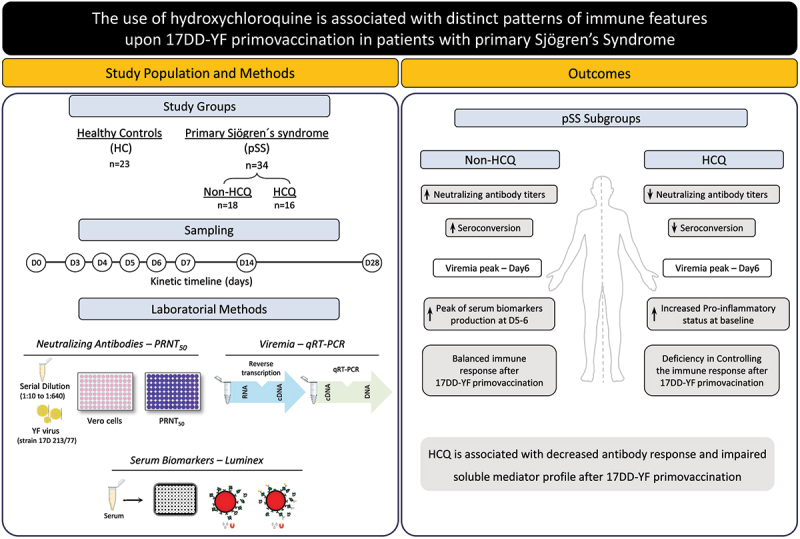
ABSTRACT
The present study aimed at investigating whether the hydroxychloroquine (HCQ) treatment would impact the neutralizing antibody production, viremia levels and the kinetics of serum soluble mediators upon planned 17DD-Yellow Fever (YF) primovaccination (Bio-Manguinhos-FIOCRUZ) of primary Sjögren’s syndrome (pSS). A total of 34 pSS patients and 23 healthy controls (HC) were enrolled. The pSS group was further categorized according to the use of HCQ (HCQ and Non-HCQ). The YF-plaque reduction neutralization test (PRNT ≥1:50), YF viremia (RNAnemia) and serum biomarkers analyses were performed at baseline and subsequent time-points (Day0/Day3–4/Day5–6/Day7/Day14-D28). The pSS group showed PRNT titers and seropositivity rates similar to those observed for HC (GeoMean = 238 vs 440, p = .11; 82% vs 96%, p = .13). However, the HCQ subgroup exhibited lower seroconversion rates as compared to HC (GeoMean = 161 vs 440, p = .04; 69% vs 96%, p = .02) and Non-HQC (GeoMean = 161 vs 337, p = .582; 69% vs 94%, p = .049). No differences in YF viremia were observed amongst subgroups. Serum biomarkers analyses demonstrated that HCQ subgroup exhibited increased levels of CCL2, CXL10, IL-6, IFN-γ, IL1-Ra, IL-9, IL-10, and IL-2 at baseline and displayed a consistent increase of several biomarkers along the kinetics timeline up to D14–28. These results indicated that HCQ subgroup exhibited a deficiency in assembling YF-specific immune response elicited by 17DD-YF primovaccination as compared to Non-HCQ subgroup. Our findings suggested that hydroxychloroquine is associated with a decrease in the humoral immune response after 17DD-YF primovaccination.
KEYWORDS: Primary Sjögren’s syndrome, hydroxychloroquine, 17DD-YF vaccine, humoral immunity, serum biomarkers
Graphical abstract
Introduction
Yellow fever (YF) is an infectious illness caused by a Flavivirus (Flaviviridae family). The disease is endemic in South America and Africa, and it is transmitted to humans by mosquitos from genera Haemogogus, Sabethes and Aedes.1 Based on the surveillance of the cases worldwide, the disease incidence is considered underestimated. The disease continues to cause several outbreaks in endemic areas leading to a higher mortality rate (>50% in severe cases). The most effective measure to minimize this epidemic scenario is the large-scale vaccination coverage.2
The live-attenuated YF vaccine was developed in 1937 and currently, there are three 17D substrains in production; 17DD manufactured in Brazil, 17D–213 manufactured in Russia, and 17D–204 manufactured in China, France, Senegal, and the United States.3 The 17D and 17DD-YF vaccines are both safe and highly immunogenic, producing protective immunity in roughly 95% of healthy individuals after a single dose.3
Studies showed that patients with autoimmune diseases (AID) and those who are undergoing immunomodulatory therapy display a suboptimal immunologic response after vaccination, when compared to healthy subjects.4–6 In this context, live-attenuated vaccines should be used with caution in AID populations because of the risk of adverse events (AE) following vaccination. In this regard, our group has developed a series of prospective non-interventional studies to assess the safety and immunogenicity of planned 17DD-YF primovaccination in AID patients. In general, 17DD-YF primovaccination is safe and immunogenic in AID patients, however, some features may interfere in the specific response to 17DD-YF vaccine, such as the type of disease, immunotherapy schemes, immunological status prior vaccination as well as the kinetics of serum soluble mediators.5,7,8
Aiming at expanding the previous reports of 17DD-YF vaccination in AID patients, the present study was carried out as a complementary study to address in more details the response of patients with primary Sjögren’s Syndrome (pSS) to 17DD-YF primovaccination. Primary Sjögren’s Syndrome is a chronic and systemic autoimmune disease with a prevalence of 0.17% in a major Brazilian metropolitan area,9 and it is the second most common autoimmune rheumatic condition in the world.10 The disease is characterized by lymphocytic infiltration of the exocrine glands, resulting in dysfunction and destruction.10 In some cases, sicca syndrome can be associated with systemic sclerosis, where the implementation of scleroderma-specific autoantibodies and capilaroscopy may be useful for differential diagnosis.11 In this context, the innate and adaptive immune systems are involved from the onset of the disease to the maintenance of the clinical manifestations.12
The pSS treatment is determined on a case-by-case basis according to disease activity as well as the presence and extent of extraglandular manifestations.13 Hydroxychloroquine (HCQ) is one of the most immunomodulatory drugs used in the pSS treatment. This drug has a good safety profile with minimal side effects.9,14 It has been shown that HCQ has antiviral properties, which interfere with signaling of endosomal Toll-like receptors as well as in autophagy activity, resulting in a reduction in antigen processing and presentation, and leading to a decrease in inflammatory cytokine secretion.15
To our knowledge, this is the first study investigating whether the use of HCQ would impact the overall performance of planned 17DD-YF primovaccination in pSS patients. The primary aim of the present study was to determine the impact of the use of HCQ on the neutralizing antibody production as well as the timeline kinetics of viremia and the profile of serum soluble mediators in pSS patients upon planned 17DD-YF primovaccination, categorized according to the use of Non-HCQ or HCQ immunotherapy.
Study population, material and methods
Study population
This is a prospective non-interventional investigation carried out between March 2017 and July 2017, during the Brazilian YF vaccination campaign coordinated by the Espírito Santo State Government. The study was submitted and approved by the HUCAM-EBSERH/UFES ethical committee (C.A.A.E 65,910,317.00000.5071) and was registered at the Brazilian Clinical Trials Registration Platform (UTN# U1111–1217–6672). The approval report from the ethical review committee board states that all the present investigation fulfills the principles of the Helsinki declaration and the 466/2012 resolution from the Brazilian National Health Council for research involving human subjects.
The goal of the present study was to determine the impact of HCQ use on the overall performance of planned 17DD-YF primovaccination addressing the safety and immunogenicity of planned 17DD-YF primovaccination (Bio-Manguinhos-FIOCRUZ) in pSS patients.
The patients were recruited as a convenience sampling at the Rheumatology Outpatient Clinic of the Hospital Universitário Cassiano Antônio Moraes (HUCAM/EBSERH) of the Federal University of Espírito Santo (UFES) in Vitória, Espírito Santo State, Brazil. During routine medical appointment, pSS patients were invited to participate in the study. Healthcare providers informed the patient with sufficient information as to clarify the risks and benefits of their participation in the study, before making their decision. Informed consent was obtained from all participants prior to inclusion in the study. The informed consent contains the following information: i) justification, aims and procedures; ii) risks and benefits; iii) details about the biological specimens to be collected and their use in the research; iv) guarantee of confidentiality; v) medical assistance follow-up; vi) freedom to refuse to participate in the study and withdraw the consent at any time; vii) participation costs, refund and compensation for possible damages; viii) signed informed consent.
