Abstract
Objectives:
The US Food and Drug Administration (FDA) has regulatory authority for modified risk tobacco product advertising claims. To guide future regulatory efforts, we investigated how variations in modified risk claim advertisements influence consumer perceptions of product risk claims for Camel Snus.
Methods:
Young people and adults (15–65), including current, never, and former smokers, were randomised to view one of five Camel Snus print advertisements as part of a web-based survey. Four of the advertisements presented information related to nitrosamine content of snus using four formats: (1) text, (2) a bar chart, (3) a text/testimonial and (4) a bar chart/testimonial. The fifth format, used as a control, was a current advertisement for Camel Snus without the explicit claims made about nitrosamine content. After viewing advertisements for all products, participants were asked which product they would be most interested in trying.
Results:
Participants exposed to advertisements that contained an explicit reduced risk message agreed the advertising claim for that product posed fewer health risks than cigarettes. However, advertisements containing the reduced risk messages were also viewed as containing less truthful information and respondents were more sceptical of the information presented. Advertisement claim format was not associated with selecting snus over the other tobacco products, nor was it associated with purchase intentions.
Conclusion:
The results of this research indicate that consumers respond to reduced risk messages, though perhaps not in the direct way anticipated. We found no significant differences by advertisement format (numerical, graphical, testimonial).
Keywords: Advertising, consumer behaviour, modified risk, Snus, tobacco
Introduction
Low-nitrosamine smokeless tobacco (LN-ST) products may pose lower health risks than traditional tobacco cigarettes (Levy et al., 2004), although many smokers still perceive the risks of cigarettes and oral tobacco to be equivalent (Dave et al., 2013; Levy et al., 2004; O’Connor et al., 2011). In the USA, the demand for LN-ST products has grown in recent years, especially among specific subgroups including young adult men, rural residents and those of lower educational attainment (Biener et al., 2011; Chang et al., 2016; Delnevo et al., 2014). However, in the USA, the demand for LN-ST products is relatively low, perhaps because smokers and those using traditional ST products are unaware of the lower health risks (Biener et al., 2016; Kiviniemi et al., 2015; Wackowski et al., 2015).
Public health communications that convey factually accurate and easily interpretable information about the lower risks of LN-ST product use relative to cigarettes may help to inform smokers about lower risk alternatives (Kozlowski and Abrams 2016; Kozlowski and Sweanor 2016). Tobacco harm reduction is a strategy that seeks to encourage users who are unwilling or unable to quit using tobacco products to switch to products that pose significantly fewer health risks. To ensure the viability of this approach, consumers must be provided with accurate information about the relative risk of alternative tobacco products (Kaufman et al., 2014; Levy et al., 2004). At the same time, there are substantial concerns that allowing tobacco products to claim relative health advantages over cigarettes could encourage use by non-users, including young people, who might be then susceptible to moving to cigarettes, or also encouraging relapse to tobacco use or forestalling cessation (Gartner et al., 2007; Hatsukami et al., 2004; Tomar, 2007; Tomar et al., 2009). These concerns are non-trivial given the history of the tobacco industry illustrates a pattern of deceptive practices, such as marketing tobacco products that resemble Nicotine Replacement Therapy (Kostygina et al., 2016), and utilising regulatory loopholes to undermine tobacco control policies (Peeters and Gilmore, 2013; Tan et al., 2013).
The US Food and Drug Administration (FDA) has regulatory authority over claims of modified risk for tobacco products (Public Law 111–31, HR 1256, 2016) and must apply a public health standard to its determinations, including potential impacts of allowing marketing claims on both current tobacco users as well as non-users. A US Institute of Medicine programme unit of the National Academies committee (Institute of Medicine of the National Academies, 2012) recommended that evidence supporting modified risk claims should include not only toxicology and abuse liability findings but consumer perceptions of the product, including messaging. The process of moving from lack of awareness of a product to trying a novel potentially modified risk product includes reactions to both product messaging (broadly encompassing knowledge, attitudes, beliefs, product expectancies and risk perceptions) and responses to product use (such as nicotine and sensory effects) (McCaffery et al., 2012).
There are many ways to communicate health information to tobacco product consumers, and approaches may differ subtly. For example, research on cigarette advertising has reported on how the use of colours in packaging and products (e.g., white space on the package, white tipping paper on filters), the use of product descriptors (e.g. ‘light’, ‘smooth’, ‘natural’, ‘organic’) and the use of imagery in an ad (e.g., lifestyle themes, technology theme) can influence consumer product risk perceptions (Bansal et al., 2011; Moodie et al., 2015; O’Connor et al., 2015; Rees et al., 2009). A more explicit approach commonly used in making product health claims involves the use of an actor/model/actual user to explain the benefits of using the product. This form of advertising is common in direct-to-consumer advertising for medications, including smoking cessation medications, and previous research has investigated this form of communication in the context of corrective statements (Smith et al., 2011; Wakefield et al., 2002). Another direct method of communicating health information is to use a ‘boxed’ claim, wherein the claim is contained in a visually distinct portion of the advertisement. These are a common format for ‘qualified’ health claims for foods and dietary supplements (RJ Reynolds Tobacco Company, 2003). Previous research has shown that a box containing ‘corrective’ information within a print advertisement for modified risk tobacco products (MRTPs) can limit consumers’ inclination to believe that these products are safer than combustion cigarettes in terms of health risk and a reduction in risk of cancer (Ellwood et al., 2010). Similarly, the degree of risk or harm reduction can also be represented numerically (i.e. a 50% reduction) or graphically (i.e. by bar charts or other graphics), and previous research indicates consumers understand well-designed graphical presentations better (Ancker et al., 2006; Biener et al., 2007; Kapsak et al., 2008; McCaffery et al., 2012).
Only a few studies have attempted to test how different risk messages impact on consumer responses to a MRTP (Mays et al., 2015; Popova and Ling, 2014; Popova et al., 2014). Other studies have examined how the presentation of prescription drug facts can influence comprehension of risk and benefit data to improve decision making among consumers (Schwartz and Woloshin, 2013). We are not aware of any studies that have tested directly how the format of a reduced risk health claim message for a tobacco product impacts consumer risk perceptions. Furthermore, evaluations of advertisement claim format should be evaluated among populations of both youths and adults because of their potentially different profiles of risk perception and future use intentions (US Department of Health and Human Services, 2012; US Department of Health and Human Services, 2014). Both smokers and non-smokers were included because of different motives regarding future use intentions: advertisement format may encourage smokers to seek reduced risk alternatives to smoking, while advertisement format may motivate future use intentions among non-smokers. This study evaluated the effects of four modified risk claim advertising formats compared with a no-claim control on consumers’ perceptions of product risk and future use intentions. The primary objectives were to (1) evaluate perceptions of health risks about LN-ST based on advertisement claim format among smokers and non-smokers and (2) evaluate the relationship between advertisement claim format, and interest in use and purchase intentions, among smokers and non-smokers. Parallel analyses were conducted among samples of youths and adults.
