Abstract
Background
Aortic aneurysms, particularly those affecting the ascending aorta, pose significant health risks due to their potential to cause life-threatening complications such as rupture and dissection. While the etiology of ascending aortic aneurysms has traditionally been associated with non-inflammatory processes, emerging evidence suggests a potential role of inflammation in their development.
Methods
This study investigates the relationship between inflammatory markers and ascending aortic aneurysms, focusing on high-sensitivity C-reactive protein (hs-CRP) and the monocyte-to-HDL ratio (MHR). A total of 135 patients with ascending aortic aneurysms and 40 control subjects underwent comprehensive evaluations, including echocardiography, computed tomography imaging, and serum biomarker measurements.
Results
The results indicate significantly elevated levels of hs-CRP and MHR in patients with ascending aortic aneurysms compared to the control group, suggesting a potential inflammatory component in the pathogenesis of these aneurysms. However, the precise mechanisms underlying this association remain to be elucidated.
Conclusion
Despite limitations such as the cross-sectional study design and relatively small sample size, this study provides valuable insights into the potential involvement of inflammation in ascending aortic aneurysms. Further research, including longitudinal studies and histopathological analysis of aortic tissue, is warranted to confirm these findings and explore the utility of inflammatory markers as diagnostic and prognostic indicators in this patient population.
Keywords: ascending aortic aneurysm, inflammation, hs-CRP, monocyte-to-HDL ratio
Introduction
Thoracic aortic aneurysms (TAA) have an estimated incidence of at least 5–10 per 100,000 person-years.1 The cause, natural history, and treatment vary depending on the location of the TAA. Aortic root or ascending aortic aneurysms are most common types (approximately 60%), followed by aneurysms of the descending aorta (approximately 35%) and aortic arch (<10).2 There are many causes of aneurysm, like heritable disorders, genetic disorders, and degenerative, mechanical, and infectious diseases. Both Abdominal and ascending aortic aneurysms share the same risk factors, including male gender, smoking, age, history of myocardial infarction, hypertension, low high-density lipoprotein (HDL) cholesterol concentration, and a high concentration of total serum cholesterol.3,4 Besides these well-known risk factors, primary hyperaldosteronism is an uncommon risk factor for acute aortic dissection.5 The importance of ascending aortic aneurysms resides in their capacity to induce life-threatening complications and the requirement for efficient management strategies. Ascending aortic aneurysms have a high likelihood of causing serious health problems and death. If not properly treated, there is a risk of life-threatening complications such as rupture and dissection.6 In a recent study, 27% of patients with AAA also presented with an ascending AA, most of whom were women, and the elderly.7 These coexistences of abdominal aneurysm and ascending aneurysm bring to our mind the possibility of sharing the same etymology. The inflammation in the etymology of AAA has been demonstrated in many studies, but it is still not well known if the inflammation has a role in the development of aneurysms in the ascending aorta. The coexistence of inflammatory cells with markers of apoptotic vascular cell death in the media of ascending aortas with aneurysms and type A dissection raises the possibility that activated T cells and Macrophages may contribute to the elimination of smooth muscle cells and the degradation of the matrix associated with thoracic aortic aneurysms and dissections.8 Several studies showed the role of inflammation and the relationship between CRP and AAA.9,10 Also, it has been shown that CRP is specifically deposited on the interface between the atheroma and the damaged aortic wall in AAA, and in the same study, there was a high level of CRP in patients with ascending Aortic aneurysm.11 Since aortic aneurysms are mainly asymptomatic during their clinical course until their complications—which may be lethal—serum biomarkers for their early diagnosis are a necessity.
C-reactive protein (CRP) and high-sensitivity CRP C-reactive protein (hs-CRP) serves as indicators of widespread inflammation and have been linked to a range of health conditions, such as cardiovascular diseases. Research has demonstrated a correlation between increased levels of hs-CRP and vascular remodeling as well as the rupture of coronary atherosclerotic plaques.12 This suggests that hs-CRP plays a role in the advancement of atherosclerotic disease. Studies have shown that elevated levels of plasma D-dimer and hypersensitive C-reactive protein (hs-CRP) are indicative of aortic dissection and aneurysm.13 The aim of this study was to measure hs-CRP levels in people with isolated ascending aortic aneurysm and compare them with control subjects to assess whether this marker can be of value in this group of patients.
