Abstract
Cognitive training using a visual speed-of-processing task, called the Useful Field of View (UFOV) task, reduced dementia risk and reduced decline in activities of daily living at a 10-year follow-up in older adults. However, there was variability in the achievement of cognitive gains after cognitive training across studies, suggesting moderating factors. Learning trials of visual and verbal learning tasks recruit similar cognitive abilities and have overlapping neural correlates with speed-of-processing/working memory tasks and therefore could serve as potential moderators of cognitive training gains. This study explored the association between the Hopkins Verbal Learning Test-Revised (HVLT-R) and Brief Visuospatial Memory Test-Revised (BVMT-R) learning with a commercial UFOV task called Double Decision. Through a secondary analysis of a clinical trial, we assessed the moderation of HVLT-R and BVMT-R learning on Double Decision improvement after a 3-month speed-of-processing/attention and working memory cognitive training intervention in a sample of 75 cognitively healthy older adults. Multiple linear regressions showed that better baseline Double Decision performance was significantly associated with better BVMT-R learning (β = − .303). This association was not significant for HVLT-R learning (β = − .142). Moderation analysis showed that those with poorer BVMT-R learning improved the most on the Double Decision task after cognitive training. This suggests that healthy older adults who perform below expectations on cognitive tasks related to the training task may show the greatest training gains. Future cognitive training research studying visual speed-of-processing interventions should account for differing levels of visuospatial learning at baseline, as this could impact the magnitude of training outcomes and efficacy of the intervention.
Keywords: Healthy aging, Cognitive training, Speed of processing, Visual learning, Verbal learning
Introduction
Cognitive training interventions are studied as a method to intervene in the trajectory of cognitive decline in older adults, ultimately aiming to reduce the risk of dementia [1]. Results from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) cognitive training trial, perhaps the largest (n = ~ 2800) longitudinal randomized control trial of cognitive training, indicated an immediate improvement in performance of training tasks, and this improvement in on tasks trained was still detected in the speed-of-processing training group up to 10 years after training ended [2]. In other words, all training groups (reasoning, speed of processing, memory) immediately improved on their respective training tasks; however, the speed-of-processing training group’s improvement sustained over a 10-year period. Moreover, another study on the same clinical trial data found that the speed-of-processing training group, but not reasoning or memory training group, had an overall 29% reduced risk of dementia at the 10-year follow-up compared to control (p = 0.049), with a 10% lower hazard rate for dementia with each additional training session [3].
The training task used in the speed-of-processing group was the Useful Field of View (UFOV) task. This task targets divided attention and visual speed of processing [4, 5]. Better performance on the UFOV task has been associated with fewer motor vehicle accidents in older adults, faster timed tasks of activities of daily living, and better attention, executive functioning, and visual processing abilities [5–7]. A systematic review and meta-analysis assessing the impact of all cognitive training interventions using the UFOV task also found it to be efficacious at improving speed-of-processing and attentional abilities, as well as improving activities of daily living abilities 7 years after training ended [8, 9]. While the ACTIVE trial demonstrated the benefits of visual speed-of-processing training, reviews of the broader cognitive training literature have found that multi-domain training, including speed of processing and working memory, resulted in the most robust boost in cognitive abilities [1, 10–12].
Despite the group-level impact of cognitive training, there is still substantial individual variability evident in cognitive training gains, which may impact the reliability and reproducibility of cognitive training findings [13, 14]. Studies assessing the impact of individual differences on speed-of-processing and working memory training gains found no effects of older age, gender, everyday living abilities, motivation/self-efficacy, performance expectation, personality factors, leisure activities, or computer literacy on training gains [8, 14–17]. One such variable that may impact cognitive training gains and learning ability is overall intelligence, or general cognitive ability. Prior research has shown that those with higher performance on one cognitive measure tend to also have higher performance on other measures as well, suggesting an common cognitive ability or intelligence factor that may underlie cognitive tests [18]. When applied to cognitive training, one study found in a sample of younger adults that higher levels of fluid intelligence positively associated with the learning curve of a video game cognitive training intervention, with the implication that baseline fluid intelligence may play a role in individual training gains [19]. On the other hand, a comprehensive meta-analysis exploring the moderation of baseline cognitive performance on executive functioning interventions in studies including participants of any age and neurological status found that those with weaker baseline cognitive performance in the task trained improved the most on training tasks [23]. This has also been a consistent finding in the UFOV literature, as one study found that poorer baseline UFOV abilities were related to larger UFOV training gains in healthy older adults [15]. This meta-analysis highlighted the need for more studies assessing cognitive moderators of cognitive training gains. Despite there being greater variability in fluid cognitive performance in aging populations, baseline fluid cognitive abilities in older adults have not been well studied as a potential moderator of training gains [20, 21].
For example, the moderation of baseline cognitive abilities that are not directly related to the training task has not been studied, even though these cognitive abilities are sensitive in detecting mild cognitive impairment and dementia status. In particular, visual and verbal list learning paradigms are sensitive in differentiating healthy cognitive groups from mild cognitive impairment and Alzheimer’s disease groups in older adults [24–26]. List learning assessments involve repeated presentation of stimuli over serial trials with the goal of learning the stimuli for later recall. The learning slopes, or amount of information learned across successive trials, has been uniquely associated with psychomotor speed, working memory, and speeded and attentional aspects of executive functioning [27–31]. As prior studies suggest moderating variables of cognitive training gains often share features of the training task, the learning slope of visual and verbal list learning tasks could potentially moderate gains in speed-of-processing/working memory cognitive training interventions. For example, one study found that the learning slope on a verbal memory measure was related to 10-week memory training benefit [32].
Additionally, the neural correlates of visual and verbal learning slopes overlap with working memory, attention, and speed-of-processing tasks, such as the UFOV. Better learning slopes in a verbal list learning task have been associated with thicker cortex of the ventrolateral prefrontal cortex in a mild cognitively impaired sample, and flatter learning curves are associated with prefrontal cortex damage [33, 34]. Additionally, hippocampal and parahippocampal cortical thickness and volume are associated with better verbal and visual learning abilities [32, 33, 35]. These brain areas have also shown a structural and functional association with working memory, attention, and UFOV task performance in older adults [36–42]. Therefore, learning slope measures from visual and verbal list learning assessments could be utilizing similar neurocognitive processes as working memory and attention training tasks, particularly in the UFOV task.
