Highlights
-
•
There is a lack of research on the association between occupational carcinogens and Hepatopancreatobiliary cancers in Africa.
-
•
The predominant occupational carcinogen is aflatoxin which is associated with hepatocellular carcinoma (HCC).
-
•
Agricultural workers, especially those involved in the production, processing, and storage of maize and peanuts, appear to be the most exposed to aflatoxin.
Keywords: Hepatopancreatobiliary cancers, HCC, Occupational carcinogens, Aflatoxins, Africa
Abstract
Introduction
Hepatopancreatobiliary (HPB) cancers encompassing malignancies of the liver, pancreas, gall bladder, and bile ducts pose a significant health burden in Africa. While the association of certain occupational carcinogens in cancer is well established globally, their potential role in HPB cancers remains understudied, especially in an African context.
Aim
This systematic review delves into the association between occupational carcinogens and HPB cancer in Africa. It examines the current state of research on occupational carcinogens and HPB cancers in Africa, identifying key challenges and knowledge gaps.
Methods
This systematic review examined publications (published between 01 January 2012 and 31 May 2023) that highlight occupational carcinogens and HBP cancers in Africa. The search was conducted on electronic databases namely PubMed, Web of Science, and Africa Wide Information.
Result
Due to the lack of information on the association between occupational carcinogens and HPB cancers in Africa, as a result of the paucity of published studies, only four articles were included in this study. Hepatocellular carcinoma (HCC) was the predominant cancer associated with the occupational carcinogen, aflatoxin. Agricultural workers, especially those involved in the production and processing of maize and peanuts, appear to be the most exposed to aflatoxin.
Conclusion
Despite the sample size limitations due to the paucity of research studies on occupational carcinogens and HPB cancers in Africa, this study provides a reasonable tool for subsequent epidemiological studies. There is a need for more research on the association of occupational carcinogens and HPB cancers in Africa, especially with the growing industrialization.
Graphical abstract
List of Abbreviations
- AHRQ
The Agency for Healthcare Research and Quality
- CCA
Cholangiocarcinoma
- GBC
Gall bladder cancer
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HCC
Hepatocellular carcinoma
- HPB
Hepatobiliary cancers
- PDAC
Pancreatic Ductal Adenocarcinoma
- ROBIS
Risk of Bias In Systematic Reviews
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- PECO
Population, Exposure, Comparator, and Outcome
- PRISMA-P
Preferred reporting items for systematic reviews and meta-analyses protocols
- PDAC
Pancreatic Ductal Adenocarcinoma
- Pv
Significant value
Introduction
Tumours originating in the liver, pancreas, bile ducts, or gallbladder are referred to as hepatopancreaticobiliary (HPB) cancers. The HPB system comprises the liver, bile ducts, and gallbladder which is essential for the digestion and processing of food mostly fats, medicine, and toxins. The liver produces bile which aids in lipid degradation, and the common hepatic duct transports the bile to the gallbladder where it is stored. Liver cancer is the most common type of HPB cancer [25]. It is a fatal cancer predicted to affect over a million people annually by 2025 [28]. Hepatocellular carcinoma (HCC) is the most common type of liver cancer in sub-Saharan Africa and is prevalent in young adults [44]. It has a higher incidence rate in males than females [11] and accounts for over 90 % of cases [14]. HCC is the fifth most prevalent cancer worldwide and a leading cause of cancer-related death in Africa [13,44,45]. Some of the risk factors for this disease include hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, hepatic cirrhosis, excessive alcohol consumption, smoking, metabolic syndrome associated with obesity and diabetes mellitus, and occupational and environmental toxins [7,22]. HCC incidence is higher in Africa compared to the rest of the world due to the high prevalence of HBV and HCV infections, and diagnosis at advanced stages [45]. HCV is the leading cause of HCC in Egypt [1] while HBV is the main cause of the disease in Sub-Saharan Africa [9].
Gallbladder cancer (GBC) is uncommon and is difficult to diagnose because there are non-specific symptoms which can be mistaken as benign gallbladder disease [38]. Although this disease is quite infrequent in developed countries, it is becoming more prevalent in some developing countries in Africa such as South Africa [5,21]. Some of the predisposing factors include age, smoking, obesity, cholelithiasis (gallstone disease), and sex. It has been observed that females are more disposed than males to develop GBC due to their higher propensity to suffer from gallstone disease [36]. The current standardised treatment for GBC is cholecystectomy [21].
Pancreatic cancer is the 3rd leading cause of cancer-related deaths in both men and women combined with a 5-year survival of about 11 % [43]. PDAC is the most common type of pancreatic neoplasm, which accounts for more than 85 % of pancreatic cancer cases worldwide [39]. PDAC has non-specific symptoms at its early stages causing it to remain undetected till late stages, usually locally advanced or metastatic with a significantly worse prognosis [10,41]. Signs and symptoms include advanced age [29], family history, and inherited genetic disorders [12,16], smoking [6], dietary factors [48], chronic pancreatitis [20], obesity and diabetes [3,20]. PDAC has been associated with symptoms such as weight loss, abdominal pain, jaundice, new on-set T2DM, bloating, nausea, vomiting, steatorrhea, changes in bowel movements, pruritus, lethargy, back and shoulder pain [37].
