Abstract
Increasing reports of insecticide resistance continue to hamper the gains of vector control strategies in curbing malaria transmission. This makes identifying new insecticide targets or alternative vector control strategies necessary. CLassifier of Essentiality AcRoss EukaRyote (CLEARER), a leave-one-organism-out cross-validation machine learning classifier for essential genes, was used to predict essential genes in Anopheles gambiae and selected predicted genes experimentally validated. The CLEARER algorithm was trained on six model organisms: Caenorhabditis elegans, Drosophila melanogaster, Homo sapiens, Mus musculus, Saccharomyces cerevisiae and Schizosaccharomyces pombe, and employed to identify essential genes in An. gambiae. Of the 10,426 genes in An. gambiae, 1,946 genes (18.7%) were predicted to be Cellular Essential Genes (CEGs), 1716 (16.5%) to be Organism Essential Genes (OEGs), and 852 genes (8.2%) to be essential as both OEGs and CEGs. RNA interference (RNAi) was used to validate the top three highly expressed non-ribosomal predictions as probable vector control targets, by determining the effect of these genes on the survival of An. gambiae G3 mosquitoes. In addition, the effect of knockdown of arginase (AGAP008783) on Plasmodium berghei infection in mosquitoes was evaluated, an enzyme we computationally inferred earlier to be essential based on chokepoint analysis. Arginase and the top three genes, AGAP007406 (Elongation factor 1-alpha, Elf1), AGAP002076 (Heat shock 70kDa protein 1/8, HSP), AGAP009441 (Elongation factor 2, Elf2), had knockdown efficiencies of 91%, 75%, 63%, and 61%, respectively. While knockdown of HSP or Elf2 significantly reduced longevity of the mosquitoes (p<0.0001) compared to control groups, Elf1 or arginase knockdown had no effect on survival. However, arginase knockdown significantly reduced P. berghei oocytes counts in the midgut of mosquitoes when compared to LacZ-injected controls. The study reveals HSP and Elf2 as important contributors to mosquito survival and arginase as important for parasite development, hence placing them as possible targets for vector control.
Introduction
Vector control interventions remain potent strategies for controlling the transmission of malaria, a disease that remains a global menace [1]. These interventions include larval control methods, use of insecticide-treated nets and indoor residual spraying [2]. However, these vector control strategies are approaching the limit of their effectiveness [3], resulting in the consistently high morbidity rates reported annually [1]. Consequently, there is an urgent need to introduce innovative vector control strategies. Other vector control interventions such as RNA interference (RNAi) based biopesticides, generation of refractory mosquitoes, sterile insect techniques are currently gaining attention. However, these techniques are dependent on the identification of appropriate targets. An important tool in functional genomics which can be used to investigate and characterize promising targets is RNAi, an important gene silencing technique that provides insight into the function of a gene and can be successfully applied to investigate gene knockdown in mosquitoes [4]. Using this technique, the synthesis of proteins that play a role in the survival, fecundity, metabolism, vectorial capacity, and the behaviour of mosquitoes and insects generally, have been suppressed, thus, unveiling some of these proteins as possible targets for vector control. For example, RNAi provided insight on the role of TEP1, LRIM1, and APL1 as crucial genes for the immune response in Anopheles [5, 6]. Similarly, several proteins involved in insecticide resistance, transport of molecules, reproductive fitness, and host-seeking behaviour have been identified through RNAi. Examples include ABCG4 [7], CYP 450s [8], c-Jun N-terminal kinase (JNK) pathway components [9], Aquaporin 3 [10], and G protein-coupled receptors (GPCRs), which play a role in development, visual, gustatory and olfactory sensing, homeostasis, and hormonal regulation [11].
RNAi has been described to be a promising technique for pest and mosquito control [12–16]. It is an important tool for identifying potential insecticidal targets that could be explored to develop insecticides or other products to limit the burden of mosquitoes on human health [17]. The accuracy and specificity of the technique make RNA-based biopesticides to be considered as alternatives to chemical-based insecticides [18–20]. Since they are specific, unwanted effects could be reduced and thus having reduced or no effects on non-target organisms. Some targets which have been investigated as RNAi biopesticides for vector control include chitin synthase [21] and 3-hydroxykynurenine transaminase [17, 22]. Likewise interfering RNA pesticide (IRP) corresponding to the mosquito Shaker and GPCR dopamine 1 receptor (dop1) genes have been tested and found to have adulticide and larvicidal activities against different mosquito species [23, 24]. However, employing experimental techniques to screen every gene in a disease vector to identify potential targets is a tall order.
In turn, computational methods can be employed to predict essential proteins in organisms [25] Essential genes are considered to be crucial for the survival or reproductive success of an organism [26, 27]. These essential genes in disease vectors could serve as possible vector control targets. Employing computational techniques can lead to refined smaller lists of genes, which could then be validated by experimental techniques to determine their suitability for vector control. Computational techniques for predicting essential genes range from simple algorithms like chokepoint analysis in metabolic networks to more complex techniques like machine learning. Chokepoint analysis has been employed to identify possible insecticidal targets in Anopheles gambiae, a major malaria vector [28]. An example of a gene predicted as essential in An. gambiae using the chokepoint criteria is arginase, which was observed to be highly expressed in the midguts of Plasmodium berghei infected mosquitoes compared to their blood-fed counterparts [29]. Hence, arginase could contribute to the development of the parasite in the mosquito and could possibly serve as a target for vector control. However, experimental validation of these predictions remains to be done. Beder et al. [30] developed a machine learning based-technique trained on six model organisms to predict essential genes using a combination of leave one organism out cross-validation and orthology based approaches. In this present study, we (i) experimentally followed up on the computational prediction of arginase to be essential, and (ii) applied the machine learning method to predict essential genes in An. gambiae, and experimentally validated a selected short list of the predicted genes using the RNAi knockdown technique.
Methods
The machine learning method to identify essential genes in silico
We applied a modification of the CLassifier of Essentiality AcRoss EukaRyote which is a machine learning classifier for essential genes which was trained on the six model organisms: Caenorhabditis elegans, Drosophila melanogaster, Homo sapiens, Mus musculus, Saccharomyces cerevisiae and Schizosaccharomyces pombe [30]. The machine was trained on 60,381 genes, using 41,635 features based on seven different sources including protein and gene sequence, functional domains, topological features, evolution/conservation, subcellular localization, and gene sets from Gene Ontology.
Feature generation
Essential genes for An. gambiae were predicted using the same features used for the model organisms. The An. gambiae str. PEST genome (GenBank assembly accession: GCA_000005575.1) was used to generate the gene and protein sequence features. The tools seqinR [31], protr [32], CodonW (http://codonw.sourceforge.net/) and rDNAse [33] were used to calculate protein and gene sequence features. For genes with isoforms, the features were generated individually for each isoform and the median of all was calculated. seqinR provided simple protein sequence information including the number of residues, the percentage of physico-chemical classes and the theoretical isoelectric point. Most protein sequence features were obtained using protr comprising autocorrelation, conjoint triad, quasi-sequence order and pseudo amino acid composition. CodonW was used to calculate simple gene descriptors like length and GC content, frequency of optimal codons and the effective number of codons. rDNAse provided DNA descriptors such as auto covariance, pseudo nucleotide composition, and kmer frequencies (n = 2–7). Domain features were calculated using the tools from the Technical University of Denmark (http://www.cbs.dtu.dk/services/), comprising the prediction of membrane helices and beta-turns, cofactor binding, acetylation and glycosylation sites. Topology features were derived from protein-protein-associations (PPA) using the STRING v11 [34] database. These features comprised of degree, degree distribution, betweenness, closeness and clustering coefficient using the Python library NetworkX. Conservation features were calculated by the number of homologous proteins of a query protein in the complete RefSeq database [35] using PSI-BLAST [36]. As features, the number of proteins identified with e-value cutoffs from 1e−5 to 1e-100 (in 1e−5 multiplication steps) were used. An alignment coverage score (ACS) was calculated for hits with a cutoff ≤1e−30 as we described formerly [37]. Furthermore, the number of homologous sequences with a score from 0 to 0.95 in 0.05 steps were calculated. Similarly, the number of paralogous sequences were calculated. Here, blastn alignment results with an e-value cutoff ≤1e−30 were used as input for the score. Subcellular localization features were predicted by the tool DeepLoc [38]. DeepLoc assigns a score for each protein to its localization in 11 eukaryotic cell compartments. Gene set features were derived from all Gene Ontology (GO) terms present in all analyzed organisms, similar to Chen et al. [39]. Here, not only the characterization of the query gene was taken into account, but also of its neighbors in the protein association network. By this, the features were more robust against false gene set annotations. The neighbors of the query gene were assembled employing the gene network definitions of STRING v11. A Fisher’s exact test for enrichment of interaction partners was performed for each of the gene sets. The log 10 values of the P-values were used as features.
