Abstract
This review examines the correlation between plant-based diets and athletic performance, with a specific emphasis on the vital aspect of optimizing micronutrients for athletes. In light of the increasing prevalence of plant-based nutrition among athletes due to its perceived advantages in terms of health, ethics, and the environment, this study investigates the ability of these diets to satisfy the demanding nutritional requirements essential for achieving optimal performance and facilitating recovery. The article emphasizes the significance of essential micronutrients such as iron, vitamin B12, calcium, vitamin D, zinc, and omega-3 fatty acids and also addressing the challenges with their absorption and bioavailability from plant sources. The review consolidates existing scientific knowledge to propose strategies for improving micronutrient consumption, comparing the effects of supplements against whole foods, and highlighting the significance of enhancing bioavailability. The proposal supports the implementation of personalized meal planning, with the assistance of sports nutritionists or dietitians, and is substantiated by case studies showcasing the success of plant-based athletes. Future research directions examine the long-term effects of plant-based diets on micronutrient status and athletic performance, as well as developing nutritional trends and technology. The review concludes that plant-based diets can meet athletes' nutritional demands and improve peak performance while aligning with personal and ethical values with strategic planning and professional guidance. This study intends to help athletes, coaches, and nutritionists understand plant-based nutrition for enhanced athletic performance.
Keywords: Plant-based diets, Athletic performance, Sports nutrition, Bioavailability, Micronutrients, Nutritional technology, Dietary supplements
1. Introduction
The transition towards a plant-based diet is more than just a modification in dietary patterns; it signifies a significant transformation in how individuals perceive food, health, and sustainability [1]. The dietary approach which emphasizes on consuming mostly plant-based meals has become increasingly popular among diverse populations worldwide [2]. The rise of plant-based diets is driven by a combination of factors, such as a growing body of scientific evidence that highlights the health advantages of such diets, rising concerns about the environmental degradation caused by conventional animal farming, and the growing awareness of ethical concerns related to animal welfare [3,4]. Plant-based diets are distinguished by their focus on consuming whole, minimally processed foods, including fruit, vegetables, legumes, whole grains, nuts, and seeds [2,5]. This dietary approach provides individuals with the flexibility to personalize their eating habits based on their personal health goals, ethical convictions, and environmental considerations. The core of the shift towards a plant-based diet lies in the convincing evidence of information that supports its various health advantages [6]. A significant amount of research has demonstrated a robust association between the consumption of plant-based diets and a reduced risk of developing chronic diseases [7]. For example, the incorporation of a dietary pattern that is abundant in plant-based foods has been linked to reduced rates of cardiovascular disease, type 2 diabetes, obesity, and some types of cancer [8]. The observed health advantages can be ascribed to the abundant presence of dietary fiber, vitamins, minerals, and antioxidants in plant-based meals [9]. These constituents play a significant role in promoting cardiovascular health, facilitating improved digestive function, and reducing inflammation [10]. Another important benefit of dietary fiber is that it acts as a prebiotic; therefore, promoting healthy gut bacteria [11]. In addition, it is worth noting that plant-based diets generally exhibit lower levels of saturated fats and cholesterol, which are known to have a role in mitigating the risk of heart disease and fostering healthier lipid profiles [12].
In addition to the advantages for individuals, the ecological consequences of adopting a plant-based diet have become progressively undeniable [13]. Animal agriculture has a substantial environmental impact, resulting in enormous greenhouse gas emissions, deforestation, water pollution, and biodiversity loss [14]. The production and processing of meat, dairy, and eggs necessitate substantial amounts of land, water, and feed, resulting in the degradation of habitats and a significant burden on natural resources [15]. Plant-based diets, in contrast, use fewer resources and produce reduced greenhouse gas emissions, presenting a more sustainable option that alleviates the environmental consequences of food production [3]. The transition towards adopting plant-centric dietary patterns signifies an essential step in addressing global environmental issues, such as climate change and the depletion of resources [16].
The adoption of plant-based diets is significantly influenced by ethical considerations [6]. A significant number of people are attracted to adopting a plant-based diet due to their concern for animal welfare and their aspiration to advocate for more compassionate and humane treatment in the food industry [17]. The animal agriculture industry frequently employs intensive farming practices that are widely seen as harsh, including confinement, overcrowding, and various forms of mistreatment [18]. By choosing to consume plant-based meals, consumers have the ability to diminish their endorsement of these practices, so fostering a desire for a greater variety of ethically produced food alternatives [19]. The transitioning to a plant-based diet presents individuals with the ability to harmonize their dietary practices with their values and health goals, despite the inherent challenges involved [20]. The objective of this review is to conduct a thorough exploration of plant-based diet, elucidating its positive impacts on health, environmental sustainability, and ethical considerations. This review explores the concept of plant-based eating in the context of athletic performance, detailing how this dietary approach can meet the nutritional demands of athletes, enhance their performance, and support recovery. It covers the definition of plant-based diets, the specific micronutrient needs of athletes, and provides practical guidelines for optimizing nutrition to improve sport performance while considering health benefits, environmental impact, and ethical considerations. By embracing a plant-based dietary approach, individuals have the potential to make a positive impact on the sustainability, health, and compassion of our planet [21].
1.1. Rationale for plant-based diets among athletes
The emphasis on micronutrients for achieving peak athletic performance and optimal well-being is grounded in the understanding that these nutrients are foundational to numerous physiological functions critical to athletes [22]. Micronutrients, despite being required in small quantities, play outsized roles in energy metabolism, oxygen transport, bone health, muscle function, and the immune response, all of which are pivotal for athletes striving for peak performance [23,24]. For instance, iron is crucial for the formation of hemoglobin, which transports oxygen to muscles during intense physical activity, directly impacting stamina and endurance [25]. Similarly, calcium and vitamin D are indispensable for bone density and strength, protecting athletes from fractures and ensuring their skeletal system can withstand the rigors of high-impact sports [26].
Moreover, the nuanced roles of micronutrients like zinc in protein synthesis, wound healing, and immune function underscore the complexity of nutritional needs in athletic populations [27]. Omega-3 fatty acids, known for their anti-inflammatory properties, not only support cardiovascular health but also aid in recovery and muscle repair after strenuous workouts [28]. The challenge within a plant-based diet context lies not in the absence of these nutrients but in their bioavailability and the unique absorption mechanisms that may necessitate more mindful dietary planning [2]. For example, the non-heme iron found in plant sources is less readily absorbed by the body than the heme iron in animal products, but its absorption can be significantly enhanced through the strategic consumption of vitamin C-rich foods alongside iron-rich plant foods [29].
The potential for nutrient deficiencies is a concern that cannot be casually dismissed, particularly when the stakes include not only athletic performance but also long-term health implications [30]. A deficiency in one or more of these critical micronutrients can lead to anemia, weakened bone structure, compromised immune function, and decreased muscle function, among other health issues [31]. These deficiencies can subtly undermine an athlete's performance, recovery, and overall well-being, sometimes with long-lasting consequences [32]. Therefore, the strategic focus on micronutrients entails not only identifying and incorporating diverse plant-based sources of these nutrients into the diet but also understanding the interactions that enhance or inhibit their absorption [33].
Addressing these nutritional nuances with a well-informed approach can empower plant-based athletes to optimize their diet for both performance and health [34]. It involves a dynamic balance between whole foods and supplements, carefully considering the timing of nutrient intake to align with metabolic windows for maximum absorption and utilization [35]. The commitment to focusing on micronutrients reflects a broader understanding of nutrition as a critical component of athletic training, one that complements physical training and recovery protocols [36]. By ensuring a comprehensive intake of essential micronutrients, plant-based athletes can achieve their performance goals while fostering robust health, demonstrating that plant-based nutrition, when strategically managed, can be fully compatible with, and even advantageous to, athletic excellence [37]; (Fig. 1).
Fig. 1.
Rationale for Plant-Based Diets Among Athletes. This figure was created with BioRender.com (accessed on 15 April 2024).
2. Micronutrients of particular concern for plant-based athletes
The micronutrients of importance for high-performance athletes in general are vitamin D, iron, and calcium, as highlighted by the International Olympic Committee [38,39]. In addition, it is necessary to address vitamin B12, iodine, zinc, and, in some cases, omega 3 fatty acids, among other nutrients for Plant-Based Athletes (PBA) [40]. The following are some examples of minerals that are categorized as micronutrients (Table 1).
Table 1.
Plant-based sources of essential micronutrients.
| Micronutrient | Plant-Based Sources | Function | Reference |
|---|---|---|---|
| Iron | Lentils, beans, tofu, tempeh, cashews, pumpkin seeds, dark leafy greens (spinach, kale) | Oxygen transport, energy production | [41] |
| Calcium and Vitamin D | Leafy greens (collard greens, kale), soybeans, tofu, tempeh, calcium-fortified plant milks (almond, soy) | Bone health, muscle function | [42,43] |
| Zinc | Chickpeas, lentils, pumpkin seeds, cashews, quinoa, mushrooms | Wound healing, immune function | [43,44] |
| Vitamin C and E | Citrus fruit, strawberries, bell peppers, nuts, seeds, spinach, and vegetable oils | Antioxidant properties, cell membrane integrity and immune function | [45,46] |
| Vitamin B12 | Fortified plant milks, nutritional yeast (best absorbed) | Energy metabolism, nervous system function | [47] |
| Iodine | Seaweed (nori, wakame), iodized salt | Thyroid function | [48] |
| Selenium | Brazil nuts (high amounts, limit intake), lentils, sunflower seeds, whole wheat bread | Antioxidant function, thyroid function | [49,50] |
| Copper | Nuts (cashews, almonds), seeds (sunflower, pumpkin), quinoa, shiitake mushrooms | Energy production, connective tissue formation | [51,52] |
| Manganese | Nuts (almonds, cashews, brazil nuts), legumes (lentils, black beans), whole grains (oats, quinoa) | Enzyme function, antioxidant function | Manganese Deficiency (accessed on 15 April 2024). |
| Magnesium | Leafy greens (spinach, kale), legumes (beans, lentils), nuts (almonds, cashews), seeds (pumpkin, sunflower), whole grains (quinoa, brown rice) | Muscle function, energy production | [53] |
| Phosphorus | Legumes (beans, lentils), nuts (almonds, cashews), seeds (pumpkin, sunflower), whole grains (oats, quinoa) | Energy storage, bone health | Top 12 Foods That Are High in Phosphorus (accessed on 15 April 2024). |
| Potassium | Potatoes, sweet potatoes, leafy greens (spinach, kale), bananas, avocados | Muscle function, nerve function | Potassium Rich Plant Based Foods (accessed on 15 April 2024). |
| Chromium | Broccoli, potatoes, whole grains (oats, barley), nuts (cashews) | Blood sugar regulation | Chromium Supplements (accessed on 15 April 2024). |
| Molybdenum | Legumes (lentils, beans), nuts (almonds, cashews), whole grains (oats) | Enzyme function | [54] |
2.1. Iron
Iron is an essential nutrient for athletes, as it plays a pivotal role in various physiological functions that hold significant importance for individuals engaged in physical activity [55]. One of its primary roles is to transport oxygen, both as part of hemoglobin in the blood and myoglobin in muscles [56]. This is crucial for the energy production and proper functioning of the electron transport system [57]. Iron is present in significant amounts in both animal and plant-derived food sources. Athletes can experience substantial adverse consequences due to inadequate iron levels [58]. It has the potential to hinder the maximum absorption of oxygen, resulting in reduced performance and endurance [59]. Insufficient levels of iron can also impede the functioning of the immune system, rendering athletes more susceptible to various illnesses and infections [60]. Insufficient iron levels can negatively affect muscular function, hence impeding overall athletic performance [58]. Additionally, it is important to acknowledge that these consequences may manifest even in the absence of anemia [61]. There are multiple factors that contribute to an increased susceptibility of elite athletes to iron deficiency [62]. The body's iron requirements are heightened by the physical demands of rigorous training and competition [63].
In addition to other factors, athletes may encounter increased gastrointestinal blood losses, inadequate dietary iron consumption, and, in the context of female athletes, menstrual bleeding [58]. Moreover, some physical activities such as running have the potential to induce the rupture of erythrocytes, resulting in the occurrence of hemolysis and hemoglobinuria [64]. Plant-based athletes, particularly females, exhibit increased susceptibility to decreased iron stores as a result of various factors, including dietary choices, menstrual cycles, and potentially lower energy consumption [65]. Absorption of iron from plant-based sources, known as nonheme iron, is generally lower compared to heme iron found in animal foods [66]. The inhibition of iron absorption can be attributed, in part, to the presence of compounds found in plants, such as polyphenols (e.g., tannin) and phytates [67]. Consequently, it may be necessary for vegetarian athletes to ingest 1.8 times the recommended dietary iron consumption compared to nonvegetarians [68,69]. The Institute of Medicine (IOM) recommends a daily iron consumption of 32 mg for female athletes and 14 mg for male athletes who follow a plant-based diet [70]. In order to optimize the absorption of nonheme iron, plant-based athletes might implement different strategies [71]. The combination of iron-rich food items, such as black beans, with vitamin C sources, like tomatoes, has been shown to substantially enhance the absorption of iron [72]. Consuming foods high in vitamin C alongside meals that contain nonheme iron can enhance absorption by a factor of 2–6 [73]. Another method involves the preparation of acidic meals, such as tomatoes, using cast-iron skillets, hence potentially enhancing iron consumption [74]. Plant-based athletes should refrain from taking tea, coffee, and calcium supplements with iron-rich meals due to their potential to impede iron absorption [75].
Iron supplementation may be essential for female plant-based athletes in some cases [65]. Prior to starting supplementation, it is essential to ascertain iron levels by a blood test [76]. The consumption of an excessive amount of iron can result in iron overload, hence posing significant health hazards [77]. The selection of an iron supplement necessitates careful consideration of both the amount and bioavailability of iron [78]. Supplementary iron in ferrous forms, including ferrous sulfate, ferrous gluconate, ferric citrate, and ferric sulfate, has a higher degree of absorption compared to other forms such as ferric iron [79].