Methods
Participants
Participants were recruited from a consumer specialty panel maintained by Global Market Insite (http://www.gmi-mr.com/global-panel/index.php) via an invitation to respond to a survey via email. Participants included smokers and non-smokers and were eligible if they were between the ages of 14 and 65 years and provided informed consent. GMI’s ‘specialty youth panel’ complies with the Children’s Online Privacy Protection Rule (16 C.F.R. Part 312), and young people’s parents were e-mailed a statement describing the risks and benefits of participation, compensation and confidentiality prior to their child engaging in the survey. The sample targeted 2,000 adults (18–65) and 1,000 youths (aged 14–17) and compensated them 60 GMI ‘marketpoints’ (20 marketpoints = USD1) for their time. Analyses were conducted separately for youth and adults. The study protocol was approved by the Institutional Review Board at Roswell Park Cancer Institute, Buffalo, NY.
Design and procedure
Participants completed a set of questions on demographic characteristics, perceived health risks associated with tobacco use and attitudes towards ST products. Smokers completed additional questions on smoking history, nicotine dependence and intention to quit in the next 6 months. Participants were then presented with a series of advertisements for three products: American Spirit cigarettes, Nicorette gum and Camel Snus. The order of presentation of the three products was counterbalanced across subjects so the six possible orderings are presented an equal number of times and were randomly assigned to participants independent of demographic or behavioural characteristics.
Within the Camel Snus condition, participants were randomised to view one of five different message conditions (four claim conditions and one comparison) for Camel Snus. Each of the four explicit risk claim messages contained a modified message referring to nitrosamine content of the product in the following formats: (1) text only, (2) bar chart, (3) text and testimonial and (4) bar chart and testimonial. Specifically, the claim stated that snus contained 51% lower concentrations of tobacco-specific nitrosamines, NNN and NNK, compared to leading cigarettes. The testimonial conditions supplemented the direct claim with a photo and quotation from a purported user who had used the product based on this information. The comparison condition was a Camel Snus advertisement with no health claim (claim formats are presented in Figure 1).
Figure 1.
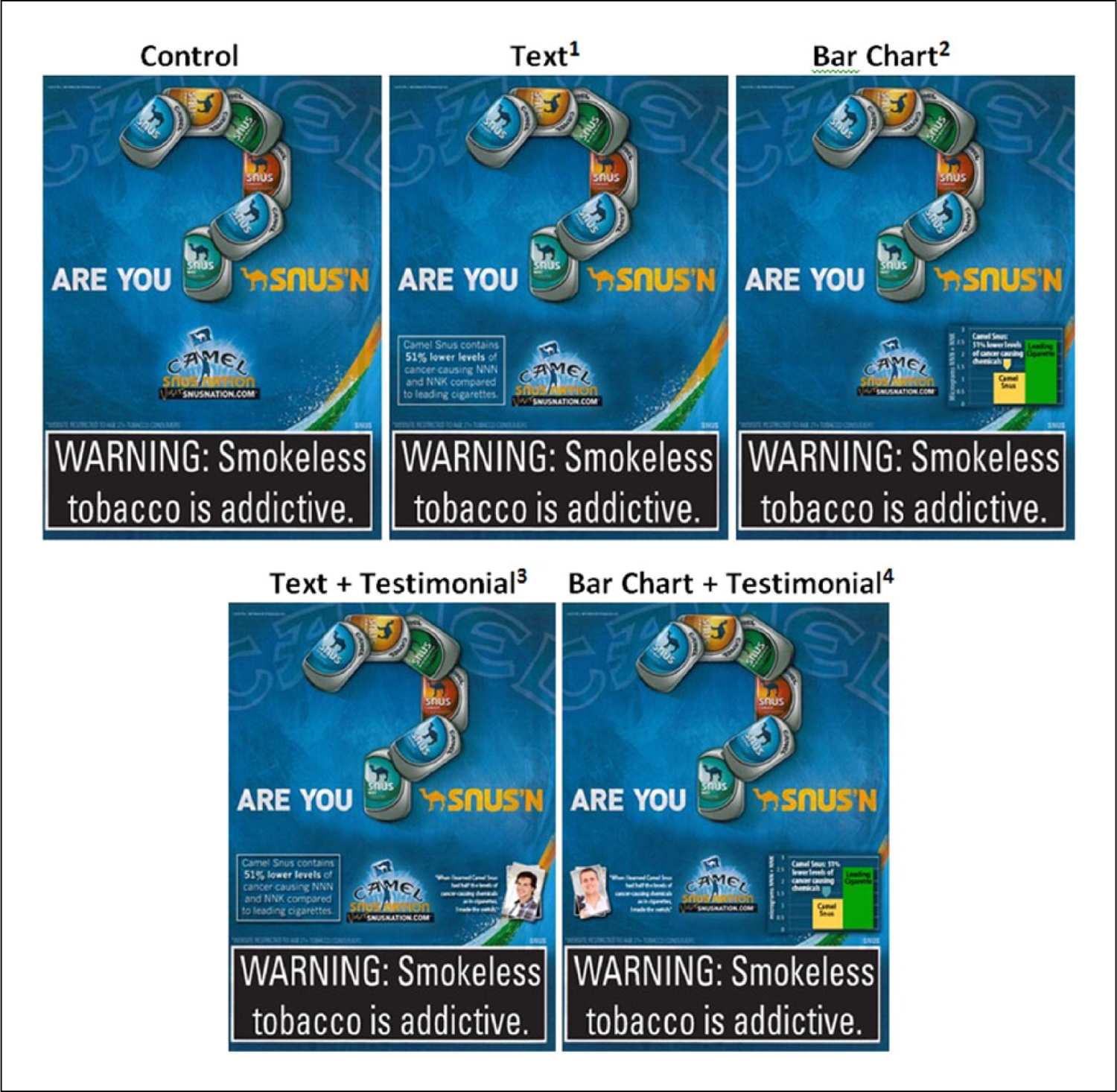
Explicit risk claim message formats.
1Text claim: ‘Camel Snus contains 51% lower levels of cancer-causing NNN and NNK compared to leading cigarettes’.
