FC with the middle occipital gyrus (MOG) and future depression symptoms for HR youth (β=-1.977, t=-5.16, p<0.001) and no significant relation for LR youth (β=0.513, t=1.92, p=0.055).
Fig. 3.
: Caudate FC Patterns at Baseline Interact with Familial Risk Status to Predict Depression Symptoms at 2-Year Follow-Up. There were positive associations between caudate FC with the a) precentral gyrus and b) supplementary motor area and subsequent depression symptoms for HR youth, whereas these relations were not significant for LR youth. There was a negative association between caudate FC with the c) middle occipital gyrus and subsequent depression symptoms for HR youth, whereas this relation was not significant for LR youth.
3.5. Nucleus Accumbens FC
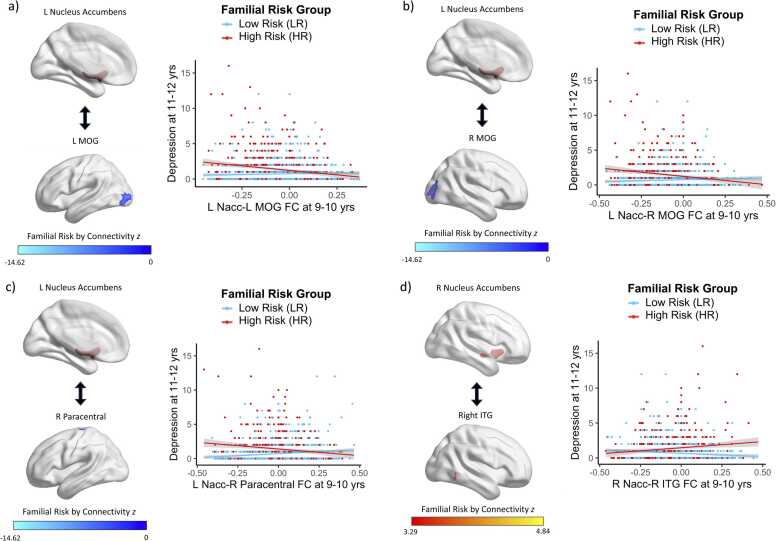
Preadolescent nucleus accumbens FC interacted with familial risk status to predict depression symptoms two years later (Fig. 4, Table S2, Table S3). Specifically, there was a negative association between nucleus accumbens FC with the left and right MOG and future depression symptoms for HR youth (β=-2.531, t=-5.77, p<0.001; β=-2.790, t=-5.97, p<0.001, respectively), whereas this relation was not significant for LR youth (β=0.175, t=0.57, p=0.571; β=0.548, t=1.65, p=0.098, respectively). Additionally, there was a negative association between nucleus accumbens FC with the paracentral gyrus and future depression symptoms for HR youth (β=-1.890, t=-4.13, p<0.001), whereas this relation was positive for LR youth (β=0.824, t=2.61, p=0.009). Finally, there was a positive association between nucleus accumbens FC with the inferior temporal gyrus (ITG) and future depression symptoms for HR youth (β=1.591, t=3.61, p<0.001), whereas this relation was negative for LR youth (β=-1.084, t=-3.26, p=0.001).
Fig. 4.
: Nucleus Accumbens FC Patterns at Baseline Interact with Familial Risk Status to Predict Depression Symptoms at 2-Year Follow-Up. There was a negative association between nucleus accumbens FC with the a) left and b) right middle occipital gyrus and subsequent depression symptoms for HR youth, whereas this relation was not significant for LR youth. There was a negative association between nucleus accumbens FC with the c) paracentral gyrus and subsequent depression symptoms for HR youth, whereas this relation was positive for LR youth. There was a positive association between nucleus accumbens FC with the d) inferior temporal gyrus and subsequent depression symptoms for HR youth, whereas this relation was negative for LR youth.
3.6. Sensitivity Analyses: Inclusion of Puberal Status as a Covariate
All findings remained significant after covarying for pubertal status (Table S4).