2.2. Calcium and vitamin D
Calcium is an essential mineral that serves a crucial function in maintaining optimal health. Approximately 99 % of this mineral occurs in teeth and bones, making it the most abundant mineral in the human body [80]. The remaining 1 % is present in the bloodstream, where it plays a crucial role in facilitating essential physiological processes such as muscle contraction, nerve transmission, and hormonal secretion. Insufficient calcium intake can result in a number of health problems such as osteoporosis, muscle cramps, and impaired nerve function [81]. Although dairy products are often linked to calcium consumption, plant-based sources can also offer an adequate amount of this essential mineral [82]. Calcium is essential for the formation and maintenance of healthy bones [83]. Over the course of our lifespan, the human body experiences continuous bone remodeling, characterized by the replacement of old bone tissue with newly formed bone tissue [84]. Sufficient calcium consumption ensures the efficient occurrence of this process, promoting optimal bone density and strength. Inadequate consumption of calcium has been associated with a decrease in bone mineralization, increasing the susceptibility to osteoporosis, a condition that is characterized by weak and brittle bones [85]. Calcium is crucial for the proper functioning of muscles, including both muscle contraction and relaxation. Upon muscle stimulation, the release of calcium ions occurs, facilitating the contraction of muscle fibers [86]. Calcium additionally facilitates the transmission of neural impulses, promoting efficient communication between nerve cells and other body tissues [87]. Inadequate calcium levels can lead to muscle cramps, spasms, and impaired nerve function [88]. Moreover, calcium is essential for the process of blood clotting, referred to as coagulation. The activation of platelets and the subsequent aggregation of platelets, facilitated by calcium, occur in response to blood vessel damage, which eventually results in the formation of a blood clot [89]. This mechanism serves to mitigate excessive bleeding while promoting the process of wound healing [90]. Inadequate calcium levels have the potential to hinder the capacity of blood clotting, resulting in prolonged bleeding [91]. Calcium plays a crucial role in the secretion of various hormones and enzymes. The stimulation of insulin secretion from the pancreas is crucial for the regulation of blood glucose levels [92]. It plays a pivotal role in insulin secretion, where intracellular calcium levels help regulate the release of insulin from the pancreatic β-cells [93,94]. This function is particularly important for athletes, as efficient insulin secretion and glucose metabolism are critical for maintaining energy levels and enhancing performance. Calcium is also essential for the secretion of hormones from the thyroid gland, which plays a crucial role in regulating metabolic processes, growth, and development [95]. It is recommended that plant-based athletes include calcium-rich foods in their dietary intake. Kale, spinach, collard greens, turnip greens, and bok choy are very nutritious leafy green vegetables that provide a rich source of calcium and other essential nutrients [96]. These vegetables possess a high calcium content and are also abundant in dietary fiber, vitamins, and minerals, so contributing to the improvement of overall health [97]. Legumes, including beans, lentils, and chickpeas, are notable for their high content of plant-based protein and substantial calcium content. Incorporating beans into the diet of athletes can enhance their calcium consumption while providing additional health advantages such as fiber, iron, and folate [98]. White beans, almonds, tahini, figs, and oranges are plant-based foods that have significantly lower calcium concentration and bioavailability [99]. Sesame seeds, chia seeds, and flaxseeds are rich sources of calcium. In addition, they offer healthy fats, protein, and other essential minerals, rendering them a nourishing addition to a plant-based diet [100]. Moreover, some plant-based milk substitutes, including soy milk, pea milk, almond milk, and oat milk, have been fortified with calcium in order to offer a comparable nutritional composition to that of conventional dairy milk [101]. Tofu and tempeh are calcium-rich soy-based food. These versatile ingredients can be utilized in a variety of culinary preparations and offer supplementary advantages like as protein, iron, and other essential amino acids [102]. Several varieties of seaweed, including kelp and wakame, are considered to be excellent source of calcium. Additionally, they possess a significant amount of iodine, a vital mineral for the proper functioning of the thyroid gland [103]. The incorporation of seaweed in athletes' diet might offer a unique calcium availability, while improving the taste and diversity of one's meals [104]. Studies have found that athletes can derive benefits from intake of calcium, above the Dietary Reference Intake (1000 mg/d for adults), with dosages ranging from 1500 to 2000 mg/d [105]. Vitamin D plays a crucial role in calcium absorption and bone health. Both calcium and vitamin D are often found together in fortified plant-based drinks, such as almond milk, soy milk, and other fortified beverages, which are vital for maintaining adequate levels in plant-based athletes. A group of soldiers undergoing military training who received calcium (2000 mg/d) and vitamin D (1000 IU/d) supplements was compared to a non-supplemented group in a randomized, double-blind, placebo-controlled trial [106]. Over the 9-week basic combat training period, the supplemented group had improved bone density and maintenance of parathyroid hormone levels (regulates calcium metabolism) compared with the non-supplemented group, which the authors speculate may translate into better bone health and lower risk of bone fractures in the future. Calcium is essential for maintaining bone health and preventing conditions such as osteoporosis and fatigue fractures. This is particularly important for individuals undergoing intense physical training, such as soldiers in basic training, where the risk of stress fractures is elevated.
If the PBA diet fails to provide calcium requirements, it is advisable to supplement with a daily dosage of 300–500 mg of calcium citrate [70]. Calcium citrate is seen as more bioavailable compared to other calcium forms included in supplements, due to its ability to be absorbed without the need for gastric acid [107]. It is advisable for plant-based athletes to refrain from consuming calcium supplements that contain bone meal, oyster shell, and shark cartilage due to the higher levels of lead present in these particular supplements [70]. Calcium supplements exhibit optimal absorption and utilization when utilized in amounts of 500 mg [108]. If the PBA takes a daily supplement of 1000 mg, the dose should be split.
2.3. Zinc
A wide variety of physiological activities rely on zinc, making it an indispensable mineral [109]. Enzymes that govern different biological activities rely on it as a cofactor, and it is involved in more than 300 enzymatic reactions itself [110]. All three of these processes—growth, development, and immune system function—require zinc [111]. It is critical to eat enough zinc-rich foods every day because the human body can't make or store zinc [112]. Enzymes that catalyze vital biological events rely on zinc as a cofactor. Many biological activities rely on these enzymes, such as the production of proteins, the maintenance and repair of DNA, the division of cells, and the healing of wounds [113]. Impaired cellular function could result from zinc deficiency, which could disrupt certain enzymatic reactions [114]. Additionally, it is involved in the development and activation of immune cells such T-cells, natural killer cells, and neutrophils, so it is vital to the immune system's efficiency [111]. It plays a role in controlling the production and activity of cytokines, which are signaling molecules that mediate immune responses [115]. Zinc loss through sweat and urine, metabolic stress caused by exercise, and inflammation that changes zinc homeostasis by encouraging zinc loss and redistribution to other tissues are just a few of the reasons why athletes have higher zinc requirements [116]. During the early years of infancy, childhood, and adolescence, zinc plays a key role in promoting healthy growth and development. It is required for DNA synthesis and cell division, which are fundamental processes for growth and development [117]. When it comes to healing wounds, zinc is important [118]. A protein that gives tissues their structural integrity, collagen, involves it in its production [119]. Zinc aids in wound healing by promoting the proliferation of epithelial cells and the development of new blood vessels. Adequate zinc levels are necessary to promote efficient healing and prevent complications [120]. The receptors for smell and taste, which are involved in the production and release of neurotransmitters that mediate these sensory functions, also require it for proper functioning. A reduced sense of smell and taste perception might result from zinc deficiency [121].
Many plant-based meals contain zinc, which, like iron, is not as bioavailable when consumed in plants as it is when obtained from animals [122]. Zinc absorption is impaired by the high phytate content in plant-based diet staples such legumes and cereals [122]. Vegetarians and vegans, in particular, may require up to 50 % more zinc than nonvegetarians, according to the Institute of Medicine (IOM) [123]. Men should consume 16.5 mg of zinc per day, while women should consume 12 mg [124]. There does not appear to be any negative health impact associated with poorer zinc status in plant-based eaters, despite the fact that they typically have lower zinc intakes and blood zinc concentrations [71]. This is probably because the body can adjust its zinc absorption and excretion rates to keep zinc levels stable. According to a 2018 review article, athletes had lower serum zinc levels despite adequate or high zinc intakes [125]. This suggests that they have higher zinc demands compared to physically inactive people. In addition, many athletes follow temporary food trends in the hopes of achieving greater results. Up to 90 % of athletes may experience low zinc levels due to following certain calorie-restrictive or food-eliminating trendy diets [126]. Due to its role in bone homeostasis, zinc deficiency in athletes can cause anorexia, significant loss of body weight, latent fatigue with decreased endurance, impaired immunological function, and, perhaps, an increased risk of osteoporosis [126]. A blood test can measure the serum zinc levels [127]. Repleting zinc stores and maybe enhancing performance in sports can be achieved by supplementation. However, zinc supplementation in an athlete without a deficiency to begin with does not appear to boost performance and may impair copper metabolism [128]. It has been proposed that zinc gluconate or zinc acetate, which are less acidic and do not induce gastrointestinal mucosal inflammation, are more tolerable when taken as supplements than zinc sulfate [129].
Legumes (e.g., beans, lentils, and chickpeas), nuts and seeds (e.g., chia, hemp, and flaxseed seeds), whole grains (e.g., quinoa, brown rice, and oats), and leafy greens (e.g., spinach, kale, and Swiss chard) can help athletes meet their zinc needs [130]. Zinc is added to some plant-based foods, like cereals and dairy substitutes. For those athletes on a plant-based diet, these fortified foods can be an easy way to get enough zinc into their diet [131].
2.4. Antioxidants: vitamin C and E
Vitamins are vital to maintain optimal health and athletic performance. Plant-based athletes must be especially aware of their vitamin consumption to avoid deficits that could impair performance and recovery [132]. Vitamin C is required for collagen formation, which helps to maintain joint, tendon, and ligament function [133]. Its antioxidant characteristics aid in the reduction of oxidative stress and the support of immunological function, both of which are essential for athletes enduring rigorous exercise [134,135]. Citrus fruit, strawberries, bell peppers, and broccoli are all plant-based sources of vitamin C [136]. It is well-absorbed in a diversified diet, but a deficiency can result in scurvy, frequent infections, and poor wound healing [137]. Vitamin E is a powerful antioxidant that protects cells from oxidative damage, which is especially beneficial for athletes [138]. It promotes cell membrane integrity and immunological activity [139]. Plant-based diets that contain Vitamin E, are nuts, seeds, spinach, and vegetable oils [46]. As a fat-soluble vitamin, it requires dietary fat to be absorbed properly. Vitamin E deficiency may lead to muscle weakness, impaired vision, and immune dysfunction [140].
2.5. Vitamin B12
One of the most important nutrients for human health, vitamin B12 (or cobalamin) serves numerous crucial functions. It is evident that sufficient amounts of vitamin B12 are critical for general health, as it aids in red blood cell formation, keep the neurological system functioning properly, facilitates DNA synthesis, energy production, methylation processes, and homocysteine metabolism [141]. Multiple problems can arise from insufficient vitamin B12. One of the most common outcomes, megaloblastic anemia, which causes weakness and fatigue, is rather common [142]. Problems with walking, mood swings, tingling, or numbness in the extremities are all examples of neurological problems that could develop [143]. Vitamin B12 insufficiency may also cause gastrointestinal problems, loss of appetite, and reduced weight [144]. Many PBAs get B12 shots. It is important to acknowledge that vitamin B12 is crucial for proper brain functioning, as it aids in faster information processing and enhances concentration levels. Patients with moderate cognitive impairment (MCI) and below-normal vitamin B12 levels have shown this [145]. Athletes should pay close attention to this since better brain function has the potential to boost performance in numerous areas, including learning new skills and maintaining focus throughout long competitions. A study investigated relationship between vitamin B12 status and muscle strength in elite male athletes. Athletes whose vitamin B12 levels were higher showed stronger muscles than those whose levels were lower, according to the findings [146]. Female athletes' exercise performance was the subject of another study that examined the effects of vitamin B12 supplements. The researchers found that supplementation considerably enhanced endurance performance and reduced fatigue during prolonged exercise [147]. Microorganisms such as bacteria and fungi synthesize the vitamin in the gastrointestinal tract of animals and are the only source of it [148]. Plants are not a reliable or adequate source of vitamin B12. The energy metabolism and general athletic performance of plant-based athletes are greatly aided by vitamin B12. Currently, 2.4 μg/d is the suggested daily requirement for vitamin B12 [149]. Because vitamin B12 is more commonly found in animal products, athletes who follow a plant-based diet must supplement their diet accordingly [150]. A PBA's vitamin B12 requirements can be fulfilled by eating fortified plant-based meals such as cereals, plant milks, and vegan burgers, crumbles, and other similar vegan animal alternatives. Supplements and fortification typically use cyanocobalamin, the most popular form of vitamin B12, due to its affordable price and good safety profile. However, methyl cobalamin is another form commonly used in supplements, which may have better absorption and retention rates in the body. For PBAs or vegans, nutritional yeast—an unleavened, deactivated form of yeast—can be a great source of protein and vitamin B12. Vitamin B12 supplements are still suggested, though, since expecting PBAs to consume nutritional yeast daily is impracticable [70,151].
PBAs can acquire the B12 they need in three different ways [70]:
-
•
Eat 2 servings per day of foods fortified with at least 2–3.5 μg of vitamin B12 each. Eat these servings at least 4 h apart to allow for optimal absorption.
-
•
Take a daily supplement providing 25–100 μg of vitamin B12.
-
•
Take a supplement providing 1000 μg of vitamin B12 twice per week.
Larger dose recommendation is due to the lower absorption rates from supplements compared to natural sources and the need to ensure adequate intake through reliable means.
2.6. Iodine
Iodine is another crucial mineral that PBAs need to be aware of. It is required for the production of thyroid hormones, which regulate various bodily functions, including metabolism, growth, and development. Ensuring sufficient iodine intake is critical for the normal functioning of the thyroid and overall health [152]. Thyroid hormones, including triiodothyronine (T3) and thyroxine (T4), are synthesized by the thyroid gland in the neck utilizing iodine. These hormones play an essential role in maintaining homeostasis in the body by controlling metabolic rate, energy production, and the development of many tissues and organs, including the central nervous system and brain [153,154]. Iodine deficiency, which occurs when the body does not have enough iodine in its blood, causes the thyroid gland to not produce adequate thyroid hormones, which in turn causes serious health problems. While sweating does lose some iodine, it is not nearly as significant as the 90 % excreted in urine from the diet [155]. Both hypothyroidism (when the thyroid gland becomes underactive and doesn't create enough thyroid hormones) and goiter (when the thyroid gland enlarges) can be caused by an inadequate consumption of iodine [156]. In regions where soil is deficient in iodine, millions of people suffer from iodine deficiency, a worldwide health problem. However, it is important to note that iodine deficiency is preventable through the consumption of iodine-rich foods or the use of iodized salt [157,158]. Vegans frequently have low amounts of iodine, although there have been instances of vegans taking too much iodine from seaweed or kelp tablets [159]. A 2018 study out of Norway examined 276 respondents' iodine levels; the subjects included vegetarians and vegans as well as people in different life stages (such as children and adolescents). The lowest scoring group was vegans, with 95 % of that group (n = 19) having insufficient iodine levels [160]. Their results are in accordance with other research studies [[161], [162], [163]]. It is crucial to ensure that the athlete consumes foods rich in iodine because measuring iodine status on an individual basis is difficult.
The PBA community must seek out new iodine sources since seafood and dairy products are already well-known to be abundant in this element. Iodine is found in seaweeds such as kelp, nori, kombu etc., which plant-based athletes can utilize. The iodine content of 1 sheet of seaweed can range between 11 % and 1989 % of the daily value, which is 150 μg/d [164]. Iodine is abundant in marine plants thus they are an excellent natural source of the mineral. One approach to be sure to get enough iodine is to use iodized salt, which provides approximately 71 μg per 1/4 teaspoon [165]. Leafy greens, potatoes, cranberries, strawberries, lentils, and chickpeas are just a few of the fruit and vegetables that can provide enough iodine. Iodine content in plant-based foods can vary widely depending on several factors, including the iodine content of the soil in which they are grown [166].
2.7. Selenium
Athletes can benefit from selenium (Se) in several ways, including improved health and performance. Protecting cells from exercise-induced oxidative stress and reducing inflammation and muscle damage, it enhances performance, strength, and endurance by acting as a potent antioxidant [167]. Se may increase mental focus and athletes may find it easier to concentrate and stay motivated even if the competition gets tougher. As a result of its immune-boosting properties, it aids athletes in fighting off diseases and infections that can compromise their performance [168]. As a biomarker for inflammation, C-reactive protein (CRP) levels in the blood are elevated in correlation with low serum selenium levels [169]. It additionally aids athletes recover quicker and function effectively by reducing muscular soreness and supporting enzyme activity [170]. It is well addressed that Se increases glutathione peroxidase production, which prevents the effect of oxidative stress in response to exercise [167]. A reduction in neurological performance was substantially linked to decreased levels of Se, according to the CHIANTI cohort study, which evaluated coordination performances among 1012 participants aged 65 or older [168].
Athletes can easily add Se to their diet by eating more foods that are naturally rich in Se or by taking supplements [130]. At the suggested dosages, it has the potential to improve athletes' overall health and performance. A systematic review of oral Se supplementation of 180 μg/day or 240 μg/day (selenium methionine) and 200 μg/day (Sodium Selenite) reported a significant drop in lipid hydroperoxide levels and an increase in glutathione peroxidase (GPx) in plasma, erythrocyte, and muscle. There was no effect on aerobic or anaerobic performance from taking Se supplements, according to the authors' conclusion [167]. A surplus of Se supplementation has the potential to induce excessive mitochondrial oxidative stress, which can have serious effects on athletes’ health [171]. Athletes can improve their health and performance by including plant-based foods such as brazil nuts, sunflower seeds, sesame seeds, flaxseeds, mushrooms, spinach, kale, broccoli, and sesame seeds in their regular diets as they are rich sources of Se [172].