Exploring this moderation is important, as the ultimate goal of cognitive training in aging is often to reduce dementia risk, and visual/verbal list learning assessments are commonly given in clinical settings to determine cognitive status [43]. Being cognizant of which potential participants or patients could have the greatest gains from cognitive training is imperative to boost the efficacy of cognitive training interventions, boost rigor in the cognitive training literature, and eventually provide tailored treatment recommendations. While visual/verbal learning abilities may moderate gains from a variety of speed-of-processing and working memory cognitive intervention tasks, we are focused here on gains from the UFOV task. Training and performance in the UFOV task have been directly associated with maintained activities of daily living and reduced dementia risk, and performance on this task improved to the greatest degree in a multidomain cognitive training intervention [2, 3, 44].
Therefore, our study objectives include (1) determining the association between learning measures of common visual/verbal list learning and memory tasks (HVLT-R and BVMT-R) and the UFOV task and (2) determining the potential moderation of visual/verbal learning on UFOV improvement after a 3-month speed-of-processing/attention and working memory cognitive training intervention in cognitively healthy older adults.
Methods
Participants
Participants were part of the National Institute on Aging funded, double-blinded randomized clinical trial, Augmenting Cognitive Training in Older Adults (ACT; NCT028511) [45]. Our primary goal was to determine if non-invasive brain stimulation, via transcranial direct current stimulation (tDCS), augmented gains of cognitive training in healthy older adults. Detailed description of the trial design are found in Woods et al. [45]. All participants were part of phase 1 of the trial. Roughly half of the sample received active tDCS, and the other half received sham tDCS.
Healthy older adults were recruited at the Universities of Florida and Arizona via local research registries, community outreach, community agencies, newspaper advertisements, public service announcements, mailings, and posted flyers. Major eligibility requirements included right handedness, age range of 65–89, no history of neurological disorders (e.g., brain injury or dementia), no history of major psychiatric illness, and no contraindications to MRI; further details are found in [45]. Cognitive status was screened via administration of the National Alzheimer’s Coordinating Center (NACC) Uniform Dataset (UDS-III) [46]. Participants were not eligible if they performed 1.5 standard deviations below the age, sex, and education-corrected mean on a general cognitive screen, or in the domains of memory, executive functioning, language, or visuospatial function. All participants provided written informed consent, and the study was approved by the Institutional Review Boards at the University of Arizona and the University of Florida. Research was carried out in accordance with institutional guidelines and the Declaration of Helsinki.
Of the 87 participants recruited for phase 1, 4 participants were not able to complete the follow-up visit due to reasons unrelated to the study (e.g., moving away), 2 participants withdrew their participation, and 1 participant experienced discomfort during testing that was unrelated to the intervention. Five additional participants were considered non-adherent to training tasks. This resulted in a final sample of 75: 36 participants in the cognitive training group and 39 in the education control group. The training groups did not differ significantly on any demographic variables (Table 1). Of the total sample, 63 (84%) identified as White, 4 (5.3%) as Black or African American, 3 (4%) as American Indian/Alaskan Native, 3 (4%) more than one race, 1 (1.3%) as Asian, and 1 (1.3%) identified as Native Hawaiian or Other Pacific Islander. Of the 7 individuals who identified as Hispanic/Latino/Latina ethnicity, 4 were White (57%), 1 (14%) was more than one race, 1 (14%) was American Indian/Alaskan Native, and 1 (14%) was Black/African American.
Table 1.
Sample demographics
| Cognitive training group (n = 36), mean ± SD (range) | Education control group (n = 39), mean ± SD (range) | Total (n = 75), mean ± SD (range) | |
|---|---|---|---|
| Age | 70.53, 3.90 (65–80) | 71.64, 5.17 (65–84) | 71.11, 4.61 (65–84) |
| Sex Ma:F | 19:17 | 22:17 | 41:34 |
| Education | 16.47, 2.30 (12–21) | 16.38, 2.20 (12–21) | 16.43, 2.23 (12–21) |
M mean, SD standard deviation, Ma male, F female
Study design
At their first visit, all participants completed screening assessments and cognitive training measures to capture pre-intervention performance. Within 60 days of their first visit, they completed a second visit that included a neurocognitive assessment battery that incorporated visual and verbal learning measures. Participants were then randomized into one of four study arms: cognitive training with active or sham tDCS or education control with active or sham tDCS. In total, cognitive training and education control participants completed sixty, 40-min training sessions over a span of 12 weeks, resulting in forty total hours of training. Active and sham tDCS groups received tDCS stimulation during 20 of their 60 training sessions using identical montages and stimulation parameters except for the duration of stimulation [45]. The active group received 20 min of 2.0 mA (30-s ramp up and down) direct current through two electrodes (area under the anode electrode was right F4, and the area under cathode electrode was left F3). Sham participants received only 30-s ramp up/down of 2.0 mA current stimulation at the beginning of the session. At the end of their training (approximately 12 weeks or 3 months later), participants returned for a post-intervention follow-up that included assessment of post-training performance. A visual flow of the study design is depicted in Fig. 1. A detailed description of full study design can be found in Woods et al. [45].
Fig. 1.
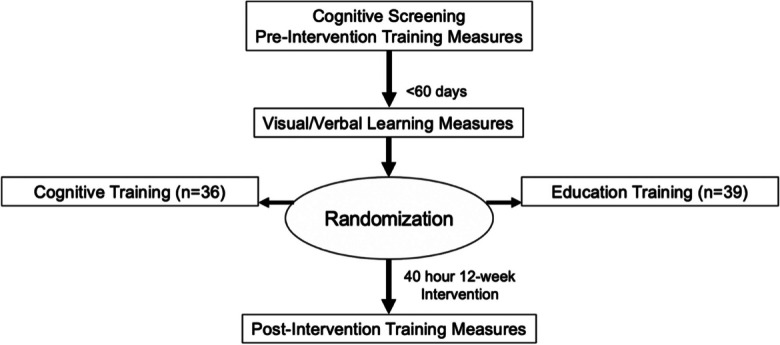
Study timeline
Randomization procedures
Randomization was performed by the clinical trial statistician. Permuted block randomization was used with block sizes of 8 and 12, and with treatment site as stratification factor. Therefore, at each site, two participants were assigned, in random order, to each one of the four conditions among the first eight participants. Three participants were assigned to each one of the four conditions among the next twelve participants, in random order.
