Abstract
Most of follicles undergo a degenerative process called follicular atresia. This process directly affects the egg production of laying hens and is regulated by external and internal factors. External factors primarily include nutrition and environmental factors. In follicular atresia, internal factors are predominantly regulated at 3 levels; organic, cellular and molecular levels. At the organic level, the hypothalamic-pituitary-ovary (HPO) axis plays an essential role in controlling follicular development. At the cellular level, gonadotropins and cytokines, as well as estrogens, bind to their receptors and activate different signaling pathways, thereby suppressing follicular atresia. By contrast, oxidative stress induces follicular atresia by increasing ROS levels. At the molecular level, granulosa cell (GC) apoptosis is not the only factor triggering follicular atresia. Autophagy is also known to give rise to atresia. Epigenetics also plays a pivotal role in regulating gene expression in processes that seem to be related to follicular atresia, such as apoptosis, autophagy, proliferation, and steroidogenesis. Among these processes, the miRNA regulation mechanism is well-studied. The current review focuses on factors that regulate follicular atresia at organic, cellular and molecular levels and evaluates the interaction network among these levels. Additionally, this review summarizes atretic follicle characteristics, in vitro modeling methods, and factors preventing follicular atresia in laying hens.
Key words: laying hen, follicular atresia, regulation factor, interaction network regulation
INTRODUCTION
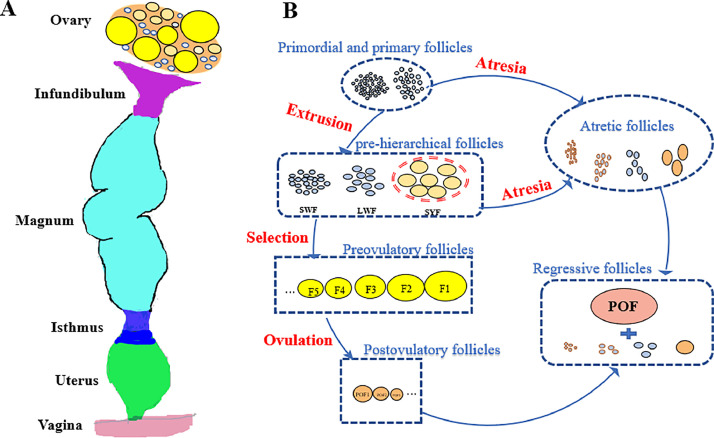
Egg production performance is the key economic aspect of the poultry industry, which is closely related to follicular development and ovulation. The reproductive tract of the laying hen is divided into the ovary and oviduct (Figure 1A). As shown in Figure 1B, the mature ovary of chicken contains numerous follicles. Based on their small to large sizes, these follicles are classified as primordial follicles (up to 80 µm), primary follicles (0.08–2 mm), pre-hierarchical follicles (2–8 mm), and preovulatory follicles (FN–F1) (Johnson, 2015). When a follicle is not selected, it undergoes to atresia. The number of pre-hierarchical follicles developing into preovulatory follicles decreases, thereby reducing egg production performance (Johnson, 1993; Yao et al., 2020). Therefore, the internal and external factors associated with follicular atresia in chickens as well as the regulatory and signaling pathways should be extensively studied.
Figure 1.
Anatomy of the reproductive tract (A) and the process of follicular development (B) in chicken. SWF, small white follicles; LWF, large white follicles: SYF, small yellow follicles: POF, postovulatory follicles.
Although studies have shown that follicular atresia may be related to varieties of internal and external factors, the regulatory mechanisms of follicular atresia are still lacking in chickens. At present, follicular atresia-inducing factors primarily include nutritional factors, environmental factors, etc (Korver, 2023). The regulation of reproductive endocrine in chickens resembles to that in mammals, which is regulated by the HPO axis (Zhao et al., 2023). According to numerous studies, follicular development requires the synergistic action of hormones and cytokines for ovulation or follicular atresia (Matsuda et al., 2012). Granulosa cells (GCs) also play a crucial role in apoptosis-initiated follicular atresia, although recent studies have reported that autophagy is also involved in follicular atresia (Bhardwaj et al., 2022).
In livestock and poultry studies, epigenetics predominantly regulates the expression of genes at transcriptional and post-transcriptional levels through non-coding RNAs, histone modifications and DNA methylation (Zhu et al., 2015). Therefore, the in-depth study of epigenetic regulatory mechanisms may have certain guiding significance for preventing follicular atresia of laying hens. In this review, we summarize the characteristics, influencing factors and regulation of follicular atresia at organic, cellular and molecular levels, so as to provide a scientific basis for improving egg production performance.
OVARIAN FOLLICULAR ATRESIA IN CHICKEN
Characteristics of Follicular Atresia
In modern commercial poultry production, follicular development and atresia in layers are garnering increased attention compared with broilers. Chicken ovaries are quite different from mammalian ovaries, because chicken ovaries have different sized follicles at all times and do not develop at the same time (Evans, 2003). Follicular atresia is a physiological or pathological process in which follicles are disintegrated, absorbed, and thus fail to develop into mature follicles (Mohd et al., 2023). During the laying period, physiological follicular atresia often occurs in pre-hierarchical follicles, whereas preovulatory follicles rarely undergo atresia except under stress (Gilbert et al., 1983). When laying hens are brooding, they exhibit maternal behavior accompanied by elevated prolactin (PRL)-induced physiological follicular atresia (Yu et al., 2023).
In poultry production, the number of atretic follicles directly affects the egg production rate. In poultry ovaries, most follicles become atretic during development, and less than 5% of the follicles transition from the primordial follicle to the mature follicle and ovulate (Yao et al., 2020). The follicle is chiefly composed the theca cell (TC) layer, GC layer, and oocytes. The TC layer is formed in the prehierarchical follicle and differentiates into 2 inner and outer layers. When a mature follicle is formed, the TC layer becomes thicker with the inner and outer layers becoming well-distinct. The GC layer is undifferentiated and flat in the primordial follicle. In the pre-hierarchical follicle, it becomes multilayered. Then, a single layer is formed as the follicle develops into a mature follicle. The oocyte remains in the primary oocyte state until mature follicle is formed, which then develops into a secondary oocyte (Perry et al., 1978).
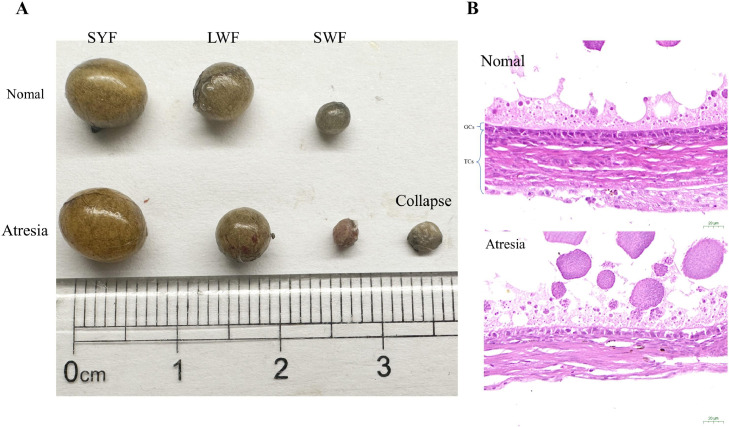
As shown in Figure 2, follicular atresia is identified by the presence of bleeding spots on its surface. These follicles deform or collapse at advanced stages (from small white follicles (SWF) to form small yellow follicles (SYF)) (Gilbert et al., 1983). Follicular atresia occurs when the proportion of apoptotic GCs in the developing follicle reaches more than 10% (Johnson et al., 1997). Histopathologically, the cell layer in the atretic follicle is loose, and part or all of the follicular cells enter the follicle cavity. Then, the follicle wall becomes wrinkled, and the follicle cavity becomes irregular and contains small tissue fragments (Yao et al., 2020). The results of transmission electron microscopy (TEM) demonstrate that the boundaries of atretic follicles are blurred, and the boundaries between the GC and TC layers are unrecognizable. The apoptotic GCs exhibit cytoplasmic accumulation, vacuoles, and contraction. Moreover, changes in organelles and accumulation of autophagosomesr were observed (Dong et al., 2022).
Figure 2.
Appearance (A) of atretic or collapsing follicles, and histopathology (B) of folicular atresia in silky black bone chicken. Data are not published from our laboratory. GCs, granulosa cells: TCs, theca cells.
Methods for Establishing Follicular Atresia Models
Atresia is a physiological or pathological process of follicular development observed in chickens during whole egg production stage (Mfoundou et al., 2021). The regulatory mechanisms underlying follicular atresia must be investigated. In vitro, subcutaneous injection of tamoxifen led to the successful construction of a follicular atresia model of chicken (Han et al., 2023). Furthermore, mercury (Hg), a highly toxic heavy metal, induces endoplasmic reticulum stress (ERS), which triggers atresia. This significantly reduces the laying performance of laying hens (Ma et al., 2022). Cadmium (Cd) induces reactive oxygen species (ROS) production and cell apoptosis to impair follicle development (Zhu et al., 2021). Fasting can also accelerate atresia to remodel the ovarian physiology, which has been used to offer avenues for further improvements in poultry production (Han et al., 2022). Hence, follicles are susceptible to abnormal atresia under various stresses, such as nutritional stress, environment stress and abused drugs. Moreover, nesting is often used as an atretic model.
Establishing an atretic follicle model in vitro can further explain the possible regulatory mechanism that improves the egg laying performance. Table 1 presents the methods of establishing a follicular atresia model. Oxidative stress models are also commonly used to study follicular atresia. For example, the H2O2-induced atretic follicle model was constructed to investigate the effect of antioxidants on oxidative stress reduction in chicken ovaries (Bao et al., 2022). The follicular atresia rate increased at the late egg laying stage in chickens. Therefore, D-galactose was used to induce GC senescence through oxidative stress (Dong et al., 2022). A serum withdrawal-induced follicular atresia model was established to investigate ERS can cause follicular atresia (Huang et al., 2022). More importantly, follicular atresia is initiated GC apoptosis, and apoptosis models are established by adding an apoptosis inducer (Zhou et al., 2022), autophagy inhibitor (Zhou et al., 2022) or mTOR inhibitor (Dong et al., 2022), which play an essential role while exploring atresia.