2.8. Copper
A number of physiological processes rely on copper, making it an important trace mineral. In addition to its roles in energy production, enzyme function, cofactor status, and iron metabolism, copper is a key antioxidant defense component [173]. Copper is an integral component of several enzymes, including cytochrome c oxidase, superoxide dismutase (SOD), dopamine beta-hydroxylase, and tyrosinase [174]. According to Ref. [175], additional Cu supplementation is needed in athletes who perform physical training regularly. Another study indicated that physical exercise decreases Cu, Mn, Se, and Zn concentrations in erythrocytes, which can contribute to a drop in athletes' performance, emphasizing the significance of these elements [176].
Cellular respiration involves cytochrome c oxidase, which plays a crucial role in the conversion of oxygen into energy [177]. SOD is an antioxidant enzyme that plays a crucial role in protecting cells against oxidative stress. Its primary function is to counteract the damaging effects of free radicals, ensuring the protection of cells [178]. Another enzyme called dopamine beta-hydroxylase that plays a role in converting dopamine into norepinephrine, a neurotransmitter that helps regulate mood and stress response. It is also essential for the metabolism of iron, helps in its absorption, transport, and storage [179]. For iron to attach to transferrin, a protein that carries iron throughout the body, it must first change from its ferrous (Fe2+) form to its ferric (Fe3+) form, a process that copper aids in Ref. [180]. Copper deficiency impairs iron metabolism, causing anemia [181]. Copper helps synthesis and maintenance of connective tissues, including collagen and elastin. It is also needed by lysyl oxidase enzyme to cross-link collagen and elastin fibers for appropriate structure and function [182].
As previously mentioned, it plays a role in the conversion of dopamine to norepinephrine. In addition, copper is necessary for the production of the neurotransmitter epinephrine and serotonin. Copper is an essential component of the antioxidant enzyme superoxide dismutase (SOD). Free radicals, highly reactive chemicals that damage cells and cause aging, cardiovascular disease, and cancer, are neutralized by SOD [183,184]. With its antioxidant properties, copper plays a vital role in protecting cells against oxidative stress, thereby supporting overall health and well-being. Plant-based foods such as nuts and seeds (cashews, almonds, sunflower seeds, sesame seeds, and pumpkin seeds), chickpeas, black beans, and kidney beans, provide a rich supply of copper [185]. However, it's worth mentioning that the bioavailability of copper from plant-based sources may be lower than that from animal-based sources.
2.9. Manganese
Manganese (Mn) is necessary for the production of energy, the development of bones, and enzyme activity, among other biological processes [186]. Despite its little amount required, it is essential for metabolism, bone formation, antioxidant defense, and connective tissue production. Early studies suggests that Mn may have positive effects on athlete health and performance, although there is still much to learn about its specific benefits [187,188]. Manganese helps break down carbohydrates, proteins, and lipids for energy production [189]. It helps the body use energy more efficiently, which may boost endurance during long workouts and competitions. It also produces neurotransmitters that may boost mental focus and coordination during physical activities [190,191]. Due of its importance in bone production, various research studies reported Mn and bone health [192]. Athletes need strong bones to avoid injury and recover faster. Compared to healthy women, osteoporotic women have low serum Mn levels [193]. During strenuous exercise, Mn may protect cells from ROS damage, according to different studies. MnSOD, an antioxidant enzyme, also requires manganese for proper functioning. MnSOD neutralizes metabolic free radicals and protects cells from oxidative damage. In mitochondrial oxidative stress, Mn superoxide dismutase (MnSOD) is essential for ROS scavenging [194,195].
In a clinical trial, athletes were found to have higher basal manganese (Mn) levels in their blood and urine compared to sedentary individuals [195]. This finding suggests enhanced Mn absorption in athletes, potentially due to increased dietary intake or higher metabolic demands. However, another study indicated that sedentary subjects had higher urine Mn levels, which could be attributed to lower iron levels commonly found in athletes leading to increased Mn absorption [188]. These studies highlight the complex interplay between Mn and iron metabolism, which can vary based on physical activity levels and nutritional status. There is limited evidence regarding Mn and athletic performance, however, athletes should be evaluated for micronutrient deficiencies on a regular basis. While additional research is required, the current evidence suggests that Mn may be beneficial for athletes who seek to optimize their performance and health [168]. Connective structures like bones and cartilage require manganese. It activates glycosaminoglycan-producing enzymes, which are crucial to connective tissues [196]. Manganese insufficiency may reduce glucose tolerance and increase diabetes risk [197]. Mn in an athlete's diet may boost performance and is found in many foods, but plant-based sources are richer [195]. Manganese-rich plants include whole grains, legumes, nuts, seeds, and dark leafy greens [198].
2.10. Magnesium
Magnesium (Mg) is an important mineral that is known to play a vital part in both overall health and athletic performance. Magnesium is essential for athletes since it can boost energy, reduce fatigue, and even improve muscle performance [199]. A vital element, magnesium is involved in many different biological processes in the human body. It participates in more than 300 enzymatic reactions that support several physiological functions, including blood pressure regulation, muscle and nerve function, DNA synthesis, and energy production [200]. Athletes are taking magnesium more often due to its many advantages in helping them stay physically healthy and perform at their best [201,202]. By increasing ATP availability, which is best described as the gold energy stores of cells, magnesium contributes to improve energy levels. A deficiency could lead to ATP depletion, which would be stressful and reduce performance all over [203]. Athletes can experience enhanced endurance and higher energy by regularly consuming magnesium, which can improve ATP production [204]. Additionally, the mineral is critical for maintaining muscle function and reducing fatigue. It has been known to foster both muscle contraction and relaxation, which enhances muscle control and performance [205]. Moreover, it reduces the accumulation of lactic acid in the muscles, which may reduce pain during exercise and speed up recovery [206]. It also offers a host of other advantages that promote physical health. It supports the cardiovascular system, reduces stress, controls blood sugar, and enhances the quality of sleep. Athletes taking magnesium on a daily basis may experience enhanced energy output, decreased fatigue, and increased physical health, all of which will help them perform to the best of their abilities [130,207]. For males and females aged 14 to over 70, the Recommended Dietary Allowance (RDA) is 400–420 and 310–320 mg/day, respectively [208].
Magnesium plays a crucial role in both strength training and cardiorespiratory processes, demonstrating a reciprocal relationship with exercise. Research indicates that adequate magnesium levels are essential for muscle contraction, energy production, and overall cardiovascular health. Exercise, in turn, can affect magnesium metabolism and requirements in athletes [209]. Exercise controls Mg distribution and usage. According to Veronese et al., 2014, daily magnesium oxide supplementation for 12 weeks seemed to improve physical performance in healthy elderly women. Their findings suggest a role for magnesium supplementation in preventing or delaying the age-related decline in physical performance [210]. Training transfers Mg to energy-producing regions [211]. Serum Mg may be transported to RBCs or muscle to support exercise during prolonged activity. Short-term exercise may lower plasma/serum volume and raise serum Mg [212]. Male athletes' muscular performance is positively associated with serum Mg levels. Research also suggests that Mg deficiency may impair neuromuscular function and cause muscle cramps. Peak exercise performance may require more magnesium for active persons than inactive people [213]. Weightlifters with low Mg levels may have ineffective energy metabolism and decreased endurance [190,214]. Higher Mg consumption is linked to enhanced cardiorespiratory function and lower oxygen demand during aerobic exercise [215]. Most studies [[216], [217], [218]] reported that 500 mg/day Mg had little impact on exercise performance in athletes, unless there was a deficit. Athletes with insufficient amounts of magnesium are not protected against inflammatory reactions, which may increase the risk of hypertension, atherosclerosis, diabetes mellitus, osteoporosis, and cancer rates [219]. Several research studies evaluated the relationship between magnesium status/supplementation and exercise performance and found a direct association between magnesium demand and increased levels of physical activity [220]. A study of 16 physically active males given 300 mg/day Mg supplementation for 14 days or a control group found no direct impact on exercise performance [218]. Athletes may acquire magnesium from plant-based diets such as leafy greens, nuts, seeds, avocado, bananas, soy products, seaweed, and certain fruit [221].
2.11. Phosphorus
Phosphorus is essential for maintaining physiological function and improving athletic performance. As an essential mineral, phosphorus plays an important role in a variety of physiological processes that athletes rely on [190]. Firstly, it is an essential component of adenosine triphosphate (ATP), the fundamental energy currency in cells. During strenuous activity, ATP is rapidly depleted and must be replenished to maintain muscular contractions. Phosphorus helps regenerate ATP, allowing athletes to retain high levels of energy and endurance [222,223]. Furthermore, phosphorus is a crucial building block of DNA and RNA, which is required for protein synthesis and cell repair. It also aids in the maintenance of acid-base balance, which promotes normal muscle function and reduces the risk of muscle fatigue and cramping [224,225]. Furthermore, phosphorus plays a role in bone mineralization, which helps to grow and strengthen bones, which is especially significant for athletes who are prone to stress fractures and injuries [226,227]. Adequate phosphorus levels are required for proper cognitive function, memory, and neurological health. As a result, athletes must ensure an appropriate intake of phosphorus through a well-balanced diet or supplementation in order to improve their performance, maintain energy levels, support muscular function, and maintain overall health [228,229]. The Recommended Dietary Allowance (RDA) for phosphorus is 700 mg per day for both adult males and females [230].
While phosphorus is generally associated with animal-based foods, numerous plant-based sources contain considerable levels of this vital mineral. For example, one cup of cooked lentils contains around 356 mg of phosphorus, which is around 36 % of the required daily consumption for adults. One cup of cooked quinoa contains 281 mg of phosphorus, which is roughly 28 % of the recommended daily intake. A cup of cooked spinach contains around 84 mg of phosphorus. Soybeans give around 261 mg/cup [231,232]. Avocados, coconut, potatoes, and mushrooms are also phosphorus-rich plant foods [233].
2.12. Potassium
Potassium (K) is a vital nutrient for the health and performance of athletes. It is an essential nutrient for human health and is required for numerous physiological functions. While the general recommendation for adults is to limit sodium intake to 2000 mg/day and ensure a minimum potassium intake of 3510 mg/day, these guidelines may not adequately reflect the needs of athletes. Athletes experience significant electrolyte loss through sweating, which can increase their requirements for sodium and potassium to maintain optimal performance and prevent muscle cramps, dehydration, and hyponatremia. Studies suggest that athletes may benefit from higher sodium intake, particularly in endurance sports where sodium loss is pronounced [234,235]. It plays a crucial role in muscle contractions and helps the body regulate fluid balance, blood pressure, and heart rate [236]. Potassium helps to maintain fluid equilibrium both within and outside of cells. It works together with sodium, another electrolyte, to control fluid levels in the body. This balance is critical for athletes to maintain healthy blood pressure because a sufficient quantity of potassium counteracts the blood pressure-raising effects of sodium [237,238]. In addition to muscle contractions, its role in nerve function and electrolyte balance is useful for athletes who sweat throughout a long practice or game [239]. Furthermore, proper potassium levels might assist athletes avoid injuries and sustain energy levels. The heart is a muscle that requires potassium for normal electrical signaling and rhythm. Potassium ions travel in and out of cardiac cells, creating electrical impulses that regulate the heart's contraction. Adequate potassium levels are critical for athletes to maintain a normal heart rate and avoid arrhythmias [240,241]. Potassium is an excellent source of energy for athletes. It helps in reducing the accumulation of lactic acid in muscles, which can contribute to fatigue during intense exercise, and supports maintaining a healthy metabolism [242,243]. It also aids in the breakdown of carbohydrates, which helps keep energy levels high throughout strenuous activity [130,243]. It is unknown whether potassium supplementation lessens the occurrence of muscle cramps in athletes. It should be noted that there has been no evidence of ergogenic effects [244]. While potassium deficiency is relatively rare, it is critical to maintain an appropriate intake of this mineral through a well-balanced diet rich in plant-based foods like dried fruit (raisins, apricots), beans, lentils, potatoes, winter squash, spinach, broccoli, beet greens, avocado and banana [245,246].
2.13. Chromium
Chromium is an essential trace mineral for optimal health and athletic performance. Chromium is critical for athletes because of its role in blood sugar regulation [247]. Chromium helps metabolize proteins, carbohydrates, and fats more efficiently. It aids athletes in maintaining energy levels throughout strenuous activity by encouraging stable blood sugar levels, which improves endurance and prevents energy collapse. Furthermore, chromium promotes muscle growth and repair through improving insulin sensitivity, which allows the body to absorb amino acids for muscle cell growth and repair [248,249].
Furthermore, chromium promotes the body's natural production of collagen, a protein critical to the health and strength of tendons, ligaments, and bones [250]. Overall, incorporating chromium in an athlete's diet and supplement routine can improve their health, performance, and overall well-being [190,251]. Chromium is marketed to athletes as a biologically active component that enhances glucose tolerance, commonly known as the glucose tolerance factor (GTF), and controls and stimulates insulin activity [248]. Thus, insulin regulates glucose, lipid, and protein metabolism through chromium reserves [247]. Excessive loss and low intake may necessitate extra chromium in athletes. Chromium supplementation may be beneficial. Chromium supplementation improves athletic performance by restoring insulin function. Increased insulin function may have minimal anabolic benefits, and organic chromium consumption may result in short-term anabolic gains that must be confirmed [247,252].
Chromium deficiency is uncommon in people who follow a well-balanced diet, and the majority of people acquire enough chromium from whole grains, meat, and vegetables. The recommended dietary allowance (RDA) for chromium varies by age and sex, although it typically ranges from 20 to 35 μg per day for adults [253,254]. Some studies like [255,256] suggested that chromium supplementation may improve body composition by improving fat loss and lean muscle mass increase. However, other researchers have found inconsistent benefits [257,258]. Chromium supplementation has also been shown to have uncertain results on exercise performance. It's important to note that the potential benefits of chromium supplementation, if any, may be more evident in individuals who are deficient in chromium or have specific medical conditions. In general, athletes and active people ought to consume a well-balanced diet that includes a variety of nutritious foods. Adequate calorie intake, macronutrient balance, and achieving the recommended daily intake of vital nutrients are all critical for good health and performance [259,260].
2.14. Molybdenum
Molybdenum (Mo) is an indispensable element for several biological processes in the human body, although being required in minute amounts. It plays a crucial role in the functioning of enzymes that are involved in the metabolism of amino acids, breakdown of toxins, and production of energy [261]. Mo also plays a vital role in sustaining general health by supporting the nervous system and the immune system [262,263]. Mo is essential for athletes as it facilitates the metabolic process of amino acids, thereby providing energy for physical exertion, and contributes to detoxification by eliminating toxic byproducts produced during exercise. Furthermore, its support to the nervous system ensures proper muscular functionality and coordination, hence enhancing sports performance and overall health [264,265]. A study conducted by (Muñoz et al., 2019) evaluated changes in Mo levels in the blood serum and urine of athletes after engaging in prolonged exercise till complete exhaustion. It indicates that Mo is essential for maintaining certain physiological equilibriums that are critical during high exertion [195]. Another study by Spillman and Haymes (1986) discuss the role of Mo as a cofactor for enzymes that play a role in energy metabolism, potentially affecting an athlete's performance and health. According to their study, this relationship highlights the significance of Mo in energy-related processes that are essential for athletic performance [266]. Nieman (1991) investigated the process of converting purines into uric acid through enzymatic activity, which is accelerated by Mo, which is associated to muscle performance and recovery. This highlights another aspect of how Mo enhances athletic performance, specifically in muscle recovery post-exertion [267].