2Bar chart: ‘Camel Snus: 51% lower levels of cancer causing chemicals’.
3Text claim and testimonial: ‘Camel Snus contains 51% lower levels of cancer-causing NNN and NNK compared to leading cigarettes’ and ‘When I learned Camel Snus had half the levels of cancer-causing chemicals as in cigarettes, I made the switch’.
4Bar chart and testimonial: ‘When I learned Camel Snus had half the levels of cancer-causing chemicals as in cigarettes, I made the switch’ and ‘51% lower levels of cancer causing chemicals’.
The study was designed to simulate how consumers might view a specific health claim presented for a tobacco product done in a way that would comply with Institute of Medicine programme unit of the National Academies recommendations, the Family Smoking Prevention and Tobacco Control Act, and any guidance on MRTP warnings. These do not give much guidance in terms of claim format other than a need to demonstrate that a claim is true and not misleading. The claims cannot be attributed to FDA, as they are not making the claim. Rather, the manufacturers are responsible for making these claims. The goal of this design was to evaluate how this might work given the real-world legal constraints placed on making these types of claims.
Each advertisement was presented individually for 15 seconds. Following each advertisement, participants were asked to complete a brief assessment of their reactions, including their perception of health risk, attitudes and interest in future use. At the conclusion of the procedure, participants were provided with a debriefing form that included information about the health effects of tobacco and information about quitting assistance.
Measures
Smoking status.
For adults, smoking status was determined based on responses to the following questions: (1) Have you ever smoked a cigarette, even a few puffs? (2) Have you smoked at least 100 cigarettes in your lifetime? (3) Do you now smoke cigarettes every day, some days or not at all? Current smoker was defined as someone who reported currently smoking every day or some days and reported having smoked at least 100 cigarettes. Ever/former smoker was defined as someone who may have had a puff or had smoked 100 cigarettes, but reported not currently smoking at all. A never smoker was defined as someone who reported never having even a puff of a cigarette. The rationale behind categorising smoking status in this manner was to keep the ‘never’ group a category of true never smokers. This same rationale also applies to the current smokers. For young people, those who responded yes to the question ‘(1) Have you ever smoked a cigarette, even a few puffs?’ were classified as ‘ever users’ and those who responded no as ‘never users’.
ST use.
Respondents were asked, ‘Do you now use dip, moist snuff, or chewing tobacco?’ This was used as the measure of current ST use.
Snus awareness and use.
Respondents were asked, ‘New types of smokeless tobacco products are now available that come in teabag-like pouches that are put in the mouth, under the lip. They do not involve chewing, spitting, or smoking. Have you heard of any products like this?’ Respondents who selected ‘yes’ were classified as ‘aware’. Those who said yes were asked to report whether they had used the product within the past 12 months, even one time, and if so, when. These two variables were then combined to form a measure where those who reported they were ‘unaware’ were counted among non-users.
Attitudes towards product advertising.
We used two measures to evaluate attitudes (truthfulness and scepticism) towards the advertisement. On a scale with five response options ranging from ‘not at all likely’ to ‘extremely likely’, participants were asked, ‘How likely is it that the advertisement you just saw contained truthful information?’ On a scale with five response options ranging from ‘not at all skeptical’ to ‘extremely skeptical’, participants were asked, ‘How skeptical are you about the truthfulness of the advertisement?’ The survey response options were such that less truthful and more sceptical run in opposite directions on a 1–5 scale (less truthful = lower value; more sceptical = higher value). The two measures were highly correlated (Spearman’s rho = −.519; p < .01). We reverse coded scepticism so that less truthful and more sceptical ran in the same direction and summed the two measures.
Perception of health risk claims.
Participants reported their perception of the health risk claims of Camel Snus advertising using the following response options:
The advertisement suggests or implies that there are no health risks from Camel Snus,
The advertisement suggests of implies that the health risks from Camel Snus are less than other tobacco products,
The advertisement suggests or implies that the health risks from Camel Snus are more than other tobacco products, and
The advertisement suggests of implies that the health risks from Camel Snus are the same as other tobacco products.
Interest in trying and intention to purchase Snus.
To gauge interest in snus as a product and the potential for trial as a modified risk product, respondents were asked, ‘which of the products would you be most interested in trying?’ Response options included the following: American Spirit, Camel Snus, Nicorette. Those who selected ‘Camel Snus’ were coded as 1; all others were coded as 0 to create a dichotomous dependent variable for interest in snus. Following product selection, respondents who selected snus were also asked, ‘How likely are you to purchase Camel Snus in the next month?’ on an 11-point scale. Responses were recoded as ‘no interest in purchasing snus’ vs ‘at least some interest in purchasing snus’.
Analyses
Data were analysed using SAS V 9.4. Data analysis was conducted separately by age (under 18 vs 18 and older). Chi-square statistics and t-tests were used to examine group differences on demographic variables by smoking status. Analysis of covariance (ANCOVA) was used to assess effect of advertisement claim format on attitudes and towards snus. Logistic regression was used to identify correlates of interest in trying snus and perceptions of health risk claims. Each of the multivariable models controlled for awareness and prior use of snus, sex, age (for adults), and race/ethnicity.
Results
Adult analyses
Sample demographics and tobacco use patterns.
The sample was evenly distributed in terms of male and female participation, while a higher proportion of the sample was aged 35 and older, compared to those who were under 35; a greater percentage (66%) described themselves as White, and more than three quarters of the sample reported at least some college education (Table 1). Over one-fifth (23%) of the adult sample were current smokers, 39% ever/former smokers and 29% never smokers. Most (84%) of the sample had never used ST products, and 16% had used them in the past 30 days or reported current use. Older respondents were more likely than young adults (18–34) to smoke, χ2(4, n = 2,007) = 82.41, p < .001. Women were more likely to be never smokers than men, χ2(2, n = 2,007) = 25.86, p < .001. Significant differences were also present by smoking status for race/ethnicity, χ2(8, n = 2,007) = 36.41, p < .001; education, χ2(6, n = 2,007) = 55.02, p < .001; and prior ST use, χ2(4, n = 2,007) = 157.86, p < .001.
Table 1.