Methods
Study Population
We enrolled a total of 135 consecutive patients diagnosed with ascending aortic aneurysm (Asc AA) with a mean age of 57.1 ± 7.3 years, of which 76 (80%) were men. Additionally, 40 control subjects with a mean age of 54.2 ± 6.8 years, including 8 (20%) men, were recruited from the outpatient cardiology department at Ordu State Hospital. All patients underwent an echocardiography examination conducted by two independent cardiologists. The ascending aorta was assessed in transthoracic parasternal long-axis view. Patients with a diameter above 40 mm were included in the patient group (95 patients), while those with a diameter below this threshold were included in the control group (40 patients). Full blood count and biochemical tests were performed on the day of the outpatient visit, and demographic patient characteristics were obtained through interviews, including gender, age, smoking history, hypertension, cardiovascular medication (including statins), family history, presence of diabetes mellitus (use of insulin or oral antidiabetic drugs), and coronary artery disease status.
CT Imaging Techniques
All patients underwent computed tomography (CT) imaging. The diameter of the ascending aorta was independently determined from the echocardiography results by a radiologist. For each examination, the radiologist generated double-oblique short-axis views through the proximal aorta using an independent imaging workstation (Vital Images workstations, Vitrea 6.0.1 3D analysis software, Minnetonka, MN). Measurements were manually obtained from these images to determine the largest diameter at the annulus, sinuses (cusp to cusp), Sino tubular junction, and mid-ascending aorta.
Determination of Serum CRP
Venous blood samples were drawn on the day of the echocardiography examination and immediately centrifuged for 10 minutes at 1200 rpm and at 4°C. Serum samples were stored at −20°C until analysis. The highly sensitive (hs-CRP) IMMULITE CRP method (Diagnostic Product Corporation) with a detection limit of 0.10 mg/L was used to measure the levels of C-reactive protein (CRP). Patients were excluded if there was evidence of acute illness or infection, active inflammatory conditions, malignancy, or corticosteroid use.
Exclusion Criteria
Patients with any valve diseases, such as bicuspid valve, aortic valve regurgitation, and aortic valve stenosis, were excluded from the study. Only patients with isolated ascending aorta aneurysms were included.
Results
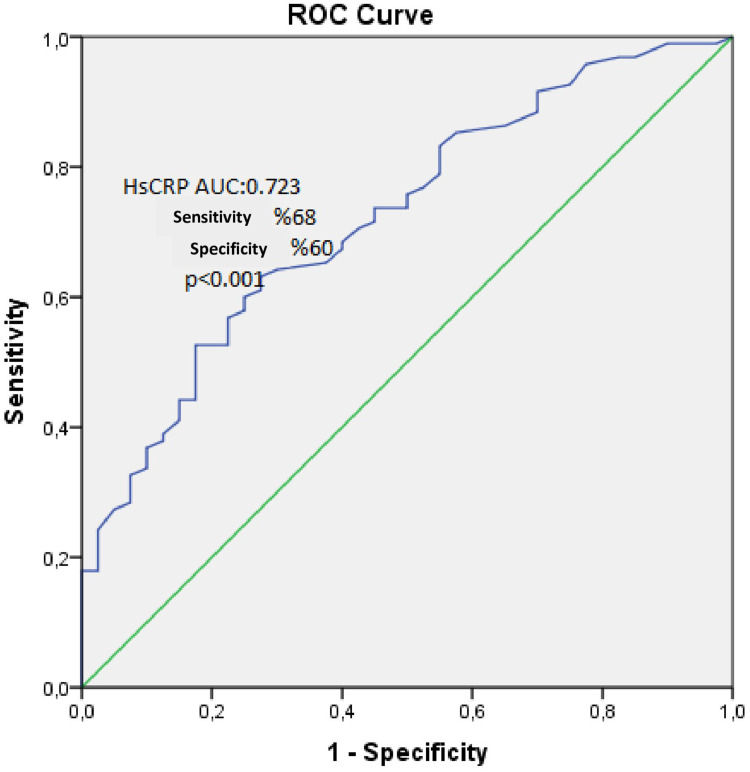
The baseline characteristics of the AA+ and AA- patient groups were summarized in Table 1. A total of 135 patients were included in the analysis, comprising 95 (70.3%) AA+ patients with a mean age of 57.0±7.3 years, of whom 76 (80%) were men, and 40 (29.7%) control subjects with a mean age of 54.2±6.8 years, of whom 8 (20%) were men. No statistically significant differences were observed between the groups in terms of antihypertensive drug usage, β-blocker usage, hyperlipidemia, coronary artery disease, hypertension, or diabetes mellitus. However, several parameters differed significantly between the AA+ and control groups. Some of the things that were more common in AA+ patients than in the control group were gender (p=0.006), age (p=0.04), creatinine levels (0.86±0.13 vs 0.81±0.12; p=0.043), hs-CRP levels (4.0±2.4 vs 2.2±1.8; p<0.001), WBC count (7.0±1.6 vs 