While rare, Mo deficiency can have a significant negative effect on an athlete's performance and health. Mo, an essential trace mineral, is involved in energy metabolism, sulfur metabolism, detoxification, antioxidant defense, and nitrogen homeostasis [268]. Athletes may suffer from impaired muscle strength, energy production, and recovery as well as increased susceptibility to oxidative stress and toxins if they don't consume enough Mo. A balanced diet high in sources of Mo, such as whole grains, legumes, nuts, and leafy green vegetables, is essential for maintaining both overall health and optimal athletic performance [269,270]. Seeking advice from a trained dietician or healthcare expert can provide tailored recommendations for preserving sufficient amounts of Mo. The recommended daily consumption of Mo is dependent upon age, gender, and stage of life. The recommended daily allowance (RDA) for adults is 45 μg; however, infants, kids, and pregnant and lactating women may require a little bit more [271]. To ensure an adequate intake of Mo, consuming a varied, well-balanced diet that is high in plant-based foods is important. By adding a range of foods high in this trace element to their diets, athletes can satisfy their Mo needs for optimum health and performance. Legumes (lentils, beans, and peas), whole grains (oats, barley, and brown rice), nuts and seeds (almonds, sunflower seeds, and peanuts), and leafy green vegetables (spinach, kale, and broccoli) are some excellent sources of Mo [272,273]. Consuming animal products such as organ meats, lamb, and beef can also increase the amount of Mo in the diet. Athletes can ensure that they are getting enough Mo in their diets to support energy metabolism, muscle strength, detoxification processes, and overall athletic performance by incorporating a variety of these foods into their meals and snacks [274,275].
3. Strategies for optimizing micronutrient intake
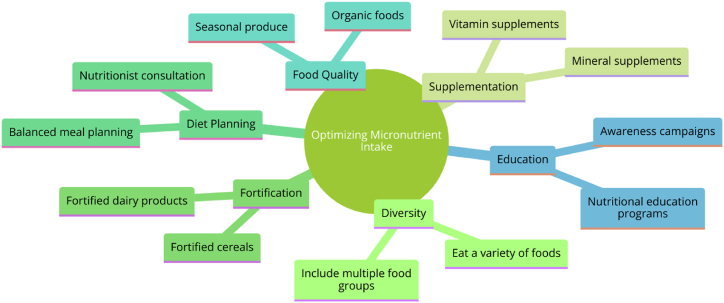
For athletes following plant-based diets, optimizing micronutrient intake requires a multifaceted strategy that strikes a balance between whole food consumption and judicious supplementation (Fig. 2; Table 2). This balance is important because, although whole foods provide a complex matrix of nutrients, fiber, and phytochemicals that are beneficial for health and performance, plant-based diets may not include enough of some micronutrients or they may contain them in less readily available forms [71,276]. Hence, supplementation becomes a focused strategy to fill nutritional gaps and guarantee athletes acquire the daily requirements for maximum performance and recovery [277]. Evidence-based research should serve as a basis for supplementation guidelines, which should consider the individual's eating habits, physical requirements, and any existing nutrient deficiencies. To guarantee the body can effectively utilize the nutrients offered, it is essential to use high-quality products that have undergone independent testing for purity and potency and will help avoid contaminants [278,279]. Optimizing intake also involves enhancing the bioavailability of micronutrients derived from plants. Scientific evidence supports dietary strategies that include soaking and sprouting legumes and grains to reduce phytic acid, which can inhibit the absorption of minerals like iron and zinc, combining fat-soluble vitamins with healthy fats to improve their uptake, and incorporating vitamin C-rich foods to improve iron absorption [280,281]. Additionally, current studies indicate that the time of nutrient intake may affect the bioavailability and effectiveness of certain nutrients. For instance, eating foods high in calcium apart from foods high in iron may avoid the competitive restriction of absorption that happens when these elements are consumed at the same time. These tactics highlight how crucial it is to consider when and how to eat food in order to optimize the nutritional advantages of a plant-based diet [282,283].
Fig. 2.
Comprehensive Strategies for Optimizing Micronutrient Intake. This figure was created with Diagrams: Show Me (accessed on April 04, 2024).
Table 2.
Strategies for enhancing micronutrient intake in plant-based diets.
| Strategy | Target Micronutrients | Description | Benefits | Implementation | References |
|---|---|---|---|---|---|
| Fortification | Iron, Calcium, Zinc, Vitamin B12, Iodine, Selenium | Adding nutrients to commonly consumed plant-based foods like cereals, plant milk, and breads | Ensures adequate intake regardless of dietary restrictions | Food manufacturers incorporate essential micronutrients into staple plant-based products, monitored by health authorities for compliance and effectiveness. | [284,285] |
| Dietary Diversity | All listed | Consuming a variety of plant foods to cover a broader spectrum of nutrients | Reduces the risk of nutrient deficiencies | Educate on the importance of a colorful plate; promote varied diets through dietary guidelines and public health campaigns. | [286,287] |
| Use of Bioavailability Enhancers | Iron, Zinc, Calcium | Including foods high in vitamin C, organic acids, or certain probiotics to enhance absorption | Increases the efficiency of nutrient uptake from the diet | Combine foods rich in enhancers (like citrus fruit or fermented products) with meals rich in target nutrients. | [131,288] |
| Supplementation | Vitamin B12, Iodine, Selenium, Vitamin D | Taking dietary supplements to ensure adequate intake of nutrients that are scarce in plants | Direct and controlled intake of specific nutrients | Supplements should be taken under healthcare guidance to tailor to individual nutritional needs, especially for exclusive plant-based dieters. | [289,290] |
| Soil and Crop Management | Selenium, Zinc, Copper, Manganese, Magnesium, Phosphorus, Potassium | Enhancing soil nutrient content to improve the nutritional value of plant foods | Improves overall food quality and nutrient content | Implement agricultural practices that enhance soil health and nutrient content, like organic fertilization and crop rotation. | [291] |
| Cooking Techniques | Iron, Zinc, Magnesium, Phosphorus | Applying cooking methods that reduce antinutrients like phytates and oxalates | Enhances mineral availability and absorption | Encourage practices such as soaking, sprouting, and fermenting grains and legumes; promote the use of iron utensils for cooking acidic foods. | [78] |
| Genetic Biofortification | Iron, Zinc, Selenium, Vitamin A | Developing crop varieties with higher nutrient densities through breeding or genetic modification | Increases intrinsic nutrient content of plant foods | Collaborate with agricultural research institutions to develop and distribute nutrient-enhanced crop varieties. | [292] |
It is impossible to overestimate the importance of supplements in a plant-based athletic diet, particularly for nutrients like vitamin B12, vitamin D, long-chain omega-3 fatty acids (EPA and DHA), and potentially even zinc and iodine that are naturally challenging to obtain in sufficient amounts from plant sources alone [293,294]. A thorough dietary assessment is frequently the first step in determining whether supplements are necessary. Blood tests may also be used to identify particular deficiencies. Supplementation guidelines ought to take into account lifestyle factors, training intensity, and the individual needs of the athlete [295,296]. The science of nutrition and supplementation continually evolves, so it's also critical to keep up with the most recent research studies. For example, the effectiveness of algae-based omega-3 supplements as a plant-based source of EPA and DHA is a noteworthy field of research, providing vegetarians and vegans with a sustainable and efficient substitute for fish oil [297,298].
It is important to comprehend the many vitamin and mineral forms, as well as their individual absorption rates and bioavailability, in order to choose the right supplements. For example, vitamin B12 is best absorbed in the methyl cobalamin form, while iron supplements are available as ferrous or ferric forms, with some evidence suggesting better absorption rates for certain types [299,300]. Effectiveness of supplementation can also be affected by when it is taken; for best absorption, fat-soluble vitamins (A, D, E, and K) should be taken with meals that include fat. In order to guarantee compliance with doping regulations and product safety, athletes should also look for items that are devoid of prohibited compounds and have certifications from reliable third-party testing organizations [301,302].
In short, optimizing the consumption of micronutrients in plant-based athletes necessitates a thorough strategy that includes evidence-based supplementation, thoughtful meal planning, and an understanding of the factors affecting nutrient bioavailability. Plant-based athletes can support their training demands, recovery, and overall health by following these suggestions, which will ensure that their dietary pattern positively impacts their long-term well-being and athletic goals [303,304]. Collaborating with plant-based and sports nutrition specialists can help receive personalized advice and adjustments based on evolving unique health metrics, training regimens, and latest research in nutritional science [305].
4. Supplementation: when diet Isn't enough
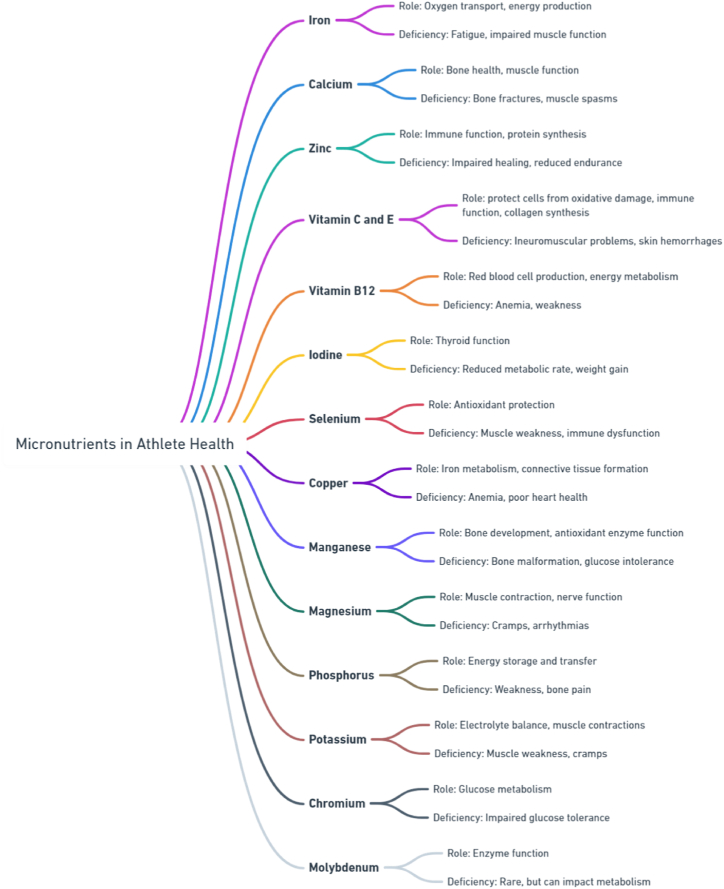
Even though most of the nutrients needed for athletic performance and health in general may be met by a well-planned plant-based diet, there are instances where diet alone won't cut it. Vitamin B12, iron, calcium, vitamin D, and omega-3 fatty acids are just a few examples of vital micronutrients that might be difficult to get enough of from plant-based diets alone [71]. To ensure athletes maintain optimal health and perform at their peak, supplementing becomes a key strategy to fill in nutritional deficiencies (Fig. 3). It is essential for athletes to incorporate foods that are high in micronutrients into their diet on a daily basis [306]. A typical day might start with a breakfast smoothie enriched with hemp, flaxseeds, or chia seeds to boost omega-3 fatty acid levels, coupled with spinach or kale for a hit of iron and calcium. Midday snacks like mixed nuts or fortified plant yogurts can give athlete a boost of micronutrients, while dinners like lentil stews or quinoa salads can give an adequate dosage of iron and plant-based protein. Such dietary diversity ensures a comprehensive spectrum of essential micronutrients [307,308].
Fig. 3.
Comprehensive Overview of Micronutrient Functions and Deficiencies. This figure was created with Whimsical.com (accessed on May 03, 2024).
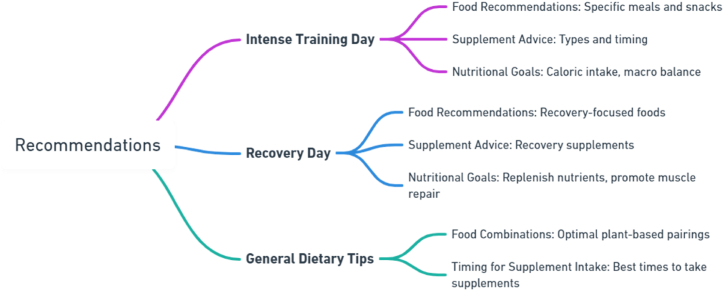
Another important aspect of a healthy diet plan, especially for athletes who have hectic lives, is meal preparation. By cooking a variety ofvegetables, legumes, and whole grains ahead of time, may save time assembling meals throughout the week and easily customize them to meet their energy and recovery needs. Meals on days of intense training might be more carbohydrate-centric to restore glycogen stores, whereas meals on days of recovery could be more fatty and protein-centric to repair muscles. Personalized meal planning is essential because the way to nutritional optimization is quite specific. Athletes can benefit greatly from the customized advice of sports nutritionists or dietitians in this area, since they can consider each player's unique training objectives, food limitations, and nutritional preferences. These experts can advise on how to maximize nutrient absorption by combining foods and can tell them when and which supplements they might need to make sure their diet is complete [23,24,309].
Powerful guides can also be found in the experiences of plant-based athletes who have successfully negotiated their dietary trajectory. The case studies, which include strength trainers and marathon runners, show how a plant-based diet combined with targeted supplementation and expert nutritional advice may support intense training needs [310,311]. These real-world examples not only show that it is possible to achieve athletic success on a plant-based diet, but they also offer others a useful guide that demonstrates how customized dietary approaches can effectively address the intricate nutritional requirements of athletes [275,312]; (Fig. 4).
Fig. 4.
Dietary Recommendations for Plant-Based Athletes. This figure was created with Whimsical.com (accessed on 05 April 2024).
5. Future directions in research and practice
Finding new avenues for study and application is crucial as the field of plant-based nutrition continues to develop in relation to both overall health and sports performance. Even while there is increasing evidence that plant-based diets are beneficial for athletes, there are still a lot of unanswered questions about the best nutritional approaches, especially when it comes to micronutrient status and how it affects performance, recovery, and long-term health. The long-term impact of plant-based diets on athletes' micronutrient status are an important subject for further research. Longitudinal research is required to completely comprehend how these diets impact nutritional status, performance, adaptation to training, and recovery over time, even if short-term trials have produced encouraging results. Furthermore, a potential field of study is the influence of gut bacteria on augmenting or impeding the bioavailability of micronutrients derived from plants. A better understanding of the relationship between nutrition absorption, gut health, and diet may result in more customized and effective dietary recommendations for athletes (Table 3).
Table 3.
