Abstract
In 2022, the global prevalence of erectile dysfunction (ED) was estimated to be at least 150 million cases. This number is greatly suspected to be underestimate as most men withhold information about ED. Also, about 15% of world population have infertility troubles, and male factors are responsible for almost half of these cases. Studies have shown that the quality of semen has decreased in the past several decades owing to various health factors and environmental toxicants. The current medical interventions involve the inhibition of phosphodiesterase 5 which suffer from serious side effects and costly. One of the popular and most sought interventions are the natural and nutritional remedies as they are foods in essence and potentially with no harm to the body. Therefore, the goal of this paper is to provide a review of the most common nutritional aphrodisiacs with increasing libido and fertility highlighting the potential active constituents as well as the underlying mechanisms.
Keywords: Erectile dysfunction, Infertility, Testosterone, Aphrodisiac foods, Aromatase inhibitors
Graphical abstract
Highlights
-
•
Biochemistry of erection and the implicating risk factors of erectile dysfunction are discussed.
-
•
Numerous foods are capable of alleviating erectile dysfunction.
-
•
Some foods boosts testosterone synthesis, secretion and action.
-
•
Other foods can block aromatase action which prevent testosterone conversion into estrogen.
1. Introduction
Sexual desire is not merely about physical release; it is a powerful force that shapes our relationships and bonds us with our partners. Actually, sexual desire is a gauge of partner value, i.e. sexual desire serves as a "critical indicator" of a partner's value as a mate. It acts like an internal compass, guiding us toward relationships that are worth sustaining. When we desire our partner, it motivates us to invest in behaviors that strengthen the relationship over time. Whether it is sacrificing for their happiness or expressing love, sexual desire plays a pivotal role. For men, sex is akin to hunger. Just as we crave food, men yearn for sexual fulfillment. Each intimate encounter holds the promise of surprise and delight—a sensory exploration that tantalizes the mind. However, external factors such as relationship conflicts can dampen this appetite. Moreover, sexuality infuses relationships with energy and excitement. It propels men to pursue their life's purpose and work while also pursuing their partner. The anticipation of sexual rewards at day's end keeps them going, like a thrilling adventure waiting to unfold (Grabski et al., 2023; Mollaioli et al., 2020). Sexual health is a multifaceted process orchestrated by intricate interactions among neurological, endocrine, and vascular systems (Jannini, 2017). Both male and female sexual dysfunctions pose significant medical and psychological challenges. These dysfunctions not only impact physical health but also have adverse effects on emotional well-being. The impairment of sexual function can detrimentally influence self-esteem, body image, interpersonal relationships, and overall physical health, including fertility. Understanding these complexities is crucial for addressing sexual health comprehensively (Gerra et al., 2015).
1.1. Epidemiology of erectile dysfunction
In 1995, approximately 152 million men worldwide experienced erectile dysfunction (ED). Projections for 2025 indicate a prevalence of around 322 million, representing an increase of nearly 170 million men. Notably, the most significant projected growth is in the developing world, specifically in Africa, Asia, and South America (Aytaç and Krane, 1999). In 2022, the global prevalence of ED was estimated to be at least 150 million cases (Kohn et al., 2022). This number is greatly suspected to be underestimate as most men withhold information about ED (Mulhall et al., 2018). Many couples, about 15%, have trouble conceiving a child, and male factors are responsible for almost half of these cases (Légaré et al., 2014). Studies have shown that the quality of semen has decreased in the past several decades (Levine et al., 2017), but this trend varies across regions and populations. There is a lot of debate about whether the quality of sperm is getting worse around the world (Mann et al., 2020). Different studies have shown different results, with some finding a downward trend in semen parameters and others not (Huang et al., 2017). A systematic review concluded that sperm counts in western countries dropped by half or more from 1973 to 2011, and the cause was unknown for about a third of the cases (Fainberg and Kashanian, 2019; Levine et al., 2017). Another study looked back at nearly 120.000 men and their total motile sperm count from 2002 to 2017 and saw a decrease of around 10% over the last 16 years (Tiegs et al., 2019). The reasons for this deterioration in semen quality are probably complex and may involve changes in lifestyle and behavior as well as possible exposure to chemicals that can interfere with homeostatic reproductive function (Bundhun et al., 2019; Hayden et al., 2018; Salas-Huetos et al., 2017).
In an amusing study, the prevalence of poisoned google search results of erectile dysfunction medications redirecting to illegal internet pharmacies was scrutinized in 12 European nations. It turned out that prevalence was highest in Spain (38.8%), followed by Hungary (32.5%), Italy (28.8%), and France (23.1%), whilst the lowest was found in Finland (7.5%), Croatia (6.3%), and Bulgaria (1.3%), as per data recorded in November 2020 (Fittler et al., 2022). During the COVID-19 pandemic, global Google searches for “erectile dysfunction” have steadily risen. The median search volume during this period was 80 (IQR: 78–83), which represents an 8.1% increase compared to the pre-COVID period (median: 74; IQR: 71–76; p < 0.001) (Mattiuzzi et al., 2022). Such increase is not surprising given that ED and COVID-19 have some mutual predisposing factors such as defects of vascular integrity, cardiovascular dysfunction and unresolved inflammation (Adeyemi et al., 2022).
1.2. Risk factors
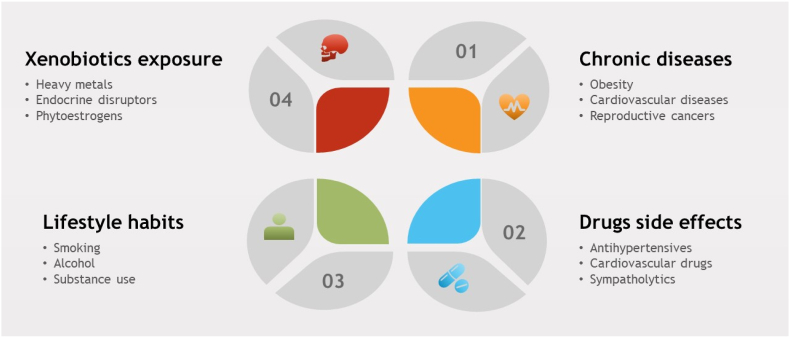
The male sexual response cycle comprises three key components: libido (desire), erectile function, and sexual activity. Various factors can disrupt this cycle, leading to ED. These factors include psychological elements, surgical procedures, trauma, vascular diseases, and neurological conditions. Additionally, lifestyle modifications, chronic diseases, environmental pollutants, and drug side effects are implicated as risk factors for ED in men (L. Chen et al., 2019). Notably, anti-hypertensive drugs are commonly associated with impotence (Tsoutsos and Kotsis, 2023). In addition, Chronic diseases such as cardiovascular disorders (Caretta et al., 2013), obesity (Rastrelli et al., 2019), hypertension and ischemia (Hudec et al., 2018), dyslipidemia, insulin resistance (Defeudis et al., 2022), chronic kidney diseases (Pizzol et al., 2021), and certain types of cancers in the reproductive system (Ma et al., 2021) contribute to ED. Lifestyle changes and dietary adjustments also impact the incidence of chronic diseases and their associated sexual complications. Additionally, environmental endocrine disruptors, heavy metals, and radiation negatively affect male sexual health (Fig. 1) (Nimbi et al., 2020). Understanding these multifaceted influences is crucial for addressing male sexual health comprehensively.
Fig. 1.
The most common risk factors of ED along with some examples.
1.3. Pathophysiology of erectile dysfunction
Erectile dysfunction (ED) refers to the persistent inability to achieve or maintain a penile erection sufficient for satisfactory sexual performance. It has a significant global prevalence and profoundly impacts quality of life. ED arises from disruptions in either neural or vascular pathways involved in erection (Romano et al., 2022). Neurogenic ED can stem from conditions such as spinal cord injury, multiple sclerosis, Parkinson's disease, lumbar disc disease, traumatic brain injury, and diabetes. Upper motor neuron lesions (above spinal nerve T10) do not cause local changes in the penis but can affect central nervous system control of erection. By contrast, sacral lesions (S2–S4 are typically responsible for reflexogenic erections) cause functional and structural alterations due to decreased innervation. The functional change resulting from such injuries is the reduction in NO load that is available to the smooth muscle. The structural changes center on apoptosis of the smooth muscle and endothelial cells of the blood vessels, as well as upregulation of fibrogenetic cytokines that lead to collagenization of the smooth muscle. These changes result in veno-occlusive dysfunction (venous leak). Vascular disease and endothelial dysfunction lead to erectile dysfunction through reduced blood inflow, arterial insufficiency, or arterial stenosis. Vasculogenic erectile dysfunction is by far the most common etiology of organic erectile dysfunction. Although age is an independent risk factor for ED, approximately one-third of 70-year-old men report no erectile difficulties (Mirone et al., 2023).
ED is a prevalent condition affecting the male reproductive system, significantly impacting the quality of life for both patients and their partners. Currently, ED is recognized as a multifaceted disorder with social, psychological, and physiological components. The primary approach for managing ED is oral PDE5 inhibitors (PDE5i), which offer advantages such as safety, efficacy, and non-invasiveness. However, alternative treatments—including intracavernosal injections, hormonal replacement therapy, vacuum erection devices, and penile prosthesis implantation (Table 1)—are viable options for patients with organic ED or those who do not tolerate PDE5i (C.-M. Wang et al., 2023). Unfortunately, all these current interventions suffer from short duration, being addictive, and can lead to serios side effects involving dysrhythmias, penile lesions and pain, and may require re-operation and removal (Argiolas et al., 2023).
Table 1.
Current treatments of ED along with mechanism of action and some examples.
| Treatment | Mechanism of Action | Additional Description | Pharmaceutical formula | Ref. |
|---|---|---|---|---|
| PDE5i | Inhibit PDE5 enzyme increasing cGMP and subsequent NO release | 1st generation: Sildenafil, Vardenafil, Tadalafil 2nd generation: Avanafil, Mirodenafil, lodenafil Udenafil |
Tablets | (Greenberg et al., 2019; Liao et al., 2019) |
| Intracavernosal agents | 1. Prostaglandin E1 (Alprostadil) stimulates cAMP release 2. Non-selective PDE5i (Papaverine) 3. Non-selective α-adrenergic antagonist (Phentolamine) 4. Synthetic VIP (Aviptadil) |
Available as single injection or combination formula like Bimix (2 drugs), Trimix (3 drugs) or Quadmix (4 drugs). | Injections | (Burnett et al., 2018; Elena et al., 2023) |
| Penile prosthesis implant | 1. Non-inflatable (malleable) implant composed of rods layers 2. Inflatable implant (usually triple layers) consists of a reservoir, pair of cylinders and a pump |
Boston Scientific AMS 700 series and Coloplast Titan series are the dominant 3-piece inflatable penile prostheses in the market | Implant and/pump | (Goodstein and Jenkins, 2023; Pang et al., 2022) |
| Penile revascularization surgery | Anastomosis of the inferior epigastric artery to the dorsal penile arteries and/or the deep dorsal vein | Eligible are only men <55 years showing no diabetes history with proven isolated arterial stenosis, with no generalized vascular burden | n/a | (Hsieh et al., 2020; Zuckerman et al., 2012) |
PDE5—phosphodiesterase type 5; cGMP—cyclic guanosine monophosphate; NO—nitric oxide; cAMP—cyclic adenosine monophosphate; VIP—Vasoactive Intestinal Peptide; n/a: not applicable.
1.4. Testosterone physiology
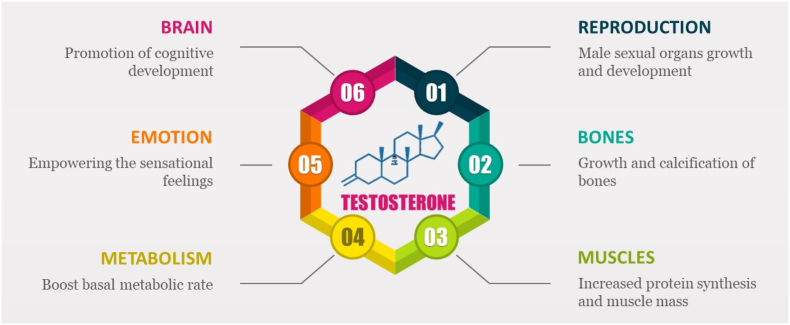
Testosterone, along with its potent metabolite dihydrotestosterone (DHT), serves as the primary androgens in the circulation of mature male mammals, including humans. These hormones play crucial roles in various biological processes, particularly in the development and maintenance of secondary male characteristics. Additionally, they are essential for reproductive functions, body composition, and musculoskeletal health as summarized Fig. 2. As the principal anabolic steroid, testosterone enhances protein production and activates both anabolic and anti-catabolic mechanisms in skeletal muscle and neuronal tissue. Consequently, it contributes to increased muscle strength, power, endurance, and hypertrophy in a dose-dependent manner (Bhasin et al., 2021; Hackett et al., 2017; Kraemer et al., 2017).
Fig. 2.
Different actions of testosterone on different organs of the body.