Table 1.
Methods for inducing follicular atresia in vitro and in vivo.
| Species | Age | Drug and treatment methods | Times | Function | Reference |
|---|---|---|---|---|---|
| Tianfu green shell laying hens | 22-wk-old | 6 mg/0.3 mL ethanol/kg per body weight Tamoxifen | 6 d | estrogen receptor modulator | (Han et al., 2023) |
| white leghorn layers | 32-wk-old | fasting | 5 d | nutritional stress | (Han et al., 2022) |
| Hyline-Brown laying hens | 42-wk-old | 27 mg/kg Hg | 10 wk | highly toxic heavy metal | (Ma et al., 2022) |
| Hyline brown laying hens and GCs of LWF | 38-wk-old | 7.5, 15, 30 and 60 mg/kg Cd, cells were treated with 0, 1, 5, 10, 15, 20, 30, 40 μM Cd | 10 wk, 24 or 48 h, | highly toxic heavy metal | (Zhu et al., 2021) |
| SWF from Hyline white hens | D280 and D580 hens | 1 mM H2O2 | 72 h | oxidative stress induced aging | (Bao et al., 2022; Chen et al., 2024) |
| SWF from Hyline white hens | D280 and D580 hens | 10 mg/mL D-galactose | 72 h | oxidative stress induced aging | (Bai et al., 2024) |
| GCs from SYF in Hyline white hens | D280 | 0, 12.5, 25, 50, 100, 200 mM D-galactose | 12 h or 24 h | oxidative stress | (Dong et al., 2022) |
| GCs from SYF in Hyline white hens | D280 | 50 μM nimustine hydrochloride | 24 h | apoptosis inducer | (Zhou et al., 2022) |
| GCs from SYF in Hyline white hens | 300-day-old | 0, 0.5, 5, 50, 500 nM Rapamycin | 4 h, 8 h or 12 h | mTOR inhibitor | (Dong et al., 2022) |
| GCs from SYF in Hyline white hens | D280 or D580 | 10 mM 3-methyladenine | 1 h | autophagy inhibitor | (Zhou et al., 2022) |
Follicular Atresia Inducing Factors
The laying performance of hens has been improved through advancements in genetic, environmental and nutritional factors (Korver, 2023). Simultaneously, chickens during the laying stage are prone to oxidative stress, which leads to follicle atresia. This may be the key reason for the reduction in egg production by laying hens under stress (Wang et al., 2021).
Adequate dietary energy guarantees a better reproductive performance of laying hens. Glucose and fatty acids are primary energy sources for ensuring rapid follicle development in chicken ovaries (Seol et al., 2006). Hence, dietary energy levels meet needs of weight gain, and bone and reproductive system development (Bermudez et al., 2023). During prolonged fasting, chick germ cells are lost and the number of primordial follicles is ultimately reduced (Chen et al., 2023). Moreover, food deprivation for 5 d induces follicle atresia and decreases gonadotropin levels, which causes a pause in egg laying at the peak of egg production (Socha and Hrabia, 2019; Han et al., 2022). However, overfeeding leads to excessive gain in chickens, and metabolic diseases, and renders GCs susceptible to apoptosis and necrosis. This further results in a sharp decline in the egg production rate or even the death of chickens and ultimately causes huge economic losses (Chen et al., 2006; Walzem and Chen, 2014). Furthermore, ad libitum feeding exacerbates earlier differentiation and steroidogenic competency of GC layer, which results in excessive and disorganized follicular growth and ultimately follicular atresia (Francoeur et al., 2021). These findings unveiled that the laying performance of laying hens varies at different nutrient levels may be because of a combination of factors, including atresia caused by changes in the levels of gonadotropins or ovarian hormones and GC viability (Onagbesan et al., 2006).
Energy restriction decreases GC apoptosis, follicular atresia, ovarian regression, and plasma estrogen concentrations to augment metabolic dysregulation and enhance the reproductive performance of chickens (Pan et al., 2014). Six-wk-old Hyline brown laying hens were divided into 3 groups and fed diets with a similar nutrition but different nitrogen corrected apparent metabolized energy (AMEn). AMEn levels in the energy restriction groups were 80% and 90% of those in the control group. This treatment was administered for 11 wk. The results indicated that energy restriction during the growing phase affects body weight uniformity and increases the total egg number per hen throughout the laying period (age: 18–72 wk) (Lu et al., 2023). According to Xie et al. (Xie et al., 2012), the hen group fed ad libitum for 2 wk developed dyslipidemia, stratified follicular atresia, and 34% lower egg production compared with the energy restriction group (30% restriction) at 26 to 28 wk. These results showed that energy restriction adjusts follicular atresia and follicular development.
Light is a key of environmental factor affecting follicular development. Light is sensed by deep brain photoreceptors, which then simulates the hypothalamus to secrete the gonadotropin-releasing hormone (GnRH) and activates the HPO axis, thereby regulating gonadal maturation and follicular development in chickens. Light can also be perceived by retinal photoreceptors, which influences melatonin secretion and antioxidant activity (Rozenboim et al., 2022). A long photoperiod accelerates ovarian atrophy and follicular atresia in the poultry (Xu et al., 2022), whereas the small-to-large follicular transition becomes slow and atresia reduces under the short photoperiod (Dandekar et al., 2001). Meanwhile, shading induces oxidative stress in the ovary of laying hens, thereby reducing the efficiency of egg production (Cheng et al., 2021).
Heat stress affects the laying performance and health status of laying hens. Li et al. (2020) suggested that heat stress induces apoptosis by activating FasL/Fas and the tumor necrosis factor-α (TNF-α) pathway, which reduces the number of preovulatory follicles, whereas increases the number of atretic follicles in laying hens. Plasma progesterone, testosterone, and estrogen levels were significantly reduced in chickens exposed to heat stress. In GCs, the mRNA expression of steroidogenic- related enzymes was also significantly reduced (Rozenboim et al., 2007). Collectively, high temperature exposure results in impaired GC functions and steroidogenic dysfunction in the egg follicles of laying hens, thereby reducing their egg production performance (Yan et al., 2022).
As industries developed, heavy metal pollution has affected follicular development and led to follicular atresia to varying degrees. Cd is a chemical pollutant and is often reported in feed. It controls the cell cycle, proliferation, and apoptosis to induce follicular atresia in chickens (Zhu et al., 2021). Hg is also deposited in ovaries, and promotes follicular atresia (Ma et al., 2018). Excessive dietary plumbum (Pb) lead can cause oxidative stress by disrupting the Nrf2-Keap1 signaling pathway, which results in follicular atresia in chicken ovaries (Ma et al., 2020).
Aging is often accompanied by a decrease in the ovarian function, and the laying rate of 580-day-old hens was significantly lower than that of 280-day-old hens (Bai et al., 2024). Age-related oxidative stress can decrease antioxidant capacity and increase autophagy and apoptosis in the ovary of laying hens, thereby resulting in follicular atresia (Chen et al., 2024). Hence, follicular atresia increases with age which decreased fertility in laying hens.
Regulation of the HPO Axis
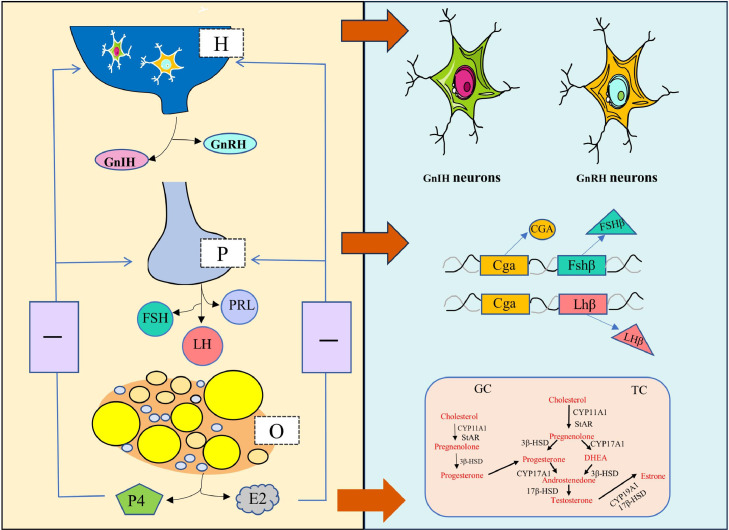
Abnormal functioning or disorders of the HPO axis induce follicular atresia in the ovary. The hypothalamus is the regulatory center of reproductive hormone homeostasis and controls follicular development. As shown in Figure 3, in the hypothalamus, gonadotropin-inhibitory hormone (GnIH) neurons secrete GnIH to inhibit the secretion of the luteinizing hormone (LH) and follicle-stimulating hormone (FSH). GnRH neurons are stimulated to secrete GnRH, GnRH receptors on the pituitary gland bind to the secreted GnRH and promote pituitary cells to secrete LH and FSH. LH and FSH comprise same α subunits, but distinct β subunits, which are closely related to egg production performance (Proudman et al., 1999). Additionally, the PRL secretion of birds is primarily composed of the PRL- releasing factors and PRL-inhibiting factors that inhibits FSH and LH (Wilkanowska et al., 2014; Du et al., 2020). Interestingly, GnRH directly binds with its receptor and inhibits GC proliferation and promotes apoptosis in vitro (Kimura, 1992). Then, the ovary receives the signal to secrete estrogen and progesterone. Both these hormones, through negative feedback precision regulation, control GnRH, LH and FSH concentrations.