Cholangiocarcinoma (CCA) is a cancer of the bile duct and could occur either in the main bile duct within the liver (intrahepatic) or outside the liver (extrahepatic). Risk factors of CCA include chemical exposure, biliary inflammation, chronic hepatic inflammation, and hepatic cirrhosis. For instance, company workers who are exposed to high concentrations of industrial chemicals such as 1,2-dichloropropane and dichloromethane, which are carcinogenic compounds, have been observed to develop CCA more frequently [31].
Exposure to occupational-derived toxins is one of the major causes of cancer worldwide with approximately 660,000 work-related cancer deaths annually [47]. Occupational safety standards and regulations vary in different African countries due to insufficient implementation of safety measures, inadequate training, and a lack of awareness among workers about the risks associated with occupational carcinogens [33]. Many African countries have significant informal sectors and small-scale industries where safety regulations are often lacking. Workers in these sectors may face increased exposure to occupational carcinogens without proper protection or awareness. Epidemiological research has shown that aflatoxin B1, a toxin food contaminant is a major risk factor for HPB cancers, especially HCC [16,17]. Although the impact of aflatoxins in carcinogenesis is underreported in Africa, globally, 4.5 billion people are exposed to aflatoxins which may result in HCC [18]. Aflatoxin-induced HCC is prevalent in sub-Saharan Africa due to limited resources to implement aflatoxin control strategies, especially in the agricultural sectors [27].
HPB cancers are fast becoming highly epidemic in Africa, and the burden of these diseases is underreported due to a lack of a comprehensive database and a shortage of resources. This systematic review aimed to provide a comprehensive overview of the relationship between occupational carcinogen exposure and HPB cancers in an African context by identifying key occupational carcinogens. Furthermore, we describe the implications of these carcinogens and highlight the need for more research to be conducted to enable the appropriate public health interventions.
Methods
Study selection
A systematic review was conducted from August 2022 to May 2023 (Prospero ID: CRD42019132493). The preferred reporting items for systematic reviews and meta-analyses protocols (PRISMA-P) checklist was used to ensure best practices for transparency in reporting our systematic review methodology [42]. Articles dating back to the last 10 years were included (2012–2023). The articles found were used to broaden the search by looking at the references and most emerging abstracts and studies were selected using the Rayyan tool [19]. The reference list of all the studies considered was also screened for more potentially relevant papers.
Eligibility criteria
The inclusion of articles into the study was based on the date of article publication (published between 01 January 2012 to 31 May 2023 – a 10-year range), English articles that focused on occupational carcinogens, and those that focused on HBP cancers, focused on the human population within Africa. Articles were excluded if they were published before 2012, focused on carcinogens other than occupational, were not conducted on human populations within Africa, and were review articles. The PECO (Population, Exposure, Comparator, and Outcome) outline was adapted and used to formulate our eligibility criteria as described below:
Population
Our population of interest consisted of Africans who had been exposed to carcinogens through occupational means.
Exposure
Any occupational carcinogen in the following list was considered a relevant exposure: carcinogen, occupational carcinogen, aflatoxins, alcohol consumption, 1,2-DCP OR 1,2-Dichloropropane, hepatitis B virus OR HBV, hepatitis C virus OR HCV, plutonium, thorium-232, thorium-232 and decay products, tobacco smoking, androgenic steroids, anabolic steroids, vinyl chloride, inorganic arsenic compounds, arsenic, dichlorodiphenyltrichloroethane OR DDT, betel quid, betel quid without tobacco, dichloromethane OR methylene chloride OR methylene dichloride, x-ray radiation, trichloroethylene, Schistosoma japonicum, gamma radiation, ionising radiation, and 2,3,7,8-tetrachlorodibenzo-p-dioxin OR 2,3,7,8-tetrachlorodibenzo-para-dioxin OR TCDD.
Comparator
Our study did not require a comparator however, in the case where an article did include a comparator, it was individuals unexposed to occupational carcinogens.
Outcome
The outcome of interest was patients diagnosed with HPB cancer, that is cancer of the liver, gallbladder, bile duct, and/or pancreas.
Information sources
The search was conducted on three electronic databases namely PubMed, Web of Science, and Africa Wide Information. Reference lists for articles selected to be in the systematic review were searched to identify potential articles that may have been missed from the database search.