Cognitive training procedures
All cognitive training was web-based and completed via laptop computer. Cognitive training consisted of four tasks targeting attention/speed-of-processing and four tasks targeting working memory from the Posit Science Brain HQ suite (www.brainhq.com; Posit Science, San Francisco, CA) via its research portal. Training tasks are commercially available at www.positscience.com. The following description is also detailed in Hardcastle et al. [44]. All training tasks adapt for increasing difficulty unique to that task by increasing the number of items to be remembered, shortening presentation time, or increasing the number of distractors. Participants were asked to complete four tasks per day, spending 10 min per task. During this time, participants completed “levels” on each task, and it takes 15–20 levels to complete 40 min of training, on average. When the 10-min limit was met, a timer built into the portal allowed the participant to move to the next task. Presentation of the tasks was counterbalanced and randomized so that participants were exposed to training tasks equally over the 12-week training period, with different tasks each day. Training performance was monitored for adherence, and interventionists were available for remediation strategies throughout the study to ensure participants reached their targeted training dose. For this study, adherence was defined as completing greater than or equal to 80% of total expected levels. See Table 2 for a list and description of training tasks, adapted from Hardcastle et al. [44].
Table 2.
Cognitive training subtests
| Subtest description | |
|---|---|
| Attention/speed of processing | |
| Hawk Eye | Participant must quickly identify a target object among distractors presented for varying amount of time |
| Divided Attention | Participant must quickly match colors, shapes, and/or fill patterns while ignoring distractor information |
| Target Tracker | Participant must accurately track several items moving around the screen amidst distractor items |
| Double Decision | Participants must to correctly identify a target object in the center of the screen, while correctly locating a simultaneously presented target object in the periphery among distractors |
| Working memory | |
| To Do List | Participants must to remember auditorily presented instructions |
| Memory Grid | Participants must match spatially distributed cards quickly |
| Auditory Ace | Participant is presented with auditory information about a playing card and must decide if the current card matches the card a specific number of cards back (auditory n-back) |
| Card Shark | Participant is presented with a playing card must decide if the current card matches the card a specified number of cards back (visual n-back) |
Education training procedures
Participants were asked to watch 40-min National Geographic Channel educational videos covering a range of topics (e.g., history, nature, wildlife). Each video was unique to that day of training. To ensure active engagement, participants were asked and reminded at the end of each video to answer questions regarding the content of videos found in a binder provided to them. Questions were returned to intervention coordinators and served as a gauge of training adherence; greater than or equal to 80% of questions correct was considered adherent. As with the cognitive intervention group, if necessary, remediation strategies were discussed to ensure participants met their training dose. See Hardcastle et al. [44] for further details.
Double Decision assessment
UFOV performance was measured via a commercially available task program from POSIT Science Brain HQ (www.brainhq.com), titled “Double Decision.” This task incorporates all elements of the classic UFOV task and is effective as a speed-of-processing intervention [47, 48]. To assess Double Decision performance, the Double Decision cognitive training task was administered at a moderate difficulty level, with 7 distractors at the screening and the post-intervention visit as part of the pre- and post-intervention training measure assessment. Therefore, this outcome measure will be referred to as the “Double Decision assessment” for clarity. For this task, participants were shown a screen (as seen in Fig. 2) for varying amounts of time. After the objects on the screen disappear, participants were asked to correctly discriminate between objects in the center of the screen (truck or car) while also correctly locating a simultaneously presented target object in the periphery among distractors (Route 66 sign). Presentation times varied based on the participant’s previous answer; if a participant answered correctly, then presentation times were shortened. The outcome variable was the log10 transformed average of presentation times of correct trials. While this Double Decision assessment task is similar in methodology to the Double Decision training task, the training task utilized a more complex background, had adaptive presentation times and numbers of distractors, and had differing levels of similarities between targets and distractors. Thus, performance on the Double Decision assessment task served as a measure of proximal improvement, or improvement on performance of the training tasks.
Fig. 2.

Still frame of POSIT Double Decision assessment at moderate difficulty. Reproduced/adapted from POSIT Brain HQ, used with permission
Visual and verbal learning
Verbal learning was assessed via the HVLT-R [49]. This measure assesses both learning and memory; however, only the learning portion of this task was used in this study. This assessment consists of three learning trials. For each learning trial, participants are read a list of 12 words (3 semantic groups containing 4 words each). Immediately after hearing the list of words, participants are asked to verbally recall as many words as they remember. Scoring includes one point per correct word recalled, totaling to 12 possible points per learning trial.
Visual learning was assessed via the BVMT-R [50]. This measure assesses both learning and memory; however, here only the learning portion of this task is used in this study. This assessment consists of three learning trials. For each learning trial, an array of 6 geometric line drawings are presented to the participant for 10 s. After presentation, the stimuli are taken away and the participant is asked to draw as many figures as they remember and in their proper locations on a blank sheet of paper. Scoring for each figure ranges from 0 to 2 depending on the accuracy and location of each figure. This results in a total score that can range from 0 to 12 for each learning trial.
Learning was quantified as the HVLT-R and BVMT-R learning ratio scores [24, 51]. This learning ratio differs from the traditional learning score (trial 3 total minus trial 1 total) because it accounts for information learned on trial 1. Therefore, it is more sensitive and specific than the traditional learning score at differentiating groups with normal cognition, mild cognitive impairment, and Alzheimer’s disease [24]. The equation for the HVLT-R and BVMT-R learning ratio is as follows:
Statistical analysis
All statistical analyses were completed via SPSS version 27. Per the primary aim of the ACT study, participants in phase 1 were randomly assigned to active or sham tDCS groups. As tDCS group effects were not of interest in this study, a blinded binary covariate of tDCS group was included as an additional moderator in moderation statistical models. tDCS group was also counterbalanced across cognitive training and education control groups. Therefore, we did not anticipate that tDCS group would play a significant role in our findings. Study site, age, sex, and education were included as covariates in all statistical models, as these variables impact cognitive performance [52–54].
