Abstract
Gamma-2 herpesviruses encode a homolog of mammalian D-type cyclins. The v-cyclin encoded by murine gammaherpesvirus 68 (γHV68) induces cell cycle progression and is an oncogene (L. F. van Dyk, J. L. Hess, J. D. Katz, M. Jacoby, S. H. Speck, and H. W. Virgin IV, J. Virol. 73:5110–5122, 1999). However, the role of the pro-proliferative v-cyclins in gamma-2 herpesvirus pathogenesis is not known. Here we report the generation and characterization of a γHV68 v-cyclin mutant (v-cyclin.LacZ) that is unable to express a functional v-cyclin protein. Notably, although the γHV68 v-cyclin is expressed from an early-late lytic transcript, v-cyclin.LacZ replicated normally in fibroblasts in vitro and during acute infection in the spleen, liver, and lungs in vivo. Moreover, v-cyclin.LacZ exhibited wild-type (wt) virulence in mice with severe combined immunodeficiency. In addition, in a model of γHV68-induced chronic disease in mice lacking the gamma interferon receptor (IFNγR−/−), v-cyclin.LacZ virus was similar to wt γHV68 in terms of the incidence of mortality and vasculitis. Further analysis revealed that the frequencies of splenocytes and peritoneal cells harboring the latent γHV68 genome in normal and B-cell-deficient mice infected with wt γHV68 or v-cyclin.LacZ were very similar. However, v-cyclin.LacZ was significantly compromised in its capacity to reactivate from latency. This phenotype was conclusively mapped to the v-cyclin gene by (i) generating a marker rescue virus (v-cyclin.MR) from the v-cyclin.LacZ mutant, which restored the frequency of cells in which virus reactivated from latency to the levels observed with wt γHV68; and (ii) generating a second v-cyclin mutant virus containing a translation stop codon within the v-cyclin gene (v-cyclin.stop), which was compromised in reactivation from latency. These studies demonstrate that despite expression as a lytic cycle gene, the pro-proliferative γHV68 v-cyclin is not required for γHV68 replication either in vitro or during acute infection in vivo but rather is a critical determinant of reactivation from latency.
Murine gammaherpesvirus 68 (γHV68; also referred to as MHV68) infection of mice is a developing small-animal model for analysis of gammaherpesvirus pathogenesis (reviewed in references 18, 19, 45, 46, 64, and 69). γHV68 is a gamma-2 herpesvirus which is closely related to the human and primate gammaherpesviruses Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV), and herpesvirus saimiri (HVS) (22, 67). Acute γHV68 infection of laboratory mice is cleared by 2 to 3 weeks postinfection, with the concomitant establishment of a presumably life-long latent infection. Notably, several γHV68 genes that are also present in HVS, KSHV, and/or EBV have been identified as candidate latent genes by transcriptional analysis of latently infected mice (68). These genes include those encoding the viral bcl-2 (v-bcl2) homolog and the viral G-protein-coupled receptor (v-GPCR) homolog as well as gene 73, which in KSHV encodes the latency-associated nuclear antigen (LANA) (11, 35, 50). In addition, KSHV, HVS, and γHV68 have in common a complement-regulatory protein homolog (1, 2, 26, 33, 67), and in both HVS and γHV68 this protein occurs in membrane-bound and soluble isoforms (26, 33). These studies argue strongly that γHV68 shares mechanisms of pathogenesis with other gammaherpesviruses.
Manipulation of the host cell cycle is a shared feature of gammaherpesviruses, and one focus for this regulation is the D-type cyclins. EBV infection (or expression of the EBV latency-associated membrane protein 1) upregulates expression of host cyclin D2 (3, 9, 63). In contrast, the gamma-2 herpesviruses HVS (32, 47), KSHV (2, 47, 52), and γHV68 (67) all contain open reading frames (ORFs) predicted to encode homologs of mammalian D-type cyclins. The viral cyclins (v-cyclins) exhibit 25 to 31% identity to mammalian D-type cyclins and 26 to 32% identity to each other, and they are positionally conserved in the gamma-2 herpesvirus genomes (67). Notably, the highest level of sequence conservation among these homologs is within the cyclin box, a domain demonstrated to be essential for cyclin-dependent kinase (cdk) binding (29, 31, 37, 38, 43, 51).
The biochemistry of the KSHV and HVS proteins has been intensively studied. The KSHV and HVS v-cyclins, in conjunction with cdk's, have been demonstrated to phosphorylate the retinoblastoma protein (pRb) and promote cell cycle progression, like their mammalian homologs (2, 8). The KSHV and HVS v-cyclins predominantly bind cdk6. Overexpression of the KSHV v-cyclin in cells expressing high levels of cdk6 results in apoptosis (48), suggesting that there may be other viral genes responsible for preventing apoptosis (e.g., v-bcl2). Several unusual properties of the v-cyclins have also been noted. The v-cyclin ORFs lack an LXCXE motif present in mammalian cyclin D proteins and thought to be important in direct binding to pRb (20, 25). The HVS and KSHV v-cyclins are able to bind multiple cdk's, rather than being restricted to binding cdk4 and cdk6 (27, 32, 39), and have a broadened substrate range, being capable of phosphorylating both pRb and histone H1 in vitro. In addition, the HVS and KSHV v-cyclin–cdk complexes are resistant to regulation by the cellular cyclin/cdk inhibitors of both the INK4 and Kip1 families (24, 41, 65). The resistance to inhibitors impacts not only the v-cyclin–cdk complex itself but also the activity of other cyclin-cdk's by means of decreased availability and redistribution of the inhibitors (42). The KSHV v-cyclin is resistant to inhibition by both p21 and p27 but is only known to phosphorylate p27, which is then actively degraded (24, 41). The HVS v-cyclin structure has been determined (56, 60), and although this analysis revealed conservation of mammalian cyclin structure in regions critical for interaction with cdk's, differences in other regions of the v-cyclin structure may explain some of the noted functional differences.
We have previously shown that the γHV68 v-cyclin promotes cell cycle progression in primary lymphocytes and can function as an oncogene in transgenic mice (66). Thus, the known gamma-2 herpesvirus v-cyclins have all been shown to promote cell cycle progression (66). While much is known about the functional properties of the v-cyclins, their role in the pathogenesis of gamma-2 herpesviruses has not been defined. Here we present a detailed analysis of acute and latent infection in mice, using a recombinant γHV68 mutant lacking a functional v-cyclin gene.
MATERIALS AND METHODS
Viruses and tissue culture.
γHV68 clone WUMS (ATCC VR1465) was passaged and grown, and the titer was determined as previously described (13, 70, 71). NIH 3T12 cells and murine embryonic fibroblasts (MEFs) were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 2 mM l-glutamine. MEFs were obtained from BALB/c or C57BL/6 mouse embryos as described previously (70).
Generation of mutant γHV68 viruses.
All recombinant viruses were generated by homologous recombination following cotransfection of NIH 3T12 cells with viral DNA and gene-targeting plasmid DNA as previously described (13). The parental genomic subclone for the targeting plasmids was constructed by insertion of the 3,723-bp BamHI-BsrGI fragment of γHV68 (bp 101654 to 105377; WUMS sequence [67]), containing gene 72 and flanking sequence, into the corresponding sites of Litmus 38 (New England Biolabs, Beverley, Mass.) to generate a plasmid called pL3700. The mutant targeting vector (pORF72-LacZ) was generated by inserting a β-galactosidase expression cassette driven by the human cytomegalovirus (HCMV) immediate-early promoter-enhancer (HindIII-XmaI fragment from pHCMV-LacZ-MP-1; a kind gift of Paul Olivo [unpublished]) into pL3700. The plasmid pORF72-LacZ contains the LacZ expression cassette in place of 475 bp of gene 72 (v-cyclin), beginning with the ATG defining the translation start site of the ORF (from the NcoI site at bp 103179 to the NsiI site at bp 102704). The plasmid pL3700-stop was generated by insertion of a 16-bp stop linker containing a unique HpaI site (15) at the PmlI site (bp 103021). All targeting plasmids were sequenced across mutations and cloning junctions and purified on cesium chloride gradients prior to transfection (54).
The mutant virus v-cyclin.LacZ was generated by calcium phosphate cotransfection of NIH 3T12 cells with γHV68 genomic DNA (2 μg) and pORF72-LacZ plasmid linearized by digestion with NheI (2 μg). Virus was harvested 5 to 8 days posttransfection, aliquoted, and stored at −80°C. Recombinant virus was then identified by blue plaque morphology and purified as described previously (13). Briefly, recombinant viruses were identified as individual blue plaques after X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) staining of infected NIH 3T12 monolayers and isolated from methyl cellulose-overlaid monolayers (49).