Search strategy
A combination of three groups of search terms was used and they were split by carcinogen type, cancer type, and location. The terms used for carcinogen type were: carcinogen, occupational carcinogen, aflatoxins, alcohol consumption, 1,2-DCP OR 1,2-Dichloropropane, plutonium, thorium-232, thorium-232 and decay products, androgenic steroids, anabolic steroids, vinyl chloride, inorganic arsenic compounds, arsenic, dichlorodiphenyltrichloroethane OR DDT, betel quid, betel quid without tobacco, dichloromethane OR methylene chloride OR methylene dichloride, x-ray radiation, trichloroethylene, Schistosoma Japonicum, gamma radiation, ionising radiation, and 2,3,7,8-Tetrachlorodibenzo-p-dioxin OR 2,3,7,8-Tetrachlorodibenzo-para-dioxin OR TCDD. The terms used for cancer type were: “liver cancer” OR “hepatocellular carcinoma” OR “intrahepatic cellular carcinoma” OR “extrahepatic cellular carcinoma” OR HCC OR IHCC OR EHCC, “gallbladder cancer” OR “gallbladder carcinoma” OR “gallbladder adenocarcinoma” OR “gallbladder squamous cell carcinoma” OR “gallbladder neuroendocrine tumour” OR GBC, cholangiocarcinoma OR CCA, and “pancreatic cancer” OR “pancreas cancer” OR “pancreatic neuroendocrine tumour” OR “pancreatic duct adenocarcinoma” OR “pancreatic ductal adenocarcinoma” OR PDAC. The terms used for location were: Africa OR “Africa South of the Sahara” OR “sub-Saharan Africa” OR “Africa” OR “African” OR “Angola” OR “Benin” OR “Botswana” OR “Burkina Faso” OR “Burundi” OR “Cameroon” OR “Cape Verde” OR “Central African Republic” OR “Chad” OR “Comoros” OR “Congo” OR “Congo-Brazzaville” OR “Republic of Congo” OR “Democratic Republic of the Congo” OR “Djibouti” OR “Eritrea” OR “Ethiopia” OR “Equatorial Guinea” OR “Gabon” OR “Gambia” OR “Ghana” OR “Guinea” OR “Guinea Bissau” OR “Ivory Coast” OR “Cote d'lvoire” OR “Kenya” OR “Lesotho” OR “Liberia” OR “Malawi” OR “Mali” OR “Mozambique” OR “Madagascar” OR “Mauritania” OR “Namibia” OR “Niger” OR “Nigeria” OR “Rwanda” OR “Senegal” OR “Sierra Leone” OR “Seychelles” OR “Somalia” OR “South Africa” OR “Sudan” OR “South Sudan” OR “Swaziland” OR “Tanzania” OR “Togo” OR “Uganda” OR “Zambia” OR “Zimbabwe.”
Selection process
All the selected articles were imported to a reference management software designed for systematic reviews, known as Rayyan. Duplicate publications were removed, and two reviewers independently screened the titles and abstracts of all the identified articles. Full texts of all the relevant articles were retrieved and reviewed in detail by the two reviewers independently. Disagreements were resolved by the senior researchers and by discussion.
Data collection process
Two reviewers independently performed data extraction of the included studies using a standardised data extraction tool developed in Excel by the team, after which both reviewers checked each other's version for comprehensiveness and accuracy. Discrepancies were resolved by the senior researchers. Data on the following items were extracted; SN/DOI, authors, year of publication, the year(s) the study was conducted, article title, journal name, type of study, country of study, aim of study, inclusion and exclusion criteria, sample size, age and gender of study participants, type of carcinogen, cancer type, and outcome (morbidity). Two other independent reviewers then sampled a subset of the articles and cross-checked the data extracted to verify that the correct information was extracted. STATA V.17 was used to conduct all the analyses.
Data management
There was only one outcome for which data was sought and that was the presence of HPB cancers. No assumptions were made regarding missing or unclear information.
Risk of bias assessment
The risk of bias arising from missing results in the synthesis was assessed using the Risk of Bias In Systematic Reviews (ROBIS) tool. All three stages of the tool were used. In the first stage, the relevance was assessed. Thereafter, the concerns of the review process (particularly with the study eligibility criteria, identification and selection of studies, data collection study appraisal, synthesis, and findings), judging the risk of bias stemming from the way review findings were interpreted and whether limitations were identified. Two independent reviewers assessed the risk of bias and disagreements were resolved through discussion and a third reviewer made the final decision for the assessment.
Effect measures and synthesis methods
The effect measure for the outcome (diagnosis with HPB cancers) included the percentage or proportion (frequency), mean, mean difference, odds ratio, risk ratio, associated risk, Wald test, or a simple statement of ‘increased risk’ or ‘decreased risk’.
To determine whether studies were eligible for synthesis, the outcome measure for each study was identified and if it corresponded with our required outcome then the study was reserved for use. No data conversions were conducted and some summary statistics were missing. Findings from the systematic review were presented in a table and forest plots for visual display of the syntheses and heterogeneity.
Reporting bias assessment
The Agency for Healthcare Research and Quality (AHRQ) tool was used to assess reporting bias in our systematic review.
Certainty assessment
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) tool was used to assess the certainty in the body of evidence for our outcome.