6.3±1.2; p=0.014), and MHR (13.1±3.96 vs 9.69±3.88; p<0.001). AA+ patients had lower HDL levels (42.2±7.2 vs 50.4±11.9; p<0.001), RDW (12.22±1.68 vs 12.74±0.94; p=0.012), MPV (7.89±1.55 vs 9.99±1.54; p<0.001), and platelet count (246±62 vs 274±66; p=0.019) than controls. Age, gender, creatinine, hs-CRP, LDL, HDL, WBC count, RDW, MPV, and MHR were some of the variables that were significantly linked to AA in a univariate analysis (Table 2). Hs-CRP (OR 1.527, 95% CI 1.117 to 2.086, p=0.008), MPV (OR 0.381, 95% CI 0.250 to 0.582, p<0.001), and platelet count (OR 0.980, 95% CI 0.968 to 0.993, p=0.002) were still able to predict AA on their own (Table 3). Furthermore, in ROC analysis, a cutoff value of 2.15 for hs-CRP demonstrated a sensitivity of 68% and a specificity of 60% for predicting AA patients (AUC: 0.723, p<0.001) (Figure 1). These findings underscore the importance of hs-CRP, MPV, and platelet count as potential biomarkers for identifying AA patients. Correlation analyses were performed. The findings showed that hs-CRP levels were significantly positively related to the presence of AA (r = 0.45, p < 0.001). This means that higher hs-CRP levels are linked to a higher chance of having AA. Similarly, MHR was also positively correlated with AA (r = 0.39, p < 0.001), suggesting that elevated MHR is associated with AA. These correlations suggest a potential causal relationship in which inflammatory processes, as indicated by elevated hs-CRP, may contribute to the development or progression of AA. The association of MHR with AA could reflect the interplay between inflammation and metabolic health, indicating a more complex underlying pathophysiology. However, more research is required to definitively establish causation.
Table 1.
Baseline Characteristics and Laboratory Findings
| Aa(+) | Aa(-) | P | |
|---|---|---|---|
| Age | 57.0±7.3 | 54.2±6.8 | 0.04 |
| Creatinine | 0.86±0.13 | 0.81±0.12 | 0.043 |
| Gender(male) | %80 | %20 | 0.006 |
| Antihypertensive drug usage | %71.6 | %28.4 | 0.549 |
| B-blocker usage | %73.6 | %26.4 | 0.511 |
| Hyperlipidemia | %71.2 | %28.8 | 0.812 |
| Coronary artery disease | %63.2 | %36.8 | 0.458 |
| Hypertension | %71.4 | %28.6 | 0.699 |
| Diabetes mellitus | %70.4 | %29.6 | 1.000 |
| hs-CRP(mg/l) | 4.0±2.4 | 2.2±1.8 | <0.001 |
| Fasting glucose (mg/dl) | 101.9±18.4 | 99.1±13.7 | 0.518 |
| Hba1c(%) | 5.7±0.4 | 5.7±0.3 | 0.754 |
| Total cholesterol(mg/dl) | 205.99±±39.11 | 205.78±43.95 | 0.978 |
| LDL (MG/DL) | 132.15±34.08 | 128.55±34.98 | 0.579 |
| HDL(MG/DL) | 42.2±7.2 | 50.4±11.9 | <0.001 |
| Triglycerides | 169±86 | 147±70 | 0.112 |
| ALT(U/L) | 27.43±20.3 | 25.0±11.6 | 0.687 |
| AST(U/L) | 24.27±9.63 | 25.38±11.22 | 0.979 |
| GGT(U/L) | 25.89±9.82 | 25.35±9.48 | 0.767 |
| Uric acid(mg/dl) | 5.51±1.25 | 5.17±1.17 | 0.148 |
| WBC(103/µL) | 7.0±1.6 | 6.3±1.2 | 0.014 |
| Hemoglobin(g/dl) | 14.21±1.43 | 13.95±1.63 | 0.361 |
| RDW(%) | 12.22±1.68 | 12.74±0.94 | 0.012 |
| MCV(FL) | 86.49±3.98 | 86.47±4.13 | 0.972 |
| Neutrophil(103/µL) | 3.9±1.2 | 3.5±0.9 | 0.09 |
| Lymphocyte(103/µL) | 2.3±0.6 | 2.1±0.5 | 0.374 |
| Neutrophil/ lymphocyte ratio | 1.8±0.6 | 1.7±0.5 | 0.591 |
| MPV(FL) | 7.89±1.55 | 9.99±1.54 | <0.001 |
| Platelet(103/µL) | 246±62 | 274±66 | 0.019 |
| MHR | 13.1±3.96 | 9.69±3.88 | <0.001 |
Notes: Bold P-values indicate statistical significance. Data are given as mean ±SD, median (interquartile range) or n (%).