The v-cyclin marker rescue (v-cyclin.MR) virus was generated by cotransfection of v-cyclin.LacZ viral DNA and pL3700 plasmid DNA followed by identification and selection of white plaques. The v-cyclin.stop virus was generated by cotransfection of v-cyclin.LacZ viral DNA and pL3700-stop plasmid DNA followed by identification and selection of white plaques. Both v-cyclin.MR and v-cyclin.stop viruses were generated by cotransfection of v-cyclin.LacZ genomic DNA (1.5 μg) with circular pL3700 (1.5 μg) or pL3700-stop (1.5 μg), respectively, using Superfect transfection reagent (Gibco BRL) according to the manufacturer's recommendations. All cotransfections of NIH 3T12 cells were done in six-well plates. Viral stocks were purified to homogeneity by selection for blue or white morphology and further characterized by Southern blot analyses as described previously (13). Mutant viruses were confirmed as such by immunoblotting of infected cells for v-cyclin expression. Viral stocks were generated from NIH 3T12 cells infected at a multiplicity of infection (MOI) of 0.05 and harvested at 50% cytopathic effect (CPE). Viral stocks were homogenized, clarified by centrifugation, aliquoted, and stored at −80°C. Titers of all viruses were determined by plaque assay at least three independent times.
Southern blotting.
Viral DNA for Southern blot analysis and for transfection was generated by infection of NIH 3T12 cells at an MOI of 0.5. Infected-cell cultures were harvested at 50% CPE, between days 4 and 6 postinfection, and DNA was prepared as previously described (67). One microgram of viral DNA was digested with either EcoRI, BamHI, or NsiI plus NotI. EcoRI and BamHI digests result in diagnostic fragments for the v-cyclin region, and the NsiI-NotI digestion is diagnostic for deletions at the left end of the viral genome (13). Digests were electrophoresed on 1% agarose gels and transferred by alkaline transfer to Nytran nylon membranes (Turboblot; Schleicher & Schuell, Keene, N.H.) according to the manufacturer's recommendations. Southern blots were probed with the 3,723-bp BamHI-BsrGI fragment of pL3700 for analysis of the v-cyclin region or with a 2,926-bp HindIII-EcoRV fragment of the HindIII G plasmid containing sequences from gene 6 (encoding a single-stranded DNA-binding protein) for evaluation of the left end of the viral genome (23). The probe was 32P labeled by random primer extension according to the manufacturer's recommendations (Megaprime DNA labeling kit; Amersham International). Blots were hybridized at 68°C and washed with two changes of 2× SSC–0.1% sodium dodecyl sulfate (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) followed by two changes of 0.5× SSC–0.1% sodium dodecyl sulfate prior to exposure to film (54).
Immunoblotting.
Polyclonal rabbit antiserum (Cocalico, Reamstown, Pa.) was generated by using purified bacterially expressed v-cyclin protein as described previously (66). Whole-cell lysates for use in immunoblot analyses were made by resuspending frozen infected-cell pellets in 1× reducing loading sample buffer and boiling for 10 min. A total of 2.5 × 105 cell equivalents was loaded per lane on 15% polyacrylamide gels and transferred to a Hybond N membrane (Amersham). Blots were blocked for 45 min in phosphate-buffered saline containing 5% nonfat milk, 5% normal goat serum, and 0.05% Tween 20 and then incubated with polyclonal rabbit anti-v-cyclin serum (1:2,000 dilution) overnight at room temperature. Blots were washed three times in phosphate-buffered saline containing 0.05% Tween 20, incubated in horseradish peroxidase-conjugated donkey anti-rabbit antiserum (1:5,000 dilution; Jackson Immunoresearch), washed three times with PBS containing 0.05% Tween 20, and developed with the ECL chemiluminescence reagent (Amersham). A monoclonal antibody against β-actin (1:1,000 dilution; Sigma catalog no. AC74), used to monitor loading of cell extracts, was detected with horseradish peroxidase-conjugated donkey anti-mouse antiserum (1:5,000 dilution; Jackson Immunoresearch).
Plaque assays and determination of viral titers.
Plaque assays were performed on NIH 3T12 cells as previously described (13). Briefly, organs for which virus titers were to be determined were thawed and homogenized with a Ten Broek tissue grinder prior to dilution and plating onto NIH 3T12 cells. Infection was performed at 37°C for 1 h before cells were overlaid with medium containing Noble agar. All titers were determined in parallel with a laboratory standard stock of known titer. The limit of detection of the assay used is 50 PFU per organ.
In vitro growth.
Viral growth in vitro was determined by infection of NIH 3T12 cells at an MOI of 5 PFU per cell, with removal of the inoculum after 1 h of infection to measure a single cycle of virus replication, or at 0.05 PFU per cell to measure multiple cycles of virus replication. Cells and supernatants were collected at various times postinfection and frozen at −80°C. Samples were then subjected to four cycles of freezing and thawing, and virus was then quantitated by plaque assay (sensitivity, 50 PFU). Growth of wild-type (wt) γHV68 in contact-inhibited MEFs was compared with that of v-cyclin.LacZ by infecting MEF monolayers with 1 PFU per well in 96-well plates. Four independent sets of dilutions of each virus were plated in 24 wells of 96-well plates and scored for appearance of CPE. Six wells from each dilution were harvested for determination of virus titers by plaque assay.
Mice, infections, and organ harvests.
Gamma interferon receptor-deficient (IFNγR−/−) mice on a 129 background were obtained from Michel Aguet (44). B-cell-deficient mice (B6μMT; C57BL/6J-Igh-6tm1Cgn) (36) were obtained from Jackson Laboratories (Bar Harbor, Maine). C.B-17 severe combined immunodeficient (SCID) mice (5) were obtained from Emil Unanue and used between 3 to 5 months of age. Mice were bred and maintained at Washington University School of Medicine in accordance with all federal and university policies. C57BL/6 mice were purchased from Jackson Laboratories. Mice were age and sex matched and were used between 7 to 10 weeks unless otherwise stated. Mice were anesthetized with metofane prior to infection or sacrifice by cervical dislocation. Mice were infected by intraperitoneal (i.p.) injection with 106 PFU of virus (unless otherwise stated) in 0.5 ml of complete DMEM. Upon sacrifice, organs for which virus titers were to be determined were placed in 1 ml of DMEM on ice and frozen at −80°C. Peritoneal exudate cells (PECs) were harvested by peritoneal lavage with 10 ml of DMEM. Virulence in C.B-17 SCID mice was determined by infection via i.p. injection with medium alone or containing 101, 103, or 106 PFU of virus, and survival was monitored daily.
Induction of arteritis was executed as previously published (71). Briefly, IFNγR−/− mice between 7 to 9 weeks of age were infected i.p. with 2 × 107 PFU of wt γHV68 or v-cyclin.LacZ. At the time of death or sacrifice, the heart and lungs were removed en bloc. The spleen, liver, and kidneys were also harvested. Tissues were fixed in 10% phosphate-buffered formalin, paraffin embedded, serial sectioned, and stained with hematoxylin and eosin. Slides were independently read by L.V.D., H.W.V., and S. Kapadia, who were blinded to their identities. Aortic lesions were scored as follows: 0, normal artery; 1, mild intimal and adventitial thickening with minimal infiltrates; 2, pronounced intimal and adventitial mononuclear infiltrates but no neutrophilic infiltrates; 3, pronounced intimal and adventitial mononuclear infiltrates and neutrophilic infiltrates at the adventitial and medial borders; 4, pronounced intimal and adventitial mononuclear infiltrates and presence of neutrophilic infiltrates in the media; and 5, severe lesions with extensive neutrophilic infiltrates and/or necrosis in the media (A. J. Dal Canto, H. W. Virgin IV, and S. H. Speck, submitted for publication). Samples from the same mice were examined for the presence of fibrosis or atrophy of the spleen as previously described (21).
Quantitation of cells harboring the γHV68 genome.
The frequency of cells harboring the γHV68 genome was determined by a limiting-dilution nested-PCR assay that amplifies γHV68 gene 50 sequences with approximately single-copy sensitivity, as described previously (72, 73). Briefly, PECs and splenocytes were harvested from latently infected mice and frozen in 10% dimethyl sulfoxide at −80°C. Cells were thawed, counted, and resuspended in an isotonic buffer. A limiting-dilution series of cells harvested from latently infected mice was then generated and plated in 96-well PCR plates (Costar). Uninfected NIH 3T12 cells were added to provide a constant cell number of 104 cells per well. Cells were then lysed with proteinase K at 56°C for 6 h, and the lysate was heated inactivated at 95°C for 15 min prior to PCR amplification. One microliter of the 20-μl first-round PCR product served as a template for the second round of PCR amplification. Products were analyzed by ethidium bromide staining of a 1.5% agarose gel. Ten PCRs were analyzed for each cell dilution, with six dilutions per sample per experiment. Control reactions of uninfected cells (negative control; six reactions/plate) or plasmid DNA (pBamHIN) of known copy number (positive control; six reactions/plate each of 10, 1, and 0.1 copy of plasmid DNA) were included in each experiment. There were no false-positive PCRs in any analyses reported here, and all assays demonstrated approximately a one-copy sensitivity for plasmid DNA.