Defining the gold standard
Essentiality information was derived from the six species: C. elegans, D. melanogaster, H. sapiens, M. musculus, S. cerevisiae and S. pombe. For D. melanogaster, H. sapiens and M. musculus, screening data was collected from screens of cellular essential genes (CEG) and organismal essential genes (OEG). For C. elegans, and the yeasts only CEG screening data was available. This essentiality information was derived from Online GEne Essentiality (OGEE) [40] and Database of Essential Genes (DEG) [41] databases and the literature (for more details see [30]). For genes with different essentially status in different screens, a majority voting was performed. For human cell line screens, a gene had to be studied in at least five experiments as described formerly by Guo et al. [42].
Normalization, feature reduction and machine learning
Data analysis was performed using R. Values of each feature were z-transformed and each value was rounded to deciles. For feature selection and learning, the data was randomly split into training (80%) and testing (20% of the data). Using the training set, feature selection was performed in two steps: first, LASSO was applied using the glmnet package [43] (cv.glmnet function, alpha = 1, type.measure = ‘auc’). In the second step, collinearity was reduced by removing highly correlating features with Pearson correlation coefficients r ≥ 0.70. Next, class imbalances were addressed during training using SMOTE [44]. The classifiers were trained using Random Forest (RF) from the caret [45] package. For RF, tuneLength in the train function was set to 3 resulting in three predictors randomly sampled at each split. For each organism, a stratified randomized 5-fold cross-validation was performed in which feature selection, parameter tuning and training of the classifiers was done using 80% of the data. 20% of the data was used for testing the performance. Leave-one-organism-out cross-validation: For each individual species (five species for CEG, four for OEG predictions), five machines were trained. Essential genes for the left-out species were predicted with machines trained on the according CEG or OEG data sets of the other organisms. Thereby the classifiers for each (non-left out) species provided an essentiality prediction score between zero and one and the average of these scores was used for the prediction of a gene to be essential in the left-out species. TPM values from an RNA-seq dataset (E-MTAB-9241) were used to aid selection of predicted genes for the experimental phase [46, 47].
Experimental methods
Mosquito handling
An. gambiae G3 strain was maintained at 27–29°C and 75–90% relative humidity with a 12:12 light-dark photoperiod. Adults were provided with 5% sucrose solution ad libitum [48]. For reproduction, female mosquitoes were fed on BALB/c mice for 30 min. Egg dishes were placed in cages 48 h post-blood-feeding and retrieved 24 h later. Mosquitoes were reared according to MR4 protocol [49]. Eggs were bleached with 1% bleach and rinsed three times with 0.05 g/L salted deionized water upon egg collections. Bleached eggs were hatched on the following day in a tray containing 500 mL of 0.05g/L salted deionized water and larva food to a final concentration of 0.02%. Splitting of L1 larvae was carried out either in the evening of the day of hatching or the day after. Larvae were split into trays, with each tray containing approximately 250 larvae in 500 mL of 0.05 g/L salted deionized water and larva food to a final concentration of 0.02%. A volume of food (3–10 mL) was added to the tray daily depending on the larvae stage. Larvae food (50 g) comprised a mixture of tuna fish meal (20g), liver powder (20g), and vitamin mix (10g). An aliquot of larvae food (1 g) was dissolved in 50 mL of water (yielding 2%), and appropriate volumes were taken from this. Upon pupation, pupae were collected into cups having clean water and placed in a cage to allow the emergence of adults.
Mice handling
BALB/c mice were used as P. berghei vertebrate hosts and in blood-feeding mosquitoes for reproduction. The mice were maintained in the animal facilities of the University of Camerino, Camerino, Italy. All animal rearing and handling was carried out according to the Italian Legislative Decree (116 of 10/27/92) on the “use and protection of laboratory animals” and in agreement with the European Directive 2010/63/UE. The experimentation was approved by the Ethical Committee of University of Camerino. According to the method of Cappelli et al. [50], BALB/c mice were maintained at 24°C, fed on standard laboratory mice pellets (Mucedola S.r.l., Milano, Italy) and provided with tap water ad libitum. Mice were anesthetized using a mixture of 10 mg/mL prequillan (ATI-srl), 20 mg/mL sedaxylan (Dechra) and 1X Phosphate-buffered saline (PBS). Each mouse was injected with 0.1 mL of this mixture intraperitoneally and used to feed mosquitoes 15 mins after injection.
Primers
Primers with T7 tail for dsRNA synthesis were designed using the E-RNAi website (https://www.dkfz.de/signaling/e-rnai3/). All qPCR primers were designed using primer wizard in Benchling (www.benchling.com), which is powered by Primer3 (https://primer3.org/). Care was taken to ensure that designed primers were target gene specific. Primers were synthesized by Metabion (www.metabion.com). All primers used in this study are provided in S1 Table.
RNA interference
Total RNA extraction from a pool of five whole mosquitoes was carried out using RNAzol RT (Sigma) according to the manufacturer’s instructions. Complementary DNA (cDNA, 10 μL) was synthesized from 500 ng total RNA using PrimeScript RT reagent kit (Takara Bio) according to the manufacturer’s instruction and incubated at 37 ℃ for 15 min, then 5 ℃ for 5 s. A fragment from each target gene was amplified using cDNA and target specific primers having the T7 promoter tag sequence TAATACGACTCACTATAGGG incorporated into their 5’ end (to enable in vitro transcription by T7 polymerase). PCR fragment of LacZ served as a control and was synthetized from E. coli expressing LacZ using LacZ specific primers. All primers used are provided in S1 Table. An aliquot of this PCR reaction was used as a template for dsRNA synthesis in vitro using TranscriptAid T7 High Yield Transcription Kit (Thermo Scientific) and purified following the manufacturer’s instruction. DNA was removed by DNase I digestion, while all proteins and free nucleotides were removed by phenol chloroform extraction according to the manufacturer’s protocol. dsRNA was eluted with DEPC treated water and its concentration measured using Nanodrop 1000 spectrometer (Thermo Fisher Scientific, USA). Gel electrophoresis (1% TBE agarose) was performed on an aliquot of the PCR products to confirm that products of the expected size were synthesized for each gene. Similarly, aliquots of the synthesized dsRNA were evaluated on a 2% TBE agarose gel.
Mosquito injection
Two to three-day-old female mosquitoes were anaesthetized on ice and injected with 138 nL (5 μg/μL) target-specific dsRNA or LacZ control dsRNA using a Drummond Nanoject II Automatic Nanoliter Injector (3-000-205-A, Drummond Scientific, Broomall, PA, USA) and glass microcapillary injection needles according to the method described by Mancini et al. [48]. The microcapillary injection needles used were obtained by using Flaming/Brown Micropipette Puller System Model P-1000 (Sutter Instruments Company, Novato, CA, USA) to pull glass capillaries (BF150-86-10, Sutter Instruments, Novato, CA, USA). For survival experiments, anesthetized mosquitoes injected with 1X PBS were used as a handling control. Mosquitoes not subjected to injection, but had undergone cold anesthetization, were used as an untreated control. Mosquitoes which died within 24 hours post-injection were discarded from the analysis. Fifteen mosquitoes were injected to determine knockdown efficiency by quantitative-PCR (qPCR) analysis (5 mosquitoes per replicate, 3 replicates), while 70 female mosquitoes per treatment were used for longevity analysis (70 mosquitoes per replicate, 4 replicates). For P. berghei infection, 200 female mosquitoes per treatment were injected. After injections, mosquitoes were maintained in the insectary under standard conditions.
