Vancomycin resistant enterococci are important nosocomial pathogens with variable epidemiological characteristics. Infections are becoming increasingly common in American hospitals, although it is rare for healthy people to carry the bacterium. By contrast, infection with vancomycin resistant enterococci is uncommon in European hospitals but many healthy people carry the bacterium. We describe an outbreak of vancomycin resistant enterococci infection in a Dutch hospital that was controlled by costly, labour intensive infection control measures.1 We also discuss what should be done to prevent vancomycin resistant enterococci becoming endemic in European hospitals.
Summary points
Vancomycin resistant enterococci have low virulence but infection is difficult to treat
Infection is endemic in US hospitals but rare in European hospitals
An outbreak in a Dutch hospital was halted by strict infection control measures
These measures were expensive and at times stopped new admissions and surgery
It is unclear whether such strict measures are justified, and European guidelines are needed
Patterns of vancomycin resistant enterococci infection
In the United States, the incidence of infection with vancomycin resistant enterococci has increased greatly in the past 10 years. In the national nosocomial infection surveillance study, the proportion of vancomycin resistant enterococci among enterococcal bloodstream infections rose from 0% in 1989 to 25.9% in 1999.2 Although the initial rise was observed in intensive care patients, infections are also common among patients treated in general hospital wards.2
Enterococci have a low virulence but can cause serious infections in immunocompromised patients, such as transplant recipients or those in intensive care units.3 Infection usually occurs in the abdominal cavity or urinary tract, and the attributable mortality may be as high as 30%,4 although these figures have not been replicated.5,6 New antibiotics (such as linezolid) that are effective against vancomycin resistant enterococci are available, but development of resistance has already been reported.7 Limiting further spread of vancomycin resistant enterococci has therefore become an important issue in the United States.8
In Europe, outbreaks of vancomycin resistant enterococci occur infrequently and are rarely accompanied by serious infections. This is despite the presence of a large reservoir of asymptomatic carriers of vancomycin resistant enterococci in the community. A European study found vancomycin resistant enterococci in less than 3% of enterococcal isolates from blood cultures.9 In the Netherlands, however, 5-10% of healthy people are colonised with vancomycin resistant enterococci.10,11
The high community reservoir in Europe has been linked to the use of avoparcin. This glycopeptide is closely related to vancomycin and has been used as a growth promoter in the livestock industry in Europe but not the United States. In the Netherlands, about 80 000 kg of avoparcin was used yearly until 1997; this compares with about 1500 kg of vancomycin used to treat humans in 1996.12 Avoparcin has a strong selective effect for vancomycin resistant enterococci, resulting in high levels of carriage in the enterococcal flora of exposed animals and their environment.11,13,14 The link between use of avoparcin and the community reservoir is supported by:
Absence of vancomycin resistant enterococci among healthy people in the United States15
Outbreak in Utrecht
The University Medical Centre Utrecht is a 1042 bed teaching hospital. The department of internal medicine and dermatology has 170 beds in six general wards and a medical intensive care unit with 10 beds. It is independently financed for a set number of patients based on the numbers treated in the unit in the preceding year, irrespective of their length of stay. No additional funds are provided if more patients are admitted than are budgeted for, and the budget is cut if it does not admit the agreed number of patients.
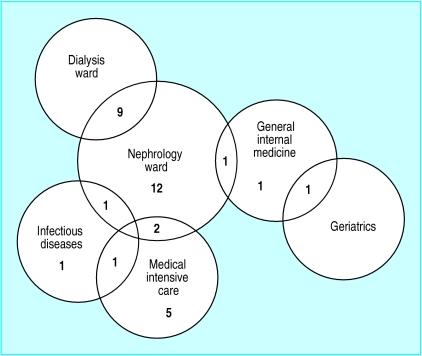
In May 2000, an outbreak of vancomycin resistant enterococci occurred in a non-university hospital 20 km from Utrecht.19 Two of the presumed source patients had been treated in our hospital, and we carried out surveillance of their contacts. Between June and September 2000, surveillance cultures identified 43 patients in the hospital who were colonised with vancomycin resistant enterococci, and isolates of 34 belonged to the same clone. This so called epidemic strain was not found in any of the 600 faecal samples obtained from contacts who had not been admitted to hospital. Figure 1 shows that most of the infected patients were in the nephrology ward (800 admissions a year; mean length of stay 12 days) or the medical intensive care unit (600 admissions a year; mean length of stay 5 days).
Figure 1.
Distribution of patients with epidemic strain of vancomycin resistant Enterococcus faecium within wards of department of internal medicine and dermatology
The epidemic enterococcal strain contained the variant esp gene, which is thought to facilitate transmission.20 The department implemented infection control measures only for patients infected with the epidemic strain. These measures included the use of gloves and gowns by healthcare workers, rigorous hand hygiene, single use of all equipment, and disinfection of rooms after patients were discharged. All patients in the affected wards had weekly faecal cultures to detect colonisation with the epidemic strain and use of vancomycin was limited as much as possible.