Sample demographics and tobacco use among adults (N = 2,007).
| Overall | Never smoker (28.8%) | Ever/former smoker (39.4%) | Daily/nondaily smoker (22.8%) | Chi-square | ||
|---|---|---|---|---|---|---|
| Age | 18–34 | 23.7 | 34.7 | 16.6 | 22.6 | 82.41 |
| 35–50 | 36.0 | 35.8 | 34.0 | 38.6 | ||
| 51–65 | 40.3 | 29.5 | 49.4 | 38.8 | ||
| Sex | Male | 48.7 | 40.1 | 50.8 | 54.0 | 25.86 |
| Female | 51.3 | 59.9 | 49.2 | 46.0 | ||
| Snus | Yes | 39.6 | 24.2 | 42.5 | 49.9 | 88.72 |
| awareness | No | 60.4 | 75.8 | 57.5 | 50.1 | |
| Snus use | Past 12 month use | 6.0 | 0.9 | 3.3 | 14.1 | 111.53 |
| Never use snus | 94.0 | 99.1 | 96.7 | 85.9 | ||
| Race/ethnicity | White, non-Hispanic | 65.5 | 60.6 | 70.4 | 63.9 | 36.41 |
| Black, non-Hispanic | 13.3 | 16.4 | 11.5 | 12.6 | ||
| Asian/Pacific Islander | 4.9 | 7.4 | 3.7 | 4.1 | ||
| Hispanic | 14.1 | 1.7 | 3.0 | 1.7 | ||
| Other | 2.2 | 13.8 | 11.4 | 17.7 | ||
| Education | Less than High School | 2.0 | 3.6 | 1.0 | 1.7 | 55.02 |
| High School Grad | 16.3 | 14.3 | 14.4 | 20.6 | ||
| Some College | 40.7 | 34.0 | 41.1 | 46.2 | ||
| College Degree or Higher | 41.0 | 48.0 | 43.5 | 31.6 | ||
| ST use | Never User | 83.6 | 97.2 | 81.4 | 73.8 | 157.86 |
| Former User/Trier | 10.7 | 1.9 | 14.7 | 13.8 | ||
| Current User | 5.7 | 0.9 | 3.9 | 12.4 |
ST: smokeless tobacco.
Chi-square tests showed all variables differed by smoking status at the p < .001 level.
Awareness and interest in snus.
Overall, 40% of adult participants respond reported awareness of snus, and of those who reported awareness, 6% reported use of snus. Awareness varied by smoking status, χ2(2, n = 2,007) = 88.70, p < .001, with 50% of current smokers, 43% of ever smokers and 24% of non-smokers knowing of snus. No differences in awareness were present by age. There were no significant interactions by smoking status on snus interest. Few respondents (9.1%) selected Camel Snus as the product that they were most interested in trying, compared to the products displayed in the other advertisements.
Objective 1: attitudes about advertising and perception of health risk claims.
Results are presented for all five of the advertisement types together. Predictors of correctly understanding the health risk claim were analysed in a multinomial logistic regression model predicting choosing the correct response to the health risks question (‘the advertisement suggests or implies that the health risks of Camel Snus are less than other tobacco products’), with advertisement type as the main predictor, adjusted for age, sex, race, smoking status, and awareness of snus products. Participants were less likely to choose any of the incorrect responses if they saw a claim relative to the no-claim control (testimonial and bar chart advertisement odds ratio [OR] = .21; 95% confidence interval [CI]: .12–.37; written and testimonial advertisement OR = .30; 95% CI: .18–.52; bar chart advertisement OR = .29; 95% CI: .17–.51; written advertisement OR = .33; 95% CI: .19–.56). Odds ratios and 95% CIs for this model are presented in Table 2.
Table 2.
Multinomial logistic regression model predicting knowledge of the health risks of Camel Snus among adults.
| No risk | Less risk | Same risk | More risk | |
|---|---|---|---|---|
| Control | REF | REF | REF | REF |
| Written | .33 (.19–.56) | REF | .13 (.09–.19) | .18 (.01–.31) |
| Bar chart | .30 (.17–.51) | REF | .15 (.11–.21) | .20 (.12–.33) |
| Written + Testimonial | .30 (.18–.52) | REF | .10 (.07–.14) | .25 (.16–.41) |
| Bar + Testimonial | .21 (.12–.37) | REF | .11 (.08–.16) | .22 (.13–.36) |
Smokers reported less scepticism of the advertisement content than never smokers and ever/former smokers (F = 7.56, p < .001, partial η2 = .008), and never smokers reported that the advertisement implied more risk compared with other tobacco products than ever/former smokers (F = 5.34, p < .005, partial η2 = .005). No differences by smoking status were present on the overall truthfulness of advertisement content. In a combined measure of truthfulness and scepticism (Figure 2), where higher values represented less scepticism towards the advertisement content and a belief that the content was more truthful, smokers were less sceptical overall towards the content and believe that the advertisement was more truthful when compared to non-smokers (F = 5.71, p = .003, partial η2 = .006). There was no significant interaction between advertisement type and smoking status (F = 1.64, p = .108, partial η2 = .007). There was an overall significant main effect of advertisement type (F = 13.30, p < .001, partial η2 = .026). Post hoc analysis examining pairwise comparisons between the no-claim control advertisement and the advertisements containing actual claim content showed significant mean differences (at the .05 level) for measures of truthfulness, scepticism, and the combined measure of truthfulness and scepticism. However, none of the advertisements containing actual claim content differed significantly from one another.
Figure 2.
