Abstract
Background and Objectives
The results of the ULTRA trial showed that ultra-early and short-term treatment with tranexamic acid (TXA) does not improve clinical outcome after aneurysmal subarachnoid hemorrhage (aSAH). Possibly, the lack of a beneficial effect in all patients with aSAH is masked by antagonistic effects of TXA in certain subgroups. In this post hoc subgroup analysis, we investigated the effect of TXA on clinical outcome in patients with good-grade and poor-grade aSAH.
Methods
The ULTRA trial was a multicenter, prospective, randomized, controlled, open-label trial with blinded outcome assessment. Participants received ultra-early and short-term TXA in addition to usual care or usual care only. This post hoc subgroup analysis included only ULTRA participants with confirmed aSAH and available World Federation of Neurosurgical Societies (WFNS) grade on admission. Patients were categorized into those with good-grade (WFNS 1–3) and poor-grade (WFNS 4–5) aSAH. The primary outcome was clinical outcome assessed by the modified Rankin scale (mRS). Odds ratios (ORs) and adjusted ORs (aORs) with 95% CIs were calculated using ordinal regression analyses. Analyses were performed using the as-treated principle. In all patients with aSAH, no significant effect modification of TXA on clinical outcome was observed for admission WFNS grade (p = 0.10).
Results
Of the 812 ULTRA participants, 473 patients had (58%; N = 232 TXA, N = 241 usual care) good-grade and 339 (42%; N = 162 TXA, N = 176 usual care) patients had poor-grade aSAH. In patients with good-grade aSAH, the TXA group had worse clinical outcomes (OR: 0.67, 95% CI 0.48–0.94, aOR 0.68, 95% CI 0.48–0.94) compared with the usual care group. In patients with poor-grade aSAH, clinical outcomes were comparable between treatment groups (OR: 1.04, 95% CI 0.70–1.55, aOR 1.05, 95% CI 0.70–1.56).
Discussion
This post hoc subgroup analysis provides another important argument against the use of TXA treatment in patients with aSAH, by showing worse clinical outcomes in patients with good-grade aSAH treated with TXA and no clinical benefit of TXA in patients with poor-grade aSAH, compared with patients treated with usual care.
Trial Registration Information
ClinicalTrials.gov (NCT02684812; submission date February 18, 2016, first patient enrollment on July 24, 2013).
Classification of Evidence
This study provides Class II evidence that tranexamic acid, given for <24 hours within the first 24 hours, does not improve the 6-month outcome in good-grade or poor initial-grade aneurysmal SAH.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease with high morbidity and mortality. The occurrence of rebleeding is one of the most important factors associated with poor outcome.1 Risk factors of rebleeding include poor neurologic condition on admission, large aneurysm size, and elevated systolic blood pressure.1,2 In the past, antifibrinolytics have been shown to decrease the occurrence of rebleeding; however, a positive effect on clinical outcome could not be established.3,4 The ULTRA trial investigated ultra-early and short-term tranexamic acid (TXA) administration after SAH. The trial was designed to end the decade-long debate on the efficacy of antifibrinolytics on clinical outcome after SAH. The recently published results of the ULTRA trial showed no clinical benefit of TXA after 6 months.5,6 Moreover, patients in the TXA group less often had excellent clinical outcome compared with those in the usual care group. This has led to significant alterations in international guidelines on the management of SAH, which now advise against the routine use of TXA.7,8
Hypothetically, the lack of overall effect of TXA in all patients with SAH may be explained by different effects of TXA in certain subgroups, for example, patients with good and poor neurologic condition on admission. Early brain injury is defined as the initial injury in the first 72 hours after aSAH.9,10 The pathophysiology of early brain injury includes the formation of microthrombi. Theoretically, reduced degradation of microthrombi by TXA may delay or hinder recovery of early brain injury and, thereby, might have a negative effect on clinical outcome. Because patients with poor-grade aSAH have more severe early brain injury, especially these patients may be negatively influenced by administration of TXA. Because the detrimental impact of rebleedings in patients with good-grade aSAH is generally much larger than in patients who are already in a poor condition, one may expect a beneficial effect of TXA on clinical outcome in good-grade patients. Therefore, the primary research question in this post hoc subgroup analysis of the ULTRA trial is as follows: does ultra-early and short-term TXA treatment improve clinical outcome at 6 months in patients with good-grade and poor-grade aSAH?