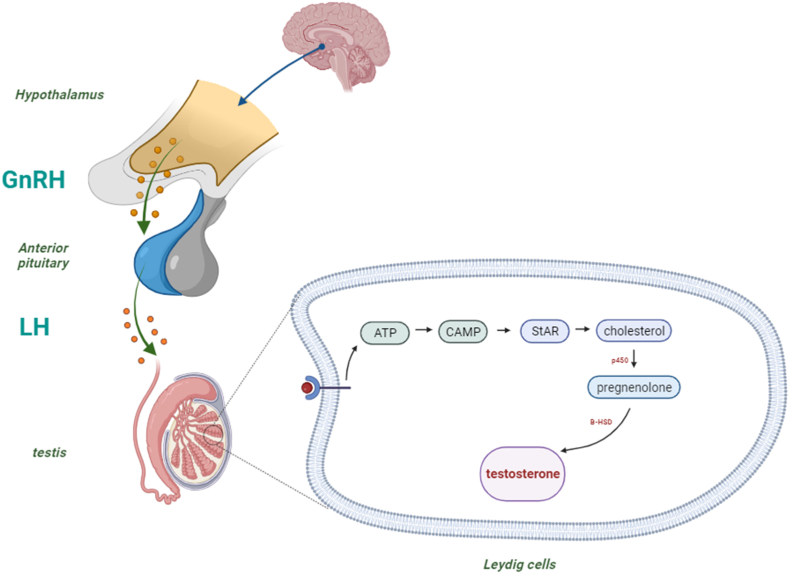
Spermatogenesis is the process of making sperm cells, which are the male reproductive cells that can fertilize an egg cell from a female. Spermatogenesis is a complex and coordinated process that involves many steps and stages, and it happens continuously throughout a male's life. Spermatogenesis depends on a system of hormones that control and regulate the activity of the testes, which are the organs where sperm are produced (Neto et al., 2016; M. Wang et al., 2018). Testosterone synthesis is strictly governed by the hypothalamic-anterior pituitary-gonadal axis. Gonadotropin-releasing hormone (GnRH), originating from the hypothalamus, promotes luteinizing hormone (LH) release from the anterior pituitary gland. LH, in turn, activates Leydig cells within the testes to produce testosterone as illustrated in Fig. 3. Elevated circulating testosterone levels result in negative feedback inhibition of GnRH and LH release. GnRH itself is modulated by various hypothalamic neuropeptides (Hohl, 2023).
Fig. 3.
Mechanism of testosterone synthesis and secretion mediated by hormonal regulation. StAR: steroidogenic acute regulatory protein, β-HSD: β -hydroxysteroid dehydrogenase.
Androgens exert their classical biological effects primarily through binding to the androgen receptor (AR). Dihydrotestosterone (DHT) binds even more strongly to the same AR than testosterone, making it approximately five times more potent in terms of androgenic activity. The AR complex undergoes structural changes, allowing it to translocate into the cell nucleus and directly bind specific target DNA sequences, leading to specific genes transcription. The AR complex functions as a transcription factor itself (Kraemer et al., 2020). Testosterone can also be converted to estradiol (E2) by the aromatase enzyme, thereby activating specific estrogen receptors. In humans, bone, adipose tissue, and the brain primarily experience testosterone effects via aromatization to E2 (Gharahdaghi et al., 2020). Aromatase, a member of the cytochrome P450 enzyme superfamily, catalyzes the conversion of androstenedione and testosterone into aromatic estrogenic steroids—estrone and estradiol, respectively. Hence, the inhibition of aromatase activity can elevate androgen concentrations. While numerous natural substances have been scientifically tested or suggested as aromatase inhibitors, certain pharmaceutical compounds with non-medical use are considered illegal (Raciti et al., 2023).
1.5. Biochemistry of erection
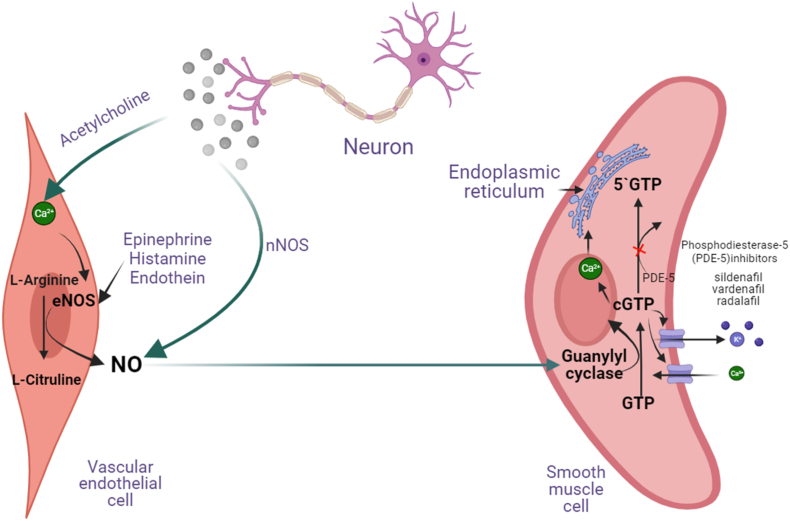
The penis has a lot of soft muscle tissues called the corpus cavernosum (CC) that can fill up with blood when a person is aroused. This happens because sexual stimulation makes the CC endothelial cells release a neurotransmitter nitric oxide (NO) that relaxes the muscle causing vasodilation. NO is made by NO synthase (NOS) from the basic amino acid arginine. NO then activates another enzyme called guanylate cyclase transforms guanosine triphosphate (GTP) into cyclic GMP (cGMP) (Fig. 4). cGMP, in turn, triggers a protein called cGMP-dependent protein kinase that changes the activity of several proteins that control how the muscle relaxes. Some of these proteins are: K+ and Ca2+ channels, myosin light-chain phosphatase, inositol triphosphate receptor, among others. The main effect of cGMP and cGMP dependent protein kinase activation is to lower the amount of Ca2+ inside the muscle cells, which makes them relax, and let more blood flow (vasodilation) into the penis, causing an erection as depicted in Fig. 4 (Agarwal et al., 2006; Melis and Argiolas, 2021).
Fig. 4.
Biochemical interactions among neurons, vascular system and testes leading to penile erection.
Moreover, testosterone was also reported to up-regulate NO signaling via increased NOS2 expression and, on the other hand, contribute to the down-regulation of cGMP signaling in Leydig cells. Altogether, testosterone-induced modulation of NO-cGMP signaling could be considered as a potent autocrine regulator of testicular steroidogenesis (Andric et al., 2010).
The treatment of erectile dysfunction (ED) has been greatly improved by the use of drugs that inhibit the enzyme phosphodiesterase 5 (PDE5), such as sildenafil, tadalafil and vardenafil. However, these drugs are not without drawbacks, as they can cause unwanted side effects, be expensive to purchase, and interact negatively with other medications. Therefore, there is a need to explore alternative ways to treat ED that are safer, cheaper and more biocompatible (Ismail and El-Sakka, 2016). This where nutritional intervention comes into play.
1.6. Nutritional intervention
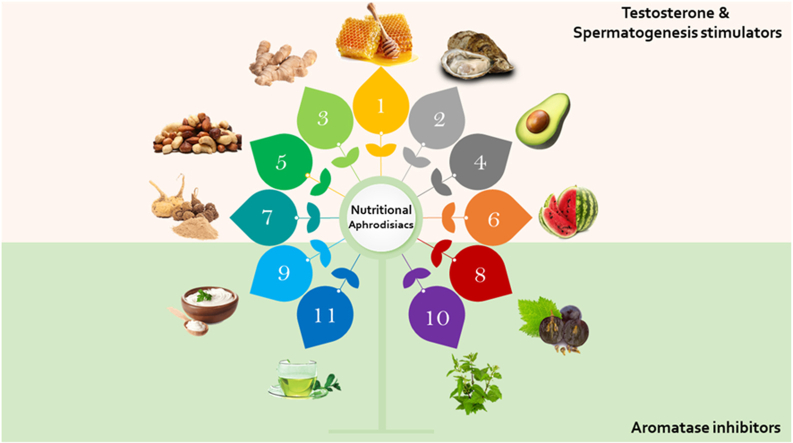
1.6.1. Foods affecting testosterone and spermatogenesis
1.6.1.1. Honey
In general, honey is a miracle natural food product with a long history of human consumption. It primarily contains sugars like fructose (around 38%) and glucose (about 32%), along with maltose (approximately 7.1%) and small amounts of sucrose, maltodextrin, and other sugars (roughly 1.5%) (Zubair and Aziz, 2015). Additionally, honey includes various components such as amino acids, enzymes like invertase, catalase, α-and β-glucosidase, acid phosphatase, glucose oxidase, and diastase, as well as water-soluble vitamins such as pyridoxine, thiamine, riboflavin, niacin, and pantothenic acid, and macrominerals including iron, calcium, copper, magnesium, manganese, zinc, and phosphorus. It also contains carotenoids and aromatic substances. Furthermore, honey is a rich source of natural antioxidants, including flavonoids and phenolic acids, which possess a wide array of biological properties, making honey a whole food (da Silva et al., 2016).
1.6.1.2. Pharmacological action
In their study, Mohamed et al. (2012) administered daily oral honey supplementation (1.2 g/kg) to rats exposed to cigarette smoke. Remarkably, the honey-fed rats exhibited positive outcomes in erectile function. Also, their ability to attain and sustain penile erection improved significantly, positively impacting sexual behaviors. These findings suggest that honey may serve as a protective agent against CS-induced adverse effects in male rats, potentially aiding in the treatment of ED. Age-related reductions in testosterone levels are often associated with erection problems. In male rats, administration of 10% honey led to increased testosterone levels (Gholami et al., 2018). Another study demonstrated that Persian honey supplementation after testis injury due to ischemia-reperfusion significantly elevated sperm counts and testosterone levels (Atangwho et al., 2020).
Furthermore, oral supplementation of 5% Palestinian honey for 20 days had intriguing effects involving increased epididymal sperm count, while testicular sorbitol dehydrogenase activity was enhanced. Simultaneously, lactate dehydrogenase activity decreased. Sperm motility relies on energy sources like adenosine triphosphate (ATP). Sorbitol dehydrogenase converts sorbitol into fructose, a crucial monosaccharide processed via the glycolytic pathway to produce ATP (Cao et al., 2009). Indeed, the degree of secretion and increase in circulating testosterone upon administration of honey depends mainly on the type of the honey as well as the dosage ingested (Khan et al., 2017). Honey act via different mechanisms to boost the men's libido including the (i) boosting of circulating testosterone, (ii) promotion of the LH which ultimately result in testosterone synthesis and secretion, (iii) braking the activity of aromatase which degrades testosterone and converts it into the E2, and (iv) serve as powerful antioxidants scavenging the free radicals within the testicular tissues (Banihani, 2019). It should be noted that, the natural bioactive compounds such as chrysin, caffeic acid, vanillic acid, þ-coumaric acid, syringic acid, ferulic acid, quercetin, myricetin, kaempferol, pinobanksin, chrysin, pinocembrin, ellagic acid, 3-hydroxybenzoic acid, galangin, rosmarinic acid, hesperetin, gallic acid mediate the aforementioned actions of honey (Zaid et al., 2021).
1.6.1.3. Watermelon
The watermelon (Citrullus lanatus) is a tropical fruit originating from a ground plant of the Cucurbitaceae family, and it holds significant economic significance as it is cultivated worldwide. It is highly regarded for its nutritional value, refreshing properties, and low-calorie content. The interior of the watermelon is typically brightly colored and contains substantial amounts of beta carotene and lycopene, both of which are well-known for their antioxidant properties. Extracts derived from watermelon have diverse applications, including their use as antihypertensive, anti-inflammatory, and antimicrobial agents (Tabiri, 2016). Each part of the watermelon exhibits distinct compositional features. For instance, the seeds serve as excellent sources of protein and can be incorporated into dietary plans and food preparations. The extracted seed oil primarily consists of linoleic acid, palmitic acid, and stearic acid, and it also contains phytochemical compounds such as lycopene, saponins, flavonoids, alkaloids, oxalates, and tannins (Neglo et al., 2021).
1.6.1.4. Pharmacological action
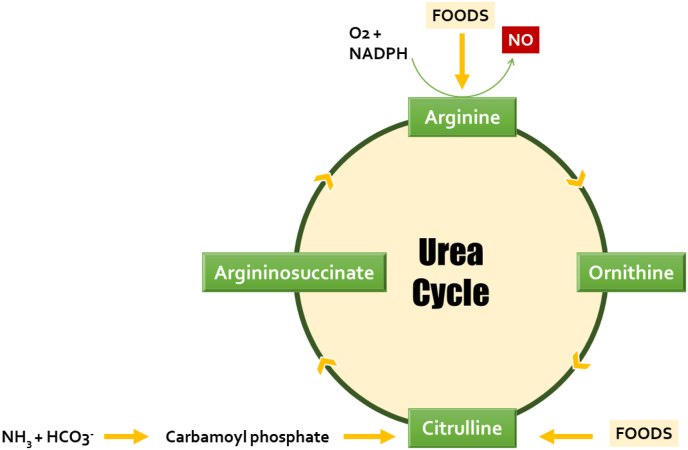
Watermelon enhances male fertility as it was reported for improving semen quality, reversing ED, enhancing testicular redox status, as well as improving gonadotropin secretion. These activities have been linked to their constituents as it contains vitamins and phytochemicals such as phenols and certain flavonoids that contribute to their antioxidant properties (Damilare and Rotdelmwa, 2022). Watermelon also contains large amounts of antioxidant vitamins such as vitamin E and vitamin C which can limit the invasive actions of free radicals within testicular niche (Choudhary, 2015). Many animal models were used to evaluate the enhancing actions of watermelon extracts and are summarized in Table 2. To add, watermelon is a rich source of the non-canonical amino acid citrulline. This amino acid is metabolized via urea cycle to arginine which is a precursor for the synthesis of NO via NOS as depicted in Fig. 5 consequently, watermelon consumption increases the blood flow to the testis as a result of the vasodilation action generated by NO (Collins et al., 2007).