3.7. Sensitivity Analyses: Examination of Other Youth Psychiatric Symptoms (Besides Depression)
All interactions remained significant when covarying for baseline total problems and follow-up total problems (Table S5) when predicting follow-up depression symptoms, indicating that findings are likely not driven by individuals being more symptomatic generally. Additionally, all interactions significantly predicted follow-up internalizing symptoms (when covarying for baseline internalizing and follow-up externalizing symptoms; Table S6), suggesting that the observed findings may extend to internalizing symptoms more broadly.
3.8. Sensitivity Analyses: Inclusion of Additional Demographic Variables as Covariates
Because the HR and LR groups differed in youth race and ethnicity, parental marital status, and household income, we conducted sensitivity analyses to examine whether results could be better explained by these differences. All reported clusters remained significant after inclusion of these potential confounds, with all effects of interest in the same direction.
4. Discussion
The present study sought to examine how associations between emotion- and reward-related neural circuitry during preadolescence and future depression symptoms during the transition to adolescence varied based on whether youth had parents with (HR) or without (LR) depression histories. We found that, compared to LR youth, HR youth exhibited stronger associations between preadolescent resting-state FC and depression symptoms two years later. More specifically, amygdala FC with regions within later-developing association networks (e.g., attention networks) was predictive of future depression symptoms, whereas striatal FC with regions within earlier-developing sensorimotor networks (e.g., visual, sensory/somatomotor networks) was predictive of future depression symptoms. Additionally, the majority of FC results involved earlier-developing regions, specifically within visual and sensory/somatomotor networks. Findings remained significant when covarying for other psychiatric symptoms (e.g., total problems), indicating that results were likely not driven by youth being more symptomatic generally. Further, findings were similar when predicting adolescent internalizing symptoms, indicating that observed findings may also extend to internalizing symptoms and disorders more broadly. Taken together, our study suggests that preadolescent amygdala and striatal circuitry may be clinically-useful FC markers of adolescent-onset depression, specifically for healthy youth at high familial risk for depression.
Overall, HR youth exhibited stronger associations between preadolescent amygdala and striatal FC and adolescent depression symptoms compared to LR youth, indicating that amygdala and striatal FC may be particularly informative prospective markers of depression for youth who have parents with depression histories. These findings align with extant research showing alterations in amygdala and striatal connectivity among HR youth who later develop depression (Fischer, 2022, Pawlak et al., 2022, Hirshfeld-Becker, 2019, Shapero, 2019, Fischer, 2018). Our study revealed differential FC between these regions with other areas involved in emotion and facial processing, cognition (e.g., selective attention, executive functioning), and visual processing, including the MTG, STG, MOG, ITG, paracentral gyrus, precentral gyrus, and precuneus (Corbetta and Shulman, 2002, Fox, 2006, Tu, 2013, Bush, 1998, Bari and Robbins, 2013, Hu, 2017, Cavanna and Trimble, 2006).
We found that HR youth exhibited stronger associations between preadolescent amygdala FC and adolescent depression symptoms compared to LR youth. Amygdala FC was particularly predictive with regions acting within later-developing association regions, such as the MTG and STG (generally part of attention networks). The MTG plays a role in emotional processing, selective attention, and working memory (Corbetta and Shulman, 2002, Fox, 2006); thus weaker amygdala-MTG FC predicting future depression symptoms among HR (but not LR) youth may reflect difficulties in emotion processing and emotion-regulating cognition that predispose youth to develop depression symptoms during adolescence. Additionally, we found that stronger amygdala-STG FC predicted elevated future depression symptoms among HR (but not LR) youth. Given that functional connections between the STG and amygdala have been shown to be associated with trait anxiety (Wang, 2021), our results may also reflect heightened risk for anxiety, which in turn has been shown to increase risk for depression and internalizing symptoms broadly (Horn and Wuyek, 2010).