Results
Article selection
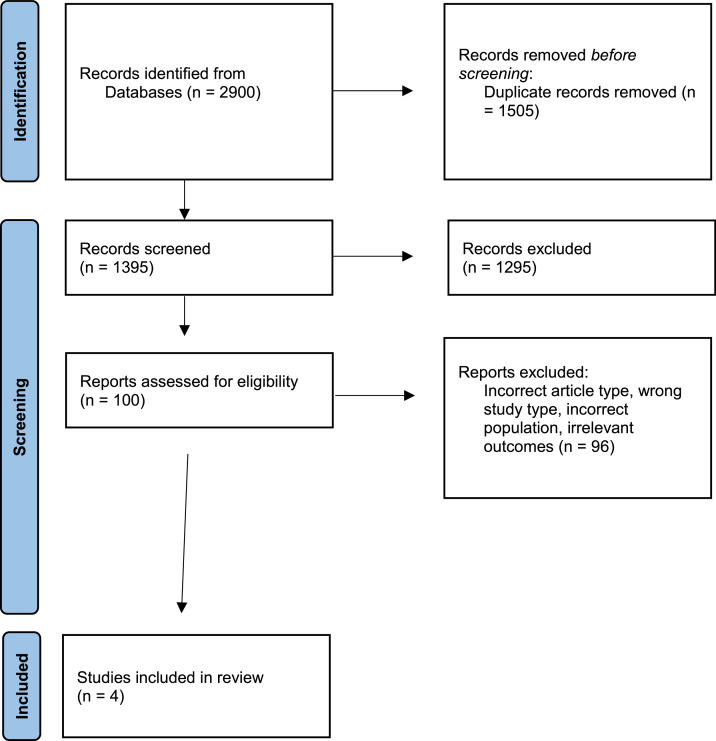
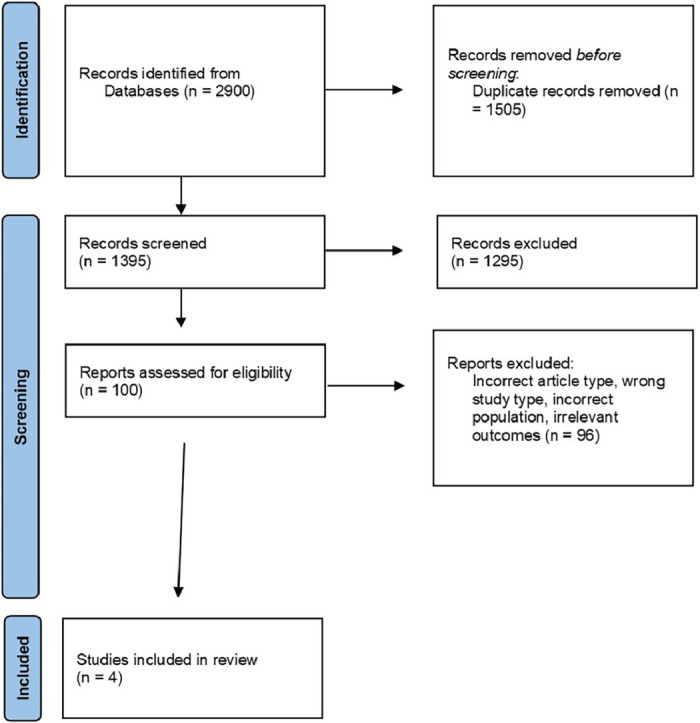
The study search and selection strategy is shown in the flow chart in Fig. 1. The preliminary literature search identified 2900 articles matching the initial search criteria. After screening, four articles were included in the descriptive systematic review.
Fig. 1.
The PRISMA flow diagram of the study selection process.
Study characteristics
Only four studies matched the inclusion criteria. The studies were from Egypt, Somalia, Malawi and Cameroon (Table 1). Due to the paucity of information on HPB cancers in Africa, the comparison between HCC cancers and others could not be achieved. The main characteristics of the included studies are shown in Table 2. Aflatoxins were the most common occupational carcinogen reported in this study and have been linked to liver cancer.
Table 1.
Summary of the included studies.
| SN | Title | Authors | Year of publication | study setting-country | Study design |
|---|---|---|---|---|---|
| 1.DOI:10.1007/s12029–013–9573- 8 | New Insights on Non-B non-C Hepatocellular Carcinoma in Mid Delta Region, Egypt | Abdel Raouf Abou El Azm & Mohamed Yousef et al., | 2014 | Egypt | Consecutive series |
| 2.DOI:10.1021/acs.jafc.8b05141 | Occurrence and Human-Health Impacts of Mycotoxins in Somalia | Ewa Wielogorska et al., | 2019 | Somalia | Descriptive study |
| 3.DOI: 10.3920/WMJ2018.2346 | Probabilistic dietary based estimation of the burden of aflatoxin-induced hepatocellular carcinoma among adult Malawians |
Matumba et al., | 2019 | Malawi | Case study |
| 4. DOI: 10.4314/ahs.v13i3.28 | Environmental exposure to carcinogens in northwestern Cameroon | Nsagha et al., | 2013 | Cameroon | Descriptive observational study |
Table 2.