Ex vivo reactivation from latency.
The frequency of cells capable of reactivating virus from latency was determined by a limiting-dilution ex vivo reactivation assay, as previously described (70, 73). Briefly, PECs and splenocytes were harvested from infected mice between days 42 and 45 postinfection, and single-cell suspensions were generated. Cells were resuspended in complete DMEM and plated in a twofold-dilution series (starting at 105 splenocytes per well and at 8 × 104 to 16 × 104 PECs per well) onto MEF monolayers in 96-well tissue culture plates. Wells were scored microscopically for CPE at 21 days postplating. Some wells were replated onto fresh MEF monolayers for final determination of infectious-virus titers, particularly wells containing large numbers of cells for which viral CPE was difficult to discern. Twenty-four wells were plated per dilution, and 12 dilutions were plated per experimental sample. Preformed infectious virus was detected by plating parallel cell samples, which had been subjected to mechanical disruption, onto MEF monolayers. Mechanically disrupted samples contained <1% live cells, and thus the presence of preformed virus, rather than viral reactivation from latently infected cells, could be detected (70, 72, 73).
Statistical methods.
All data was analyzed by using GraphPad Prism software (GraphPad Software, San Diego, Calif.). Survival data were plotted and statistically analyzed with the Mantel-Haenszel test; to correct for the number of postexperimental comparisons in a conservative way, we multiplied calculated P values by the total number of comparisons between survival curves (significant P values are indicated in the relevant figure legends). Titer data were statistically analyzed with the nonparametric Mann-Whitney test. Severity of aortitis was scored by three independent blinded readers and analyzed with the nonparametric Mann-Whitney test. The frequencies of reactivation and genome-positive cells were statistically analyzed by paired t test. Frequencies of reactivation and viral-genome-positive cells were obtained from the cell number at which 63% of the wells scored positive for either reactivating virus or the presence of the viral genome based on the Poisson distribution; data were subjected to nonlinear-regression analysis to obtain the single-cell frequency for each limiting-dilution analysis.
RESULTS
Targeted disruption of the γHV68 v-cyclin gene.
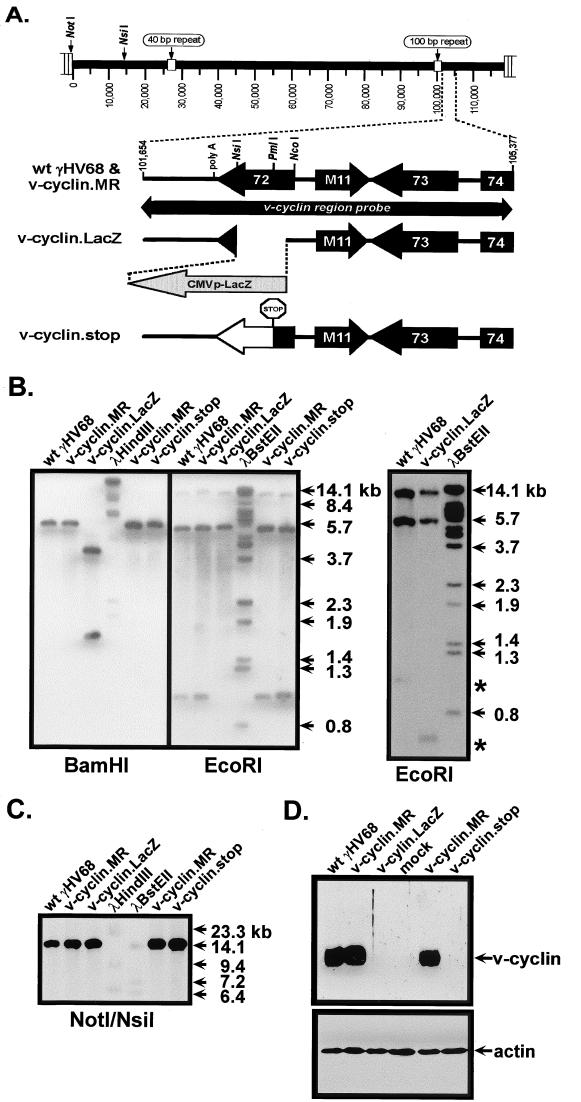
The v-cyclin-deficient γHV68 v-cyclin.LacZ was generated by insertion of a LacZ expression cassette, containing the β-galactosidase gene under the control of the HCMV immediate-early promoter-enhancer (see Materials and Methods). The LacZ expression cassette insertion replaced 475 bp of γHV68 sequence, from bp 103179 to 102704 (WUMS sequence [67]), beginning at the predicted translation initiation codon (Fig. 1A). This deletion-insertion does not disrupt any other known viral coding sequences. v-cyclin.LacZ was identified by blue plaque morphology and plaque purified to homogeneity. The structure of v-cyclin.LacZ was confirmed by Southern blot analyses of EcoRI- and BamHI-digested viral DNA probed with a 32P-labeled fragment spanning the region of the viral genome containing the v-cyclin gene (Fig. 1A and B) (the probe contained viral sequences from bp 101654 to 105377). As expected, the v-cyclin region probe hybridized to 3.7- and 1.6-kb BamHI fragments with v-cyclin.LacZ, compared to a 5.2-kb BamHI fragment with wt γHV68, and to 10.8-, 5.2-, and 0.5-kb EcoRI fragments with v-cyclin.LacZ virus, compared to 10.8-, 5.2-, and 0.9-kb fragments with wt γHV68 (Fig. 1B).
FIG. 1.
Genomic structures of v-cyclin.LacZ and v-cyclin.stop mutants. (A) Shown is a schematic representation of the region of the γHV68 genome encoding the v-cyclin gene (gene 72). Mutations in the v-cyclin gene, as well as the marker rescue virus, were generated as described in Materials and Methods. Genome coordinates of the surrounding ORFs in wt γHV68 region are as follows: 100-bp repeat region, bp 98981 to 101170; ORF72, bp 103181 to 102426; M11 (v-bcl-2), bp 103418 to 103930; ORF73, bp 104868 to 103927; and ORF74 (v-GPCR), bp 105057 to 106067. Genome coordinates are based on the γHV68 WUMS sequence (67). The cyclin region probe is depicted as a bar below the wt γHV68 schematic and spans bp 101654 to 105377. Also shown, at the left end of the viral genome, are the locations of the NotI and NsiI sites used to diagnose for the presence of deletions in this region of the genome (see panel C, below). (B) Southern analysis of wt γHV68, v-cyclin.LacZ, v-cyclin.stop, and v-cyclin.MR viruses. Purified genomic viral DNA was digested with BamHI or EcoRI, electrophoresed, blotted, and hybridized with the cyclin region probe. 32P-labeled molecular size markers were included on each Southern blot, and the locations of positions of size standards are indicated. The Southern blot shown in the right-hand panel demonstrates the presence of the predicted 0.5-kb diagnostic EcoRI band in the v-cyclin.LacZ mutant, which was run off the blot shown in the left-hand panel. (C) To assess the integrity of the left end of the viral genome, purified viral DNA was digested with NotI and NsiI, electrophoresed, blotted, and probed with a fragment of the viral genome containing gene 6 (bp 11100 to 14026). The locations of the NotI and NsiI sites at the left end of the genome are depicted in panel A. (D) Immunoblot analysis of v-cyclin protein expression in NIH 3T12 cells. NIH 3T12 fibroblasts were lytically infected with wt γHV68, v-cyclin.LacZ, v-cyclin.stop, or v-cyclin.MR or were mock infected, and cell lysates collected at 24 h postinfection were probed with a polyclonal rabbit antiserum to the v-cyclin (upper panel). The blot was reprobed with a monoclonal antibody against β-actin (Sigma catalog no. AC74) (lower panel).
Because we had previously observed spontaneous deletions at the left end of the γHV68 genome during the generation of mutant viruses (13), additional Southern blot analyses were performed to confirm the integrity of the left end of the viral genome (Fig. 1C). Viral DNA was digested with NotI and NsiI (Fig. 1A) and probed with a fragment of the γHV68 genome containing ORF6 (bp 11100 to 14026). The hybridization of the probe to a 14.8-kb fragment with wt and mutant viruses (Fig. 1C), and the lack of smaller fragments corresponding to deletions, demonstrated the integrity of the left end of the viral genome.