Table 2.
Some in vivo studies confirming the beneficial roles of watermleon and its extracts on fertility.
| Animal used | Part used and form of extract | Route of administration | Dosage | Duration of Treatment | Actions | Ref |
|---|---|---|---|---|---|---|
| Wistar rats | Fruit; aqueous extract | Orally | 100 and 200 mg/kg | 30 days |
|
Daramola et al. (2018) |
| Wistar rats | Seed; crude powder and ethanolic extract | Orally | 200 mg/kg | 28 days |
|
(Akhuetie et al., 2018) |
| Wistar rats | Rind; Hydro- methanolic Extract | Orally | 500 mg/kg | 42 days |
|
(Kolawole et al., 2019) |
| Wistar rats | Rind; ethanolic extract | Orally | 100, 200 and 400 mg/kg | 52 days |
|
(Pratama et al., 2021) |
| albino rats | Seed; ethanolic extract | Orally | 200 mg/kg | 8 weeks |
|
(Odo et al., 2021) |
↑: increased; ↓: decreased; FSH: follicle-stimulating hormone; SOD: superoxide dismutase; MDA: malondialdehyde.
Fig. 5.
entry points of some foods to the urea cycle to utilize arginine for nitric oxide synthesis.
1.6.1.5. Ginger
Ginger (Zingiber officinale) is deemed a flowering plant whose rhizome (plant stem) is most commonly used as spices (Eliopoulos, 2007). It has been widely deployed for managing a variety of illnesses owing to its medicinal benefits. It was reported that ginger greatly alleviated hyperglycemia-induced oxidative stress, inflammation and apoptosis and, hence, protected rats against diabetic nephropathy complications (Al Hroob et al., 2018) and, thus, is a therapeutic option tested in many clinical trials (Zhu et al., 2018). Besides diabetes, cancer (S.-Y. Chen et al., 2018; Nachvak et al., 2022), Alzheimer's disease (Kim et al., 2018; Schepici et al., 2021), cerebrovascular events (Cerdá et al., 2022; Fakhri et al., 2021), and depression (Lin et al., 2020).
1.6.1.6. Pharmacological action
There are many in vivo models tested the potential improving role of ginger (in diet), aqueous extract, methanolic extract, or pure compounds isolated from ginger including gingerol, zingerone (Banihani, 2018). Many of these studies reported the boosting actions of ginger directly on circulating testosterone concentrations as listed in Table 3.
Table 3.
Some evidences of the beneficial action of ginger, ginger extracts and isolated compounds on testosterone of animal models.
| Extract/compound | Dose | Duration | Population | Effect on Serum Testosterone | Ref. |
|---|---|---|---|---|---|
| Aqueous extract of ginger | 600 mg/kg | 8 days | SIDR | ↑ | Kamtchouing et al. (2002) |
| Methanolic roots extract | 100 and 200 mg/kg | 65 days | AIDR | ↑ | Shalaby and Hamowieh (2010) |
| Aqueous extract of roots | 150 and 300 mg/kg | 65 days | AIDR | ↑ | Shalaby and Hamowieh (2010) |
| Ginger | 4% of the diet | 2 weeks | Hypertensive male rats | ↑ | Akinyemi et al. (2015) |
| Zingerone | 20 mg/kg | 8 weeks | SIDR | – | Eid et al. (2017) |
| geraniol | 200 mg/kg | 8 weeks | SIDR | – | Eid et al. (2017) |
| 6-gingerol | 75 mg/kg | 8 weeks | SIDR | – | Eid et al. (2017) |
| Fresh ginger roots | 1.5 g/15 g of diet | 30 days | AIDR | ↑ | Ghlissi et al. (2013) |
↑ indicate elevation; - indicate no change; SIDR: Streptozotocin-induced diabetic rats; AIDR: Alloxan-induced diabetic rats.
Mechanistically, ginger can enhance the libido via various aspects. For example, it significantly neutralizes reactive oxygen species (ROS), diminishes systemic inflammation, increase the serum levels of LH, and decreases the levels of 3β-hydroxysteroid dehydrogenase (3βHSD), steroidogenic acute regulatory protein (StAR), 17β-estradiol (N. Li et al., 2022; Raoufi et al., 2023). In addition, Ghareib et al. concluded that 6-gingerol, the active constituent of ginger, is able to activate cGMP and greatly enhance the synthesis and liberation of NO. This will ensure the vasodilation and, consequently, more blood flow to the testes (Ghareib et al., 2015).
1.6.1.7. Sea foods
Oysters, as a seafood, have been thought to significantly improve the male sexual function for thousands of years. Oysters, revered in traditional Chinese medicine for their potential to address deficiencies and enhance yang, also serve as aphrodisiacs in Western cultures. Dried oyster meat boasts protein content of 45–52%, fat content of 7–11%, and sugar content of 19–38%. Additionally, it is rich in taurine, vitamins, and minerals, rendering it an excellent candidate for bioactive peptide development. Enzymatic hydrolysis effectively transforms proteins into bio-absorbable active peptides, optimizing the efficient utilization of marine proteins (Asha et al., 2016; Miao et al., 2018; Q. Wang et al., 2014).
1.6.1.8. Pharmacological action
It has been demonstrated that the oligopeptides derived from oyster significantly improved cyclophosphamide-induced partial androgen deficiency of the aging male through the triggering of the continuous production of testosterone within 6 weeks (Jin et al., 2021). Similarly, the enzymatic hydrolysate of oyster was tested on improving ED in hemicastrated male rats. The results showed that in a dose-dependent manner, oyster hydrolysate reduced the latency of penile erection induced by electrical stimulation, demonstrating its potential in alleviating ED. Further exploration revealed that oyster hydrolysate significantly elevates serum levels of testosterone, luteinizing hormone, and nitric oxide (NO). These effects resemble those observed in the normal control group, suggesting that oyster hydrolysate stimulates testosterone synthesis by promoting luteinizing hormone production, subsequently enhancing NO content via the NO-cGMP signaling pathway. The underlying mechanism involve the release of NO as a response of testosterone action, activating the guanylyl cyclase (H.-Y. Wang et al., 2020). Moreover, some authors underlie the huge amounts of zinc found in oyster are the major drivers for the synthesis of testosterone (Astuti et al., 2019; Brenowitz, 2013). Collectively, oyster zinc can first instigate the testosterone biosynthesis whereas the peptides results in the production of NO which act as vasodilator, increasing the blow flow to penis alleviating ED.
Additionally, fish oil is famous for increasing men's libido traditionally. Some nutrients, such as ω-3 fatty acids, antioxidants (primarily, vitamin E, vitamin C, β-carotene, selenium, zinc, cryptoxanthin, and lycopene), and other vitamins (for example, vitamin D and folate) that are found in a healthy diet can improve semen quality (Stanhiser et al., 2022). Eating fish, shellfish, other seafood, poultry, cereals, vegetables, fruits, low-fat dairy, and skim milk can have positive effects on various aspects of semen (Stanhiser et al., 2020). However, fish and fish liver oil also have other important nutrients, such as retinoic acid (that is, vitamin A), that can enhance semen quality.10 On the other hand, diets that have a lot of processed meats, soy foods, potatoes, full-fat and total dairy products, cheese, coffee, alcohol, sugar-sweetened beverages, and sweets can worsen semen quality (Gaskins and Chavarro, 2018). In Denmark, fish oil supplements were given to young men followed by testing semen quality and reproductive hormone levels. It turned out that fish oil supplementation was correlated in a dose-response manner with increased semen volume, higher total sperm count, larger testicular size, a higher free testosterone to LH ratio, and lower FSH and LH levels in comparison with negative controls (Jensen et al., 2020). The potential mechanism involve the stimulation of production and release of NO from endothelial cells into smooth muscle cells, causing vasodilation and increasing the blood flow to the testis (Falsig et al., 2019). Furthermore, pre-clinical evidence reported that omega-3 (ω-3) polyunsaturated fatty acids (PUFAs), in particular, docosahexaenoic acid (DHA) have been proven to affect testosterone biosynthesis even in overweight and obese males (Abbott et al., 2020).
1.6.1.9. Ginseng
Ginseng, a plant known for its slow growth and fleshy roots, has a history rooted in traditional East Asian medicine where it has been valued for its adaptogenic qualities that enhance physical performance, vitality, and resilience against stress and aging. Ginseng, whether used alone or as part of dietary supplements, is widely recognized for its potential therapeutic effects across various health conditions (Roychoudhury et al., 2022). Notably, ginseng-containing compounds rank among the most popular and best-selling herbal medicines globally. Specifically, ginseng has gained attention as a potential remedy for ED, a condition where a man struggles to achieve or maintain an erection sufficient for sexual activity (Koppula et al., 2023). The effects of aphrodisiacs actions of ginseng on in vivo animal models were studied extensively and provided in Table 4.
Table 4.
A summary of In vivo studies showcasing the potential aphrodisiac activities of ginseng.
| In vivo model | Ginseng dose | Parameters assessed | Main outcome | Ref. |
|---|---|---|---|---|
| Doxorubicin-induced testicular damage in rats | 100 & 200 mg/kg | Antioxidant enzymes, sex hormone receptors, pro-inflammatory cytokines and autophagy | Protected chemotherapeutic-mediated testicular damage | Cha et al. (2018) |
| Subacute immobilization stress-induced testicular damage in rats | 100 & 200 mg/kg | Blood chemistry, sperm kinematic values, expression spermatogenesis-related proteins, sex hormone receptors, antioxidant-related enzymes | Blocked stress-caused male sterility | (S.-H. Lee et al., 2019) |
| Lithium hydrochloride and pilocarpine induced temporal lobe epilepsy rat model | 150 mg/kg | Sperm kinematics and apoptosis signaling | alleviated germ cells apoptosis, enhanced seminiferous tubule anatomy | Ganjkhani et al. (2019) |
| Heat stress-exposed rats | 200 mg/kg | Sperm kinematics and serum lipid metabolism marker, testicular antioxidant enzymes, inflammatory cytokines, sex hormonal receptors, and spermatogenesis-related transcription factors | attenuated heat stress-induced changes in all parameters examined | Kopalli et al. (2019) |
| D-Galactose-induced mice | 50 & 250 mg/kg | Oxidative stress parameters, inflammatory markers | Amerliorated D-Gal-triggered reproductive injury via modulating MAPKs signaling | (Q. Zhang et al., 2021) |
| Mice model | Oligopeptides from ginseng (250 mg/kg); Combined ginseng and oyster (62.5 mg/kg and 60 mg/kg). | Sexual functional parameters | Raised serum NO, testosterone, and corpus cavernosum cGMP levels while diminished the corpus cavernosum phosphodiesterase-5 level | (D. Li et al., 2021) |
For example, one research has highlighted ginseng's aphrodisiac attributes and its potential to enhance sexual function in men. For instance, a study conducted in 2012 involving 119 men with mild-to-moderate ED demonstrated that ginseng berry extract notably improved overall sexual function (Choi et al., 2013). In a more recent double-blind, randomized controlled trial, the effect of carob and ginseng supplements on semen analysis parameters, sexual function, and sex hormones in infertile men was explored. The carob group showed a significant increase in normal sperm counts and testosterone levels, while the ginseng group demonstrated improvements in sperm motility and serum levels of FSH and LH. These findings suggest that carob and ginseng supplements may have a positive effect on semen analysis parameters and sex hormones in infertile men. The study's results are consistent with previous research that has shown the beneficial effects of carob and ginseng on male reproductive health (Pilehvari et al., 2024).
In a Cochrane systematic review published in 2022, the aphrodisiac properties of ginseng were concluded to be insignificant. Indeed, ginseng was demonstrated to have effects on ED when compared to placebo, appears to be trivial based on the Erectile Function Domain of the International Index of Erectile Function (IIEF)-15 instrument. The mean difference was 3.52 (95% confidence interval [CI] 1.79 to 5.25) across 3 studies with low certainty of evidence. However, ginseng may have little to no effect on adverse events compared to placebo (risk ratio [RR] 1.45, 95% CI 0.69 to 3.03) based on 7 studies. On the flip side, the impact on sexual experience was positively prominent. Ginseng may improve men's self-reported ability to have intercourse (RR 2.55, 95% CI 1.76 to 3.69) based on 6 studies. However, its effect on men's satisfaction with intercourse (Intercourse Satisfaction Domain of IIEF-15) is also trivial (MD 1.19, 95% CI 0.41 to 1.97) across 3 studies (H. W. Lee et al., 2022).
The mechanisms behind ginseng's sexual-enhancing properties are linked to its capacity to elevate estrogen receptor beta (ERβ) levels in the brain, potentially counteracting stress through antiapoptotic and antioxidative actions. Notably, ginsenoside, a ginseng saponin, has shown efficacy in enhancing libido and sexual potency by modulating the NO/cGMP pathway, indicating its potential in managing erectile dysfunction. The bioactive constituents of ginseng, particularly ginsenosides, play a pivotal role in driving its therapeutic effects. It is essential for individuals to scrutinize the composition of ginseng products to ensure their quality and medicinal efficacy before use.