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol of the ULTRA trial has been previously published.11,12 In brief, the ULTRA trial was a randomized, controlled, multicenter, open-label trial with blinded outcome assessment. The trial was conducted in 8 treatment centers and 16 referral hospitals between July 24, 2013, and January 20, 2020. The study was performed in accordance with the principles of the Declaration of Helsinki and International Conference of Harmonization guidelines for Good Clinical Practice and was registered on ClinicalTrials.gov (NCT02684812). The ethics committee of the Amsterdam University Medical Center (Amsterdam UMC, Amsterdam, the Netherlands) approved the trial protocol (2012_160#2012370). A description of the informed consent procedure has been previously published.11,12
Patients and Intervention
For this post hoc study of the ULTRA trial (NCT02684812), we excluded ULTRA participants without confirmed causative intracranial aneurysm on CT angiography and/or digital subtraction angiography. Patients were allocated (1:1 randomization, stratified by treatment center) to either ultra-early (immediately after SAH diagnosis and within 24 hours of ictus) and short-term (until aneurysm treatment or maximally 24 hours) TXA in addition to usual care or usual care only.11 Patients, investigators, and health care providers were not masked to study drug assignment. For the purpose of this study, patients were categorized into patients with good-grade (WFNS I-III) and poor-grade (WFNS IV-V) aSAH based on the WFNS grade. The WFNS grade was scored based on the Glasgow coma scale score and presence or absence of motor impairment on admission at the referral hospital or, in case of immediate presentation at the treatment center, at the treatment center. Patients of whom the WFNS grade classification was unavailable due to an incomplete neurologic assessment on admission were excluded.
Data Collection
We used prospectively collected baseline characteristics: age, sex, medication use (platelet inhibitors, anticoagulation, and antihypertensive drugs), WFNS grade10 on admission, Fisher grade13 on first noncontrast head CT, and aneurysm location. Other collected data included the following: treatment modality (endovascular, clipping, and none); information on timing of TXA administration and aneurysm treatment; and the occurrence of common complications following aSAH: rebleeding (CT-proven and all rebleedings), hydrocephalus, delayed cerebral ischemia, thromboembolic complications during endovascular treatment, cerebral infarction related to clipping procedure, per-procedural rupture, extracranial thrombosis, hemorrhagic complications, severe hyponatremia, pneumonia, infectious meningitis, urinary tract infection, seizures, delirium, and the mRS score and mortality at 6 months after aSAH (eMethods, page 1–2).5,6
Outcomes
The primary outcome was the mRS score, ranging from 0 (no symptoms) to 6 (death), at 6 months after aSAH. Secondary outcomes included good (mRS 0–3) and excellent (mRS 0–2) clinical outcomes 6 months after aSAH, all-cause mortality at 30 days and 6 months after aSAH, and the aforementioned complications following aSAH.
Statistical Analysis
All analyses were performed for patients with good-grade (WFNS I-III) and poor-grade (WFNS IV-V) aSAH separately. Because the selection of subgroups undoes the randomization, we deemed it more informative and methodologically robust to use the as-treated principle. Patients who actually received TXA treatment in addition to usual care were assigned to the “TXA group” and patients who received usual care only were assigned to the “usual care group.” Hereby, we aimed to provide a more accurate representation of the impact of TXA on clinical outcome in patients with good-grade and poor-grade aSAH. Normality of continuous data was tested using the Shapiro-Wilk test (statistic test threshold of 0.9). Baseline characteristics were compared between the TXA group and usual care group using the Chi-Square test, independent sample t test, Fisher exact test, or Mann-Whitney U test, depending on the distribution of the data. The primary outcome was analyzed using ordinal regression analysis. Secondary outcomes were analyzed using logistic regression analyses. Odds ratios (ORs) with corresponding 95% confidence interval (CIs) were calculated. For the primary outcome, good clinical outcome, excellent clinical outcome, and mortality at 30 days and 6 months, adjusted ORs (aOR) with 95% CIs were calculated adjusting for treatment center and for baseline characteristics with a p value <0.2. Effect modification of TXA by admission WFNS grade was tested using ordinal regression analyses with clinical outcome as dependent variable and as-treated treatment group (TXA or usual care), admission WFNS grade, and an interaction term (as-treated treatment group * admission WFNS grade) as independent variables, using all patients with aSAH. If the calculated p value of the interaction term was <0.05, effect modification was considered significant. Analyses were also performed in patient groups according to the intention-to-treat principle, in which patients were analyzed according to the group they were allocated to by randomization. Missing data were handled using a pairwise deletion method, in which incomplete cases were deleted on an analysis-by-analysis basis, and frequencies were calculated using the total number of patients with available data. Statistical analyses were performed using the IBM SPSS Statistics version 28 software (IBM Corporation, Armonk, NY).
Data Availability
The authors have reported all relevant data used to conduct the research. All data requests should be submitted to the Principal Investigator (DV) for consideration. Access to anonymized data may be granted following review.
