Chemopreventive agents show promise for preventing and reversing cancer development
Chemoprevention of cancer aims to prevent, arrest, or reverse either the initiation phase of carcinogenesis or the progression of neoplastic cells to cancer. It has been an active area of research for several decades; the use of retinoids to prevent cancer of the head and neck is a notable example.1 Chemoprevention is widely used and readily accepted by doctors and patients in the form of drugs that lower cholesterol concentrations and blood pressure to reduce the risk of cardiovascular disease. It can also be used in some apparently healthy people at risk of cancer to prevent or reduce their risk of developing invasive disease. The biomedical community needs to recognise and advocate approaches to prevent cancer with the same enthusiasm that it currently directs towards treating it.
Summary points
Cancer is a multistage disease, not a single event, and doctors should emphasise cancer prevention in addition to cancer treatment and cure
Chemoprevention with naturally occurring (many dietary) and synthetic agents shows promise for preventing, arresting, and reversing cancer development
Chemopreventive agents must have low toxicities compared with chemotherapeutic agents used in cancer patients
Physicians should identify patients at high risk of cancer who might benefit from participation in chemoprevention trials
Validation of surrogate endpoint biomarkers for clinical cancer is essential to reduce size and duration of chemoprevention trials
Methods
I searched the databases PubMed and CANCERLIT for the period from 1 January 1996 to 31 July 2001 using the key words “chemoprevention” and “neoplasms.” I used recent reviews identified by these searches, plus several archived journal articles and textbooks on chemoprevention available at the US National Library of Medicine, to develop an overview of cancer chemoprevention.
Identifying suitable chemopreventive agents
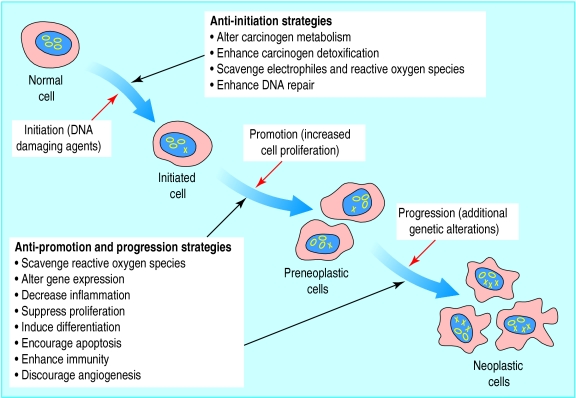
Research into chemoprevention uses a systematic strategy that begins by surveying the results of epidemiological, laboratory, and clinical research for compounds, both naturally occurring and synthetic, that seem to inhibit carcinogenesis. Many compounds, belonging to diverse structural and functional chemical classes, have been identified as potential chemopreventive agents. These include vitamins and minerals (such as folate, vitamin E, vitamin D, calcium, and selenium); naturally occurring phytochemicals (such as curcumin, genistein, indole-3-carbinol, and l-perillyl alcohol); and synthetic compounds (such as retinoids, selective oestrogen receptor modulators, and cyclo-oxygenase-2 inhibitors) (see table A on bmj.com). Several of these potential agents have been investigated in studies of chemoprevention of colorectal cancer.2 Chemopreventive agents might reduce the cancer risk through various mechanisms and different stages of carcinogenesis (fig 1).3,4
Figure 1.
Multistage carcinogenesis: processes and prevention strategies. The initiation stage is characterised by the conversion of a normal cell to an initiated cell in response to DNA damaging agents (genetic damage indicated by an X). The promotion stage is characterised by the transformation of an initiated cell into a population of preneoplastic cells, a result of alterations in gene expression and cell proliferation. The progression stage involves the transformation of the preneoplastic cells to a neoplastic cell population as a result of additional genetic alterations. (Adapted from Hursting et al (1999)4 with authors' permission)
Evidence from epidemiological and laboratory studies
Epidemiological studies into diet and cancer development are invaluable for giving clues about which dietary components may be effective chemopreventive agents.5 One review of more than 250 case-control and cohort studies found that data overwhelmingly supported an inverse association between intake of fruit and vegetables and cancer risk, with associations more consistently observed for vegetables than for fruit.6 Numerous components found in fruit and vegetables might contribute to their ability to reduce the risk of cancer, including dietary fibre, micronutrients, and various phytochemicals, as well as interactions among the components.