Figure 3.
Regulation of reproductive hormones of the HPO axis in chicken. H, hypothalamic: P, pituitary: O, ovary: P4, progesterone; E2, estrogen.
The steroid hormone synthesis pathway includes the transport of cholesterol from the liver to the ovary. This cholesterol is used by GCs to synthesize progesterone, and by TCs to synthesize estrogen (Zhao et al., 2023). The specific underlying mechanism is that SR-BI/CD36 drives the transport of extracellular cholesterol for steroidogenesis. Cholesterol delivery to the mitochondrial matrix is regulated by the mitochondrial targeted steroidogenic acute regulatory protein (StAR). Then, cholesterol is further converted to pregnenolone via cytochrome P450scc (CYP11A1). 3β-Hydroxysteroid dehydrogenase (3β-HSD) catalyzes the conversion of pregnenolone to progesterone, which is subsequently converted to androstenedione by cytochrome P450 17A1 (CYP17A1). Finally, P450 aromatase (CYP19A1) and 17β-HSD convert androstenedione and testosterone to estrogen. As atresia progresses, aromatase enzyme activities and consequently complete steroid production is rapidly lost (Armstrong, 1985).
Follicular atresia may be associated with abnormal levels of reproductive hormones, ovarian dysfunction, and hypothalamic dysfunction (Mikhael et al., 2019). Following hypothalamic dysfunction, GnRH secretion is inhibited or overstimulated, which leads to insufficient or excessive FSH and LH secretion by the pituitary gland. However, when GnRH binds to receptors on GCs, apoptosis is promoted, which leads to follicular atresia. The FSH concentration is the highest in high-producing laying hens, which is sufficient for hens to lay eggs once per day (Prastiya et al., 2022). The reduction in FSH receptor (FSHR) and LH receptor (LHR) expression in the ovary lead to a decreased responsiveness to FSH and LH, respectively, thereby causing a failure of normal follicular development. Moreover, the progesterone added induces follicular atresia, whereas, in vivo elevation in estrogen levels reduces the rate of follicular atresia (Manikkam and Rajamahendran, 1997). The difference is that in vitro, progesterone and estrogen levels are reduced by inhibiting the synthesis of steroid hormones in the ovary. Furthermore, nutritional and environmental factors regulate the function of the HPO axis (Zhao et al., 2023). These results validated that the HPO axis plays an essential role in regulating the follicle fate.
Regulation of Hormones and Cytokines In GCs
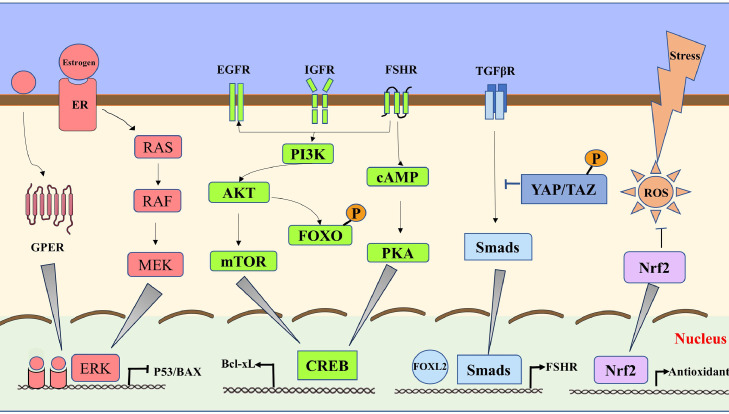
Follicular atresia is a noninflammatory spontaneous process that occurs during follicular development. It generally results from the lack of cytokines or hormones that control cell survival or death (Matsuda et al., 2012). Follicular atresia in poultry is closely related to the regulation of GCs, TCs, and oocytes. However, in atretic follicles, GCs are more sensitive than TCs. In summary, Figure 4 presents cell survival factors and signals in GCs. Further research is required to explore specific mechanisms in chickens.
Figure 4.
Cell survival factors and signals in GCs.
Role of Reproductive Hormones
During follicular development, various reproductive hormones are crucial players in atresia regulation. FSH (Matsuda et al., 2012) and estrogen (Xu et al., 2022) are the primary regulators of follicular atresia. Yang et al. (2023) reported that FSH and LH content in poultry were significantly increased in the healthy follicles compared with the atresia follicles. FSH primarily induces progesterone synthesis and LHR expression in the GCs of prehierarchical follicles, but after GC transitions to the differentiation stage, hierarchical follicles become insensitive to FSH (Calvo et al., 1981). FSH promotes GC proliferation and reduces apoptosis to retain the development of pre-hierarchical follicles via the AKT pathway, so as to promote cell survival and prevent atresia (Johnson, 2003; Guo et al., 2019). In chickens, the PI3K/Akt signaling pathway phosphorylates forkhead box (FOXOs) family. FOXOs drive apoptosis after dephosphorylation (Cui et al., 2019).
FSH could also stimulate the production of intracellular cyclic adenosine monophosphate (cAMP) and activation of the protein kinase A (PKA) signaling pathway by binding to FSHR, thereby significantly increasing Bcl-X (LONG) and ultimately developing resistance to follicular atresia in ovaries (Johnson et al., 1999; Johnson and Lee, 2016). Interplay of PI3K and PKA promotes the phosphorylation of the cAMP response element-binding protein (CREB). CREB is a transcription factor regulating target gene transcription (Sirotkin and Grossmann, 2003; Chen et al., 2007; Kilanowska et al., 2022). In addition, the vasculature area was significantly lower in atretic follicles than in healthy follicles. FSH enhances the development of pre-hierarchical follicles by increasing the expression of hypoxia-inducible factor (HIF), thereby augmenting angiogenesis and yolk deposition (Ma et al., 2020). Overall, FSH regulates steroidogenesis, and GC proliferation or apoptosis through P13K/AKT/FoxO3a, mTOR pathways and cAMP/PKA in laying hens.
Estrogens are involved in many ovarian physiological processes, including promoting primordial follicle development (Zhao et al., 2017), maintaining the cell phenotype, and stimulating cell proliferation (Britt and Findlay, 2002). Estrogen receptors (ERs) is a ligand-activated transcription factor regulating cellular responses to estrogens and target gene transcription. ERα and ERβ are 2 types of estrogen nuclear receptors. The ERα gene expression level was significantly enhanced compared with the ERβ gene expression level in GCs (Liu et al., 2015). ERα, thus is a pivotal player in follicular development. Many studies have summarized that G-protein-coupled ER (GPER) is a membrane ER that exerts the estrogen function (Li et al., 2022). Instead, ERs can directly interact with signaling proteins, such as the renin-angiotensin system (RAS), residual adversarial fusion (RAF), and extracellular regulated kinase (ERK), to activate the RAS/RAF/ERK pathway. This pathway then regulates specific gene expression to accelerate GC proliferation and inhibit apoptosis through ERK phosphorylation (Hong and Choi, 2018; Xu et al., 2018; Yang et al., 2023). Moreover, estrogen levels affect yolk formation in the liver (Tramunt et al., 2021), and calcium deposition in the oviduct and bone, thereby regulating follicular development, and albumin and egg shell production (Bain et al., 2016; Hanlon et al., 2022). Overall, atretic follicles produce less estrogen, and estrogen administration decreases the number of atretic follicles (Yoshimura and Tamura, 1986). Estrogen also affects non-gonadal tissues, thereby regulating egg formation, liver metabolism, bone physiology, and integration of other physiological processes.
Role of Cytokines
Hens are born with 480,000 oocytes in their ovaries and sexually mature with approximately 12,000 oocytes. However, only a few hundred oocytes mature and ovulate. Cytokine regulation is crucial for this process (Onagbesan et al., 2009). Insulin-like growth factors (IGFs) are peptides categorized into IGF1 and IGF2, which bind to the same receptor. The functions of IGFs include steroid hormone synthesis, cell proliferation and inhibition of cell apoptosis in chicken ovaries. Decreased IGF1 levels in the serum and follicles are associated with decreased egg production by laying hens (Xin et al., 2022). Therefore, treatment of GCs with IGF1 induces rapid AKT phosphorylation. Subsequently, apoptotic progression of GCs is prevented through the PI3K-AKT signaling pathway (Johnson et al., 2001). In short, a reduction in atretic follicle number is in part caused by the resistance of GCs to apoptotic signals through IGF.
The epidermal growth factor (EGF) family has several members, including EGF, TGF-α, heparin-binding EGF, amphiregulin, betacellulin and epiregulin. All these members bind to a common receptor, epidermal growth factor receptor (EGFR). EGF regulates progesterone secretion in GCs, and cell proliferation and apoptosis in the follicle germinal disc region (Volentine et al., 1998). Similarly, the addition of TGF-α to culture GCs induces rapid AKT phosphorylation. Subsequently, oligonucleosome formation in GCs is prevented through the PI3K-AKT signaling pathway (Johnson et al., 2001).
TGF-β binds to cell membrane receptors and activates SMA and mother against decapentaplegic (MAD)-related proteins (SMADs). It then enters the nucleus and cooperates with Forkhead Box L2 (FOXL2) to bind with DNA and regulate various cellular functions of follicular atresia (Qin et al., 2015). The hippo pathway signaling that prevents premature GC differentiation can avoid this phenomenon (Zhu et al., 2023). FSHR mRNA expression was enhanced by adding TGF-β to GCs after 20 h culture (Woods and Johnson, 2005). TGF-β also signals through non-Smad pathways, such as the MAPK and PI3K/AKT pathways, that regulate various cellular functions, including proliferation, differentiation, and apoptosis. These results suggested that this signaling pathway is related to follicular atresia, but it has both relieving and promoting effects.