Assessment of the impact of occupational carcinogens.
| Participants and sample size | Age (years) Mean/range? |
Gender | Occupation | Cancer type | Carcinogen | Results | |
|---|---|---|---|---|---|---|---|
| 1 | 281 HCC patients and 20 healthy volunteers | HCC =185(M) 57(F) non-B and non-C 37(M) 2(F) | Farmers, workers in pesticide and fertilizer industries | HCC | Tobacco: Patients smoked about two packets (≥40 cigarettes) a day for 10 years or one packet daily for 20 + years. Organophosphorus pesticide exposure (industry/farming/residence), fertilizer exposure |
Exposure: Tobacco smoking = pV 0.048 Exposure: Organophosphorous Pesticides = 13.829(0.001) Exposure to pesticides (pV=0.001) are risk of HCC occurence Exposure: Fertilizers = 0.000 (0.994) |
|
| 2 | N/A | Farmers | Liver cancer | Aflatoxin | Exposure = AFB1 (associated risk = 75 cancers per 100 000 people for white maize), (associated risk = 31 cancers per 100 000 people for yellow maize) | ||
| 3 | 274 households | N/A | Farmers | HCC | Aflatoxin (through maize, groundnuts, both maize and groundnuts) | Exposure = Maize (whole population) Mean = 1.86, SD = 6.66, P5=0.03, P50 =0.47, P90=3.91, P95=7.24, Exposure = Groundnuts only (whole population) Mean = 1.26, SD = 2.72, P5=0.04, P50 =0.5, P90=2.94, P95=4.77 Exposure = Maize and groundnuts (whole population) Mean = 3.1, SD = 6.85, P5=0.22, P50 =1.45, P90=6.62, P95=10.57 |
|
| 4 | 42 people | 19–61 | 34 (81.0 %) men and 8(19.0 %) women | Farmers, factory workers, health workers, teachers, students | Liver cancer | Coal tar, Wood dust, fibres, chemicals, hair saloon products, fumes, radiation, extreme ultraviolet rays, smoke, and various metals. | Coal tar 35(83.3) was the most exposed carcinogen, followed by fibres 34(81.0) chemicals25(59.5) and fumes 25(59.5). |
*HCC: hepatocellular carcinoma, AFB1: Aflatoxin B1, non-B; non-hepatitis B virus, non-c: non-hepatitis C virus.
NA: not available.
Study quality assessment
The quality criteria were adopted from the Joanna Briggs Institute (JBI) critical appraisal tool, which was amended based on the study design of the included studies [15]. Quality questions relevant to this study design were adopted and amended with special attention given to the objectives of the study and outcomes. These included four questions are; (1) Is the question clearly and explicitly stated? (2) Were the inclusion criteria appropriate for the review question? (3) Were the outcome(s) appropriately linked to the variables? and (4) Were the specific outcomes determined appropriately? This refers to whether the studies ensured accuracy and consistency. Each question was rated for 0= No or Not applicable, 1= unclear and 2= Yes. Studies that scored between 6 and 8 were regarded as high quality and included in the study.
Discussion
To the best of our knowledge, this is the first descriptive systematic review of the effect of occupational carcinogens on HPB cancers in Africa. Although there are many studies on other races and populations [17,27], little is known about the African population. Up-to-date data on the association of occupational carcinogens in HPB cancers are scarce due to poor data records and medical systems in Africa. This systematic review provides a comprehensive overview of the relationship between occupational carcinogen exposure and HPB cancers in an African context by identifying aflatoxin as a crucial occupational carcinogen.
Agricultural workers, especially those involved in the production, processing, and storage of maize and peanuts, are at a heightened risk of exposure. These workers come into direct contact with aflatoxin-contaminated crops without proper protective measures leading to increased exposure and HCC risk [2] especially in countries such as Somalia where their staple foods include wheat, sorghum, and maize [49]. Industrial workers in Europe, Asia, and North America are the most exposed to these carcinogens and have an elevated risk of developing HPB cancer, such as pancreatic cancer [4]. Furthermore, constant exposure to pesticides and fertilizers made from phosphates has increased exposure to carcinogens, hence increasing HCC risk. These chemicals could induce HCC by direct hepatoxicity-inducing oxidative stress [8].
The male gender is the most affected in this study. According to the latest global cancer statistics in Africa, the male sex is predominantly affected by liver cancer [46]. This could be due to the protective role of oestrogen in liver disease progression in females [32] or via the inhibition of the proinflammatory effect of myeloid differentiation 88 (MyD88) mediated-secretion of interleukin 6 (IL-6) [34]. Also, the occupations in question are usually male-dominated. Although the age range was between 19 and 61 years, this study did not specifically show age gaps that are most affected.
Liver cancer, specifically HCC, is the predominant cancer affected by occupational carcinogens in Africa and this could be due to the prevalence of rural areas and occupations [11]. Mozambique has been shown to have the highest incidence of HCC in the world [46]. Regions such as Taiwan have shown a decline in HCC due to reduced aflatoxin exposure [26].
Aflatoxin is the predominant carcinogen which is a major co-factor of HCC in Africa. Africa faces a significant burden of aflatoxin-related health issues due to factors such as climate, poor agricultural practices, and inadequate storage facilities. High temperatures and humidity coupled with poor harvest handling and storage conditions create an environment conducive for aflatoxin production in foodstuffs. Aflatoxin metabolites have been shown to promote tumour suppressor p53 gene mutation and epidemiological results have demonstrated that it is one of the main contributors to HCC onset [17,30]. Recent studies have shown that the population aflatoxin-related HCC risk is approximately 17 % [24]. Reducing the aflatoxin-related risk could reduce the HCC incidence by 23 % [26]. In the Western region of the world, chlorinated hydrocarbons, pesticides, and polycyclic aromatic hydrocarbons, among others, are the predominant occupational carcinogens associated with HPB cancer [4].