Plant derived foods contain thousands of chemically dissimilar phytochemicals, many of which have been investigated in studies in vitro and in vivo to determine their effects on cancer risk and their related mechanisms of action.7–9 In one study, for example, diallyl sulphide (found in allium vegetables such as garlic and onion) seemed to suppress cell division in human colon tumour cells by interfering with the cell cycle; cells remained in the inactive G phase instead of moving to the M phase, where mitosis occurs (fig 2).10 In another example, soybean phytochemicals (such as genistein) may inhibit the growth of prostate tumours through reduced cell proliferation and angiogenesis and increased apoptosis.11,12
Figure 2.
Garlic (Allium sativum) contains chemicals that suppress cell division in human colon tumour cells by interfering with the cell cycle
Evidence from systematic evaluation of agent classes
Chemopreventive agents can be identified by systematic evaluation of classes of agents that act at specific molecular targets, using laboratory assays to characterise their mechanisms of action with respect to cancer. Some agents that are studied by these so called mechanistic assays are signal transduction modulators, hormone modulators, and anti-inflammatories (which inhibit promotion and progression of neoplasia), antimutagens (which inhibit initiation), and antioxidants (which inhibit initiation and promotion).13 Such systematic evaluations can provide additional information on the chemopreventive potential of phytochemicals initially identified through epidemiological and laboratory research.
Evidence from cancer treatment
Strategies developed for the treatment of cancer have provided indications for the potential chemopreventive value of certain agents used in treatment—for example, finasteride (a 5-α-reductase inhibitor used to treat benign prostatic hyperplasia) for prostate cancer and tamoxifen (a selective oestrogen receptor modulator) for breast cancer. Finasteride is being tested in a prostate cancer prevention trial in about 18 000 men aged over 55 whose concentrations of prostate specific antigen (PSA) are lower than 3 ng/ml and in whom a digital rectal examination was negative.14 The trial is designed to determine whether daily doses of finasteride can reduce the incidence of cancer over seven years.
A breast cancer prevention trial was initiated in 1992 in response to data from trials in women with early breast cancer that indicated that treatment with tamoxifen resulted in a significant decrease (40-50%) of contralateral breast cancer.15 The trial, conducted with more than 13 000 women at increased risk of breast cancer because of age or other risk factors, was unblinded in 1998 when it was found that women who took tamoxifen daily for five years had a 49% reduced risk of breast cancer compared with those taking placebo.16 An ongoing study of tamoxifen and raloxifene aims to determine whether raloxifene, also a selective oestrogen receptor modulator, is as effective as tamoxifen in reducing the risk of breast cancer in postmenopausal women at high risk.17
The effects of agents such as tamoxifen underscore the sometimes vague boundary between prevention and treatment of cancer, an issue complicated by new findings in molecular biology that blur the distinctions between premalignant and malignant lesions.17
Preclinical testing of suitable agents
The preclinical development of chemopreventive agents includes an initial assessment of their efficacy using in vitro and cell based mechanistic assays and in vivo screens in animal models of carcinogenesis that are representative of human cancers and exhibit precancerous lesions (see table B on bmj.com). The most promising agents are characterised more fully in the animal models to evaluate, for example, dose-response curves, dosing regimens, and combinations with other agents tested.13 Compounds that show high efficacy and low toxicity in animal studies are considered for testing in humans. Potential chemopreventive agents selected for testing in people at high risk of developing cancer must have low toxicities compared with the drugs used to treat existing cancer.
Clinical chemoprevention trials
Phase I clinical trials are generally conducted in a limited number of healthy subjects. They determine the dose related safety and efficacy of an agent and its pharmacokinetic variables, including absorption, distribution, metabolism, and excretion.