Molecular Mechanisms of the Interaction Between Autophagy and Apoptosis
GC proliferation and differentiation provide survival molecules for follicular development, while also initiating apoptosis and autophagy, leading to follicular atresia. Therefore, apoptosis and autophagy largely determine follicle fate (Matsuda et al., 2012).
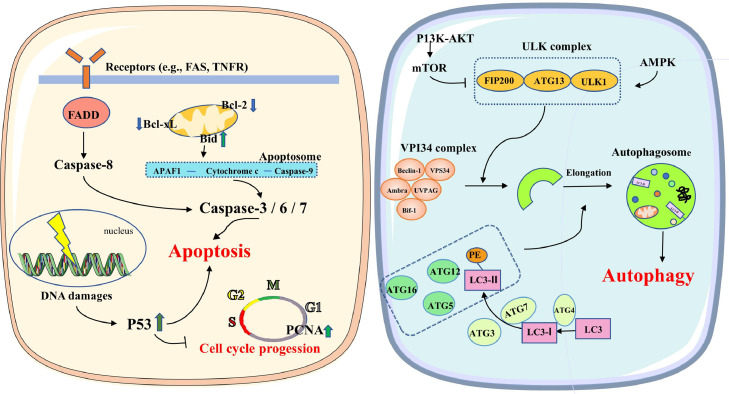
As follicles become atresia, mitotic cell populations are reduced, whereas apoptotic cells are increased (Kitamura et al., 2002). Apoptosis can be passively initiated through the loss of reproductive hormones and support of cytokines (e.g., FSH, LH, and EGF), and by destruction of the germinal disc region (Yao et al., 1998), or actively initiated via one or more death domain-containing receptors. Figure 5 illustrates the apoptotic pathway. The combination of FASL and FAS or TNF and its receptor can induce Fas-Associated protein with Death Domain (FADD) to activate the caspase-8 protein, and then activate the Caspase cascade, thereby leading to GC apoptosis (Johnson and Bridgham, 2002). Death receptor-6 (DR6) is one of the TNF receptor family members, which were found to regulate follicular atresia within the chicken ovary (Bridgham et al., 2001). BCL-2 family proteins can be classified as pro-apoptotic proteins (such as Bax, Bad and Bid) and anti-apoptotic proteins (such as BCL-2 and BCL-xl) (Johnson et al., 1996). The mitochondrial membrane is the main action site for BCL-2 family proteins. Whether the apoptotic pathway will be activated depends on the interaction between anti-apoptotic/pro-apoptotic proteins. When the apoptotic pathway is activated, cytochrome C is released from the mitochondria into the cytoplasm. It then binds to Apaf-1 and induces Apaf-1 oligopolization, thereby initiating the formation of apoptotic bodies. This complex then binds caspase-9 and promotes caspase-3 recruitment after self-activation (Johnson et al., 2007). Stress increases DNA damage, disintegrates the Golgi complex, arrests cell cycle progression, and increases P53 activation-induced apoptosis and autophagy (Zhou et al., 2022). These results suggested that apoptosis is separated into intrinsic and extrinsic pathways to control cell death.
Figure 5.
Apoptosis and autophagy signaling pathways.
TEM and RNA-seq analysis of follicles have revealed that autophagy participates in follicular atresia and broodiness of poultry (Yu et al., 2016). Autophagy is a protective mechanism against antioxidant stress and apoptosis in cells (Jiang et al., 2023). However, excessive autophagy can cause cell death (Bhardwaj et al., 2022). In autophagy, a conserved cellular process, macromolecules and organelles are transported to lysosomes for degradation and recycling. Based on the mechanism and function, autophagy is of different types, including macro-autophagy, micro-autophagy and chaperone-mediated autophagy (Tesseraud et al., 2021). Figure 5 presents the macro-autophagy pathway. In the first stage of autophagy, the ULP complex and VPI34 complex induce autophagosome formation, followed by the activation of 2 ubiquitin-like systems. Microtubule-Associated Protein 1 Light Chain 3 (LC3-I) is coupled with phosphatidylethanolamine to form LC3-II, which forms an 800 kDa complex with ATG12, ATG16 and ATG5, thereby achieving autophagosome elongation for degradation (Piekarski et al., 2014). In conclusion, under certain circumstances (a shortage of nutritional factors), autophagy is a stress adaptation and avoids cell death. By contrast, in extreme cases (fasting for a long period), excessive autophagy will be a cell death pathway affecting follicular atresia in chickens (Zhou et al., 2019).
The autophagy-maintained imbalance leads to follicular atresia in chicken ovaries. In fact, apoptosis and autophagy appear to coexist in different situations, and the 2 different cell death processes share common proteins (e.g., Bcl2 and P53) (Maiuri et al., 2007; Tasdemir et al., 2008). It accounts for the increased expression of autophagy– and apoptosis–related genes in atretic follicles. Consequently, the apoptosis–autophagy crosstalk is complex and can involve multiple signaling pathways and regulatory mechanisms.
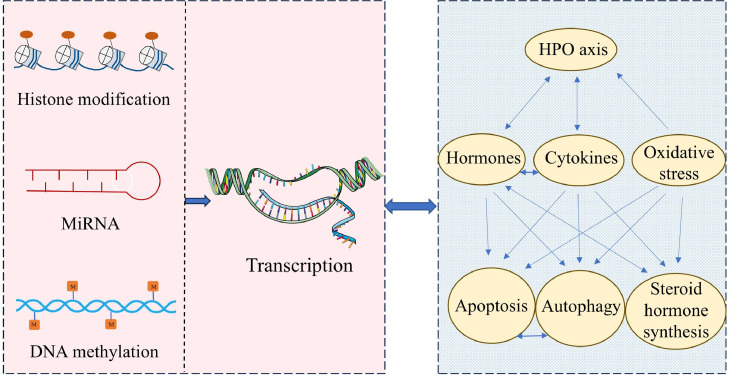
Epigenetic Modifications of Gene Expression Associated With Follicular Atresia
Epigenetic modifications cause inheritable changes in gene expression without inducing changes in the DNA sequence which included but are not limited to DNA methylation, histone modification, and non-coding RNA regulation. Follicular atresia involves many complex epigenetic events essential for reproductive performance, including transcriptional activation and repression (Pan et al., 2012). The mechanism through which epigenetic regulation affects follicular atresia is not completely established in chickens. Still, some studies have revealed mechanisms underlying epigenetic modifications involved in cell apoptosis and autophagy and provided evidence of epigenetic regulation of steroidogenesis in avian species (Zhu et al., 2015). In addition, Fig. 6 illustrates the interaction network regulates follicular atresia at different levels.
Figure 6.
Epigenetic regulations of genes expression in follicular atresia and interaction network at organic, cellular, and molecular levels.
Non-Coding RNA
The non-coding RNA is a type of gene post-transcriptional expression regulator. It is a pivotal player in physiological processes such as cell proliferation, differentiation and apoptosis (He et al., 2021). Numerous studies on the regulatory effects of non-coding RNA on apoptosis of laying hens have mainly focused on microRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA).
MiRNAs are short non-coding RNAs (18–22 nt) and are expressed in various tissues, including muscles, ovaries, adipose tissues, livers, and immunological organs. MiRNAs primarily regulates gene expression after transcription, and this has been widely studied in follicular atresia in poultry (Zhang et al., 2019). For example, miR-34a-5p increased in atretic follicles, and accelerated autophagy and apoptosis of chicken GCs in vitro. These results confirmed that miR-34a-5p activates the Hippo-YAP signaling pathway by targeting LEF1 to participate in the autophagy and apoptosis of GCs (Han et al., 2023). Simultaneously, MiRNA-129-1-3p attenuated H2O2-induced oxidative stress of GCs by inactivating the MCU signal axis (Zhu et al., 2023). The other species of miRNAs are presented in Table 2.
Table 2.
Species and function of miRNAs in chicken GCs.
| miRNA | Target gene | Function | Pathway | Reference |
|---|---|---|---|---|
| miR-181b-5p | RAP1B | increases lipid deposition and progesterone synthesis | RAP1B/ERK1/2 Pathway | (Lin et al., 2023) |
| miR-30a-5p | Beclin1 | inhibits GC death | / | (He et al., 2022) |
| miR-138-5p | SIRT1 | promotes apoptosis | / | (Yu et al., 2023) |
| miR-146a-5p | DEGS1 | suppresses cell growth, lipid deposition, and progesterone biosynthesis | AKT signaling | (Tang et al., 2024) |
| miR-122-5p | MAPK | regulates proliferation and apoptosis | / | (Zhang et al., 2022) |
| miR-146b-3p | AKT1 | regulates autophagy and apoptosis | PI3K/AKT signaling pathway | (Wei et al., 2022) |
| miR-204 | TRPM3 | regulates autophagy and apoptosis | AMPK/ULK pathway. | (Cui et al., 2020) |
| miR-128-3p | YWHAB and PPAR-γ | promotes GC apoptosis, reduced lipid deposition, and hormone biosynthesis | 14-3-3β/FoxO, PPAR-γ/LPL signaling pathways | (Ning et al., 2023) |
| miR-140-3p | AMH | promotes proliferation and hormone synthesis | / | (Zhang et al., 2023) |
| miR-23b-3p | GDF9 | inhibits proliferation and steroid hormone synthesis | / | (Wei et al., 2022) |
| miR-27b-3p | CYP1B1 | inhibits estrogen secretion of goose granulosa cells | AMPK signaling pathway | (Hu et al., 2023) |
LncRNAs are long non-coding RNAs having more than 200 nucleotides. They lack a protein coding function and play an indispensable role in follicular atresia (Meng et al., 2021). The LncRNA regulation mechanism of follicular atresia was explored by comparing the follicles between brooding and laying broilers (Yu et al., 2023). The study identified 124 and 147 lncRNAs specifically expressed in the follicles of brooding and laying broilers, respectively. LncRNA MSTRG.5970.28, as a follicular development-related lncRNA, can inhibit follicular development by inhibiting GC proliferation and promoting GC apoptosis in poultry (Zhao et al., 2023).