We constructed a marker rescue virus (v-cyclin.MR) in which a targeting vector containing wt v-cyclin sequences was used to regenerate wt virus from the v-cyclin.LacZ mutant (Fig. 1A). v-cyclin.MR was identified by white plaque selection and plaque purified to homogeneity. Southern blot analysis of v-cyclin.MR (Fig. 1B and C) demonstrated that its hybridization pattern was identical to that of wt γHV68. When experiments demonstrated a phenotypic difference between wt γHV68 and v-cyclin.LacZ, we repeated them with the v-cyclin.MR virus, thereby excluding the possible contribution of spurious mutations at distal sites to observed phenotypic differences (see below).
To rule out the possibility that the LacZ expression cassette contributed to phenotypic changes (e.g., by altering expression of adjacent genes), as we have previously shown can occur with some γHV68 mutants (13), we replaced the LacZ expression cassette with a v-cyclin gene containing a translation stop codon at the PmlI site in the v-cyclin ORF (Fig. 1A, v-cyclin .stop). This introduced a translation stop codon at the beginning of the highly conserved cyclin box and thus is predicted to encode a nonfunctional 53-amino-acid truncated v-cyclin protein. Southern blot analyses were performed to confirm the genotype of the v-cyclin.stop virus (Fig. 1B and C).
Equal numbers of NIH 3T12 cells infected with wt γHV68, v-cyclin.LacZ, v-cyclin.MR, or v-cyclin.stop and mock-infected cells were analyzed by immunoblotting with a rabbit polyclonal antiserum generated against bacterially expressed v-cyclin (66). The v-cyclin protein was readily detected in extracts prepared from cells infected with wt γHV68 or v-cyclin.MR virus, but not in those of v-cyclin.LacZ-, v-cyclin.stop-, or mock-infected cells (Fig. 1D). Subsequent incubation of the immunoblot with a monoclonal antibody against β-actin demonstrated equal loading of cellular extract in all lanes (Fig. 1D).
γHV68 v-cyclin.LacZ replicates normally in immortalized and primary mouse fibroblasts in vitro.
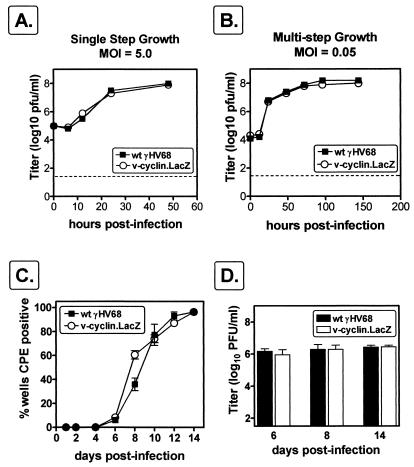
We have previously shown that the v-cyclin transcript and protein are abundantly expressed during virus replication in murine fibroblasts (66) and may therefore be important for lytic-virus replication. We therefore compared the growth of wt γHV68 to that of v-cyclin.LacZ in a single round of replication and in multiple rounds of replication in NIH 3T12 fibroblasts. In both single and multiple cycles of replication, v-cyclin.LacZ replication was indistinguishable from that of wt γHV68 (Fig. 2A and B), demonstrating that v-cyclin is not required for efficient replication in an immortalized murine cell line.
FIG. 2.
γHV68-cyclin.LacZ grows similarly to wt γHV68. NIH 3T12 cells were infected with 5.0 (A) or 0.05 (B) PFU of wt γHV68 (closed squares) or v-cyclin.LacZ (open circles) per cell, samples were harvested at the times indicated, and virus was quantitated by plaque assay. Data are representative of two independent experiments. The dotted line indicates the sensitivity of the plaque assay (50 PFU). The inoculum was removed 1 h postinfection and replaced with fresh medium to measure single-step growth (A) or was left in place to measure multistep growth (B). (C) Viral growth in MEFs. MEF monolayers in 96-well plates were infected with 1 PFU (MOI, ca. 0.0001) of wt γHV68 or v-cyclin.LacZ. The CPE in 24 wells per virus was recorded over the course of 15 days postinfection. Results are the means of values for four independent dilution series of wt γHV68 and v-cyclin.LacZ (the standard errors of the means [SEM] are indicated by error bars). (D) Six wells of MEFs infected with 1 PFU of wt γHV68 or v-cyclin.LacZ were harvested at days 6, 8, and 14 for determination of viral titer by plaque assay. Results are the means of values for six wells each of wt γHV68 and v-cyclin.LacZ at each time point (SEMs are indicated by error bars).
We considered the possibility that replication in immortal fibroblast cell lines does not reflect the phenotype of v-cyclin.LacZ in primary cells. Thus, we infected contact-inhibited primary MEFs 1 week after they had reached confluence, using a very low MOI (≈0.0001). MEFs were infected with one PFU of wt or v-cyclin.LacZ virus per well of 96-well plates (∼10,000 cells/well), and CPE was monitored over the course of 15 days. There was no difference between growth of wt γHV68 and growth of v-cyclin.LacZ as determined by measuring the induction of CPE (Fig. 2C). To quantitate the amount of virus produced, six wells each of wt γHV68- or v-cyclin.LacZ-infected cells were harvested at days 6, 8, and 14. The amount of virus produced by infection of MEFs with wt γHV68 was equivalent, by plaque assay, to that produced by infection with v-cyclin.LacZ (Fig. 2D). Thus, the v-cyclin is not essential for virus replication in either immortal or primary mouse fibroblasts.
The γHV68 v-cyclin is dispensable for acute infection in vivo, virulence in SCID mice, and induction of chronic pathology in IFNγR−/− mice.
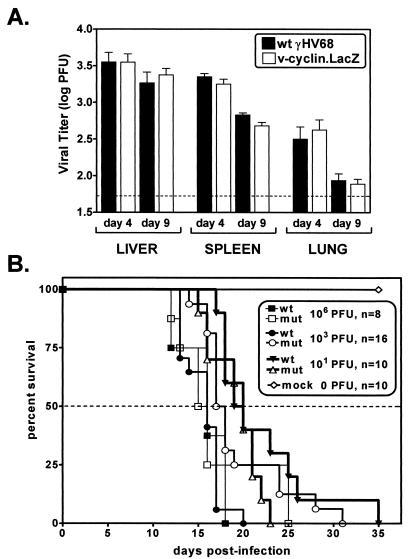
We assessed the impact of knocking out the v-cyclin gene on acute and persistent γHV68 infection by comparing wt and mutant viruses in three experimental settings known to involve virus replication in tissues: (i) acute virus replication at early times after i.p. virus inoculation; (ii) kinetics of lethality in C.B-17 SCID mice (5); and (iii) induction of elastic arteritis and mortality in IFNγR−/− mice (71). Acute virus replication in vivo was assessed by determining viral titers in the spleens, livers, and lungs of C57BL/6 (B6) mice 4 or 9 days after infection with 106 PFU of wt γHV68 or v-cyclin.LacZ. Consistent with the in vitro replication data, there were no significant differences in acute virus replication in these organs (Fig. 3A). As an independent measure of virus replication in vivo, the virulence of wt γHV68 was compared to that of v-cyclin.LacZ by monitoring the kinetics of lethality in SCID mice. SCID mice were infected with 101, 103, or 106 PFU of wt γHV68 or v-cyclin.LacZ, and survival was monitored (Fig. 3B). At each dose, wt γHV68 and v-cyclin.LacZ had very similar 50% survival times, and, as expected, higher doses resulted in earlier lethality. There were some differences in the survival times of a few infected SCID mice, but no consistent trend was observed (Fig. 3B). A few wt γHV68-infected mice survived longer than any v-cyclin.LacZ-infected mice at 10 PFU, while a few v-cyclin.LacZ-infected mice survived longer than any wt γHV68-infected mice at 103 and 106 PFU. Thus, wt γHV68 and v-cyclin.LacZ demonstrated little or no difference in acute-phase growth in vivo, as determined by measuring titers of virus in organs of C57Bl/6 mice or virulence in SCID mice, and as such the v-cyclin gene was deemed dispensable for acute virus replication in vivo.
FIG. 3.
In vivo growth and virulence. (A) Viral titers in spleens, livers, and lungs of mice 4 and 9 days after i.p. infection. C57BL/6 mice were infected with 106 PFU of wt γHV68 (closed bars) or v-cyclin.LacZ (open bars). Data from two independent experiments with seven to nine mice total per time point were pooled (the standard errors of the means are indicated by error bars). (B) Virulence of wt γHV68 and v-cyclin.LacZ in SCID mice. C.B-17 SCID mice were infected by i.p. injection with 106, 103, or 101 PFU of wt γHV68 (closed symbols) or v-cyclin.LacZ (open symbols), and mortality was recorded over the course of the experiment. Data from two independent experiments were pooled, and the total number of mice at each dose is indicated. mut, mutant; mock, mock-infected mouse data.