Results
Patients
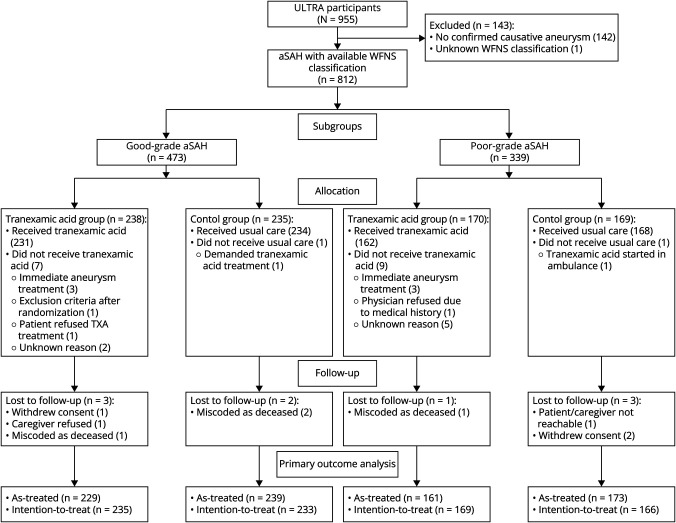
The ULTRA trial enrolled 955 participants, of whom 813 had aSAH. One patient with Glasgow coma scale score 13 could not be classified into good-grade or poor-grade aSAH due to missing information about focal neurologic impairment and was therefore excluded from this analysis. The remaining 812 patients were classified into those with good-grade (N = 473, 58%) or poor-grade (N = 339, 42%) aSAH. The mRS score at follow-up was available in 803 (99%) patients (Figure 1).
Figure 1. Flow Diagram of Included Patients.
Intervention
In 1 patient, allocated to the TXA group, it was uncertain whether TXA was administered, and this patient was therefore excluded from the as-treated analysis. Of patients with good-grade aSAH, 232 (49%) received TXA treatment in addition to usual care and 241 (51%) received usual care only. TXA treatment was administered within a median of 78 (interquartile range [IQR] 50–139) minutes after confirmation of SAH. Aneurysm treatment was performed within a median of 15 (IQR 7–20) hours after SAH diagnosis. Of patients with poor-grade aSAH, 162 (48%) received TXA treatment in addition to usual care and 176 (52%) received usual care only. TXA treatment was administered at a median of 70 (IQR 47–124) minutes after confirmation of SAH. Aneurysm treatment was performed at a median of 12 (IQR 4–23) hours after SAH diagnosis. Baseline characteristics did not differ between treatment groups in patients with good-grade and poor-grade aSAH (Table 1). In the intention-to-treat analyses of baseline characteristics, only Fisher grade significantly differ between both treatment groups (eTable 1).
Table 1.
Baseline Characteristics of Patients With Good-Grade and Poor-Grade aSAH According to the As-Treated Principle
| Good-grade aSAH WFNS I-III (N = 473) |
Poor-grade aSAH WFNS IV-V (N = 338) |
|||||
| TXA group (N = 232) | Usual care group (N = 241) | p Value | TXA group (N = 162) | Usual care group (N = 176) | p Value | |
| Age, mean (SD) | 58 (13) | 58 (13) | 0.79 | 59 (13) | 59 (12) | 0.77 |
| Female | 163 (70) | 169 (70) | 1.00 | 122 (75) | 122 (69) | 0.23 |
| Fisher grade | 0.44 | 0.16 | ||||
| II | 20 (9) | 14 (6) | 1 (1) | 1 (1) | ||
| III | 79 (34) | 90 (37) | 19 (12) | 34 (19) | ||
| IV | 135 (57) | 137 (57) | 142 (88) | 141 (80) | ||
| Medication use | ||||||
| Platelet inhibitor | 24 (10) | 24 (10) | 0.88 | 24 (15) | 25 (14) | 0.88 |
| Anticoagulation | 8 (4) | 7 (3) | 0.80 | 5 (3) | 7 (4) | 0.77 |
| Antihypertensive drugs | 58 (25) | 54 (22) | 0.52 | 36 (23) | 40 (23) | 1.00 |
| None of the above | 156 (68) | 170 (71) | 0.49 | 112 (70) | 118 (67) | 0.64 |
| Location of aneurysm | 0.24 | 0.41 | ||||
| Anterior circulation | 183 (79) | 202 (84) | 134 (83) | 139 (79) | ||
| Posterior circulation | 48 (21) | 39 (16) | 28 (17) | 37 (21) | ||
| Treatment modality | 0.79 | 0.55 | ||||
| Endovascular | 166 (72) | 168 (70) | 98 (61) | 97 (55) | ||
| Clipping | 50 (22) | 58 (24) | 30 (19) | 36 (21) | ||
| None | 16 (7) | 15 (6) | 33 (21) | 43 (24) | ||
Data presented as n (%), unless noted otherwise. Percentages may not total 100 because of rounding. The as-treated analyses included 811 patients (n = 1 patient of whom it was unknown whether TXA was administered or not). Missing data in patients with good grade aSAH: medication use, N = 1 missing. Missing data in patients with poor-grade aSAH: medication use, N = 3 missing; location of aneurysm, N = 1 missing; and treatment modality, N = 1 missing.