1.6.1.10. Nuts
Nuts such as walnuts are a food that has many beneficial properties for human health (Holt et al., 2015). Almond (Prunus dulcis Mill) is the most consumed tree nut throughout the world. North America is the largest producer of almonds, pistachios (Pistachia vera L.), and walnuts (Juglans regia), while the Middle East countries produce mostly cashews (Anacardium occidentale) and hazelnuts (Corylus avellana) (Laddha et al., 2020). According to the EuroFir food composition databases, walnuts have more n-3 fatty acids than other nuts in the Balkan region. Walnuts are also rich in α-linolenic acid (ALA), which is a type of ω-3 fatty acid. Maguire and his colleagues showed that walnuts have a lot of ALA (11.6% of total) compared to other nuts like hazelnuts and almonds (Gervasi et al., 2021; H. Zhang et al., 2018). The amount of ALA to linoleic acid, which is another type of fatty acid, is high among all the tree nuts. This means that walnuts have a good balance of ω-6 and ω-3 fatty acids (Alasalvar et al., 2020).
1.6.1.11. Pharmacological action
The efficacy of nuts for reproductive male functions were examined in in vivo animal models. For example, alcohol-exposed rats had lower body and testicular weights, worse semen quality, lower levels of vitamins C and E, altered hormone profiles, and damaged testicular structure than the control animals. However, giving tiger nuts to the rats restored the testicular health, semen quality, and antioxidant enzymes in different degrees depending on the amount. Therefore, Tiger nuts can reverse the harmful effect of alcohol on the testis in different ways depending on the dose (Gbotolorun et al., 2022). Older male rats had more damage in their testicles and worse semen quality. A diet with hazelnut added improved the testicular health, semen quality, oxidative stress in the seminal plasma and plasma, vitamin E in the seminal plasma, and testosterone levels in the plasma in both groups. This work shows that a diet with hazelnut added can greatly improve the testicular antioxidant function and semen quality in older male rats (Kara et al., 2019).
Two studies have tested how eating nuts affects sperm quality in humans (Robbins et al., 2012; Salas-Huetos et al., 2018). The first study by Robbins and others found that eating 75 g of walnuts every day for 12 weeks improved the health, movement, and shape of sperm in 117 healthy men who ate Western-style diets. The authors traced these improvements to the increase in blood ω-6 PUFAs and ALA, a plant-based ω-3 PUFA, and suggested some possible ways that these fats could affect sperm (ALA is a good indicator of walnut intake (Garcia-Aloy et al., 2019; Petrović-Oggiano et al., 2020)). The second study, the FERTINUTS trial, by Salas-Huetos et al. looked at the effect of eating different nuts (almonds, hazelnuts, and walnuts) on changes in normal sperm measures, and explored several possible mechanisms. A total of 119 healthy men of reproductive age who ate Western-style diets were divided into two groups, one that ate 60 g of mixed nuts every day and one that did not eat any nuts. Eating nuts significantly increased the total number of sperm and their health, movement, and shape, and these findings were explained by a decrease in sperm DNA damage. Eating nuts was also linked to a lower expression level of the micro-RNA hsa-miR-34b-3p and to different methylation patterns in 36 regions of the genome between the start and the end of the trial (Salas-Huetos et al., 2021). These studies show that sperm epigenome mechanisms can change with diet.
1.6.1.12. probiotics
Probiotics are live microorganisms that can confer health benefits to the host when consumed in adequate amounts. Probiotics have been widely studied for their effects on various aspects of human health, such as gastrointestinal, cardiovascular, and immune functions (Natarajan and Bhatt, 2020). However, the potential role of probiotics in enhancing male fertility has received less attention, despite the growing evidence that the gut microbiome can influence the reproductive system (Franasiak and Scott, 2015; Tett et al., 2021).
1.6.1.13. Pharmacological action
A study tested how three kinds of Lactobacilli (Lactobacillus brevis, Lactobacillus salivarius, and Lactobacillus plantarum) could help human sperm in the lab. They found that these bacteria could protect sperm from harmful oxygen molecules when there were problems in the vagina. This could make it easier for the woman to get pregnant (Barbonetti et al., 2011). This was the first study finding a correlation between probiotics and spermatozoa physiology. Then after, several studies have suggested that probiotics can increase testosterone levels in men by modulating the gut microbiome and its interactions with the endocrine system. For example, a randomized controlled trial involving 40 healthy men found that supplementation with a probiotic strain of Lactobacillus reuteri for 12 weeks significantly increased serum free testosterone levels compared to placebo (Younis and Mahasneh, 2020). Another randomized controlled trial involving 72 overweight men found that supplementation with a probiotic mixture containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactococcus lactis for 12 weeks significantly increased serum total testosterone levels compared to placebo (Corbett et al., 2021). The mechanisms by which probiotics increase testosterone levels are not fully understood, but may involve reducing intestinal inflammation, enhancing intestinal barrier function, modulating the activity of steroidogenic enzymes, altering the metabolism of bile acids and phytoestrogens, and influencing the hypothalamic-pituitary-gonadal axis (H. Wang et al., 2022).
Semen quality is another important indicator of male fertility that reflects the quantity and quality of sperm cells. Semen quality can be affected by various factors, such as oxidative stress, infection, inflammation, lifestyle habits, and environmental exposures. Poor semen quality can lead to reduced sperm motility, morphology, viability, and concentration, which can impair fertilization and pregnancy outcomes. Therefore, improving semen quality is a key goal for enhancing male fertility (H. Wang et al., 2022).
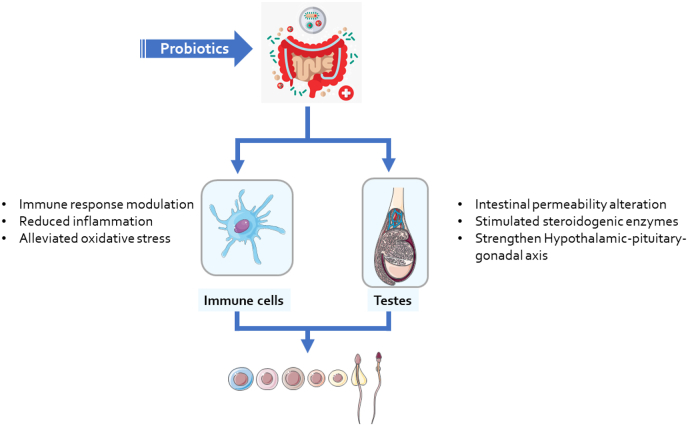
Several studies have suggested that probiotics can improve semen quality in men by modulating the gut microbiome and its interactions with the reproductive system as summarized in Table 5. For example, a randomized controlled trial involving 100 infertile men found that supplementation with a probiotic strain of Lactobacillus plantarum for 12 weeks significantly improved sperm motility, morphology, viability, and concentration compared to placebo (Rahimiyan-Heravan et al., 2020). Another randomized controlled trial involving 50 infertile men found that supplementation with a probiotic strain of Lactobacillus rhamnosus for 12 weeks significantly improved sperm motility and morphology compared to placebo (Dardmeh et al., 2017). The mechanisms by which probiotics improve semen quality are not fully elucidated, but may involve reducing oxidative stress and DNA damage in sperm cells, enhancing antioxidant defenses in seminal plasma, modulating immune responses and cytokine production in the reproductive tract and influencing the expression of microRNAs and epigenetic markers in sperm cells (see Fig. 6) (Akram et al., 2023; Y. Zhang et al., 2023).
Table 5.
A selection of probiotics with their positive actions of on male reproductive functions.
| Bacteria | Target | Outcome | Ref. |
|---|---|---|---|
| Proteobacteria | Sperm | ↑ male seminal hyperviscosity | Monteiro et al. (2018) |
| L. casei CGMCC 1.570 | Sperm | ↑ spermatozoa motility and mitochondrial activity in boars | (J. Zhang et al., 2020) |
| L. plantarum TW1-1 | Testes | Restored DEHP-induced testis injury in mice | Tian et al. (2019) |
| PRO-Men Hyperbiotics | Prostate | ↓ inflammation of recurrent prostatitis in men | Pacifici et al. (2021) |
| L. rhamnosus GG | Prostate cancer cells | ↓ prostate cancer cell viability | Celebioglu (2021) |
| Fecal microbiota transplantation | Testes | ↑ spermatogenesis and sperm quality in mice | (P. Zhang et al., 2021) |
↑: improved; ↓: reduced.
Fig. 6.
summary of the mechanisms of probiotics-mediated spermatogenesis and immune modulation.
1.6.1.14. Avocado
Avocado, scientifically known as Persea americana, is a distinctive fruit renowned for its exceptional nutritional profile, establishing it as a favored ingredient in diverse culinary preparations globally. This fruit is abundant in essential macronutrients, vitamins, minerals, and bioactive compounds, offering numerous health advantages (Punia Bangar et al., 2022). An average avocado comprises approximately 73% water, 15% fat, 8.5% carbohydrates (primarily fibers), and 2% protein. The monounsaturated fatty acids present in avocados, notably oleic acid, play a pivotal role in mitigating inflammation and potentially exerting protective effects against cancer. Avocados also serve as a notable source of vital vitamins and minerals, encompassing B-vitamins, vitamin K, potassium, copper, vitamin E, and vitamin C. They boast significant folate levels crucial for cellular growth and metabolism, rendering avocados particularly advantageous for expectant mothers. Furthermore, avocados are rich in dietary fiber, delivering 24% of the daily value (DV) per 100 g serving. Dietary fiber is indispensable for appetite regulation, nurturing beneficial gut bacteria, and diminishing the risk of various ailments like heart disease, stroke, obesity, type 2 diabetes, and depression. Additionally, avocado seeds exhibit a wealth of polysaccharides, proteins, lipids, vitamins, minerals, and other bioactive compounds, positioning them as a valuable asset for diverse industrial applications. Their elevated content of monounsaturated fats, particularly oleic acid, alongside their rich vitamin, mineral, and fiber content, underscores their significance as a beneficial dietary inclusion (Ford et al., 2023).
The sexual-enhancing attributes of avocados are associated with several factors. Avocados are recognized as aphrodisiacs due to their high phytonutrient content, which can boost sexual pleasure, desire, and attraction. Furthermore, avocados contain omega-3 and other fatty acids that promote mental clarity and enhance imagination abilities. In addition, avocados are rich in Vitamin E and zinc, which are crucial for sperm quality and male fertility (Ali et al., 2022). Vitamin E enhances sperm quality, while zinc is essential for testosterone production and improving fertility. Biochemically, Monounsaturated fats and zinc are essential for the anabolism of steroids including testosterone whereas the vitamins such as ascorbic acid and tocopherol protect sexual organs from ongoing oxidative stress and reduce the consequent inflammation (Olabiyi and Ajayi, 2022). Therefore, avocados are known to have aphrodisiac effects, increase intercourse duration, treat premature ejaculation, enhance virile qualities, and contribute to an overall better sexual life (Maurya, 2022).
A recent study investigated the ameliorative effect of ethanolic fruit extracts of Perssea americana and Prunus dulcis on caffeine-induced testicular damage in male albino rats. The research involved 60 rats, which were divided into 12 groups. The results showed that rats treated with 400 mg/kg of Prunus dulcis or Persea americana fruit extract for 7, 14, and 21 days had significantly higher plasma luteinizing hormone levels compared to the negative control group. Additionally, there was evidence of regeneration of damaged testicular tissues in the extract-treated rats. The study suggests that P. dulcis and P. americana fruit extracts have a testiculo-curative effect on caffeine-induced testicular damage in rats (Otobo et al., 2022).
1.7. Aromatase inhibitors
Aromatase is an enzyme that belongs to a large group of enzymes known as P450, and it has the code name CYP19A1 (Bulun et al., 2004). Aromatase is found in different parts of the human body, such as the ovaries, testes, fat, placenta, liver, brain and bone (Brooks et al., 2020; Kalicińska et al., 2020). Aromatase is important for making estrogen, which is a female sex hormone. In the testes, aromatase is made by different types of cells, such as Leydig cells, Sertoli cells and germ cells. Germ cells are the cells that become sperm cells. Aromatase is also made in the epididymis, which is a tube that stores and transports sperm cells (Carreau et al., 2010).
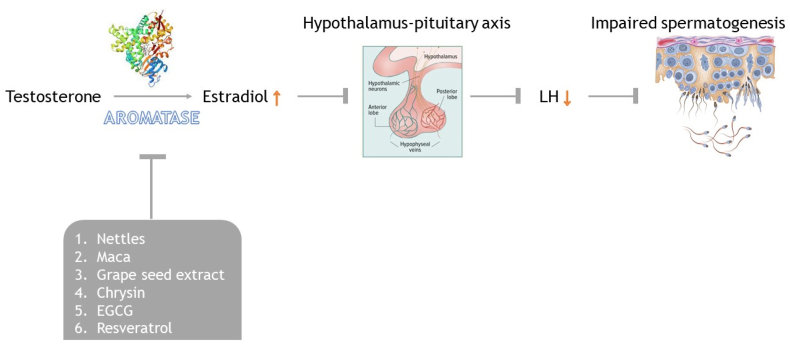
Aromatase inhibitors are compounds that can inhibit the activity of the aromatase enzyme, which is responsible for the conversion of androgens (testosterone) to estrogens (E2). Inhibiting this enzyme can be a potential treatment for male infertility, as it can help to increase testosterone levels and improve semen parameters (Fig. 7) (de Ronde and de Jong, 2011). There are both natural and pharmaceutical aromatase inhibitors that can be used for this purpose (Korani, 2023). Here are some natural inhibitors of the aromatase enzyme that have been studied for their potential to treat male infertility.