At inclusion, all participants have received a log with information about YF vaccine-related adverse events and were instructed to register, on a daily basis, any symptoms observed from day 1 of vaccination throughout the 28 days follow-up after 17DD-YF vaccination. In order to overcome the subjectiveness of patient log reports, the symptoms recorded in the log were double-checked by a trained nurse/physician during weekly medical visits. Adverse events were classified according to the WHO guidelines.16 Healthy controls were also enrolled as a convenience sampling at the HUCAM/EBSERH during routine YF immunization. The pSS patients included in the present study fulfilled the international classification criteria for primary Sjögren’s Syndrome diagnosis according to the American College of Rheumatology/European League Against Rheumatism consensus.17
The exclusion criteria comprised: previous records of YF vaccination; participants with positive PRNT results (≥1:50) at study baseline; subjects who received other vaccine within 30-days interval; patients with medical warning to not receive the YF vaccine; subjects who refused to participate in the study protocol; patients with primary immunodeficiency or other immunosuppression causes: HIV carriers with records of CD4+ T-cell counts lower than 200 cells/mm3 or results of lymphocyte counts lower than 500 cells/mm3, patients with previous history of organ transplantation or neoplasia; and patients who received high levels of immunosuppressive treatment with antiproliferative drugs including (cyclophosphamide), mycophenolate mofetil, calcineurin inhibitors (tacrolimus, cyclosporine, sirolimus), prednisone ≥20 mg/day, methotrexate >20 mg/week, azathioprine >2 mg/kg/day.
The demographic characteristics, clinical features and immunotherapy schemes of participants included in the study are described in Table 1. The present study enrolled a total of 57 volunteers, including pSS patients (n = 34) and healthy controls (HC) without diagnosis of autoimmune diseases (n = 23). The pSS group was composed of females, age ranging from 23 to 92 years-old (median age = 56.5; IQR = 47.0–65.0). A control group comprised of 23 healthy volunteers, 16 females and 7 males, age ranging from 24 to 73 years-old (median age = 63.5, IQR = 53.0–67.3). The median age was similar in HC and pSS groups (p = .2612), with a female predominance in pSS as compared to HC (70% vs 100%, p = .0006). The pSS group was further categorized according to the use of HCQ, referred as: Non-HCQ (n = 18, age raging from 33 to 92, median = 59.5, IQR = 52.0–68.3) and HCQ (n = 16, age raging from 23 to 69, median = 53.5, IQR = 40.5–59.8). The median age was similar in Non-HCQ as compared to HCQ group (p = .0709) (Table 1).
Table 1.
Demographic characteristics, clinical features and immunotherapy schemes of patients with primary Sjogren’s syndrome and healthy controls.
| Parameters | Study Groups |
pSS subgroups |
||||
|---|---|---|---|---|---|---|
| HC (n = 23) | pSS (n = 34) | p value | Non-HCQ (n = 18) | HCQ (n = 16) | p value | |
| Age*, median (IQR) | 63.5 (53.0–67.3) | 56.5 (47.0–65.0) | .2612 | 59.5 (52.0–68.3) | 53.5 (40.5–59.8) | .0709 |
| Sex (female), n (%) | 16 (70%) | 34 (100%) | .0006 | 18 (100%) | 16 (100%) | >.9999 |
| ESSDAI (mean ± SD) | n/a | 0.38 ± 0.82 | - | 0.36 0.74 | 0.40 ± 0.91 | .8900 |
| Drugs | ||||||
| MTX | n/a | 6 (21%) | - | 3 (16%) | 3 (25%) | .5486 |
| SSA | n/a | 1 (3%) | - | 0 (0%) | 1 (6%) | .2817 |
| MTX/LFN | n/a | 1 (3%) | - | 0 (0%) | 1 (6%) | .2817 |
* Age is expressed in median with interquartile range (IQR); HC = healthy controls; pSS = patients with primary Sjögren’s Syndrome; Non-HCQ = pSS not using hydroxychloroquine; HCQ = pSS using hydroxychloroquine; ESSDAI = EULAR Sjögren’s Syndrome disease activity index; n/a = not applicable; SD = standard deviation. MTX = Methotrexate; SSA = Sulfasalazine; LFN = Leflunomide. Age was compared by Mann-Whitney test. Sex and Drugs were compared by Chi-square. In all cases, significant difference was considered at p ≤ .05 and underscored by bold underlined format.
Clinical data
Baseline clinical records including disease activity score according to EULAR Sjögren’s Syndrome disease activity index (ESSDAI)18 and the current use of synthetic and biological disease-modifying anti-rheumatic drugs (DMARDs) were assessed using the medical records. All pSS patients were in remission or under low disease activity and were advised by their rheumatologist advice to receive planned 17DD-YF primovaccination. Disease activity index (ESSDAI) of pSS patients was 0.38 0.82, which was similar in both subgroups (Non-HCQ = 0.36 0.74 vs HCQ = 0.4 0.91; p = .8900). Immunosuppressive therapy or biological therapy was interrupted before 17DD-YF primovaccination, according to the Brazilian recommendation on the safety and effectiveness of YF vaccination in patients with chronic immune-mediated inflammatory diseases.19 In the Non-HCQ group, 16% were under Methotrexate treatment. In the HCQ group, 25% were treated with Methotrexate, 6% with Sulfasalazine and 6% with Leflunomide (Table 1).
Serum samples
Whole blood samples were collected by venipuncture in vacuum tubes without anticoagulant and processed within 12 h. Serum specimens were obtained by centrifugation at 1,400× g, 4°C for 15 min, aliquoted and stored at −80°C until used for laboratorial analysis. Samples were collected at consecutive time-points, comprising: prior vaccination (D0) as well as D3, D4, D5, D6, D7, D14 and D28 after 17DD primovaccination. Samples collected at D0 and D28 were used for the analysis of YF-specific neutralizing antibodies test (PRNT). Samples collected for the quantification of YF-viral RNAnemia along the kinetic timeline were obtained using a cross-sectional design of 2 time-points for each volunteer, comprising: (D3 and D6) or (D4 and D7) or (D5 and D14).
YF-specific neutralizing antibody test (PRNT)
The neutralizing antibody titers were measured by the Plaque Reduction Neutralization Test (PRNT) at Laboratório de Tecnologia Virológica, Bio-Manguinhos (LATEV, FIOCRUZ-RJ, Brazil) according to Simões et al., 2012.20 Briefly, heat-inactivated serum samples (56°C, 30 min) were submitted to 2-fold serial dilution, starting at 1:5 up to 1:640, in 96-well tissue culture plates and tested in triplicates. Thereafter, each well received a suspension of the YF virus (strain 17D 213/77) and after the neutralization step, a suspension of Vero cells was added. After six days of incubation phase, the monolayers were fixed and stained. The antibody neutralization titer was defined as the reciprocal of serum dilution capable to reducing 50% of plaque numbers (PRNT50) as compared with the plaque counts obtained for the virus control. Seropositivity was considered using the reciprocal of serum dilution 1:50 dilution as the cutoff. The seroconversion ratio was defined as the proportion of individuals with PRNT values above the cutoff edge (1:50) at D28 after 17DD-YF primovaccination.
Quantification of YF-viral RNAnemia
The YF-viral RNAnemia was quantified by qRT-PCR assays at the Laboratório de Tecnologia Virológica, Bio-Manguinhos (LATEV, FIOCRUZ-RJ, Brazil). In summary, the YF RNA was extracted from serum samples using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The qRT-PCR assays were directed to NS5 region of YF virus genome, as described by Mantel et al., 2008.21 Reverse transcription reaction was carried out with random primers in 20 μL of the extracted RNA added to 20 μL of High-Capacity cDNA Reverse Transcription mix. The thermal conditions were 50°C, 5 min; 95°C, 20 s and 40 cycles of 95°C, 3 s, and 60°C, 33 s. The standard curve was created by cloning an 83-bp sequence from the NS5 region into a commercial cloning vector, following the manufacturer’s instructions. The standard curve was constructed using a ten-fold serial dilution of standard plasmid containing NS5 gene of yellow fever virus. The results were expressed as mean copies/mL based on the standard curve used as a reference to quantify the YF-viral RNAnemia of each tested sample. The limit of detection (LOD) for the qRT-PCR assays was 6.25 copies/µL previously validated at LATEV, FIOCRUZ-RJ, Brazil.