Other occupational carcinogens such as organophosphorus pesticides, tobacco, fumes, and others such as radiation, coal tar, in decreasing potency order, have been associated with HPB cancers [23,35]. Pesticides may enter the body during work hours via inhalation and are metabolised by the liver causing metabolic and biochemical liver damage and ultimately increasing the risk of HCC [40].
Prevention and mitigation
The impact of occupational carcinogens on HPB cancers in Africa is a complex issue influenced by various factors including socioeconomic conditions, healthcare access, and lifestyle factors [17]. Implementing targeted interventions in crucial areas such as public education on the risks of aflatoxin exposure and campaigns promoting healthy lifestyles may mitigate occupational carcinogen exposure. Implementing screening programs and raising awareness about early symptoms of HPB cancer can facilitate early detection and improve treatment outcomes. Improving healthcare infrastructure, training healthcare professionals, and enhancing access to diagnostic and treatment facilities are essential for the effective management of HPB cancers in Africa. Efforts to mitigate the risk include implementing and enforcing occupational safety regulations, enhancing occupational safety regulations, promoting awareness among workers and employers, providing protective equipment, and promoting regular check-ups for early detection and intervention. Encouraging the adoption of good agricultural practices such as proper drying, storage, and pest management techniques can help minimise aflatoxin contamination in crops. Establishing efficient and reliable aflatoxin monitoring systems can help identify contaminated food products before they reach consumers enabling timely intervention. Supporting research efforts to develop improved crop varieties with enhanced resistance to aflatoxin-producing fungi and exploring innovative detection and mitigation technologies. Additionally, the quality of epidemiological data available must be improved and up-to-date cancer registries should be established and coordinated throughout Africa.
Recommendations
There is a huge knowledge gap surrounding research in occupational carcinogens and HPB cancers in Africa. Several factors contribute to the shortage of research including; limited funding, lack of awareness, inadequate data collection, reluctance to change practices due to concerns about cost and economic viability, and finally limited expertise in occupational health and cancer further impeding progress in this field.
To address the lack of research on occupational carcinogens and their link to HPB cancer in Africa, concerted efforts are required such as increased funding and capacity building to promote the training of researchers, healthcare professionals, and occupational health experts to understand better and address the issues. Raising awareness among workers, employers, and policymakers about the risks posed by occupational carcinogens and the importance of preventive measures [17]. Establishing and strengthening cancer registries to facilitate data collection and analysis will enable researchers to gain better insight. Also encouraging collaboration between international research institutions and African counterparts to share knowledge and expertise in studying occupational carcinogens.
Conclusion
This systematic review demonstrates the lapses in research studies relating to occupational carcinogens in Africa. Despite the limited articles, this study provides a reasonable tool for subsequent epidemiological studies and follow-up. Awareness campaigns on health hazards of occupational exposures to carcinogens could be vital in improving research participation and data collection. Implementing aflatoxin biocontrol management strategies within African countries is beneficial towards improving overall health.
CRediT authorship contribution statement
Nnenna Elebo: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Mafuno Grace Mpinganjira: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Pavan Baichan: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. John Devar: Writing – review & editing, Validation, Investigation, Data curation. Jones Omoshoro-Jones: Writing – review & editing, Writing – original draft, Validation, Investigation, Data curation. Joel Msafiri Francis: . Martin Smith: Writing – review & editing, Supervision, Methodology, Investigation. Ekene Emmanuel Nweke: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics statement
The review required no ethical clearance.
Acknowledgements
The authors would like to acknowledge Prof Gill Nelson for thoroughly going through the article and providing valuable comments
Funding
E.E.N is supported by the National Research Foundation grant (Grant number: 138367).
References
- 1.Abdelaziz A.O., Elbaz T.M., Shousha H.I., Ibrahim M.M., Rahman El-Shazli M.A., Abdelmaksoud A.H., Aziz O.A., Zaki H.A., Elattar I.A., Nabeel M.M. Survival and prognostic factors for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Asian Pac. J. Cancer Prev. 2014;15:3915–3920. doi: 10.7314/apjcp.2014.15.9.3915. [DOI] [PubMed] [Google Scholar]