-
1.
Nettle root or nettle leaves are often used to make prostate medication. Nettles contain compounds that act as natural estrogen blockers. Both the plant extract and the isolated polysaccharides target mainly aromatase but it also seems that sex hormone binding globulin, epidermal growth factor and prostate steroid membrane receptors are involved in the anti-prostatic effect (Chrubasik et al., 2007; Khalafi-Kheydani et al., 2022).
-
2.
Maca is a cruciferous plant that originates in Peru. Maca (Lepidium meyenii) extract is derived from the plant roots is rich in of flavonolignans as well as glucosinolates that have been demonstrated to block aromatase activity and the prostate tumorigenesis (Bai et al., 2015; Soundararajan and Kim, 2018).
-
3.
Grape seed extract has been shown to act as an aromatase inhibitor, or estrogen blocker, in both men suffering from ED as well as postmenopausal women at high risk for breast cancer. The main active ingredient for this bioactivity is procyanidin dimers (Kijima et al., 2006).
-
4.
Chrysin is a flavonoid present in passionflower, honey, and bee propolis. The enzyme-inhibiting activity of chrysin is well-documented and, thus, has been utilized not only for fertility treatment but also for hormone-dependent breast cancer and as an adjuvant therapy for estrogen-dependent diseases (Balam et al., 2020).
-
5.
Epigallocatechin gallate (EGCG), the active compound of green tea, has been assessed in an epidemiological study inferring aromatase inhibition through changes in estradiol levels. Results demonstrated that estradiol levels were lower in people taking higher amounts of EGCG [147]. However, EGCG, along with other constituents of green tea, had no significant modulation of circulating testosterone (Samavat et al., 2019).
-
6.
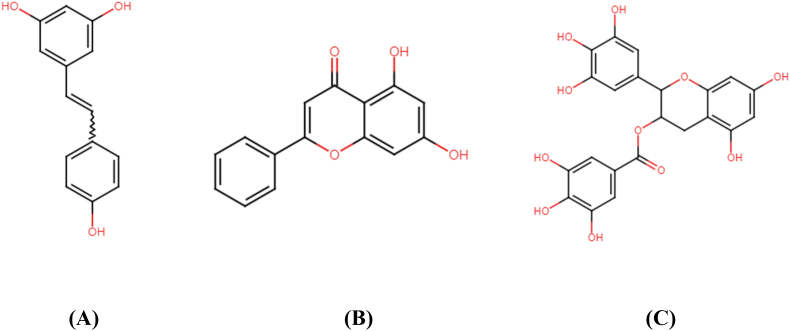
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a naturally occurring polyphenol present in many fruits and foods such as peanuts, blueberries and cranberries, as well as the grapes skin, displayed significant suppression of aromatase activity in adipocytes in vitro. Additionally, it also serves as a key inhibitor for steroid metabolism (Chottanapund et al., 2014; Poschner et al., 2018). The chemical structures of resveratrol, chyrsin and EGCG are provided in Fig. 8.
-
7.
Probiotics can indirectly result in lower aromatase activity. Accumulating evidence show that probiotics intake is correlated with weight loss and reduced obesity. In turn, reduced obesity reduces the biosynthesis and secretion of aromatase which would result in sustained testosterone levels and actions (Colleluori et al., 2020; Park et al., 2023).
Fig. 7.
Role of aromatase and its inhibition of male spermatogensis.
Fig. 8.
Chemical structure of resveratrol (A), chrysin (B) and EGCG (C).
In addition to the increased testosterone production, secretion and alleviating its degradation by aromatase, scavenging the ROS through the intake of antioxidants is also beneficial in this context. Food-rich antioxidants have been shown to have a positive impact on ED by enhancing vascular health and reducing oxidative stress. These antioxidants, found in fruits, vegetables, and other dietary sources, help protect blood vessels from damage and promote the production of nitric oxide, a key molecule in achieving and maintaining an erection. Studies suggest that a diet rich in antioxidants can lead to improved erectile function. For example, a study found that a diet high in antioxidants was associated with a reduced risk of ED. Also, other reports showed that increased consumption of antioxidants led to improved endothelial function, which is crucial for healthy blood flow and erectile function. These findings highlight the potential of dietary antioxidants in mitigating ED (Su et al., 2022; Q. Zhang et al., 2011). Food ingredients such as cocoa, green tea and gloves are among the highest in antioxidants. This positive action of antioxidants is attributable to the overwhelming spread of ROS both systemically and locally (in the male reproductive system context) (Sansone et al., 2017).
2. Conclusion and future remarks
In conclusion, erectile dysfunction and male infertility are major health problems that affect millions of men worldwide. The current pharmacological treatments are not satisfactory, as they have serious side effects, high costs and drug interactions. Therefore, many men seek natural and nutritional remedies that can enhance their sexual performance and reproductive health. These remedies are based on the consumption of aphrodisiac foods that have proven benefits for libido and fertility. This paper reviewed some of these superfoods that increase testosterone synthesis, secretion and spermatogenesis, their active constituents and their mechanisms of action. Also, the most prominent aromatase inhibitors were also listed. The future researches should be directed to the involvement of these foods in clinical trials and deeply unveil their mechanism of action. Moreover, the synergistic actions of antioxidants and testosterone boosters or aromatase blockers should be examined in vivo setup.
Funding
No funding was acquired for conducting the current study.
CRediT authorship contribution statement
Haitham Al-Madhagi: Conceptualization, design, drafting, manuscript and, Formal analysis. Abd Alraouf Tarabishi: illustration, Visualization, and critical review.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Quancai Sun
Data availability
No data was used for the research described in the article.
References
- Abbott K., Burrows T.L., Acharya S., Thota R.N., Garg M.L. Dietary supplementation with docosahexaenoic acid rich fish oil increases circulating levels of testosterone in overweight and obese men. Prostagl. Leukot. Essent. Fat. Acids. 2020;163 doi: 10.1016/j.plefa.2020.102204. [DOI] [PubMed] [Google Scholar]
- Adeyemi D.H., Odetayo A.F., Hamed M.A., Akhigbe R.E. Impact of COVID 19 on erectile function. Aging Male. 2022;25(1):202–216. doi: 10.1080/13685538.2022.2104833. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Nandipati K.C., Sharma R.K., Zippe C.D., Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J. Androl. 2006;27(3):335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- Akhuetie J.O., Oyeyemi M.O., Oloye A.A. Studies on crude powder and ethanolic extract of Citrullus lanatus seeds: phytochemical analysis, effects on haemogram and some reproductive characteristics of male albino rats. Niger. Vet. J. 2018;39(2):133. doi: 10.4314/nvj.v39i2.5. [DOI] [Google Scholar]
- Akinyemi A.J., Adedara I.A., Thome G.R., Morsch V.M., Rovani M.T., Mujica L.K.S., Duarte T., Duarte M., Oboh G., Schetinger M.R.C. Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicol Rep. 2015;2:1357–1366. doi: 10.1016/j.toxrep.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram M., Ali S.A., Kaul G. Probiotic and prebiotic supplementation ameliorates chronic restraint stress-induced male reproductive dysfunction. Food Funct. 2023;14(18):8558–8574. doi: 10.1039/D3FO03153E. [DOI] [PubMed] [Google Scholar]
- Al Hroob A.M., Abukhalil M.H., Alghonmeen R.D., Mahmoud A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2018;106:381–389. doi: 10.1016/j.biopha.2018.06.148. [DOI] [PubMed] [Google Scholar]
- Alasalvar C., Salvadó J.-S., Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020;314 doi: 10.1016/j.foodchem.2020.126192. [DOI] [PubMed] [Google Scholar]
- Ali M., Farag B., Hussein H., Fahmy S. Sexual activity, semen characteristics and testosterone levels in mature male rabbits treated with hormonal and non-hormonal preparations. Egypt. J. Anim. Prod. 2022;59(2):79–85. doi: 10.21608/ejap.2022.131455.1039. [DOI] [Google Scholar]
- Andric S.A., Janjic M.M., Stojkov N.J., Kostic T.S. Testosterone-induced modulation of nitric oxide-cGMP signaling pathway and androgenesis in the rat Leydig cells. Biol. Reprod. 2010;83(3):434–442. doi: 10.1095/biolreprod.110.083626. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Argiolas F.M., Argiolas G., Melis M.R. Erectile dysfunction: treatments, advances and new therapeutic strategies. Brain Sci. 2023;13(5) doi: 10.3390/brainsci13050802. Article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha K.K., Remya Kumari K.R., Ashok Kumar K., Chatterjee N.S., Anandan R., Mathew S. Sequence determination of an antioxidant peptide obtained by enzymatic hydrolysis of oyster Crassostrea madrasensis (preston) Int. J. Pept. Res. Therapeut. 2016;22(3):421–433. doi: 10.1007/s10989-016-9521-0. [DOI] [Google Scholar]
- Astuti P., Airin C.M., Sarmin S., Nururrozi A., Harimurti S. Effect of shell as natural testosterone boosters in Sprague Dawley rats. Vet. World. 2019;12(10):1677–1681. doi: 10.14202/vetworld.2019.1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atangwho I.J., Ibeneme C.E., Egbung G.E., Ibeneme E., Eno M.A., Nwankpa P. Effect of long-term feeding of the Obudu natural honey and table sugar-sweetened diets on obesity and pro-inflammatory biomarkers in rats. BMC Nutrition. 2020;6:3. doi: 10.1186/s40795-019-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytaç Mckinlay, Krane The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- Bai N, He K, Roller M, Lai CS, Bai L, Pan MH. Flavonolignans and other constituents from Lepidium meyenii with activities in anti-inflammation and human cancer cell lines. Journal of Agricultural and Food Chemistry. 2015 Mar 11;63(9) doi: 10.1021/acs.jafc.5b00219. 2458–63. Epub 2015 Feb 25. PMID: 25667964. [DOI] [PubMed] [Google Scholar]
- Balam F.H., Ahmadi Z.S., Ghorbani A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): a systematic review. Heliyon. 2020;6(3) doi: 10.1016/j.heliyon.2020.e03557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihani S.A. Ginger and testosterone. Biomolecules. 2018;8(4) doi: 10.3390/biom8040119. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihani S.A. Mechanisms of honey on testosterone levels. Heliyon. 2019;5(7) doi: 10.1016/j.heliyon.2019.e02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbonetti A., Cinque B., Vassallo M.R.C., Mineo S., Francavilla S., Cifone M.G., Francavilla F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011;95(8):2485–2488. doi: 10.1016/j.fertnstert.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Hatfield D.L., Hoffman J.R., Kraemer W.J., Labotz M., Phillips S.M., Ratamess N.A. Anabolic-androgenic steroid use in sports, health, and society. Med. Sci. Sports Exerc. 2021;53(8):1778–1794. doi: 10.1249/MSS.0000000000002670. [DOI] [PubMed] [Google Scholar]
- Brenowitz E.A. Testosterone and brain-derived neurotrophic factor interactions in the avian song control system. Neuroscience. 2013;239:115–123. doi: 10.1016/j.neuroscience.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D.C., Coon V J.S., Ercan C.M., Xu X., Dong H., Levine J.E., Bulun S.E., Zhao H. Brain aromatase and the regulation of sexual activity in male mice. Endocrinology. 2020;161(10) doi: 10.1210/endocr/bqaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun S.E., Takayama K., Suzuki T., Sasano H., Yilmaz B., Sebastian S. Organization of the human aromatase p450 (CYP19) gene. Semin. Reprod. Med. 2004;22(1):5–9. doi: 10.1055/s-2004-823022. [DOI] [PubMed] [Google Scholar]
- Bundhun P.K., Janoo G., Bhurtu A., Teeluck A.R., Soogund M.Z.S., Pursun M., Huang F. Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Publ. Health. 2019;19(1):36. doi: 10.1186/s12889-018-6319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A.L., Nehra A., Breau R.H., Culkin D.J., Faraday M.M., Hakim L.S., Heidelbaugh J., Khera M., McVary K.T., Miner M.M., Nelson C.J., Sadeghi-Nejad H., Seftel A.D., Shindel A.W. Erectile dysfunction: AUA guideline. J. Urol. 2018;200(3):633–641. doi: 10.1016/j.juro.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Cao W., Aghajanian H.K., Haig-Ladewig L.A., Gerton G.L. Sorbitol can fuel mouse sperm motility and protein tyrosine phosphorylation via sorbitol dehydrogenase. Biol. Reprod. 2009;80(1):124–133. doi: 10.1095/biolreprod.108.068882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta N., Feltrin G., Tarantini G., D'Agostino C., Tona F., Schipilliti M., Selice R., Minicuci N., Gerosa G., Foresta C. Erectile dysfunction, penile atherosclerosis, and coronary artery vasculopathy in heart transplant recipients. J. Sex. Med. 2013;10(9):2295–2302. doi: 10.1111/jsm.12233. [DOI] [PubMed] [Google Scholar]