Outcomes
Patients With Good-Grade aSAH
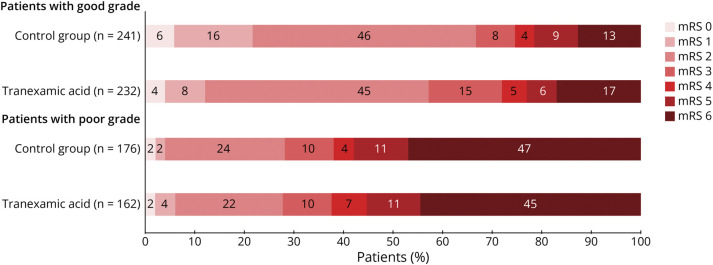
The mRS score at 6 months after aSAH was available for 468 of 473 (99%) patients with good-grade aSAH. The overall distribution of mRS scores at 6 months showed that patients in the TXA group had worse clinical outcome than patients in the usual care group (OR 0.67, 95% CI 0.48–0.94; aOR, adjusted for treatment center, 0.68, 95% CI 0.48–0.94 (Figure 2). None of the baseline characteristics had a p value <0.20; therefore, no adjustment was made for baseline characteristics. Patients in the TXA group less often had excellent clinical outcome than patients in the usual care group (133/232, 58% vs 161/241, 67%; aOR, adjusted for treatment center, 0.67, 95% CI 0.46–0.97; Table 2). Other secondary outcomes and the occurrence of rebleeding and ischemic/thrombotic complications did not differ significantly between treatment groups (Tables 2 and 3). Hydrocephalus more frequently occurred in the TXA group (138/232, 60%) than in the usual care group (121/241, 50%), OR 1.46, 95% CI 1.01–2.10. The frequency of interventions for hydrocephalus, including lumbar punction, external lumbar drainage, external ventricular drainage, and permanent ventriculoperitoneal shunting, did not significantly differ between the treatment groups (eTable 2).
Figure 2. Distribution of Modified Rankin Scale Score at 6 Months in Both Treatment Groups, Separately Analyzed for Patients With Good-Grade and Poor-Grade aSAH, Using the As-Treated Principle.
Stacked bar chart of scores on the modified Rankin scale (0–6). A score of 0 indicates no symptoms, 1 no clinically significant disability, 2 slight disability (patient is able to look after own affairs without assistance, but is unable to perform all previous activities), 3 moderate disability (patient requires some help but is able to walk unassisted), 4 moderately severe disability (patient is unable to attend to bodily needs without assistance and unable to walk unassisted), 5 severe disability (patient requires constant nursing care and attention), and 6 death. In good-grade patients, the overall distribution of the mRS scores 6 months after aSAH with ordinal regression analysis differed significantly between the TXA and usual care groups, showing worse mRS scores in the TXA group (OR 0.67, 95% CI 0.48–0.94; aOR 0.68, 95% CI 0.48–0.94, adjusted for treatment center). In poor-grade patients, the overall distribution of mRS scores 6 months after aSAH with ordinal regression analysis showed no significant differences between the TXA and usual care groups, OR 1.04, 95% CI 0.70–1.55, neither after adjustment for treatment center, aOR 1.05, 95% CI 0.70–1.56. aSAH = aneurysmal subarachnoid hemorrhage; mRS = modified Rankin scale; TXA = tranexamic acid.
Table 2.