CircRNAs are new type of non-coding RNAs. They have also been studied in follicular development and atresia in laying hens. In a study, the circRNA profiles of GCs were constructed, which facilitated the further analysis of egg production performance (Wang et al., 2022). The study demonstrated that ssc-circINHA-001 regulated the expression of miRNA cluster-enhanced inhibin subunit β through the circRNA-microRNA-INHBA regulatory axis, thereby resisting GC apoptosis and follicular atresia.
Histone Modifications
Histone modifications are major epigenetic regulations that prominently modifies gene transcription through acetylation, methylation, ubiquitination, and phosphorylation at the N-terminus of histones (Kouzarides, 2007). The reversible process of histone acetylation is regulated by histone deacetylase and the histone acetylation enzyme. Histone acetylation promotes transcription, whereas histone deacetylation promotes gene silencing or repression (Sun et al., 2012). Histone methylation is the most stable histone modification. Single, double, and trimethylation of lysine (K) side chains of proteins H3 and H4 are crucial for physiological processes such as follicular and oocyte development (Shen et al., 2017). After reproductive maturity, higher levels of histone H3K27 acetylation and lower levels of H3K36 trimethylation were associated with the increased abundance of ERα mRNA transcripts in chicken ovaries (90 d vs. 160 d) (Guo et al., 2020). In mice ovarian GCs, the occupancy of active histone modifications H3K9ac regulates the expression of genes associated with PPARγ and PGC1α signaling pathways to promote steroidogenesis (Ye et al., 2021).
Histone modifications play a major role in apoptosis and autophagy of GCs. H3K27me3 transcriptionally represses RUNX1, whereas RUNX1 acts as a transcription factor regulating hormone synthesis and cell apoptosis and proliferation (Zhong et al., 2020). Similarly, H3k27me3 mediated miR143 controls follicular atresia by regulating apoptosis and steroid production (Zhong et al., 2020). Moreover, silent information regulator 1 (SIRT1) and acetyl-CoA maintain histone acetylation and induce autophagy gene expression. By contrast, histone demethylation inhibits autophagy in ovaries (Zhou et al., 2019). These results suggested that histone modification regulates gene expression in follicular development and atresia in chickens.
DNA Methylation
In DNA methylation, DNA methyltransferases convert 5-methyl cytosine to cytosine in the presence of the methyl donor S–adenosine methionine (Chen and Zhang, 2020). CpG islands in the promoter region are methylated to repress the gene transcription. By contrast, when CpG islands are not methylated, downstream genes are activated (Moore et al., 2013). In a study, feeding betaine reduced mRNA expression of DNA methyltransferase (DNMT) 1, DNMT3a, and DNMT3b, and decreased DNA methylation in the LHR promoter region which increased the hormone sensitivity of laying hen ovaries as well as DNA methylation in the promoter region of caspase-3. The underlying mechanism may be anti-apoptosis, which thus improved egg production performance (Guo et al., 2023). Zhu et al. (2015) provided a novel insight that DNA methylation in the proximal promoters of StAR and CYP11A1 differs in various follicles. These findings implied that DNA methylation regulates the transcription of GC function-related genes to adjust follicular atresia.
Prevention of Follicular Atresia
In chickens, stimulation (environmental and nutritional factors) or aging can lead to ROS accumulation, which results in oxidative stress. Oxidative stress participates in follicular atresia (Bai et al., 2023). Many studies have reported that antioxidants can counteract oxidative stress by increasing the activity of antioxidant enzymes, reversing the tryptophan-kynurenine pathway, upregulating nuclear factor E2-related factor 2 (Nrf2) and SIRT1 gene expression, and decreasing the expression of FoxO1 and P53 (Wang et al., 2022), thereby reducing the follicular atresia rate (Jia et al., 2016; Wu et al., 2023). For example, the bioactive lignan honokiol alleviates oxidative stress through the SIRT3/AMPK pathway in aging laying hens (Chen et al., 2024). Melatonin is often used to prevent follicular atresia through anti-stress and apoptosis mechanisms (Hao et al., 2024; Hao et al., 2024). Meanwhile, the mechanism underlying oxidative stress-induced sharp decline in the laying rate of layers is autophagy disorders in GCs (Zhou et al., 2022). Recent studies have shown that nobilecin improves follicle aging by inhibiting oxidative stress and promoting autophagy in chickens. Furthermore, spermidine induces autophagy, thereby protecting against oxidative stress and apoptosis, and finally alleviating follicular atresia in geese (Jiang et al., 2023). These results thus suggest that research on methods for preventing follicular atresia is underway as it would increase the laying rate and prolong the laying time of laying hens.
CONCLUSIONS
In this review, we discussed internal and external factors that regulate follicular atresia (Table 3). Some fundamental questions related to follicular atresia to be answered are how dynamic changes in chicken follicular atresia are addressed and which is the first messenger that regulates gene expression and signaling pathways at various levels. Thus, differences between follicular atresia at different stages or in different species need to be urgently investigated to improve our understanding of underlying mechanisms, promote follicular development, and avoid follicular pathological atresia. Moreover, research concerning the role of epigenetics in follicular atresia is currently lacking. Many regulatory mechanisms remain to be elucidated. On combing with new technologies such as third-generation sequencing, single-cell sequencing, Cleavage Under Targets and Tagmentation (CUT&Tag), and ATAC-seq, the mechanism underlying follicular atresia can be better explored at the organic, cellular and molecular levels.
Table 3.
Internal and external factors regulating follicular atresia.
| Factor | Type | Regulations | Mechanisms |
|---|---|---|---|
| Energy level | nutrition | fasting induces follicle atresia; Over feeding disorganizes follicular growth to become atresia; Energy restriction adjusts follicular atresia | controlled gonadotropins or ovarian hormones levels and the viability of GCs |
| Light | environment | long-photoperiod accelerates follicle atresia; short-photoperiod reduces follicle atresia | activated the HPO axis and affected melatonin secretion |
| Heat stress | temperature | increases atretic follicles | impaired GCs functions and steroidogenic dysfunction |
| Chemical pollutants | environment | induces follicular atresia | controlled cell cycle, cell proliferation or apoptosis and caused oxidative stress |
| Aging | physiological factors | induces follicular atresia | decreased antioxidant capacity and increased autophagy and apoptosis |
| HPO axis | organic level | regulates follicle fate (selected or atresia) | controled the synthesis and secreted of sex hormone |
| FSH | hormone | enhances follicular development | promoted the GCs proliferation and reduces apoptosis through P13K/AKT/FoxO3a, mTOR pathways and cAMP/PKA pathways |
| Estrogens | hormone | decreases the number of atretic follicles | regulated the expression of specific genes to accelerate GCs proliferation and inhibit apoptosis through RAS/RAF/ERK pathway |
| IGF | cytokines | reduces follicular atresia | regulated steroid hormone synthesis, cell proliferation and inhibit apoptosis through P13K-AKT pathway |
| EGF | cytokines | reduces follicular atresia | regulated the secretion of progesterone in GCs, cell proliferation and apoptosis through P13K-AKT pathway |
| TGF-β | cytokines | relieves or promoted follicular atresia | regulated proliferation, differentiation and apoptosis |
| Apoptosis autophagy | programmed death | regulates follicular atresia | through apoptosis and autophagy signaling pathways |
| Non-coding RNA | Epigenetic modifications | participates follicular atresia | regulated gene expression after transcription |
| Histone Modification | Epigenetic modifications | regulates gene expression in chicken follicular development and atresia | modified the transcription of genes through acetylation, methylation, ubiquitination, and phosphorylation at the N-terminus of histones |
| DNA Methylation | Epigenetic modifications | adjusts follicle atresia | repressed the transcription of genes |
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the grant of National Natural Science Foundation of China (grant number 31960690 and 32360867). We are grateful to colleagues (College of Animal Science and Technology, Jiangxi Agricultural University) for their help in the review.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103893.