- Carreau S., Wolczynski S., Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 2010;365(1546):1571–1579. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebioglu H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021;203(3):1221–1229. doi: 10.1007/s00203-021-02200-1. [DOI] [PubMed] [Google Scholar]
- Cerdá B., Marhuenda J., Arcusa R., Villaño D., Ballester P., Zafrilla P., Cerdá B., Marhuenda J., Arcusa R., Villaño D., Ballester P., Zafrilla P. Current Topics in Functional Food. IntechOpen; 2022. Ginger in the prevention of cardiovascular diseases. [DOI] [Google Scholar]
- Cha K.-M., Kopalli S.R., Han S.Y., Lee S.-H., Jeong M.-S., Cho J.Y., Han C.-G., Lee S.-H., Kim S.-N., Kim J.-C., Kim S.-K. Korean red ginseng attenuates doxorubicin-induced testicular dysfunction in rats by modulating inflammatory, oxidative, and autophagy responses. J. Funct.Foods. 2018;40:736–743. doi: 10.1016/j.jff.2017.12.008. [DOI] [Google Scholar]
- Chen L., Shi G., Huang D., Li Y., Ma C., Shi M., Su B., Shi G. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Chen S.-Y., Lee Y.-R., Hsieh M.-C., Omar H.A., Teng Y.-N., Lin C.-Y., Hung J.-H. Enhancing the anticancer activity of antrodia cinnamomea in hepatocellular carcinoma cells via cocultivation with ginger: the impact on cancer cell survival pathways. Front. Pharmacol. 2018;9:780. doi: 10.3389/fphar.2018.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.D., Park C.W., Jang J., Kim S.H., Jeon H.Y., Kim W.G., Lee S.J., Chung W.S. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled, double-blind clinical study. Int. J. Impot. Res. 2013;25(2):45–50. doi: 10.1038/ijir.2012.45. [DOI] [PubMed] [Google Scholar]
- Chottanapund S., Van Duursen M.B.M., Navasumrit P., Hunsonti P., Timtavorn S., Ruchirawat M., Van den Berg M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. Vitro: An International Journal Published in Association with BIBRA. 2014;28(7):1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Choudhary B.R. 2015. Phytochemicals and Antioxidants in Watermelon (Citrullus lanatus) Genotypes under Hot Arid Region.http://krishi.icar.gov.in/jspui/handle/123456789/2257 [Google Scholar]
- Chrubasik J.E., Roufogalis B.D., Wagner H., Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2007;14(7–8):568–579. doi: 10.1016/j.phymed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Colleluori G., Chen R., Turin C.G., Vigevano F., Qualls C., Johnson B., Mediwala S., Villareal D.T., Armamento-Villareal R. Aromatase inhibitors plus weight loss improves the hormonal profile of obese hypogonadal men without causing major side effects. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.K., Wu G., Perkins-Veazie P., Spears K., Claypool P.L., Baker R.A., Clevidence B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition. 2007;23(3):261–266. doi: 10.1016/j.nut.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Corbett G., Crosby D., McAuliffe F. Probiotic therapy in couples with infertility: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;256:95–100. doi: 10.1016/j.ejogrb.2020.10.054. [DOI] [PubMed] [Google Scholar]
- da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Damilare R., Rotdelmwa M. Impact of watermelon (citrallus lanatus) on male fertility. JBRA Assisted Reproduction. 2022 doi: 10.5935/1518-0557.20220075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daramola O.O., Oyeyemi W.A., Beka F.U., Ofutet E.A. Protective effects of aqueous extract of Citrullus lanatus fruit on reproductive functions and antioxidant activities in arsenic-treated male wistar rats. Afr. J. Biomed. Res. 2018;21(1) doi: 10.4314/ajbr.v21i1. Article 1. [DOI] [Google Scholar]
- Dardmeh F., Alipour H., Gazerani P., van der Horst G., Brandsborg E., Nielsen H.I. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ronde W., de Jong F.H. Aromatase inhibitors in men: effects and therapeutic options. Reprod. Biol. Endocrinol. : RB&E. 2011;9:93. doi: 10.1186/1477-7827-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeudis G., Mazzilli R., Tenuta M., Rossini G., Zamponi V., Olana S., Faggiano A., Pozzilli P., Isidori A.M., Gianfrilli D. Erectile dysfunction and diabetes: a melting pot of circumstances and treatments. Diabetes Metabol. Res. Rev. 2022;38(2):e3494. doi: 10.1002/dmrr.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid B.G., Mosli H., Hasan A., El-Bassossy H.M. Ginger ingredients alleviate diabetic prostatic complications: effect on oxidative stress and fibrosis. Evid. base Compl. Alternative Med.: eCAM. 2017;2017 doi: 10.1155/2017/6090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena B.W., Zachary M., Haritha P., Graham B.A., Wayne H.J.G. Current status of intracavernosal injection therapy in erectile dysfunction. Expet Opin. Pharmacother. 2023;24(8):925–933. doi: 10.1080/14656566.2023.2204189. [DOI] [PubMed] [Google Scholar]
- Eliopoulos C. Ginger: more than a great spice. Director. 2007;15(1):46–47. [PubMed] [Google Scholar]
- Fainberg J., Kashanian J.A. Recent advances in understanding and managing male infertility. F1000Research. 2019;8 doi: 10.12688/f1000research.17076.1. F1000 Faculty Rev-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhri S., Patra J.K., Das S.K., Das G., Majnooni M.B., Farzaei M.H. Ginger and heart health: from mechanisms to therapeutics. Curr. Mol. Pharmacol. 2021;14(6):943–959. doi: 10.2174/1874467213666201209105005. [DOI] [PubMed] [Google Scholar]
- Falsig A.-M.L., Gleerup C.S., Knudsen U.B. The influence of omega-3 fatty acids on semen quality markers: a systematic PRISMA review. Andrology. 2019;7(6):794–803. doi: 10.1111/andr.12649. [DOI] [PubMed] [Google Scholar]
- Fittler A., Paczolai P., Ashraf A.R., Pourhashemi A., Iványi P. Prevalence of poisoned google search results of erectile dysfunction medications redirecting to illegal internet pharmacies: data analysis study. J. Med. Internet Res. 2022;24(11) doi: 10.2196/38957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford N.A., Spagnuolo P., Kraft J., Bauer E. Nutritional composition of hass avocado pulp. Foods. 2023;12(13):2516. doi: 10.3390/foods12132516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franasiak J.M., Scott R.T. Reproductive tract microbiome in assisted reproductive technologies. Fertil. Steril. 2015;104(6):1364–1371. doi: 10.1016/j.fertnstert.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Ganjkhani M., Nourozi S., Bigonah R., Rostami A., Shokri S. Ameliorating impacts of ginseng on the apoptosis of spermatogenic cells and sperm quality in temporal lobe epilepsy rat model treated with valproate. Andrologia. 2019;51(9) doi: 10.1111/and.13348. [DOI] [PubMed] [Google Scholar]
- Garcia-Aloy M., Hulshof P.J.M., Estruel-Amades S., Osté M.C.J., Lankinen M., Geleijnse J.M., de Goede J., Ulaszewska M., Mattivi F., Bakker S.J.L., Schwab U., Andres-Lacueva C. Biomarkers of food intake for nuts and vegetable oils: an extensive literature search. Genes & Nutrition. 2019;14:7. doi: 10.1186/s12263-019-0628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins A.J., Chavarro J.E. Diet and fertility: a review. Am. J. Obstet. Gynecol. 2018;218(4):379–389. doi: 10.1016/j.ajog.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbotolorun S.C., Salako A.A., Ogunlade B. Tiger nut: antidote for alcohol-induced testicular toxicity in male Sprague-Dawley rats. JBRA Assisted Reproduction. 2022;26(2):222–231. doi: 10.5935/1518-0557.20210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G., Manfredini M., Somaini L., Maremmani I., Leonardi C., Donnini C. Sexual dysfunction in men receiving methadone maintenance treatment: clinical history and psychobiological correlates. Eur. Addiction Res. 2015;22(3):163–175. doi: 10.1159/000441470. [DOI] [PubMed] [Google Scholar]
- Gervasi T., Barreca D., Laganà G., Mandalari G. Health benefits related to tree nut consumption and their bioactive compounds. Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115960. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharahdaghi N., Phillips B.E., Szewczyk N.J., Smith K., Wilkinson D.J., Atherton P.J. Links between testosterone, oestrogen, and the growth hormone/insulin-like growth factor Axis and resistance exercise muscle adaptations. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.621226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareib S.A., El-Bassossy H.M., Elberry A.A., Azhar A., Watson M.L., Banjar Z.M. 6-Gingerol alleviates exaggerated vasoconstriction in diabetic rat aorta through direct vasodilation and nitric oxide generation. Drug Des. Dev. Ther. 2015;9:6019–6026. doi: 10.2147/DDDT.S94346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghlissi Z., Atheymen R., Boujbiha M.A., Sahnoun Z., Makni Ayedi F., Zeghal K., El Feki A., Hakim A. Antioxidant and androgenic effects of dietary ginger on reproductive function of male diabetic rats. Int. J. Food Sci. Nutr. 2013;64(8):974–978. doi: 10.3109/09637486.2013.812618. [DOI] [PubMed] [Google Scholar]
- Gholami M., Abbaszadeh A., Khanipour Khayat Z., Anbari K., Baharvand P., Gharravi A.M. Honey improves spermatogenesis and hormone secretion in testicular ischaemia-reperfusion-induced injury in rats. Andrologia. 2018;50(1) doi: 10.1111/and.12804. [DOI] [PubMed] [Google Scholar]
- Goodstein T., Jenkins L.C. A narrative review on malleable and inflatable penile implants: choosing the right implant for the right patient. Int. J. Impot. Res. 2023;35(7):623–628. doi: 10.1038/s41443-023-00765-7. [DOI] [PubMed] [Google Scholar]
- Grabski B., Kasparek K., Koziara K., Mijas M. Erectile problems in polish straight, bisexual, and gay men: does sexual identity really matter? J. Sex. Res. 2023;60(4):473–483. doi: 10.1080/00224499.2022.2074952. [DOI] [PubMed] [Google Scholar]
- Greenberg D.R., Richardson M.T., Tijerina J.D., Bass M.B., Eisenberg M.L. The quality of systematic reviews and meta-analyses in erectile dysfunction treatment and management published in the sexual medicine literature. J. Sex. Med. 2019;16(3):394–401. doi: 10.1016/j.jsxm.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Hackett G., Kirby M., Edwards D., Jones T.H., Wylie K., Ossei-Gerning N., David J., Muneer A. British society for sexual medicine guidelines on adult testosterone deficiency, with statements for UK practice. J. Sex. Med. 2017;14(12):1504–1523. doi: 10.1016/j.jsxm.2017.10.067. [DOI] [PubMed] [Google Scholar]
- Hayden R.P., Flannigan R., Schlegel P.N. The role of lifestyle in male infertility: diet, physical activity, and body habitus. Curr. Urol. Rep. 2018;19(7):56. doi: 10.1007/s11934-018-0805-0. [DOI] [PubMed] [Google Scholar]
- Hohl A. Springer Nature; 2023. Testosterone: from Basic to Clinical Aspects. [Google Scholar]
- Holt R.R., Yim S.J., Shearer G.C., Hackman R.M., Djurica D., Newman J.W., Shindel A.W., Keen C.L. Effects of short-term walnut consumption on human microvascular function and its relationship to plasma epoxide content. J. Nutr. Biochem. 2015;26(12):1458–1466. doi: 10.1016/j.jnutbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Hsieh C.-H., Hsu G.-L., Chang S.-J., Yang S.S.-D., Liu S.-P., Hsieh J.-T. Surgical niche for the treatment of erectile dysfunction. Int. J. Urol. 2020;27(2):117–133. doi: 10.1111/iju.14157. [DOI] [PubMed] [Google Scholar]
- Huang C., Li B., Xu K., Liu D., Hu J., Yang Y., Nie H., Fan L., Zhu W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017;107(1):83–88.e2. doi: 10.1016/j.fertnstert.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Hudec S., Spacek M., Hutyra M., Moravec O., Taborsky M. Sexual activity and cardiovascular disease, erectile dysfunction as a predictor of ischemic heart disease. Cor Vasa. 2018;60(3):e296–e305. doi: 10.1016/j.crvasa.2017.08.006. [DOI] [Google Scholar]
- Ismail E.A., El-Sakka A.I. Innovative trends and perspectives for erectile dysfunction treatment: a systematic review. Arab Journal of Urology. 2016;14(2):84–93. doi: 10.1016/j.aju.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannini E.A. SM = SM: the interface of systems medicine and sexual medicine for facing non-communicable diseases in a gender-dependent manner. Sexual Medicine Reviews. 2017;5(3):349–364. doi: 10.1016/j.sxmr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Jensen T.K., Priskorn L., Holmboe S.A., Nassan F.L., Andersson A.-M., Dalgård C., Petersen J.H., Chavarro J.E., Jørgensen N. Associations of fish oil supplement use with testicular function in young men. JAMA Netw. Open. 2020;3(1) doi: 10.1001/jamanetworkopen.2019.19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q., Ma Y., Shi W., Wang J., Zhao R., Zhang H., Wu M., Liu W. Oyster oligopeptide improving cyclophosphamide-induced partial androgen deficiency of the aging male by promotion of testosterone synthesis. Geriatr. Gerontol. Int. 2021;21(2):268–275. doi: 10.1111/ggi.14129. [DOI] [PubMed] [Google Scholar]