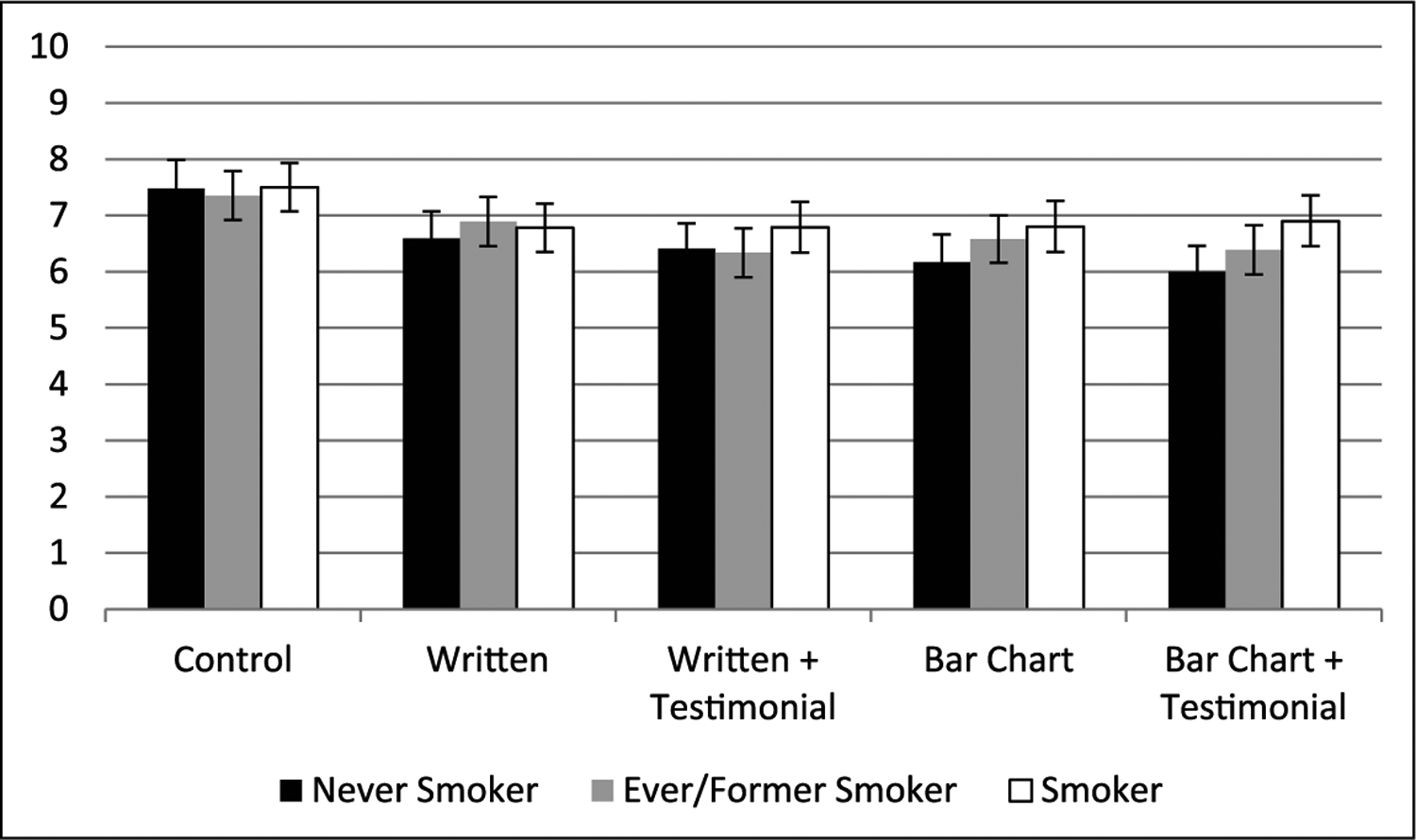
Degree of scepticism and truthfulness of ad content among adults (higher values indicate less sceptical/more truthful).
Error bars represent 95% confidence intervals.
Objective 2: effect of claim format on interest in trying and intention to purchase.
Advertisement claim format was not associated with selecting snus over the other tobacco products (American Spirit or Nicorette) (p = .684), though being male rather than female (OR: 3.17, CI: 2.21–4.56), younger rather than older (18–34: OR: 3.11, CI 2.02–4.78; 35–50: OR: 1.60, CI: 1.05–2.44) Hispanic rather than non-Hispanic (OR: 2.28, CI: 1.51–3.42) and snus use in the past 12 months rather than no past 12 month snus use (OR: 2.79, CI: 1.65–4.72) increased odds of selecting snus. Among those who were more interested in trying snus (N = 183), we evaluated intention to purchase snus in the next month. Advertisement claim format was not associated with purchase intentions; however, current smoking (OR: 21.98, CI: 6.39–75.61) and past 12 month snus use (OR: 6.56, CI: 1.90–22.57) were significantly associated with increased odds of purchasing snus in the next month when compared to non-current smokers and non-snus users.
Youth analyses
Sample demographics and tobacco use patterns.
The youth sample comprised mostly 16- and 17-year-olds, with a higher proportion of females; a greater percentage (74%) described themselves as White (Table 3). One-quarter of youth reported having tried cigarettes, at least a few puffs, and 10% reported currently smoking some days or every day. No significant differences in smoking status were identified by sex or race, though 15-year-olds were less likely than 16- and 17-year-olds to report having tried cigarettes, χ2(2, n = 1,000) = 13.13, p < .001, and report ST use, χ2(2, n = 1,000) = 114.26, p < .001.
Table 3.
Sample demographics and tobacco use among youth (N = 1,000).
| All | Tried cigarettes (24.8%) | Never tried cigarettes (75.2%) | Chi-square | ||
|---|---|---|---|---|---|
| Age | 15 | 27.9 | 19.0 | 30.9 | 13.13* |
| 16 | 33.8 | 37.9 | 32.4 | ||
| 17 | 38.3 | 43.1 | 36.7 | ||
| Sex | Male | 43.4 | 45.2 | 43.4 | 0.248 |
| Female | 56.6 | 54.8 | 56.6 | ||
| Race/ethnicity | White, non-Hispanic | 73.5 | 77.4 | 72.2 | 6.974 |
| Black, non-Hispanic | 8.5 | 8.5 | 8.5 | ||
| Asian/Pacific Islander | 6.4 | 3.2 | 7.4 | ||
| Hispanic | 8.6 | 2.0 | 3.3 | ||
| Other | 3.0 | 8.9 | 8.5 | ||
| Snus awareness | Yes | 27.9 | 35.5 | 64.5 | 23.68* |
| No | 72.1 | 20.7 | 79.3 | ||
| Snus use | Past 12 month use | 3.0 | 10.1 | 0.7 | 56.82* |
| Never use snus | 97.0 | 89.9 | 99.3 | ||
| ST use | Never User | 93.3 | 78.6 | 98.1 | 114.26* |
| Former User/Trier | 3.6 | 10.9 | 1.2 | ||
| Current User | 3.1 | 10.5 | 0.7 |
ST: smokeless tobacco.
Significant at p < .001 level.
Awareness and interest in snus.
Overall, 28% of young people reported awareness of snus. Those who had tried cigarettes were more likely to be aware of, χ2(1, n = 1,000) = 23.68, p < .001, and use snus, χ2(1, n = 1,000) = 56.82, p < .001, than those who had not tried cigarettes. In all, 7% reported that Camel Snus was the product that they were most interested in trying and having tried cigarettes was associated with selecting snus, χ2(1, n = 1,000) = 7.76, p < .007. Among those who selected snus, 25% (N = 18) expressed a fair to certain interest in purchasing the product within the next month.
Objective 1: attitudes about advertising and perception of health risk claims.