Our study found that weaker striatal FC (i.e., caudate/nucleus accumbens) during preadolescence predicted elevated depression symptoms during adolescence among HR (but not LR) youth. Striatal FC was primarily associated with regions that are part of earlier-developing somatomotor networks, such as the MOG, precentral gyrus, SMA, paracentral gyrus, and ITG, which are typically part of visual and sensory/somatomotor networks. The MOG is involved in face-sensitive attention processing (Tu, 2013), and there is evidence of decreased neural responses in this region among depressed individuals (Teng, 2018); thus weaker FC between striatal regions with the MOG being linked to elevated future depression symptoms among HR youth may reflect a predisposition towards a selective bias in processing emotion- and reward-related information. Moreover, HR (but not LR) youth exhibited positive associations between caudate-precentral gyrus/SMA FC and future depression symptoms, which is consistent with studies revealing alterations in these regions in individuals with major depression (Peng, 2015) as well as those at high familial risk for depression (Papmeyer, 2015). Furthermore, weaker nucleus accumbens-paracentral gyrus FC was related to elevated future depression symptoms among HR youth, whereas stronger FC was associated with elevated future depression symptoms among LR youth. The paracentral gyrus is part of the parietal lobe and is crucial for somatomotor and emotion processing (Cavanna and Trimble, 2006), and reductions in gray matter volume in this region have been observed in depressed individuals (Lee, 2018). Our results suggest that having a parent with depression may be associated with alterations in youths’ ability to process somatomotor and emotion information. Finally, we observed that stronger nucleus accumbens-ITG FC was associated with elevated future depression symptoms among HR youth, whereas weaker nucleus accumbens-ITG FC was associated with elevated future depression symptoms among LR youth. The ITG is involved in affective stimuli processing (Hu, 2017), and alterations in ITG volume have been observed among individuals with major depression (Kocsis, 2021), including positive correlations between ITG volume and depressive symptoms (Li, 2010). Thus, our findings suggest that HR youth may be more sensitive to processing affective stimuli as compared to LR youth.
Interestingly, the majority of depression-predictive FC results involved regions within visual and sensory/somatomotor networks, which typically mature earlier in development. Association networks are still undergoing significant development within the age range of our sample (i.e., 9–10 years). By contrast, sensorimotor networks may have reached greater relative “maturity” by this age, and therefore may be more likely to differentially shape future symptom development as a function of familial risk status. Thus, it is possible that as neurodevelopment progresses within association networks, they may begin to be more predictive of future symptomology at older ages (e.g., 14–18) due to divergent developmental processes. It is important for future work to investigate whether later-developing (versus earlier-developing) FC during adolescence/young adulthood predicts future symptomology differentially based on familial risk status. Nonetheless, although the majority of our results involved striatal FC with earlier-developing networks, our study also revealed patterns of amygdala FC with later-developing networks that were predictive of future depression symptoms among HR youth. Taken together, findings from our study align with extant evidence that identifies altered amygdala and striatal circuitry as potential biomarkers for the future onset and course of depression among HR youth (Fischer, 2022, Pawlak et al., 2022, Hirshfeld-Becker, 2019, Shapero, 2019, Fischer, 2018).
It is important to note that the effect sizes observed in the present study are relatively small. However, these effect sizes are similar to the effect sizes observed in other ABCD fMRI studies (e.g., (Owens, 2021)). The ABCD Study has a large enough sample size to enable the reliable detection of small effect sizes (Dick, 2021). ABCD analyses are also expected to yield small effect sizes in part because of the study’s diverse demographic sample; in other words, effect sizes may be more “diluted” because of the complex background and contextual variables (Karcher and Barch, 2021). For example, in the context of this study, it is possible that our approach to operationalizing familial risk (which is based on a single item, as discussed further below) is likely to exhibit significant between-subject variability, likely reflecting a combination of both familial risk for depression and other non-specific risk factors. This limited specificity and increased variability in the expression of the risk phenotype may further contribute to this effect size “dilution.” Additionally, small effect sizes observed in ABCD neuroimaging studies may reflect the fact that many real-world associations are truly small (Dick, 2021). Thus, in nature, the pre-adolescent brain may indeed exert small effects on future depression outcomes among youth at high familial risk. This theory aligns with the understanding that depression is a result of numerous different neurobiological, environmental, and psychological variables (Ho, 2022). Many small effects can be considered clinically and behaviorally meaningful (Funder and Ozer, 2019); therefore, additional research is needed to further investigate the clinical and behavioral effects of small effect sizes detected in the brain that predict the future development of depression among youth at high familial risk.