Future research directions in plant-based diets and athletic performance.
| Research Area | Current Knowledge | Gaps | Proposed Studies | Potential Impact |
|---|---|---|---|---|
| Comparing Diet Types | Research has focused on the differences in performance among omnivore, vegetarian, and vegan athletes [293]. | Limited comparative studies across athletic disciplines. | Longitudinal studies comparing dietary impacts on performance metrics. | Enhanced dietary guidelines tailored for specific sports and performance goals. |
| Nutrient Optimization | Importance of micronutrient intake and muscle protein synthesis noted, but lacking practical strategies [313]. | Lack of detailed understanding of micronutrient absorption. | Controlled trials on supplementation strategies and diet formats. | Optimized dietary strategies enhancing performance and recovery. |
| Technological Advancements | Interest in integrating precision farming and nanotechnologies into nutrition strategies for athletes [314]. | Research on application and effectiveness of technologies in nutrition. | Pilot projects using technologies in meal planning and monitoring. | Innovative nutrition approaches potentially enhancing recovery and performance. |
| Diet Composition | Investigation into optimal macronutrient distribution for various athletic performances [315]. | No consensus on macronutrient ratios and timing related to training. | Comparative research on diet patterns and their effects on performance metrics. | Refined dietary recommendations that can be personalized for individual needs. |
Paying attention to new developments in plant-based nutrition, like the production of fortified meals and novel plant-based protein sources, is also crucial. The capacity of athletes to achieve their nutritional demands may be greatly impacted by these improvements, especially for those nutrients that are typically difficult to obtain from plant-based sources. Plant-based athletic nutrition may undergo a revolutionary shift with the introduction of lab-grown meats and algae-based supplements, which provide sustainable and bioavailable amounts of vital minerals like iron, B12, and omega-3 fatty acids. The growing personalization of diet based on metabolic, genetic, and microbiome profiling is another trend to take into account. With further advancements in research, it might be possible to develop highly customized plant-based diets that maximize an athlete's performance and micronutrient status according to their unique biological composition. This strategy could reduce some of the difficulties that now come with making sure that a plant-based diet provides an appropriate intake of all necessary components. Furthermore, the intersection of technology and nutrition, facilitated by wearables and apps, opens up new possibilities for monitoring and enhancing micronutrient consumption and comprehending its instantaneous impacts on performance indicators. By bridging the knowledge gap between research and practice, these tools may enable athletes to make informed decisions regarding their supplementing and food plans. Future studies and applications in plant-based nutrition for athletes present several chances to close current knowledge gaps and take advantage of developing trends. Through the advancement of research on the relationship between micronutrient status and athletic performance and plant-based diets, as well as the incorporation of cutting-edge technologies and customized nutrition strategies, we can enhance our ability to assist athletes in reaching their performance objectives while preserving their health.
6. Conclusion: thriving on a plant-based diet as an athlete
A clear path to achieving and even exceeding performance goals while following a diet that is in line with ethical, environmental, and health values has been made evident by the investigation of plant-based diets in the context of athletic performance. The key takeaway is that the demanding needs of athletic training and competition may be completely supported by a carefully designed, micronutrient-rich plant-based diet. This conclusion is not only theoretical; it is backed by an increasing amount of scientific evidence as well as personal accounts of athletes who thrived on a plant-based diet. It emphasizes how plant-based diets have the potential to be a powerful nutritional tool as well as a stimulant for reaching peak physical fitness and optimal health outcomes. However, it takes more than just purpose to perform at this level on a plant-based diet. It necessitates a dedication to lifelong learning, a desire to try new things, and a readiness to interact with nutrition specialists. The field of nutrition science is always changing, providing fresh perspectives and methods for improving and optimizing dietary practices. Therefore, athletes need to continue to learn about their bodies and their sport, and they need to be prepared to modify their nutrition plans in response to new research, changing training requirements, and the individual reactions of their bodies to different diets. Working with dietitians or sports nutritionists, particularly those with experience in plant-based nutrition, can offer athletes vital support in navigating the complexity of nutrition to achieve the best balance for their particular needs. In summary, the evidence clearly supports that plant-based diets are feasible for athletes who want to maximize their overall health, performance, and recovery. The journey toward optimal performance using plant-based nutrition is both a personal and scientific endeavor, blending the art of listening to one's body with the science of nutrition. Athletes can achieve their objectives and set the standard for proving the significant influence of diet on performance by adopting a comprehensive approach to diet planning, staying up to date on the latest research, and consulting a specialist.
Funding
This study was sponsored by the Zhejiang Provincial Natural Science Foundation of China for Distinguished Young Scholars (LR22A020002), Zhejiang Provincial Key Research and Development Program of China (2021C03130), Zhejiang Provincial Natural Science Foundation (LTGY23H040003), Ningbo key R&D Program (2022Z196), Research Academy of Medicine Combining Sports, Ningbo (No.2023001), the Project of NINGBO Leading Medical &Health Discipline (No.2022-F15, No.2022-F22), Ningbo Natural Science Foundation (20221JCGY010532, 20221JCGY010607), Public Welfare Science & Technology Project of Ningbo, China (2021S134), and K. C. Wong Magna Fund in Ningbo University, Zhejiang Rehabilitation Medical Association Scientific Research Special Fund (ZKKY2023001).
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Asma Ayaz: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Wajid Zaman: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Zsolt Radák: Writing – review & editing, Validation, Resources, Investigation. Yaodong Gu: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used AI-assisted writing technologies, including OpenAI's GPT-4 model, to assist in generating and refining the manuscript. These tools were utilized to enhance the clarity, coherence, and organization of the content, particularly in structuring complex concepts and synthesizing extensive research findings. After using these AI-assisted tools, the authors thoroughly reviewed and edited the manuscript to ensure accuracy, relevance, and adherence to academic standards. The authors affirm that they take full responsibility for the content of the publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Asma Ayaz, Email: asma@nbu.edu.cn.
Wajid Zaman, Email: wajidzaman@yu.ac.kr.
Zsolt Radák, Email: radak@tf.hu.
Yaodong Gu, Email: guyaodong@nbu.edu.cn.
References
- 1.Vinnari M., Vinnari E. A framework for sustainability transition: The case of plant-based diets. J. Agric. Environ. Ethics. 2014;27:369–396. [Google Scholar]
- 2.Alcorta A., et al. Foods for plant-based diets: challenges and innovations. Foods. 2021;10(2):293. doi: 10.3390/foods10020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Vliet S., Kronberg S.L., Provenza F.D. Plant-based meats, human health, and climate change. Front. Sustain. Food Syst. 2020;4:555088. [Google Scholar]
- 4.Fehér A., et al. A comprehensive Review of the Benefits of and the Barriers to the Switch to a plant-based diet. Sustainability. 2020;(10):12. [Google Scholar]
- 5.Gehring J., et al. Consumption of ultra-processed foods by pesco-vegetarians, vegetarians, and vegans: associations with duration and age at diet initiation. J. Nutr. 2021;151(1):120–131. doi: 10.1093/jn/nxaa196. [DOI] [PubMed] [Google Scholar]
- 6.Graça J., Oliveira A., Calheiros M.M. Meat, beyond the plate. Data-driven hypotheses for understanding consumer willingness to adopt a more plant-based diet. Appetite. 2015;90:80–90. doi: 10.1016/j.appet.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Qian F., et al. Association between plant-based dietary patterns and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA Intern. Med. 2019;179(10):1335–1344. doi: 10.1001/jamainternmed.2019.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Remón A., et al. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Critical reviews in food science. 2018;58(2):262–296. doi: 10.1080/10408398.2016.1158690. [DOI] [PubMed] [Google Scholar]
- 9.Ayaz Asma, Wajid Zaman, Zsolt Radák, Yaodong Gu Harmony in Motion: Unraveling the Nexus of Sports, Plant-Based Nutrition, and Antioxidants for Peak Performance. Antioxidants. 2024;13(4):437. doi: 10.3390/antiox13040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deledda A., et al. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants. 2021;10(5):708. doi: 10.3390/antiox10050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trautwein E.A., McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 2020;12(9):2671. doi: 10.3390/nu12092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña-Jorquera H., et al. Plant-based nutrition: Exploring health benefits for atherosclerosis, chronic diseases, and metabolic syndrome—a comprehensive review. Nutrients. 2023;15(14):3244. doi: 10.3390/nu15143244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark M., Hill J., Tilman D. The diet, health, and environment trilemma. Annual Review of Environment Resources. 2018;43:109–134. [Google Scholar]
- 14.Leip A., et al. Impacts of European livestock production: nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015;10(11):115004. [Google Scholar]
- 15.Gerber P.J., et al. Environmental impacts of beef production: review of challenges and perspectives for durability. Meat Sci. 2015;109:2–12. doi: 10.1016/j.meatsci.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Zavaglia A., Mejuto J.C., Simal-Gandara J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020;134 doi: 10.1016/j.foodres.2020.109256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese J. Beacon Press; 2018. The End of Animal Farming: How Scientists, Entrepreneurs, and Activists Are Building an Animal-free Food System. [Google Scholar]
- 18.Leighton P. The harms of industrial food production: how modern agriculture, livestock rearing and food processing contribute to disease, environmental degradation and worker exploitation. The Palgrave handbook of social harm. 2021:199–225. [Google Scholar]
- 19.Onwezen M.C., et al. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite. 2021;159 doi: 10.1016/j.appet.2020.105058. [DOI] [PubMed] [Google Scholar]
- 20.Biesbroek S., et al. Toward healthy and sustainable diets for the 21st century: importance of sociocultural and economic considerations. Proceedings of the National Academy of Sciences. 2023;120(26) doi: 10.1073/pnas.2219272120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kevany K.M., Baur G., Wang G.C. Shifting food systems: increasing well-being through plant-based approaches. Explore. 2018;14(6):435–442. doi: 10.1016/j.explore.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Martín-Rodríguez A., et al. Advances in understanding the interplay between dietary practices, body composition, and sports performance in athletes. Nutrients. 2024;16(4):571. doi: 10.3390/nu16040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas D.T., Erdman K.A., Burke L.M. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016;48(3):543–568. doi: 10.1249/MSS.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 24.Guest N.S., et al. Sport nutrigenomics: personalized nutrition for athletic performance. Front. Nutr. 2019;6:433157. doi: 10.3389/fnut.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner L. Feeling Sluggish and Low on Energy? You’re Probably Missing One of These Key Nutrients: if you aren’t getting enough of these six vitamins and minerals, then you’re letting your diet sap your stamina. Better Nutr. 2022 https://link.gale.com/apps/doc/A751130251/HRCA?u=anon~efc552e8&sid=googleScholar&xid=9333e851 [Google Scholar]
- 26.Schuiling K.D., Robinia K., Nye R. Osteoporosis update. J. Midwifery Wom. Health. 2011;56(6):615–627. doi: 10.1111/j.1542-2011.2011.00135.x. [DOI] [PubMed] [Google Scholar]
- 27.Woźniak K., et al. Nutrition strategies for optimizing performance and health in young athletes. Journal of education. Health. 2024;60:11–33. [Google Scholar]
- 28.Gammone M.A., et al. Omega-3 polyunsaturated fatty acids: benefits and endpoints in sport. Nutrients. 2019;11(1):46. doi: 10.3390/nu11010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Firdose K., Firdose N. Iron Metabolism-A Double-Edged Sword. IntechOpen; 2021. Dietary iron. [Google Scholar]
- 30.Joe S., Joe E., Rowley L.L. Consequences of physical health and mental illness risks for academic achievement in grades K–12. Rev. Res. Educ. 2009;33(1):283–309. [Google Scholar]
- 31.Godswill A.G., et al. Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: a systematic review. International Journal of Food Sciences. 2020;3(1):1–32. [Google Scholar]
- 32.Gowers C. University of Essex; 2023. An Investigation of Energy Availability and Relative Energy Deficiency in Sport in an Athletic Population. [Google Scholar]
- 33.Nair M.K., Augustine L.F., Konapur A. Food-based interventions to modify diet quality and diversity to address multiple micronutrient deficiency. Front. Public Health. 2016;3 doi: 10.3389/fpubh.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva P., et al. Nutrition and food literacy: framing the challenges to health communication. Nutrients. 2023;15(22):4708. doi: 10.3390/nu15224708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin K.G., Seebohar B. Human Kinetics; 2011. Performance Nutrition: Applying the Science of Nutrient Timing. [Google Scholar]
- 36.Bermon S., et al. Consensus statement immunonutrition and exercise. Exerc. Immunol. Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 37.Batavia A. Developing interventions to address priorities: food, dietary supplements, lifestyle, and referrals. Integrative Functional Medical Nutrition Therapy: Principles Practices. 2020:715–742. [Google Scholar]
- 38.Baranauskas M., et al. Nutritional habits among high-performance endurance athletes. Medicina. 2015;51(6):351–362. doi: 10.1016/j.medici.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Beck K.L., et al. Micronutrients and athletic performance: a review. Food Chem. Toxicol. 2021;158:112618. doi: 10.1016/j.fct.2021.112618. [DOI] [PubMed] [Google Scholar]
- 40.Littlejohn P.T., et al. Multiple micronutrient deficiencies alter energy metabolism in host and gut microbiome in an early-life murine model. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1151670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw K.A., et al. Benefits of a plant-based diet and considerations for the athlete. European journal of applied physiology. 2022;122(5):1163–1178. doi: 10.1007/s00421-022-04902-w. [DOI] [PubMed] [Google Scholar]
- 42.Firmansyah A., Prasetya M.R.A. The nutrition needs of adolescent athletes: a systematic review. Jurnal SPORTIF: Jurnal Penelitian Pembelajaran. 2021;7(3):400–418. [Google Scholar]
- 43.Yerzhankyzy Y.Y., Bekbolatkyzy S.Z., Kazys M. Comparative evaluation of actual nutrition practices and macroand micronutrients consumption of athletes in a range of Sport types. Science for Education Today. 2018;8(1):205–222. [Google Scholar]
- 44.Serra M.C., Beavers K.M. Essential and nonessential micronutrients and sport. Nutritional Supplements in Sports and Exercise. 2015:77–103. [Google Scholar]
- 45.García-Closas R., et al. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004;91(6):1005–1011. doi: 10.1079/bjn20041130. [DOI] [PubMed] [Google Scholar]