Real-time quantitative PCR (RT-qPCR)
RNA was extracted from whole mosquito samples at intervals of 24 h post-dsRNA injection– 24 h, 48 h, 72 h and 96 h for the arginase experiment. For the experiments of HSP, Elf2 and Elf1, RNA was extracted from whole mosquito samples three days post-dsRNA injection. RNA was reverse-transcribed into cDNA using iScript™ gDNA Clear cDNA Synthesis Kit (BioRad). Real-time quantitative PCR was conducted using 4 μL of HOT FIREPol® EvaGreen® qPCR Supermix (Solis Biodyne, Estonia), 2 μL of template (cDNA), 2.5 μL of 1 μM Primer mix (combined forward and reverse primers) which was made up to a final volume of 20 μL using nuclease-free water. PCR amplification was performed by preheating the reaction to 95°C for 12 min, followed by 40 PCR cycles (95°C for 30 s, 60˚C for 30 s, and 74˚C for 30 s), a melt curve run from 65–95 ℃, with an increase in temperature by 0.5 ℃/cycle every 5 s. An. gambiae ribosomal S7 gene was used as an internal reference gene for the normalization of each target gene, and the results were normalized further against the LacZ injected control group. Relative expression levels of the genes were reported as 2-ΔΔCT [51]. The amplification efficiency of all qPCR primers used was determined.
Survival assay
Longevity was assessed to determine whether knockdown of HSP, Elf2 or Elf1 affected An. gambiae survival. Longevity was evaluated using a total of 280 female mosquitoes per treatment (70 mosquitoes per replicate, 4 replicates). The rate of survival was monitored until 100% mortality was reached for all six treatments (HSP dsRNA, Elf2 dsRNA, Elf1 dsRNA, LacZ dsRNA, PBS, and not injected).
For arginase knockdown, longevity was assessed using a total of 140 female mosquitoes per treatment (70 mosquitoes per replicate, 2 replicates). The setup included three treatment groups–Arg dsRNA, LacZ dsRNA, and the not injected treatment groups. All mosquitoes, including those in the not injected treatment groups, were exposed to a naïve blood meal from mice 48 h post-dsRNA injection. A blood meal was introduced because an effect of knockdown of these genes on P. berghei development was hypothesized. The rate of survival was monitored until 19 days post-blood meal. Survival analyses were performed using the Kaplan–Meier method [52, 53] and significance between groups determined by Log-rank (Mantel-Cox) tests. Graphs were plotted using the ggsurvplot function in the R-programming software [54].
P. berghei infection
The murine malaria parasite, P. berghei was used in this study as a model organism for investigation of human malaria. Three mice were infected with P. berghei (GFPCON, PbGFPCON) from frozen capillary stocks diluted in 200 μL of PBS (7.2 pH) through intraperitoneal injection. The level of parasitemia in mice was determined four days after infection using slides with methanol fixation of air-dried blood smears taken from the tail of the mice, followed by staining with 15% (w/v) Giemsa solution. Parasitemia was counted under an optical microscope (Olympus CX21). The mouse with the best parasitemia was selected as the donor mouse for passaging to recipient mice. Infection of recipient mice was carried out according to the method of Cappelli et al. [50] with slight modification. Eight-week-old female mice (18–25 g) were infected with P. berghei directly by an intraperitoneal injection of 5 x 106 infected erythrocytes (from a donor mouse) diluted in 200 μL of PBS (7.2 pH). Parasitemia and gametocytemia were determined in a 15% Giemsa-stained blood smear obtained from recipient infected mice three days after infection using an optical microscope. Recipient mice were used in feeding mosquitoes when parasitemia was between 8 and 11%, and gametocytemia was between 1.2 and 3.8. Prior to feeding of mosquitoes with mice, mice were anesthetized using a mixture of 10 mg/mL prequillan, 20 mg/mL sedaxylan and 1X PBS, the mice were used to feed mosquitoes 15 mins after being anesthetized.
Non-fed and partially fed females were removed from the cage. To allow parasite development, mosquitoes were kept at 19 ℃ and 70% humidity. Midguts from a total of 50 mosquitoes per treatment group were dissected 10 days post-blood meal (PBM) to confirm the presence of oocysts using fluorescent microscopy. The number of oocytes in each midgut was counted and compared to the LacZ control.
Statistical analysis
Statistical analysis on gene expression data and oocytes count data were performed at a 95% confidence interval in GraphPad Prism 5. Relative gene expression data were presented as mean ± SEM, and statistical significance was determined by one-way analysis of variance (when comparing more than two groups) and Bonferroni post hoc test. Where only two groups were compared, the paired T-test was used. Data from survival analysis were analyzed using the Kaplan–Meier method [52], presented as mean ± confidence interval, and a Log-rank test was used to determine whether survival curves were statistically significant between the target-specific dsRNA treated groups and control groups. Survival curves were plotted using R software. Oocytes count data from infection experiments was presented as median on a dot plot, with each dot representing the number of oocytes/midgut. Statistical significance was between oocytes count from LacZ and the treatment group was determined by a Mann Whitney test.
Results
Machine learning results
Predictions were carried on a total of 10426 genes in An. gambiae. Using the CLEARER approach, 1946 genes (18.7%) were predicted to be CEGs, 1716 (16.5%) to be OEGs and 852 genes (8.2%) to be essential as both OEGs and CEGs. Using the orthology based approach, only 249 (2.4%) genes were predicted to be essential. Combining both CLEARER and orthology based approach, only 94 genes (0.9%) were predicted to be essential (Fig 1). The results of the predictions for all 10426 genes in An. gambiae are provided in S1 File.
Fig 1. Venn diagram of the number of predicted essential An. gambiae genes by CLEARER and orthology algorithm.
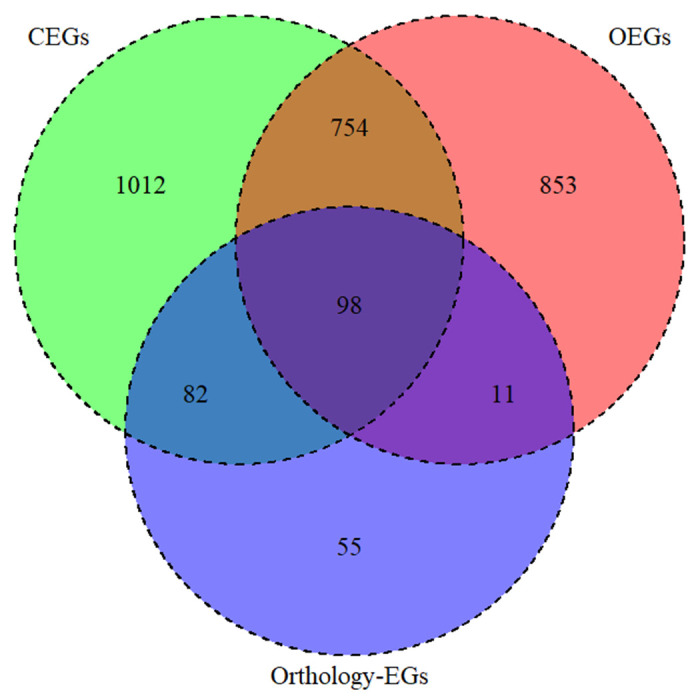
CEGs: Cellular essential genes, OEGs: Organism essential genes, Orthology-EGs: Orthology essential genes.
Some of the genes predicted as essential based on the three approaches had very low prediction scores based on the CLEARER approach (<0.2). To proceed further, we focused on the essentiality scores from CLEARER, selecting the top 250 ranked genes, which belonged to the top 2.5% of the scores. We proposed that an essential gene in addition to being essential in our predictions, should be highly expressed. Only thirteen of the genes belonging to the top 2.5% had high gene expression values (i.e., mean TPM > 1000), suggesting they are highly expressed in all conditions. The median, 25th and 75th percentile TPM values of all genes are provided in S1 File. We experimentally validated the top 3 highly expressed predicted genes which were non-ribosomal genes. These genes are presented in Table 1.
Table 1. Selected predicted genes for experimental validation.
| Gene | AGAP007406 | AGAP002076 | AGAP009441 |
|---|---|---|---|
| Uniprot annotation | Q7PT29 (Elongation factor 1-alpha) | A7UVK8 (Heat shock 70kDa protein 1/8) | Q7PTN2 (Elongation factor 2) |
| Essentiality score | 0.38759 | 0.26499 | 0.60084 |
| CE prediction | Essential | Essential | Essential |
| OE prediction | Essential | Essential | Essential |
| Essential orthologs | 6 | 14 | 14 |
| Non-essential orthologs | 11 | 52 | 6 |
| Orthology Prediction | Non-essential | Non-essential | Essential |
| TPM (Mean) | 7919.616 | 3462.944 | 3025.191 |
| TPM (Median) | 8114.321 | 3602.958 | 3051.977 |
| TPM (25th Percentile) | 6072.29 | 2342.043 | 2166.012 |
| TPM (75 Percentile) | 10231.74 | 4560.174 | 4026.935 |
| Drosophila melanogaster Ortholog | FBgn0000557 | FBgn0266599 | FBgn0000559 |
| OGEE Essentiality of Drosophila melanogaster Ortholog | Conditionally essential | Conditionally essential | Essential |
Essentiality score is based on CLEARER prediction; Essential/Non-essential orthologs are based on OGEE and DEG database; CE prediction: Cellular essentiality prediction; OE prediction: Organism essentiality prediction.