Primary and Secondary Outcomes for Patients With Good-Grade and Poor-Grade aSAH According to the As-Treated Principle
| Good-grade aSAH WFNS I-III (N = 473) |
Poor-grade aSAH WFNS IV-V (N = 338) |
|||||
| TXA group (N = 232) | Usual care group (N = 241) | OR (95% CI) aOR (95% CI) | TXA group (N = 162) | Usual care group (N = 176) | OR (95% CI) aOR (95% CI) | |
| Primary outcome | ||||||
| mRS scores at 6 moa | 0.67 (0.48–0.94) 0.68 (0.48–0.94) |
1.04 (0.70–1.55) 1.05 (0.70–1.56) |
||||
| mRS 0 | 10 (4) | 13 (6) | 3 (2) | 3 (2) | ||
| mRS 1 | 19 (8) | 38 (16) | 6 (4) | 3 (2) | ||
| mRS 2 | 104 (45) | 110 (46) | 35 (22) | 42 (24) | ||
| mRS 3 | 34 (15) | 18 (8) | 16 (10) | 17 (10) | ||
| mRS 4 | 11 (5) | 9 (4) | 11 (7) | 7 (4) | ||
| mRS 5 | 13 (6) | 21 (9) | 18 (11) | 19 (11) | ||
| mRS 6 | 38 (17) | 30 (13) | 72 (45) | 82 (47) | ||
| Secondary outcomes | ||||||
| Good clinical outcomea | 167 (73) | 179 (75) | 0.90 (0.60–1.36) 0.90 (0.60–1.36) |
60 (37) | 65 (38) | 0.99 (0.63–1.54) 0.98 (0.63–1.56) |
| Excellent clinical outcomea | 133 (58) | 161 (67) | 0.67 (0.46–0.98) 0.67 (0.46–0.97) |
44 (27) | 48 (28) | 0.98 (0.61–1.58) 0.97 (0.59–1.59) |
| All-cause mortality at 30 d | 30 (13) | 27 (11) | 1.18 (0.68–2.05) 1.18 (0.67–2.07) |
62 (38) | 72 (41) | 0.90 (0.58–1.39) 0.92 (0.59–1.44) |
| All-cause mortality at 6 mo | 38 (16) | 30 (12) | 1.38 (0.82–2.31) 1.39 (0.82–2.34) |
72 (44) | 82 (47) | 0.92 (0.60–1.41) 0.93 (0.60–1.44) |
Data presented as n (%), unless noted otherwise. Percentages may not total 100 because of rounding. Odds ratio (OR), adjusted odds ratio (aOR; adjusted for treatment center in both good-grade and poor-grade patients), 95% confidence interval (95% CI). The as-treated analyses included 811 patients (n = 1 patient of whom it was unknown whether TXA was administered or not).
In good-grade aSAH group: N = 5 patients lost to follow-up (N = 229 in the TXA group and N = 239 in the usual care group). In poor-grade aSAH group: N = 4 patients lost to follow-up (N = 161 in the TXA group and N = 173 in the usual care group).
Table 3.
Frequency of Adverse Events of Patients With Good-Grade and Poor-Grade aSAH According to the As-Treated Principle
| Good-grade aSAH WFNS I-III (N = 473) |
Poor-grade aSAH WFNS IV-V (N = 338) |
|||||
| TXA group (N = 232) | Usual care group (N = 241) | OR (95% CI) | TXA group (N = 162) | Usual care group (N = 176) | OR (95% CI) | |
| All rebleedings before aneurysm treatment | 24 (10) | 31 (13) | 0.78 (0.44–1.38) | 22 (14) | 34 (19) | 0.66 (0.37–1.18) |
| CT-proven rebleedings before aneurysm treatment | 23 (10) | 29 (12) | 0.80 (0.45–1.44) | 15 (9) | 28 (16) | 0.54 (0.28–1.05) |
| Hydrocephalus | 138 (60) | 121 (50) | 1.46 (1.01–2.10) | 112 (69) | 126 (72) | 0.89 (0.56–1.42) |
| Delayed cerebral ischemia | 60 (26) | 60 (25) | 1.05 (0.70–1.59) | 38 (24) | 48 (27) | 0.81 (0.50–1.33) |
| Thromboembolic complications during endovascular treatment | 18 (11) | 24 (14) | 0.73 (0.38–1.40) | 11 (11) | 9 (9) | 1.24 (0.49–3.13) |
| Cerebral infarction related to clipping procedure | 14 (28) | 9 (16) | 2.12 (0.83–5.43) | 8 (27) | 9 (14) | 1.05 (0.35–3.18) |
| Per-procedural rupture | ||||||
| Coiling | 8 (5) | 7 (4) | 1.17 (0.41–3.29) | 7 (7) | 6 (6) | 1.17 (0.38–3.61) |
| Clipping | 9 (18) | 15 (26) | 0.63 (0.25–1.60) | 7 (23) | 8 (22) | 1.07 (0.34–3.38) |
| Extracranial thrombosisa | 3 (1) | 1 (0) | 3.14 (0.33–30.5) | 2 (1) | 6 (3) | 0.35 (0.70–1.78) |
| Hemorrhagic complication | 14 (6) | 18 (8) | 0.80 (0.39–1.64) | 12 (8) | 16 (9) | 0.81 (0.37–1.76) |
| Severe hyponatraemia | 7 (3) | 7 (3) | 1.04 (0.36–3.01) | 4 (3) | 1 (1) | 4.43 (0.49–40.1) |
| Pneumonia | 19 (8) | 30 (12) | 0.63 (0.34–1.15) | 32 (20) | 38 (22) | 0.89 (0.53–1.52) |
| Infectious meningitis | 18 (8) | 19 (8) | 0.98 (0.50–1.92) | 9 (6) | 13 (7) | 0.74 (0.31–1.78) |
| Urinary tract infection | 21 (9) | 33 (14) | 0.63 (0.35–1.12) | 15 (9) | 9 (5) | 1.89 (0.81–4.46) |
| Seizure | 24 (10) | 24 (10) | 1.04 (0.57–1.90) | 28 (17) | 17 (10) | 1.95 (1.03–3.73) |
| Delirium | 31 (13) | 33 (14) | 0.97 (0.57–1.65) | 23 (14) | 23 (13) | 1.10 (0.59–2.05) |
Data presented as n (%), unless noted otherwise. Odds ratio (OR), adjusted odds ratio (aOR), 95% confidence interval (95% CI). The as-treated analyses included 811 patients (n = 1 patient of whom it was unknown whether TXA was administered or not).