Analysis of serum immunological biomarkers
The levels of serum soluble mediators were measured using the Luminex Bio-plex platform at Flow Cytometry Facility of Instituto René Rachou (IRR, FIOCRUZ-MG, Brazil). A high-throughput microbeads array (Bio-Plex Pro™ Human Cytokine 27-plex Assay, Bio-Rad Laboratories, Hercules, CA, USA) was used to determine the levels of chemokines (CXCL8; CCL11; CCL3; CCL4; CCL2; CCL5; CXCL10), pro-inflammatory cytokines (IL-1β; IL-6; TNF-α; IL-12; IFN-γ; IL-15; IL-17), (IL-1Ra; IL-4; IL-5; IL-9; IL-10; IL-13) and growth factors (FGF-basic; PDGF; VEGF; G-CSF; GM-CSF; IL-2; IL-7). Sample processing and data acquisition were carried out by a trained technician using the Luminex 200 System and the Bioplex Manager Software. The final concentration of each serum biomarker was estimated by a 5-parameter logistic regression according to the standard curve inserted on each experimental batch, and the results were expressed in pg/mL.
Statistical analysis
Descriptive statistical analyses were performed in Prism 8.0.2 software (GraphPad Software, San Diego, USA). Comparative analysis of adverse events post-17DD-YF primovaccination was carried out by Chi-square test. Analysis of PRNT titers and basal levels of serum soluble mediators were carried out by Student t test and Mann-Whitney test, respectively. Multiple comparative analyses of PRNT titers and YF-viral RNAnemia at the peak of viremia were performed by One-Way variance analysis (ANOVA) or Kruskal-Wallis test followed by Tukey or Dunn’s post-test for pairwise comparisons. The analysis of the area under the curve (AUC) for cumulative RNAnemia along the kinetics timeline as well as the viremia kinetic curve profiles were carried out by Kolmogorov-Smirnov (KS) test. In all cases, a threshold of p < .05 was considered for statistical significance.
The serum soluble mediator signatures were assembled, along the kinetics timeline, to outline the panoramic profile of these biomarkers. Initially, the biomarker levels originally expressed as continuous values (pg/mL) were converted into categorical data (percentual proportion, %). The global median for each serum soluble mediator was defined as the threshold to identify the proportion of subjects (%) with biomarker levels above the cutoff edges. Heatmaps were constructed considering the proportion of subjects with biomarker levels above the global median cutoff at each time-point. A color key was used to underscore the proportion of subjects with increased biomarkers levels in percentiles: 10th (green), 50th (black) and 90th (red).
Correlation analysis was used to define the connections between the serum soluble mediators and integrative correlation matrices were constructed. The Cytoscape software platform (version 3.10.1) was employed to construct the integrative matrices. The clusters were distributed according to the type of serum soluble mediator. For this purpose, Pearson and Spearman correlation tests were used to obtain the “r” scores at p < .05. Serum soluble mediators involving at least 10 strong correlations were underscored by dark connecting edges. All the edges are represented as curves and in bundle following the description of Cornelissen et al., 2008.22 The amount of connectivity amongst attributes was calculated for each group along the kinetics timeline.
Results
Safety of the 17DD-YF vaccine
The occurrence of adverse events in both groups was monitored based on the weekly medical visit and patient log reports up to 28 days after 17DD-YF primovaccination. According to the WHO guidelines for causality assessment of an adverse event following immunization,16 only mild adverse events were reported for the cohort under study. The analysis of local (pain, lymphedema, general edema, warmth at the inoculation site) and systemic (fever, malaise, headache, myalgia, arthralgia, back pain, diarrhea, abdominal pain, nausea, emesis, pruritus, dyspnea, cough, and weakness) adverse events demonstrated similar rates in pSS patients and HC (2% vs 8% and 38% vs 21%, p = .14 and p = .10, respectively). Detailed description of adverse events post 17DD-YF primovaccination in pSS, categorized according to the use of HCQ immunotherapy are presented in Supplementary Table S1. Regarding any differences in arthralgia report, data analysis demonstrated similar rates in Non-HCQ and HCQ group (33% vs 31%, p = >.9999 and 44% vs 50%, p = >.9999; respectively).
Neutralizing antibody titers and seropositivity rates in primary Sjögren’s syndrome patients and healthy controls upon 17DD-YF primovaccination
The YF-specific neutralizing antibodies titers and the seropositive rates in pSS patients and healthy controls upon 17DD-YF primovaccination were measured, and the results are presented in Figure 1. The PRNT geometric mean titers (GeoMean) and seropositivity rates at D28 were considered as primary endpoint to evaluate the impact of the use of HCQ on the neutralizing antibody production. Data analysis demonstrated that both PRNT GeoMean and seropositivity rates were similar in pSS as compared to HC group (GeoMean = 238(142–400) vs 440(291–665), p = .1193; 82% vs 96%, p = .1334) (Figure 1).
Figure 1.
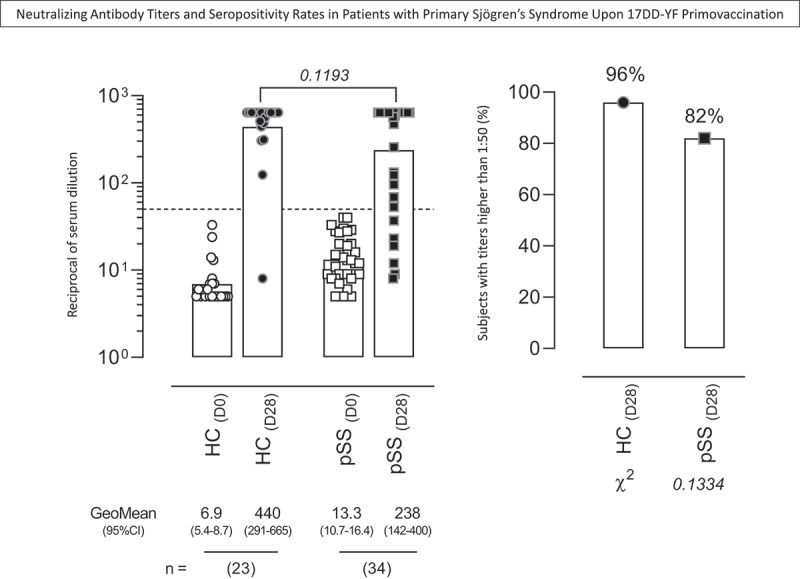
Neutralizing antibody titers and seropositivity rates in patients with primary Sjögren’s Syndrome upon 17DD-YF primovaccination. The YF-specific neutralizing antibodies were measured by plaque reduction neutralization test – PRNT [20] in serum samples from patients with primary Sjögren’s Syndrome (pSS, D0 =  , D28 =
, D28 =  , n = 34) and healthy controls (HC, D0 =
, n = 34) and healthy controls (HC, D0 =  ,D28 =
,D28 =  , n = 23). Data are shown as scattering of individual values of reciprocal of serum dilution over column charts representing geometric mean titers (95%CI). The seropositivity rates are shown as frequency (%) of subjects with titers higher than 1:50 (dashed line).Comparative analysis of PRNT titers and seropositive rates (pSS vs HC) were assessed by Student t test and Chi-square test, respectively. In all cases, significant differences were considered at p < .05.
, n = 23). Data are shown as scattering of individual values of reciprocal of serum dilution over column charts representing geometric mean titers (95%CI). The seropositivity rates are shown as frequency (%) of subjects with titers higher than 1:50 (dashed line).Comparative analysis of PRNT titers and seropositive rates (pSS vs HC) were assessed by Student t test and Chi-square test, respectively. In all cases, significant differences were considered at p < .05.
To assess the impact of the use of HCQ in YF-specific neutralizing antibody titers and the seropositivity rates upon 17DD-YF primovaccination, the pSS patients were classified according to the use of HCQ (HCQ and Non-HCQ group, respectively) (Figure 2). The results showed that HCQ exhibited lower PRNT GeoMean and seroconversion rates as compared to HC (GeoMean = 161 vs 440, p = .0465; 69% vs 96%, p = .0220). Conversely, the Non-HQC group showed similar PRNT GeoMean and seroconversion rates as compared to HC (GeoMean = 337 vs 440, p = .4290; 94% vs 96%, p = .8586). Although the PRNT titers observed in HCQ were similar to that detected in Non-HCQ (GeoMean = 161 vs 337, p = .2648), significantly lower seropositivity rate was observed in HCQ as compared to Non-HCQ (69% vs 94%, p = .0498) (Figure 2).
Figure 2.
