Abstract
Background/Purpose:
Stereotactic body radiation therapy (SBRT) is standard for patients with inoperable early-stage NSCLC. We hypothesized that SBRT for sarcoma pulmonary metastases would achieve high rates of local control with acceptable toxicity and that patients with oligometastatic disease may achieve prolonged survival following SBRT.
Materials/Methods:
This retrospective review included consecutive patients at our institution treated with SBRT for sarcoma pulmonary metastases. Cumulative incidence of local failure (LF) was estimated using a competing risks framework.
Results:
We identified 66 patients treated to 95 pulmonary metastases with SBRT. The median follow-up from the time of SBRT was 36 months (95% CI 34 – 53 months). The cumulative incidence of LF at 12 and 24 months was 3.1% (95% CI 0.9 – 10.6%) and 7.4% (95% CI 4.0% - 13.9%), respectively. The 12- and 24-month overall survival was 74% (95% CI 64 – 86%) and 49% (38 – 63%), respectively. Oligometastatic disease, intrathoracic only disease, and performance status were associated with improved survival on univariable analysis. Three patients had grade 2 pneumonitis, and one patient had grade 2 esophagitis. No patients had ≥ grade 3+ toxicities.
Conclusion:
To the best of our knowledge, this is the largest series of patients treated with SBRT for pulmonary sarcoma metastases. We observed that SBRT offers an effective alternative to surgical resection with excellent local control and low proportions of toxicity.
Introduction
Sarcomas are a heterogenous group of mesenchymal malignancies with a diverse set of genetic and epigenetic alterations and clinical characteristics. 1,2 Approximately 80% of cases arise from soft tissue, and the remainder arise from bone.3,4 Sarcomas have a propensity for dissemination to the lungs at diagnosis5 and at time of metastatic recurrence, with up to 35% of patients eventually developing pulmonary metastases.5–7
Among patients with pulmonary metastases who have a limited burden of metastatic disease, surgical resection is the standard of care for operable patients8 and can achieve excellent local control and prolonged survival in select patients. 9–11
Stereotactic body radiation therapy (SBRT) is an important alternative approach for management of pulmonary sarcoma metastases depending on patient performance status, patient preference, medical operability and resectability of the metastatic lesion. SBRT delivers a high dose of ablative radiation and relies on a number of technological advances in radiation therapy, including reproducible positioning systems, tumor motion control, sophisticated treatment planning and image guidance to validate patient positioning and accurately localize the tumor. 12 SBRT is the standard of care for medically inoperable patients with early-stage lung cancer, with 3-year local control rates exceeding 95%.13,14 The clinical applications of SBRT have since expanded to metastatic disease.15–17 There is growing experience using SBRT for pulmonary metastases.18–21
However, there is an absence of histology-specific data on SBRT for lung metastases, which is particularly significant given the rarity and heterogeneity of bone and soft tissue sarcomas. In addition, there is an absence of safety data for combining SBRT in combination with newer systemic sarcoma therapies, including oral tyrosine kinase inhibitors and immune checkpoint inhibitors.
To our knowledge, this is the largest series of consecutive patients treated with SBRT for sarcoma pulmonary metastases, and we assess histology-specific local control and evaluate the safety of SBRT with systemic therapy.
Methods
Patient and Study Details
This study was approved by the Institutional Review Board at our institution. We identified all consecutive patients who received SBRT for sarcomas with pulmonary metastases at our institution between 2011 and 2021. SBRT was defined as image-guided radiotherapy delivered in between 1 and 8 fractions with a BED of ≥ 48. Following SBRT, patients were typically monitored with repeat chest CT per the discretion of the treating clinician. Patients who did not receive cross-sectional imaging follow-up were excluded from the analysis.
Each treated lesion was classified by location as ultracentral, central, and peripheral. Lesions were classified as ultracentral if the gross tumor was abutting or directly invading the trachea or proximal bronchial tree.11 Lesions were classified as central if the gross tumor volume (GTV) overlapped with a 2 cm expansion of the proximal bronchial tree. All other lesions were classified as peripheral in location.
We evaluated the timing of systemic therapy receipt relative to SBRT. For oral systemic therapies, our institutional practice generally is to hold oral therapies on the days of SBRT and for 3–5 days after the completion of SBRT. For intravenous (IV) therapies, we evaluated the timing of the IV infusion relative to the nearest fraction of SBRT (either before or after the IV therapy).
Patients with multiple courses of SBRT for pulmonary metastases were classified according to their status during the first course of treatment. Patients with ≤ 3 total sites of disease were classified as oligometastatic. Patients with polymetastatic disease (> 3 sites of metastatic disease) with progression of < 3 sites of disease and other sites of disease controlled were defined as oligoprogressive. Patients designated with intrathoracic only disease had disease confined to the lungs, pleura, and/or thoracic lymph nodes.
SBRT Technique
Our institutional approach to lung SBRT has been previously described. 22,23 Briefly, patients were immobilized on a custom Alpha Cradle mold and underwent a 4D-CT simulation. Among patients planned with a 4D-CT, an internal target volume was created based on gross tumor identified on the free-breathing CT with adjustments based on the 4-dimensional CT scan. A clinical target volume was created using a 2- or 3-mm expansion to account for microscopic disease spread. A 5 mm expansion was used to create a planning target volume. IMRT was utilized for treatment planning. Per our institutional practices, peripheral lesions are generally treated with a 3-fraction regimen (18 Gy x 3), lesions within 1 cm of the chest wall are treated with a 4-fraction regimen (12 Gy x 4), lesions abutting the diaphragm, heart, or with gross tumor within 2 cm of the proximal bronchial tree are treated with a 5-fraction regimen (10 Gy x 5), and lesions abutting proximal airways or the esophagus are treated with an 8-fraction regimen (7.5 Gy x 8). However, dose and fractionation are ultimately selected by the treating clinician who may consider additional factors including lesion size and prior treatment. Image guidance was utilized for all patients with kilo-voltage cone-beam CT imaging taken immediately prior to treatment and matched to the tumor on the planning CT.
Statistical Analysis
We evaluated local failure (LF) from the time of completion of SBRT. Follow-up imaging was compared to the SBRT treatment plan to assess for local failure. Lesions progressing within the PTV or at the margin of the PTV on two consecutive follow-up CT scans were classified as local failure, consistent with methodology utilized in Rusthoven et al19. When available, PET imaging was also used to assess for local failure. PET avidity comparable or greater to pre-treatment avidity supported the classification of local failure. The date of local failure was backdated to the earliest scan showing progression. Fisher’s tests were used to evaluate associations between BED and lesion size as well as lesion location. We did not adjust for clustering due to multiple lesions from the same patient.