As in the adult analyses, results are presented for all five advertisement types, together. Results are presented for all five of the advertisement types together. Predictors of correctly understanding the health risk claim were analysed in a multinomial logistic regression model predicting choosing the correct response to the health risks question (‘the advertisement suggests or implies that the health risks of Camel Snus are less than other tobacco products’), with advertisement type as the main predictor, adjusted for age, sex, race, smoking status, and awareness of snus products. Across all claim conditions, participants were less likely to choose any of the incorrect responses if they saw any of the claims relative to the no-claim control (testimonial and bar chart advertisement OR = .28; 95% CI: .13–.60) (written and testimonial advertisement OR = .27; 95% CI: .13–0.) (bar chart advertisement OR = .20; 95% CI: .09–.45) (written advertisement OR = .34; 95% CI: .17–.70). Odds ratios and 95% CIs for this model are presented in Table 4.
Table 4.
Multinomial logistic regression model predicting knowledge of the health risks of Camel Snus among youth.
| No risk | Less risk | Same risk | More risk | |
|---|---|---|---|---|
| Control | REF | REF | REF | REF |
| Written | .34 (.17–.70) | REF | .12 (.07–.20) | .26 (.13–.49) |
| Bar chart | .20 (.09–.45) | REF | .16 (.10–.25) | .16 (.08–.33) |
| Written + Testimonial | .27 (.13–.56) | REF | .08 (.04–.13) | .18 (.09–.36) |
| Bar + Testimonial | .28 (.13–.60) | REF | .14 (.09–.23) | .16 (.08–.33) |
Ever use of cigarettes was associated with less scepticism of the advertisement content than never users (F = 7.94, p < .005, partial η2 = .026). All advertisements that contained an explicit reduced risk message, except the text-only message, were associated with increased scepticism (F = 15.79, p < .001, partial η2 = .031) of advertisement content compared with the no-claim control. Each of the four advertisements with a reduced risk message was viewed as less truthful (F = 8.43, p < .001, partial η2 = .017), and implied less risk (F = 28.11, p < .001, partial η2 = .054) than the no-claim control. Post hoc analysis examining pairwise comparisons between the no-claim control advertisement and the advertisements containing actual claim content showed significant mean differences (at the .05 level) for measures of truthfulness, scepticism and the combined measure of truthfulness and scepticism. However, none of the advertisements containing actual claim content differed significantly from one another.
In a combined measure of truthfulness and scepticism (Figure 3), where higher values represented less scepticism towards the advertisement content and a belief that the content was more truthful, ever smokers were more likely than never smokers to be less sceptical towards the content and believe that the advertisement was more truthful (F = 5.10, p = .024, partial η2 = .005). There was no significant interaction between advertisement type and smoking status (F = .717, p = .581, partial η2 = .003). There was an overall significant main effect of advertisement type (F = 10.45, p < .001, partial η2 = .041).
Figure 3.
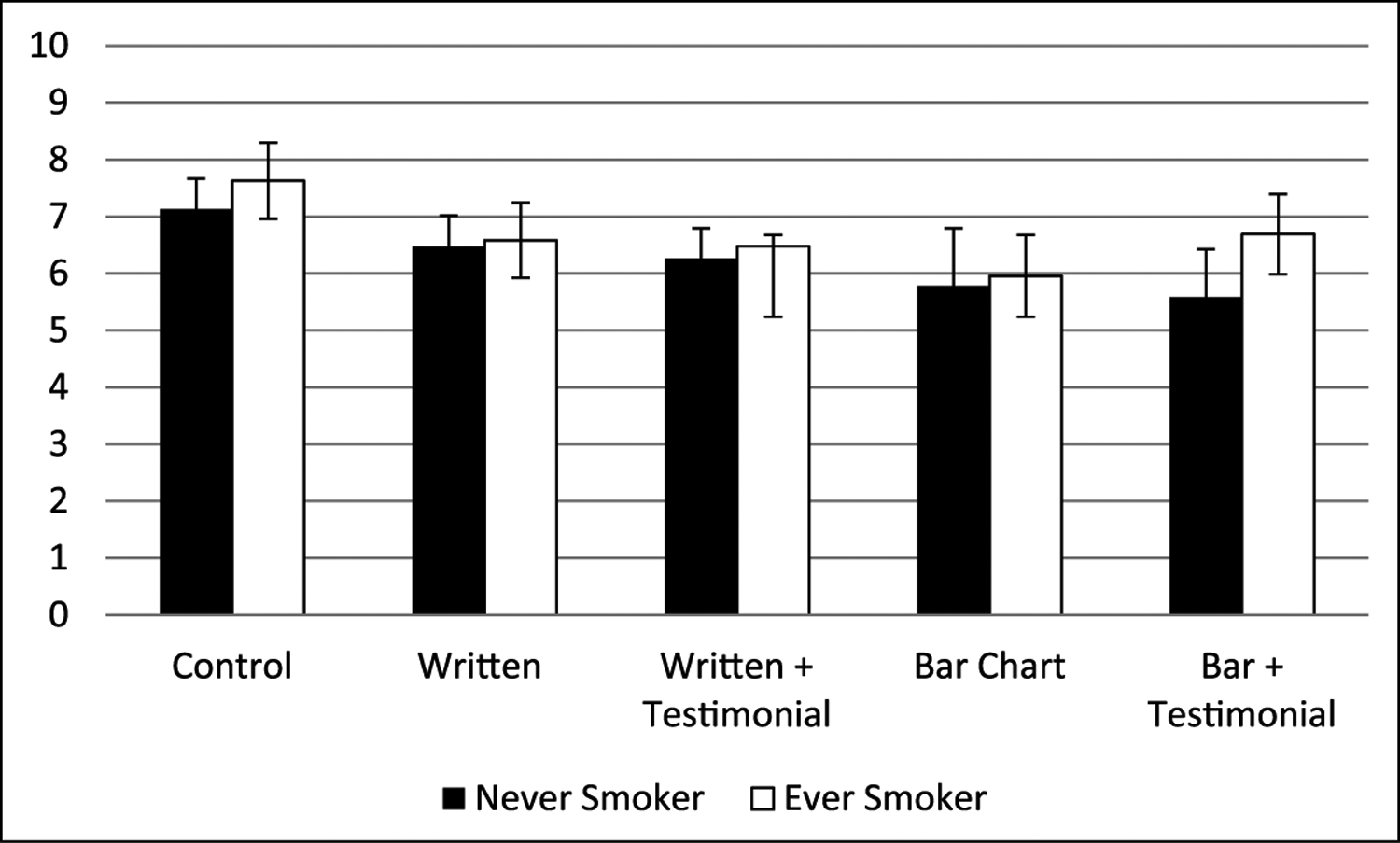
Degree of scepticism and truth of ad content among youth (higher values indicate less sceptical/more truthful).
Error bars represent 95% confidence intervals.