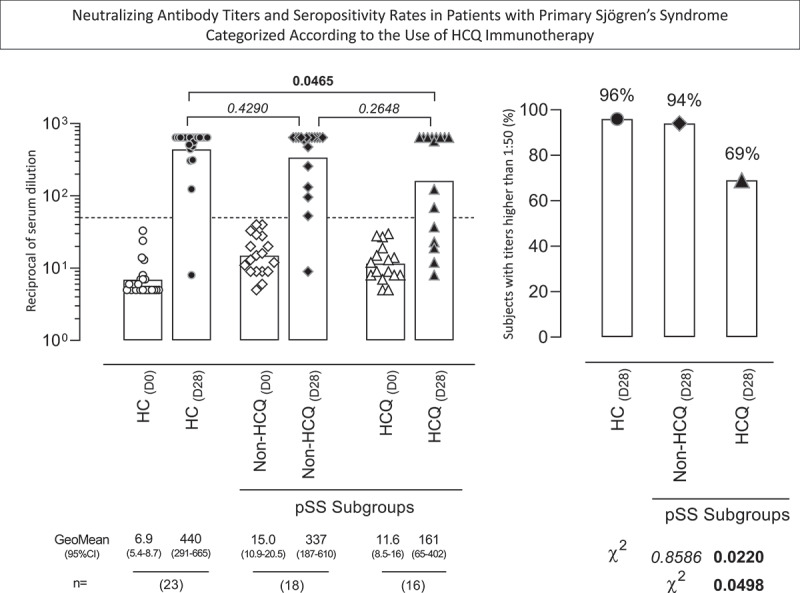
Neutralizing antibody titers and seropositivity rates in patients with primary Sjögren’s Syndrome categorized according to the use of HCQ immunotherapy. The YF-specific neutralizing antibodies were measured by plaque reduction neutralization test – PRNT [20] in serum samples from patients with primary Sjögren’s Syndrome (pSS), categorized according to the use HCQ immunotherapy, referred as: Non-HCQ (D0 =  , D28 =
, D28 =  , n = 18) or HCQ (D0 =
, n = 18) or HCQ (D0 =  , D28 =
, D28 =  , n = 16) and samples from healthy controls (HC, D0 =
, n = 16) and samples from healthy controls (HC, D0 =  , D28 =
, D28 =  , n = 23). Data are shown as scattering of individual values of reciprocal of serum dilution over column charts representing geometric mean titers (95% CI). The seropositivity rates are shown as frequency (%) of subjects with titers higher than 1:50 (dashed line). Comparative analysis of PRNT titers and seropositive rates (pSS subgroups vs HC) were assessed by Student t test and Chi-square test, respectively. In all cases, significant differences were considered at p < .05 and highlighted by bold format.
, n = 23). Data are shown as scattering of individual values of reciprocal of serum dilution over column charts representing geometric mean titers (95% CI). The seropositivity rates are shown as frequency (%) of subjects with titers higher than 1:50 (dashed line). Comparative analysis of PRNT titers and seropositive rates (pSS subgroups vs HC) were assessed by Student t test and Chi-square test, respectively. In all cases, significant differences were considered at p < .05 and highlighted by bold format.
Considering that 5 out of 16 pSS patients amongst HCQ subgroup were using other medications (MTX = 3, MTX/LFN = 1, SSA = 1) additional analysis was carried out to evaluate whether the use of other medications was associated with lower antibody response observed in HCQ. Data analysis demonstrated that the use of additional medications did not impact the neutralizing antibody response in HCQ as compared to HCQ + other medications [GeoMean = 154 (47–506) vs 179 (20–1,592), p = .8571] Data not shown.
Further analysis was carried out to verify whether age at primovaccination impacted the PRNT titers. Correlation analyses were carried out and the results presented in the Supplementary Figure S1. No correlation was observed between age and PRNT titers in both pSS subgroups (Supplementary Figure S1).
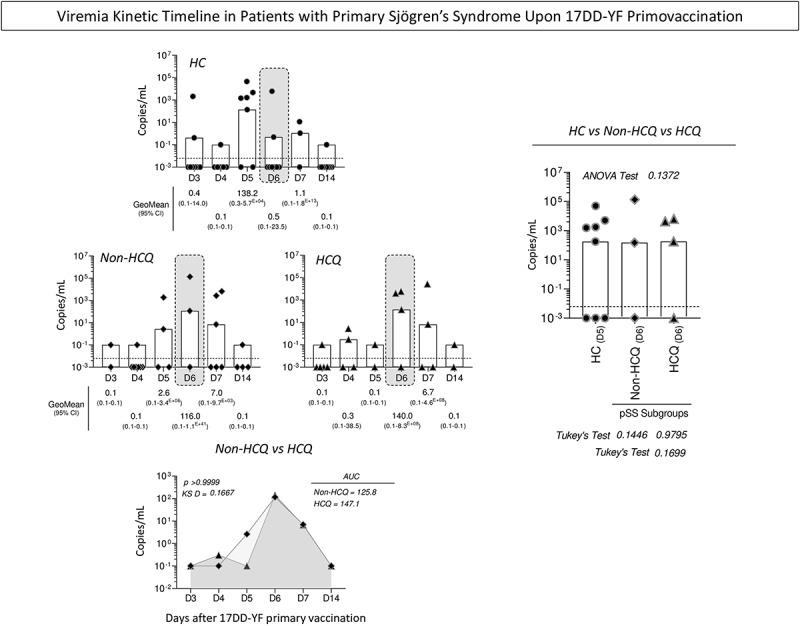
Viremia kinetics in primary Sjögren’s syndrome patients and healthy controls upon 17DD-YF primovaccination
The YF-viral RNAnemia along kinetics viremia was measured, and the results were expressed as mean copies/mL (Figure 3). The results demonstrated that all pSS patients, regardless of the use of HCQ, exhibited a peak of viremia at D6, representing a slightly late viremia peak as compared to HC group (D5). Comparative analysis of the AUC between the viremia Non-HCQ vs HCQ kinetics curves did not show significant differences. Moreover, no differences were observed in the analysis of viremia levels at peak (Figure 3). Aiming at further characterizing whether age at primovaccination impacted the peak of viremia, correlation analyses were carried out and the results presented in the Supplementary Figure S1. No correlation was observed between age and viremia levels at peak RNAnemia (D6) in both pSS subgroups (Supplementary Figure S1). The analysis of cumulative RNAnemia for individual samples was also carried out along the kinetics timeline according to age. Comparative analysis between Non-HCQ vs HCQ subgroups did not demonstrate significant difference (p = .4366; KS D = 0.3250) for the AUC of cumulative RNAnemia (Supplementary Figure S1).
Figure 3.
Viremia kinetic timeline in patients with Sjögren’s Syndrome upon 17DD-YF primovaccination.The viremia levels were measured by qRT-PCR assay in serum samples from patients with Sjögren’sSyndrome (pSS), categorized according to the use of HCQ immunotherapy, referred as: Non-HCQ ( , n = 18) or HCQ (
, n = 18) or HCQ ( , n = 16) and healthy controls (HC,
, n = 16) and healthy controls (HC,  , n = 23) at distinct time points upon 17DD-YF primovaccination (D3,D4, D5, D6, D7, and D14). Data are shown as GeoMean of copies/mL (95%CI) along the kinetics timeline. Dashed line represents the limit of detection (6.25 copies/µL). Undetectable levels were computed as 0.001 copies/mL. Viremia kinetic curves are shown as GeoMean of copies/mL in overlayed line charts. Comparative analysis of viremia levels at the day of viremia peak (gray rectangles) was carried out by ANOVA followed by Tukey’s post-test and the viremia kinetic curves compared by Kolmogorov-Smirnov (KS) test. In all cases, significant differences at were considered at p < .05.
, n = 23) at distinct time points upon 17DD-YF primovaccination (D3,D4, D5, D6, D7, and D14). Data are shown as GeoMean of copies/mL (95%CI) along the kinetics timeline. Dashed line represents the limit of detection (6.25 copies/µL). Undetectable levels were computed as 0.001 copies/mL. Viremia kinetic curves are shown as GeoMean of copies/mL in overlayed line charts. Comparative analysis of viremia levels at the day of viremia peak (gray rectangles) was carried out by ANOVA followed by Tukey’s post-test and the viremia kinetic curves compared by Kolmogorov-Smirnov (KS) test. In all cases, significant differences at were considered at p < .05.
Serum biomarkers in primary Sjögren’s syndrome patients categorized according to the use of HCQ immunotherapy at baseline
In a complementary analysis, intending to investigate whether the basal profile of serum soluble mediators was associated with the HCQ immunotherapy, the pSS patients were categorized according to the use of HCQ, and the biomarkers were measured at baseline (D0). Overall, the results reveal that HCQ group exhibited an increase in the levels of CCL2, CXL10, IL-6, IFN-γ, IL1-Ra, IL-9, IL-10 and IL-2 (Figure 4). Detail statistical analysis of serum soluble mediators in pSS patients further categorized according to the use of HCQ is provided in the Supplementary Table S2.
Figure 4.