Cumulative incidence of LF was estimated using a competing risks framework where death without the event of interest was a competing event. Univariable (UVA) subdistribution hazards competing risks regression adjusted for clustering due to multiple courses per patient was used to generate confidence intervals for cumulative incidence estimates and to examine associations between LF and variables of interest. The models would not converge for some variables due to 0 events in one category. For these variables, non-parametric cumulative incidence plots were generated, and Gray’s tests were used to test for differences between groups. We also evaluated toxicity and overall survival (OS) from the time of the last fraction of SBRT. OS was estimated using the Kaplan-Meier method and compared across groups using the Log-rank test and Cox proportional hazards regression.
Pulmonary toxicity was assessed using CTCAE v 5.0. Among patients with multiple lesions treated with metachronous SBRT, toxicity was censored at the subsequent course of SBRT for a pulmonary metastasis. The biologically effective dose (BED) was determined assuming an alpha/beta ratio of 10 Gy for pulmonary metastases. In this report, all statistical computations were performed, and all output was generated, using SAS Software Version 9.4 (The SAS Institute, Cary, NC) and R version 4.2.2 with the tidyverse (v1.3.2), gtsummary (v1.6.2), cmprsk (v2.2.11) and survival (v3.4.0) packages [refs].
Results
We identified 66 patients treated with SBRT to 95 pulmonary metastases treated. 35 (53%) were men, 33 (50%) had intrathoracic only disease, and 36 (55%) had oligometastatic disease (Table 1). The most common disease histology was leiomyosarcoma (n = 19, 29%), undifferentiated pleomorphic sarcoma (n = 10, 15%), liposarcoma (n = 7, 11%), and osteosarcoma (n = 7, 11%). Almost all patients had received prior systemic therapy (92%; n = 61), while 35 (53%) patients had prior surgical resection, and 4 patients (4%) had prior radiofrequency ablation.
Table 1.
Baseline patient characteristics
| No. (%) | |
|---|---|
| Total | 66 |
| Sex | |
| Male | 31 (48%) |
| Female | 35 (52%) |
| Age, median (years) | 58 |
| KPS | |
| 90 – 100 | 39 (59%) |
| 70 – 80 | 26 (39%) |
| ≤ 60 | 1 (1%) |
| Oligometastatic* | |
| Yes | 36 (55%) |
| No | 30 (45%) |
| Intrathoracic only disease* | |
| Yes | 33 (50%) |
| No | 33 (50%) |
| Prior systemic therapy* | |
| Yes | 61 (92%) |
| No | 5 (8%) |
| Courses of SBRT, median (range) | |
| Histology** | |
| Leiomyosarcoma | 19 (29%) |
| Undifferentiated pleomorphic sarcoma | 10 (15%) |
| Liposarcoma | 7 (11%) |
| Osteosarcoma | 7 (11%) |
| Synovial | 5 (8%) |
| Other | 18 (27%) |
At time of first course of lung SBRT
Other disease histologies include: Ewing (n = 4), chondrosarcoma (n = 3), myxofibrosarcoma (n = 2), glomangiosarcoma (n = 2), epithelioid (n = 2), peripheral nerve sheath (n = 2), alveolar (n = 1), undifferentiated with epithelioid and myxoid features (n = 1), perivascular epithelioid cell Tumor (n = 1)
The median number of treated pulmonary metastases per patient was 1 (range: 1 – 5), and 13 patients had synchronous SBRT to more than one pulmonary metastasis simultaneously. At the time of the first course of SBRT, 59% (n = 39) of patients had a Karnofsky Performance Status (KPS) of 90 or 100, whereas 39% (n = 26) had a KPS of 70 or 80, and 1 patient (1%) had a KPS of 60.
A total of 20 patients (30%) were treated with SBRT within 21 days of IV systemic therapy that was continued before and after SBRT (median: 5 days, range: 0 – 20 days) including immune checkpoint inhibitors (n = 4) (Supplemental Table 1). Nine patients took oral systemic therapy within 1 week of SBRT that was continued before and after the day of SBRT delivery (Supplemental Table 2).
The characteristics of treated lesions are listed in Table 2. Among the 95 treated pulmonary metastases, 21 (22%) metastases were ultracentral, 28 (29%) were central, and the remaining 46 (48%) were peripheral in location. The most common dose and fractionation was 50 Gy in 5 fractions. The median GTV volume was 10.4 cm3 (range: 0.2 – 375.8 cm3). Smaller lesions (with a GTV ≤ median) were more likely to have been treated with a BED ≥ 100 Gy compared to larger lesions (p<0.001; Fisher’s exact test). Lesion location (peripheral versus non-peripheral) was not associated with receipt of a BED of ≥ = 100 Gy (p = 0.2; Fisher’s exact test).
Table 2.