Assumptions of normality for linear regression were checked. Then, linear regression models were conducted predicting baseline Double Decision assessment performance from HVLT-R and BVMT-R learning ratio scores separately, controlling for study site, age, sex, and education.
To assess for moderation, a repeated measure moderation analysis was performed via the MEMORE 2.1 (Mediation and Moderation analysis for repeated measures designs) macro tool in SPSS [55]. Moderation models were performed on the cognitive training group and education control groups separately and assessed the moderation of baseline HVLT-R and BVMT-R learning ratio scores on Double Decision assessment performance from baseline (pre-intervention) to 12-week (post-intervention) follow-up. Moderation variables were residuals controlling for study site, age, sex, and education. A conceptual diagram of the moderation model is found in Fig. 3.
Fig. 3.
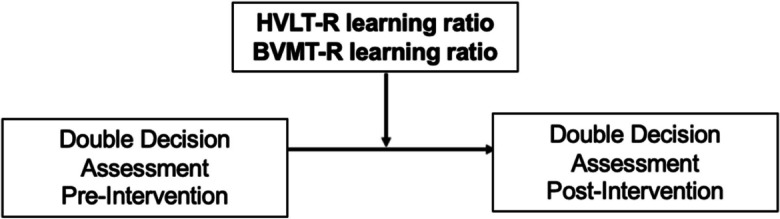
Diagram of moderation model
Results
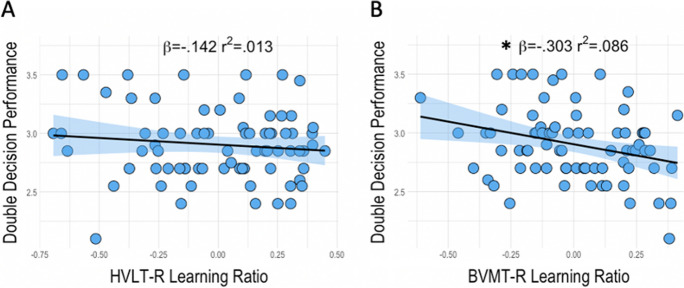
Linear regressions assessing the relationship between baseline Double Decision assessment and visual and verbal learning ratio scores found no significant association between Double Decision assessment performance and HVLT-R learning ratio (β = − 0.142, t = − 1.190, p = 0.238). BVMT-R learning ratio, however, did predict Double Decision assessment in that better learning was associated with faster Double Decision assessment performance (β = − 0.303, t = − 2.753, p = 0.008) (Fig. 4). Covariates of study site, age, sex, and education did not predict Double Decision assessment performance in either regression model (p > 0.05).
Fig. 4.
Regressions of HVLT-R learning ratio (A) and BVMT-R learning ratio (B) with Double Decision assessment performance with 95% confidence Intervals; r.2 reflects variance explained from the partial correlation between predictors and Double Decision assessment performance; β = standardized beta; x-axis = residual predictors controlling for covariates; y-axis = Double Decision performance; *p < .05
Repeated measure moderation models (with 5000 bootstrapping iterations) assessed the moderation of HVLT-R and BVMT-R learning ratios on Double Decision assessment performance change from pre- to post-intervention for cognitive training and education control groups separately. HVLT-R learning ratio moderation models did not predict a significant amount of variance in Double Decision assessment change from pre- to post-intervention in the education control [F(2,36) = 0.8321, p = 0.4433, r2 = 0.0442] or in the cognitive training groups [F(2,33) = 0.4706, p = 0.6288, r2 = 0.0277]. As a moderator, HVLT-R learning ratio did not significantly moderate this relationship for cognitive training or education control groups (p > 0.05).
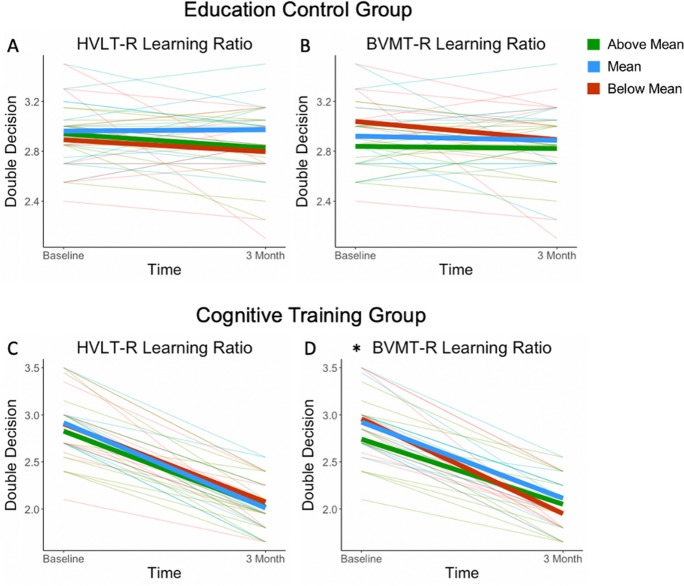
BVMT-R learning ratio moderation models did not predict a significant amount of variance in Double Decision assessment change from pre- to post-intervention in the education control group [F(2,36) = 1.3345, p = 0.2760, r2 = 0.0690] nor did BVMT-R learning ratio moderate this relationship significantly (p > 0.05). For the cognitive training group, the BVMT-R learning ratio moderation model did predict a significant amount of variance in Double Decision assessment change from pre- to post-intervention [F(2,36) = 6.5734, p = 0.0040, r2 = 0.2049] and BVMT-R learning ratio significantly moderated this relationship (p < 0.01). For each unit increase in BVMT-R learning ratio, there was a 0.6173 unit decrease in the difference from pre- to post-intervention performance. For interpretation, this moderation suggests that cognitive training is less effective for individuals with a higher BVMT-R learning ratio and is more effective for individuals with a lower BVMT-R learning ratio. Individuals with a lower BVMT-R learning ratio improved more on the Double Decision assessment task after cognitive intervention (Table 3).