- 46.Flory S., Birringer M., Frank J. In: Bioavailability and Metabolism of Vitamin E, in Vitamin E in Human Health. Weber P., et al., editors. Springer International Publishing: Cham; 2019. pp. 31–41. [Google Scholar]
- 47.Smith S.M., Lane H.W. Nutritional biochemistry of space flight. Life Support Biosph. Sci. 1999;6(1):5–8. [PubMed] [Google Scholar]
- 48.Bhattacharjee S., Islam G.M.R. Seaweed antioxidants as novel ingredients for better health and food quality: Bangladesh prospective. Proc Pak Acad Sci. 2014;51:215–233. [Google Scholar]
- 49.Jędrzejczak R., Ręczajska W., Szteke B. Selenium in food of plant origin in Poland. Puławy-Olsztyn Alina Kabata-Pendias. Krystyna Skibniewska. 2006:316. May, 2006. [Google Scholar]
- 50.Tapiero H., Townsend D.M., Tew K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003;57(3–4):134–144. doi: 10.1016/s0753-3322(03)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunturu S., Dharmarajan T.S. Copper and zinc. Geriatric Gastroenterology. 2020:1–17. [Google Scholar]
- 52.Tapiero H., Townsend D.á, Tew K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003;57(9):386–398. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtois D., et al. Magnesium enrichment and distribution in plants. Isot. Environ. Health Stud. 2003;39(4):273–279. doi: 10.1080/10256010310001621137. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser B.N., et al. The role of molybdenum in agricultural plant production. Ann. Bot. 2005;96(5):745–754. doi: 10.1093/aob/mci226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peeling P., et al. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008;103:381–391. doi: 10.1007/s00421-008-0726-6. [DOI] [PubMed] [Google Scholar]
- 56.Grassi B., et al. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J. Appl. Physiol. 2003;95(1):149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- 57.Kracke F., Vassilev I., Krömer J.O. Microbial electron transport and energy conservation–the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim M., et al. Iron considerations for the athlete: a narrative review. Eur. J. Appl. Physiol. 2019;119:1463–1478. doi: 10.1007/s00421-019-04157-y. [DOI] [PubMed] [Google Scholar]
- 59.Clement D.B., Sawchuk L.L. Iron status and sports performance. Sports Med. 1984;1:65–74. [Google Scholar]
- 60.Damian M.-T., et al. Anemia in sports: a narrative review. Life. 2021;11(9):987. doi: 10.3390/life11090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCormick R., et al. Refining treatment strategies for iron deficient athletes. Sports Med. 2020;50(12):2111–2123. doi: 10.1007/s40279-020-01360-2. [DOI] [PubMed] [Google Scholar]
- 62.Chatard J.-C., et al. Anaemia and iron deficiency in athletes: practical recommendations for treatment. Sports Med. 1999;27:229–240. doi: 10.2165/00007256-199927040-00003. [DOI] [PubMed] [Google Scholar]
- 63.Lukaski H.C. Vitamin and mineral status: effects on physical performance. Nutrition. 2004;20(7–8):632–644. doi: 10.1016/j.nut.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Shaskey D.J., Green G.A. Sports haematology. Sports Med. 2000;29:27–38. doi: 10.2165/00007256-200029010-00003. [DOI] [PubMed] [Google Scholar]
- 65.Holmes M., Willoughby D.S. The influence of vegetarian diets on iron metabolism and supplementation in female athletes. Journal of Exercise Nutrition. 2018;1(1) [Google Scholar]
- 66.Hunt J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003;78(3):633S–639S. doi: 10.1093/ajcn/78.3.633S. [DOI] [PubMed] [Google Scholar]
- 67.Gillooly M., et al. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983;49(3):331–342. doi: 10.1079/bjn19830042. [DOI] [PubMed] [Google Scholar]
- 68.Berning J.R. vol. 19. The Encyclopaedia of Sports Medicine: An IOC Medical Commission Publication; 2013. The Vegetarian Athlete; pp. 382–391. [Google Scholar]
- 69.Craig W.J., Mangels A.R. Position of the American dietetic association: vegetarian diets. J. Am. Diet Assoc. 2009;109(7):1266. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 70.Heller S. Micronutrient needs of athletes eating plant-based diets. Nutr. Today. 2019;54(1):23–30. [Google Scholar]
- 71.Craig W.J., et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients. 2021;13(11):4144. doi: 10.3390/nu13114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson B. Food-based approaches for combating iron deficiency. Nutritional anemia. 2007;337:1–21. [Google Scholar]
- 73.Lynch S.R., Cook J.D. Interaction of vitamin C and iron. Ann NY Acad Sci. 1980;355(1):32–44. doi: 10.1111/j.1749-6632.1980.tb21325.x. [DOI] [PubMed] [Google Scholar]
- 74.Jones A.L. Red Lightning Books; 2020. Modern Cast Iron: the Complete Guide to Selecting, Seasoning, Cooking, and More. [Google Scholar]
- 75.Kardasis W., et al. The IRONy in athletic performance. Nutrients. 2023;15(23):4945. doi: 10.3390/nu15234945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stoffel N.U., et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–1239. doi: 10.3324/haematol.2019.220830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hider R.C., Kong X. Iron: effect of overload and deficiency. Interrelations between essential metal ions human diseases. 2013:229–294. doi: 10.1007/978-94-007-7500-8_8. [DOI] [PubMed] [Google Scholar]
- 78.Hurrell R., Egli I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010;91(5):1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 79.Brise H., Hallberg L. Absorbability of different iron compounds. Acta Med. Scand. 1962;171(Suppl 376):23–38. doi: 10.1111/j.0954-6820.1962.tb18680.x. [DOI] [PubMed] [Google Scholar]
- 80.Beto J.A. The role of calcium in human aging. Clinical nutrition research. 2015;4(1):1. doi: 10.7762/cnr.2015.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Theobald H.E. Dietary calcium and health. Nutr. Bull. 2005;30(3):237–277. [Google Scholar]
- 82.Geller S.G., et al. Investigating knowledge on calcium and preferences for dairy vs. plant-based alternatives. Journal of Healthy Eating Active Living. 2022;2(2):60. [PMC free article] [PubMed] [Google Scholar]
- 83.Cashman K. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002;87(S2):S169–S177. doi: 10.1079/BJNBJN/2002534. [DOI] [PubMed] [Google Scholar]
- 84.Parfitt A.J.C.T.I. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif. Tissue Int. 1984;36:S37–S45. doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- 85.Fonseca H., et al. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44:37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 86.Berchtold M.W., Brinkmeier H., Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000;80(3):1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 87.Berridge M. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 88.Benavides Damm T, Egli M. Calcium’s role in mechanotransduction during muscle development. Cellular Physiology and Biochemistry. 2014;33(2):249–272. doi: 10.1159/000356667. [DOI] [PubMed] [Google Scholar]
- 89.Periayah M.H., et al. Mechanism action of platelets and crucial blood coagulation pathways in hemostasis. International journal of hematology-oncology. 2017;11(4):319. [PMC free article] [PubMed] [Google Scholar]
- 90.Sang Y., et al. Interplay between platelets and coagulation. Blood Rev. 2021;46:100733. doi: 10.1016/j.blre.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palta S., Saroa R., Palta A.J.I.j.o.a. Overview of the coagulation system. 2014;58(5):515–523. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quesada I., et al. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J. Endocrinol. 2008;199(1):5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 93.Karamali M., et al. The effects of calcium, vitamins D and K co-supplementation on markers of insulin metabolism and lipid profiles in vitamin D-deficient women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes. 2017;125(5):316–321. doi: 10.1055/s-0043-104530. [DOI] [PubMed] [Google Scholar]
- 94.Um C.Y., et al. Circulating insulin-like growth factor-related biomarkers: correlates and responses to calcium supplementation in colorectal adenoma patients. Mol. Carcinog. 2017;56(9):2127–2134. doi: 10.1002/mc.22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dev N., Sankar J., Vinay M. Functions of thyroid hormones. Thyroid Disorders: Basic Science Clinical Practice. 2016:11–25. [Google Scholar]
- 96.Anderson K., et al. Improving Women's Health across the Lifespan. CRC Press; 2021. Optimal nutrition for women; pp. 3–40. [Google Scholar]
- 97.Liu R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013;4(3):384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogerson D. Vegan diets: practical advice for athletes and exercisers. Sports Nutr. Rev. J. 2017;14:1–15. doi: 10.1186/s12970-017-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McClements I.F., McClements D.J. Designing healthier plant-based foods: fortification, digestion, and bioavailability. Food Res. Int. 2023;169:112853. doi: 10.1016/j.foodres.2023.112853. [DOI] [PubMed] [Google Scholar]
- 100.Chandran A.S., Suri S., Choudhary P. Sustainable plant protein: a recent overview of sources, extraction techniques and utilization ways. Sustainable Food Technology. 2023;1(4):466–483. [Google Scholar]
- 101.Mäkinen O.E., et al. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Critical reviews in food science. 2016;56(3):339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- 102.Ali N. 16 soybean Processing and utilization. The soybean. 2010:345. [Google Scholar]
- 103.Dijck-Brouwer D.J., et al. Thyroidal and extrathyroidal requirements for iodine and selenium: a combined evolutionary and (Patho) Physiological approach. Nutrients. 2022;14(19):3886. doi: 10.3390/nu14193886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shannon E., Abu-Ghannam N. Seaweeds as nutraceuticals for health and nutrition. Phycologia. 2019;58(5):563–577. [Google Scholar]
- 105.Tenforde A.S., et al. Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: a review of the literature. Pm. 2010;2(10):945–949. doi: 10.1016/j.pmrj.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 106.Gaffney-Stomberg E., et al. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: a randomized, double-blind, placebo controlled trial. Bone. 2014;68:46–56. doi: 10.1016/j.bone.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 107.Rosenbloom C. Academy of Nutrition and Dietetics; 2012. Sports Nutrition: A Practice Manual for Professionals. [Google Scholar]
- 108.Straub D.A. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr. Clin. Pract. 2007;22(3):286–296. doi: 10.1177/0115426507022003286. [DOI] [PubMed] [Google Scholar]
- 109.Sharma A., et al. Zinc–an indispensable micronutrient. Physiol. Mol. Biol. Plants. 2013;19:11–20. doi: 10.1007/s12298-012-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCall K.A., Huang C.-c., Fierke C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130(5):1437S–1446S. doi: 10.1093/jn/130.5.1437S. [DOI] [PubMed] [Google Scholar]
- 111.Bonaventura P., et al. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14(4):277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 112.Tripathi P. Blue Rose Publishers; 2024. Food Nutrition in Human Science. [Google Scholar]
- 113.Osredkar J., Sustar N. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol s. 2011;3(2161):495. [Google Scholar]
- 114.Kloubert V., Rink L. function, Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015;6(10):3195–3204. doi: 10.1039/c5fo00630a. [DOI] [PubMed] [Google Scholar]
- 115.Prasad A.S. Effects of zinc deficiency on immune functions. J. Trace Elem. Exp. Med. 2000;13(1):1–20. [Google Scholar]
- 116.Chu A., Samman S. Zinc homeostasis in exercise: implications for physical performance. Vitam. Miner. 2014;3:e132. [Google Scholar]
- 117.Bhowmik D., Chiranjib K., Kumar S. A potential medicinal importance of zinc in human health and chronic. Int J Pharm. 2010;1(1):5–11. [Google Scholar]
- 118.Lin P.-H., et al. Zinc in wound healing modulation. Nutrients. 2017;10(1):16. doi: 10.3390/nu10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chasapis C.T., et al. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020;94:1443–1460. doi: 10.1007/s00204-020-02702-9. [DOI] [PubMed] [Google Scholar]
- 120.Lansdown, Alan BG, Ursula Mirastschijski, Nicky Stubbs, Elizabeth Scanlon, and Magnus S. Ågren, Zinc in wound healing: theoretical, experimental, and clinical aspects, Wound repair and regeneration 15(1) (2007) 2-16. [DOI] [PubMed]
- 121.Yagi T., et al. The role of zinc in the treatment of taste disorders. Recent Patents on Food. Nutrition Agriculture. 2013;5(1):44–51. doi: 10.2174/2212798411305010007. [DOI] [PubMed] [Google Scholar]
- 122.Gibson R.S., Raboy V., King J.C. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018;76(11):793–804. doi: 10.1093/nutrit/nuy028. [DOI] [PubMed] [Google Scholar]
- 123.Freeland-Graves J.H., et al. Global diversity of dietary intakes and standards for zinc, iron, and copper. J. Trace Elem. Med. Biol. 2020;61 doi: 10.1016/j.jtemb.2020.126515. [DOI] [PubMed] [Google Scholar]
- 124.Wada L., Turnlund J.R., King J.C.J.N.r. Zinc utilization in young men fed adequate and low zinc intakes. J. Nutr. 1985;115(10):1345–1354. doi: 10.1093/jn/115.10.1345. [DOI] [PubMed] [Google Scholar]
- 125.Heffernan S.M., et al. The role of mineral and trace element supplementation in exercise and athletic performance: a systematic review. Nutrients. 2019;11(3):696. doi: 10.3390/nu11030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Micheletti A., Rossi R., Rufini S. Zinc status in athletes: relation to diet and exercise. Sports Med. 2001;31:577–582. doi: 10.2165/00007256-200131080-00002. [DOI] [PubMed] [Google Scholar]
- 127.Gau J.-T., Ebersbacher C., Kao T.-C. Serum zinc concentrations of adults in an outpatient clinic and risk factors associated with zinc deficiency. J. Osteopath. Med. 2020;120(11):796–805. doi: 10.7556/jaoa.2020.138. [DOI] [PubMed] [Google Scholar]
- 128.Lukaski H.C. Micronutrients (magnesium, zinc, and copper): are mineral supplements needed for athletes? International Journal of Sport Nutrition and Exercise Metabolism. 1995;5(s1):S74–S83. [PubMed] [Google Scholar]
- 129.Brits I. 2012. International Pharmacopoeia Monographs for Zinc Acetate and Zinc Gluconate Active Pharmaceutical Ingredients Used in the Treatment of Paediatric Diarrhoea. [Google Scholar]
- 130.Larson-Meyer D.E., Woolf K., Burke L. Assessment of nutrient status in athletes and the need for supplementation. Int. J. Sport Nutr. Exerc. Metabol. 2018;28(2):139–158. doi: 10.1123/ijsnem.2017-0338. [DOI] [PubMed] [Google Scholar]
- 131.Gibson R.S., et al. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010;31(2_suppl2):S134–S146. doi: 10.1177/15648265100312S206. [DOI] [PubMed] [Google Scholar]
- 132.Larson-Meyer D.E., Ruscigno M. Human Kinetics Publishers; 2019. Plant-Based Sports Nutrition: Expert Fueling Strategies for Training, Recovery, and Performance. [Google Scholar]
- 133.Hakimi O., et al. 2014. Ascorbic Acid Is Essential for Significant Collagen Deposition by Human Tenocytes in Vitro. [Google Scholar]
- 134.Lykkesfeldt J. On the effect of vitamin C intake on human health: how to (mis) interprete the clinical evidence. Redox Biol. 2020;34:101532. doi: 10.1016/j.redox.2020.101532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Higgins M.R., Izadi A., Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int. J. Environ. Res. Publ. Health. 2020:17. doi: 10.3390/ijerph17228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yalcin H., Çapar T.D. In: Minimally Processed Refrigerated Fruits and Vegetables. Yildiz F., Wiley R.C., editors. Springer US; Boston, MA: 2017. Bioactive compounds of fruits and vegetables; pp. 723–745. [Google Scholar]
- 137.Aranceta, Javier, Lluís Serra-Majem, Carmen Pérez-Rodrigo, Juan Llopis, José Mataix, Lourdes Ribas, Rafael Tojo, and Josep A. Tur, Vitamins in Spanish food patterns: the eVe Study, Public health nutrition 4(6a) (2001) 1317-1323. [DOI] [PubMed]
- 138.Takanami Y., et al. vol. 29. 2000. pp. 73–83. (Vitamin E Supplementation and Endurance Exercise: Are There Benefits? Sports Medicine). [DOI] [PubMed] [Google Scholar]
- 139.Kang J.H., et al. A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 2006;166(22):2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 140.Aslam A., et al. Vitamin E deficiency induced neurological disease in common variable immunodeficiency: two cases and a review of the literature of vitamin E deficiency. Clin. Immunol. 2004;112(1):24–29. doi: 10.1016/j.clim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Butola L.K., et al. Vitamin B12-do you know everything. J. Evol. Med. Dent. Sci. 2020;9(42):3139–3147. [Google Scholar]