Our study has several limitations. We used a theory-driven approach and selected connectivity with specific ROIs based on prior work on the neural markers of familial risk for depression (Gotlib, 2010, Frost Bellgowan, 2015, Chai, 2016, Chai, 2015, Fischer, 2022, Morgan, 2019, Morgan, 2022, Olino, 2014, Singh, 2018, Holt-Gosselin, 2023). However, other potentially informative circuits were not examined that may be predictive of future mental health outcomes; as such, future work could utilize data-driven, exploratory approaches. Another important limitation to acknowledge is that family depression history was measured using a single item for each family member. Nevertheless, the relative ease and time-efficient manner of collecting these data facilitated the collection of such data from a large sample, which represents a key strength of this work. It is important to note that depression symptoms on average were relatively low at the 2-year follow-up among HR and LR youth. This is due in part by design, as we purposely selected only youth who had no current or prior psychiatric diagnosis at the initial visit because we were interested in identifying vulnerability markers in the brain prior to the onset of any psychopathology. Additionally, it is possible that the 2-year follow-up interval is too short to capture significant increases in depression. Given that depression onset typically peaks during late adolescence/early adulthood (Kessler, 2007), future studies examining later follow-up time points (e.g., 5-, 6-year) will be crucial. Because the ABCD Study is an ongoing 10-year study, it will be feasible to examine these later follow-up time points as the data become available. Nevertheless, clinical symptoms at the 2-year follow-up are helpful to examine because this period, although relatively short, may more readily capture early-onset depression, which is generally associated with worse functional and symptom outcomes (compared to later onset, (Zisook, 2007)). An additional limitation is that it is possible that some youth in the LR group had parents who developed depression between the initial visit (i.e., at 9–10 years) and follow-up (i.e., at 11–12 years). The family history measure was only collected at baseline, and therefore we are unable to confirm whether the youth in the LR group had parents who continued to be free of any psychiatric problems during this period. Furthermore, we examined FC at one time point; however, it is recommended that future studies examine the relations among longitudinal FC trajectories of emotion- and reward-related circuitry, familial risk status, and mental health outcomes. Finally, while our study focused on neurobiological markers of risk for depression for HR and LR youth, it is important for future work to simultaneously consider environmental influences such as early life adversity, which has been linked with alterations in brain development (Tierney and Nelson, 2009), risk for depression (Goff and Tottenham, 2015), and neural changes in individuals at high familial risk for depression (Carballedo, 2012). This work will be essential for enhancing understanding of how the complex interactions between the brain and environment contribute to future depression risk for vulnerable youth.
Despite these limitations, the current study has numerous strengths. This is the largest longitudinal study to date to elucidate differential neural markers that predict future depression symptoms as a function of familial risk status in a preadolescent sample. Additionally, the examination of youth who did not meet diagnostic criteria for any psychiatric disorder at baseline enables the identification of pre-existing vulnerability factors for the later development of psychopathology. Utilizing this type of sample helps to distinguish between the observed neural correlates reflecting pre-existing risk factors versus correlates of psychiatric symptoms. Third, our design had a group with low familial risk for depression, which is important for understanding how family depression history can differentially relate to brain-symptom associations. Lastly, neural circuitry was examined within an age range (i.e., 9–10 years) that precedes adolescence, which marks a period of heightened risk for psychopathology. Neural circuitry was collected from a sample with a narrow age range at baseline (i.e., 9–10 years) and follow-up (i.e., 11–12 years), whereas prior work examined larger age ranges (e.g., 8–14) and had inconsistent follow-up time points (e.g., 3±2 years after baseline). The narrow age range and consistent follow-up schedule examined by our study enable greater precision in regard to the timing of when neural vulnerability markers may emerge during development.