Table 3.
Moderation models
| b | t (36) | p | Confidence interval | |
|---|---|---|---|---|
| Education control group | ||||
| HVLT-R learning ratio | − 0.0309 | − 0.1840 | 0.8550 | − 0.3720–0.3101 |
| BVMT-R learning ratio | − 0.2550 | − 1.0786 | 0.2879 | − 0.7343–0.2244 |
| Cognitive training group | t (33) | |||
| HVLT-R learning ratio | − 0.1144 | − 0.7549 | 0.4557 | − 0.4228–0.1939 |
| BVMT-R learning ratio | − 0.6173 | − 3.5555 | *0.0012 | − 0.9705– − 0.2641 |
*p < .01
Importantly, the tDCS group did not significantly moderate Double Decision assessment change in any moderation model. Therefore, to probe the BVMT-R learning ratio moderation in the cognitive training group, a simple slope method was utilized irrespective of tDCS group. The simple slope analysis estimated the effect of BVMT-R learning ratio on Double Decision assessment gains at the mean ± 1 standard deviation (SD) above and below the mean of the BVMT-R learning ratio. We found that individuals whose BVMT-R learning ratio performance was 1 SD below the mean were expected to improve by 0.9967 unit increase (log-transformed milliseconds) on Double Decision assessment, individuals who performed at the mean were expected to improve by 0.8417 unit increase on Double Decision assessment, and individuals 1 SD above the mean were expected to improve by 0.6866 unit increase on Double Decision assessment. All of these effects are significant (p < 0.0001; Table 4). To summarize, at all levels of BVMT-R learning ratio scores, participants were expected to improve significantly on Double Decision assessment. However, participants performing at or below the mean on BVMT-R learning ratio were expected to have greater improvement on the Double Decision assessment, compared to above the mean (Fig. 5).
Table 4.
Probing moderation of BVMT-R learning ratio
| Effect | t (34) | p | Confidence interval | |
|---|---|---|---|---|
| 1 SD above | 0.9967 | 16.3894 | < .0001 | 0.8731–1.1203 |
| Mean | 0.8417 | 19.7124 | < .0001 | 0.7549–0.9284 |
| 1 SD below | 0.6866 | 11.2912 | < .0001 | 0.5631–0.8102 |
SD standard deviation
Fig. 5.
Moderation of education control group HVLT-R (A) and BVMT-R (B) learning ratio and cognitive training group HVLT-R (C) and BVMT-R (D) learning ratio on Double Decision assessment performance from baseline to 3-month follow-up. As Double Decision performance output is in milliseconds, lower scores reflect better performance. “Above Mean” reflects those who performed .5 standard deviations and above the mean, “Below Mean” reflects those who performed − .5 standard deviations and below the mean, and “Mean” reflects all other participants on the respective moderation variable. *p < .01 moderation
Discussion
There is evidence that working memory and attention/speed-of-processing cognitive training interventions may briefly improve cognitive performance and reduce the risk of dementia, although more research is needed to confirm these findings [2, 3, 8, 12]. However, few studies have explored cognitive moderating variables of cognitive training gains in an aging population, even though there is significant variability in fluid cognitive abilities in older adults [20, 21]. Furthering our understanding of variables that moderate improvements from cognitive training interventions could contribute to the reproducibility and rigor of the cognitive training literature and improve the efficacy of cognitive training interventions, which will eventually aid in providing tailored treatment recommendations in a clinical setting. Therefore, this study explored the association of the Double Decision task with visual and verbal learning measures at baseline, and the moderation of visual and verbal learning measures on Double Decision assessment changes after 12 weeks of working memory and attention/speed-of-processing cognitive training intervention in healthy older adults.
We found that at baseline, only BVMT-R learning ratio was associated with Double Decision performance, in that better visuospatial learning was associated with faster performance. There was no significant association with the HVLT-R learning ratio. It was foreseeable that BVMT-R learning ratio would predict Double Decision assessment performance, as these tasks share similar cognitive processes of quickly learning timed presentations of visual stimuli and immediate recall of visual and spatial details. Quick processing and learning of visual stimuli is something that is not a central component of the HVLT-R learning trials. The Double Decision task also has a strong processing speed component [5, 15]. Interestingly, one prior study also shows that processing speed abilities account for a significant amount of unique variance in BVMT-R learning [56]. The authors proposed that this association represented the underlying component of speeded learning required in BVMT-R learning trials [56]. Our findings corroborate and expand upon this prior work by showing that visual learning is also associated with a visuospatial speeded divided attention task. A meta-analysis of the literature assessing the cognitive correlates of the UFOV task found associations with visual speed, executive functioning, attention, and visual memory abilities [5]. However, most such studies reported only on visual-based cognitive tasks; components of verbal and visual learning were not assessed. Therefore, our findings help to fill this gap and suggest that visuospatial, but not verbal, learning ability is also a component associated with the Double Decision or UFOV performance.
Our findings also map onto the known neural correlates of visual and verbal learning. Of the very few studies assessing the functional neural correlates of BVMT-R learning, one study found that right prefrontal cortical activity was associated with BVMT-R learning trials in a sample of amnestic mild cognitively impaired older adults [57]; visuospatial learning requires parietal lobe function [58]. These are also areas that are functionally and structurally involved in Double Decision performance [38, 39, 59]. On the contrary, functional neural correlates of HVLT-R learning primarily involve the fusiform gyrus, hippocampus, and temporal areas [33, 60]. While temporal brain regions do show an association with Double Decision performance and BVMT-R learning, prior research shows that these neural correlates tend to be lateralized to the right hemisphere, while neural correlates of verbal list learning typically favor the left hemisphere [35, 38, 61]. The inherent language component of HVLT-R learning may be an explanation as to why it does not predict performance in the predominately visual function-based Double Decision task.