- 142.Green R., Mitra A.D. Megaloblastic anemias: nutritional and other causes. Medical Clinics. 2017;101(2):297–317. doi: 10.1016/j.mcna.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 143.Healton E.B., et al. Neurologic aspects of cobalamin deficiency. Medicine. 1991;70(4):229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 144.Stabler S.P., Allen R.H. Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr. 2004;24:299–326. doi: 10.1146/annurev.nutr.24.012003.132440. [DOI] [PubMed] [Google Scholar]
- 145.Tardy A.-L., et al. Vitamins and minerals for energy, fatigue and cognition: a narrative review of the biochemical and clinical evidence. Nutrients. 2020;12(1):228. doi: 10.3390/nu12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saifalddin D.L., et al. The effect of eight weeks of walking exercise and folate supplementation on plasma homocysteine levels in elderly non-athletes. Indonesian Journal of Sport Science and Coaching. 2023;5(1):64–73. [Google Scholar]
- 147.Woolf K., Manore M.M. B-vitamins and exercise: does exercise alter requirements? International journal of sport nutrition and exercise metabolism. 2006;16(5):453–484. doi: 10.1123/ijsnem.16.5.453. [DOI] [PubMed] [Google Scholar]
- 148.Joint F.A.O. 2002. Human Vitamin and Mineral Requirements. [Google Scholar]
- 149.Ortigues-Marty I., et al. Nutritional value of meat: the influence of nutrition and physical activity on vitamin B12 concentrations in ruminant tissues. Reprod. Nutr. Dev. 2005;45(4):453–467. doi: 10.1051/rnd:2005038. [DOI] [PubMed] [Google Scholar]
- 150.Niklewicz A., et al. The importance of vitamin B12 for individuals choosing plant-based diets. Eur. J. Nutr. 2023;62(3):1551–1559. doi: 10.1007/s00394-022-03025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rathee S., et al. Nanofortification of vitamin B-complex in food matrix: need, regulations, and prospects. Food Chem.: Molecular Sciences. 2022;4:100100. doi: 10.1016/j.fochms.2022.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ahad F., Ganie S.A. Iodine, Iodine metabolism and Iodine deficiency disorders revisited. Indian journal of endocrinology and metabolism. 2010;14(1):13–17. [PMC free article] [PubMed] [Google Scholar]
- 153.Benvenga S., et al. Thyroid gland: anatomy and physiology. Encyclopedia of Endocrine Diseases. 2018;4:382–390. [Google Scholar]
- 154.Ahmed O.M., et al. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2008;26(2):147–209. doi: 10.1016/j.ijdevneu.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 155.Mao I., Chen M.-L., Ko Y.-C. Electrolyte loss in sweat and iodine deficiency in a hot environment. Arch. Environ. Health. 2001;56(3):271–277. doi: 10.1080/00039890109604453. [DOI] [PubMed] [Google Scholar]
- 156.Kiran M.A., Kumar Y.N. Research on natural remedies of hyperthyroidism and hypothyroidism and IDENITIFY new symptoms, Treatment Regimen and Role of Doctor of Pharmacy. World Journal of Pharmaceutical Research. 6(1) 2016:890–920. [Google Scholar]
- 157.Biban B.G., Lichiardopol C. Iodine deficiency. still a global problem? Current health sciences journal. 2017;43(2):103. doi: 10.12865/CHSJ.43.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kapil U. Health consequences of iodine deficiency. Sultan Qaboos University Medical Journal. 2007;7(3):267. [PMC free article] [PubMed] [Google Scholar]
- 159.Smyth P.P. Iodine, seaweed, and the thyroid. Eur. Thyroid J. 2021;10(2):101–108. doi: 10.1159/000512971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brantsæter A.L., et al. Inadequate iodine intake in population groups defined by age, life stage and vegetarian dietary practice in a Norwegian convenience sample. Nutrients. 2018;10(2):230. doi: 10.3390/nu10020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sobiecki J.G., et al. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition–Oxford study. Nutr. Res. 2016;36(5):464–477. doi: 10.1016/j.nutres.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leung A.M., et al. Iodine status and thyroid function of Boston-area vegetarians and vegans. The Journal of clinical endocrinology and metabolism. 2011;96(8):E1303. doi: 10.1210/jc.2011-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elorinne A.-L., et al. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nicol K., et al. Iodine and plant-based diets–a narrative review and calculation of iodine content. British journal of nutrition. 2023:1–28. doi: 10.1017/S0007114523001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dasgupta P.K., Liu Y., Dyke J.V. Iodine nutrition: iodine content of iodized salt in the United States. Environmental science & technology. 2008;42(4):1315–1323. doi: 10.1021/es0719071. [DOI] [PubMed] [Google Scholar]
- 166.Gharibzahedi S.M.T., Jafari S.M. The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017;62:119–132. [Google Scholar]
- 167.Fernández-Lázaro D., et al. The role of selenium mineral trace element in exercise: antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients. 2020;12(6):1790. doi: 10.3390/nu12061790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ghazzawi H.A., et al. Exploring the relationship between micronutrients and athletic performance: a comprehensive scientific systematic review of the literature in sports medicine. Sports. 2023;11(6):109. doi: 10.3390/sports11060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brodska H., et al. Biomarkers in critically ill patients with systemic inflammatory response syndrome or sepsis supplemented with high-dose selenium. J. Trace Elem. Med. Biol. 2015;31:25–32. doi: 10.1016/j.jtemb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 170.Calder P.C. vol. 72. 2013. Feeding the immune system; pp. 299–309. (Proceedings of the Nutrition Society). 3. [DOI] [PubMed] [Google Scholar]
- 171.Keane K. 2014. Impact of High Intensity Interval Training (HIIT) And/or Selenium (Se) Supplementation on Oxidative Stress and Antioxidant Status in Active Females. [Google Scholar]
- 172.Vesanto Melina R., Brenda Davis R. John Wiley & Sons; 2008. Becoming Vegetarian: the Complete Guide to Adopting a Healthy Vegetarian Diet. [Google Scholar]
- 173.Mezzaroba L., et al. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology. 2019;74:230–241. doi: 10.1016/j.neuro.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 174.Gaetke L.M., Chow C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 175.Toro-Román V., et al. Copper concentration in erythrocytes, platelets, plasma, serum and urine: influence of physical training. Sports Nutr. Rev. J. 2021;18:1–8. doi: 10.1186/s12970-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Siquier-Coll J., et al. Serum, erythrocyte and urinary concentrations of iron, copper, selenium and zinc do not change during an incremental test to exhaustion in either normothermic or hyperthermic conditions. J. Therm. Biol. 2019;86:102425. doi: 10.1016/j.jtherbio.2019.102425. [DOI] [PubMed] [Google Scholar]
- 177.Wikstrom M., Krab K., Sharma V. Oxygen activation and energy conservation by cytochrome c oxidase. Chemical reviews. 2018;118(5):2469–2490. doi: 10.1021/acs.chemrev.7b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Birben E., et al. Oxidative stress and antioxidant defense. World allergy organization journal. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gonzalez-Lopez E., Vrana K.E. Dopamine beta-hydroxylase and its genetic variants in human health and disease. J. Neurochem. 2020;152(2):157–181. doi: 10.1111/jnc.14893. [DOI] [PubMed] [Google Scholar]
- 180.Dutt S., Hamza I., Bartnikas T.B. Molecular mechanisms of iron and heme metabolism. Annu. Rev. Nutr. 2022;42:311–335. doi: 10.1146/annurev-nutr-062320-112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Myint Z.W., et al. Copper deficiency anemia. Ann. Hematol. 2018;97:1527–1534. doi: 10.1007/s00277-018-3407-5. [DOI] [PubMed] [Google Scholar]
- 182.Vallet S.D., Ricard-Blum S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019;63(3):349–364. doi: 10.1042/EBC20180050. [DOI] [PubMed] [Google Scholar]
- 183.Liaw K.-Y., et al. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am. J. Gastroenterol. 1997;92(12) [PubMed] [Google Scholar]
- 184.Harris E.D. Copper as a cofactor and regulator of copper, zinc superoxide dismutase. J. Nutr. 1992;122:636–640. doi: 10.1093/jn/122.suppl_3.636. [DOI] [PubMed] [Google Scholar]
- 185.Khalid W., et al. Functional constituents of plant-based foods boost immunity against acute and chronic disorders. Open Life Sci. 2022;17(1):1075–1093. doi: 10.1515/biol-2022-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen P., Bornhorst J., Aschner M.A. Manganese metabolism in humans. Front. Biosci. 2018;23:1655–1679. doi: 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- 187.Nasolodin V.V., Gladkikh I.P., Dvorkin V.A. Effect of vitamin supplementation on the metabolism of iron, copper and manganese in athletes. Gigiena i Sanitariia. 1993;6:31–33. [PubMed] [Google Scholar]
- 188.Maynar M., et al. Erythrocyte concentrations of chromium, copper, manganese, molybdenum, selenium and zinc in subjects with different physical training levels. Sports Nutr. Rev. J. 2020;17:1–9. doi: 10.1186/s12970-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chandra S., Roychoudhury A. Role of selenium and manganese in mitigating oxidative damages. Protective chemical agents in the amelioration of plant abiotic stress: Biochemical and Molecular Perspectives. 2020:597–621. [Google Scholar]
- 190.Speich M., Pineau A., Ballereau F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin. Chim. Acta. 2001;312(1–2):1–11. doi: 10.1016/s0009-8981(01)00598-8. [DOI] [PubMed] [Google Scholar]
- 191.Zatta P., et al. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res. Bull. 2003;62(1):15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 192.Rondanelli M., et al. Essentiality of manganese for bone health: an overview and update. Nat. Prod. Commun. 2021;16(5) [Google Scholar]
- 193.Wang C., et al. Relationship between blood manganese and bone mineral density and bone mineral content in adults: a population-based cross-sectional study. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0276551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dahlquist D.T., et al. Effects of macro-and micronutrients on exercise-induced hepcidin response in highly trained endurance athletes. Applied Physiology. Nutrition, and Metabolism. 2017;42(10):1036–1043. doi: 10.1139/apnm-2017-0207. [DOI] [PubMed] [Google Scholar]
- 195.Muñoz D., et al. Effect of an acute exercise until exhaustion on the serum and urinary concentrations of cobalt, copper, and manganese among well-trained athletes. Biol. Trace Elem. Res. 2019;189:387–394. doi: 10.1007/s12011-018-1500-1. [DOI] [PubMed] [Google Scholar]
- 196.Kalisińska E., Budis H. 2019. Manganese, Mn. Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments: an Ecotoxicological Assessment of the Northern Hemisphere; pp. 213–246. [Google Scholar]
- 197.Walter R.M., Jr., et al. Copper, zinc, manganese, and magnesium status and complications of diabetes mellitus. Diabetes Care. 1991;14(11):1050–1056. doi: 10.2337/diacare.14.11.1050. [DOI] [PubMed] [Google Scholar]
- 198.Mishra A., et al. Importance of dietary supplements to the health. Curr. Nutr. Food Sci. 2021;17(6):583–600. [Google Scholar]
- 199.Zhang Y., et al. Can magnesium enhance exercise performance? Nutrients. 2017;9(9):946. doi: 10.3390/nu9090946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Baaij J.H.F., Hoenderop J.G.J., Bindels R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 201.Welch A.A., Skinner J., Hickson M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK biobank cohort. Nutrients. 2017;9(11):1189. doi: 10.3390/nu9111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Welch A.A., et al. Dietary magnesium is positively associated with skeletal muscle power and indices of muscle mass and may attenuate the association between circulating C-reactive protein and muscle mass in women. J. Bone Miner. Res. 2016;31(2):317–325. doi: 10.1002/jbmr.2692. [DOI] [PubMed] [Google Scholar]
- 203.Al Alawi A.M., Majoni S.W., Falhammar H. Magnesium and human health: perspectives and research directions. International Journal of Endocrinology. 2018:9041694. doi: 10.1155/2018/9041694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bohl C.H., Volpe S.L. Magnesium and exercise. Crit. Rev. Food Sci. Nutr. 2002;42(6):533–563. doi: 10.1080/20024091054247. [DOI] [PubMed] [Google Scholar]
- 205.Liu Y., et al. Magnesium supplementation enhances mTOR signalling to facilitate myogenic differentiation and improve aged muscle performance. Bone. 2021;146 doi: 10.1016/j.bone.2021.115886. [DOI] [PubMed] [Google Scholar]
- 206.Reno A.M., et al. Effects of magnesium supplementation on muscle soreness and performance. J. Strength Condit Res. 2022;36(8):2198–2203. doi: 10.1519/JSC.0000000000003827. [DOI] [PubMed] [Google Scholar]
- 207.Cloyd J. The top 6 essential health benefits of magnesium that you should know. Nutrition. 2023 https://www.rupahealth.com/post/the-top-6-therapeutic-uses-of-magnesium-you-need-to-know [Google Scholar]
- 208.Efsa Panel on Dietetic Products N. and Allergies, Scientific opinion on dietary reference values for magnesium. EFSA J. 2015;13(7):4186. [Google Scholar]
- 209.Volpe S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013;4(3):378S–383S. doi: 10.3945/an.112.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Veronese N., et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am. J. Clin. Nutr. 2014;100(3):974–981. doi: 10.3945/ajcn.113.080168. [DOI] [PubMed] [Google Scholar]
- 211.Yamanaka R., et al. Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 2016;6(1):30027. doi: 10.1038/srep30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nielsen F.H., Lukaski H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006;19(3):180–189. [PubMed] [Google Scholar]
- 213.Rude R.K. Magnesium deficiency: a cause of heterogenous disease in humans. J. Bone Miner. Res. 1998;13(4):749–758. doi: 10.1359/jbmr.1998.13.4.749. [DOI] [PubMed] [Google Scholar]
- 214.Brilla L.R., Haley T.F. Effect of magnesium supplementation on strength training in humans. J. Am. Coll. Nutr. 1992;11(3):326–329. doi: 10.1080/07315724.1992.10718233. [DOI] [PubMed] [Google Scholar]
- 215.Lukaski H.C. Magnesium, zinc, and chromium nutrition and athletic performance. Can. J. Appl. Physiol. 2001;26(S1):S13–S22. doi: 10.1139/h2001-038. [DOI] [PubMed] [Google Scholar]
- 216.Pollock N., et al. An 8-year analysis of magnesium status in elite international track & field athletes. J. Am. Coll. Nutr. 2020;39(5):443–449. doi: 10.1080/07315724.2019.1691953. [DOI] [PubMed] [Google Scholar]
- 217.Czaja J., et al. Evaluation for magnesium and vitamin B6 supplementation among Polish elite athletes. Roczniki Państwowego Zakładu Higieny. 2011;62(4) [PubMed] [Google Scholar]
- 218.Kass L.S., Skinner P., Poeira F. A pilot study on the effects of magnesium supplementation with high and low habitual dietary magnesium intake on resting and recovery from aerobic and resistance exercise and systolic blood pressure. J. Sports Sci. Med. 2013;12(1):144. [PMC free article] [PubMed] [Google Scholar]
- 219.Michalak M., et al. Bioactive compounds for skin health: a review. Nutrients. 2021;13(1):203. doi: 10.3390/nu13010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Veasey R.C., et al. The effects of supplementation with a vitamin and mineral complex with guaraná prior to fasted exercise on affect, exertion, cognitive performance, and substrate metabolism: a randomized controlled trial. Nutrients. 2015;7(8):6109–6127. doi: 10.3390/nu7085272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bae Y.-J., Kim M.-H., Choi M.-K. Analysis of magnesium contents in commonly consumed foods and evaluation of its daily intake in Korean independent-living subjects. Biol. Trace Elem. Res. 2010;135:182–199. doi: 10.1007/s12011-009-8511-x. [DOI] [PubMed] [Google Scholar]
- 222.Hargreaves M., Spriet L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020;2(9):817–828. doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 223.Cooke M., Wu S.S.X. Nutrition for Sport, Exercise and Performance. Routledge; 2020. Energy for sport and exercise; pp. 23–37. [Google Scholar]
- 224.Walsh C.T. The chemical biology of phosphorus. Royal Society of Chemistry. 2020;13 [Google Scholar]
- 225.Kiela P.R., Radhakrishnan V.M., Ghishan F.K. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Elsevier; 2017. Phosphorus: basic nutritional aspects; pp. 413–427. [Google Scholar]
- 226.Nattiv A., Armsey T.D. Stress injury to bone in the female athlete. Clin. Sports Med. 1997;16(2):197–224. doi: 10.1016/s0278-5919(05)70017-x. [DOI] [PubMed] [Google Scholar]
- 227.Anderson J.J.B., et al. Nutrition in Exercise and Sport. third ed. CRC Press; 2022. Nutrition and bone in physical activity and sport; pp. 219–244. [Google Scholar]