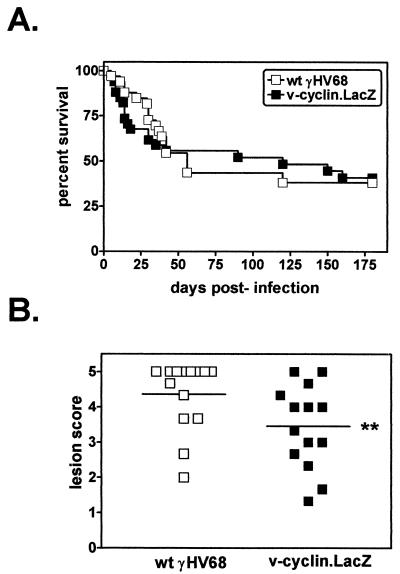
γHV68 induces chronic pathology, including aortitis, splenic fibrosis, and mortality, in IFNγR−/− mice (21, 71). IFNγR−/− mice infected with 2 × 107 PFU of wt γHV68 or v-cyclin.LacZ were monitored for survival and histopathologic indications of splenic pathology and large-elastic-vessel arteritis. Rates of survival of IFNγR−/− mice infected with wt γHV68 or v-cyclin.LacZ revealed no significant difference in virus-induced mortality over the course of three independent experiments (Fig. 4A). Analysis of splenic pathology also demonstrated no detectable difference in fibrosis/atrophy between mice infected with wt γHV68 and v-cyclin.LacZ-infected mice (data not shown). Similarly, the incidence of arteritis in the great elastic vessels in IFNγR−/− mice infected with wt γHV68 (80%; 31 of 39 infected mice) did not differ significantly from that of v-cyclin.LacZ-infected mice (84%; 32 of 38 infected mice). However, microscopic analysis of the arteritic lesions revealed a slight difference in severity of the arteritic lesions (see Materials and Methods). Aortic lesions in v-cyclin.LacZ-infected IFNγR−/− mice were slightly less severe than those in wt γHV68-infected IFNγR−/− mice (Fig. 4B) (P = 0.0326). It should be noted that the difference between histology scores of 3 and 5 is the extent of neutrophilic infiltration and necrosis in the media of the aorta (Dal Canto et al., submitted). Thus, the slight decrease in severity of the aortic lesions induced by v-cyclin.LacZ indicates that this mutant virus has a subtle defect in the induction of chronic pathology in the great elastic vessels.
FIG. 4.
Analysis of chronic infection of the vascular system in IFNγR−/− mice. (A) Survival of wt γHV68- or v-cyclin.LacZ-infected IFNγR−/− mice. IFNγR−/− mice were infected with 2 × 107 PFU of wt γHV68 (open squares) or v-cyclin.LacZ (closed squares), and mortality was recorded over the course of the experiment. Data from three independent experiments were compiled (wt γHV68, n = 37 mice; v-cyclin.LacZ, n = 38 mice). (B) Severity of arteritic lesions at the base of the aorta in wt γHV68- or v-cyclin.LacZ-infected IFNγR−/− mice. The histologic scoring criteria are described in detail elsewhere (Dal Canto et al., submitted). In this scale, 0 represents no lesion and 5 represents the most severe lesion. Comparison of wt γHV68 (open squares)- and v-cyclin.LacZ (closed squares)-induced lesions is depicted by individual points and means (horizontal lines). ∗∗, v-cyclin.LacZ-induced lesions were slightly less severe than wt γHV68-induced lesions (P = 0.0326.)
The γHV68 v-cyclin is dispensable for establishment of latency in vivo but is critical for reactivation from latency.
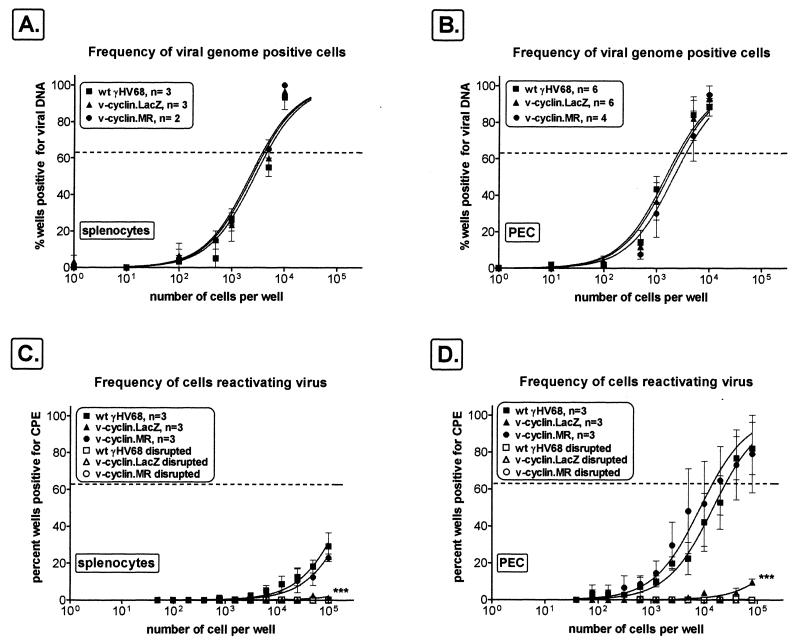
The experiments detailed above demonstrated that the v-cyclin is not required for acute or persistent virus replication in vivo. We next examined the ability of v-cyclin.LacZ to establish latency in vivo. γHV68 establishes latency in macrophages and B cells (72), and both PECs and splenocytes harbor latent virus (73). To monitor the establishment of latency, we measured the frequency of viral-genome-positive cells in PECs and splenocytes 42 days postinfection, using a limiting-dilution nested-PCR assay capable of detecting a single copy of the viral genome in a background of 104 uninfected cells (72, 73). As discussed below, acute virus replication was cleared from the spleen and peritoneum by 16 days postinfection (70). Splenocytes isolated from wt γHV68-, v-cyclin.MR-, or v-cyclin.LacZ-infected C57Bl/6 mice contained equivalent frequencies of viral-genome-positive cells (∼1 in 4,000 cells) (Fig. 5A). C57Bl/6 PECs from wt γHV68-, v-cyclin.MR-, or v-cyclin.LacZ-infected mice also demonstrated very similar frequencies of viral-genome-positive cells (∼1 in 3,000 cells for wt γHV68 and v-cyclin.MR and ∼1 in 4,000 cells for v-cyclin.LacZ) (Fig. 5B). Therefore, the ability of the v-cyclin.LacZ to establish latency was not significantly different from that of wt γHV68 in either splenocytes or PECs of infected C57Bl/6 mice.
FIG. 5.
The v-cyclin.LacZ mutant establishes latency but fails to reactivate efficiently from C57Bl/6 mice. C57Bl/6 mice were infected i.p. with 106 PFU of v-cyclin.LacZ (triangles), wt γHV68 (squares), or v-cyclin.MR (circles) virus, and cells were harvested 42 days postinfection for quantitation of the frequency of viral genome positive cells (A and B) and the frequency of cells reactivating virus (C and D). (A and B) Latently infected splenocytes or PECs were analyzed for the frequency of viral-genome-positive cells by limiting-dilution nested PCR. Ten PCRs were performed per cell dilution for each experiment, and PCR controls were run within each experiment. The numbers of individual experiments are indicated, and the standard errors of the means are shown (error bars). (C and D) Limiting-dilution ex vivo reactivation analyses using the same populations of splenocytes and PECs as for the experiments shown in panels A and B. Intact (live) cells were plated on MEF indicator monolayers to determine the frequency of ex vivo reactivation (closed symbols). In parallel, samples of each cell population were mechanically disrupted to measure preformed infectious virus (open symbols), which was demonstrated to be absent. Curve fit lines were derived by nonlinear-regression analysis, and symbols represent means and SEMs (error bars) of data from individual experiments as indicated. The dashed line is at 63%, the value which was used to calculate the frequency of genome-positive cells or the frequency of reactivating cells as indicated by a Poisson distribution. Data represent independent experiments as indicated, with each experiment containing cells pooled from four to seven mice. ∗∗∗, frequencies of reactivation for v-cyclin.LacZ and v-cyclin.MR were statistically different (PECs, P = 0.003; splenocytes, P = 0.043).