Missing data in poor grade aSAH: delayed cerebral ischemia, N = 1 missing, cerebral infarction related to clipping procedure, N = 1 missing, and hemorrhagic complication, N = 1 missing.
Extracranial thrombosis includes deep venous thrombosis and pulmonary embolism.
In the intention-to-treat analyses, the OR for hydrocephalus was not statistically significant (OR 1.38, 95% CI 0.97–2.00). Other results were similar to the as-treated analyses (eTable 3–4 and eFigure 1).
Patients With Poor-Grade aSAH
The mRS score at 6 months after aSAH was obtained for 334 of 339 (99%) patients with poor-grade aSAH. The overall distribution of mRS scores at 6 months showed no significant differences between the TXA and usual care groups (OR: 1.04, 95% CI 0.70–1.55; aOR, adjusted for treatment center: 1.05, 95% CI 0.70–1.56; Figure 2). None of the baseline characteristics had a p value <0.20; therefore, no adjustment was made for baseline characteristics. Secondary outcomes also did not differ significantly (Table 2). Patients in the TXA group more frequently had seizures than patients in the usual care group (28/162, 17% vs 17/176, 10%; OR 1.95, 95% CI 1.03–3.73; Table 3). The occurrence of rebleeding, ischemic/thrombotic, and all other complications were not different between treatment groups. The intention-to-treat analyses showed similar results as the as-treated analyses (eTables 3–4 and eFigure 1). In all patients with aSAH, no significant effect modification of TXA on clinical outcome was observed for admission WFNS grade (p = 0.10).
Discussion
This post hoc study shows worse clinical outcome in patients with good-grade aSAH treated with TXA and no clinical benefit of TXA treatment in patients with poor-grade aSAH, compared with patients treated with usual care. The recently published results of the ULTRA trial have further elucidated the decade-long debate on the efficacy of antifibrinolytics on clinical outcome after aSAH by showing no clinical benefit of ultra-early and short-term TXA treatment. Possibly, an overall effect was masked by different effects in certain subgroups of patients. We hypothesized a harmful effect of TXA in patients with poor-grade aSAH and a beneficial effect of TXA in patients with good-grade aSAH. However, the results of our post hoc subgroup analyses show the contrary: TXA seems to be harmful in patients with good-grade aSAH, based on worse mRS scores and less excellent clinical outcome in patients treated with TXA compared with patients treated with usual care. This could not be explained by differences in the occurrence of rebleeding or ischemic/thromboembolic complications. In patients with poor-grade aSAH, clinical outcome did not differ significantly between treatment groups.
Evaluation of common complications following aSAH in patients with good-grade aSAH revealed a higher occurrence of hydrocephalus in the TXA group. The mechanism of action of TXA is to prevent rebleeding by reducing blood clot breakdown. Hypothetically, a reduction of blood clot breakdown in the subarachnoid space may impede absorption of the CSF and, as a consequence, may lead to hydrocephalus. In 2022, a systematic review with meta-analyses, including the ULTRA trial and 6 other randomized controlled trials, showed a significantly 13% higher pooled incidence of hydrocephalus in patients with SAH treated with TXA compared with that in controls.14 On the contrary, a 2022 Cochrane review, including the ULTRA trial and 5 other randomized controlled trials, showed a RR of 1.09, 95% CI 0.9 to 1.20 for hydrocephalus in patients treated with antifibrinolytic treatment vs controls.4 Whether the observed higher occurrence of hydrocephalus in patients with good-grade aSAH using TXA is clinically relevant cannot be concluded based on our results. In patients with poor-grade aSAH, the incidence of hydrocephalus is known to be high already (in ULTRA participants with aSAH: 64% in the TXA group and 59% in the usual care group6; in patients with poor-grade aSAH: 69% in the TXA and 72% in the usual care group). The relatively high incidence of hydrocephalus in patients with poor-grade aSAH could mask an additional harmful effect of tranexamic acid.