- 228.Goolsby M.A., Boniquit N. Bone health in athletes: the role of exercise, nutrition, and hormones. Sports health. 2017;9(2):108–117. doi: 10.1177/1941738116677732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9(7):568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Adatorwovor R., Roggenkamp K., Anderson J.J.B. Intakes of calcium and phosphorus and calculated calcium-to-phosphorus ratios of older adults: NHANES 2005–2006 data. Nutrients. 2015;7(11):9633–9639. doi: 10.3390/nu7115492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Aykroyd W.R., Doughty J., Walker A.F. vol. 20. Food & Agriculture Org; 1982. (Legumes in Human Nutrition). [PubMed] [Google Scholar]
- 232.Sheldon M. Food and its Uses: Spinach. https://foodmedcenter.org/food-and-its-uses-spinach/
- 233.St-Jules D.E., et al. Examining the proportion of dietary phosphorus from plants, animals, and food additives excreted in urine. J. Ren. Nutr. 2017;27(2):78–83. doi: 10.1053/j.jrn.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shirreffs S.M., Sawka M.N. Fluid and electrolyte needs for training, competition, and recovery. J. Sports Sci. 2011;29(sup1):S39–S46. doi: 10.1080/02640414.2011.614269. [DOI] [PubMed] [Google Scholar]
- 235.Nimmo M.A., Ekblom B. Fatigue and illness in athletes. J. Sports Sci. 2007;25(Suppl 1):S93–S102. doi: 10.1080/02640410701607379. [DOI] [PubMed] [Google Scholar]
- 236.Williams M.H. Dietary supplements and sports performance: minerals. Sports Nutr. Rev. J. 2005;2:1–7. doi: 10.1186/1550-2783-2-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carlson G.P. Elsevier; 1997. Fluid, Electrolyte, and Acid-Base Balance, in Clinical Biochemistry of Domestic Animals; pp. 485–516. [Google Scholar]
- 238.Darrow D.C., Yannet H. The changes in the distribution of body water accompanying increase and decrease in extracellular electrolyte. J. Clin. Investig. 1935;14(2):266–275. doi: 10.1172/JCI100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Meyer F., et al. Fluid balance and dehydration in the young athlete: assessment considerations and effects on health and performance. Am. J. Lifestyle Med. 2012;6(6):489–501. [Google Scholar]
- 240.Mielgo Ayuso J., et al. Evaluation of nutritional status and energy expenditure in athletes. Nutr. Hosp. 2015;31(Supl. 3):227–236. doi: 10.3305/nh.2015.31.sup3.8770. [DOI] [PubMed] [Google Scholar]
- 241.Sejersted O.M., Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000;80(4):1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- 242.Lindinger M.I., Cairns S.P. Regulation of muscle potassium: exercise performance, fatigue and health implications. Eur. J. Appl. Physiol. 2021;121(3):721–748. doi: 10.1007/s00421-020-04546-8. [DOI] [PubMed] [Google Scholar]
- 243.Cairns S.P. Lactic acid and exercise performance: culprit or friend? Sports Med. 2006;36:279–291. doi: 10.2165/00007256-200636040-00001. [DOI] [PubMed] [Google Scholar]
- 244.Kamińska J., et al. Does the minerals content and osmolarity of the fluids taken during exercise by female field hockey players influence on the indicators of water-electrolyte and acid-basic balance? Nutrients. 2021;13(2):505. doi: 10.3390/nu13020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ryan M. VeloPress; 2012. Sports Nutrition for Endurance Athletes. [Google Scholar]
- 246.Vainio H., Bianchini F. vol. 8. IARC; 2003. (Fruit and Vegetables). [Google Scholar]
- 247.Lefavi R.G., et al. Efficacy of chromium supplementation in athletes; emphasis on anabolism. Int. J. Sport Nutr. Exerc. Metabol. 1992;2(2):111–122. doi: 10.1123/ijsn.2.2.111. [DOI] [PubMed] [Google Scholar]
- 248.Vincent J.B. The biochemistry of chromium. J. Nutr. 2000;130(4):715–718. doi: 10.1093/jn/130.4.715. [DOI] [PubMed] [Google Scholar]
- 249.Genchi G., et al. The double face of metals: the intriguing case of chromium. Appl. Sci. 2021;11(2):638. [Google Scholar]
- 250.Wu C.-H., et al. Production and characterization of lucrative hypoglycemic collagen-peptide-chromium from tilapia scale. Process Biochemistry. 2022;115:10–18. [Google Scholar]
- 251.Lukaski H.C. Magnesium, zinc, and chromium nutriture and physical activity. Am. J. Clin. Nutr. 2000;72(2):585S–593S. doi: 10.1093/ajcn/72.2.585S. [DOI] [PubMed] [Google Scholar]
- 252.Clancy S.P., et al. Effects of chromium picolinate supplementation on body composition, strength, and urinary chromium loss in football players. Int. J. Sport Nutr. Exerc. Metabol. 1994;4(2):142–153. doi: 10.1123/ijsn.4.2.142. [DOI] [PubMed] [Google Scholar]
- 253.Yaman B. Health effects of chromium and its concentrations in cereal foods together with sulfur. Exposure and Health. 2020;12(2):153–161. [Google Scholar]
- 254.Koubová E., et al. Dietary intakes of minerals, essential and toxic trace elements for adults from Eragrostis tef L: a nutritional assessment. Nutrients. 2018;10(4):479. doi: 10.3390/nu10040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Willoughby D., Hewlings S., Kalman D. Body composition changes in weight loss: strategies and supplementation for maintaining lean body mass, a brief review. Nutrients. 2018;10(12):1876. doi: 10.3390/nu10121876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vincent J.B. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 2003;33:213–230. doi: 10.2165/00007256-200333030-00004. [DOI] [PubMed] [Google Scholar]
- 257.Anderson R.A. Effects of chromium on body composition and weight loss. Nutr. Rev. 1998;56(9):266–270. doi: 10.1111/j.1753-4887.1998.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 258.Lukaski H.C., et al. Chromium supplementation and resistance training: effects on body composition, strength, and trace element status of men. Am. J. Clin. Nutr. 1996;63(6):954–965. doi: 10.1093/ajcn/63.6.954. [DOI] [PubMed] [Google Scholar]
- 259.Maret W. Chromium supplementation in human health, metabolic syndrome, and diabetes. Met. Ions Life Sci. 2019;19:231–251. doi: 10.1515/9783110527872-015. [DOI] [PubMed] [Google Scholar]
- 260.Bytomski J.R. Fueling for performance. Sports health. 2018;10(1):47–53. doi: 10.1177/1941738117743913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Imran M., et al. Molybdenum-induced effects on nitrogen metabolism enzymes and elemental profile of winter wheat (Triticum aestivum L.) under different nitrogen sources. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20123009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mendel R.R. Metabolism of molybdenum. Metallomics and the Cell. 2013:503–528. doi: 10.1007/978-94-007-5561-1_15. [DOI] [PubMed] [Google Scholar]
- 263.Jomova K., et al. Essential metals in health and disease. Chem. Biol. Interact. 2022;367 doi: 10.1016/j.cbi.2022.110173. [DOI] [PubMed] [Google Scholar]
- 264.Margaritis I. Training, changes in nutritional requirements and dietary support of physical exercise. Nutrition and skeletal muscle. 2019:151–182. [Google Scholar]
- 265.Fissn, R.B.K.P.F . Lulu; 2019. Essentials of Exercise & Sport Nutrition: Science to Practice. com) [Google Scholar]
- 266.Spillman D.M., Haymes E.M. Glycogen, diet, and the athlete. Health Educ. 1986;17(5):68–70. [PubMed] [Google Scholar]
- 267.Nieman D.C. Fitness and sports medicine: an introduction. Med. Sci. Sports Exerc. 1991;23(5) [Google Scholar]
- 268.Maynar M., et al. Influence of an acute exercise until exhaustion on serum and urinary concentrations of molybdenum, selenium, and zinc in athletes. Biol. Trace Elem. Res. 2018;186:361–369. doi: 10.1007/s12011-018-1327-9. [DOI] [PubMed] [Google Scholar]
- 269.Sahdev, A. and K. Kanta, THE IMPORTANCE OF VITAMINS AND MINERALS TO THE ENERGY CYCLE AND ONE'S OVERALL HEALTH AND WELL-BEING.
- 270.Manore M., Meyer N.L., Thompson J. Human Kinetics; 2009. Sport Nutrition for Health and Performance. [Google Scholar]
- 271.Standing Committee on the Scientific Evaluation of Dietary Reference, I., et al . National Academies Press; 2002. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. [PubMed] [Google Scholar]
- 272.Seifert M., et al. The biological and toxicological importance of molybdenum in the environment and in the nutrition of plants, animals and man: Part III. Molybdenum content of the food. Acta Aliment. 2009;38(4):471–481. [Google Scholar]
- 273.Nieder R., et al. Microelements and their role in human health. Soil components and human health. 2018:317–374. [Google Scholar]
- 274.MacLachlan D.J., et al. Arsenic, cadmium, cobalt, copper, lead, mercury, molybdenum, selenium and zinc concentrations in liver, kidney and muscle in Australian sheep. J. Food Compos. Anal. 2016;50:97–107. [Google Scholar]
- 275.Dunford M. American Dietetic Associati; 2006. Sports Nutrition: A Practice Manual for Professionals. [Google Scholar]
- 276.Smith, JohnEric W., Megan E. Holmes, and Matthew J. McAllister, Nutritional considerations for performance in young athletes, Journal of Sports Medicine 2015(1) (2015) 734649. [DOI] [PMC free article] [PubMed]
- 277.Garthe I., Maughan R.J. Athletes and supplements: prevalence and perspectives. Int. J. Sport Nutr. Exerc. Metabol. 2018;28(2):126–138. doi: 10.1123/ijsnem.2017-0429. [DOI] [PubMed] [Google Scholar]
- 278.Franz M.J., et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25(1):148–198. doi: 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- 279.Tapsell L.C., et al. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv. Nutr. 2016;7(3):445–454. doi: 10.3945/an.115.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gupta R.K., Gangoliya S.S., Singh N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of food science and technology. 2015;52:676–684. doi: 10.1007/s13197-013-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lestienne I., et al. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005;89(3):421–425. [Google Scholar]
- 282.SandstroÈm B. Micronutrient interactions: effects on absorption and bioavailability. Br. J. Nutr. 2001;85(S2):S181–S185. [PubMed] [Google Scholar]
- 283.Nabrzyski M. CRC Press; 2006. Functional Role of Some Minerals in Foods, in Mineral Components in Foods; pp. 123–161. [Google Scholar]
- 284.Aksoylu Özbek Z., Taşkın B., Sözeri Atik D. Springer; 2023. Fortification of Plant-Based Food Analogs, in Plant-Based Foods: Ingredients, Technology and Health Aspects; pp. 35–72. [Google Scholar]
- 285.Grasso A.C., et al. The potential of food fortification as an enabler of more environmentally sustainable, nutritionally adequate diets. Nutrients. 2023;15(11):2473. doi: 10.3390/nu15112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Freeland-Graves, Jeanne H., Namrata Sanjeevi, Jane J. Lee. Global perspectives on trace element requirements. Journal of Trace Elements in Medicine and Biology. 2015;31:135–141. doi: 10.1016/j.jtemb.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 287.Jakše B., et al. Nutrient and food intake of participants in a whole-food plant-based lifestyle program. J. Am. Coll. Nutr. 2021;40(4):333–348. doi: 10.1080/07315724.2020.1778584. [DOI] [PubMed] [Google Scholar]
- 288.Zhang Y.Y., et al. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: a review. Compr. Rev. Food Sci. Food Saf. 2021;20(1):652–685. doi: 10.1111/1541-4337.12669. [DOI] [PubMed] [Google Scholar]
- 289.Tonheim L.E., Dietary intake and consumption of plant-based substitutes in vegans, vegetarians and pescatarians in Norway. (2021). Master thesishttps://oda.oslomet.no/oda-xmlui/handle/11250/2770750.
- 290.Kuriyan R., et al. The effects of regular consumption of a multiple micronutrient fortified milk beverage on the micronutrient status of school children and on their mental and physical performance. Clinical Nutrition. 2016;35(1):190–198. doi: 10.1016/j.clnu.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 291.Singh V., et al. Effect of tillage practices and residue management on soil quality and crop yield under maize (Zea mays)—based cropping system in Mollisol. Indian J. Agric. Sci. 2011;81(11):1019. [Google Scholar]
- 292.Saini P.K., et al. Biofortification of iron, zinc, and iodine in crops using transgenic techniques. Opsearch: American Journal of Open Research. 2022;1(2):48–61. [Google Scholar]
- 293.Lynch H., Johnston C., Wharton C. Plant-based diets: considerations for environmental impact, protein quality, and exercise performance. Nutrients. 2018;10(12):1841. doi: 10.3390/nu10121841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Weiss K., et al. Performance improvement in sport through vitamin D-a narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022;26(21):7756–7770. doi: 10.26355/eurrev_202211_30124. [DOI] [PubMed] [Google Scholar]
- 295.Bailey R.L., West K.P., Jr., Black R.E. The epidemiology of global micronutrient deficiencies. Annals of nutrition and metabolism. 2015;66(Suppl. 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 296.Brindle E., et al. Measurement of micronutrient deficiency associated biomarkers in dried blood spots using a multiplexed immunoarray. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kerksick C.M., et al. ISSN exercise & sports nutrition review update: research & recommendations. Sports Nutr. Rev. J. 2018;15:1–57. doi: 10.1186/s12970-018-0242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang A., et al. Microalgae as a mainstream food ingredient: demand and supply perspective. Microalgae biotechnology for food, health and high value products. 2020:29–79. [Google Scholar]
- 299.Fernández-Bañares F., Monzón H., Forné M. A short review of malabsorption and anemia. World J. Gastroenterol.: WJG. 2009;15(37):4644. doi: 10.3748/wjg.15.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Koury M.J., Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004;24:105–131. doi: 10.1146/annurev.nutr.24.012003.132306. [DOI] [PubMed] [Google Scholar]
- 301.Jeanes Y.M., et al. The absorption of vitamin E is influenced by the amount of fat in a meal and the food matrix. Br. J. Nutr. 2004;92(4):575–579. doi: 10.1079/bjn20041249. [DOI] [PubMed] [Google Scholar]
- 302.Booth S.L., Centurelli M.A. Vitamin K: a practical guide to the dietary management of patients on warfarin. Nutr. Rev. 1999;57(9):288–296. doi: 10.1111/j.1753-4887.1999.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 303.Jenner S. 2020. Dietary Intakes, Nutrition Knowledge and the Factors Influencing Dietary Behaviours and Food Choices of Professional Australian Football Athletes. [Google Scholar]
- 304.Shaw K.A. 2023. Biofortification of Plant-Based Food Products and Applications to the Athlete. [Google Scholar]
- 305.Arenas-Jal M., et al. Trends in the food and sports nutrition industry: a review. Critical reviews in food science and nutrition. 2020;60(14):2405–2421. doi: 10.1080/10408398.2019.1643287. [DOI] [PubMed] [Google Scholar]
- 306.Volpe S.L. Micronutrient requirements for athletes. Clin. Sports Med. 2007;26(1):119–130. doi: 10.1016/j.csm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 307.Dandrea-Russert Nichole. Hatherleigh Press; 2023. R.D.N., the Vegan Athlete's Nutrition Handbook: the Essential Guide for Plant-Based Performance. [Google Scholar]
- 308.Greene B., Stewart B. Ulysses Press; 2013. The Vegan Athlete: Maximizing Your Health and Fitness while Maintaining a Compassionate Lifestyle. [Google Scholar]
- 309.Thomas D.T., Erdman K.A., Burke L.M. Position of the Academy of nutrition and dietetics, dietitians of Canada, and the American college of sports medicine: nutrition and athletic performance. J. Acad. Nutr. Diet. 2016;116(3):501–528. doi: 10.1016/j.jand.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 310.Vinci D.M., et al. Nutrition supplementation practices and nutrition knowledge of ultramarathon runners. Med. Sci. Sports Exerc. 2004;36(5) [Google Scholar]
- 311.Ulery J.A., et al. Associations between health status, training level, motivations for exercise, and supplement use among recreational runners: 839. Med. Sci. Sports Exerc. 2021;53(8S) doi: 10.1080/19390211.2021.1910395. [DOI] [PubMed] [Google Scholar]
- 312.Amawi A., et al. Athletes' nutritional demands: a narrative review of nutritional requirements. Frontiers in Nutrition. 2024;10 doi: 10.3389/fnut.2023.1331854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kaufman M., et al. Popular dietary trends' impact on athletic performance: a critical analysis review. Nutrients. 2023;15(16):3511. doi: 10.3390/nu15163511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kulhánek M., et al. Plant nutrition—new methods based on the lessons of history: a review. Plants. 2023;12(24):4150. doi: 10.3390/plants12244150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Monti K.S. Optimizing nutrition in plant-based diets. JAAPA. 2022;35(4):39–42. doi: 10.1097/01.JAA.0000823180.17959.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.