As soon as a case of vancomycin resistant enterococci infection occurred on a ward, patients were divided into three categories: those who were definitely infected; those who were possibly infected; and newly admitted patients, who were considered uninfected. Uninfected patients were nursed in disinfected rooms only, and the three cohorts were kept separate. Nursing staff were, as far as possible, divided between the three cohorts to minimise cross contamination.
Patients classified as possibly colonised were declared negative after three subsequent faecal cultures had been free of vancomycin resistant enterococci. If the epidemic strain was detected in a patient in the negative cohort, the whole cohort was reclassified as possibly infected. Because of space limitations, the policy meant that at times we could not admit new patients to the ward. Transfer of patients to other wards was limited and patients were discharged as soon as possible.
Financial impact of outbreak
Implementation of the infection control measures meant that both the general wards and the intensive care unit had to turn away new admissions on three occasions. This resulted in a loss of 37 admissions for the general wards and 11 admissions for the intensive care unit compared with the same periods in the previous year. This represents a loss of €35 500 (£22 200). It is unclear if the closure to new admissions affected patient care or outcome. During this period, 1543 surveillance cultures were obtained (£13 800), and the costs for gloves, gowns, and disinfection procedures amounted to £1060, £3130, and £8100 respectively. The cost of molecular typing was £7300. As a result, the total costs for the first four months of the outbreak were £55 590 (less than 1% of the total operating costs of the department).
Despite all efforts, this outbreak was not completely controlled over four months, although the number of new positive patients decreased. The outbreak has been attributed to a clone with extreme abilities for transmission.20 However, so far no serious infections have occurred.
Variable professional response
The need to close wards to new admissions and cancel routine surgery because no intensive care beds were available caused considerable debate among the hospital's physicians. Some argued that strict infection control measures were not necessary because enterococci have low virulence; patients' need for surgery should therefore have priority. Others thought that the stringent measures were essential to counter the threat of endemicity of colonisation with vancomycin resistant enterococci. A further problem emerged when colonised patients were well enough to be discharged to non-university hospitals or nursing homes closer to home. Some of the institutions refused to accept these patients because they thought that they would be unable to implement full infection control measures and they feared a spread of vancomycin resistant enterococci infection in their own units.21
Wider implications
The outbreak of vancomycin resistant enterococci has similarities to the early outbreaks of methicillin resistant Staphylococcus aureus in the Netherlands in the early 1980s. At this time a strategy of “search and destroy” was followed, which effectively prevented spread of methicillin resistant S aureus and subsequent infections in Dutch hospitals.22 There are, however, important epidemiological differences between methicillin resistant S aureus and vancomycin resistant enterococci. Methicillin resistant S aureus is more virulent and there is no community reservoir of carriers. Patients at risk of infection can be identified and treated and colonisation eradicated.
The outbreaks of vancomycin resistant enterococci have created a new dilemma. With the American experience in mind, many doctors may agree that spread of these multiple resistant bacteria should be prevented. Half hearted infection control measures are likely to result in further nosocomial spread, which will eventually result in an increase in infections. On the other hand, a rigorous infection and control policy will result in extensive patient discomfort, increased workload for healthcare workers, and substantial extra costs for healthcare organisations. Whether such a full scale effort should be made to control a micro-organism that is considerably less virulent than methicillin resistant S aureus is debatable.
We believe that national, and possibly European, guidelines to control the spread of vancomycin resistant enterococci should be drawn up because patients are often transferred between hospitals. The large community reservoir of vancomycin resistant enterococci in the Netherlands means that a strategy based on the results of genotyping, as practised in our hospital, is likely to be preferable. Such a strategy will, however, require more epidemiological data on the nature of hospital outbreaks. If the genotypes of vancomycin resistant enterococci responsible for most hospital outbreaks can be identified, reliable and rapid genotyping methods must be developed (which will certainly generate extra costs) and a reference centre established. The alternative is to implement rigorous infection control measures for all patients found to be carrying vancomycin resistant enterococci. This could be up to 5% of patients.
In the United States, guidelines to limit further spread of vancomycin resistant enterococci were formulated when colonisation was already endemic in many hospitals.23 Although these guidelines have proved sufficient to control outbreaks and reduce endemicity,24,25 the incidence of infection is still increasing.2 We therefore propose that a European consensus on control measures should be reached and guidelines drawn up on how to approach hospital outbreaks before these bacteria become endemic in Europe's hospitals.
Figure 2.