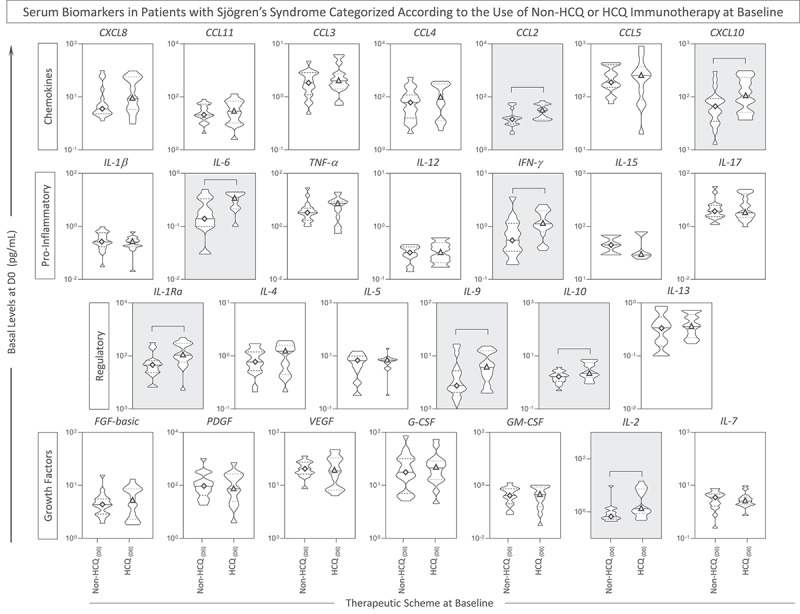
Serum soluble mediators in patients with Sjögren’sSyndrome categorized according to the use of HCQ immunotherapy. The levels of serum soluble mediators were measured by high-throughput microbeads array in serum samples from patients with Sjögren’s Syndrome (pSS), categorized according to the use of immunotherapy, referred as: Non-HCQ (D0 =  , n = 18) or HCQ (D0 =
, n = 18) or HCQ (D0 =  , n = 16) prior 17DD-YF primovaccination. The levels of chemokines(CXCL8, CCL11, CCL3, CCL4, CCL2, CCL5, CXCL10), pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-12, IFN-γ, IL-15, IL-17),regulatory cytokines (IL-1Ra, IL-4, IL-5, IL-9, IL-10, IL-13) and growth factors (FGF-basic, PDGF, VEGF, G-CSF, GM-CSF, IL-2 and IL-7) are shown as violin plot distribution (min and max) of serum concentrations (pg/mL) underscoring the median (continuous line) and 25th and 75th interquartile range (dashedlines). Significant differences at p < .05 are identified by connecting line and underscored by gray background.
, n = 16) prior 17DD-YF primovaccination. The levels of chemokines(CXCL8, CCL11, CCL3, CCL4, CCL2, CCL5, CXCL10), pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IL-12, IFN-γ, IL-15, IL-17),regulatory cytokines (IL-1Ra, IL-4, IL-5, IL-9, IL-10, IL-13) and growth factors (FGF-basic, PDGF, VEGF, G-CSF, GM-CSF, IL-2 and IL-7) are shown as violin plot distribution (min and max) of serum concentrations (pg/mL) underscoring the median (continuous line) and 25th and 75th interquartile range (dashedlines). Significant differences at p < .05 are identified by connecting line and underscored by gray background.
Kinetics biomarker signatures in primary Sjögren’s syndrome patients and healthy controls upon 17DD-YF primovaccination
To better understand the dynamics of serum soluble mediators upon 17DD-YF primovaccination and the connection with the use of HCQ in pSS patients, signatures were built (Figure 5).
Figure 5.
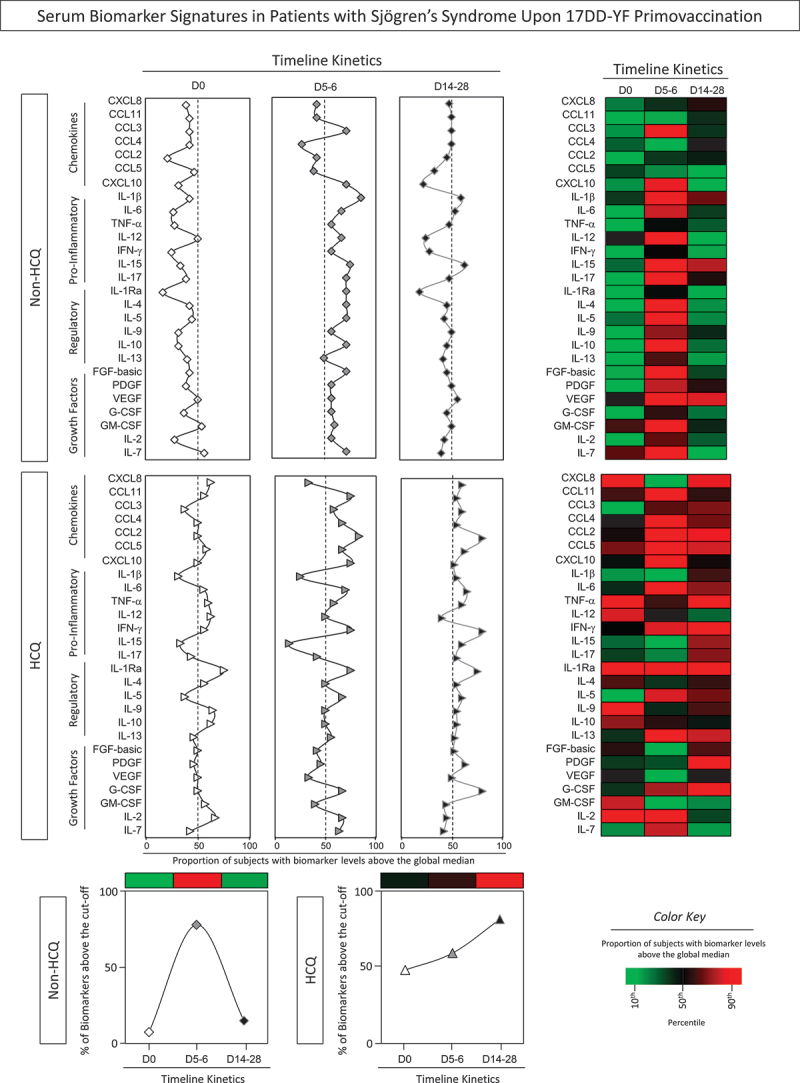
Serum biomarker signatures in patients with Sjögren’s Syndrome upon 17DD-YF primovaccination. The levels of serum soluble mediators were measured by high-throughput microbeads array in serum samples from patients with Sjögren’s Syndrome (pSS), categorized according to the use of HCQ immunotherapy, referred as: Non-HCQ (D0 =  , D5-6 =
, D5-6 =  , D14-28 =
, D14-28 =  , n = 18) or HCQ (D0 =
, n = 18) or HCQ (D0 =  , D5-6 =
, D5-6 =  , D14-28 =
, D14-28 =  , n = 16) prior 17DD-YF primovaccination. Serum biomarker signatures were assembled converting the biomarker levels originally express as continuous values (pg/mL) into categorial data, and the results reported as proportion of subjects (%) with biomarker levels above the global median cutoff defined for each serum soluble mediator. Signature curves were assembled to identify the biomarkers with proportion of subjects above the 50th percentile (dashed line). Heatmaps were constructed considering the proportion of subjects with biomarker levels above the global median cutoff at each time point (D0, D5-6 and D14-28). Color key underscores the proportion of subjects with increased biomarkers levels in percentiles: 10th (green), 50th (black) and 90th (red). Line charts show the total number of biomarkers above the global median cutoff.
, n = 16) prior 17DD-YF primovaccination. Serum biomarker signatures were assembled converting the biomarker levels originally express as continuous values (pg/mL) into categorial data, and the results reported as proportion of subjects (%) with biomarker levels above the global median cutoff defined for each serum soluble mediator. Signature curves were assembled to identify the biomarkers with proportion of subjects above the 50th percentile (dashed line). Heatmaps were constructed considering the proportion of subjects with biomarker levels above the global median cutoff at each time point (D0, D5-6 and D14-28). Color key underscores the proportion of subjects with increased biomarkers levels in percentiles: 10th (green), 50th (black) and 90th (red). Line charts show the total number of biomarkers above the global median cutoff.