In the youth sample, neither truthfulness nor scepticism were significantly associated (p < .001) with selecting snus, nor were they associated with intent to purchase snus.
Objective 2: effect of claim format on product interest and intention to purchase.
Claim style was not associated with selecting snus, though being male rather than female (OR: 2.11, CI: 1.26–3.53)’ Asian/Pacific Islander (OR: 2.567, CI: 1.06–6.25), Hispanic (OR: 3.78, CI: 1.36–10.46) or another race (3.41, CI: 1.59–7.31) compared to White; and reporting awareness compared to those who reported they were not aware of snus (OR: 2.13, CI: 1.22–3.71); and snus use in the past 12 months compared to non-snus users (OR: 2.76, CI: 1.63–4.69) increased the odds of selecting snus as the product with most interest in trying. Among youth who selected snus (N = 73), 25% (N = 18) reported good to certain intentions to purchase snus within the upcoming month.
Discussion
Findings from this study show that participants exposed to advertisements that contained an explicit modified risk message rated that product as posing fewer health risks than traditional cigarettes. However, the specific advertisement format (text only, graph only, combined text and testimonial, combined graph and testimonial) did not differentially impact risk perceptions about snus. Given existing literature suggesting we should expect to find differences by format (Ancker et al., 2006; Biener et al., 2007; Kapsak et al., 2008; McCaffery et al., 2012), this null finding was a surprise. A possible reason may be that the nitrosamine claim itself was not particularly salient – nitrosamines are fairly obscure (5% awareness), (Hall et al., 2014) even though they are strong carcinogens and directly linked to smoking-attributable cancers (Hatsukami et al., 2015; Hecht, 2014). Results may have differed with claims about other constituents, or with a claim of disease risk reduction, issues which future studies can address.
Respondents rated advertisements containing the explicit reduced risk messages as less truthful and were more sceptical of the information that was presented. Given the well-publicised history of deception on the part of the tobacco industry (US District Court 2006) and decades of health promotion around tobacco, it is perhaps not surprising to find high levels of scepticism towards claims about tobacco products. Studies of health claims for tobacco products should include metrics of scepticism and perceived truthfulness, as it cannot be assumed that claims will be believed, whether factually supported or not.
Among adults, smoking and prior experiences with snus were associated with interest in snus. This may suggest that the message is reaching the intended population (those who might be more open to using snus) and that smokers are more likely to pay attention to the reduced risk messages. A similar general pattern was found among youth. In both cases, we did not find that non-smokers were likely to report a future intention to purchase snus, contrary to findings reported by Mays et al. (2015). This suggests that participants who had already made up their minds about trying snus (smokers not interested in switching, non-smokers not interested in trying and people with low snus awareness) were only influenced somewhat by the messages. This is not a limitation, but rather it is noteworthy and might provide an indication of the power of messaging. Specifically, inclusion of a reduced risk message may not have the anticipated effect of increasing snus substitution, but instead may have the opposite effect of increasing scepticism.
Some limitations should be considered when interpreting the results from this research. The measure of snus interest is a three-level choice and we lack the data to verify whether the choice in this context reflects real-world choices. Future research to validate this measurement approach would be useful. Additionally, these three choices were forced. In other words, there was no ‘none of the above’ option. When we received the data, we noted that almost all non-smokers selected nicotine gum, such that it became a de facto ‘none of the above’ for that group. This is limiting in that it might not reflect a true feeling about a particular choice. Overall, awareness of and interest in snus was low in both the adult (40%) and youth sample (<30%). Very few never smokers where aware of or had ever used snus in the past, and most said they had no interest in trying snus. This is consistent with the broader literature on snus – sales and prevalence have remained low for the past several years, despite marketing and advertising campaigns by the tobacco industry (Biener et al., 2016). Our data were derived from a consumer opt-in Internet panel and are not reflective of the population as whole. For example, the sample used in these analyses report a higher level of education and these data have a higher prevalence of smoking than would be found in the US population. Second, we were unable to evaluate whether consumers actually intend to purchase snus for harm-reduction purposes and lack prospective data that would shed light on behavioural outcomes. In this experiment, the overall low interest in snus is a major limiting factor. Showing the advertisement once may not make much of a difference in those who are not predisposed to try Camel Snus.
Implications for tobacco regulation
The results of this research do indicate that consumers respond to reduced risk messages, though perhaps not in the direct way anticipated. We found no significant differences by advertisement format (numerical, graphical, testimonial). Both adults and the young people in our sample reported that the advertisements containing the reduced exposure message indicated that the product posed less harm to the consumer than smoking, but also expressed scepticism about this messaging. Current smoking status did not moderate the effect of the advertising claims on interest in snus. From a public health policy perspective, it would have been ideal if we observed reduced risk advertising claims spurring interest in snus among current cigarette smokers while having no effect among non-smokers.
Study findings suggest that including reduced exposure claims in product advertising may not necessarily lead to public health gains. Future research should evaluate how other claim and messaging formats influence behavioural decision making and changes in product use patterns over time.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided by a grant from the US National Institutes of Health (U19CA157345).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K. Michael Cummings has received grant funding from Pfizer, Inc. to study the impact of a hospital based tobacco cessation intervention and has also served as an expert witness in litigation filed against the tobacco industry.
References
- Ancker JS, Senathirajah Y, Kukafka R, et al. (2006) Design features of graphs in health risk communication: A systematic review. Journal of the American Medical Informatics Association 13(6): 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal-Travers M, Hammond D, Smith P, et al. (2011) The impact of cigarette pack design, descriptors, and warning labels on risk perception in the U.S. American Journal of Preventive Medicine 40(6): 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal-Travers M, O’Connor R, Fix BV, et al. (2011) What do cigarette pack colors communicate to smokers in the U.S.? American Journal of Preventive Medicine 40(6): 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Bogen K and Connolly G (2007) Impact of corrective health information on consumers’ perceptions of ‘reduced exposure’ tobacco products. Tobacco Control 16(5): 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Mccausland K, Curry L, et al. (2011) Prevalence of trial of Snus products among adult smokers. American Journal of Public Health 101(10): 1874–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Roman AM, McInerney SA, et al. (2016) Snus use and rejection in the USA. Tobacco Control 25(4): 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Levy DT and Meza R (2016) Trends and factors related to smokeless tobacco use in the united states. Nicotine & Tobacco Research 18: 1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave DM and Saffer H (2013) Demand for smokeless tobacco: Role of advertising. Journal of Health Economics 32: 682–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnevo CD, Wackowski OA, Giovenco DP, et al. (2012) Examining market trends in the United States smokeless tobacco use: 2005–2011. Tobacco Control 23(2): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood KC, Trumbo PR and Kavanaugh CJ (2010) How the US food and drug administration evaluates the scientific evidence for health claims. Nutrition Reviews 68(2): 114–121. [DOI] [PubMed] [Google Scholar]
- Gartner CE, Hall WD, Chapman S, et al. (2007) Should the health community promote smokeless tobacco (Snus) as a harm reduction measure? PLoS Medicine 4: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MG, Ribisl KM and Brewer NT (2013) Smokers’ and nonsmokers’ beliefs about harmful tobacco constituents: Implications for FDA communication efforts. Nicotine & Tobacco Research 16(3): 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Lemmonds C and Tomar SL (2004) Smokeless tobacco use: Harm reduction or induction approach? Preventive Medicine 38(3): 309–317. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Stepanov I, Severson H, et al. (2015) Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prevention Research 8(1): 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS (2014) It is time to regulate carcinogenic tobacco-specific nitrosamines in cigarette tobacco. Cancer Prevention Research 7(7): 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies (2012) Scientific standards for studies on modified risk tobacco products. Available at: https://www.nationalacademies.org/hmd/~/media/Files/Activity%20Files/PublicHealth/Modified-Risk-Tobacco/MRTP_rb.pdf (accessed 7 September 2016).