D KUNKEL AND M SCHOUTEN
Electron microscope image of enterococci
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Bonten MJM, Mascini EM, Willems R, Timmer GJ, Gaillard CA, Vandenbroucke-Grauls CM. Wat te doen als men vancomycine-resistente enterokokken aantreft? Ned Tijdschr Geneeskd. 2000;144:2545–2549. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990-May 1999, issued June 1999. Am J Infect Control. 1999;27:520–532. doi: 10.1016/s0196-6553(99)70031-3. [DOI] [PubMed] [Google Scholar]
- 3.Moellering RC. Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992;14:1173–1178. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 4.Edmond MB, Ober JF, Dawson JD, Weinbaum DL, Wenzel RP. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin Infect Dis. 1996;23:1234–1239. doi: 10.1093/clinids/23.6.1234. [DOI] [PubMed] [Google Scholar]
- 5.Shay DK, Maloney SA, Montecalvo M, Banerjee S, Wormser GP, Arduino MJ, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995;172:993–1000. doi: 10.1093/infdis/172.4.993. [DOI] [PubMed] [Google Scholar]
- 6.Garbutt JM, Ventrapragada M, Littenebrg B, Mundy LM. Association between resistance to vancomycin and death in cases of Enterococcus faecium bacteremia. Clin Infect Dis. 2000;30:466–472. doi: 10.1086/313694. [DOI] [PubMed] [Google Scholar]
- 7.Gonzales RD, Schreckenberger PC, Graham MB, Kelkar S, DenBesten K, Quin JP. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet. 2001;357:1179. doi: 10.1016/S0140-6736(00)04376-2. [DOI] [PubMed] [Google Scholar]
- 8.Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 9.Schouten M, Hoogkamp-Korstanje J, Meis J, Voss A. Prevalence of vancomycin-resistant enterococci in Europe. Eur J Clin Microbiol Infect Dis. 2000;19:816–822. doi: 10.1007/s100960000390. [DOI] [PubMed] [Google Scholar]
- 10.Endtz HP, van den Braak N, van Belkum A, Kluytmans JA, Koeleman JG, Spanjaard L, et al. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in the Netherlands. J Clin Microbiol. 1997;35:3026–3031. doi: 10.1128/jcm.35.12.3026-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Bogaard AE, Mertens P, London NH, Stobberingh EE. High prevalence of colonization with vancomycin- and pristinamycin-resistant enterococci in healthy humans and pigs in the Netherlands: is the addition of antibiotics to animal feeds to blame? J Antimicrob Chemother. 1997;40:454–456. doi: 10.1093/jac/40.3.454. [DOI] [PubMed] [Google Scholar]
- 12.Van den Bogaard AE, Bruinsma N, Stobberingh EE. The effect of banning avoparcin on VRE carriage in the Netherlands. J Antimicrob Chemother. 2000;46:146–147. doi: 10.1093/jac/46.1.146. [DOI] [PubMed] [Google Scholar]
- 13.Devriese LA, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, et al. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates J, Jordens JZ, Griffiths DT. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34:507–516. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 15.Coque TM, Tomayko JF, Ricke SC, Okhuysen PC, Murray BE. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Bogaard AE, Jensen LB, Stobberingh EE. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med. 1997;337:1558–1559. doi: 10.1056/NEJM199711203372117. [DOI] [PubMed] [Google Scholar]
- 17.Stobberingh EE, van den Bogaard A, London N, Driessen C, Top J, Willems R. Enterococci with glycopeptide resistance in turkeys, turkey farmers, turkey slaughterers, and (sub)urban residents in the south of the Netherlands: evidence for transmission of vancomycin resistance from animals to humans? Antimicrob Agents Chemother. 1999;43:2215–2221. doi: 10.1128/aac.43.9.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klare I, Badstübner D, Konstabel C, Böhme G, Claus H, Witte W. Decreased incidence of vanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microbial Drug Resistance. 1999;5:45–52. doi: 10.1089/mdr.1999.5.45. [DOI] [PubMed] [Google Scholar]
- 19.Van der Steen LF, Bonten MJM, van Kregten E, Harssema-Poot JJC, Willems R, Gaillard CA. Uitbraak van vancomycine-resistente Enterococcus faecium op een afdeling nefrologie. Ned Tijdschr Geneeskd. 2000;144:2568–2572. [PubMed] [Google Scholar]
- 20.Willems R, Homan W, Top J, van Santen-Verheuvel M, Tribe D, Manzioros X, et al. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet. 2001;357:853–855. doi: 10.1016/S0140-6736(00)04205-7. [DOI] [PubMed] [Google Scholar]
- 21.Mascini E, Bonten M, Troelstra A, Verhoef J. De melaatsen van de nieuwe eeuw. Medisch Contact. 2001;56:1008–1011. [Google Scholar]
- 22.Verhoef J, Beaujean D, Blok H, Baars A, Meyler A, van der Werken C, et al. A Dutch approach to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1999;18:461–466. doi: 10.1007/s100960050324. [DOI] [PubMed] [Google Scholar]
- 23.Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 24.Montecalvo MA, Jarvis WR, Uman J, Shay DK, Petrullo C, Rodney K, et al. Infection control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med. 1999;131:269–272. doi: 10.7326/0003-4819-131-4-199908170-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ostrowsky BE, Trick WE, Sohn AH, Quirk SB, Holt S, Carson LA, et al. Control of vancomycin-resistant enterococcus in health care facilities in a region. N Engl J Med. 2001;344:1427–1433. doi: 10.1056/NEJM200105103441903. [DOI] [PubMed] [Google Scholar]