Phase II clinical trials evaluate the efficacy of an agent in a larger group of subjects at high risk of certain cancers. Important objectives include identifying biochemical, genetic, molecular, cellular, or histological biomarkers of cancer that can be used to estimate possible neoplastic progression and determining whether the chemopreventive agent can affect the modulation of the identified biomarker(s).
Phase III clinical trials, conducted either in populations at high risk of specific cancers or in subjects from the general population, are usually randomised, controlled, large scale trials conducted primarily to determine the efficacy of the intervention.18 The selenium and vitamin E clinical trial, for example, is a phase III trial to test vitamin E and selenium, individually and in combination, in 32 000 middle aged men with normal prostate specific antigen concentrations. The primary end point will be prostate cancer diagnosed by community practices, and the trial is projected to last 12 years, including seven years of intervention and five years of follow up.19 The Division of Cancer Prevention of the US National Cancer Institute is currently sponsoring more than 65 phase I, II, and III chemoprevention trials (table 1).
Table 1.
Selected ongoing phase I, II, and III cancer prevention trials sponsored by the US National Cancer Institute
| Target organ | Agent |
|---|---|
| Phase I trials | |
| Breast | l-Perillyl alcohol |
| Selective oestrogen receptor modulators-3 (2 trials) | |
| Soy isoflavones | |
| Colon | Curcumin |
| Ursodiol | |
| Lung | Phenethyl isothiocyanate |
| l-Selenomethionine and vitamin E | |
| Prostate | Lycopene (3 trials) |
| Soy isoflavones | |
| Skin | Epigallocatechin gallate and polyphenon E |
| Phase II trials | |
| Anogenital warts, human papillomavirus, HIV | Indole-3-carbinol |
| Barrett's oesophagus | Celecoxib |
| 2-Difluoromethylornithine | |
| Bladder | Celecoxib |
| Breast | 2-Difluoromethylornithine (2 trials) |
| Exemestane | |
| l-Perillyl alcohol | |
| Selective oestrogen receptor modulators-3 (2 trials) | |
| Tamoxifen (2 trials) | |
| Tamoxifen and N-(4-hydroxyphenyl) retinamide (2 trials) | |
| Targretin | |
| Cervix | 9-cis-Retinoic acid |
| 2-Difluoromethylornithine | |
| N-(4-hydroxyphenyl) retinamide | |
| Colon | Celecoxib (2 trials) |
| Celecoxib and 2-difluoromethylornithine | |
| 2-Difluoromethylornithine and sulindac | |
| Folic acid | |
| Vitamin D and calcium | |
| Endometrium | Medroxyprogesterone v depo-provera |
| Liver | Oltipraz |
| Lung | Anetholetrithione |
| Budesonide | |
| Mouth | Celecoxib |
| N-(4-hydroxyphenyl) retinamide | |
| Ovary | N-(4-hydroxyphenyl) retinamide and oral contraceptive |
| Prostate | Celecoxib |
| 2-Difluoromethylornithine | |
| 2-Difluoromethylornithine and casodex | |
| Flutamide | |
| Flutamide and luprolide | |
| Flutamide and toremifene | |
| N-(4-hydroxyphenyl) retinamide | |
| Selenised yeast | |
| Soy (dietary) | |
| Soy isoflavones | |
| Vitamin D analogue | |
| Skin | Celecoxib |
| Polyphenon E (wartheal) | |
| Sulindac | |
| Phase III trials | |
| Bladder | 2-Difluoromethylornithine |
| N-(4-hydroxyphenyl) retinamide | |
| Breast | Raloxifene and tamoxifen |
| Colon | Celecoxib |
| Oesophagus | l-Selenomethionine and celecoxib |
| Prostate | Finasteride |
| Selenomethionine | |
| Selenium and vitamin E | |
| Skin | 2-Difluoromethylornithine |
| N-(4-hydroxyphenyl) retinamide | |
Study designs and findings for several phase III trials have been summarised.20 The outcomes of the α tocopherol, β carotene cancer prevention study and the β carotene and retinol efficacy trial highlight the difficulty in identifying single dietary components as chemopreventive agents.21,22 Epidemiological data that linked high intakes of food containing β carotene (such as certain vegetables and fruits) to reduced risk of lung cancer provided strong support for clinical interventions to test the chemopreventive effect of β carotene supplements on the risk of lung cancer. Results from both studies, however, indicated harmful effects for both β carotene (a vitamin A precursor) and retinol (vitamin A) in terms of an increased incidence