While AGAP009441 was predicted to be essential by all three methods (belonging to one of the 98 genes in Fig 1), AGAP007406 and AGAP002076 were predicted to be essential as both CEGs and OEGs, but not essential by orthology based approach (thus, belonging to the 754 genes in Fig 1). However, both genes had 14/66 (21%) and 6/17 (35%) essential orthologs, respectively and their D. melanogaster ortholog are conditionally essential based on OGEE Essentiality.
Effect of knockdown of arginase on survival of mosquitoes and P. berghei development
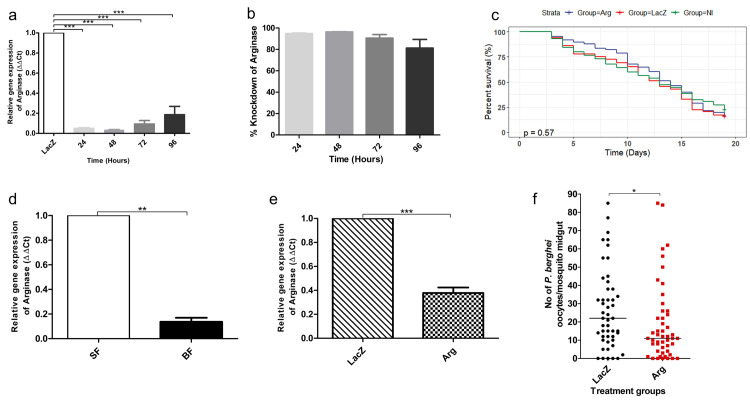
The primer efficiency of all qPCR primers used in this study is provided in S2 Table. Mosquitoes injected with arginase dsRNA had a significant reduction (p<0.001) in the expression of arginase at 24-, 48-, 72- and 96-hours post-injection compared to control groups injected with LacZ dsRNA (Fig 2A). Percentage knockdown (%KD) was ≥ 80% at all time intervals considered (94.83%, 96.37%, 90.53%, and 81.21% at 24-, 48-, 72- and 96-hours, respectively) (Fig 2B). Despite the observed strong silencing of arginase, knockdown of arginase had no significant effect on the survival of the mosquitoes compared to the LacZ-injected (p = 0.39) and not injected (NI) controls (p = 0.86) for a period of 19 days post-blood meal (Fig 2C). Survival of mosquitoes did not differ significantly (p = 0.57) among the three groups. To investigate possible reasons why no effect on survival was observed despite the strong silencing, gene expression levels of arginase in mosquitoes fed with blood compared to those fed with sugar were determined (Fig 2D). Likewise, expression levels of arginase in mosquitoes fed on a blood meal 48 h after arginase or lacZ dsRNA treatment were evaluated to determine if blood feeding reverses/masks the knockdown effect of arginase dsRNA treatment earlier observed (Fig 2E). This was done because the mosquitoes in the survival experiments were given blood meal 48 h after dsRNA injection since arginase was considered a possible target for the abrogation of P. berghei development in the mosquitoes. It was observed that arginase levels were significantly reduced (p<0.01) in naïve blood-fed mosquitoes 24 h after a blood meal compared to their sugar-fed counterparts (%KD = 62%) (Fig 2D). Likewise, arginase levels were greatly reduced (p<0.001) in blood-fed arginase dsRNA-treated mosquitoes compared to their blood-fed LacZ dsRNA-treated counterparts 24 h after blood meal (Fig 2E). This showed that blood feeding did not mask or revert gene silencing. Since arginase levels are reduced during a naïve blood meal, arginase might not be essential for the survival of mosquitoes. Silencing of arginase was noted to significantly reduce (p = 0.020) the number of Plasmodium oocytes counts in the midgut of mosquitoes compared to the LacZ dsRNA injected control group (Fig 2F).
Fig 2.
(a) Relative gene expression of arginase at 24, 48, 72 and 96 h after dsRNA injection in An. gambiae G3 mosquitoes (n = 6) (b) Percentage knockdown of arginase at 24, 48, 72 and 96 h after dsRNA injection in An. gambiae G3 mosquitoes (n = 6) (c) Percentage survival of female An. gambiae blood-fed at 48H after knockdown of arginase (d) Relative gene expression of arginase in not injected blood-fed (BF) An. gambiae G3 compared to not injected sugar-fed (SF) 24 h after blood feeding (n = 3) (e) Relative gene expression of arginase in Blood fed (BF) An. gambiae G3 mosquitoes injected with LacZ dsRNA or Arg dsRNA at 72 h post dsRNA injection (n = 5) (Blood feeding carried out 48 h after injection) (f) P. berghei oocytes counts 10 days post infection in female An. gambiae fed with blood of parasitized mice 48 h after knockdown of arginase (n = 49). NI: not injected, Arg: arginase, SF: sugar fed, BF: blood fed. Blood feeding did not mask the knockdown effect of Arg-dsRNA. Relative gene expression of arginase for each time point were calibrated against their respective LacZ injected group at the same time interval. Arg: arginase. *** = p< 0.001, ** = p< 0.01, * = p< 0.05.
Effect of knockdown of HSP, Elf2 and Elf1 on survival of An. gambiae
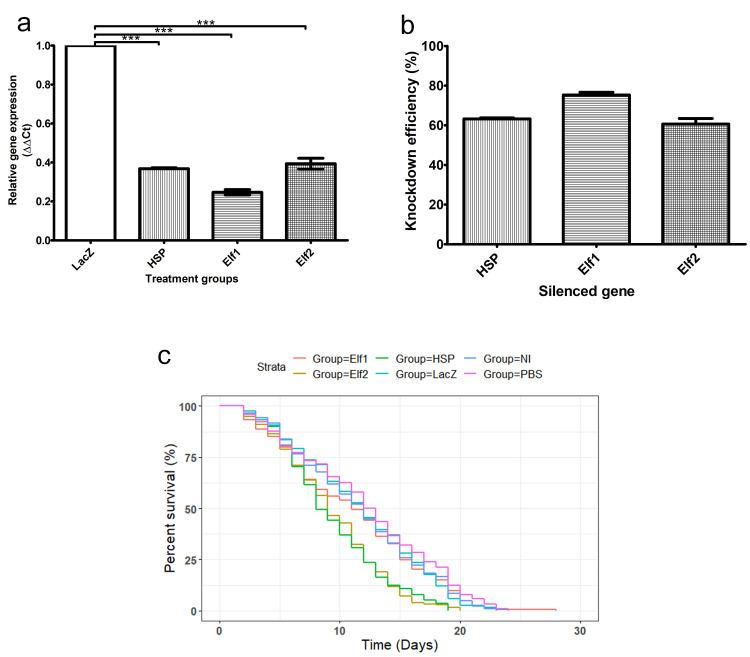
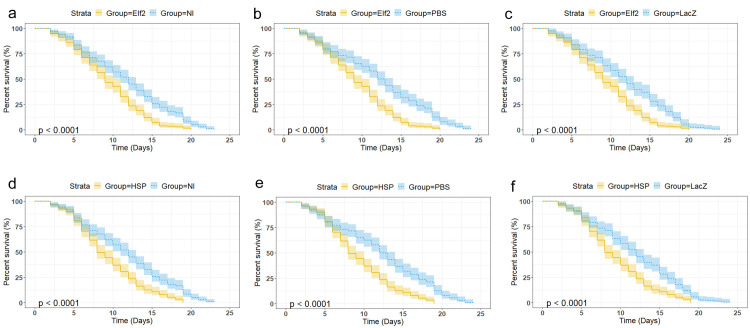
An. gambiae G3 mosquitoes injected with either HSP, Elf2 or Elf1 dsRNA had a significant reduction (p<0.001) in the expression of the respective gene 72 h post-injection compared to control groups injected with LacZ dsRNA (Fig 3A). Percentage knockdown (%KD) in HSP, Elf2 and Elf1 dsRNA injected mosquitoes were 63%, 61%, and 75%, respectively (Fig 3B). Although Elf1 dsRNA injection resulted in 75% knockdown of the gene, survival of Elf1 dsRNA injected mosquitoes did not differ significantly (p>0.05) when compared to the not injected, PBS injected, LacZ injected mosquitoes (Fig 3C). However, knockdown of Elf2 or HSP significantly decreased (p<0.0001) the survival of the mosquitoes when compared to either not injected, PBS injected or LacZ dsRNA injected controls (Fig 4A–4F).