Abbreviations: Aa, aortic aneurysm; ALT, alanin aminotransferaz; AST, aspartat aminotransferaz; Hba1c, hemoglobin a1c; HDL, high density lipoprotein cholesterol; hs-CRP, high sensitive c reactive protein; LDL, low density lipoprotein cholesterol; MCV, mean corpuscular volume; MHR, monocyte/hdl; MPV, mean platelet volume; RDW, red cell distribution width; WBC, white blood cell.
Table 2.
Univariate Logistic Regression Analysis
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.053 | 1.001–1.107 | 0.047 |
| Creatinine | 1.202 | 1.05–1.606 | 0.046 |
| Gender | 0.35 | 0.163–0.751 | 0.007 |
| HDL (mg/dL) | 0.905 | 0.862–0.951 | <0.001 |
| Hs-CRP(mg/L) | 1.467 | 1.202–1.790 | <0.001 |
| MHR | 1.276 | 1.132–1.438 | <0.001 |
| MPV(fL) | 0.458 | 0.344–0.610 | <0.001 |
| RDW(%) | 0.746 | 0.547–1017 | 0.063 |
| Platelet(103/µL) | 0.993 | 0.988–0.999 | 0.025 |
| WBC(103/µL) | 1.397 | 1.069–1.826 | 0.014 |
Note: Bold P-values indicate statistical significance.
Abbreviations: hs-CRP, High Sensitive C Reactive Protein; HDL, High Density Lipoprotein Cholesterol; RDW, Red Cell Distribution Width; MPV, Mean Platelet Volume; WBC, White Blood Cell.
Table 3.
Predictors of Aortic Aneurysm in Multivariate Logistic Regression Analyses
| OR | 95% CI | p-value | |
|---|---|---|---|
| Hs-CRP(mg/L) | 1.527 | 1.117–2.086 | 0.008 |
| MPV(fL) | 0.381 | 0.250–0.582 | <0.001 |
| Platelet(103/µL) | 0.980 | 0.968–0.993 | 0.002 |
Note: Bold P-values indicate statistical significance.
Abbreviations: OR, odds ratio; CI, confidence interval; MPV, Mean Platelet Volume.
Figure 1.
The ROC curve analysis of hs-CRP in predicting ascending aortic aneurysms.