The data analysis revealed that at baseline the Non-HCQ group displayed a reduced production of most biomarkers at baseline, except for GM-CSF and IL-7. However, at D5–6, a different pattern was observed with an increase of several biomarkers, characterizing a complex response at the peak of viremia. At D14–28, the biomarker profile was characterized by few serum soluble mediators above the global median cutoff. On the other hand, the HCQ group exhibited an opposed profile displaying a consistent increase of several biomarkers along the kinetics timeline. This pattern extends to D14–28, indicating a possible deficit in deflating the inflammatory condition along time post-vaccination. Heatmaps corroborate these distinct patterns, revealing that the Non-HCQ group only showed a significant increase in biomarker levels at D5–6 after 17DD-YF primovaccination. On the other hand, HCQ group exhibited increased levels of several biomarkers in all time-points of kinetics timeline (Figure 5a).
This deficiency in deflating the immune response observed in the HCQ group was reinforced by the proportion of prominent levels of biomarkers that reached the peak at D14–28, while the Non-HCQ group showed a peak of biomarker production was at D5–6 (Figure 5b).
Serum biomarker integrative networks in primary Sjögren’s syndrome patients upon 17DD-YF primovaccination
Intending to visualize the systemic relationship between distinct serum soluble mediators along the kinetics timeline (D0, D5–6 and D14–28) of 17DD-YF-primovaccinated pSS patients, serum biomarker integrative networks were built, and the findings are shown in Figure 6. The network matrices were built to highlight the significant strong correlations as well as the biomarkers involved in more than 10 neighborhood connections.
Figure 6.
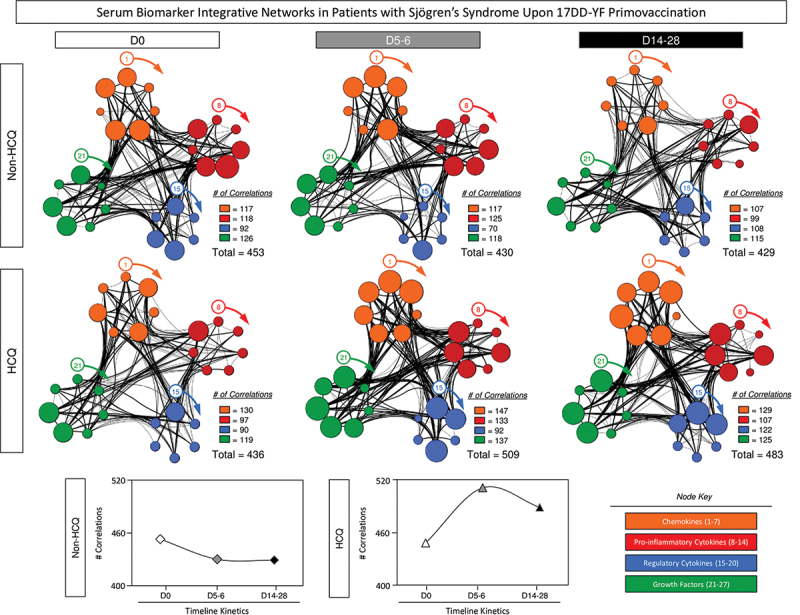
Serum biomarker integrative networks in patients with Sjögren’s Syndrome upon 17DD-YF primovaccination. The levels of serum soluble mediators were measured by high-throughput microbeads array in serum samples from patients with Sjögren’s Syndrome (pSS), categorized according to the use of HCQ immunotherapy, referred as: Non-HCQ (D0 =  , D5-6 =
, D5-6 =  , D14-28 =
, D14-28 =  , n = 18) or HCQ (D0 =
, n = 18) or HCQ (D0 =  , D5-6 =
, D5-6 =  , D14-28 =
, D14-28 =  , n=16) prior 17DD-YF primovaccination. The integrative networks were assembled based on Pearson and Spearman “r” scores between chemokines (
, n=16) prior 17DD-YF primovaccination. The integrative networks were assembled based on Pearson and Spearman “r” scores between chemokines ( = CXCL8, CCL11, CCL3, CCL4, CCL2, CCL5, CXCL10), pro-inflammatory cytokines (
= CXCL8, CCL11, CCL3, CCL4, CCL2, CCL5, CXCL10), pro-inflammatory cytokines ( = IL-1β, IL-6, TNF-α, IL-12, IFN-γ, IL-15, IL-17), regulatory cytokines (
= IL-1β, IL-6, TNF-α, IL-12, IFN-γ, IL-15, IL-17), regulatory cytokines ( = IL-1Ra, IL-4, IL-5, IL-9, IL-10, IL-13) and growth factors (
= IL-1Ra, IL-4, IL-5, IL-9, IL-10, IL-13) and growth factors ( = FGF-basic, PDGF, VEGF, G-CSF, GM-CSF, IL-2 and IL-7). The networks were created considering only significant correlations (p < .05). Connecting edges show weak/moderate (0.1 to 0.67, thin lines) and strong “r” scores (≥ 0.67, bold lines) between pairs of attributes. Line charts show the total number of connections between the biomarkers.
= FGF-basic, PDGF, VEGF, G-CSF, GM-CSF, IL-2 and IL-7). The networks were created considering only significant correlations (p < .05). Connecting edges show weak/moderate (0.1 to 0.67, thin lines) and strong “r” scores (≥ 0.67, bold lines) between pairs of attributes. Line charts show the total number of connections between the biomarkers.
The results reveal that Non-HCQ group, at baseline (D0), presented a complex network involving a high number of correlations between pairs of serum soluble biomarkers (n = 453 at baseline/D0). Along the kinetics timeline, the number of correlations decreased (n = 430 at D5–6; n = 429 at D14–28), however, the complexity of the network at D5–6 remained corresponding to that found at D0. Furthermore, a reduction in the network complexity was observed at D14–28, which demonstrated the capacity of these patients in achieving a balance in immune response after 17DD-YF primovaccination.
A distinct network profile was observed for HCQ group. At baseline (D0), serum soluble mediators displayed lower number of strong correlations (n = 436 at baseline/D0). At D5–6, an increase in the neighborhood connectivity was observed reaching the total of 509 with a decline to 483 connections at D14–28 (Figure 6). In contrast with the decrease in network complexity showed in Non-HCQ, the use of HCQ by pSS patients seem to influence the constant inflammatory condition. These data are in accordance to those described in the Figure 5.
Discussion
Our data showed that patients with pSS had PRNT titers and seroconversion rates similar to healthy controls, but geometric mean titers were 46% lower than in healthy controls. Similarly, another study by our group already showed that patients with autoimmune diseases presented late seroconversion profiles according to kinetics timelines of PRNT than HC, particularly those with Spondylarthritis (73%) and Systemic Lupus Erythematosus (73%).7
In addition to the previous autoimmune disease reports, factors such as inflammatory status prior vaccination and use of medications can interfere with the immune response.7,8 Therefore, aiming to minimize confounding bias, all patients with Sjögren’s Syndrome included in this study had low disease activity, as assessed ESSDAI.18 Moreover, drugs associated with high immunosuppression are known to impair immune response to vaccination and increased risk of adverse events (AE).6,19 Previous biological DMARD therapy decreased the humoral immune response, even after washing out 5 half-lives.8 Patients taking medications such as antiproliferative drugs, including cyclophosphamide, mycophenolate mofetil, azathioprine, calcineurin inhibitors, biological therapy and prednisone ≥20 mg/day should avoid receiving live virus vaccines.8,19 However, HCQ is not on that list of medications.
We evaluated the influence of medications on vaccine response. In this analysis, HCQ group exhibited lower seroconversion rates as compared to healthy controls (69% vs 96%, p = .02) and Non-HQC (69% vs 94%, p = .049).