- 2.Agbetiameh D., Ortega-Beltran A., Awuah R.T., Atehnkeng J., Cotty P.J., Bandyopadhyay R. Prevalence of aflatoxin contamination in maize and groundnut in ghana: population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018;102:764–772. doi: 10.1094/PDIS-05-17-0749-RE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen D.K., Korc M., Petersen G.M., Eibl G., Li D., Rickels M.R., Chari S.T., Abbruzzese J.L. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66:1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreotti G., Silverman D.T. Occupational risk factors and pancreatic cancer: a review of recent findings. Mol. Carcinog. 2012;51:98–108. doi: 10.1002/mc.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baichan P., Naicker P., Augustine T.N., Smith M., Candy G., Devar J., Nweke E.E. Proteomic analysis identifies dysregulated proteins and associated molecular pathways in a cohort of gallbladder cancer patients of African ancestry. Clin. Proteomics. 2023;20:8. doi: 10.1186/s12014-023-09399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosetti C., Lucenteforte E., Silverman D.T., Petersen G., Bracci P.M., Ji B.T., Negri E., Li D., Risch H.A., Olson S.H., Gallinger S., Miller A.B., Bueno-de-Mesquita H.B., Talamini R., Polesel J., Ghadirian P., Baghurst P.A., Zatonski W., Fontham E., Bamlet W.R., Holly E.A., Bertuccio P., Gao Y.T., Hassan M., Yu H., Kurtz R.C., Cotterchio M., Su J., Maisonneuve P., Duell E.J., Boffetta P., La Vecchia C. Cigarette smoking and pancreatic cancer: an analysis from the international pancreatic cancer case-control consortium (Panc4) Annals of Oncol. 2012;23:1880–1888. doi: 10.1093/annonc/mdr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budny A., Kozłowski P., Kamińska M., Jankiewicz M., Kolak A., Budny B., Budny W., Niemunis-Sawicka J., Szczypiór G., Kurniawka B., Burdan F. [Epidemiology and risk factors of hepatocellular carcinoma] Pol. Merkur. Lekarski. 2017;43:133–139. [PubMed] [Google Scholar]
- 8.Cotrim H.P., Andrade Z.A., Parana R., Portugal M., Lyra L.G., Freitas L.A.R. Nonalcoholic steatohepatitis: a toxic liver disease in industrial workers. Liver. 1999;19:299–304. doi: 10.1111/j.1478-3231.1999.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 9.de Martel C., Maucort-Boulch D., Plummer M., Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elebo N., Omoshoro-Jones J., Fru P.N., Devar J., De Wet van Zyl C., Vorster B.C., Smith M., Cacciatore S., Zerbini L.F., Candy G., Nweke E.E. Serum metabolomic and lipoprotein profiling of pancreatic ductal adenocarcinoma patients of African Ancestry. Metabolites. 2021;11 doi: 10.3390/metabo11100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Kassas M., Elbadry M. Hepatocellular carcinoma in Africa: challenges and opportunities. Front. Med. (Lausanne) 2022;9 doi: 10.3389/fmed.2022.899420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez E., La Vecchia C., D'Avanzo B., Negri E., Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol. Biomarkers Prev. 1994;3:209–212. [PubMed] [Google Scholar]
- 13.Galle P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.-L., Schirmacher P., Vilgrain V. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Ganne-Carrié N., Nahon P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019;70:284–293. doi: 10.1016/j.jhep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Gebremedhin M., Semahegn A., Usmael T., Tesfaye G. Unsafe abortion and associated factors among reproductive aged women in Sub-Saharan Africa: a protocol for a systematic review and meta-analysis. Syst. Rev. 2018;7:130. doi: 10.1186/s13643-018-0775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groopman J., Scholl P., Wang J. Epidemiology of human aflatoxin exposures and their relationship to liver cancer. Prog. Clin. Biol. Res. 1996;395:211–222. [PubMed] [Google Scholar]
- 17.Hamid A., Selim Tesfamariam, Goitom I., Zhang Y., Zhang Z. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention (review) Oncol. Lett. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid A., Selim Tesfamariam, Goitom I., Zhang Y., Zhang Z. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention (Review) Oncol. Lett. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison H., Griffin S.J., Kuhn I., Usher-Smith J.A. Software tools to support title and abstract screening for systematic reviews in healthcare: an evaluation. BMC. Med. Res. Methodol. 2020;20:7. doi: 10.1186/s12874-020-0897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iordache S., Săftoiu A., Cazacu S., Gheonea D.I., Dumitrescu D., Popescu C., Ciurea T. Endoscopic ultrasound approach of pancreatic cancer in chronic pancreatitis patients in a tertiary referral centre. J. Gastrointestin. Liver. Dis. 2008;17:279–284. [PubMed] [Google Scholar]
- 21.Khan Z.A., Khan M.U., Brand M. Gallbladder cancer in Africa: a higher than expected rate in a “low-risk” population. Surgery. 2022;171:855–858. doi: 10.1016/j.surg.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Kole C., Charalampakis N., Tsakatikas S., Vailas M., Moris D., Gkotsis E., Kykalos S., Karamouzis M.V., Schizas D. Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers. (Basel) 2020;12 doi: 10.3390/cancers12102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Davis D. Cancer in the workplace: the case for prevention. Environ. Sci. Policy Sustain. Dev. 1981;23:25–37. doi: 10.1080/00139157.1981.9928735. [DOI] [Google Scholar]