Appendix. Supplementary materials
REFERENCES
- Armstrong D.G. Changes in aromatase activity in small ovarian follicles of the domestic fowl (Gallus domesticus) during growth and atresia. J. Endocrinol. 1985;105:297–301. doi: 10.1677/joe.0.1050297. [DOI] [PubMed] [Google Scholar]
- Bai J., Wang X., Chen Y., Yuan Q., Yang Z., Mi Y., Zhang C. Nobiletin ameliorates aging of chicken ovarian prehierarchical follicles by suppressing oxidative stress and promoting autophagy. Cells. 2024;13:415. doi: 10.3390/cells13050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K., Hao E., Huang C.X., Yue Q.X., Wang D.H., Shi L., Chen Y.F., Chen H., Huang R.L. Melatonin alleviates ovarian function damage and oxidative stress induced by dexamethasone in the laying hens through FOXO1 signaling pathway. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. what are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao T., Yao J., Zhou S., Ma Y., Dong J., Zhang C., Mi Y. Naringin prevents follicular atresia by inhibiting oxidative stress in the aging chicken. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez B., Ishii T., Wu Y.H., Carpenter R.D., Sherk V.D. Energy balance and bone health: a nutrient availability perspective. Curr. Osteoporos. Rep. 2023;21:77–84. doi: 10.1007/s11914-022-00765-4. [DOI] [PubMed] [Google Scholar]
- Bhardwaj J.K., Paliwal A., Saraf P., Sachdeva S.N. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J. Cell. Physiol. 2022;237:1157–1170. doi: 10.1002/jcp.30613. [DOI] [PubMed] [Google Scholar]
- Bridgham J.T., Bobe J., Goetz F.W., Johnson A.L. Conservation of death receptor-6 in avian and piscine vertebrates. Biochem. Biophys. Res. Commun. 2001;284:1109–1115. doi: 10.1006/bbrc.2001.5093. [DOI] [PubMed] [Google Scholar]
- Britt K.L., Findlay J.K. Estrogen actions in the ovary revisited. J. Endocrinol. 2002;175:269–276. doi: 10.1677/joe.0.1750269. [DOI] [PubMed] [Google Scholar]
- Calvo F.O., Wang S.C., Bahr J.M. LH-stimulable adenylyl cyclase activity during the ovulatory cycle in granulosa cells of the three largest follicles and the postovulatory follicle of the domestic hen (Gallus domesticus) Biol. Reprod. 1981;25:805–912. doi: 10.1095/biolreprod25.4.805. [DOI] [PubMed] [Google Scholar]
- Chen J., Pu L., Niu Y., Tian K., Jia X., Zhang L., Lu Y. Prolonged fasting induces significant germ cell loss in chickens after hatching. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.E., McMurtry J.P., Walzem R.L. Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poult. Sci. 2006;85:70–81. doi: 10.1093/ps/85.1.70. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Hsiao P.W., Lee M.T., Mason J.I., Ke F.C., Hwang J.J. Interplay of PI3K and cAMP/PKA signaling, and rapamycin-hypersensitivity in TGFbeta1 enhancement of FSH-stimulated steroidogenesis in rat ovarian granulosa cells. J. Endocrinol. 2007;192:405–419. doi: 10.1677/JOE-06-0076. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yang Z., Bai J., Wang X., Yuan Q., Mi Y., Zhang C. Bioactive lignan honokiol alleviates ovarian oxidative stress in aging laying chickens by regulating SIRT3/AMPK pathway. Antioxidants (Basel) 2024;13:377. doi: 10.3390/antiox13030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang Y. Role of mammalian DNA methyltransferases in development. Annu. Rev. Biochem. 2020;89:135–158. doi: 10.1146/annurev-biochem-103019-102815. [DOI] [PubMed] [Google Scholar]
- Cheng S.B., Li X.Q., Wang J.X., Wu Y., Li P., Pi J.S. The effect of light on follicular development in laying hens. Anim. Biosci. 2021;34:1766–1775. doi: 10.5713/ab.20.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C., Han S., Yin H., Luo B., Shen X., Yang F., Liu Z., Zhu Q., Li D., Wang Y. FOXO3 is expressed in ovarian tissues and acts as an apoptosis initiator in granulosa cells of chickens. Biomed. Res. Int. 2019;2019:6902906. doi: 10.1155/2019/6902906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Liu L., Kwame A.F., Zhu Q., Wang Y., Li D., Shu G., Tian Y., Zhao X. High expression of miR-204 in chicken atrophic ovaries promotes granulosa cell apoptosis and inhibits autophagy. Front. Cell. Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.580072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar D.S., Devkar R.V., Ramachandran A.V. Effect of short photoperiod on organ growth kinetics and serum hormone profile in pullets of domestic fowl, Gallus gallus domesticus. Indian J. Exp. Biol. 2001;39:230–237. [PubMed] [Google Scholar]
- Dong J., Guo C., Zhou S., Zhao A., Li J., Mi Y., Zhang C. Leukemia inhibitory factor prevents chicken follicular atresia through PI3K/AKT and Stat3 signaling pathways. Mol. Cell. Endocrinol. 2022;543 doi: 10.1016/j.mce.2021.111550. [DOI] [PubMed] [Google Scholar]
- Du Y., Liu L., He Y., Dou T., Jia J., Ge C. Endocrine and genetic factors affecting egg laying performance in chickens: a review. Br. Poult. Sci. 2020;61:538–549. doi: 10.1080/00071668.2020.1758299. [DOI] [PubMed] [Google Scholar]
- Evans A.C. Characteristics of ovarian follicle development in domestic animals. Reprod. Domest. Anim. 2003;38:240–246. doi: 10.1046/j.1439-0531.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- Francoeur L., Stephens C.S., Johnson P.A. Ad libitum feeding in broiler breeder hens alters the transcriptome of granulosa cells of pre-hierarchal follicles. Animals (Basel) 2021;11:2706. doi: 10.3390/ani11092706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.B., Perry M.M., Waddington D., Hardie M.A. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus) J. Reprod. Fertil. 1983;69:221–227. doi: 10.1530/jrf.0.0690221. [DOI] [PubMed] [Google Scholar]
- Guo C., Zhang G., Lin X., Zhao D., Zhang C., Mi Y. Reciprocal stimulating effects of bFGF and FSH on chicken primordial follicle activation through AKT and ERK pathway. Theriogenology. 2019;132:27–35. doi: 10.1016/j.theriogenology.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Guo F., Jing M., Zhang A., Yi J., Zhang Y. Effects of dietary betaine on the laying performance, antioxidant capacity, and uterus and ovary function of laying hens at the late stage of production. Animals (Basel) 2023;13:3283. doi: 10.3390/ani13203283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Chen Y., Chen Q., Guo X., Yuan Z., Kang L., Jiang Y. Epigenetic changes associated with increased estrogen receptor alpha mRNA transcript abundance during reproductive maturation in chicken ovaries. Anim. Reprod. Sci. 2020;214 doi: 10.1016/j.anireprosci.2020.106287. [DOI] [PubMed] [Google Scholar]
- Han S., Wang J., Cui C., Yu C., Zhang Y., Li D., Ma M., Du H., Jiang X., Zhu Q., Yang C., Yin H. Fibromodulin is involved in autophagy and apoptosis of granulosa cells affecting the follicular atresia in chicken. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Zhao X., Zhang Y., Amevor F.K., Tan B., Ma M., Kang H., Wang J., Zhu Q., Yin H., Cui C. MiR-34a-5p promotes autophagy and apoptosis of ovarian granulosa cells via the Hippo-YAP signaling pathway by targeting LEF1 in chicken. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C., Ziezold C.J., Bedecarrats G.Y. The diverse roles of 17beta-estradiol in non-gonadal tissues and its consequential impact on reproduction in laying and broiler breeder hens. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.942790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao E.Y., Liu X.L., Chen X.Y., Xue H., Su B.F., Chen Y.F., Wang D.H., Shi L., Bai K., Hou F., Hou J.K., Bao H.L., Chen H. Melatonin alleviates endoplasmic reticulum stress and follicular granulosa cell apoptosis by regulating ATF4 to activate mTOR signaling pathway in chickens. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2024.103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Wang K., Gao Y., Wang C., Li L., Liao Y., Hu K., Liang M. Roles of noncoding RNA in reproduction. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.777510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Li D., Tian Y., Wei Q., Amevor F.K., Sun C., Yu C., Yang C., Du H., Jiang X., Ma M., Cui C., Zhang Z., Tian K., Zhang Y., Zhu Q., Yin H. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J. Anim. Sci. Biotechnol. 2022;13:55. doi: 10.1186/s40104-022-00697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Choi Y. Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep. 2018;51:225–229. doi: 10.5483/BMBRep.2018.51.5.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Rong Y., Deng Y., Li L., Hu J., Yuan X., He H., Li L., Wang J. miR-27b-3p inhibits estrogen secretion of goose granulosa cells by targeting CYP1B1 through the AMPK signaling pathway. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Hou Y., Li H., Wu H., Hu J., Lu Y., Liu X. Endoplasmic reticulum stress is involved in small white follicular atresia in chicken ovaries. Theriogenology. 2022;184:140–152. doi: 10.1016/j.theriogenology.2022.03.012. [DOI] [PubMed] [Google Scholar]
- Jia Y., Yang M., Zhu K., Wang L., Song Y., Wang J., Qin W., Xu Z., Chen Y., Liu G. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 2016;6:39799. doi: 10.1038/srep39799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Wang X., Zhou X., Wang Z., Li S., Sun Q., Jiang Y., Ji C., Ling W., An X., Kang B. Spermidine alleviating oxidative stress and apoptosis by inducing autophagy of granulosa cells in Sichuan white geese. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Regulation of follicle differentiation by gonadotropins and growth factors. Poult. Sci. 1993;72:867–873. doi: 10.3382/ps.0720867. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim. Reprod. Sci. 2003;78:185–201. doi: 10.1016/s0378-4320(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Chapter 28 - Reproduction in the female. Scanes C.G., editor. Chapter 28 - Reproduction in the femalePages 635–665 in Sturkie's Avian Physiology. 2015 [Google Scholar]