In the moderation analyses, only BVMT-R learning ratio moderated Double Decision gains in the cognitive training group. Specifically, individuals in the cognitive training group who had poorer BVMT-R learning had larger gains in Double Decision performance at the 3-month follow-up, and there was not a significant moderation of HVLT-R learning ratio in Double Decision gains. This pattern of findings suggests that improvements in Double Decision performance are specific to visuospatial, rather than verbal, learning abilities, and gains in cognitive training tasks are moderated by abilities that are associated with the task at baseline. This finding also suggests that healthy older adults who perform below expectations on cognitive tasks still hold the capacity to improve performance via cognitive training interventions. Our findings are also consistent with work from prior research from Ball and colleagues [15], which found that baseline speed-of-processing/executive functioning abilities were associated with gains in UFOV performance after speed-of-processing cognitive training intervention. Of note, Ball et al. [15] did not find an association between Benton Visual Retention or other visual memory measures and UFOV training gain. When considering our findings, this suggests that gains in UFOV/Double Decision may only be related to the speeded learning portion of visuospatial tasks, and not visual memory.
In healthy older adults, the pattern of poorer performers improving the most after training has been observed in other studies. For example, Ball and colleagues [15] also found that those with the slower UFOV performance and impaired processing speed at baseline had the greatest trajectory of improvement over time. Additionally, in a sample of healthy older adults undergoing working memory cognitive training, Faraza and colleagues [62] found that those with higher baseline working memory were less likely to have maintenance of training gains. The authors suggest that in healthy older adults, this could be due to a ceiling effect in high performers that limits training gain potential. However, Mondini and colleagues [63] found that even in a sample of demented older adults, less cognitive reserve was related to greater gains in cognitive training. These findings suggest that poorer functioning may predict greater training gains across the cognitive functioning spectrum in aging. This contradicts prior research in younger adults showing that higher intelligence and fluid cognitive abilities are associated with greater training gains [19], and serves to expand the current understanding of the impact of baseline fluid cognition on training gains in healthy older adults. However, future research will be needed to assess whether this pattern is evident in those with aging associated cognitive impairment.
Summary and limitations
Our results show that in healthy older adults, better visuospatial learning abilities predict faster Double Decision performance. We also found that those with poorer visuospatial learning at baseline improved the most on Double Decision assessment after a 3-month working memory and attention/speed-of-processing cognitive training intervention. These findings contribute to understanding the cognitive measures that may predict who will benefit most from multidomain cognitive training interventions.
The findings of this study should be interpreted within the context of a few limitations. First, there is limited demographic generalizability of our findings. The demographics of our sample reflect highly educated and predominately non-Hispanic White individuals. In 2021, the United States Census reported that only 32.9% of the population had obtained a bachelor’s degree or higher (https://www.census.gov/quickfacts/fact/table/US/PST045221). Therefore, our sample reflects an select portion of the American population that presumably has higher levels of cognitive reserve. Our results may also not be generalizable to systemically marginalized racial/ethnic groups, which may be at a higher risk of cognitive decline due to social determinants of health, disparities in access to culturally appropriate medical care, and/or racial biases in cognitive screening tools [64–67].
A second limitation includes the variables of interest in this study. The primary focus was on the Double Decision task, the task with the largest magnitude of improvement compared to the other training tasks [44]. However, there was also significant improvement on almost all other training tasks in the cognitive training group [44]. Different associations and moderations may be evident when exploring other training variables.
Further, this study only assessed single assessment baseline levels of learning ability as a moderating factor for Double Decision gain. As prior research has shown intelligence is highly correlated with learning abilities in healthy adults, a future study could explore if overall intelligence is another moderating factor in cognitive training gains with serial assessment to confirm that baseline cognitive abilities are reliable and do not reflect statistical anomalies (e.g., regression to the mean). Future research could also explore whether baseline Double Decision performance predicts gains in Double Decision, as prior research suggests that better baseline performance in the training task is associated with more learning of that same task during training [22].
Mood can also impact cognitive performance, and more specifically greater depression levels can reduce processing speed abilities in older adults [68]. Despite this association, there are few studies directly assessing the moderating influence of subclinical depression levels on cognitive training gains in cognitively healthy older adults. One study did find that cognitive interventions improved depression levels in clinically depressed individuals, so it plausible that this association could be bidirectional [69]. As increased depression symptoms can be common in aging, further research exploring moderating factors of cognitive training gains should include mood and/or depression levels [70].
Finally, a portion of individuals in this sample received active tDCS stimulation during cognitive training. tDCS group assignment was counterbalanced across training and education control groups and was included as a binary covariate in all relevant analyses. However, it is possible that tDCS group allocation could have impacted our findings.
Future research
Our findings suggest many avenues for future research. For example, it would be important to explore moderating factors of working memory cognitive training tasks, as some research has found that having poorer baseline working memory/executive function capacity predicted less training gains over time, which is a direction opposite to what this study found [16]. Future research should also explore whether baseline cognitive performance predicts training gains over more timepoints and longitudinal trajectories, as this would better inform our understanding of the interaction of baseline cognitive abilities, cognitive training, and dementia risk.
The Double Decision task is related to many aspects of everyday functioning that decline in dementia, such as driving and management of medication and finances [6, 7]. Thus, future research could explore the association of BVMT-R learning ratio with the functional abilities that are associated with Double Decision performance. Better understanding the predictive value of the BVMT-R learning ratio and everyday functional abilities should be clinically useful, as one study suggested that speeded visuospatial processing and learning tasks were more sensitive to aging than verbal task counterparts, and a literature review concluded that the addition of visuospatial measures to neurocognitive assessments could improve accuracy of dementia diagnosis [71, 72]. Overall, future cognitive training research studying improvement in visual speed-of-processing trained abilities should account for differing levels of visuospatial functioning at baseline, as this would likely impact the magnitude of training outcomes. This study is an important addition to the growing body of literature on cognitive training in older adults, which like all highly specific areas of cognitive research are vulnerable to publication bias. More studies are needed to more reliably understand the cognitive changes as a result of cognitive training interventions in older adults. The Preventing Alzheimer’s Disease with Cognitive Training (PACT) trial is one such ongoing multi-site randomized clinical intervention trial that will help better elucidate these exact questions by directly assessing the impact of cognitive training interventions on mild cognitive impairment and dementia risk [73].