Fig 3.
(a) Relative gene expression of HSP, Elf1, Elf2 72 h after dsRNA injection in An. gambiae G3 (n = 3) (b) Percentage knockdown of HSP, Elf1, Elf2 72 h after dsRNA injection in An. gambiae G3 (n = 3) (c) Percentage survival of female An. gambiae in NI, LacZ, PBS, HSP, Elf1 or Elf2 treated groups after knockdown. HSP: Heat shock 70kDa protein 1/8, Elf2: Elongation factor 2, Elf1: Elongation factor 1-alpha, NI: not injected, PBS: PBS injected, LacZ: LacZ injected. %KD of HSP, Elf1 and Elf2 were 63, 75 and 61%, respectively. *** = p< 0.001.
Fig 4.
Percentage survival of female An. gambiae (a) NI compared to Elf2 dsRNA injected (b) PBS injected compared to Elf2 dsRNA injected (c) LacZ dsRNA injected compared to Elf2 dsRNA injected (d) NI compared to HSP dsRNA injected (f) LacZ dsRNA injected compared to HSP dsRNA injected (g) PBS injected compared to HSP dsRNA injected. Lines represent mean (n = 4) while bands represent confidence interval. HSP: Heat shock 70kDa protein 1/8, Elf2: Elongation factor 2, NI: Not injected, PBS: PBS injected, LacZ: LacZ injected.
Discussion
Arginase was considered a possible target for disrupting P. berghei development in the mosquitoes due to its observed increased expression in midgut upon P. berghei infection [29]. Longevity assay was carried out for arginase to evaluate the suitability of the gene knockdown for the Plasmodium infection experiment. The mosquitoes needed to survive longer, at least 10 days to allow time for Plasmodium infection, development, and assessment of Plasmodium oocytes counts. Despite the strong knockdown achieved by arginase gene-silencing, no effect on longevity of mosquitoes was noted in two longevity assay replicates (Fig 2A–2C). These two replicates did not show any variance. In both replicates, the survival of mosquitoes following knockdown of arginase was comparable to the control group. The study suggests that arginase might not be important for the survival of mosquitoes, which may be reasoned by the observation that its expression is significantly reduced during blood feeding (Fig 2D and 2E). Hence, the Plasmodium infection study was carried out. Similarly, no effect on survival due to arginase knockdown was observed during infection studies. In turn, knockdown of arginase resulted in a significant reduction in P. berghei oocytes counts per midgut at day 10 post-infection (Fig 2F), suggesting that knockdown of arginase hampers the development of P. berghei. Arginase competes with nitric oxide synthase for the same substrate, arginine. Parasites, e.g., trypanosomes, have been reported to evade nitric oxide production in the host by activating the production of host arginase [55]. This has been observed to result in a depletion of l-arginine, resulting in reduced levels of cytotoxic nitric oxide and enhanced production of polyamines required for parasite growth [56]. Although this complete mechanism has not been elucidated in An. gambiae, it is proposed that knockdown of arginase might result in increased abundance of nitric oxide, thereby enhancing parasite clearance [57–59]. However, this must be further investigated. In addition, arginase metabolizes arginine to produce ornithine, which is a precursor for polyamine synthesis. It has been shown that polyamines modulate Plasmodium infection [58, 59], hence, knockdown of arginase may prevent their synthesis thereby reducing parasite load [57]. Since the knockdown of arginase did not affect survival, it might be useful to investigate the effect of a complete knockout of arginase on the development of Plasmodium. Arginase shares 42.0% (E-value: 4e-87) protein sequence identity with its mitochondrial ortholog in human (P78540) and 45.4% (E-value: 3e-87) to the cytosolic human ortholog (P05089) although no significant similarity was found between their nucleotide sequences. Hence, from this study, arginase might represent a good target to explore for transmission-blocking in An. gambiae, with consideration made to developing highly selective inhibitors. The incomplete parasite clearance observed as a result of arginase knockdown could be because knockdown transiently reduces expression of genes and does not completely prevent protein synthesis, hence some arginase would be available. Likewise, immune response in mosquitoes to parasite invasion is complex, involving interaction of many proteins and cell types. The timing and intensity of these interactions can result in different outcomes [60]. Simultaneous knockdown of multiple proteins that influence these responses, resulting in enhanced parasite clearance might be necessary to achieve complete clearance.
Knockdown of Elongation factor 1-alpha, Elf1 (AGAP007406) did not affect the survival of mosquitoes despite the strong knockdown observed upon Elf1 dsRNA treatment (Fig 3A–3C). This observed result might be explained by the presence of an isoform for Elongation factor 1-alpha (AGAP003541) in An. gambiae. AGAP003541 had very low TPM values (<1) in the RNA-seq data used in this study, as compared to AGAP007406 that had TPM values of ≈8000 (see S1 File), hence AGAP007406 was considered to be the major isoform and was targeted by RNAi. Since dsRNA was designed to specifically target AGAP007406, increased expression of its isoform AGAP003541 might be triggered to perform the necessary function of the gene product, thereby counteracting the effect of silencing the AGAP007406 isoform. Elf1 is a housekeeping gene with a GTP binding protein product necessary for peptide elongation during protein translation [61], hence it is an important hub in protein networks [62]. Inhibition of eukaryotic translation elongation factor 1 alpha 1 by Nannocystin Ax has been reported to inhibit translation of new proteins and downregulate cyclin D1, inducing G1 cell cycle arrest in colon cancer cells [61]. This suggests the importance of this gene for cellular survival. To further investigate the essentiality of Elongation factor 1-alpha in mosquitoes, it would be necessary to design dsRNA that would target conserved regions between the two genes or use chemical inhibitors.
Knockdown of Elongation factor 2 (Elf2) significantly reduced the survival of An. gambiae (Figs 3C, 4A–4C). The Elf2 is a GTP-binding protein essential for protein synthesis. It catalyses the translocation of 2 tRNAs, and mRNA on the ribosome following peptidyl transfer [63]. Unlike Elf1, Elf2 is encoded by a single gene [64]. Phosphorylation of Elf2 leads to its inactivation, consequently downregulating translation and reducing peptide chain elongation [65]. Considering this crucial role of Elf2 and the uniqueness of its gene, its knockdown would result in reduced synthesis of proteins in the mosquitoes, which ultimately results in the death of the mosquito. Knockdown of Elf2 in mice downregulated expression and synthesis of proteins involved in histone and chromatin binding DNA helicase activity, while synthesis of ribosomal proteins was upregulated [64]. This suggests that Elf2 is indispensable for cell division. It has also been reported that Fragment A of Diphtheria toxin, which is produced by Corynebacterium diphtheriae, inhibits protein synthesis through ADP ribosylation of Elf2 (ADP ribosylation leads to inactivation). Diphtheria toxin causes diphtheria, which results in 5 to 10% death in C. diphtheriae infected patients, and the mortality rate might be up to 20% in children < 5 years or adults > 40 years [66]. Hence, inhibition of Elf2 may lead to death. While it is essential to investigate the effect of the knockdown on the transcriptome of the mosquitoes to identify the mechanism by which the observed death occurred, it is suggested that reducing levels of cell cycle proteins and other crucial proteins might contribute to the observed death. Elf2 has 78.7% (E-value: 0.0) protein sequence identity with its ortholog in human (P13639) and 79.5 gene sequence identity (E-value: 2.5e-94) to the human eukaryotic translation elongation factor 2 gene (EEF2) (NM_001961.4). Hence, caution must be taken in developing inhibitors that can be used as insecticide molecules against this target. Identifying unique and specific features in the protein of mosquitoes compared to humans can aid the development of highly specific inhibitors for this target [67]. For example, studies have shown that selective acetylcholinesterase inhibitors can be designed for An. gambiae by targeting an unpaired cysteine residue present in the mosquito but absent in humans [68, 69]. Sequence alignment of Elf2 amino acid residues from An. gambiae (AGAP009441), An. stephensi (ASTEI20_042603), An. funestus (AFUN2_002633), Aedes aegypti (AAEL004500), Ae. albopictus (AALFPA_058151), Culex quinquefasciatus (CQUJHB017554) and humans (NP_001952.1) provide evidence that selective insecticide development might be possible (S1 Fig). The sequence alignment reveals specific amino residues conserved across the mosquito species but not in human, as well as some residues conserved in the anopheline mosquitoes only. When compared to humans, there is a unique sequence region (TNPDQRD) present in all mosquito sequences aligned which is absent in the human sequence. Such unique residues, among others (in S1 Fig) could be exploited for the development of selective and specific insecticides that are not toxic to humans [67], once their functional role have been better studied. Hence, Elf2 might represent a good target to explore for insecticide development. Similarly, this gene could be a good candidate for RNAi-based pesticide vector control strategies.