- 24.Lemoine M., Thursz M.R. Battlefield against hepatitis B infection and HCC in Africa. J. Hepatol. 2017;66:645–654. doi: 10.1016/j.jhep.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Liu, C.-Y., Chen, K.-F., Chen, P.-J., 2015. Treatment of liver cancer. cold spring harb perspect med 5, a021535. 10.1101/cshperspect.a021535. [DOI] [PMC free article] [PubMed]
- 26.Liu Y., Chang C.-C.H., Marsh G.M., Wu F. Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur. J. Cancer. 2012;48:2125–2136. doi: 10.1016/j.ejca.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan Liu, Felicia Wu. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 29.Malvezzi M., Carioli G., Bertuccio P., Rosso T., Boffetta P., Levi F., La Vecchia C., Negri E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Annal. Oncol. 2016;27:725–731. doi: 10.1093/annonc/mdw022. [DOI] [PubMed] [Google Scholar]
- 30.McLean M., Dutton M.F. Cellular interactions and metabolism of aflatoxin: an update. Pharmacol. Ther. 1995;65:163–192. doi: 10.1016/0163-7258(94)00054-7. [DOI] [PubMed] [Google Scholar]
- 31.Mimaki S., Totsuka Y., Suzuki Y., Nakai C., Goto M., Kojima M., Arakawa H., Takemura S., Tanaka S., Marubashi S., Kinoshita M., Matsuda T., Shibata T., Nakagama H., Ochiai A., Kubo S., Nakamori S., Esumi H., Tsuchihara K. Hypermutation and unique mutational signatures of occupational cholangiocarcinoma in printing workers exposed to haloalkanes. Carcinogenesis. 2016;37:817–826. doi: 10.1093/carcin/bgw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montella M., D'Arena G., Crispo A., Capunzo M., Nocerino F., Grimaldi M., Barbieri A., D'Ursi A.M., Tecce M.F., Amore A., Galdiero M., Ciliberto G., Giudice A. Role of sex hormones in the development and progression of hepatitis b virus-associated hepatocellular carcinoma. Int. J. Endocrinol. 2015. 2015 doi: 10.1155/2015/854530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mossburg S., Agore A., Nkimbeng M., Commodore-Mensah Y. Occupational hazards among healthcare workers in africa: a systematic review. Ann. Glob. Health. 2019;85:78. doi: 10.5334/aogh.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naugler W.E., Sakurai T., Kim S., Maeda S., Kim K., Elsharkawy A.M., Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science (1979) 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 35.Nsagha D., Sasco A., Assob J.C.N., Njunda A., Shey C., Kamga H.L.F. Environmental exposure to carcinogens in northwestern Cameroon. Afr. Health Sci. 2013;13:718–724. doi: 10.4314/ahs.v13i3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitt S.C., Jin L.X., Hall B.L., Strasberg S.M., Pitt H.A. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann. Surg. 2014;260 doi: 10.1097/SLA.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 37.Porta M., Fabregat X., Malats N., Guarner L., Carrato A., de Miguel A., Ruiz L., Jariod M., Costafreda S., Coll S., Alguacil J., Corominas J.M., Solà R., Salas A., Real F.X. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin. Translat. Oncol. 2005;7:189–197. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 38.Prasad N., Sen S. Gall bladder carcinoma: the facts and the mimics. Egypt. J. Radiol. Nucl. Med. 2021;52:1. doi: 10.1186/s43055-020-00386-w. [DOI] [Google Scholar]
- 39.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 40.Saad-Hussein A., Beshir S., Taha M.M., Shahy E.M., Shaheen W., Abdel-Shafy E.A., Thabet E. Early prediction of liver carcinogenicity due to occupational exposure to pesticides. Mutation Res. Gene. Toxicol. Environ. Mutagen. 2019;838:46–53. doi: 10.1016/j.mrgentox.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Sarantis P., Koustas E., Papadimitropoulou A., Papavassiliou A.G., Karamouzis M.V. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020;12:173–181. doi: 10.4251/wjgo.v12.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 43.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 44.Spearman C.W., Dusheiko G., Jonas E., Abdo A., Afihene M., Cunha L., Desalegn H., Kassianides C., Katsidzira L., Kramvis A., Lam P., Lesi O.A., Micah E.A., Musabeyezu E., Ndow G., Nnabuchi C.V., Ocama P., Okeke E., Rwegasha J., Shewaye A.B., Some F.F., Tzeuton C., Sonderup M.W. Hepatocellular carcinoma: measures to improve the outlook in sub-Saharan Africa. Lancet Gastroenterol. Hepatol. 2022;7:1036–1048. doi: 10.1016/S2468-1253(22)00041-3. [DOI] [PubMed] [Google Scholar]
- 45.Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N., Cowie B., Forouzanfour M.H., Groeger J., Hanafiah K.M., Jacobsen K.H., James S.L., MacLachlan J., Malekzadeh R., Martin N.K., Mokdad A.A., Mokdad A.H., Murray C.J.L., Plass D., Rana S., Rein D.B., Richardus J.H., Sanabria J., Saylan M., Shahraz, So S., Vlassov S., Weiderpass V.V., Wiersma E., Younis S.T., Yu M., El Sayed Zaki C., Cooke M. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 47.Takala J. Eliminating occupational cancer. Ind. Health. 2015;53:307–309. doi: 10.2486/indhealth.53-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y.-T., Gou Y.-W., Jin W.-W., Xiao M., Fang H.-Y. Association between alcohol intake and the risk of pancreatic cancer: a dose–response meta-analysis of cohort studies. BMC. Cancer. 2016;16:212. doi: 10.1186/s12885-016-2241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wielogorska E., Mooney M., Eskola M., Ezekiel C.N., Stranska M., Krska R., Elliott C. Occurrence and human-health impacts of mycotoxins in Somalia. J. Agric. Food Chem. 2019;67:2052–2060. doi: 10.1021/acs.jafc.8b05141. [DOI] [PubMed] [Google Scholar]