Data availability
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board (DSMB) in the context of a phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).
Acknowledgements
We would like to thank all of our participants for their time and research assistants for their hard work and instrumental role in making this manuscript possible.
Funding
The study was financially supported by the National Institute of Aging/National Institutes of Health (T32AG020499, K01AG050707, R01AG054077, P30AG019610, and T32AG061892), the University of Florida Center for Cognitive Aging and Memory Clinical Translational Research, the state of Arizona and Arizona Department of Health Services, the McKnight Brain Research Foundation, and National Heart, Lung, and Blood Institute (T32HL134621).
Declarations
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kueider AM, Bichay K, Rebok G. Cognitive training for older adults: what is it and does it work. Cent Ageing Am Inst Res. 2014;1–8.
- 2.Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimers Dement Transl Res Clin Interv. 2017;3:603–611. doi: 10.1016/j.trci.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball KK, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71–79. [PubMed] [Google Scholar]
- 5.Woutersen K, Guadron L, van den Berg AV, Boonstra FN, Theelen T, Goossens J. A meta-analysis of perceptual and cognitive functions involved in useful-field-of-view test performance. J Vis. 2017;17:11. doi: 10.1167/17.14.11. [DOI] [PubMed] [Google Scholar]
- 6.Aust F, Edwards JD. Incremental validity of Useful Field of View subtests for the prediction of instrumental activities of daily living. J Clin Exp Neuropsychol. 2016;38:497–515. doi: 10.1080/13803395.2015.1125453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci. 2005;82:724–731. doi: 10.1097/01.opx.0000175009.08626.65. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JD, Fausto BA, Tetlow AM, Corona RT, Valdés EG. Systematic review and meta-analyses of useful field of view cognitive training. Neurosci Biobehav Rev. 2018;84:72–91. doi: 10.1016/j.neubiorev.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Fausto B, Tetlow A, Corona R, Valdés E, Edwards J. Useful field of view cognitive training improves older adults’ everyday function. Innov Aging. 2018;2:680–680. doi: 10.1093/geroni/igy023.2532. [DOI] [Google Scholar]
- 10.Cheng Y, Wu W, Feng W, Wang J, Chen Y, Shen Y, et al. The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med. 2012;10:30. doi: 10.1186/1741-7015-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PLoS ONE. 2012;7:e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mewborn CM, Lindbergh CA, Stephen ML. Cognitive interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: a systematic review and meta-analysis of randomized-controlled trials. Neuropsychol Rev. 2017;27:403–439. doi: 10.1007/s11065-017-9350-8. [DOI] [PubMed] [Google Scholar]
- 13.Buitenweg J, Murre J, Ridderinkhof KR. Brain training in progress: a review of trainability in healthy seniors. Front Hum Neurosci. 2012;6:1–11. [DOI] [PMC free article] [PubMed]
- 14.Guye S, De Simoni C, von Bastian CC. Do individual differences predict change in cognitive training performance? A latent growth curve modeling approach. J Cogn Enhanc. 2017;1:374–393. doi: 10.1007/s41465-017-0049-9. [DOI] [Google Scholar]
- 15.Ball KK, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol Ser B. 2007;62:19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 16.Matysiak O, Kroemeke A, Brzezicka A. Working memory capacity as a predictor of cognitive training efficacy in the elderly population. Front Aging Neurosci. 2019;11:126–126. doi: 10.3389/fnagi.2019.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review Dev Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- 18.Tremont G, Hoffman RG, Scott JG, Adams RL. Effect of intellectual level on neuropsychological test performance: a response to Dodrill (1997) Clin Neuropsychol. 1998;12:560–567. doi: 10.1076/clin.12.4.560.7238. [DOI] [Google Scholar]
- 19.Lee H, Boot WR, Baniqued PL, Voss MW, Prakash RS, Basak C, et al. The relationship between intelligence and training gains is moderated by training strategy. PLoS ONE. 2015;10:e0123259. doi: 10.1371/journal.pone.0123259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen H, Mackinnon A, Jorm A, Henderson A, Scott L, Korten A. Age differences and interindividual variation in cognition in community-dwelling elderly. Psychol Aging. 1994;9:381. doi: 10.1037/0882-7974.9.3.381. [DOI] [PubMed] [Google Scholar]
- 21.De Felice S, Holland CA. Intra-individual variability across fluid cognition can reveal qualitatively different cognitive styles of the aging brain. Front Psychol. 2018;9:1–16. [DOI] [PMC free article] [PubMed]
- 22.Zinke K, Zeintl M, Rose NS, Putzmann J, Pydde A, Kliegel M. Working memory training and transfer in older adults: effects of age, baseline performance, and training gains. Dev Psychol. 2014;50:304. doi: 10.1037/a0032982. [DOI] [PubMed] [Google Scholar]
- 23.Traut HJ, Guild RM, Munakata Y. Why does cognitive training yield inconsistent benefits? A meta-analysis of individual differences in baseline cognitive abilities and training outcomes. Front Psychol. 2021;12:1–20. [DOI] [PMC free article] [PubMed]
- 24.Hammers DB, Suhrie K, Dixon A, Gradwohl BD, Duff K, Spencer RJ. Validation of HVLT-R, BVMT-R, and RBANS learning slope scores along the Alzheimer’s continuum. Arch Clin Neuropsychol. 2022;37:78–90. doi: 10.1093/arclin/acab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havlík F, Mana J, Dušek P, Jech R, Růžička E, Kopeček M, et al. Brief Visuospatial Memory Test-Revised: normative data and clinical utility of learning indices in Parkinson’s disease. J Clin Exp Neuropsychol. 2020;42:1099–1110. doi: 10.1080/13803395.2020.1845303. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman V, Emrani S, Matusz EF, Miller D, Garrett KD, Gifford KA, et al. Visual and verbal serial list learning in patients with statistically-determined mild cognitive impairment. Innov Aging. 2019;3:1–12. 10.1093/geroni/igz009. [DOI] [PMC free article] [PubMed]