Characteristics of SBRT treatment for pulmonary sarcoma metastases
| No. (%) | |
|---|---|
| No. of lesions | 95 |
| Location | |
| Ultracentral | 21 (22%) |
| Central | 28 (29%) |
| Peripheral | 46 (48%) |
| Dose/fractionation (BED in Gy) | |
| 30 Gy/5 fractions (48) | 16 (17%) |
| 27 Gy/3 fractions (51.3) | 5 (5%) |
| 35 Gy/5 fractions (59.5) | 5 (5%) |
| 30 Gy/3 fx (60) | 4 (4%) |
| 40 Gy/5 fractions (72) | 9 (9%) |
| 24 Gy/1 fx (81.6) | 1 (1%) |
| 44 Gy/5 fx (82.72) | 1 (1%) |
| 45 Gy/4 fractions (85.5) | 3 (3%) |
| 50 Gy/5 fractions (100) | 32 (32%) |
| 60 Gy/8 fractions (105) | 7 (7%) |
| 48 Gy/4 fractions (105.6) | 5 (5%) |
| 54 Gy/3 fractions (151.2) | 7 (7%) |
| Gross tumor volume | |
| Median (range) | 10.4 (0.2 – 375.8) |
BED: Biologically Effective dose
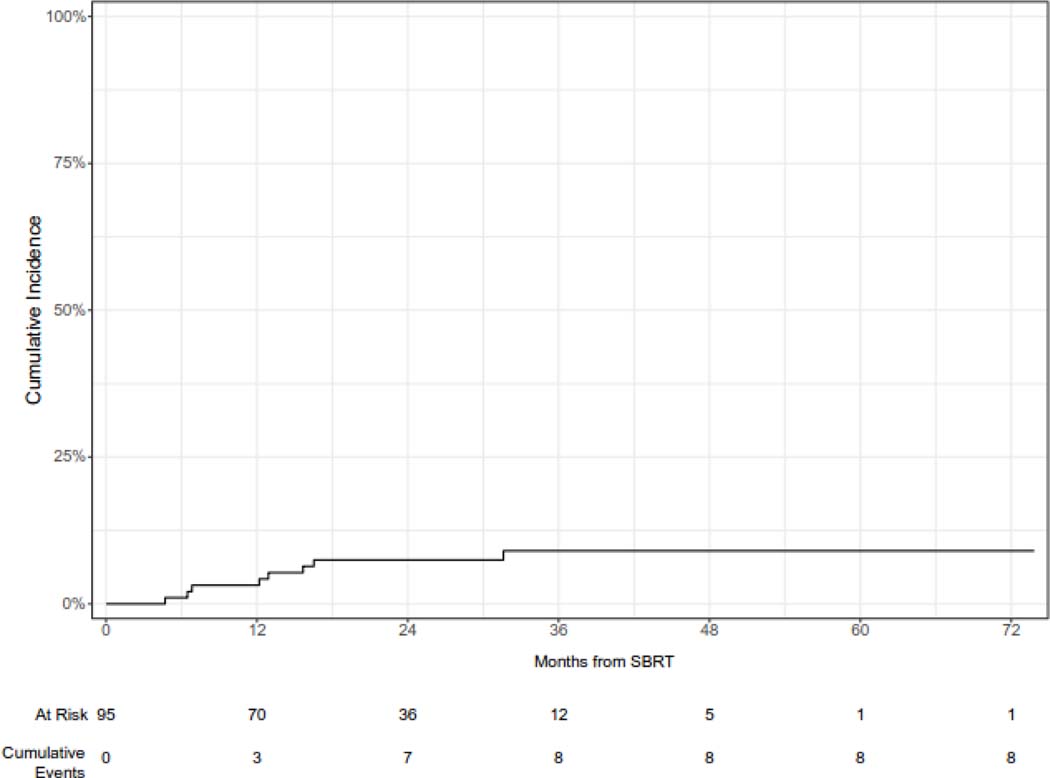
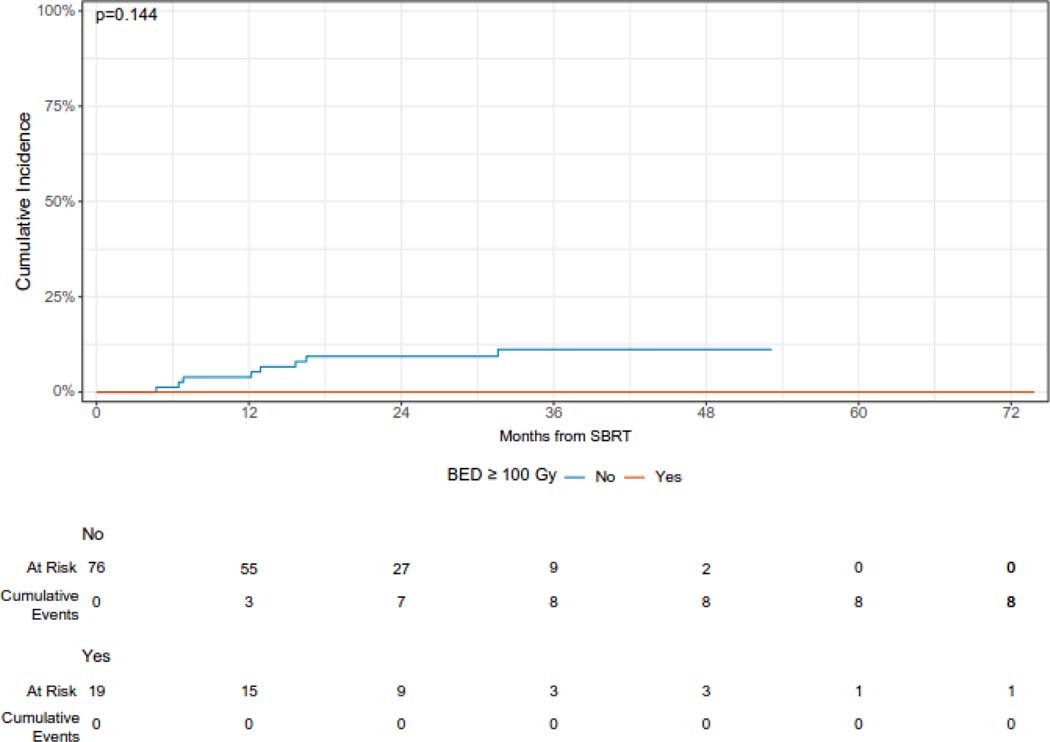
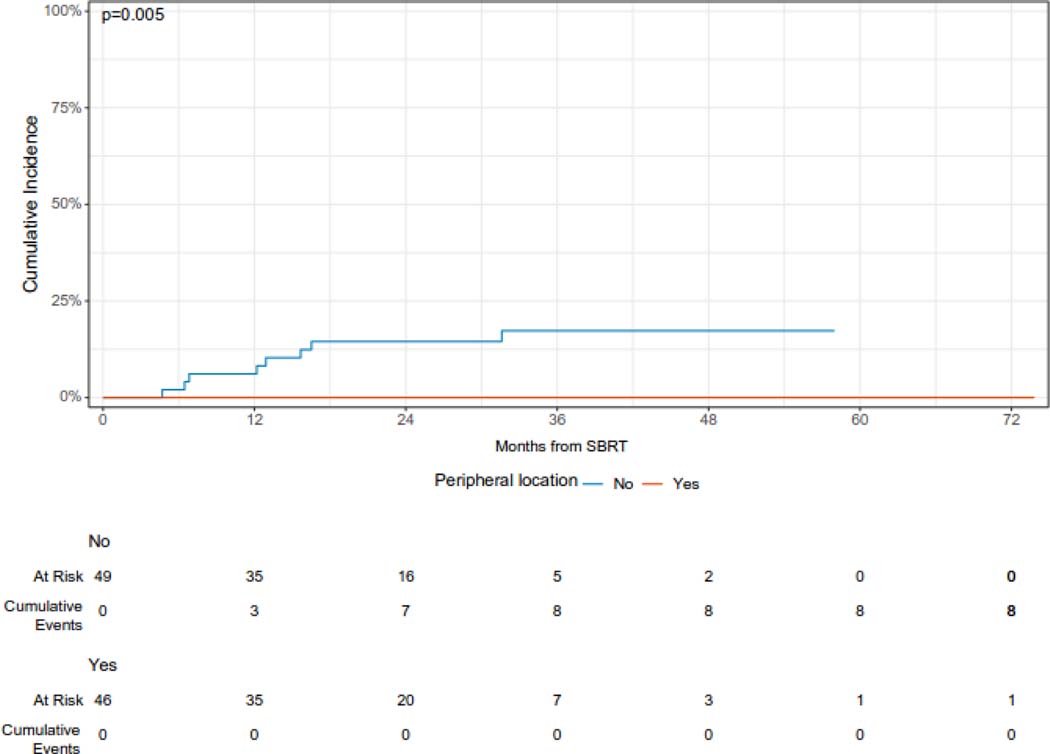
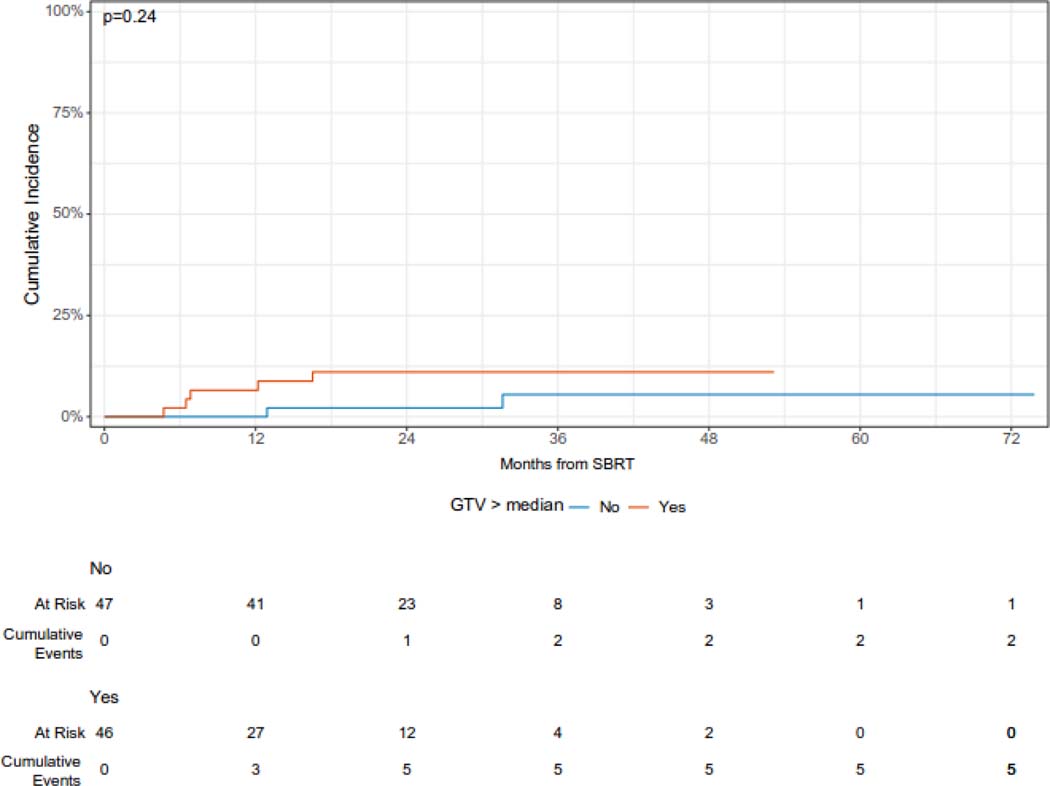
The median follow-up from the time of SBRT was 36 months (95% CI 34 – 53 months). The cumulative incidence of local failure at 12 and 24 months was 3.1% (95% CI 0.9 – 10.6%) and 7.4% (95% CI 4.0% - 13.9%), respectively (Figure 1A). Larger lesions (with gross tumor volume ≥ median) had a higher cumulative incidence of LF as compared with smaller (gross tumor volume < median) lesions [CI at 24 months (95% CI): 10.9 (5.1 – 23.1) vs. 2.2 (0.3 – 13.7), but this difference was not statistically significant (p = 0.24). Similarly, lesions treated with a BED of ≥ =100 Gy had a reduced risk of local failure which was not statistically significant, while peripheral lesions had a significantly reduced 24-month cumulative incidence of local failure (Figure 1B, 1C, 1D). Histology was not significantly associated with the cumulative incidence of local failure (Supplementary Figure 1). One patient who developed recurrence 5 months after SBRT was treated with salvage reirradiation. No other patients received local salvage therapy. All local failures were diagnosed radiographically without biopsy.
Figure 1A.
Cumulative incidence of local failure following of sarcoma pulmonary metastases.
Figure 1B.
Cumulative incidence of local failure following of sarcoma pulmonary metastases according to BED.
Figure 1C.
Cumulative incidence of local failure following of sarcoma pulmonary metastases according to location.
Figure 1D.
Cumulative incidence of local failure following or sarcoma pulmonary metastases according to size of lesion.