of lung cancer in cigarette smokers. In contrast, the physicians' health study found no significant evidence of either benefit or harm for cancer from β carotene supplementation.23 Fruit and vegetables contain numerous potential chemopreventive agents in addition to β carotene, and it is possible that β carotene is simply a marker for other protective dietary components. Such “unsuccessful” trials can, however, provide valuable leads for further research. In the α tocopherol, β carotene cancer prevention study, for example, 34% fewer cases of prostate cancer and 16% fewer cases of colorectal cancer were diagnosed in men who received vitamin E supplements.21
Biomarkers as surrogate end points in clinical chemoprevention trials
Considerable research is currently focused on identifying biomarkers as surrogate end points in place of overt cancer in cancer chemoprevention trials. Cancer is a comparatively infrequent event, and clinically overt cancer usually takes many years to develop. Clinical trials to test the effectiveness of chemopreventive agents therefore require large study populations and a long term commitment of resources. The availability of biomarkers as surrogate end points for clinical disease would allow smaller trials of shorter duration, facilitating clinical research into chemoprevention.
Acceptable biomarkers for cancer must be reliable (repeatable), highly sensitive and specific, quantitative, readily obtained by non-invasive methods, part of the causal pathway for disease, capable of being modulated by the chemopreventive agent, and have high predictive value for clinical disease.24 Table 2 shows examples of potential biomarkers that are being evaluated as surrogate end points in phase II and III trials sponsored by the National Cancer Institute's Division of Cancer Prevention. The use of presurgical models, in which a chemopreventive agent is administered for several weeks before surgery, is an innovative approach to identifying possible biomarkers and evaluating the effects of agents on these. For example, phase II trials of finasteride and lycopene have been conducted in patients before they had radical prostatectomies, and patients with early breast cancer are being recruited to a presurgical intervention with tamoxifen and fenretinide.25–27 Finasteride did not exhibit any chemopreventive effect on potential biomarkers in prostate tissue at the dose given.25 Lycopene, however, significantly reduced the extent of diffuse high grade prostatic intraepithelial neoplasia.26
Table 2.
Potential surrogate end points being evaluated in phase II and III chemoprevention trials sponsored by the National Cancer Institute (adapted from Kelloff et al (2000)21
| Cancer site | End points | ||
|---|---|---|---|
| Primary | Histopathology | Other | |
| Prostate | Prevention or regression of prostatic intraepithelial neoplasia | Nuclear morphometry (nuclear texture and shape), size and No of nucleoli, DNA ploidy | Proliferation (MIB-1, PCNA), apoptosis (No of apoptotic bodies, transglutaminase, bcl-2 gene), differentiation LewisY antigen, androgen receptor expression), cell regulatory molecules (c-erbB-2 gene, TGFβ, p53 gene), invasion or metastasis (angiogenesis, PSA) |
| Breast | Prevention or regression of hyperplasia or ductal carcinoma in situ | Mammographic density, nuclear morphometry, DNA ploidy | Proliferation (MIB-1, PCNA), apoptosis (bcl-2), differentiation (sialyl Tn-antigen), cell regulatory molecules (oestrogen receptor, EGFR, c-erbB-2, IGF-I, p53) |
| Colon | Prevention of colorectal adenoma | Nuclear morphometry (nuclear texture and shape), size and No of nucleoli, DNA ploidy | Proliferation (BrdU uptake, MIB-1, PCNA, ratio of proliferation to apoptosis), apoptosis (apoptotic bodies by confocal laser microscopy, TUNEL assay), differentiation (LewisY antigen, sialyl Tn-antigen, apomucins), cell regulatory molecules (p53) |
| Lung | Regression of bronchial dysplasia | Nuclear morphometry (pleomorphism), DNA ploidy | Proliferation (PCNA), apoptosis (bcl-2), cell regulatory molecules (telomerase, EGFR, p53) |
| Bladder | Prevention of new tumour | DNA ploidy | Proliferation (PCNA), differentiation (G-actin), cell regulatory molecules (EGFR) |