- Johnson A.L., Ratajczak C., Haugen M.J., Liu H.K., Woods D.C. Tumor necrosis factor-related apoptosis inducing ligand expression and activity in hen granulosa cells. Reproduction. 2007;133:609–616. doi: 10.1530/REP-06-0287. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T., Witty J.P., Tilly J.L. Susceptibility of avian ovarian granulosa cells to apoptosis is dependent upon stage of follicle development and is related to endogenous levels of bcl-xlong gene expression. Endocrinology. 1996;137:2059–2066. doi: 10.1210/endo.137.5.8612548. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T., Witty J.P., Tilly J.L. Expression of bcl-2 and nr-13 in hen ovarian follicles during development. Biol. Reprod. 1997;57:1096–1103. doi: 10.1095/biolreprod57.5.1096. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T., Swenson J.A. Activation of the Akt/protein kinase B signaling pathway is associated with granulosa cell survival. Biol. Reprod. 2001;64:1566–1574. doi: 10.1095/biolreprod64.5.1566. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T., Jensen T. Bcl-X(LONG) protein expression and phosphorylation in granulosa cells. Endocrinology. 1999;140:4521–4529. doi: 10.1210/endo.140.10.7022. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Lee J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016;95:108–114. doi: 10.3382/ps/pev318. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Bridgham J.T. Caspase-mediated apoptosis in the vertebrate ovary. Reproduction. 2002;124:19–27. doi: 10.1530/rep.0.1240019. [DOI] [PubMed] [Google Scholar]
- Kilanowska A., Ziolkowska A., Stasiak P., Gibas-Dorna M. cAMP-dependent signaling and ovarian cancer. Cells. 2022;11:3835. doi: 10.3390/cells11233835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A. [Effect of gonadotropin releasing hormone agonist (Gn-RHa) on steroidogenesis in human and rat ovaries] Nihon Sanka Fujinka Gakkai Zasshi. 1992;44:1261–1268. [PubMed] [Google Scholar]
- Kitamura A., Yoshimura Y., Okamoto T. Changes in the populations of mitotic and apoptotic cells in white follicles during atresia in hens. Poult. Sci. 2002;81:408–413. doi: 10.1093/ps/81.3.408. [DOI] [PubMed] [Google Scholar]
- Korver D.R. Review: Current challenges in poultry nutrition, health, and welfare. Animal. 2023;17(Suppl) doi: 10.1016/j.animal.2023.100755. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Li G.M., Liu L.P., Yin B., Liu Y.Y., Dong W.W., Gong S., Zhang J., Tan J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-alpha systems. Poult. Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yang Y., Wang H., Jiang Z., Ma H. Genistein accelerates glucose catabolism via activation the GPER-mediated cAMP/PKA-AMPK signaling pathway in broiler chickens. Life Sci. 2022;303 doi: 10.1016/j.lfs.2022.120676. [DOI] [PubMed] [Google Scholar]
- Lin Z., Gong Y., Sun H., Yang C., Tang Y., Yin L., Zhang D., Wang Y., Yu C., Liu Y. Lipid deposition and progesterone synthesis are increased by miR-181b-5p through RAP1B/ERK1/2 pathway in chicken granulosa cells. J. Agric. Food Chem. 2023;71:12910–12924. doi: 10.1021/acs.jafc.3c03178. [DOI] [PubMed] [Google Scholar]
- Liu L., Li D., Gilbert E.R., Xiao Q., Zhao X., Wang Y., Yin H., Zhu Q. Effect of monochromatic light on expression of estrogen receptor (ER) and progesterone receptor (PR) in ovarian follicles of chicken. Plos One. 2015;10 doi: 10.1371/journal.pone.0144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Wang Q., Wang K.H., Ma M., Wang X.G., Guo J., Dou T.C., Hu Y.P., Li Y.F., Yang Z., Qu L. Effects of energy restriction during growing phase on the productive performance of Hyline Brown laying hens aged 6 to 72 wk. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Cheng B., Li Y., Wu Q., Wang Y., Chai X., Ren A. Protective effect of nano-selenium on mercury-induced prehierarchical follicular atresia in laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Yao J., Zhou S., Mi Y., Tan X., Zhang C. Enhancing effect of FSH on follicular development through yolk formation and deposition in the low-yield laying chickens. Theriogenology. 2020;157:418–430. doi: 10.1016/j.theriogenology.2020.07.012. [DOI] [PubMed] [Google Scholar]
- Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Manikkam M., Rajamahendran R. Progesterone-induced atresia of the proestrous dominant follicle in the bovine ovary: changes in diameter, insulin-like growth factor system, aromatase activity, steroid hormones, and apoptotic index. Biol. Reprod. 1997;57:580–587. doi: 10.1095/biolreprod57.3.580. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Inoue N., Manabe N., Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012;58:44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- Meng L., Zhao K., Wang C.C., Tao J., Wu Z., Teerds K., Zhang S. Characterization of long non-coding RNA profiles in porcine granulosa cells of healthy and atretic antral follicles: implications for a potential role in apoptosis. Int. J. Mol. Sci. 2021;22:2677. doi: 10.3390/ijms22052677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mfoundou J.D.L., Guo Y.J., Liu M.M., Ran X.R., Fu D.H., Yan Z.Q., Li M.N., Wang X.R. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhael S., Punjala-Patel A., Gavrilova-Jordan L. Hypothalamic-pituitary-ovarian axis disorders impacting female fertility. Biomedicines. 2019;7:5. doi: 10.3390/biomedicines7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd K.I., Saleem R., Choudhary O.P., Singh I. Posthatch developmental changes in the ovary with emphasis on follicular development and atresia in the native chicken (Uttara fowl) of Uttarakhand, India. Anat. Histol. Embryol. 2024;53:e12977. doi: 10.1111/ahe.12977. [DOI] [PubMed] [Google Scholar]
- Moore L.D., Le T., Fan G. DNA methylation and its basic function. Neuropsychopharmacol. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Z., Deng X., Li L., Feng J., Du X., Amevor F.K., Tian Y., Li L., Rao Y., Yi Z., Du X., Cui Z., Zhao X. miR-128-3p regulates chicken granulosa cell function via 14-3-3beta/FoxO and PPAR-gamma/LPL signaling pathways. Int. J. Biol. Macromol. 2023;241 doi: 10.1016/j.ijbiomac.2023.124654. [DOI] [PubMed] [Google Scholar]
- Onagbesan O.M., Metayer S., Tona K., Williams J., Decuypere E., Bruggeman V. Effects of genotype and feed allowance on plasma luteinizing hormones, follicle-stimulating hormones, progesterone, estradiol levels, follicle differentiation, and egg production rates of broiler breeder hens. Poult. Sci. 2006;85:1245–1258. doi: 10.1093/ps/85.7.1245. [DOI] [PubMed] [Google Scholar]
- Onagbesan O., Bruggeman V., Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim. Reprod. Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Pan Y.E., Liu Z.C., Chang C.J., Huang Y.F., Lai C.Y., Walzem R.L., Chen S.E. Feed restriction ameliorates metabolic dysregulation and improves reproductive performance of meat-type country chickens. Anim. Reprod. Sci. 2014;151:229–236. doi: 10.1016/j.anireprosci.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Pan Z., Zhang J., Li Q., Li Y., Shi F., Xie Z., Liu H. Current advances in epigenetic modification and alteration during mammalian ovarian folliculogenesis. J. Genet. Genomics. 2012;39:111–123. doi: 10.1016/j.jgg.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Perry M.M., Gilbert A.B., Evans A.J. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J. Anat. 1978;125(Pt 3):481–497. [PMC free article] [PubMed] [Google Scholar]
- Piekarski A., Khaldi S., Greene E., Lassiter K., Mason J.G., Anthony N., Bottje W., Dridi S. Tissue distribution, gender- and genotype-dependent expression of autophagy-related genes in avian species. Plos One. 2014;9 doi: 10.1371/journal.pone.0112449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prastiya R.A., Madyawati S.P., Sari S.Y., Nugroho A.P. Effect of follicle-stimulating hormone and luteinizing hormone levels on egg-laying frequency in hens. Vet. World. 2022;15:2890–2895. doi: 10.14202/vetworld.2022.2890-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudman J.A., Vandesande F., Berghman L.R. Immunohistochemical evidence that follicle-stimulating hormone and luteinizing hormone reside in separate cells in the chicken pituitary. Biol. Reprod. 1999;60:1324–1328. doi: 10.1095/biolreprod60.6.1324. [DOI] [PubMed] [Google Scholar]
- Qin N., Fan X.C., Xu X.X., Tyasi T.L., Li S.J., Zhang Y.Y., Wei M.L., Xu R.F. Cooperative effects of FOXL2 with the members of TGF-beta superfamily on FSH receptor mRNA expression and granulosa cell proliferation from hen prehierarchical follicles. Plos One. 2015;10 doi: 10.1371/journal.pone.0141062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenboim I., Tako E., Gal-Garber O., Proudman J.A., Uni Z. The effect of heat stress on ovarian function of laying hens. Poult. Sci. 2007;86:1760–1765. doi: 10.1093/ps/86.8.1760. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Bartman J., Avital C.N., Mobarkey N., Zaguri S., El H.M., Chaiseha Y., Marco A. Targeted differential photostimulation alters reproductive activities of domestic birds. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.1040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol H.S., Sato K., Murakami H., Toyomizu M., Akiba Y. Changes in gene expression involved in energy utilization during chicken follicle development. Anim. Reprod. Sci. 2006;95:283–294. doi: 10.1016/j.anireprosci.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Shen H., Xu W., Lan F. Histone lysine demethylases in mammalian embryonic development. Exp. Mol. Med. 2017;49:e325. doi: 10.1038/emm.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin A.V., Grossmann R. Role of tyrosine kinase- and MAP kinase-dependent intracellular mechanisms in control of ovarian functions in the domestic fowl (Gallus domesticus) and in mediating effects of IGF-II. J. Reprod. Dev. 2003;49:99–106. doi: 10.1262/jrd.49.99. [DOI] [PubMed] [Google Scholar]
- Socha J.K., Hrabia A. Response of the chicken ovary to GH treatment during a pause in laying induced by fasting. Domest. Anim. Endocrinol. 2019;69:84–95. doi: 10.1016/j.domaniend.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Sun W.J., Zhou X., Zheng J.H., Lu M.D., Nie J.Y., Yang X.J., Zheng Z.Q. Histone acetyltransferases and deacetylases: molecular and clinical implications to gastrointestinal carcinogenesis. Acta Biochim. Biophys. Sin. (Shanghai) 2012;44:80–91. doi: 10.1093/abbs/gmr113. [DOI] [PubMed] [Google Scholar]