Heat shock 70kDa protein 1/8 (HSP) significantly reduced the survival of An. gambiae (Figs 3C, 4D–4F). HSP is a molecular chaperone for protein folding, and its induction has been reported to suppress o’nyong-nyong virus (ONNV) infection. Consequently, its knockdown enhances ONNV replication in Anopheles coluzzii [70]. Subsequently, when ONNV was coinjected with respective dsRNA targeting HSP, survival was observed to be greatly reduced compared to control groups in which ONNV was coinjected with β-galactosidase. When dsRNA targeting HSP alone was injected in mosquitoes, reduced survival was observed, although not as high as in combination with ONVV. This shows that both the reduced HSP levels and its subsequent effect in increasing ONNV level together resulted in an increased mortality rate [70]. Hence, the finding in this study further evidences the essentiality of HSP for the survival of mosquitoes.
Further studies to evaluate the effect of HSP on Plasmodium development would give further insight into how HSP could be manipulated to hamper malaria transmission. HSP has also been shown to be upregulated in DDT-resistant An. funetus in Benin [71]. Similarly, AALB008255 in An. albimanus, which is identical to HSP in its carboxyl end was found to be downregulated in P. berghei infection in the mosquito [72]. There is a need to further investigate the effect of knockdown of this gene on insecticide resistance as well as Plasmodium infection. HSP has 80% identity with heat shock protein family A (HSP70) member 1A and 1B in human (NM_005345.6, and NM_005346.6) and 72.6% with heat shock cognate 71kDA protein isoform 1 in humans (NM_006597.6). Hence, caution should be taken if it is considered a target for insecticide development. Sequence alignment of HSP70 amino acid residues from An. gambiae (AGAP002076), An. stephensi (ASTEI20_036817), An. funestus (AFUN2_003795), Ae. aegypti (AAEL019403), Ae. albopictus (AALFPA_044680), Culex quinquefasciatus (CQUJHB018229) and humans (NP_006588.1 or NP_005336.3) provide evidence that selective insecticide development might be possible (S2 Fig). The sequence alignment reveals specific amino residues conserved across the mosquito species but not in human, as well as some residues conserved in these anopheline mosquitoes only. When compared to humans, there is a unique sequence region (APGAG) present in all mosquito sequences aligned that is absent in the human sequences. Such unique residues, among others (in S2 Fig) could be exploited for the development of selective and specific insecticides that are not toxic to humans [67].
The machine learning approach in this study is based on predicting essential genes across eukaryotes at organismal and cellular levels using CLEARER and an orthology based approach. The approach has proven to be useful for predictions of essential genes in Tribolium castaenum, some of which were experimentally validated to be essential [30]. The essential genes/proteins in this study could serve as probable targets for selective insecticide development, exploiting unique insect specific amino residues present in the targets compared to their human orthologs. Also, they could be targeted for RNAi biopesticide vector control strategies. In addition, these essential genes can be targeted for gene drive vector control strategies such as Cleave and rescue [73, 74], home and rescue gene drive [75].
Other studies are ongoing to develop other models that are conditionally essential. For example, a machine learning model to predict essential development stage and immune response genes in Drosophila melanogaster has been developed [76]. This model could be expanded to An. gambiae to predict conditionally essential genes that could be tested experimentally in the future. Still, a limitation of such prediction studies is, of course, that they typically come along with lists of predictions containing several false positives and missing false negatives. This makes it mandatory to follow up with experimental validations to reduce the false positives. Besides this, the analyses performed in this study was carried out using An. gambiae infected with P. berghei. As a future aspect, it would be intriguing observing perturbation studies in An. gambiae infected with P. falciparum.
Conclusion
The machine learning approach was based on data of six model organisms and was applied to genome data of An. gambiae in a top-down approach. It led to new findings independent of prior expert knowledge, making it a valuable alternative to conservative ways to screen for novel targets in vector control. Of the four genes tested in this study, three were observed to be possible targets for vector control. These three genes were non-redundant. This elucidates the importance of combining computational techniques with experimental techniques in finding targets as non-redundant predicted targets might play a crucial for survival or immunity in the organism. This study provides evidence that HSP and Elf2 are important for survival of An. gambiae, as such, they could serve as possible targets for insecticide development or RNAi-based biopesticides. Similarly, knockdown of arginase was observed to reduce P. berghei oocytes count in An. gambiae, suggesting arginase as a possible transmission-blocking target in mosquitoes. As such, they could be exploited as targets for disease control.
Supporting information
The asterisk sign (*) indicates positions that have single and conserved amino acid residues. The full colon sign (:) indicates conservation between amino acid residues of strongly similar properties. The dot sign (.) indicates conservation between amino acid residues of weakly similar properties. Residues in red boxes are conserved across all mosquito species aligned but not in humans. Residues in blue boxes are conserved in anopheline mosquitoes only.
(TIF)
The asterisk sign (*) indicates positions that have single and conserved amino acid residues. The full colon sign (:) indicates conservation between amino acid residues of strongly similar properties. The dot sign (.) indicates conservation between amino acid residues of weakly similar properties. Residues in red boxes are conserved across all mosquito species aligned but not in humans. Residues in blue boxes are conserved in anopheline mosquitoes only. Residues in green boxes are conserved in Anopheles and Aedes mosquitoes only.
(TIF)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We appreciate Covenant University and University of Camerino for the support provided. We appreciate Prof Annette Habluetzel and Dr Matteo Valzano of University of Camerino for the guides giving on Plasmodium infection. We appreciate Dr Matthew Peirce of University of Perugia for the advice given on dsRNA injections.
Data Availability
The source code used in this study is available at https://github.com/ThomasBeder/CLEARER and archived with complete data sets and trained models at Zenodo https://doi.org/10.5281/zenodo.5557738.
Funding Statement
This research was funded by Deutsche Forschungsgemeinschaft (DFG) and Fogarty National Institutes of Health (NIH) Common Fund. 1. Deutsche Forschungsgemeinschaft (DFG) Authors: RK and EA Grant number: KO 3678/5-1 Full name: Deutsche Forschungsgemeinschaft URL: https://gepris.dfg.de/gepris/projekt/347509908 Did the sponsors or funders play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript? No 2. Fogarty National Institutes of Health (NIH) Common Fund Authors: EA Grant number: 1U2RTW010679 Full name: Fogarty National Institutes of Health (NIH) Common Fund URL: https://reporter.nih.gov/search/4JPGbrv7BEWp23Ineb9CoA/projects Did the sponsors or funders play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript? No.