Discussion
This study demonstrated that hs-CRP and the monocyte/HDL ratio are elevated in patients with ascending AA compared to the control group, suggesting a potential role for inflammation in the development of ascending AA. The etiology of ascending AA differs from that of AAA, where inflammation plays a significant role. Early 1930s researchers Erdheim et al described the etiology of ascending aortic aneurysms as medial degeneration, which is characterized by non-inflammatory lesions and a noticeable loss of smooth muscle cells and elastic fibers within the arterial media layer.14 The inflammatory process in the arterial wall encompasses multiple mechanisms, such as destructive remodeling, inflammation, angiogenesis, biomechanical wall stress, and molecular genetics.15 Observations have shown that inflammatory changes occur within the tissue of an aneurysm, including an increase in local AngII signaling and a change in the phenotype of smooth muscle cells. These changes contribute to the development of ascending aortic aneurysms.16 Recent evidence suggests a convergence in risk factors between ascending AA and AAA, indicating a potential inflammatory component in ascending aortic aneurysm development. Several studies have reported elevated inflammatory biomarkers in patients with ascending AA. For instance, Dolapoglu et al found increased CRP levels in patients with ascending AA and proposed the CRP/albumin ratio as a potential marker for rapid growth in ascending aortic aneurysms.17 Similarly, Jiyoung Yu et al reported high CRP levels in patients with ascending AA.11 Rumin HE et al found evidence that inflammatory infiltrates, especially T cells that express Fas, may play a role in TAAs, which are similar to AAAs.8 In our study, people with ascending AA had higher levels of hs-CRP and a high ratio of monocytes to HDL compared to the control group. This finding aligns with previous research showing a decrease in HDL levels in AAA patients.18 It is known that HDL can help prevent the growth of aortic aneurysms.19 The lower HDL levels in our study’s ascending AA patient group may be what caused the rise in the monocyte/HDL ratio. However, whether this finding reflects the anti-inflammatory and antioxidant effects of HDL or merely serves as a surrogate marker of cardiovascular health remains uncertain. New research suggests that HDL molecules not only take cholesterol off of macrophages, but they also stop macrophages from moving and control how activated and stuck together monocytes are, which could affect the development of ascending AA.20 The elevation of hs-CRP and MHR in ascending AA patients indicates the presence of inflammation, but whether it is a cause or a consequence remains unclear, especially considering that 63% of our patients had coronary artery disease. However, Increased concentrations of hs-CRP and MHR have been associated with the existence and advancement of aortic aneurysms, indicating its potential usefulness as a diagnostic biomarker for identifying individuals who are at risk of developing ascending aortic aneurysms.21 Further clarification would require histopathological analysis of aortic tissue, which was not feasible in our study.
Study Limitations
Despite its contributions, this study has several limitations that should be acknowledged. Firstly, its cross-sectional design limits the ability to establish causality between inflammatory markers and AA development. Longitudinal studies are needed to elucidate the temporal relationship between inflammation and AA progression. Additionally, the relatively small sample size and single-center nature of the study may limit the generalizability of the findings. Future research with larger, more diverse cohorts is warranted to validate these Results and enhance their clinical relevance. Furthermore, the lack of histopathological analysis of aortic tissue precludes a detailed understanding of the underlying inflammatory processes in AA. Incorporating histological data in future studies could provide valuable insights into the mechanisms driving AA pathogenesis. Finally, the fact that a lot of patients have other health problems at the same time, like coronary artery disease, can make it hard to figure out what the levels of inflammatory biomarkers mean. Accounting for these confounders in future analyses would help clarify the specific role of inflammation in AA development.
Conclusion
This study provides valuable insights into the potential involvement of inflammation in the development of ascending aortic aneurysms (AA). Higher amounts of hs-CRP and the monocyte-to-HDL ratio (MHR) were found in people with AA compared to the control group. This suggests that inflammation may play a part in the development of AA. The increased levels of hs-CRP and MHR indicate a substantial inflammatory contribution to the development of ascending aortic aneurysms. This suggests that chronic inflammation plays a role in the degradation of the extracellular matrix, loss of smooth muscle cells, and upregulation of proteolytic enzymes, all of which contribute to the progression of the aneurysm. These results show that more research needs to be done on the inflammatory processes that lead to AA and on how inflammatory markers might be useful as diagnostic and prognostic tools in this group of patients.