Previous studies have been undertaken to verify whether the concurrent administration of immunomodulatory drugs may affect the immune response to distinct vaccines. It has been previously shown that Methotrexate therapy impacts the seasonal influenza vaccination in rheumatoid arthritis patients, which lead to FDA recommendation of MTX discontinuation for one week following quadrivalent influenza vaccination.23 Likewise, other studies have shown that HCQ can impair humoral responses to PCV13 pneumococcal conjugate vaccine (PCV13) in patients with cutaneous lupus erythematosus under HCQ treatment.24 Conversely, reduced antibody seroconversion has been observed in subjects receiving concomitant administration of chloroquine with live oral cholera vaccine (CVD103-HgR)25 as well as to rabies vaccine.26 However, no effect of prophylactic administration of chloroquine on seroconversion upon YF-vaccination in healthy travelers to YFV and malaria co-endemic areas.27,28 Hydroxychloroquine is a valuable disease-modifying anti-rheumatic drug (DMARD) in rheumatology, especially in systemic lupus erythematosus and pSS upon medical indication. As HCQ is not qualified as a potent immunomodulatory drug and, therefore, in most cases of vaccination, HCQ therapy withdraw is not required. However, considering that HCQ may have antiviral properties mediated by its endosomal maturation inhibitory activity, the concurrent administration of HCQ may affect the immune response to live-attenuated viral vaccines. Another point to be considered is that the prophylactic or chronic HCQ administration may have distinct impact on the immune response to vaccine. Therefore, subjects undertaking chloroquine for a prolonged period (e.g., patients with rheumatic diseases or residents of malaria endemic areas) might present impaired antibody response to YF-vaccine.27 Our findings corroborate this hypothesis since lower levels of neutralizing antibodies were observed in HCQ group as compared to healthy controls. It is important to mention that lower levels of neutralizing antibodies do not necessarily mean lower levels of protection. Previous studies in experimental models have demonstrated that neutralizing antibodies alone in titers ≥40, before challenge, is fully protective against challenge with YF virus, while titers of 10–20 provided partial protection.29
The precise mechanism of action of hydroxychloroquine and impact in immune response is unclear. Chloroquine could adversely affect the antibody response to YF-vaccine through distinct mechanisms: by directly impacting viral replication or interfering in immunological mechanisms required for antibody production.27 It has been shown that HCQ decrease the replication of several viruses such as dengue virus, chikungunya virus, influenza A virus, zika virus, and severe acute respiratory syndrome coronavirus (SARS-CoV-2) in in vitro models.30 Moreover, the impact of chloroquine in reducing the YFV replication has been also documented.31 The HCQ may also suppress crucial immunological functions mediated by macrophages, which are required for antigen processing and presentation, and therefore, interfering in the production of cytokines.32 Novel functions of HCQ such as down-regulation of T follicular helper cells (Tfh) have been reported in patients with rheumatoid arthritis.33 Tfh cells are vital for the development of germinal centers, promoting the differentiation of B-cells into memory and plasma cells, ultimately affecting the antibody response.34 A recent study has reported that Tfh cells are elevated in peripheral blood of patients with primary Sjögren’s syndrome.35 We hypothesize that one of the putative mechanisms associated with lower seroconversion upon 17DD-YF primovaccination in pSS patients involved the impact of HCQ on Tfh cells in addition to other impaired immunological responses. We have observed that the HCQ group had a dysfunctional immune response, with an increase of IFN-γ and IL-10 levels compared to the controls and Non-HCQ group, which endorse previous results that suggested a poorer seroconversion status on patients with autoimmune diseases due to an asynchronous biomarker production upon 17DD-YF vaccination.7
The analysis of viremia levels and kinetics timeline did not support the differences observed in seroconversion amongst HCQ subgroups. The analysis of immune mediators demonstrated that HCQ group presented a delayed increase in serum biomarkers that reached the peak at D14–28 as compared to Non-HCQ that exhibited the peak of biomarker production at D5–6, likewise the profile previously reported for healthy controls.7 It is possible that changes in the soluble mediators response play a relevant role guiding the neutralizing antibody response in pSS patients. It has been shown that 17DD-YF primovaccination induced an increase of pro-inflammatory cytokines followed by a decline around 14–28 days.7 The HCQ group exhibited an increase in the levels of cytokines at baseline, exhibiting an asynchronous cytokine response with the viremia peak and a deficient immunomodulatory profile after vaccination. This finding agrees with a previous study showing that patients with autoimmune diseases exhibited asynchronous viremia/soluble mediator response as the putative mechanism underling the inefficient seroconversion.7
This study has some limitations. The small number of patients enrolled in the present investigation besides the inclusion of patients in remission or under low disease activity suggested that our findings should not be directly extrapolated to all pSS patients without considering these clinical particularities. Moreover, the sample size did not allow addressing the impact of specific DMARDs (MTX, SSA, LFN) on the 17DD-YF vaccination. It should be highlighted, however, that primary Sjögren’s Syndrome is a quite rare disorder within the Brazilian population. Another limitation was the use of a restricted range of serum dilutions to test the neutralizing antibodies (1:5 to 1:640). The use of higher serum dilutions could allow a more precise comparison between groups. Despite that, it was possible to show a significant difference in the neutralizing antibody levels after vaccination in pSS patients using HCQ as compared to healthy controls.
Our findings suggested that the use use of HCQ is associated with low neutralizing antibody response after 17DD-YF primovaccination. However, it is important to mention that HCQ is a safe and efficient drug, being one of the most prescribed medications for several autoimmune diseases such as Lupus, Rheumatoid arthritis, Sjögren’s Syndrome, and Myopathies. Additional studies are needed to further evaluate the immunological events underlying the impact of HCQ on the humoral and cellular response to live virus vaccines.
Supplementary Material
Acknowledgments
The authors thank the Hospital Universitário Cassiano Antônio Moraes/EBSERH/UFES for support and use of its facilities. The study was carried out by students enrolled at the Programa de Pós-graduação em Saúde Coletiva (UFES) and the Programa de Pós-graduação em Ciências da Saúde (FIOCRUZ-Minas), supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors thank the Program for Technological Development in Tools for Health-RPT-FIOCRUZ for using the flow cytometry facilities. OAMF, ATC received PQ fellowships from CNPq and are research fellows from FAPEAM (PVN-II, PRÓ-ESTADO Program #005/2019. OAMF is a research fellow from Universidade do Estado do Amazonas-UEA (PROVISIT No. 005/2023-PROPESP/UEA).
Funding Statement
This study was funded by a grant from the Programa Nacional de Imunizações (PNI/SVS), Ministério da Saúde do Brasil, OCAMO Project (Fundo Nacional da Saúde, TED 93/2019, 22/11/2019). The study was also supported by Fundação de Apoio à Pesquisa do Espírito Santo (FAPES) e Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Biomanguinhos/FIOCRUZ, Conselho Nacional de Desenvolvimento Científico e Tecnológico– CNPq and Sociedade de Reumatologia do Espírito Santo (SORES).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
KLLLM, IACR, OAMF and VV conceived and designed the research protocol. OAMF, ATC, FFSTF and VV were responsible for acquiring the financing. LMHM composed the advisory committee. KLLLM, IACR, LGRA, IRM, STM, PCMR, EVS, APEG, MFB, SMBL, WDS, ASA, RTS, JFAS, JPBS, ACCA, ATC, VPM, OAMF and VV collected samples and conducted the experiments. KLLLM, IACR, SMBL, WDS, ASA, RTS, JFAS, ACCA, and VPM acquired the data. KLLLM, IACR, SMBL, ACCA, VPM, JGCdR, ATC, OAMF and VV analyzed and interpreted the data. KLLLM, IACR, JGCdR, ACCA, OAMF and VV edited and wrote the manuscript. All authors have read, reviewed, and approved the final version of the manuscript.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2318814.