- Kalicińska E., Wojtas K., Majda J., Zacharski M., Skiba J., Śliwowski J., Banasiak W., Ponikowski P., Jankowska E.A. Expression of sex steroid receptors and aromatase in adipose tissue in different body regions in men with coronary artery disease with and without ischemic systolic heart failure. Aging Male: The Official Journal of the International Society for the Study of the Aging Male. 2020;23(2):141–153. doi: 10.1080/13685538.2018.1494144. [DOI] [PubMed] [Google Scholar]
- Kamtchouing P., Mbongue Fandio G.Y., Dimo T., Jatsa H.B. Evaluation of androgenic activity of Zingiber officinale and Pentadiplandra brazzeana in male rats. Asian J. Androl. 2002;4(4):299–301. [PubMed] [Google Scholar]
- Kara H., Orem A., Yulug E., Yucesan F.B., Kerimoglu G., Yaman S.O., Bodur A., Turedi S., Alasalvar C. Hazelnut consumption improves testicular antioxidant function and semen quality in young and old male rats. Food Chem. 2019;294:1–8. doi: 10.1016/j.foodchem.2019.04.087. [DOI] [PubMed] [Google Scholar]
- Khalafi-Kheydani A., Mahmoodi H., Sadat Z., Azizi-Fini I. The effect of nettle root extract on urinary problems in older men with benign prostatic hyperplasia: a randomized clinical trial. J. Herb. Med. 2022;34 doi: 10.1016/j.hermed.2022.100568. [DOI] [Google Scholar]
- Khan R.U., Naz S., Abudabos A.M. Towards a better understanding of the therapeutic applications and corresponding mechanisms of action of honey. Environ. Sci. Pollut. Control Ser. 2017;24(36):27755–27766. doi: 10.1007/s11356-017-0567-0. [DOI] [PubMed] [Google Scholar]
- Kijima I., Phung S., Hur G., Kwok S.-L., Chen S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006;66(11):5960–5967. doi: 10.1158/0008-5472.CAN-06-0053. [DOI] [PubMed] [Google Scholar]
- Kim C.-Y., Seo Y., Lee C., Park G.H., Jang J.-H. Neuroprotective effect and molecular mechanism of [6]-Gingerol against scopolamine-induced amnesia in C57BL/6 mice. Evid. base Compl. Alternative Med.: eCAM. 2018;2018 doi: 10.1155/2018/8941564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn T.P., Rajanahally S., Hellstrom W.J.G., Hsieh T.-C., Raheem O.A. Global trends in prevalence, treatments, and costs of penile prosthesis for erectile dysfunction in men. European Urology Focus. 2022;8(3):803–813. doi: 10.1016/j.euf.2021.05.003. [DOI] [PubMed] [Google Scholar]
- Kolawole T., Adienbo O., Dapper V. Ameliorative effects of hydromethanolic extract of Citrullus lanatus (watermelon) rind on semen parameters, reproductive hormones and testicular oxidative status following nicotine administration in male Wistar rats. Niger. J. Physiol. Sci. 2019;34(1):1. doi: 10.4314/njps.v34i1.12. [DOI] [PubMed] [Google Scholar]
- Kopalli S.R., Cha K.-M., Hwang S.-Y., Jeong M.-S., Kim S.-K. Korean Red Ginseng (Panax ginseng Meyer) with enriched Rg3 ameliorates chronic intermittent heat stress-induced testicular damage in rats via multifunctional approach. Journal of Ginseng Research. 2019;43(1):135–142. doi: 10.1016/j.jgr.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula S., Kopalli S.R., Kang H.H., Kim S.-K. Benefits of panax ginseng on male reproductive systems: a comprehensive review. Food Supplements and Biomaterials for Health. 2023;3(4) doi: 10.52361/fsbh.2023.3.e32. [DOI] [Google Scholar]
- Korani M. Aromatase inhibitors in male: a literature review. Medicina Clínica Práctica. 2023;6(1) doi: 10.1016/j.mcpsp.2022.100356. [DOI] [Google Scholar]
- Kraemer W.J., Ratamess N.A., Hymer W.C., Nindl B.C., Fragala M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020;11:33. doi: 10.3389/fendo.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer W.J., Ratamess N.A., Nindl B.C. Recovery responses of testosterone, growth hormone, and IGF-1 after resistance exercise. J. Appl. Physiol. 2017;122(3):549–558. doi: 10.1152/japplphysiol.00599.2016. [DOI] [PubMed] [Google Scholar]
- Laddha A.P., Adki K.M., Gaikwad A.B., Kulkarni Y.A. In: Nuts and Seeds in Health and Disease Prevention. second ed. Preedy V.R., Watson R.R., editors. Academic Press; 2020. Chapter 32—beneficial effects of nuts from India in cardiovascular disorders; pp. 453–469. [DOI] [Google Scholar]
- Lee H.W., Lee M.S., Kim T.-H., Alraek T., Zaslawski C., Kim J.W., Moon D.G. Ginseng for erectile dysfunction: a Cochrane systematic review. The World Journal of Men’s Health. 2022;40(2):264–269. doi: 10.5534/wjmh.210071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Choi K.-H., Cha K.-M., Hwang S.-Y., Park U.-K., Jeong M.-S., Hong J.-Y., Han C.-K., In G., Kopalli S.R., Kim S.-K. Protective effects of Korean Red Ginseng against sub-acute immobilization stress-induced testicular damage in experimental rats. Journal of Ginseng Research. 2019;43(1):125–134. doi: 10.1016/j.jgr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Légaré C., Droit A., Fournier F., Bourassa S., Force A., Cloutier F., Tremblay R., Sullivan R. Investigation of male infertility using quantitative comparative proteomics. J. Proteome Res. 2014;13(12):5403–5414. doi: 10.1021/pr501031x. [DOI] [PubMed] [Google Scholar]
- Levine H., Jørgensen N., Martino-Andrade A., Mendiola J., Weksler-Derri D., Mindlis I., Pinotti R., Swan S.H. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum. Reprod. Update. 2017;23(6):646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Ren J., He L., Sun J., Liu P., Li Y. Combined effects of oligopeptides isolated from panax ginseng C.A. Meyer and Ostrea gigas thunberg on sexual function in male mice. Int. J. Environ. Res. Publ. Health. 2021;18(5):2349. doi: 10.3390/ijerph18052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Xing Y., Sultan A.H., Raeeszadeh M., Akbari A., Liu H. Ginger (zingiber officinale roscoe) improves ethanol-induced reproductive dysfunction by enhancing steroidogenesis and inhibiting oxidative stress and inflammation. Braz. Arch. Biol. Technol. 2022;64 doi: 10.1590/1678-4324-2021210035. [DOI] [Google Scholar]
- Liao X., Qiu S., Bao Y., Wang W., Yang L., Wei Q. Comparative efficacy and safety of phosphodiesterase type 5 inhibitors for erectile dysfunction in diabetic men: a Bayesian network meta-analysis of randomized controlled trials. World J. Urol. 2019;37(6):1061–1074. doi: 10.1007/s00345-018-2583-1. [DOI] [PubMed] [Google Scholar]
- Lin Y.-F., Zhu J.-F., Chen Y.-D., Sheng J.-L., He J.-J., Zhang S.-Y., Jin X.-Q. [Effect of ginger-separated moxibustion on fatigue, sleep quality and depression in patients with chronic fatigue syndrome: a randomized controlled trial] Zhongguo Zhen Jiu = Chinese Acupuncture & Moxibustion. 2020;40(8):816–820. doi: 10.13703/j.0255-2930.20190722-k0001. [DOI] [PubMed] [Google Scholar]
- Ma C., Su H., Li H. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.627891. https://www.frontiersin.org/articles/10.3389/fonc.2020.627891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann U., Shiff B., Patel P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020;30(3):296–301. doi: 10.1097/MOU.0000000000000745. [DOI] [PubMed] [Google Scholar]
- Mattiuzzi C., Lippi G., Henry B.M. Estimating the global prevalence of erectile dysfunction during the COVID-19 pandemic. Aging Male. 2022;25(1):255–256. doi: 10.1080/13685538.2022.2120981. [DOI] [PubMed] [Google Scholar]
- Maurya N.K. Recent Advances in Male Reproductive System. IntechOpen; 2022. Libido boosting functional foods. [DOI] [Google Scholar]
- Melis M.R., Argiolas A. Erectile function and sexual behavior: a review of the role of nitric oxide in the central nervous system. Biomolecules. 2021;11(12):1866. doi: 10.3390/biom11121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Liao W., Kang M., Jia Y., Wang Q., Duan S., Xiao S., Cao Y., Ji H. Anti-fatigue and anti-oxidant activities of oyster (Ostrea rivularis) hydrolysate prepared by compound protease. Food Funct. 2018;9(12):6577–6585. doi: 10.1039/C8FO01879K. [DOI] [PubMed] [Google Scholar]
- Mirone V., Fusco F., Cirillo L., Napolitano L. In: Practical Clinical Andrology. Bettocchi C., Busetto G.M., Carrieri G., Cormio L., editors. Springer International Publishing; 2023. Erectile dysfunction: from pathophysiology to clinical assessment; pp. 25–33. [DOI] [Google Scholar]
- Mohamed M., Sulaiman S.A., Jaafar H., Sirajudeen K.N.S. Effect of different doses of Malaysian honey on reproductive parameters in adult male rats. Andrologia. 2012;44(s1):182–186. doi: 10.1111/j.1439-0272.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- Mollaioli D., Ciocca G., Limoncin E., Di Sante S., Gravina G.L., Carosa E., Lenzi A., Jannini E.A.F. Lifestyles and sexuality in men and women: the gender perspective in sexual medicine. Reprod. Biol. Endocrinol. 2020;18(1):10. doi: 10.1186/s12958-019-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C., Marques P.I., Cavadas B., Damião I., Almeida V., Barros N., Barros A., Carvalho F., Gomes S., Seixas S. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am. J. Reprod. Immunol. 2018;79(6) doi: 10.1111/aji.12838. [DOI] [PubMed] [Google Scholar]
- Mulhall J.P., Trost L.W., Brannigan R.E., Kurtz E.G., Redmon J.B., Chiles K.A., Lightner D.J., Miner M.M., Murad M.H., Nelson C.J., Platz E.A., Ramanathan L.V., Lewis R.W. Evaluation and management of testosterone deficiency: AUA guideline. J. Urol. 2018;200(2):423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- Nachvak S.M., Soleimani D., Rahimi M., Azizi A., Moradinazar M., Rouhani M.H., Halashi B., Abbasi A., Miryan M. Ginger as an anticolorectal cancer spice: a systematic review of in vitro to clinical evidence. Food Sci. Nutr. 2022;11(2):651–660. doi: 10.1002/fsn3.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A., Bhatt A.S. Microbes and microbiomes in 2020 and beyond. Nat. Commun. 2020;11(1):4988. doi: 10.1038/s41467-020-18850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neglo D., Tettey C.O., Essuman E.K., Kortei N.K., Boakye A.A., Hunkpe G., Amarh F., Kwashie P., Devi W.S. Comparative antioxidant and antimicrobial activities of the peels, rind, pulp and seeds of watermelon (Citrullus lanatus) fruit. Scientific African. 2021;11 doi: 10.1016/j.sciaf.2020.e00582. [DOI] [Google Scholar]
- Neto F.T.L., Bach P.V., Najari B.B., Li P.S., Goldstein M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Nimbi F.M., Tripodi F., Rossi R., Navarro-Cremades F., Simonelli C. Male sexual desire: an overview of biological, psychological, sexual, relational, and cultural factors influencing desire. Sexual Medicine Reviews. 2020;8(1):59–91. doi: 10.1016/j.sxmr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Odo R., Uchendu C., Okeke S. Protective effects of Citrullus lanatus seed ethanol extract on aluminum chloride-induced testosterone, testicular and hematological changes in an experimental male rat model. Vet. Res. Forum. 2021;12(1) doi: 10.30466/vrf.2020.104327.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabiyi A.A., Ajayi K. Diet, herbs and erectile function: a good friendship. Andrologia. 2022;54(6) doi: 10.1111/and.14424. [DOI] [PubMed] [Google Scholar]
- Otobo M.B., Akaninwor J.O., Amadi B.A., Wellington E.O. Ameliorative effect of ethanolic fruit extract of Perssea americana and Prunus dulcis on caffein-testicular damage in male wistar albino rats. Int. J. Biochem. Res. Rev. 2022:35–49. doi: 10.9734/ijbcrr/2022/v31i630331. [DOI] [Google Scholar]
- Pacifici L., Santacroce L., Dipalma G., Haxhirexha K., Topi S., Cantore S., Altini V., Pacifici A., Vito D.D., Pettini F., Cascella G., Saini R., Scacco S., Ballini A., Inchingolo F. Gender medicine: the impact of probiotics on male patients. La Clinica Terapeutica. 2021;172(1) doi: 10.7417/CT.2021.2274. Article 1. [DOI] [PubMed] [Google Scholar]
- Pang K.H., Muneer A., Alnajjar H.M. A systematic review of penile prosthesis insertion in patients with spinal cord injury. Sexual Medicine Reviews. 2022;10(3):468–477. doi: 10.1016/j.sxmr.2022.01.004. [DOI] [PubMed] [Google Scholar]