Given that infection with wt γHV68, v-cyclin.MR, and v-cyclin.LacZ resulted in equivalent establishment of latency, we determined the abilities of these infected cells to reactivate the virus from latency in an ex vivo reactivation assay (70, 72, 73). Splenocytes and PECs were analyzed by limiting-dilution reactivation analysis on MEF indicator cells (Fig. 5C and D). Viral CPE on the MEF monolayers was scored 21 days after explanation. Notably, splenocytes isolated from wt γHV68- or v-cyclin.MR-infected C57Bl/6 mice exhibited a low but detectable frequency of viral reactivation (∼1 in 200,000 cells) (Fig. 5C). However, no reactivation was observed with splenocytes harvested from v-cyclin.LacZ-infected C57Bl/6 mice (Fig. 5C). This impairment in v-cyclin.LacZ reactivation was readily apparent in latently infected PECs, for which the frequency of virus reactivation for both wt γHV68 (∼1 in 15,000 cells)- and v-cyclin.MR (∼1 in 25,000 cells)-infected mice was easily measured, while there was only a very low level of detectable virus reactivation for PECs harvested from v-cyclin.LacZ-infected mice (<1 in 106 cells) (Fig. 5D). Thus, the v-cyclin.LacZ virus was severely impaired for reactivation from latency in normal C57Bl/6 mice.
We have previously reported that γHV68-infected B-cell-deficient mice have altered latency (70, 73). These mice have a slightly elevated (approximately sixfold) frequency of viral-genome-positive cells in PECs compared to C57Bl/6 control mice. In the spleen, the frequencies of viral-genome-positive cells in B-cell-deficient and C57Bl/6 control mice are very similar. However, the frequency of γHV68 reactivation in splenocytes and PECs of infected B-cell-deficient mice was 50- to 100-fold higher than that for infected C57Bl/6 mice at day 42 postinfection.
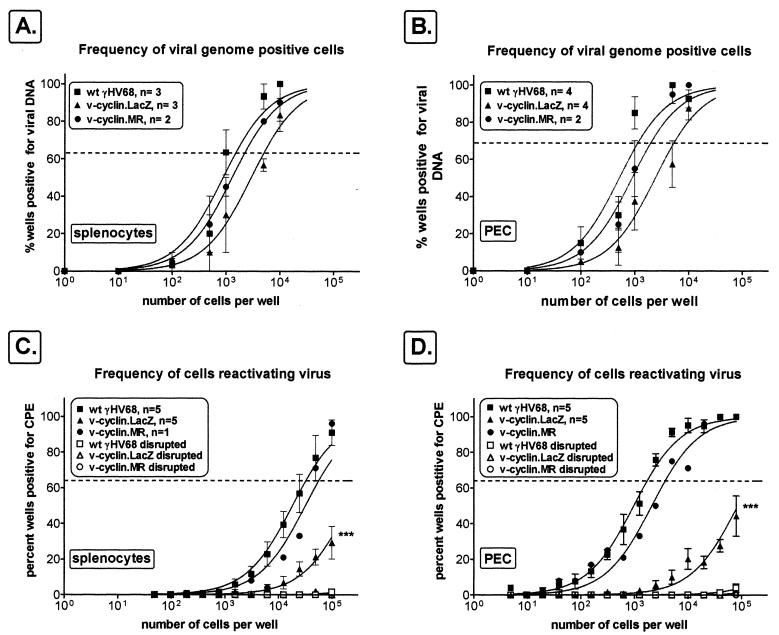
To determine whether the v-cyclin plays an important role in virus reactivation in these immunocompromised mice, we compared the frequencies of viral-genome-positive PECs and splenocytes and the frequencies of cells reactivating virus in B-cell-deficient mice infected with wt γHV68, v-cyclin.MR, or v-cyclin.LacZ. In B-cell-deficient mice at day 42 postinfection, the frequencies of v-cyclin.LacZ genome-positive cells in the spleen (∼1 in 5,000 cells) and PECs (∼1 in 4,500 cells) were slightly lower than the frequencies observed in mice infected with wt γHV68 (spleen, ∼1 in 1,500 cells; PECs, ∼1 in 700 cells) or v-cyclin.MR (spleen, ∼1 in 2,200 cells; PECs, ∼1 in 1,200 cells) (Fig. 6A and B). Consistent with the results obtained in infected C57Bl/6 mice, the frequency of splenocyte virus reactivation was significantly lower in B-cell-deficient mice infected with v-cyclin.LacZ virus (∼1 in 200,000 cells) than in those infected with either wt γHV68 (∼1 in 30,000 cells) or v-cyclin.MR (∼1 in 50,000 cells) (Fig. 6C). A greater reduction in reactivation efficiency was observed in PECs harvested from B-cell-deficient mice infected with v-cyclin.LacZ (∼1 in 150,000 cells) than in mice infected with wt γHV68 (∼1 in 1,800 cells) or v-cyclin.MR (∼1 in 2,500 cells). These results demonstrated that the phenotype of the v-cyclin mutation was dominant over the enhanced-reactivation phenotype observed in B-cell-deficient mice. In addition, the decreased reactivation efficiency of the v-cyclin mutant virus in B-cell-deficient mice indicates that the reactivation defect is apparent in a latently infected non-B-cell population, which is almost exclusively macrophages in PECs of B-cell-deficient mice (72).
FIG. 6.
The v-cyclin.LacZ mutant establishes latency but fails to reactivate efficiently in cells from B-cell-deficient mice. B-cell-deficient mice were infected with 106 PFU of v-cyclin.LacZ (triangles), wt γHV68 (squares), or v-cyclin.MR (circles), and cells were harvested 42 days postinfection for quantitation of the frequency of viral-genome-positive cells (A and B) and the frequency of cells reactivating virus (C and D). (A and B) Latently infected splenocytes and PECs were analyzed for the frequency of viral-genome-positive cells by limiting-dilution nested PCR, as described in the legend to Fig. 5 and in Materials and Methods. (C and D) Cells were harvested 42 days postinfection for limiting-dilution ex vivo reactivation analysis on MEF indicator monolayers, as described in the legend to Fig. 5 and in Materials and Methods. The presence of preformed infectious virus was assessed by limiting-dilution analysis of disrupted cells, with a control being included in each experiment (open symbols). Curve fit lines were derived by nonlinear-regression analysis (standard errors of the means are shown [error bars]). The dashed line is at 63%, the value which was used to calculate the frequency of genome-positive cells (A and B) or the frequency of reactivating cells (C and D) as indicated by a Poisson distribution. Data represent independent experiments as indicated, with each experiment utilizing cells pooled from four to seven mice. ∗∗∗, differences in the frequencies of reactivation for v-cyclin.LacZ and wt γHV68 were statistically significant (PECs, P = 0.0005; splenocytes, P = 0.0162).
In splenocytes and PECs from C57Bl/6 and B-cell-deficient mice, there was never detectable preformed infectious virus, as measured by plating mechanically disrupted cells (Fig. 5C and D and 6C and D). Therefore, the detection of viral-genome-positive cells and virus-reactivating cells represents latently infected cells rather than an ongoing lytic infection.
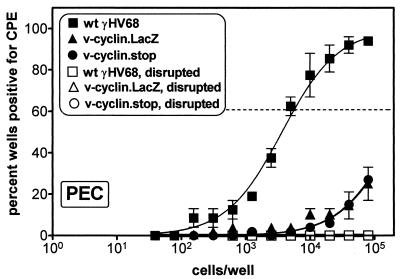
As described above, to address the concern that the insertion of the LacZ expression cassette into the v-cyclin gene might alter expression of adjacent genes, we generated a v-cyclin mutant virus (v-cyclin.stop) containing a translation stop codon at the beginning of the cyclin box (see Materials and Methods). v-cyclin.stop, as well as wt γHV68 and v-cyclin.LacZ, was used to infect C57Bl/6 mice, and PECs were analyzed in the ex vivo reactivation assay 28 days postinfection (Fig. 7). As observed with the v-cyclin.LacZ virus, there was a ∼100-fold reduction in the frequency of reactivation of v-cyclin.stop or v-cyclin.LacZ in PECs compared to wt γHV68. Thus, this analysis provides strong evidence that the observed defect in virus reactivation maps to the v-cyclin gene.
FIG. 7.
The v-cyclin.stop mutant exhibits the same reactivation phenotype as the v-cyclin.LacZ mutant. C57Bl/6 mice were infected with 106 PFU of v-cyclin.LacZ (triangles), wt γHV68 (squares), or v-cyclin.stop (circles) virus, and cells were harvested 28 days postinfection for quantitation of the frequency of virus reactivation in latently infected PECs, as described in the legend to Fig. 5 and in Materials and Methods. The data shown were pooled from two independent experiments with a total of 10 mice per virus. The diminished frequencies of reactivation of both the v-cyclin.LacZ (P = 0.0029) and v-cyclin.stop (P = 0.0023) compared to wt γHV68 were statistically significant.