A higher occurrence of seizures was observed in patients with poor-grade aSAH treated with TXA compared with those treated with usual care. Seizures following TXA administration have been repeatedly reported in studies on cardiac surgery and after accidental high-dose intrathecal TXA injections.15 This phenomenon has previously been explained by the structural similarity of TXA and both glycine and GABA receptors.16 Possibly, the seizure-inducing effect of TXA may be more pronounced in patients with poor-grade aSAH because the blood-brain barrier is often disturbed in these patients. Because poor-grade patients are associated with more early brain injury, they may therefore be more prone to TXA-induced seizures than patients with good-grade aSAH.
Strengths of this study are the large sample size, nationwide participation, blinded outcome assessment, and the negligible number of patients who were lost to follow-up. Our study has several limitations. Because this study is a post hoc analysis, it may be underpowered for the addressed research questions. Only a selection of ULTRA participants was used, which could lead to differences between treatment groups. However, baseline characteristics were evenly distributed among the treatment groups. The Netherlands is a very small and densely populated country, resulting in a relatively small time interval between aSAH ictus and aneurysm obliteration. Other countries may have larger ictus to aneurysm treatment time intervals with a subsequent higher risk of rebleeding. Therefore, this study may lack generalizability. Last, treatment and safety outcomes were not blinded.
This post hoc subgroup analysis provides another important argument against the use of TXA in patients with aSAH, by showing worse clinical outcomes in patients with good-grade aSAH treated with TXA and no clinical benefit of TXA treatment in patients with poor-grade aSAH, compared with patients treated with usual care. The use of TXA may potentially lead to more frequent hydrocephalus in patients with good-grade aSAH and seizures in patients with poor-grade aSAH support our admonition.
Acknowledgment
Foremost, the authors thank the patients participating in this trial, without whom this trial would have not been possible. The authors also thank the research teams in all participating centers who contributed to the data collection.
Glossary
- aOR
adjusted OR
- aSAH
aneurysmal subarachnoid hemorrhage
- IQR
interquartile range
- mRS
modified Rankin scale
- OR
odds ratio
- TXA
tranexamic acid
- WFNS
World Federation of Neurosurgical Societies
Appendix 1. Authors
| Name | Location | Contribution |
| Maud A. Tjerkstra, MD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| René Post, MD, PhD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Menno R. Germans, MD, PhD | Department of Neurosurgery, Clinical Neuroscience Centre, University Hospital Zurich | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and study concept or design |
| Mervyn D.I. Vergouwen, MD, PhD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Centre, University Medical Centre Utrecht | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Korne Jellema, MD, PhD | Department of Neurology, Haaglanden Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Radboud W. Koot, MD, PhD | Department of Neurosurgery, Leids University Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Nyika D. Kruyt, MD, PhD | Department of Neurology, Leids University Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Jasper F.C. Wolfs, MD, PhD | Department of Neurosurgery, Haaglanden Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Frits C. De Beer, MD | Department of Neurosurgery, ISALA Hospital | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Hans Kieft, MD, PhD | Department of Intensive Care, ISALA Hospital | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Dharmin Nanda, MD, PhD | Department of Neurosurgery, ISALA Hospital | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Bram Van Der Pol, MD, PhD | Department of Neurosurgery, Elisabeth Tweesteden Ziekenhuis | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Gerwin Roks, PhD | Department of Neurology, Elisabeth Tweesteden Ziekenhuis | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Frank De Beer, MD | Department of Neurology, Spaarne Gasthuis | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Loes J. Reichman, MD | Department of Neurology, Ziekenhuisgroep Twente | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Paul J.A.M. Brouwers, MD, PhD | Department of Neurology, Medisch Spectrum Twente | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Vincent I.H. Kwa, MD, PhD | Department of Neurology, OLVG | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Taco C. Van Der Ree, MD, PhD | Department of Neurology, Dijklander Hospital | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Henri Paul Bienfait, MD, PhD | Department of Neurology, Gelre Hospital | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Hieronymus D. Boogaarts, MD, PhD | Department of Neurosurgery, Radboud University Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Catharina J.M. Klijn, MD, PhD | Department of Neurology, Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Centre | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data |
| Victoria Visser, MD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content |
| René van den Berg, MD, PhD | Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Bert A. Coert, MD, PhD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Janneke Horn, MD, PhD | Department of Intensive Care, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and study concept or design |