References
- 1.World Health Organisation. World malaria report 2021. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- 2.Raghavendra K, Barik TK, Reddy B, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108(4):757–79. doi: 10.1007/s00436-010-2232-0 [DOI] [PubMed] [Google Scholar]
- 3.Wiltshire RM, Duman-Scheel M. Advances in oral RNAi for disease vector mosquito research and control. Curr Opin Insect Sci. 2020;40:18–23. doi: 10.1016/j.cois.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Airs PM, Bartholomay LC. RNA interference for mosquito and mosquito-borne disease control. Insects. 2017;8(1). doi: 10.3390/insects8010004 ; PubMed Central PMCID: PMC5371932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billingsley PF, George KI, Eappen AG, Harrell RA, Alford R, Li T, et al. Transient knockdown of Anopheles stephensi LRIM1 using RNAi increases Plasmodium falciparum sporozoite salivary gland infections. Malar J. 2021;20(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simões ML, Caragata EP, Dimopoulos G. Diverse host and restriction factors regulate mosquito-pathogen interactions. Trends Parasitol. 2018;34(7):603–16. doi: 10.1016/j.pt.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Negri A, Ferrari M, Nodari R, Coppa E, Mastrantonio V, Zanzani S, et al. Gene silencing through RNAi and antisense Vivo-Morpholino increases the efficacy of pyrethroids on larvae of Anopheles stephensi. Malar J. 2019;18(1):294–. doi: 10.1186/s12936-019-2925-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pondeville E, David JP, Guittard E, Maria A, Jacques JC, Ranson H, et al. Microarray and RNAi analysis of P450s in Anopheles gambiae male and female steroidogenic tissues: CYP307A1 is required for ecdysteroid synthesis. PLoS One. 2013;8(12):e79861. Epub 2013/12/11. doi: 10.1371/journal.pone.0079861 ; PubMed Central PMCID: PMC3851169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peirce MJ, Mitchell SN, Kakani EG, Scarpelli P, South A, Shaw WR, et al. JNK signaling regulates oviposition in the malaria vector Anopheles gambiae. Sci Rep. 2020;10(1):14344. Epub 2020/09/03. doi: 10.1038/s41598-020-71291-5 ; PubMed Central PMCID: PMC7462981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Tsujimoto H, Huang Y, Rasgon JL, Agre P. Aquaglyceroporin function in the malaria mosquito Anopheles gambiae. Biol Cell. 2016;108:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngai M, McDowell MA. The search for novel insecticide targets in the post-genomics era, with a specific focus on G-protein coupled receptors. Mem Inst Oswaldo Cruz. 2017;112:1–7. doi: 10.1590/0074-02760160345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catteruccia F, Levashina EA. RNAi in the malaria vector, Anopheles gambiae. Therapeutic Applications of RNAi: Springer; 2009. p. 63–75. [DOI] [PubMed] [Google Scholar]
- 13.Huvenne H, Smagghe G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol. 2010;56(3):227–35. doi: 10.1016/j.jinsphys.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Yadav M, Dahiya N, Sehrawat N. Mosquito gene targeted RNAi studies for vector control. Functional & Integrative Genomics. 2023;23(2):180. doi: 10.1007/s10142-023-01072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Cui C, Wang G, Wei G, Bai L, Li Y, et al. Engineered Gut Symbiotic Bacterium-Mediated RNAi for Effective Control of Anopheles Mosquito Larvae. Microbiology Spectrum. 2023;11(4):e01666–23. doi: 10.1128/spectrum.01666-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Deng X, Zhu Q, Wu D, Zhong J, Wen L, et al. The dsRNA delivery, targeting and application in pest control. Agronomy. 2023;13(3):714. [Google Scholar]
- 17.Fei X, Zhang Y, Ding L, Xiao S, Xie X, Li Y, et al. Development of an RNAi-based microalgal larvicide for the control of Aedes aegypti. Parasit Vectors. 2021;14(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher SJ, Reeves PT, Hoang BT, Mitter N. A perspective on RNAi-based biopesticides. Frontiers in plant science. 2020;11:51. doi: 10.3389/fpls.2020.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller R, Bálint M, Hardes K, Hollert H, Klimpel S, Knorr E, et al. RNA interference to combat the Asian tiger mosquito in Europe: A pathway from design of an innovative vector control tool to its application. Biotechnol Adv. 2023:108167. doi: 10.1016/j.biotechadv.2023.108167 [DOI] [PubMed] [Google Scholar]
- 20.Mehlhorn S, Hunnekuhl VS, Geibel S, Nauen R, Bucher G. Establishing RNAi for basic research and pest control and identification of the most efficient target genes for pest control: a brief guide. Frontiers in Zoology. 2021;18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez SBG, Guimarães-Ribeiro V, Rodriguez JVG, Dorand FA, Salles TS, Sá-Guimarães TE, et al. RNAi-based bioinsecticide for Aedes mosquito control. Sci Rep. 2019;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Wang S, Ou R, Samrakandi M, Beerntsen BT, Sayre RT. Development of an RNAi based microalgal larvicide to control mosquitoes. Malaria World J. 2013;4(6):1–7. doi: 10.5281/zenodo.10894766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hapairai LK, Mysore K, Sun L, Li P, Wang C-W, Scheel ND, et al. Characterization of an adulticidal and larvicidal interfering RNA pesticide that targets a conserved sequence in mosquito G protein-coupled dopamine 1 receptor genes. Insect Biochem Mol Biol. 2020;120:103359. doi: 10.1016/j.ibmb.2020.103359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mysore K, Hapairai LK, Sun L, Li P, Wang CW, Scheel ND, et al. Characterization of a dual-action adulticidal and larvicidal interfering RNA pesticide targeting the Shaker gene of multiple disease vector mosquitoes. PLoS Negl Trop Dis. 2020;14(7):e0008479. Epub 2020/07/21. doi: 10.1371/journal.pntd.0008479 ; PubMed Central PMCID: PMC7392347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Wang J, Zhong J, Pan Y. Predicting essential proteins based on weighted degree centrality. IEEE/ACM Trans Comput Biol Bioinform. 2013;11(2):407–18. [DOI] [PubMed] [Google Scholar]
- 26.Oyelade J, Isewon I, Uwoghiren E, Aromolaran O, Oladipupo O. In silico knockout screening of Plasmodium falciparum reactions and prediction of novel essential reactions by analysing the metabolic network. Biomed Res Int. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rancati G, Moffat J, Typas A, Pavelka N. Emerging and evolving concepts in gene essentiality. Nature Reviews Genetics. 2018;19(1):34–49. doi: 10.1038/nrg.2017.74 [DOI] [PubMed] [Google Scholar]
- 28.Adebiyi MO, Ogunlana OO, Adebiyi E, Fatumo S, Rasgon JL, editors. The Anopheles gambiae insecticidal targets made bare by in-silico analysis. International Conference on African Development Issues (CU-ICADI); 2015; African Leadership Development Centre, Covenant University Canaanland, Ota Ogun State, Nigeria. [Google Scholar]
- 29.Adedeji EO, Ogunlana OO, Fatumo S, Aromolaran OT, Beder T, Koenig R, Adebiyi E. The Central Metabolism Model of Anopheles gambiae: A Tool for Understanding Malaria Vector Biology. Biotechnological Approaches to Sustainable Development Goals: Springer; 2023. p. 229–48. [Google Scholar]
- 30.Beder T, Aromolaran O, Dönitz J, Tapanelli S, Adedeji Eunice O, Adebiyi E, et al. Identifying essential genes across eukaryotes by machine learning. NAR Genomics and Bioinformatics. 2021;3(4). doi: 10.1093/nargab/lqab110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charif D, Thioulouse J, Lobry J, Perrière G. Online synonymous codon usage analyses with the ade4 and seqinr packages. Bioinformatics. 2005;21(4):545–7. doi: 10.1093/bioinformatics/bti037 [DOI] [PubMed] [Google Scholar]
- 32.Xiao N, Cao D-S, Zhu M-F, Xu Q-S. protr/ProtrWeb: R package and web server for generating various numerical representation schemes of protein sequences. Bioinformatics. 2015;31(11):1857–9. doi: 10.1093/bioinformatics/btv042 [DOI] [PubMed] [Google Scholar]
- 33.Zhu M, Dong J, Cao D. rDNAse: generating various numerical representation schemes of DNA sequences. R package version. 2016;1(1). [Google Scholar]
- 34.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D13. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007;35(suppl_1):D61–D5. doi: 10.1093/nar/gkl842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402. Epub 1997/09/01. doi: 10.1093/nar/25.17.3389 ; PubMed Central PMCID: PMC146917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinayagam A, König R, Moormann J, Schubert F, Eils R, Glatting K-H, et al. Applying support vector machines for gene ontology based gene function prediction. BMC Bioinformatics. 2004;5(1):1–14. doi: 10.1186/1471-2105-5-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almagro Armenteros JJ, Sønderby CK, Sønderby SK, Nielsen H, Winther O. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics. 2017;33(21):3387–95. doi: 10.1093/bioinformatics/btx431 [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Zhang Z, Jiang S, Li R, Li W, Zhao C, et al. New insights on human essential genes based on integrated analysis and the construction of the HEGIAP web-based platform. Briefings in Bioinformatics. 2020;21(4):1397–410. doi: 10.1093/bib/bbz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W-H, Lu G, Chen X, Zhao X-M, Bork P. OGEE v2: an update of the online gene essentiality database with special focus on differentially essential genes in human cancer cell lines. Nucleic Acids Res. 2016:gkw1013. doi: 10.1093/nar/gkw1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo H, Lin Y, Gao F, Zhang C-T, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014;42(D1):D574–D80. doi: 10.1093/nar/gkt1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo F-B, Dong C, Hua H-L, Liu S, Luo H, Zhang H-W, et al. Accurate prediction of human essential genes using only nucleotide composition and association information. Bioinformatics. 2017;33(12):1758–64. doi: 10.1093/bioinformatics/btx055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. Journal of statistical software. 2010;33(1):1–22. Epub 2010/09/03. doi: 10.1109/TPAMI.2005.127 ; PubMed Central PMCID: PMC2929880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP. SMOTE: synthetic minority over-sampling technique. Journal of artificial intelligence research. 2002;16:321–57. [Google Scholar]