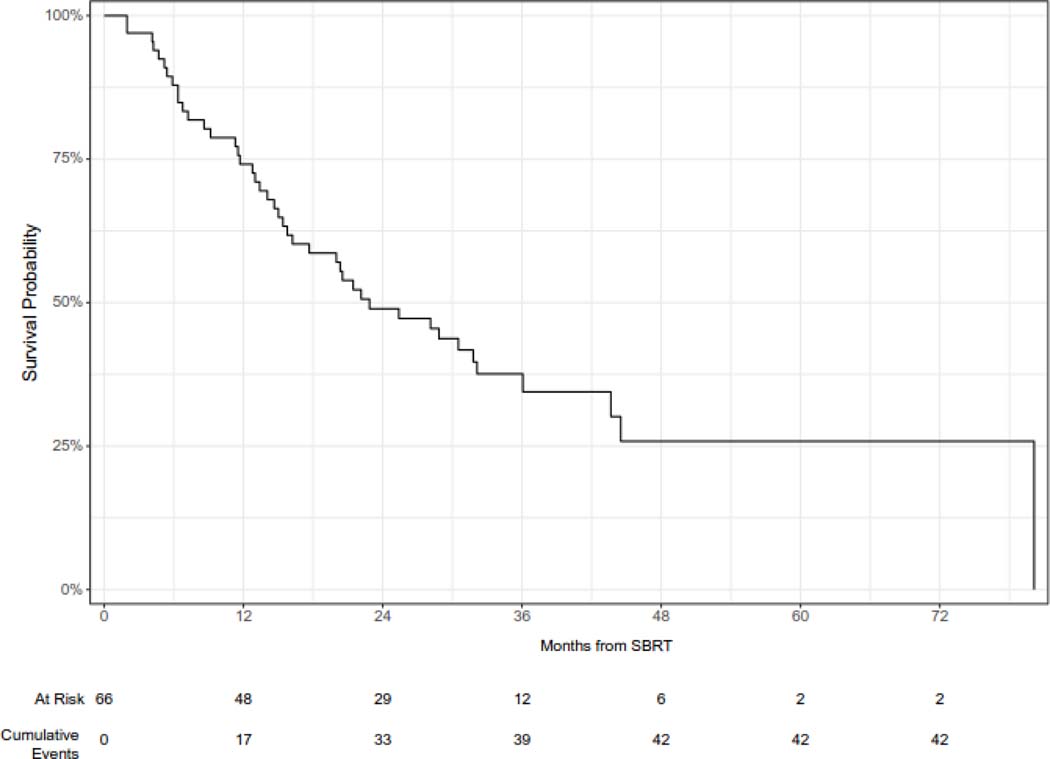
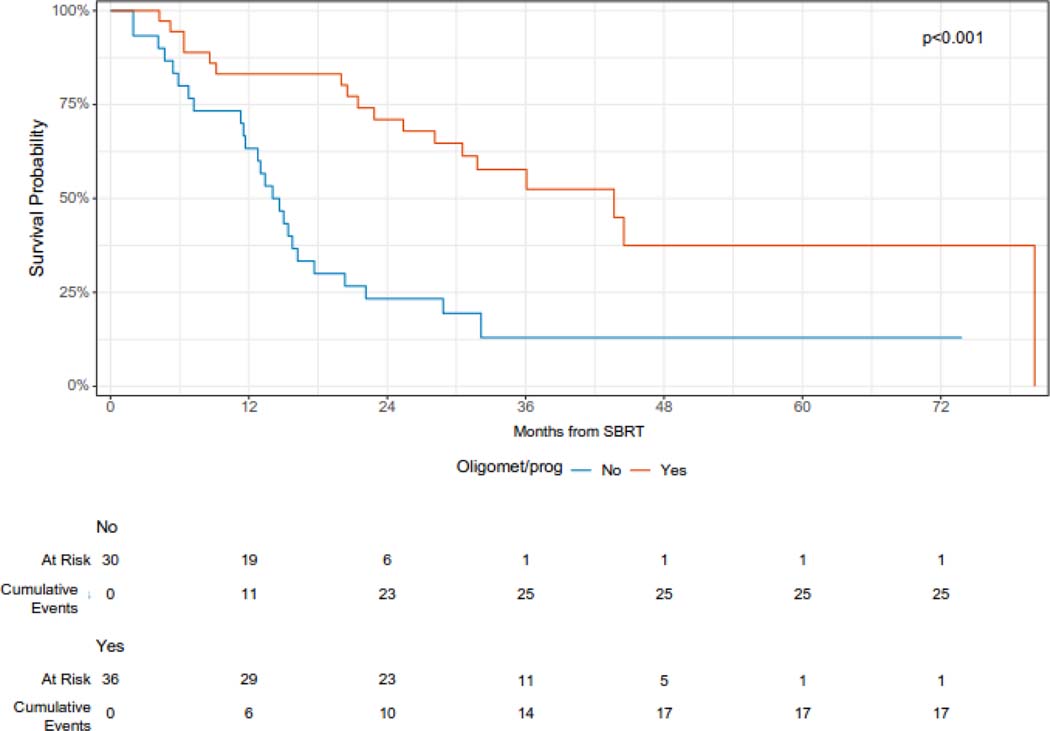
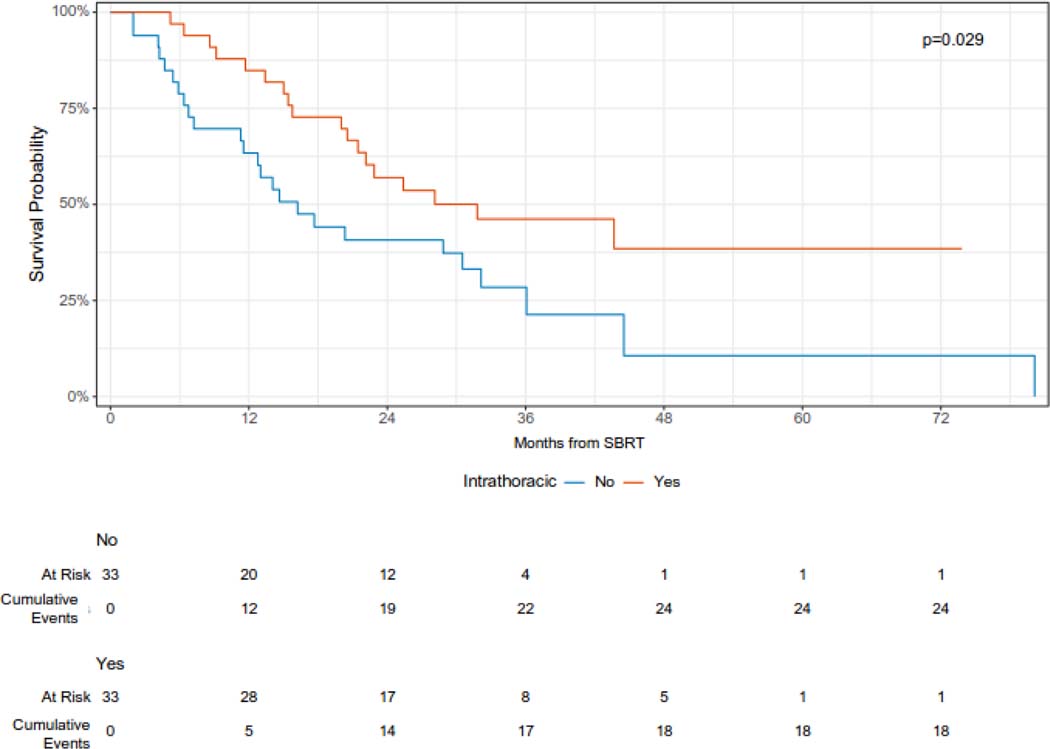
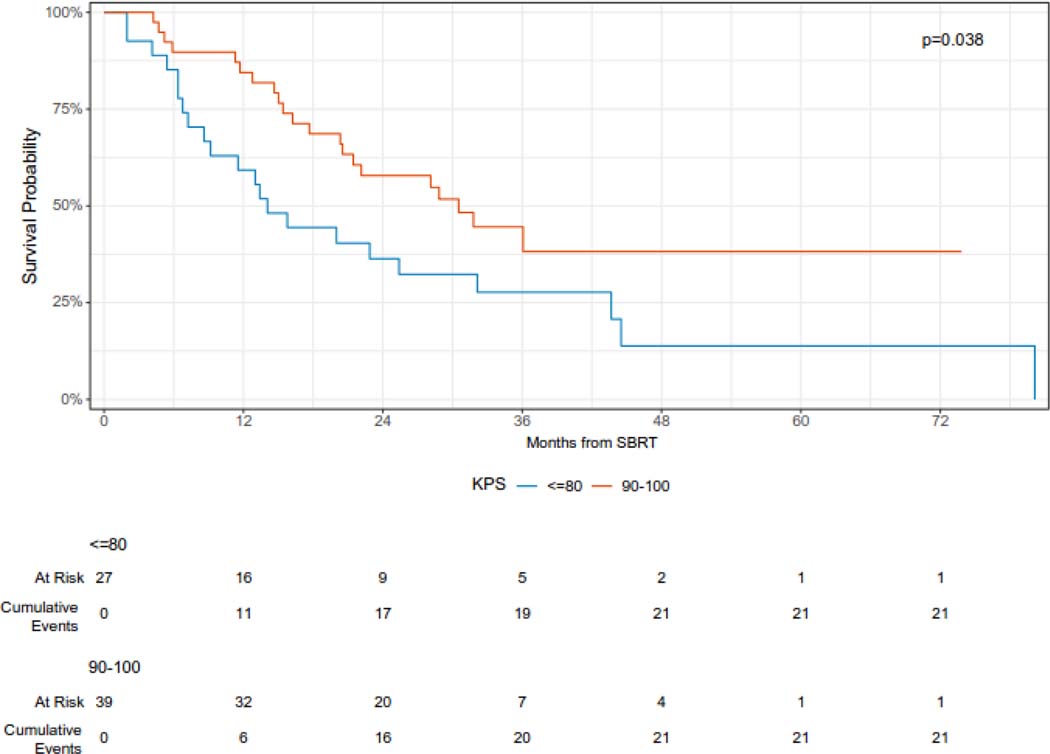
The median overall survival was 23 months (95% CI 16 – 36 months), and the 12- and 24-month overall survival was 74% (95% CI 64 – 86%) and 49% (38 – 63%), respectively (Figure 2A). On univariable analysis, oligometastatic disease at the time of SBRT was associated with improved survival (median survival 44 months vs. 14 months, log-rank p < 0.0001) (Figure 2B). In addition, intrathoracic only disease was associated with improved survival (12-month overall survival 85% vs 63%, log-rank p = 0.029) (Figure 2C). KPS 90 or 100 was also associated with improved survival (12-month overall survival 84% v 59%, log-rank p = 0.038) (Figure 2D). On univariable Cox regression analysis, oligometastatic disease (HR 0.29, 95% CI 0.16 – 0.56), intrathoracic only disease (HR 0.51, 95% CI 0.28 – 0.94), and performance status (HR 0.53, 95% CI 0.29 – 0.97) were significantly associated with OS (Supplementary Table 1).
Figure 2A.
Overall survival following SBRT for sarcoma pulmonary metastases.
Figure 2B.
Overall survival following SBRT for sarcoma pulmonary metastases according to disease burden.
Figure 2C.
Overall survival following SBRT for sarcoma pulmonary metastases according to intrathoracic versus extra-thoracic disease.
Figure 2D.
Overall survival following SBRT for sarcoma pulmonary metastases according to performance status.
Among the entire cohort of 66 patients, three patients (4.5%) had grade 2 toxicity and no patient had grade 3 or greater toxicity. The patient with grade 2 esophagitis had a central lesion treated to 45 Gy in 5 fractions. Two patients experienced grade 2 pneumonitis: a patient with metastatic liposarcoma treated to 50 Gy in 5 fractions to a peripheral metastasis, and a patient with metastatic leiomyosarcoma treated to 45 Gy in 5 fractions to a central metastasis. Neither patient with grade 2 pneumonitis received IV systemic therapy within 21 days or oral therapy within 1 week. There was no toxicity experienced among patients who had ultracentral pulmonary metastases or among patients treated synchronously to multiple metastases with SBRT.