- Tang Y., Lin Z., Liu L., Yin L., Zhang D., Yu C., Yang C., Gong Y., Wang Y., Liu Y. Attenuated AKT signaling by miR-146a-5p interferes with chicken granulosa cell proliferation, lipid deposition and progesterone biosynthesis. Theriogenology. 2024;214:370–385. doi: 10.1016/j.theriogenology.2023.11.007. [DOI] [PubMed] [Google Scholar]
- Tasdemir E., Maiuri M.C., Galluzzi L., Vitale I., Djavaheri-Mergny M., D'Amelio M., Criollo A., Morselli E., Zhu C., Harper F., Nannmark U., Samara C., Pinton P., Vicencio J.M., Carnuccio R., Moll U.M., Madeo F., Paterlini-Brechot P., Rizzuto R., Szabadkai G., Pierron G., Blomgren K., Tavernarakis N., Codogno P., Cecconi F., Kroemer G. Regulation of autophagy by cytoplasmic p53. Nat. Cell. Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseraud S., Avril P., Bonnet M., Bonnieu A., Cassar-Malek I., Chabi B., Dessauge F., Gabillard J.C., Perruchot M.H., Seiliez I. Autophagy in farm animals: current knowledge and future challenges. Autophagy. 2021;17:1809–1827. doi: 10.1080/15548627.2020.1798064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramunt B., Montagner A., Tan N.S., Gourdy P., Remignon H., Wahli W. Roles of estrogens in the healthy and diseased oviparous vertebrate liver. Metabolites. 2021;11:502. doi: 10.3390/metabo11080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volentine K.K., Yao H.H., Bahr J.M. Epidermal growth factor in the germinal disc and its potential role in follicular development in the chicken. Biol. Reprod. 1998;59:522–526. doi: 10.1095/biolreprod59.3.522. [DOI] [PubMed] [Google Scholar]
- Walzem R.L., Chen S.E. Obesity-induced dysfunctions in female reproduction: lessons from birds and mammals. Adv. Nutr. 2014;5:199–206. doi: 10.3945/an.113.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jia R., Gong H., Celi P., Zhuo Y., Ding X., Bai S., Zeng Q., Yin H., Xu S., Liu J., Mao X., Zhang K. the effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants (Basel) 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jia R., Celi P., Zhuo Y., Ding X., Zeng Q., Bai S., Xu S., Yin H., Lv L., Zhang K. Resveratrol alleviating the ovarian function under oxidative stress by alternating microbiota related tryptophan-kynurenine pathway. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.911381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Guo Z., Zi C., Wu P., Lv X., Chen L., Chen F., Zhang G., Wang J. CircRNA expression in chicken granulosa cells illuminated with red light. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Xue H., Sun C., Li J., He H., Amevor F.K., Tan B., Ma M., Tian K., Zhang Z., Zhang Y., He H., Xia L., Zhu Q., Yin H., Cui C. Gga-miR-146b-3p promotes apoptosis and attenuate autophagy by targeting AKT1 in chicken granulosa cells. Theriogenology. 2022;190:52–64. doi: 10.1016/j.theriogenology.2022.07.019. [DOI] [PubMed] [Google Scholar]
- Wilkanowska A., Mazurowski A., Mroczkowski S., Kokoszynski D. Prolactin (PRL) and prolactin receptor (PRLR) genes and their role in poultry production traits. Folia Biol (Krakow) 2014;62:1–8. doi: 10.3409/fb62_1.1. [DOI] [PubMed] [Google Scholar]
- Woods D.C., Johnson A.L. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol. Reprod. 2005;72:643–650. doi: 10.1095/biolreprod.104.033902. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou S., Zhao A., Mi Y., Zhang C. Protective effect of rutin on ferroptosis-induced oxidative stress in aging laying hens through Nrf2/HO-1 signaling. Cell Biol. Int. 2023;47:598–611. doi: 10.1002/cbin.11960. [DOI] [PubMed] [Google Scholar]
- Xie Y.L., Pan Y.E., Chang C.J., Tang P.C., Huang Y.F., Walzem R.L., Chen S.E. Palmitic acid in chicken granulosa cell death-lipotoxic mechanisms mediate reproductive inefficacy of broiler breeder hens. Theriogenology. 2012;78:1917–1928. doi: 10.1016/j.theriogenology.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Xin Q., Uyanga V.A., Jiao H., Zhao J., Wang X., Li H., Zhou Y., Lin H. Insulin-like growth factor-1 is involved in the deteriorated performance of aged laying hens. J. Anim. Sci. 2022;100:skac286. doi: 10.1093/jas/skac286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Qin N., Xu X., Sun X., Chen X., Zhao J. Inhibitory effect of SLIT2 on granulosa cell proliferation mediated by the CDC42-PAKs-ERK1/2 MAPK pathway in the prehierarchical follicles of the chicken ovary. Sci. Rep. 2018;8:9168. doi: 10.1038/s41598-018-27601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Chen S., Chen W., Zhou X., Yan F., Huang T., Wang Y., Lu H., Zhao A. Comparative analysis of the follicular transcriptome of Zhedong white geese (Anser Cygnoides) with different photoperiods. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Hu M., Gu L., Lei M., Chen Z., Zhu H., Chen R. Effect of heat stress on egg production, steroid hormone synthesis, and related gene expression in chicken preovulatory follicular granulosa cells. Animals (Basel) 2022;12:1467. doi: 10.3390/ani12111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Chen X., Liu Z., Zhao Y., Chen Y., Geng Z. Integrated transcriptome and proteome revealed that the declined expression of cell cycle-related genes associated with follicular atresia in geese. BMC Genomics. 2023;24:24. doi: 10.1186/s12864-022-09088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H.H., Volentine K.K., Bahr J.M. Destruction of the germinal disc region of an immature preovulatory chicken follicle induces atresia and apoptosis. Biol. Reprod. 1998;59:516–521. doi: 10.1095/biolreprod59.3.516. [DOI] [PubMed] [Google Scholar]
- Yao J., Ma Y., Lin X., Zhou S., Mi Y., Zhang C. The attenuating effect of the intraovarian bone morphogenetic protein 4 on age-related endoplasmic reticulum stress in chicken follicular cells. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/4175613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Zeng X., Wang S., Zeng X., Yang G., Ye C., Cai S., Chen M., Li S., Qiao S. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARgamma and PGC1alpha pathways in ovarian granulosa cells. Faseb. J. 2021;35:e21316. doi: 10.1096/fj.202000444R. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Tamura T. Effects of estradiol administration on the follicular tissue of hypophysectomized hens. Poult. Sci. 1986;65:1808–1810. doi: 10.3382/ps.0651808. [DOI] [PubMed] [Google Scholar]
- Yu C., Lin Z., Song X., Hu C., Qiu M., Yang L., Zhang Z., Pen H., Chen J., Xiong X., Xia B., Jiang X., Du H., Li Q., Zhu S., Liu S., Yang C., Liu Y. Whole transcriptome analysis reveals the key genes and noncoding RNAs related to follicular atresia in broilers. Anim. Biotechnol. 2023;34:3144–3153. doi: 10.1080/10495398.2022.2136680. [DOI] [PubMed] [Google Scholar]
- Yu J., Lou Y., He K., Yang S., Yu W., Han L., Zhao A. Goose broodiness is involved in granulosa cell autophagy and homeostatic imbalance of follicular hormones. Poult. Sci. 2016;95:1156–1164. doi: 10.3382/ps/pew006. [DOI] [PubMed] [Google Scholar]
- Zhang B.B., Li X.N., Li M.X., Sun Y.Y., Shi Y.X., Ma T.H. miR-140-3p promotes follicle granulosa cell proliferation and steroid hormone synthesis via targeting AMH in chickens. Theriogenology. 2023;202:84–92. doi: 10.1016/j.theriogenology.2023.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang G., Cui Z., Li J., Zhang D., Li Z., Lin Z., Yin H., Ran J., Wang Y., Liu Y. miR-122-5p regulates proliferation and apoptosis of chicken granulosa cells of hierarchal follicles by targeting MAPK3. Gene. 2022;824 doi: 10.1016/j.gene.2022.146397. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xu Y., Liu H., Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019;17:9. doi: 10.1186/s12958-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Lv C., Liu G., Mi Y., Zhang C. Effect of estrogen on chick primordial follicle development and activation. Cell Biol. Int. 2017;41:630–638. doi: 10.1002/cbin.10766. [DOI] [PubMed] [Google Scholar]
- Zhao X., Li H., Chen X., Wu Y., Wang L., Li J. Long non-coding RNA MSTRG.5970.28 regulates proliferation and apoptosis of goose follicle granulosa cells via the miR-133a-3p/ANOS1 pathway. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Li L., He Y., He B., Li Z., Zhang Z., Zhang H., Yuan X., Li J. Activation of steroidogenesis, anti-apoptotic activity, and proliferation in porcine granulosa cells by RUNX1 is negatively regulated by H3K27me3 transcriptional repression. Genes (Basel) 2020;11:495. doi: 10.3390/genes11050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Peng X., Mei S. Autophagy in ovarian follicular development and atresia. Int. J. Biol. Sci. 2019;15:726–737. doi: 10.7150/ijbs.30369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Zhao A., Wu Y., Mi Y., Zhang C. Protective effect of grape seed proanthocyanidins on oxidative damage of chicken follicular granulosa cells by inhibiting FoxO1-mediated autophagy. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.762228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Mao Y., Zhou W., Jiang Y. Dynamic changes in the follicular transcriptome and promoter DNA methylation pattern of steroidogenic genes in chicken follicles throughout the ovulation cycle. Plos One. 2015;10 doi: 10.1371/journal.pone.0146028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Yan M., Chen J., Li H., Zhang Y. MicroRNA-129-1-3p attenuates autophagy-dependent cell death by targeting MCU in granulosa cells of laying hens under H2O2-induced oxidative stress. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Miao S., Zhou W., Elnesr S.S., Dong X., Zou X. MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells. Ecotoxicol. Environ. Saf. 2021;214 doi: 10.1016/j.ecoenv.2021.112091. [DOI] [PubMed] [Google Scholar]
- Zhu M., Miao S., Zhou W., Elnesr S.S., Dong X., Zou X. Corrigendum to: "MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells [Ecotoxicol. Environ. Saf. 214 (2021) 112091] Ecotoxicol. Environ. Saf. 2021;217 doi: 10.1016/j.ecoenv.2021.112091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.