References
- 1.Douam F, Ploss A.. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018;26(11):913–13. doi: 10.1016/j.tim.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monath TP, Vasconcelos PF.. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Collins ND, Barrett AD. Live attenuated yellow fever 17D vaccine: a legacy vaccine still controlling outbreaks in Modern day. Curr Infect Dis Rep. 2017;19(3):14. doi: 10.1007/s11908-017-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonacio AC, Do Nascimento Pedrosa T, Borba EF, Aikawa NE, Pasoto SG, Filho JCRF, Sampaio Barros MM, Leon EP, Lombardi SCFS, Junior AM, et al. Immunogenicity and safety of primary fractional-dose yellow fever vaccine in autoimmune rheumatic diseases. PloS Negl Trop Dis. 2021;15(11):e0010002. [accessed 2021 Nov 29]. doi: 10.1371/journal.pntd.0010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valim V, Machado KLLL, Miyamoto ST, Pinto AD, Rocha PCM, Serrano EV, Dinis VG, Gouvêa SA, Dias JGF, Campi-Azevedo AC. et al. Planned yellow fever Primovaccination is Safe and immunogenic in patients with autoimmune diseases: a prospective non-interventional study. Front Immunol. 2020;11:1382. [accessed 2020 Jul 17]. doi: 10.3389/fimmu.2020.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieten RW, Goorhuis A, Jonker EFF, de Bree GJ, de Visser AW, van Genderen PJJ, Remmerswaal EBM, ten Berge IJM, Visser LG, Grobusch MP, et al. 17D yellow fever vaccine elicits comparable long-term immune responses in healthy individuals and immune-compromised patients. J Infect. 2016;72(6):713–722. doi: 10.1016/j.jinf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 7.da Costa-Rocha IA, Machado KLLL, Campi-Azevedo AC, Teixeira-Carvalho A, Peruhype-Magalhães V, de Lima SMB, Miranda EH, GF Trindade, Casagrande TZ, et al. Serum biomarker profile orchestrating the seroconversion status of patients with autoimmune diseases upon planned primary 17DD yellow fever vaccination, Sci Rep. 2021;11(1):10431. [accessed 2021 May 17]. doi: 10.1038/s41598-021-89770-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casagrande TZ, Costa-Rocha IAD, Gavi MBRO, Miyamoto ST, Martins PC, Serrano ÉV, Dinis VG, Machado KLLL, Gouvea SA, Caser LC, et al. Previous biological therapy and impairment of the IFN-γ/IL-10 axis are associated with low immune response to 17DD-YF vaccination in patients with spondyloarthritis. Vaccine. [accessed 2022 Jul 30];40(32):4580–4593. doi: 10.1016/j.vaccine.2022.05.071. Epub 2022 Jun 18. PMID: 35728990. [DOI] [PubMed] [Google Scholar]
- 9.Valim V, Zandonade E, Pereira AM, Brito Filho, O.H.D., Serrano, E V., Musso, C., Giovelli, R A., Ciconelli, R M.. Primary Sjögren’s syndrome prevalence in a major metropolitan area in Brazil. Revista Brasileira de Reumatologia (English Edition). 2013;53(1):24–34. doi: 10.1016/S2255-5021(13)70003-8. [DOI] [PubMed] [Google Scholar]
- 10.Thorne I, Sutcliffe N. Sjögren’s syndrome. Br J Hosp Med (Lond). 2017;78(8):438–442. doi: 10.12968/hmed.2017.78.8.438. [DOI] [PubMed] [Google Scholar]
- 11.Marketos N, Koulouri V, Piperi EP, Georgaki, M E., Nikitakis, N G., Mavragani, C P.. Scleroderma-specific autoantibodies: should they be included in the diagnostic work-up for Sjögren’s syndrome? Semin Arthritis Rheum. 2022;55:152026. doi: 10.1016/j.semarthrit.2022.152026 [DOI] [PubMed] [Google Scholar]
- 12.Theander E, Vasaitis L, Baecklund E, Nordmark G, Warfvinge G, Liedholm R, Brokstad K, Jonsson R, Jonsson MV. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70(8):1363–8. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The diagnosis and treatment of Sjögren’s syndrome. Dtsch Arztebl Int. 2017;114(20):354–361. doi: 10.3238/arztebl.2017.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhang T, Guo Z, Pu J, Riaz F, Feng R, Fang X, Song J, Liang Y, Wu Z. et al. The efficiency of hydroxychloroquine for the treatment of primary Sjögren’s syndrome: a systematic review and meta-analysis. Front Pharmacol. 2021;12:693796. [accessed 2021 Sep 7]. doi: 10.3389/fphar.2021.693796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gies V, Bekaddour N, Dieudonné Y, Guffroy A, Frenger Q, Gros F, Rodero MP, Herbeuval JP, Korganow, AS.. Beyond anti-viral effects of Chloroquine/Hydroxychloroquine. Front Immunol. 2020;11:1409. [accessed 2020 Jul 2]. doi: 10.3389/fimmu.2020.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. [accessed 2022 Apr 18]. https://apps.who.int/iris/handle/10665/80670.
- 17.Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ. et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69(1):35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, Gottenberg J-E, Ramos-Casals M, Dorner T, Ravaud P, et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): a user guide, RMD Open. 2015;1(1):e000022. [accessed 2015 Feb 20]. doi: 10.1136/rmdopen-2014-000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pileggi GS, Da Mota LMH, Kakehasi AM, De Souza AW, Rocha A, de Melo AKG, da Fonte CAM, Bortoletto C, Brenol CV, Marques CDL, et al. Brazilian recommendations on the safety and effectiveness of the yellow fever vaccination in patients with chronic immune-mediated inflammatory diseases, Adv Rheumatol. 2019;59(1):17. [accessed 2019 Apr 29]. doi: 10.1186/s42358-019-0056-x. [DOI] [PubMed] [Google Scholar]
- 20.Simões M, Camacho LA, Yamamura AM, Miranda EH, AC C, da Silva Freire M. Evaluation of accuracy and reliability of the plaque reduction neutralization test (micro-PRNT) in detection of yellow fever virus antibodies. Biologicals. 2012;40(6):399–404. doi: 10.1016/j.biologicals.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Aguirre M, Gulia S, Girerd-Chambaz Y, Colombani S, Moste C, Barban V. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever–dengue vaccines. J Virol Methods. 2008. Jul;151(1):40–6. doi: 10.1016/j.jviromet.2008.03.026 Epub 2008 May 22. PMID: 18501437. [DOI] [PubMed] [Google Scholar]
- 22.Cornelissen B, Zaidman A, Holten D. Execution trace analysis through massive sequence and circular bundle views. Journal Of Systems And Software. 2008;81(12):2252–68. doi: 10.1016/j.jss.2008.02.068. [DOI] [Google Scholar]
- 23.Park J, Lee Y, Shin K, Shin K, Kim MJ, Ha Y-J, Choi SR, Park JW, Kim MH, Kim JY, et al. Effect of the methotrexate discontinuation for 1 versus 2 weeks on vaccine response to seasonal influenza vaccine in rheumatoid arthritis: a noninferiority randomized controlled trial Arthritis Rheumatol. 2022;74(suppl 9). doi: 10.1002/art.42644. [DOI] [PubMed] [Google Scholar]
- 24.Pelka K, Matyja-Bednarczyk A, Wojas-Pelc A, Pastuszczak M. Hydroxychloroquine does not impair antibody response to 13-valent pneumococcal conjugate vaccine in patients with cutaneous lupus erythematosus—a pilot study. Dermatol Ther. 2021;34(4):e15013. doi: 10.1111/dth.15013. [DOI] [PubMed] [Google Scholar]
- 25.Kollaritsch H, Que JU, Kunz C, Wiedermann G, Herzog C, SJ C Jr.. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J Infect Dis. 1997. Apr;175(4):871–5. doi: 10.1086/513984. [DOI] [PubMed] [Google Scholar]
- 26.Pappaioanou M, Fishbein DB, Dreesen DW, Schwartz IK, Campbell GH, Sumner JW, Patchen LC, Brown WJ. Antibody response to preexposure human diploid-cell rabies vaccine given concurrently with chloroquine. N Engl J Med. 1986 ;314(5):280–4. doi: 10.1056/NEJM198601303140504. [DOI] [PubMed] [Google Scholar]
- 27.Tsai TF, Bolin RA, Lazuick JS, Miller KD. Chloroquine does not adversely affect the antibody response to yellow fever vaccine. J Infect Dis. 1986. Oct;154(4):726–7. doi: 10.1093/infdis/154.4.726. [DOI] [PubMed] [Google Scholar]
- 28.Barry M, Patterson JE, Tirrell S, Cullen MR, Shope RE. The effect of chloroquine prophylaxis on yellow fever vaccine antibody response: comparison of plaque reduction neutralization test and enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991. Jan;44(1):79–82. doi: 10.4269/ajtmh.1991.44.79. [DOI] [PubMed] [Google Scholar]
- 29.Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. [accessed 2011 Aug 11];29(35):6008–16. doi: 10.1016/j.vaccine.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. [accessed 2020 Aug 7];12(8):e12476. doi: 10.15252/emmm.202012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandriss MW, Schlesinger JJ. Antibody-mediated infection of P388D1 cells with 17D yellow fever virus: effects of chloroquine and cytochalasin B. J Gen Virol. 1984. Apr;65(Pt 4):791–4. doi: 10.1099/0022-1317-65-4-791. [DOI] [PubMed] [Google Scholar]
- 32.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–66. doi: 10.1038/s41584-020-0372-x [DOI] [PubMed] [Google Scholar]
- 33.Han J, Zhou Q, Li X, He J, Han Y, Jie H, He Y, Sun E. Novel function of hydroxychloroquine: down regulation of T follicular helper cells in collagen-induced arthritis. Biomed Pharmacother. 2018. Jan;97:838–843. doi: 10.1016/j.biopha.2017.10.132. [DOI] [PubMed] [Google Scholar]
- 34.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular helper T cells. Annu Rev Immunol. [accessed 2016 May 20];34(1):335–68. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 35.Dupré A, Pascaud J, Rivière E, Paoletti A, Ly B, Mingueneau M, Mariette X, Nocturne G. Association between T follicular helper cells and T peripheral helper cells with B-cell biomarkers and disease activity in primary sjögren syndrome. RMD Open. 2021. Mar;7(1):e001442. doi: 10.1136/rmdopen-2020-001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.