Discussion
In this large cohort, SBRT was demonstrated to be a safe and effective alternative to surgical resection of pulmonary metastases among patients with metastatic sarcoma. Three patients had grade 2 toxicities, and no patient had grade 3 or greater toxicity. With extended median follow-up of 36 months, we observed a 24-month cumulative incidence of local failure of 7.4%. Patients with a limited burden of metastatic disease had the potential for prolonged survival after SBRT.
In this heavily pretreated cohort, with 92% of patients having received prior systemic therapy and 53% having undergone prior resection of one or more pulmonary metastases, we observed a low incidence of toxicity with three patients having grade 2 toxicities and no grade 3 or greater toxicities. This is consistent with other retrospective studies of SBRT for sarcoma pulmonary metastases.19,24–27 Notably, this cohort contained patients treated with an oral multitargeted tyrosine kinase inhibitor, pazopanib, which was FDA approved in 2012.28 Pazopanib has been associated with rates of pneumothorax between 3% - 14% among patients with lung metastases.28–30 In our cohort of 6 patients treated with 10 courses of SBRT to pulmonary sarcoma metastases with pazopanib within 1 week of SBRT, we did not observe any cases of pneumothorax or other toxicity, consistent with evaluation of pazopanib in pediatric patients treated with radiation.31 Furthermore, we observed no toxicity in patients treated with SBRT and IV systemic therapy within 3 weeks of SBRT, including immune checkpoint inhibitors. Immune checkpoint inhibitors can achieve a durable response in advanced sarcoma32–34 and are being explored in combination with targeted therapies and chemotherapy.35,36 While these are small cohorts, the possibility that patients may receive definitive local therapy for pulmonary metastases with no or limited interruption to systemic therapy and minimal risk of toxicity is encouraging.