DISCUSSION
All known gamma-2 herpesviruses encode a v-cyclin that is well conserved in sequence and position in the viral genome. Here we have demonstrated that the v-cyclin is critical for reactivation of γHV68 but is dispensable for acute virus replication in vivo. Based on this, we propose a model in which latently infected cells that have exited the cell cycle (e.g., resting B cells and macrophages) require v-cyclin function to reenter the cell cycle, thus poising them at a stage of the cell cycle which is permissive for virus replication. This model is consistent with our current understanding of v-cyclin expression in γHV68 infection based on two studies that have analyzed v-cyclin mRNA expression in latency. v-cyclin transcription was detected by reverse transcription-PCR in 4 of 16 and 1 of 16 reactions using RNA harvested from latently infected PECs or splenocytes, respectively (68). v-cyclin expression was not detected by Northern analysis of uninduced S11 cells, a B-lymphoma cell line harboring episomal γHV68 (30). The v-cyclin protein is expressed in ex vivo reactivating cultures (data not shown); however, the small number of latently infected cells and the asynchronous nature of reactivation do not allow for clear differentiation of expression in latently infected cells, in early reactivating cells, or in the subsequent spread and lytic infection in this system. The v-cyclin is clearly expressed during lytic infection and in reactivation and may be expressed at a low level in some latently infected cells. The inability of cellular cyclin/cdk inhibitors to inhibit the KSHV and HVS v-cyclins (24, 41, 65) raises the possibility that the v-cyclins have evolved to efficiently activate cdk's, even in resting differentiated cells (61). However, the data presented here also demonstrate that this function of the v-cyclin is clearly dispensable for direct entry into the lytic cycle during virus replication in permissive cells in vitro and in vivo. This difference between the requirements for reactivation and those for direct entry into the lytic cycle may reflect (i) differences in the cell types infected, (ii) the presence of a virus-encoded redundant activity which functions during direct entry into the lytic cycle but is not appropriately expressed during virus reactivation, and/or (iii) acute virus replication in vivo primarily occurring in cycling cells.
Is the v-cyclin gene critical for reactivation from latency in other gamma-2 herpesviruses? Despite the conservation of the gamma-2 herpesvirus v-cyclins, there are differences in their expression. The HVS v-cyclin protein is expressed in transformed T cells (32), and the KSHV v-cyclin transcript is expressed in KSHV-positive cell lines established from peripheral effusion lymphomas (11, 12, 14, 16). However, the KSHV v-cyclin was not detected in one case of benign, localized Castleman's disease (40), indicating that v-cyclin expression may vary in B cells latently infected with KSHV.
The KSHV v-cyclin is expressed as part of a complex transcriptional unit. The KSHV v-cyclin gene is flanked by gene 73, which encodes a nuclear antigen expressed in the spindle cells of Kaposi's sarcoma [latency-associated nuclear antigen (LANA)] (11, 35, 50), and K13, which encodes an inhibitor of FLICE (v-FLIP) (52). The ORFs encoding LANA 73, v-cyclin, and v-FLIP are expressed under the control of a single promoter, resulting in at least three distinct alternatively spliced transcripts which are 3′ coterminal (16). There is a slight increase in the level of KSHV v-cyclin-specific mRNA upon phorbol ester induction of lytic-gene expression in some PEL cell lines (16).
In contrast to v-cyclin expression by KSHV, γHV68 lytic infection of murine fibroblasts results in strong transcription of the v-cyclin gene (66). Notably, in the γHV68 genome the M11 gene (encoding v-bcl2) is interposed, in the opposite orientation, between gene 73 and the v-cyclin gene (gene 72) (Fig. 1A) (67), suggesting that the v-cyclin gene and gene 73 are regulated independently (67). While γHV68 v-cyclin is abundantly transcribed during lytic infection, we previously detected little transcription of gene 73 or M11 during virus replication in NIH 3T12 fibroblasts (68), although others have detected gene 73 and M11 transcripts in poly(A)+ RNA from lytically infected BHK cells (62). There are other examples of genes with contrasting expression patterns in γHV68 and KSHV. The KSHV genes encoding GPCR and v-bcl2 are induced by phorbol ester and are therefore considered to be lytic genes (55) (reviewed in reference 57), whereas in γHV68 infection the GPCR (gene 74) and v-bcl2 (M11) transcripts are difficult to detect in lytically infected fibroblasts and score as latency-associated genes in a reverse transcription-PCR screen of latently infected tissue (68). However, it is important to note that the tissue culture systems used to characterize KSHV and γHV68 gene expression are quite distinct. In the case of KSHV, studies have been limited to characterizing phorbol ester induction of lytic genes in a limited number of latently infected tumor cell lines, while the characterization of γHV68 lytic-gene expression has been largely limited to virus replication in permissive fibroblast cell lines. There may be fundamental differences in the regulation of specific viral genes depending on the cell type infected and on whether reactivation from latency or direct entry into the lytic cycle is assessed. In addition, some genes associated with latency may be induced during virus replication, as has been observed for the EBV v-bcl2 gene homolog (the BHRF1 gene) (4). Thus, a great deal of caution must be taken in inferring the role(s) of a viral gene in pathogenesis based on its pattern of expression in tissue culture models. The results presented here confirm this point. Despite scoring as a lytic cycle gene in permissive fibroblasts, the γHV68 v-cyclin gene is not essential for viral replication but is key for reactivation from latency.
The requirement for the γHV68 v-cyclin for reactivation from latency may well be related to a need to cause cell cycle progression to the G1/S boundary in order to foster efficient DNA replication during virus reactivation. There is substantial precedence in other herpesvirus systems for control of cell cycle progression. With respect to host cyclin function, B-cell growth transformation by EBV is associated with upregulation of host D-type cyclins through expression of latency-associated membrane protein 1 (3, 28). The immediate-early protein ICP0 of the alphaherpesvirus herpes simplex virus type 1 interacts with host cyclin D3 (34), and mutants lacking functional ICP0 are complemented by G1/S-phase cellular factors (7). In addition, replication of both herpes simplex virus and HCMV, a betaherpesvirus, is decreased by drugs that inhibit cdk's (6, 59).
There is also growing evidence that during herpesvirus replication, lytically infected cells are arrested at a specific stage of the cell cycle. In EBV-growth-transformed B cells, the immediate-early transcription activator Zta triggers viral reactivation and also blocks cell cycle progression prior to S phase (10). Similarly, the alphaherpesvirus bovine herpesvirus 1 appears to inhibit cell cycle progression through S phase (58). Infection with the betaherpesvirus HCMV arrests cells at G1/S (and perhaps G2/M) (reviewed in references 17 and 53). Thus, control of the cell cycle, by induction of cell cycle progression and/or arrest at S phase, appears to be an important feature of alpha-, beta-, and gamma-herpesvirus replication.
Given that the γHV68 v-cyclin is expressed as an early-late gene in murine fibroblasts, it is surprising that the v-cyclin is completely dispensable for acute virus replication in vivo. This suggests that γHV68 encodes other functions that regulate the cell cycle in permissive cell types in vivo. However, the data presented here clearly document that the v-cyclin is critical for efficient reactivation from latency. The latter observation provides the first demonstration of a viral protein that appears to be specifically required for efficient reactivation from latency, and it suggests that the molecular events involved in virus reactivation in a latently infected cell are distinct from those involved in direct entry into the lytic cycle in a permissive cell type. It remains to be determined whether the v-cyclins encoded by other gamma-2 herpesviruses play a similar role during reactivation. This currently cannot be addressed for KSHV, but it could be determined for infection of primates with either HVS or the recently identified rhesus monkey rhadinovirus. Finally, the data presented here underscore the limitations of in vitro analyses of viral mutants and the importance of pathogenesis studies in determining the roles of specific herpesvirus genes.
ACKNOWLEDGMENTS
S.H.S. was supported by NIH grants CA43143, CA52004, CA58524, CA74730, and HL60090. H.W.V. was supported by grant RPG-97-134-01-MBC from the American Cancer Society and by NIH grants AI39616, CA74730, and HL60090. L.V.D. was supported by grant PF-4379 from the American Cancer Society and grant 5 T32 AI07163 from the NIH.
We thank Avril Adelman of the Washington University Medical Center Biostatistics Department for help with statistical analyses. We also thank members of the laboratories of H.W.V., S.H.S., David Leib, and Lynda Morrison for constructive comments on this research.