| Head and neck | Prevention or regression of dysplastic lesion | DNA ploidy | Proliferation (MIB-1, PCNA), cell regulatory molecules (EGFR, c-erbB-2, TGFα, TGFβ) |
| Cervix | Regression of cervical intraepithelial neoplasia | Nuclear morphometry (pleomorphism, DNA content), DNA ploidy | Proliferation (PCNA), differentiation (keratins), cell regulatory molecules (EGFR, ras gene expressions or mutations) |
| Oesophagus | Prevention or regression of Barrett's dysplasia | Nuclear morphometry (pleomorphism, DNA content), nuclear morphometry (size and No of nucleoli), DNA ploidy | Proliferation (MIB-1, PCNA), apoptosis, cell regulatory molecules (EGFR, p53) |
| Skin | Prevention or regression of actinic keratosis | No data | Proliferation (PCNA, ODC activity), cell regulatory molecules (EGFR, TGFβ, p53) |
MIB-1=monoclonal antibody, PCNA=proliferating cell nuclear antigen, TGF=transforming growth factor, PSA=prostate specific antigen, EGFR=epidermal growth factor receptor, IGF-1=insulin-like growth factor I, BrdU=5′-bromodeoxyuridine, TUNEL=terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labelling, ODC=ornithine decarboxylase.
No biomarkers have yet been validated as surrogate end points for cancer. Research is focusing on intraepithelial neoplasia, a premalignant condition exemplified by colorectal adenomas, prostatic intraepithelial neoplasia, and cervical intraepithelial neoplasia. Intraepithelial neoplasia and its associated genetic and molecular changes are currently considered to provide the best opportunities for validating surrogate endpoint biomarkers in epithelial tissues.24 The National Cancer Institute's early detection research network was established to accelerate the development and validation of biomarkers for evaluating cancer risk and detecting premalignancy. The network links centres of expertise from academia and industry and includes a centre for data management and coordination that will develop a common database for network research.
Chemoprevention and medical practice
The medical community can play an important part in cancer prevention by recognising the multistage nature of cancer development, making all patients aware of factors that increase cancer risk and ways to reduce risk, and identifying patients at high risk of cancer who might benefit from chemopreventive interventions. Primary care doctors should evaluate cancer risk even for people who seem healthy. A woman's risk of invasive breast cancer, for example, can be calculated by using the breast cancer assessment tool found at http://bcra.nci.nih.gov/brc/questions.htm Similar assessment tools are not yet available for other cancers, but risk factors for various cancers are outlined at www.cancer.org and provide some basis for assessing a patient's degree of risk for a particular cancer. Although this approach needs refinement, it allows doctors to develop an individual risk profile for cancer that may help guide preventive interventions, such as chemoprevention, and motivate patients to change their behaviour.
Additional educational resources
Journal articles
Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis 2000;21:525-30.
Decensi A, Costa A. Recent advances in cancer chemoprevention, with emphasis on breast and colorectal cancer. Eur J Cancer 2000;36:694-709.
Kelloff GJ, Crowell JA, Steele VE, Lubet RA Malone WA Boone CW, et al. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr 2000;130:467-71S.
Websites
Chemopreventive Agent Development Research Group, Division of Cancer Prevention, National Cancer Institute (www.cancer.gov/prevention/cadrg) (accessed 15 Feb 2002)
National Cancer Institute's comprehensive clinical trials database (www.cancer.gov/clinical_trials/). Includes information on cancer chemoprevention trials (accessed 15 Mar 2002)
National Cancer Institute's Division of Cancer Prevention early detection research network (http://www3.cancer.gov/prevention/cbrg/edrn/). Focuses on development and validation of biomarkers for evaluating cancer risk and detecting premalignancy (accessed 15 Mar 2002)
Supplementary Material
Footnotes
Competing interests: None declared.