The local control rate may be affected by a variety of factors, including dose, fractionation, lesion location, and disease histology. Our experience with a low cumulative incidence of LF at two years is consistent with other series reporting excellent rates of local control with SBRT. A multi-institutional retrospective study of 44 patients treated with SBRT to 56 sarcoma pulmonary metastases showed a 2-year local control rate of 90%.24 A prospective, multi-institutional phase I/II trial of SBRT for 1 – 3 lung metastases with a cumulative diameter of smaller than 7 cm and a low burden of extrathoracic disease showed two-year local control of 96%.19 Additional single institution retrospective analyses have also demonstrated local control rates in excess of 90%.25–27 While we were able to achieve BEDs in excess of 100 Gy for ultracentral lesions, local control remained improved for peripheral lesions.
Pulmonary metastasectomy is the standard of care among patients who are medically fit surgical candidates with limited systemic disease burden where complete resection of metastases is possible.37 Complete resection and limited burden of metastatic disease have correlated with improved outcomes after metastasectomy in the setting of metastatic sarcoma.38,39 In this cohort, 29% of treated lesions were classified as ultracentral due to their location abutting or invading central airways or other central structures. Resection of such ultracentral lesions often require more extensive surgical procedures that may be associated with a higher risk of morbidity.40,41 For patients who are medically unfit for surgery or have unresectable lesions involving critical structures, SBRT is an excellent alternative. SBRT also offers the advantage to limited interruption to systemic therapy, which is of particular importance in the setting of synchronous progression.
Patients with oligometastatic disease at the time of SBRT have the potential for prolonged survival after treatment, and we observed a median survival of 44 months among patients treated with SBRT for oligometastatic disease. There is a growing body of literature supporting the benefit of local therapy for select patients with oligometastatic disease.15,16,42 Optimal selection of patients for aggressive local treatment in the context of metastatic disease is an important area of research.
This is a single institution retrospective study that includes patients with a variety of sarcoma disease histologies. Patients were also treated with a wide range of dose and fractionation schemes depending on the preference of the treating clinician. Oral systemic therapy agents were held on the days of SBRT per our institutional guidelines, limiting the conclusions regarding the safety of concurrent SBRT and systemic therapies. Subgroup analyses are limited by sample size. Nonetheless, this is the largest series of patients with sarcoma pulmonary metastases treated with SBRT to date and the first to evaluate histology-specific local control and safety with newer systemic therapy agents.
Conclusion
We observed that SBRT was an effective and safe modality to achieve local control of sarcoma pulmonary metastases, including in heavily pretreated patients and those with receiving systemic therapy. SBRT is an excellent option for patients who are medically inoperable or decline surgery. Long-term survival is possible among patients with oligometastatic disease
Supplementary Material
Supplemental Figure 1. Cumulative incidence of local failure according to disease histology.
Highlights.
We observed excellent long-term disease control with SBRT and potential for prolonged survival particularly among patients with oligometastatic disease at the time of SBRT.
The 24-month cumulative incidence of local failure was 7.4% after SBRT for pulmonary sarcoma metastases
We observed low rates of toxicity, including no grade 3 or greater toxicity. This cohort included patients with ultra-central lesions and multiple pulmonary metastases treated with SBRT simultaneously.
SBRT is an important treatment modality for patients with sarcoma pulmonary metastases, particularly patients who are unfit for surgery or have unresectable metastases
Acknowledgements:
This manuscript was presented, in part, at the American Society of Radiation Oncology 2022 Annual Meeting in San Antonio, Texas.
Author Conflicts of Interest Statement:
Daphna Y. Gelblum reports research funding from Merck. Charles B. Simone, II reports Honoraria from Varian and Novocure and serves in a Leadership position for the Proton Collaborative Group, American Society for Radiation Oncology, NRG Oncology, American Radium Society, and Annals of Palliative Medicine. Annemarie F. Shepherd has stock ownership in ArcellX and Doximity. Abraham J. Wu reports research support from CivaTech Oncology, Inc, Honoraria from Nanovi A/S, serves on the Scientific Advisory Board of Simphotek, Inc, and has stock in Simphotek, Inc. Andreas Rimner reports research funding from Varian Medcial Systems, Boehringer Ingelheim, Astra Zeneca, Merck, and Pfizer and serves in Leadership Positions for the International Thymic Malignancies Group and International Mesothelioma Interest group. Narek Shaverdian reports research funding from Novartis. Daniel R. Gomez reports research funding from Astra Zeneca and Varian and receives Honoraria from Johnson and Johnson, MedLearning Gropu, and GRAIL. Sandra P. D’Angelo reports relationships with Aadi Bioscience, Inc., GlaxoSmithKline, Pfizer, Servier, GI Innovation Inc., and Adaptimmune. William D. Tap reports relationships with AmMax Bio, Inc, Atropos Therapeutics, Inc., Ayala Pharmaceuticals, Inc, Bayer, Certis Oncology Solutions, Cogent Biosciences, Inc., Connecting Humans in Health, LLC, Daiichi Sankyo, Deciphera, Eli Lilly and Company, Epizyme, Foghorn Therapeutics, Kowa Research Institute, Inc., MedPacto, Inc., Novo Holdings A/S, PER Events, LLC, PeerView Institute for Medical Education (PVI), and Servier. All other authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability:
Research data are not available at this time.