- Kapsak WR, Schmidt D, Childs NM, et al. (2008) Consumer perceptions of graded, graphic and text label presentations for qualified health claims. Critical Reviews in Food Science and Nutrition 48(3): 248–256. [DOI] [PubMed] [Google Scholar]
- Kaufman AR, Mays D, Koblitz AR, et al. (2014) Judgments, awareness, and the use of Snus among adults in the United States. Nicotine & Tobacco Research 16(10): 1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi MT and Kozlowski LT (2015) Deficiencies in public understanding about tobacco harm reduction: Results from a United States national survey. Harm Reduction Journal 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostygina G, England L and Ling P (2016) New product marketing blurs the line between nicotine replacement therapy and smokeless tobacco products. American Journal of Public Health 106(7): 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski L and Sweanor D (2016) Withholding differential risk information on legal consumer nicotine/tobacco products: The public health ethics of health information quarantines. The International Journal on Drug Policy 32: 17–23. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT and Abrams DB (2016) Obsolete tobacco control themes can be hazardous to public health: The need for updating views on absolute product risks and harm reduction. BMC Public Health 16: 432. Available at: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-3079-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Mumford EA, Cummings KM, et al. (2004) The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention 13: 2035–2042. [PubMed] [Google Scholar]
- McCaffery KJ, Dixon A, Hayen A, et al. (2012) The influence of graphic display format on the interpretations of quantitative risk information among adults with lower education and literacy: A randomized experimental study. Medical Decision Making : An International Journal of the Society for Medical Decision Making 32: 532–544. [DOI] [PubMed] [Google Scholar]
- Mays D, Moran MB, Levy DT, et al. (2015) The impact of health warning labels for Swedish Snus advertisements on young adults’ Snus perceptions and behavioral intentions. Nicotine & Tobacco Research 18(5): 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie C, Ford A, Mackintosh A, et al. (2015) Are all cigarettes just the same? Female’s perceptions of slim, coloured, aromatized and capsule cigarettes. Health Education Research 30(1): 1–12. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Bansal-Travers M, Cummings KM, et al. (2015) Filter presence and tipping paper color influence consumer perceptions of cigarettes. BMC Public Health 15: 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Norton KJ, Bansal-Travers M, et al. (2011) US smokers’ reactions to a brief trial of oral nicotine products. Harm Reduction Journal 8: 1477–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters S and Gilmore AB (2013) How online sales and promotion of snus contravenes current European union legislation. Tobacco Control 22(4): 266–273. [DOI] [PubMed] [Google Scholar]
- Peeters S and Gilmore AB (2013) Transnational tobacco company interests in smokeless tobacco in Europe: Analysis of internal industry documents and contemporary industry materials. PLoS Medicine 10(9): e1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L and Ling PM (2014) Nonsmokers’ responses to new warning labels on smokeless tobacco and electronic cigarettes: An experimental study. BMC Public Health 14: 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L, Neilands TB and Ling PM (2014) Testing messages to reduce smokers’ openness to using novel smokeless tobacco products. Tobacco Control 23: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Law 111–31 HR 1256 (2016). Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf (accessed 25 April 2016).
- Rees VW, Kreslake JM, Cummings KM, et al. (2009) Assessing consumer responses to potential reduced-exposure tobacco products: A review of tobacco industry and independent research methods. Cancer Epidemiology, Biomarkers, & Prevention 18: 3225–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RJ Reynolds Tobacco Company (2003) Comments to proposed rulemaking, fire safety standards for cigarettes. RJ Reynolds. Available at: http://tobaccodocuments.org (Bates No. 528767372) [Google Scholar]
- Schwartz LM and Woloshin S (2013) The drug facts box: Improving the communication of prescription drug information. Proceedings of the National Academy of Sciences 110(Suppl. 3): 14069–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Bansal-Travers M, Oconnor R, et al. (2011) Correcting over 50 years of tobacco industry misinformation. American Journal of Preventive Medicine 40(6): 690–698. [DOI] [PubMed] [Google Scholar]
- Tan CE, Kyriss T and Glantz SA (2013) Tobacco company efforts to influence the food and drug administration-commissioned institute of medicine report clearing the smoke: An analysis of documents released through litigation. PLoS Medicine 10(5): e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL (2007) Epidemiologic perspectives on smokeless tobacco marketing and population harm. American Journal of Preventive Medicine 33(6): S387–S397. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Fox BJ and Severson HH (2009) Is smokeless tobacco use an appropriate public health strategy for reducing societal harm from cigarette smoking? International Journal of Environmental Research and Public Health 6(1): 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2012) Preventing Tobacco Use among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- US Department of Health and Human Services (2014) The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- US District Court for the District of Columbia (2006) No. 99-CV-02496GK (Final Opinion). Available at: http://www.tobaccocontrollaws.org/files/live/litigation/2425/US_United%20States%20v.%20Philip%20Morris.pdf
- Wackowski OA and Delnevo CD (2015) Young adults’ risk perceptions of various tobacco products relative to cigarettes. Health Education & Behavior 43(3): 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M, Morley C, Horan JK, et al. (2002) The cigarette pack as image: New evidence from tobacco industry documents. Tobacco Control 11(Suppl. 1): i73–i80. [DOI] [PMC free article] [PubMed] [Google Scholar]