- Park S.-J., Sharma A., Lee H.-J. Postbiotics against obesity: perception and overview based on pre-clinical and clinical studies. Int. J. Mol. Sci. 2023;24(7):6414. doi: 10.3390/ijms24076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović-Oggiano G., Debeljak-Martačić J., Ranković S., Pokimica B., Mirić A., Glibetić M., Popović T. The effect of walnut consumption on n-3 fatty acid profile of healthy people living in a non-mediterranean west balkan country, a small scale randomized study. Nutrients. 2020;12(1):192. doi: 10.3390/nu12010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilehvari S., Masoumi S.Z., Bahar T.G., Kazemi F., Moradkhani S., Maleki P. Effect of carob and ginseng supplements on semen analysis parameters, sexual function, and sex hormones in infertile men: double-blind, randomized controlled trial study. Iran. J. Nurs. Midwifery Res. 2024;29(1):113. doi: 10.4103/ijnmr.ijnmr_460_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzol D., Xiao T., Yang L., Demurtas J., McDermott D., Garolla A., Nardelotto A., Grabovac I., Soysal P., Kazancioglu R.T., Veronese N., Smith L. Prevalence of erectile dysfunction in patients with chronic kidney disease: a systematic review and meta-analysis. Int. J. Impot. Res. 2021;33(5) doi: 10.1038/s41443-020-0295-8. Article 5. [DOI] [PubMed] [Google Scholar]
- Poschner S., Maier-Salamon A., Zehl M., Wackerlig J., Dobusch D., Meshcheryakova A., Mechtcheriakova D., Thalhammer T., Pachmann B., Jäger W. Resveratrol inhibits key steps of steroid metabolism in a human estrogen-receptor positive breast cancer model: impact on cellular proliferation. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00742. https://www.frontiersin.org/articles/10.3389/fphar.2018.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratama A.P.I., Susilowati S., Maslachah L., Ratnani H., Suprayogi T.W. THE EFFECT OF WATERMELON (Citrullus lanatus) RIND ETHANOLIC EXTRACT ON THE NUMBER OF LEYDIG, SERTOLI, AND SPERMATOGENIC CELLS OF RAT (Rattus novergicus) EXPOSED TO HEAT. Ovozoa Journal of Animal Reproduction. 2021;10(1):7. doi: 10.20473/ovz.v10i1.2021.7-11. [DOI] [Google Scholar]
- Punia Bangar S., Dunno K., Dhull S., Siroha A., Changan S., Maqsood S., Rusu A. Avocado seed discoveries: chemical composition, biological properties, and industrial food applications. Food Chem. X. 2022;16 doi: 10.1016/j.fochx.2022.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raciti L., Formica C., Raciti G., Quartarone A., Calabrò R.S. Gender and neurosteroids: implications for brain function, neuroplasticity and rehabilitation. Int. J. Mol. Sci. 2023;24(5):4758. doi: 10.3390/ijms24054758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimiyan-Heravan M., Roshangar L., Karimi P., Sefidgari-Abrasi S., Morshedi M., Saghafi-Asl M., Bavafa-Valenlia K. The potential therapeutic effects of Lactobacillus plantarum and inulin on serum and testicular reproductive markers in diabetic male rats. Diabetol. Metab. Syndrome. 2020;12:53. doi: 10.1186/s13098-020-00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoufi M.F., Farahani T.M., Jadidi E.S.M.S., Gardeshi T.M. Protective effects of ginger extract on oxidative stress and steroidogenesis-related genes in the ovary of streptozotocin-induced diabetic rats. International Journal of Medical Laboratory. 2023 doi: 10.18502/ijml.v10i3.13750. [DOI] [Google Scholar]
- Rastrelli G., Lotti F., Reisman Y., Sforza A., Maggi M., Corona G. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expet Rev. Endocrinol. Metabol. 2019;14(5):321–334. doi: 10.1080/17446651.2019.1657827. [DOI] [PubMed] [Google Scholar]
- Robbins W.A., Xun L., FitzGerald L.Z., Esguerra S., Henning S.M., Carpenter C.L. Walnuts improve semen quality in men consuming a western-style diet: randomized control dietary intervention Trial 1. Biol. Reprod. 2012;87(4):1–8. doi: 10.1095/biolreprod.112.101634. 101. [DOI] [PubMed] [Google Scholar]
- Romano L., Granata L., Fusco F., Napolitano L., Cerbone R., Priadko K., Sciorio C., Mirone V., Romano M. Sexual dysfunction in patients with chronic gastrointestinal and liver diseases: a neglected issue. Sexual Medicine Reviews. 2022;10(4):620–631. doi: 10.1016/j.sxmr.2021.02.002. [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., Choudhury B.P., Choudhury A.P., Pal M., Kosgi R., Mandal S.C. In: Herbal Biomolecules in Healthcare Applications. Mandal S.C., Nayak A.K., Dhara A.K., editors. Academic Press; 2022. Chapter 24 - herbal aphrodisiac biomolecules in the management of male reproductive and sexual problems: connecting nature with clinics; pp. 591–611. [DOI] [Google Scholar]
- Salas-Huetos A., Bulló M., Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum. Reprod. Update. 2017;23(4):371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A., James E.R., Salas-Salvadó J., Bulló M., Aston K.I., Carrell D.T., Jenkins T.G. Sperm DNA methylation changes after short-term nut supplementation in healthy men consuming a Western-style diet. Andrology. 2021;9(1):260–268. doi: 10.1111/andr.12911. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A., Moraleda R., Giardina S., Anton E., Blanco J., Salas-Salvadó J., Bulló M. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: a randomized controlled trial. Am. J. Clin. Nutr. 2018;108(5):953–962. doi: 10.1093/ajcn/nqy181. [DOI] [PubMed] [Google Scholar]
- Samavat H., Wu A.H., Ursin G., Torkelson C.J., Wang R., Yu M.C., Yee D., Kurzer M.S., Yuan J.-M. Green tea catechin extract supplementation does not influence circulating sex hormones and insulin-like growth factor Axis proteins in a randomized controlled trial of postmenopausal women at high risk of breast cancer. J. Nutr. 2019;149(4):619–627. doi: 10.1093/jn/nxy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone A., Jannini E.A., Romanelli F. In: Antioxidants in Andrology. Balercia G., Gandini L., Lenzi A., Lombardo F., editors. Springer International Publishing; 2017. Antioxidants in male sexual dysfunctions; pp. 71–79. [DOI] [Google Scholar]
- Schepici G., Contestabile V., Valeri A., Mazzon E. Ginger, a possible candidate for the treatment of dementias? Molecules. 2021;26(18):5700. doi: 10.3390/molecules26185700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M.A., Hamowieh A.R. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem. Toxicol.: An International Journal Published for the British Industrial Biological Research Association. 2010;48(10):2920–2924. doi: 10.1016/j.fct.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Kim JS. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules. 2018 Nov 15;23(11):2983. doi: 10.3390/molecules23112983. PMID: 30445746; PMCID: PMC6278308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhiser J., Jukic A.M.Z., McConnaughey D.R., Steiner A.Z. Omega-3 fatty acid supplementation and fecundability. Hum. Reprod. 2022;37(5):1037–1046. doi: 10.1093/humrep/deac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhiser J., Jukic A.M.Z., Steiner A.Z. Serum omega-3 and omega-6 fatty acid concentrations and natural fertility. Hum. Reprod. 2020;35(4):950–957. doi: 10.1093/humrep/dez305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Yang Z., Qu H., Luo C., Yuan G., Wu J., Jiao Y. Effect of antioxidants supplementation on erectile dysfunction: a systematic review and meta-analysis of randomized controlled trials. Sexual Medicine Reviews. 2022;10(4):754–763. doi: 10.1016/j.sxmr.2022.01.002. [DOI] [PubMed] [Google Scholar]
- Tabiri B. Watermelon seeds as food: nutrient composition, phytochemicals and antioxidant activity. Int. J. Nutr. Food Sci. 2016;5(2):139. doi: 10.11648/j.ijnfs.20160502.18. [DOI] [Google Scholar]
- Tett A., Pasolli E., Masetti G., Ercolini D., Segata N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021;19(9):585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Yu Z., Feng P., Ye Z., Li R., Liu J., Hu J., Kakade A., Liu P., Li X. Lactobacillus plantarum TW1-1 alleviates diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front. Cell. Infect. Microbiol. 2019;9 doi: 10.3389/fcimb.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs A.W., Landis J., Garrido N., Scott R.T., Hotaling J.M. Total motile sperm count trend over time: evaluation of semen analyses from 119,972 men from subfertile couples. Urology. 2019;132:109–116. doi: 10.1016/j.urology.2019.06.038. [DOI] [PubMed] [Google Scholar]
- Tsoutsos S., Kotsis V. Erectile dysfunction and its correlation with arterial hypertension and cardiovascular diseases. Journal of Atherosclerosis Prevention and Treatment. 2023;14(1):23–34. doi: 10.53590/japt.02.1044. [DOI] [Google Scholar]
- Wang C.-M., Wu B.-R., Xiang P., Xiao J., Hu X.-C. Management of male erectile dysfunction: from the past to the future. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xu A., Gong L., Chen Z., Zhang B., Li X. The microbiome, an important factor that is easily overlooked in male infertility. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.831272. https://www.frontiersin.org/articles/10.3389/fmicb.2022.831272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-Y., Yan Z., Liu S., Liu A.-Q., Sun J.-S. Effects of enzymatic hydrolysate of oyster on erectile function in male hemicastrated rats. Int. J. Pept. Res. Therapeut. 2020;26(4):2001–2007. doi: 10.1007/s10989-019-09989-y. [DOI] [Google Scholar]
- Wang M., Liu X., Chang G., Chen Y., An G., Yan L., Gao S., Xu Y., Cui Y., Dong J., Chen Y., Fan X., Hu Y., Song K., Zhu X., Gao Y., Yao Z., Bian S., Hou Y., et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell. 2018;23(4):599–614.e4. doi: 10.1016/j.stem.2018.08.007. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li W., He Y., Ren D., Kow F., Song L., Yu X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis) Food Chem. 2014;145:991–996. doi: 10.1016/j.foodchem.2013.08.099. [DOI] [PubMed] [Google Scholar]
- Younis N., Mahasneh A. Probiotics and the envisaged role in treating human infertility. Middle East Fertil. Soc. J. 2020;25(1):33. doi: 10.1186/s43043-020-00039-y. [DOI] [Google Scholar]
- Zaid S.S.M., Ruslee S.S., Mokhtar M.H. Protective roles of honey in reproductive health: a review. Molecules. 2021;26(11) doi: 10.3390/molecules26113322. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhao H., Zhang Y., Shen Y., Su H., Jin J., Jin Q., Wang X. Characterization of positional distribution of fatty acids and triacylglycerol molecular compositions of marine fish oils rich in omega-3 polyunsaturated fatty acids. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/3529682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu H., Yang Q., Li P., Wen Y., Han X., Li B., Jiang H., Li X. Genomic sequencing reveals the diversity of seminal bacteria and relationships to reproductive potential in boar sperm. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Feng Y., Li L., Ge W., Yu S., Hao Y., Shen W., Han X., Ma D., Yin S., Tian Y., Min L., Sun Z., Sun Q., Zhang H., Zhao Y. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut. 2021;70(1):222–225. doi: 10.1136/gutjnl-2020-320992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Radisavljevic Z.M., Siroky M.B., Azadzoi K.M. Dietary antioxidants improve arteriogenic erectile dysfunction. Int. J. Androl. 2011;34(3):225–235. doi: 10.1111/j.1365-2605.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yang C., Zhang M., Lu X., Cao W., Xie C., Li X., Wu J., Zhong C., Geng S. Protective effects of ginseng stem-leaf saponins on D-galactose-induced reproductive injury in male mice. Aging. 2021;13(6):8916–8928. doi: 10.18632/aging.202709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Hou B., Liu T., Wu Y., Wang Z. Probiotics improve polystyrene microplastics-induced male reproductive toxicity in mice by alleviating inflammatory response. Ecotoxicol. Environ. Saf. 2023;263 doi: 10.1016/j.ecoenv.2023.115248. [DOI] [PubMed] [Google Scholar]
- Zhu J., Chen H., Song Z., Wang X., Sun Z. Effects of ginger (zingiber officinale roscoe) on type 2 diabetes mellitus and components of the metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Evid. base Compl. Alternative Med.: eCAM. 2018;2018 doi: 10.1155/2018/5692962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair R., Aziz N. As smooth as honey—the historical use of honey as topical medication. JAMA Dermatology. 2015;151(10):1102. doi: 10.1001/jamadermatol.2015.1764. [DOI] [PubMed] [Google Scholar]
- Zuckerman J.M., McCammon K.A., Tisdale B.E., Colen L., Uroskie T., McAdams P., Jordan G.H. Outcome of penile revascularization for arteriogenic erectile dysfunction after pelvic fracture urethral injuries. Urology. 2012;80(6):1369–1374. doi: 10.1016/j.urology.2012.07.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.