Extra tables appear on bmj.com
References
- 1.Khuri FR, Lippman SM, Spitz MR, Lotan R, Hong WK. Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst. 1997;89:199–211. doi: 10.1093/jnci/89.3.199. [DOI] [PubMed] [Google Scholar]
- 2.Langman M, Boyle P. Chemoprevention of colorectal cancer. Gut. 1998;43:578–585. doi: 10.1136/gut.43.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelloff GJ, Sigman CC, Greenwald P. Cancer chemoprevention: progress and promise. Eur J Cancer. 1999;35:2031–2038. doi: 10.1016/s0959-8049(99)00299-3. [DOI] [PubMed] [Google Scholar]
- 4.Hursting SD, Slaga TJ, Fischer SM, DiGiovanni J, Phang JM. Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. J Natl Cancer Inst. 1999;91:215–225. doi: 10.1093/jnci/91.3.215. [DOI] [PubMed] [Google Scholar]
- 5.Chemoprevention Working Group. Prevention of cancer in the next millennium: report of the Chemoprevention Working Group to the American Association for Cancer Research. Cancer Res. 1999;59:4743–4758. [PubMed] [Google Scholar]
- 6.Smith-Warner SA, Giovannucci E. Fruit and vegetable intake and cancer. In: Heber D, Blackburn GL, Go VLW, editors. Nutritional oncology. San Diego: Academic Press; 1999. pp. 153–183. [Google Scholar]
- 7.Huang M-T, Asawa T, Ho C-T, Rosen RT, editors. Food phytochemicals for cancer prevention I—fruits and vegetables. Washington, DC: American Chemical Society; 1994. [Google Scholar]
- 8.Ho C-T, Osawa T, Huang M-T, Rosen RT, editors. Food phytochemicals for cancer prevention II—teas, spices, herbs. Washington, DC: American Chemical Society; 1994. [Google Scholar]
- 9.. Dietary phytochemicals in cancer prevention and treatment. New York: Plenum Press; 1996. [Google Scholar]
- 10.Knowles LM, Milner JA. Diallyl sulfide inhibits p34cdc2 kinase activity through changes in complex formation and phosphorylation. Carcinogenesis. 2000;21:1129–1134. [PubMed] [Google Scholar]
- 11.Zhou J-R, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 12.Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–131. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- 13.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 1999;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 14.Brawley OW, Parnes H. Prostate cancer prevention trials in the USA. Eur J Cancer. 2000;36:1312–1315. doi: 10.1016/s0959-8049(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists' Collaborative group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 16.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 17.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90:1514–1528. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald P, Kelloff G, Burch-Whitman C, Kramer BS. Chemoprevention. CA Cancer J Clin. 1995;45:31–49. doi: 10.3322/canjclin.45.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald P, Lieberman R. Chemoprevention trials for prostate cancer. In: Chung LWK, Isaaca WB, Simons JW, editors. Prostate cancer: biology, genetics, and the new therapeutics. Totowa, NJ: Humana Press; 2000. pp. 499–518. [Google Scholar]
- 20.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen OP, Huttunen JK, Albanes D Alpha-Tocopherol Beta-Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 22.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 23.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 24.Kelloff GJ, Sigman CC, Johnson KM, Boone CW, Greenwald P, Crowell JA, et al. Perspectives on surrogate end points in the development of drugs that reduce the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:127–137. [PubMed] [Google Scholar]
- 25.Urban D, Myers R, Manne U, Weiss H, Mohler J, Perkins D, et al. Evaluation of biomarker modulation by fenretinide in prostate cancer patients. Eur Urol. 1999;35:429–438. doi: 10.1159/000019875. [DOI] [PubMed] [Google Scholar]
- 26.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 27.Singletary E, Lieberman R, Atkinson N, Sneige N, Sahin A, Tolley S, et al. Novel translational model for breast cancer chemoprevention study: accrual to a presurgical intervention with tamoxifen and N-[4-hydroxyphenyl]retinamide. Cancer Epidemiol Biomarkers Prev. 2000;9:1087–1090. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.