| Charles B.L.M. Majoie, MD, PhD | Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Gabriël J.E. Rinkel, MD, PhD | Department of Neurology and Neurosurgery, UMC Utrecht Brain Centre, University Medical Centre Utrecht | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| Yvo B.W.E.M. Roos, MD, PhD | Department of Neurology, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; study concept or design |
| W. Peter Vandertop, MD, PhD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Dagmar Verbaan, MD, PhD | Department of Neurosurgery, Amsterdam UMC, University of Amsterdam | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Appendix 2. Coinvestigators
| Coinvestigators are listed at Neurology.org. |
Footnotes
Editorial, page e209327
Study Funding
The ULTRA trial was funded by the Fonds NutsOhra (project 1202-31). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Disclosure
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: M.D.I. Vergouwen reports a grant from the Dutch Heart Foundation (Clinical Established Investigator grant 2018T076); J.F.C. Wolfs reports personal fees from Consultant Nuvasive, personal fees from Zimmer Biomet, personal fees from Safe Orthopaedics, and personal fees from EIT/Johnson and Johnson, outside the submitted work; H.D. Boogaarts reports consulting fees paid to the Department of Neurosurgery, Radboud University Medical Center Nijmegen from Stryker neurovascular; R. van den Berg reports consulting fees for unrelated research and teaching activities from Cerenovus Neurovascular; C.B.L.M. Majoie reports grants from CVON/Dutch Heart Foundation, grants from European Commission, grants from Dutch Health Evaluation Program, grants from TWIN Foundation, and grants from Stryker, outside the submitted work and is a Shareholder of Nico-Lab, a company that focuses on the use of artificial intelligence for medical image analysis; G.J.E. Rinkel reports no disclosures relevant to the manuscript; Y.B.W.E.M. Roos is a minor stockholder of Nico-Lab; D. Verbaan reports funding of Fonds NutsOhra; the other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Larsen CC, Astrup J. Rebleeding after aneurysmal subarachnoid hemorrhage: a literature review. World Neurosurg. 2013;79(2):307-312. doi: 10.1016/j.wneu.2012.06.023 [DOI] [PubMed] [Google Scholar]
- 2.Guo L-M, Zhou H-Y, Xu J-W, Wang Y, Qiu Y-M, Jiang J-Y. Risk factors related to aneurysmal rebleeding. World Neurosurg. 2011;76(3-4):292-298. doi: 10.1016/j.wneu.2011.03.025 [DOI] [PubMed] [Google Scholar]
- 3.Baharoglu MI, Germans MR, Rinkel GJ, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013;2013(8):CD001245. doi: 10.1002/14651858.cd001245.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Germans MR, Dronkers WJ, Baharoglu MI, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2022;11(11):CD001245. doi: 10.1002/14651858.CD001245.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post R, Germans MR, Tjerkstra MA, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet (London, England) 2021;397(10269):112-118. doi: 10.1016/S0140-6736(20)32518-6 [DOI] [PubMed] [Google Scholar]
- 6.Tjerkstra MA, Post R, Germans MR, et al. Tranexamic acid after aneurysmal subarachnoid hemorrhage: post-hoc analysis of the ULTRA trial. Neurology. 2022;99(23):e2605-e2614. doi: 10.1212/WNL.0000000000201160 [DOI] [PubMed] [Google Scholar]
- 7.Hoh BL, Ko NU, Amin-Hanjani S, et al. 2023 guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023;54(7):e314–e370. doi: 10.1161/STR.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 8.Treggiari MM, Rabinstein AA, Busl KM, et al. Guidelines for the neurocritical care management of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2023;39(1):1-28. doi: 10.1007/s12028-023-01713-5 [DOI] [PubMed] [Google Scholar]
- 9.Rass V, Helbok R. Early brain injury after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep. 2019;19(10):78. doi: 10.1007/s11910-019-0990-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Report of World Federation of neurological Surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985-986. [DOI] [PubMed] [Google Scholar]
- 11.Germans MR, Post R, Coert BA, Rinkel GJ, Vandertop WP, Verbaan D. Ultra-early tranexamic acid after subarachnoid hemorrhage (ULTRA): study protocol for a randomized controlled trial. Trials. 2013;14(1):143. doi: 10.1186/1745-6215-14-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post R, Germans MR, Coert BA, Rinkel GJ, Vandertop WP, Verbaan D. Update of the ULtra-early TRranexamic Acid after Subarachnoid Hemorrhage (ULTRA) trial: statistical analysis plan. Trials. 2020;21(1):199. doi: 10.1186/s13063-020-4118-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1-9. doi: 10.1227/00006123-198001000-00001 [DOI] [PubMed] [Google Scholar]
- 14.Ren J, Qian D, Wu J, et al. Safety and efficacy of tranexamic acid in aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. systematic review. Front Neurol. 2022;12:710495. doi: 10.3389/fneur.2021.710495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng W, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiology Intensive Ther. 2015;47(4):339-350. doi: 10.5603/AIT.a2015.0011 [DOI] [PubMed] [Google Scholar]
- 16.de Faria JL, da Silva Brito J, Costa e Silva LT, et al. Tranexamic acid in Neurosurgery: a controversy indication—review. Neurosurg Rev. 2021;44(3):1287-1298. doi: 10.1007/s10143-020-01324-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have reported all relevant data used to conduct the research. All data requests should be submitted to the Principal Investigator (DV) for consideration. Access to anonymized data may be granted following review.