4.1. Conclusions
Taken together, our study provides novel insight regarding brain-based prognostic, preadolescent signatures for the future development of depression during adolescence among a large sample of youth at high and low familial risk for depression. Our findings indicate that alterations in amygdala/striatal FC with regions belonging to visual and sensory/somatomotor networks likely contribute to the development of depression for youth at high familial risk for depression, whereas FC within these circuits may not be as predictive for future depression symptoms for youth at low familial risk. The identification of preadolescent neural markers that predict future depression symptoms will help inform our understanding of the pathophysiology of depression. In the longer term, neurobiological markers of vulnerability could potentially serve as targets for future prevention or intervention efforts. For example, preadolescents at high familial risk for depression may benefit from preventative interventions that target the specific neural circuitry associated with familial risk for depression.
Funding and acknowledgements
This work was supported by the National Institute on Drug Abuse (U01DA041174), Jacobs Foundation Early Career Research Fellowship, and The Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Richard "Dick" Abidin Early Career Award and Grant to DGG. BHG is supported by the National Science Foundation Graduate Research Fellowship (NSF GRFP) and the National Institute of Mental Health (NIMH) Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NRSA) F31 (1F31MH131246–01). TJK is supported by Yale’s Child Study Center Postdoctoral T32 (Postdoctoral Research in Childhood Neuropsychiatric Disorders: T32-MH01826837) and a Brain & Behavior Research Foundation Young Investigator Award. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NDA Release 4.0 (DOI: http://dx.doi.org/10.15154/1523041). We are grateful to the study participants for their time and participation.
Author contributions
BHG, JJ, and DGG conceptualized the research question, hypotheses, and analytic plan presented in this manuscript. TJK, AR, TJH, AP, NB, AH, OMD, EF, and DAF preprocessed the imaging data. BHG, TJK, and KR undertook the statistical analysis of the quantified data. BHG, TJK, and DGG wrote the manuscript. BHG, TJK, KR, AR, TJH, AP, NB, AH, OMD, EF, DAF, JJ, and DGG critically reviewed the manuscript. ABCD Consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD Consortium investigators. The author is an Editorial Board Member for Developmental Cognitive Neuroscience and was not involved in the editorial review or the decision to publish this article.
Disclosures
The authors (BHG, TJK, KR, AR, THJ, AP, NB, AH, OMD, EF, DAF, JJ, DGG) have no relevant financial or non-financial interests to disclose, and no conflicts of interest.
CRediT authorship contribution statement
Kathryn Rodrigues: Writing – review & editing, Formal analysis. Jutta Joormann: Writing – review & editing, Writing – original draft, Conceptualization. Damien Fair: Formal analysis. Taylor Keding: Writing – review & editing, Formal analysis. Dylan Gee: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization. Bailey Holt-Gosselin: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization. Nora Byington: Formal analysis. Anders Perrone: Formal analysis. Eric Feczko: Formal analysis. Oscar Miranda-Dominguez: Formal analysis. Timothy J. Hendrickson: Formal analysis. Amanda Rueter: Formal analysis. Audrey Houghton: Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101400.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD data used in this report came from NDA Release 4.0 (DOI: http://dx.doi.org/10.15154/1523041).
References
- Achenbach, T.M., 2009. The Achenbach System of Emprically Based Assessment (ASEBA): Development, Findings, Theory and Applications. 2009, University of Vermont Research Center for Children, Youth, and Families.