- 45.Kuhn M. Building predictive models in R using the caret package. Journal of statistical software. 2008;28:1–26.27774042 [Google Scholar]
- 46.Raddi G, Barletta ABF, Efremova M, Ramirez JL, Cantera R, Teichmann SA, et al. Mosquito cellular immunity at single-cell resolution. Science. 2020;369(6507):1128–32. doi: 10.1126/science.abc0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raddi G, Barletta ABF, Efremova M, Ramirez JL, Cantera R, Teichmann SA, et al. bulk RNA-seq of Anopheles gambiae hemocytes, guts, and carcasses after blood-feeding, Plasmodium berghei infection, or sugar feeding. 2020. [Google Scholar]
- 48.Mancini MV, Damiani C, Short SM, Cappelli A, Ulissi U, Capone A, et al. Inhibition of Asaia in Adult Mosquitoes Causes Male-Specific Mortality and Diverse Transcriptome Changes. Pathogens. 2020;9(5):380. doi: 10.3390/pathogens9050380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedict M. Methods in Anopheles research. 2015 ed. USA: Malaria research and reference reagent resource center (MR4); 2016. [Google Scholar]
- 50.Cappelli A, Valzano M, Cecarini V, Bozic J, Rossi P, Mensah P, et al. Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit Vectors. 2019;12(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53(282):457–81. [Google Scholar]
- 53.Koletsi D, Pandis N. Survival analysis, part 2: Kaplan-Meier method and the log-rank test. Am J Orthod Dentofacial Orthop. 2017;152(4):569–71. doi: 10.1016/j.ajodo.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 54.R Core Team. R: A Language and Environment for Statistical Computing. 2021. [Google Scholar]
- 55.Bhattacharjee S. Arginase: A Prospective Regulator of Oxidative Stress During Microbial Pathogenesis. Oxidative Stress in Microbial Diseases: Springer; 2019. p. 97–109. [Google Scholar]
- 56.Vincendeau P, Gobert AP, Daulouède S, Moynet D, Mossalayi MD. Arginases in parasitic diseases. Trends Parasitol. 2003;19(1):9–12. doi: 10.1016/s1471-4922(02)00010-7 [DOI] [PubMed] [Google Scholar]
- 57.Angleró-Rodríguez YI, Blumberg BJ, Dong Y, Sandiford SL, Pike A, Clayton AM, Dimopoulos G. A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci Rep. 2016;6(1):34084. doi: 10.1038/srep34084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5(5):e1000423. doi: 10.1371/journal.ppat.1000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95(10):5700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molina-Cruz A, Canepa GE, Alves e Silva TL, Williams AE, Nagyal S, Yenkoidiok-Douti L, et al. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proceedings of the National Academy of Sciences. 2020;117(5):2597–605. doi: 10.1073/pnas.1917042117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou Y, Liu R, Xia M, Sun C, Zhong B, Yu J, et al. Nannocystin ax, an eEF1A inhibitor, induces G1 cell cycle arrest and caspase-independent apoptosis through cyclin D1 downregulation in colon cancer in vivo. Pharmacol Res. 2021;173:105870. doi: 10.1016/j.phrs.2021.105870 [DOI] [PubMed] [Google Scholar]
- 62.Zhou H, Guan Y, Feng M, Fu Y, Tachibana H, Cheng X. Evaluation on Elongation Factor 1 Alpha of Entamoeba histolytica Interaction with the Intermediate Subunit of the Gal/GalNAc Lectin and Actin in Phagocytosis. Pathogens. 2020;9(9):702. doi: 10.3390/pathogens9090702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jørgensen R, Merrill AR, Andersen G. The life and death of translation elongation factor 2. Biochem Soc Trans. 2006;34(1):1–6. doi: 10.1042/BST20060001 [DOI] [PubMed] [Google Scholar]
- 64.Gerashchenko MV, Nesterchuk MV, Smekalova EM, Paulo JA, Kowalski PS, Akulich KA, et al. Translation elongation factor 2 depletion by siRNA in mouse liver leads to mTOR-independent translational upregulation of ribosomal protein genes. Sci Rep. 2020;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor‐2 (eEF2): its regulation and peptide chain elongation. Cell biochemistry and function. 2011;29(3):227–34. doi: 10.1002/cbf.1740 [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Diphtheria. In: Atkinson W, Hamborsky J, Mcintyre La, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 9th ed. Washington DC: Public Health Foundation; 2006. p. 59–70. [Google Scholar]
- 67.Adedeji EO, Ogunlana OO, Fatumo S, Beder T, Ajamma Y, Koenig R, Adebiyi E. Anopheles metabolic proteins in malaria transmission, prevention and control: a review. Parasit Vectors. 2020;13(1):465. doi: 10.1186/s13071-020-04342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dou D, Park JG, Rana S, Madden BJ, Jiang H, Pang Y-P. Novel selective and irreversible mosquito acetylcholinesterase inhibitors for controlling malaria and other mosquito-borne diseases. Sci Rep. 2013;3:1068. doi: 10.1038/srep01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pang Y-P, Ekström F, Polsinelli GA, Gao Y, Rana S, Hua DH, et al. Selective and irreversible inhibitors of mosquito acetylcholinesterases for controlling malaria and other mosquito-borne diseases. PLoS One. 2009;4:e6851. doi: 10.1371/journal.pone.0006851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sim C, Hong YS, Tsetsarkin KA, Vanlandingham DL, Higgs S, Collins FH. Anopheles gambiae heat shock protein cognate 70B impedes o’nyong-nyong virus replication. BMC Genomics. 2007;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riveron JM, Yunta C, Ibrahim SS, Djouaka R, Irving H, Menze BD, et al. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014;15(2):R27. doi: 10.1186/gb-2014-15-2-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarado-Delgado A, Ortiz GP, Tello-López ÁT, Encarnación S, Conde R, Martínez-Batallar ÁG, et al. Infection with Plasmodium berghei ookinetes alters protein expression in the brain of Anopheles albimanus mosquitoes. Parasit Vectors. 2016;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oberhofer G, Ivy T, Hay BA. Gene drive and resilience through renewal with next generation Cleave and Rescue selfish genetic elements. Proceedings of the National Academy of Sciences. 2020;117(16):9013–21. doi: 10.1073/pnas.1921698117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oberhofer G, Ivy T, Hay BA. Split versions of Cleave and Rescue selfish genetic elements for measured self limiting gene drive. PLoS Genet. 2021;17(2):e1009385. doi: 10.1371/journal.pgen.1009385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kandul NP, Liu J, Bennett JB, Marshall JM, Akbari OS. A confinable home-and-rescue gene drive for population modification. Elife. 2021;10:e65939. doi: 10.7554/eLife.65939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aromolaran OT, Isewon I, Adedeji E, Oswald M, Adebiyi E, Koenig R, Oyelade J. Heuristic-enabled active machine learning: A case study of predicting essential developmental stage and immune response genes in Drosophila melanogaster. PLoS One. 2023;18(8):e0288023. doi: 10.1371/journal.pone.0288023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The asterisk sign (*) indicates positions that have single and conserved amino acid residues. The full colon sign (:) indicates conservation between amino acid residues of strongly similar properties. The dot sign (.) indicates conservation between amino acid residues of weakly similar properties. Residues in red boxes are conserved across all mosquito species aligned but not in humans. Residues in blue boxes are conserved in anopheline mosquitoes only.
(TIF)
The asterisk sign (*) indicates positions that have single and conserved amino acid residues. The full colon sign (:) indicates conservation between amino acid residues of strongly similar properties. The dot sign (.) indicates conservation between amino acid residues of weakly similar properties. Residues in red boxes are conserved across all mosquito species aligned but not in humans. Residues in blue boxes are conserved in anopheline mosquitoes only. Residues in green boxes are conserved in Anopheles and Aedes mosquitoes only.
(TIF)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
The source code used in this study is available at https://github.com/ThomasBeder/CLEARER and archived with complete data sets and trained models at Zenodo https://doi.org/10.5281/zenodo.5557738.