Ethical Consideration
The study adhered to the principles of the Declaration of Helsinki and was approved by the Ordu state hospital ethics committee. Participants provided informed consent, and measures were taken to ensure patient confidentiality and data protection. The protocol minimized potential biases and conflicts of interest were disclosed. The research aimed to provide valuable insights into inflammatory markers and ascending aortic aneurysms.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Kardiologia Polska. 2014;72(12):1169–1252. doi: 10.5603/KP.2014.0225 [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the E. J Am Soc Echocardiogr. 2015;28(2):119–182. [DOI] [PubMed] [Google Scholar]
- 3.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994–2001. Circulation. 2009;119(16):2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619 [DOI] [PubMed] [Google Scholar]
- 4.Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14(4):343–349. doi: 10.1093/eurpub/14.4.343 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SA, Hassan MS, Aksu F, Waberi MM, Fujeyra AM. Primary hyperaldosteronism: a rare potential risk factor for acute aortic dissection, an interesting case report. J Cardiovas Cardiol. 2024;2(1):1–3. doi: 10.61440/JCC.2024.v2.12 [DOI] [Google Scholar]
- 6.Erkin A, Asil K, Kara I, Erkengel HI. Management of a complicated redo giant dissecting aortic aneurysm. Cardiovasc J Afr. 2017;28(4):e6–8. doi: 10.5830/CVJA-2016-087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hultgren R, Larsson E, Wahlgren CM, Swedenborg J. Female and elderly abdominal aortic aneurysm patients more commonly have concurrent thoracic aortic aneurysm. Ann Vasc Surg. 2012;26(7):918–923. doi: 10.1016/j.avsg.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 8.He R, Guo DC, Estrera AL, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thoracic Cardiovasc Surg. 2006;131(3):671–678.e2. doi: 10.1016/j.jtcvs.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 9.Vainas T, Lubbers T, Stassen FR, et al. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation. 2003;107(8):1103–1105. doi: 10.1161/01.CIR.0000059938.95404.92 [DOI] [PubMed] [Google Scholar]
- 10.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6(8):543–552. doi: 10.1038/nrcardio.2009.102 [DOI] [PubMed] [Google Scholar]
- 11.Kim EN, Yu J, Lim JS, et al. Se Jin Oh CRP immunodeposition and proteomic analysis in abdominal aortic aneurysm. PLoS One. 2021;16(8):e0245361. doi: 10.1371/journal.pone.0245361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Ren D, Wang CS, Li T, Yao HC. High sensitivity C-reactive protein to prealbumin ratio measurement as a marker of the prognosis in acute coronary syndrome. Sci Rep. 2019;9(1):11583. doi: 10.1038/s41598-019-48189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan SM, Shi YH, Wang JJ, Lü FQ, Gao S. Níveis plasmáticos elevados do dímero D e da proteína C reativa hipersensíveis podem indicar desordens aórticas. Braz J Cardiovasc Surg. 2011;26(4):573–581. doi: 10.5935/1678-9741.20110047 [DOI] [Google Scholar]
- 14.Erdheim J. Medionecrosis aortae idiopathica cystica. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin. 1930;276(1):187–229. [Google Scholar]
- 15.Kirsch E, Radu N, Allaire É, Loisance D. Pathobiology of idiopathic ascending aortic aneurysms. Asian Cardiovasc Thorac Ann. 2006;14(3):254–260. doi: 10.1177/021849230601400320 [DOI] [PubMed] [Google Scholar]
- 16.Le V, Yamashiro Y, Yanagisawa H, Wagenseil J. Measuring, reversing, and modeling the mechanical changes due to the absence of fibulin-4 in mouse arteries. Biomech Model Mechanobiol. 2014;13(5):1081–1095. doi: 10.1007/s10237-014-0556-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolapoglu A, Avci E, Kiris T. The predictive value of C-reactive protein to albümin ratio for ascending aort progression in patients with ascending aortic diameter of 40–50 mm. Journal of Cardiothoracic Surgery. 2022;17(1):1–7. doi: 10.1186/s13019-022-02003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118(23):2382–2392. doi: 10.1161/CIRCULATIONAHA.108.802074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golledge J, Van Bockxmeer F, Jamrozik K, McCann M, Norman PE. Association between serum lipoproteins and abdominal aortic aneurysm. Am j Cardiol. 2010;105(10):1480–1484. doi: 10.1016/j.amjcard.2009.12.076 [DOI] [PubMed] [Google Scholar]
- 20.Açıkgöz SK, Açıkgöz E, Şensoy B, Topal S, Aydoğdu S. Monocyte to high-density lipoprotein cholesterol ratio is predictive of in-hospital and five-year mortality in ST-segment elevation myocardial infarction. Cardiol j. 2016;23(5):505–512. doi: 10.5603/CJ.a2016.0026 [DOI] [PubMed] [Google Scholar]
- 21.Acar B, Yayla Ç, Gül M, et al. Monocyte-to-hdl-cholesterol ratio is associated with ascending aorta dilatation in patients with bicuspid aortic valve. Afr Health Sci. 2021;21(1):96–104. doi: 10.4314/ahs.v21i1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]