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bush G., et al. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum. Brain Mapp. 1998;6(4):270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballedo A., et al. Early life adversity is associated with brain changes in subjects at family risk for depression. World J. Biol. Psychiatry. 2012;13(8):569–578. doi: 10.3109/15622975.2012.661079. [DOI] [PubMed] [Google Scholar]
- Casey B.J., et al. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chai X.J., et al. Functional and structural brain correlates of risk for major depression in children with familial depression. Neuroimage Clin. 2015;8:398–407. doi: 10.1016/j.nicl.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., et al. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol. Psychiatry. 2016;80(11):849–858. doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., et al. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.B., et al. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: the ABCD experience. Dev. Cogn. Neurosci. 2018;32:143–154. doi: 10.1016/j.dcn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dick A.S., et al. Meaningful associations in the adolescent brain cognitive development study. Neuroimage. 2021;239 doi: 10.1016/j.neuroimage.2021.118262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb. Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E., Conan G., Marek S., Tervo-Clemmens B., Cordova M., Doyle O., Earl E., Perrone A., Sturgeon D., Klein R., Harman G., Kilamovich D., Hermosillo R., Miranda-Dominguez O., Adebimpe A., Bertolero M., Cieslak M., Covitz S., Hendrickson T., Juliano A.C., Snider K., Moore L.A., Uriartel J., Graham A.M., Calabro F., Rosenberg M.D., Rapuano K.M., Casey B., Watts R., Hagler D., Thompson W.K., Nichols T.E., Hoffman E., Luna B., Garavan H., Satterthwaite T.D., Ewing S.F., Nagel B., Dosenbach N.U.F., Fair D.A. bioRxiv; 2021. Adolescent Brain Cognitive Development (ABCD) community MRI collection and utilities. [Google Scholar]
- Fischer A.S., et al. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. 2018;75(5):493–502. doi: 10.1001/jamapsychiatry.2017.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.S., et al. Reward-circuit biomarkers of risk and resilience in adolescent depression. J. Affect Disord. 2019;246:902–909. doi: 10.1016/j.jad.2018.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.S., et al. Intrinsic connectivity and family dynamics: striatolimbic markers of risk and resilience in youth at familial risk for mood disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7(9):855–866. doi: 10.1016/j.bpsc.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost Bellgowan J., et al. A neural substrate for behavioral inhibition in the risk for major depressive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(10):841–848. doi: 10.1016/j.jaac.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder D.C., Ozer D.J. Evaluating effect size in psychological research: Sense and nonsense. Adv. Methods Pract. Psychol. Sci. 2019;2(2):156–168. [Google Scholar]
- Geller B., et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(4):450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS Spectr. 2015;20(4):337–345. doi: 10.1017/S1092852914000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., et al. Neural processing of reward and loss in girls at risk for major depression. Arch. Gen. Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler, Jr D.J., et al. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker D.R., et al. Intrinsic functional brain connectivity predicts onset of major depression disorder in adolescence: a pilot study. Brain Connect. 2019;9(5):388–398. doi: 10.1089/brain.2018.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.C., et al. Multi-level predictors of depression symptoms in the Adolescent Brain Cognitive Development (ABCD) study. J. Child Psychol. Psychiatry. 2022 doi: 10.1111/jcpp.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Gosselin B., et al. Neural circuit markers of familial risk for depression among healthy youths in the adolescent brain cognitive development (ABCD) study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2023 doi: 10.1016/j.bpsc.2023.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn P.J., Wuyek L.A. Anxiety disorders as a risk factor for subsequent depression. Int J. Psychiatry Clin. Pr. 2010;14(4):244–247. doi: 10.3109/13651501.2010.487979. [DOI] [PubMed] [Google Scholar]
- Hu B., et al. Emotion regulating attentional control abnormalities in major depressive disorder: an event-related potential study. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-13626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., et al. Diagnostic efficiency and psychometric properties of CBCL DSM-oriented scales in a large sample of Chinese school-attending students aged 5-16. Asian J. Psychiatr. 2023;88 doi: 10.1016/j.ajp.2023.103724. [DOI] [PubMed] [Google Scholar]
- Karcher N.R., Barch D.M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2021;46(1):131–142. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., et al. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis K., et al. Voxel-based asymmetry of the regional gray matter over the inferior temporal gyrus correlates with depressive symptoms in medicated patients with major depressive disorder. Psychiatry Res. Neuroimaging. 2021;317 doi: 10.1016/j.pscychresns.2021.111378. [DOI] [PubMed] [Google Scholar]