References
- 1.Nacev BA, Sanchez-Vega F, Smith SA, et al. Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nat Commun. Jun 15 2022;13(1):3405. doi: 10.1038/s41467-022-30453-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nacev BA, Jones KB, Intlekofer AM, et al. The epigenomics of sarcoma. Nat Rev Cancer. Oct 2020;20(10):608–623. doi: 10.1038/s41568-020-0288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed, International Agency for Research on Cancer. 2020;3 [Google Scholar]
- 4.Gounder MM, Agaram NP, Trabucco SE, et al. Clinical genomic profiling in the management of patients with soft tissue and bone sarcoma. Nat Commun. Jun 15 2022;13(1):3406. doi: 10.1038/s41467-022-30496-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie-Large M, James SL, Tiessen L, Davies AM, Grimer RJ. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur J Cancer. Sep 2008;44(13):1841–5. doi: 10.1016/j.ejca.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Potter DA, Glenn J, Kinsella T, et al. Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol. Mar 1985;3(3):353–66. doi: 10.1200/JCO.1985.3.3.353 [DOI] [PubMed] [Google Scholar]
- 7.Billingsley KG, Lewis JJ, Leung DH, Casper ES, Woodruff JM, Brennan MF. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer. Jan 15 1999;85(2):389–95. [PubMed] [Google Scholar]
- 8.NCCN Gudielines Soft Tissue Sarcoma. 2021;
- 9.Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg. Nov 2011;92(5):1780–6; discussion 1786–7. doi: 10.1016/j.athoracsur.2011.05.081 [DOI] [PubMed] [Google Scholar]
- 10.Ferguson PC, Deheshi BM, Chung P, et al. Soft tissue sarcoma presenting with metastatic disease: outcome with primary surgical resection. Cancer. Jan 15 2011;117(2):372–9. doi: 10.1002/cncr.25418 [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. Jul 2015;89(1):50–6. doi: 10.1016/j.lungcan.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 12.Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. Feb 1 2010;76(2):326–32. doi: 10.1016/j.ijrobp.2009.09.042 [DOI] [PubMed] [Google Scholar]
- 13.Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol. Sep 1 2018;4(9):1287–1288. doi: 10.1001/jamaoncol.2018.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. Sep 1 2018;4(9):1263–1266. doi: 10.1001/jamaoncol.2018.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez DR, Blumenschein GR Jr.,, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. Dec 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. Jun 20 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4–10 oligometastatic tumors (SABR-COMET-10): study protocol for a randomized phase III trial. BMC Cancer. Aug 19 2019;19(1):816. doi: 10.1186/s12885-019-5977-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol. Jul 2010;5(7):1091–9. doi: 10.1097/JTO.0b013e3181de7143 [DOI] [PubMed] [Google Scholar]
- 19.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. Apr 1 2009;27(10):1572–8. doi: 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 20.Cuaron JJ, Yorke ED, Foster A, et al. Stereotactic body radiation therapy for primary lung cancers >3 centimeters. J Thorac Oncol. Nov 2013;8(11):1396–401. doi: 10.1097/JTO.0b013e3182a47181 [DOI] [PubMed] [Google Scholar]
- 21.von Reibnitz D, Shaikh F, Wu AJ, et al. Stereotactic body radiation therapy (SBRT) improves local control and overall survival compared to conventionally fractionated radiation for stage I non-small cell lung cancer (NSCLC). Acta Oncol. Nov 2018;57(11):1567–1573. doi: 10.1080/0284186X.2018.1481292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spratt DE, Wu AJ, Adeseye V, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clin Lung Cancer. May 2016;17(3):177–183 e2. doi: 10.1016/j.cllc.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sura S, Yorke E, Jackson A, Rosenzweig KE. High-dose radiotherapy for the treatment of inoperable non-small cell lung cancer. Cancer J. Jul-Aug 2007;13(4):238–42. doi: 10.1097/PPO.0b013e31813ffd7b [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig KE, Hanley J, Mah D, et al. The deep inspiration breath-hold technique in the treatment of inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. Aug 1 2000;48(1):81–7. doi: 10.1016/s0360-3016(00)00583-6 [DOI] [PubMed] [Google Scholar]
- 25.Baumann BC, Bernstein KA, DeLaney TF, et al. Multi-institutional analysis of stereotactic body radiotherapy for sarcoma pulmonary metastases: High rates of local control with favorable toxicity. J Surg Oncol. Oct 2020;122(5):877–883. doi: 10.1002/jso.26078 [DOI] [PubMed] [Google Scholar]
- 26.Soyfer V, Corn BW, Shtraus N, et al. Single-institution Experience of SBRT for Lung Metastases in Sarcoma Patients. Am J Clin Oncol. Feb 2017;40(1):83–85. doi: 10.1097/COC.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 27.Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol. Mar-Apr 2016;6(2):e27–33. doi: 10.1016/j.prro.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieber J, Streblow J, Uhlmann L, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer. Jul 2016;97:51–8. doi: 10.1016/j.lungcan.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 29.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. May 19 2012;379(9829):1879–86. doi: 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 30.Verschoor AJ, Gelderblom H. Pneumothorax as adverse event in patients with lung metastases of soft tissue sarcoma treated with pazopanib: a single reference centre case series. Clin Sarcoma Res. 2014;4:14. doi: 10.1186/2045-3329-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. May 1 2016;122(9):1408–16. doi: 10.1002/cncr.29961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broughman J. Safety of Metastasis-Directed SBRT and Concurrent Pazopanib in Pediatric Sarcoma. Int J of Radiation Oncology, Biology, & Physics. 2020;108(3) [Google Scholar]
- 33.Klemen ND, Hwang S, Bradic M, et al. Long-term Follow-up and Patterns of Response, Progression, and Hyperprogression in Patients after PD-1 Blockade in Advanced Sarcoma. Clin Cancer Res. Mar 1 2022;28(5):939–947. doi: 10.1158/1078-0432.CCR-21-3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. Nov 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. Mar 2018;19(3):416–426. doi: 10.1016/S1470-2045(18)30006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Angelo SP, Richards AL, Conley AP, et al. Pilot study of bempegaldesleukin in combination with nivolumab in patients with metastatic sarcoma. Nat Commun. Jun 16 2022;13(1):3477. doi: 10.1038/s41467-022-30874-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisdom AJ, Mowery YM, Riedel RF, Kirsch DG. Rationale and emerging strategies for immune checkpoint blockade in soft tissue sarcoma. Cancer. Oct 1 2018;124(19):3819–3829. doi: 10.1002/cncr.31517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grilley-Olson JE, Webber NP, Demos DS, Christensen JD, Kirsch DG. Multidisciplinary Management of Oligometastatic Soft Tissue Sarcoma. Am Soc Clin Oncol Educ Book. May 23 2018;38:939–948. doi: 10.1200/EDBK_200573 [DOI] [PubMed] [Google Scholar]
- 39.Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg. May 1999;229(5):602–10; discussion 610–2. doi: 10.1097/00000658-199905000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. Sep 2009;88(3):877–84; discussion 884–5. doi: 10.1016/j.athoracsur.2009.04.144 [DOI] [PubMed] [Google Scholar]
- 41.Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg. Dec 2003;76(6):1782–8. doi: 10.1016/s0003-4975(03)01243-8 [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Bao F, Zheng H, et al. Local extension at the hilum region is associated with worse long-term survival in stage I non-small cell lung cancers. Ann Thorac Surg. Feb 2012;93(2):389–96. doi: 10.1016/j.athoracsur.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 43.Rim CH, Shin IS, Park S, Lee HY. Benefits of local consolidative treatment in oligometastases of solid cancers: a stepwise-hierarchical pooled analysis and systematic review. NPJ Precis Oncol. Jan 21 2021;5(1):2. doi: 10.1038/s41698-020-00141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cumulative incidence of local failure according to disease histology.
Data Availability Statement
Research data are not available at this time.