REFERENCES
- 1.Albrecht J-C, Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. 1992;66:3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittmann S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Yaseen N, Sharma S. Latent membrane protein-1 induces cyclin D2 expression, pRb hyperphosphorylation, and loss of TGF-beta-1-mediated growth inhibition on EBV-positive B cells. J Immunol. 1995;155:1047–1056. [PubMed] [Google Scholar]
- 4.Austin P J, Flemington E, Yandava C N, Strominger J L, Speck S H. Complex transcription of the Epstein-Barr virus BamHI fragment H rightward open reading frame 1 (BHRF1) in latently and lytically infected B lymphocytes. Proc Natl Acad Sci USA. 1988;85:3678–3682. doi: 10.1073/pnas.85.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosma M, Schuler W, Bosma G. The scid mouse mutant. Curr Top Microbiol Immunol. 1988;137:197–202. doi: 10.1007/978-3-642-50059-6_29. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 7.Cai W, Schaffer P A. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannell E, Mittnacht S. Viral encoded cyclins. Semin Cancer Biol. 1999;9:221–229. doi: 10.1006/scbi.1999.0090. [DOI] [PubMed] [Google Scholar]
- 9.Cannell E J, Farrell P J, Sinclair A J. Epstein-Barr virus exploits the normal cell pathway to regulate Rb activity during the immortalisation of primary B-cells. Oncogene. 1996;13:1413–1421. [PubMed] [Google Scholar]
- 10.Cayrol C, Flemington E K. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 1996;15:2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 11.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden-Kent D, Paterson H, Weiss R A, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 13.Clambey E T, Virgin IV H W, Speck S H. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis M A, Sturzl M A, Blasig C, Schreier A, Guo H G, Reitz M, Opalenik S R, Browning P J. Expression of human herpesvirus 8-encoded cyclin D in Kaposi's sarcoma spindle cells. J Natl Cancer Inst. 1997;89:1868–1874. doi: 10.1093/jnci/89.24.1868. [DOI] [PubMed] [Google Scholar]
- 15.DeLuca N A, Schaffer P A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988;62:732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmer D, Mocarski E S. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty P C, Topham D J, Tripp R A, Cardin R D, Brooks J W, Stevenson P G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 19.Doherty P C, Tripp R A, Hamilton-Easton A M, Cardin R D, Woodland D L, Blackman M A. Tuning into immunological dissonance: an experimental model for infectious mononucleosis. Curr Opin Immunol. 1997;9:477–483. doi: 10.1016/s0952-7915(97)80098-2. [DOI] [PubMed] [Google Scholar]
- 20.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 21.Dutia B M, Clarke C J, Allen D J, Nash A A. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Efstathiou S, Ho Y M, Hall S, Styles C J, Scott S D, Gompels U A. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 23.Efstathiou S, Ho Y M, Minson A C. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 24.Ellis M, Chew Y P, Fallis L, Freddersdorf S, Boshoff C, Weiss R A, Lu X, Mittnacht S. Degradation of p27Kip cdk inhibitor triggered by Kaposi's sarcoma virus cyclin-cdk6 complex. EMBO J. 1999;18:644–653. doi: 10.1093/emboj/18.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 26.Fodor W L, Rollins S A, Bianco-Caron S, Rother R P, Guilmette E R, Burton W V, Albrecht J-C, Fleckenstein B, Squinto S P. The complement control protein homolog of herpesvirus saimiri regulates serum complement by inhibiting C3 convertase activity. J Virol. 1995;69:3889–3892. doi: 10.1128/jvi.69.6.3889-3892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollyoake M, Stuhler A, Farrell P, Gordon J, Sinclair A. The normal cell cycle activation program is exploited during the infection of quiescent B lymphocytes by Epstein-Barr virus. Cancer Res. 1995;55:4784–4787. [PubMed] [Google Scholar]
- 29.Horton L E, Templeton D J. The cyclin box and C-terminus of cyclins A and E specify CDK activation and substrate specificity. Oncogene. 1997;14:491–498. doi: 10.1038/sj.onc.1200851. [DOI] [PubMed] [Google Scholar]
- 30.Husain S M, Usherwood E J, Dyson H, Coleclough C, Coppola M A, Woodland D L, Blackman M A, Stewart J P, Sample J T. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8+ T lymphocytes. Proc Natl Acad Sci USA. 1999;96:7508–7513. doi: 10.1073/pnas.96.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich N P. Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 32.Jung J U, Stäger M, Desrosiers R C. Virus-encoded cyclin. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapadia S B, Molina H, van Berkel V, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J Virol. 1999;73:7658–7670. doi: 10.1128/jvi.73.9.7658-7670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedes D H, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J Clin Investig. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi H, Stewart E, Poon R, Adamczewski J P, Gannon J, Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell. 1992;3:1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lees E M, Harlow E. Sequences within the conserved cyclin box of human cyclin A are sufficient for binding to and activation of cdc2 kinase. Mol Cell Biol. 1993;13:1194–1201. doi: 10.1128/mcb.13.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Lee H, Yoon D-W, Albrecht J-C, Fleckenstein B, Neipel F, Jung J U. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luppi M, Barozzi P, Maiorana A, Trovato R, Marasca R, Morselli M, Cagossi K, Torelli G. Expression of cell-homologous genes of human herpesvirus-8 in human immunodeficiency virus-negative lymphoproliferative diseases. Blood. 1999;94:2931–2933. [PubMed] [Google Scholar]
- 41.Mann D J, Child E S, Swanton C, Laman H, Jones N. Modulation of p27Kip1 levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J. 1999;18:654–663. doi: 10.1093/emboj/18.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16INK4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan D O. The dynamics of cyclin dependent kinase structure. Curr Opin Cell Biol. 1996;8:767–772. doi: 10.1016/s0955-0674(96)80076-7. [DOI] [PubMed] [Google Scholar]
- 44.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 45.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 46.Nash A A, Usherwood E J, Stewart J P. Immunological features of murine gammaherpesvirus infection. Semin Virol. 1996;7:125–130. [Google Scholar]
- 47.Nicholas J, Cameron K R, Honess R W. Herpesvirus saimiri encodes homologs of G protein-coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 48.Ojala P M, Tiainen M, Salven P, Veikkola T, Castanos-Velez E, Sarid R, Biberfeld P, Makela T P. Kaposi's sarcoma-associated herpesvirus-encoded v-cyclin triggers apoptosis in cells with high levels of cyclin-dependent kinase 6. Cancer Res. 1999;59:4984–4989. [PubMed] [Google Scholar]
- 49.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 50.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S-J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo A A, Jeffrey P D, Pavletich N P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 52.Russo J J, Bohenzky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvant B S, Fortunato E A, Spector D H. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J Virol. 1998;72:3729–3741. doi: 10.1128/jvi.72.5.3729-3741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 55.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarid R, Wiezorek J S, Moore P S, Chang Y. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J Virol. 1999;73:1438–1446. doi: 10.1128/jvi.73.2.1438-1446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarid R. Gene expression of Kaposi's sarcoma-associated herpesvirus. Epstein-Barr Virus Rep. 1999;6:64–70. [Google Scholar]
- 58.Schang L M, Hossain A, Jones C. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J Virol. 1996;70:3807–3814. doi: 10.1128/jvi.70.6.3807-3814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulze-Gahmen U, Jung J U, Kim S H. Crystal structure of a viral cyclin, a positive regulator of cyclin-dependent kinase 6. Structure. 1999;7:245–254. doi: 10.1016/s0969-2126(99)80035-5. [DOI] [PubMed] [Google Scholar]
- 61.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 62.Simas J P, Swann D, Bowden R, Efstathiou S. Analysis of murine gammaherpesvirus-68 transcription during lytic and latent infection. J Gen Virol. 1999;80:75–82. doi: 10.1099/0022-1317-80-1-75. [DOI] [PubMed] [Google Scholar]
- 63.Sinclair A J, Palmero I, Holder A, Peters G, Farrell P J. Expression of cyclin D2 in Epstein-Barr virus-positive Burkitt's lymphoma cell lines is related to methylation status of the gene. J Virol. 1995;69:1292–1295. doi: 10.1128/jvi.69.2.1292-1295.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Speck S H, Virgin H W., IV Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr Opin Microbiol. 1999;2:403–409. doi: 10.1016/s1369-5274(99)80071-x. [DOI] [PubMed] [Google Scholar]
- 65.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 66.Van Dyk L F, Hess J L, Katz J D, Jacoby M, Speck S H, Virgin H W., IV The murine gammaherpesvirus 68 v-cyclin is an oncogene that promotes cell cycle progression in primary lymphocytes. J Virol. 1999;73:5110–5122. doi: 10.1128/jvi.73.6.5110-5122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Virgin H W, IV, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Virgin H W, IV, Presti R M, Li X-Y, Liu C, Speck S H. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J Virol. 1999;73:2321–2332. doi: 10.1128/jvi.73.3.2321-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Virgin H W, IV, Speck S H. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–379. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 70.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W., IV Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weck K E, Dal Canto A J, Gould J D, O'Guin A K, Roth K A, Saffitz J E, Speck S H, Virgin H W., IV Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat Med. 1997;3:1346–1353. doi: 10.1038/nm1297-1346. [DOI] [PubMed] [Google Scholar]
- 72.Weck K E, Kim S S, Virgin IV H W, Speck S H. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol. 1999;73:3273–3283. doi: 10.1128/jvi.73.4.3273-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weck K E, Kim S S, Virgin IV H W, Speck S H. B cells regulate murine gammaherpesvirus 68 latency. J Virol. 1999;73:4651–4661. doi: 10.1128/jvi.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]