- Lee F.S., et al. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346(6209):547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.M., et al. Gray matter volume reductions were associated with TPH1 polymorphisms in depressive disorder patients with suicidal attempts. Psychiatry Invest. 2018;15(12):1174–1180. doi: 10.30773/pi.2018.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G., et al. The association between pubertal status and depressive symptoms and diagnoses in adolescent females: a population-based cohort study. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.T., et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50(1):347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Lieb R., et al. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch. Gen. Psychiatry. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Luking K.R., et al. Reward processing and risk for depression across development. Trends Cogn. Sci. 2016;20(6):456–468. doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.K., et al. Differential neural responding to affective stimuli in 6- to 8-year old children at high familial risk for depression: associations with behavioral reward seeking. J. Affect Disord. 2019;257:445–453. doi: 10.1016/j.jad.2019.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.K., et al. Maternal response to positive affect moderates the impact of familial risk for depression on ventral striatal response to winning reward in 6- to 8-year-old children. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7(8):824–832. doi: 10.1016/j.bpsc.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino T.M., et al. Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev. Cogn. Neurosci. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M.M., et al. Multimethod investigation of the neurobiological basis of ADHD symptomatology in children aged 9-10: baseline data from the ABCD study. Transl. Psychiatry. 2021;11(1):64. doi: 10.1038/s41398-020-01192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papmeyer M., et al. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol. Psychiatry. 2015;78(1):58–66. doi: 10.1016/j.biopsych.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M., Bray S., Kopala-Sibley D.C. Resting state functional connectivity as a marker of internalizing disorder onset in high-risk youth. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-25805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., et al. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., et al. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petito A., et al. The Burden of Depression in Adolescents and the Importance of Early Recognition. J. Pediatr. 2020;218:265. doi: 10.1016/j.jpeds.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Power J.D., et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J.P., et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin. Exp. Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Shapero B.G., et al. Neural markers of depression risk predict the onset of depression. Psychiatry Res Neuroimaging. 2019;285:31–39. doi: 10.1016/j.pscychresns.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.K., et al. Limbic intrinsic connectivity in depressed and high-risk youth. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57(10):775–785. doi: 10.1016/j.jaac.2018.06.017. e3. [DOI] [PubMed] [Google Scholar]
- Spear L.P. Adolescent brain development and animal models. Ann. N. Y Acad. Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Teng C., et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry. 2018;18(1):370. doi: 10.1186/s12888-018-1955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney A.L., Nelson C.A. 3rd, brain development and the role of experience in the early years. Zero Three. 2009;30(2):9–13. [PMC free article] [PubMed] [Google Scholar]
- Toenders Y.J., et al. Neuroimaging predictors of onset and course of depression in childhood and adolescence: A systematic review of longitudinal studies. Dev. Cogn. Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., et al. Category-selective attention modulates unconscious processes in the middle occipital gyrus. Conscious Cogn. 2013;22(2):479–485. doi: 10.1016/j.concog.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Wang M., et al. Dysfunction of resting-state functional connectivity of amygdala subregions in drug-naive patients with generalized anxiety disorder. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.758978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D.E., et al. First episode of depression in children at low and high familial risk for depression. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(3):291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Zisook S., et al. Effect of age at onset on the course of major depressive disorder. Am. J. Psychiatry. 2007;164(10):1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD data used in this report came from NDA Release 4.0 (DOI: http://dx.doi.org/10.15154/1523041).