- 27.Duff K, Schoenberg MR, Scott JG, Adams RL. The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol. 2005;20:111–122. doi: 10.1016/j.acn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.John SE, Ritter A, Wong C, Parks CM. The roles of executive functioning, simple attention, and medial temporal lobes in early learning, late learning, and delayed recall. Aging Neuropsychol Cogn. 2022;29:400–417. doi: 10.1080/13825585.2021.2016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RN, Rosenberg AL, Morris JN, Allaire JC, McCoy KJ, Marsiske M, et al. A growth curve model of learning acquisition among cognitively normal older adults. Exp Aging Res. 2005;31:291–312. doi: 10.1080/03610730590948195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane KD, Yochim BP. Construct Validity and Extended Normative Data for Older Adults for the Brief Visuospatial Memory Test. Revised Am J Alzheimers Dis Dementiasr. 2014;29:601–606. doi: 10.1177/1533317514524812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderploeg RD, Schinka JA, Retzlaff P. Relationships between measures of auditory verbal learning and executive functioning. J Clin Exp Neuropsychol. 1994;16:243–252. doi: 10.1080/01688639408402635. [DOI] [PubMed] [Google Scholar]
- 32.Walhovd KB, Bråthen ACS, Panizzon MS, Mowinckel AM, Sørensen Ø, de Lange A-MG, et al. Within-session verbal learning slope is predictive of lifespan delayed recall, hippocampal volume, and memory training benefit, and is heritable. Sci Rep. 2020;10. 10.1038/s41598-020-78225-1. [DOI] [PMC free article] [PubMed]
- 33.Gifford KA, Phillips JS, Samuels LR, Lane EM, Bell SP, Liu D, et al. Associations between verbal learning slope and neuroimaging markers across the cognitive aging spectrum. J Int Neuropsychol Soc. 2015;21:455–467. doi: 10.1017/S1355617715000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luria A. Higher cortical functions in man. New York: Consultants Bureau Enterprises. Inc Diskuss; 1966. [Google Scholar]
- 35.Bonner-Jackson A, Mahmoud S, Miller J, Banks SJ. Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimers Res Ther. 2015;7:61. doi: 10.1186/s13195-015-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowes JR, Stroman P, Garcia A. Neural correlates of focused attention in cognitively normal older adults. World J Neurosci. 2011;1:19. doi: 10.4236/wjns.2011.12003. [DOI] [Google Scholar]
- 37.Hardcastle C, O’Shea A, Kraft JN, Albizu A, Evangelista ND, Hausman HK, et al. Contributions of hippocampal volume to cognition in healthy older adults. Front Aging Neurosci. 2020;12:1–10. [DOI] [PMC free article] [PubMed]
- 38.Kraft JN, O’Shea A, Albizu A, Evangelista ND, Hausman HK, Boutzoukas E, et al. Structural neural correlates of Double Decision performance in older adults. Front Aging Neurosci. 2020;12:278. doi: 10.3389/fnagi.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraft JN, Albizu A, O’Shea A, Hausman HK, Evangelista ND, Boutzoukas E, et al. Functional neural correlates of a Useful Field of View (UFOV)-based fMRI Task in older adults. Cereb Cortex 2021;32:1993–2012. 10.1093/cercor/bhab332. [DOI] [PMC free article] [PubMed]
- 40.Nissim NR, O’Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ. Frontal structural neural correlates of working memory performance in older adults. Front Aging Neurosci. 2017;8:328–328. doi: 10.3389/fnagi.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, et al. Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage. 2005;26:36–47. doi: 10.1016/j.neuroimage.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Zanto TP, Gazzaley A. Aging of the frontal lobe. Handb. Clin. Neurol. 2019;163:369–389. 10.1016/B978-0-12-804281-6.00020-3. [DOI] [PubMed]
- 43.Schoenberg MR, Scott JG. The little black book of neuropsychology: a syndrome-based approach. Springer; 2011.
- 44.Hardcastle C, Hausman HK, Kraft JN, Albizu A, O’Shea A, Boutzoukas EM, et al. Proximal improvement and higher-order resting state network change after multidomain cognitive training intervention in healthy older adults. GeroScience. 2022 doi: 10.1007/s11357-022-00535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods AJ, Cohen R, Marsiske M, Alexander GE, Czaja SJ, Wu S. Augmenting cognitive training in older adults (The ACT Study): design and methods of a phase III tDCS and cognitive training trial. Contemp Clin Trials. 2018;65:19–32. doi: 10.1016/j.cct.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS) Alzheimer Dis Assoc Disord. 2018;32:10–17. doi: 10.1097/WAD.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards JD, Hauser RA, O’Connor ML, Valdés EG, Zesiewicz TA, Uc EY. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. 2013;81:1284. doi: 10.1212/WNL.0b013e3182a823ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross LA, Webb CE, Whitaker C, Hicks JM, Schmidt EL, Samimy S, et al. The effects of useful field of view training on brain activity and connectivity. J Gerontol Ser B. 2019;74:1152–1162. doi: 10.1093/geronb/gby041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test – Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. doi: 10.1076/clin.12.1.43.1726. [DOI] [Google Scholar]
- 50.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8:145. doi: 10.1037/1040-3590.8.2.145. [DOI] [Google Scholar]
- 51.Spencer RJ, Gradwohl BD, Williams TF, Kordovski VM, Hammers DB. Developing learning slope scores for the repeatable battery for the assessment of neuropsychological status. Appl Neuropsychol Adult. 2020:29;584–590. 10.1080/23279095.2020.1791870. [DOI] [PubMed]
- 52.Duff K. Demographically corrected normative data for the Hopkins Verbal Learning Test-Revised and Brief Visuospatial Memory Test-Revised in an elderly sample. Appl Neuropsychol Adult. 2016;23:179–185. doi: 10.1080/23279095.2015.1030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31:166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Hooren S, Valentijn A, Bosma H, Ponds R, Van Boxtel M, Jolles J. Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 55.Montoya AK. Moderation analysis in two-instance repeated measures designs: probing methods and multiple moderator models. Behav Res Methods. 2019;51:61–82. doi: 10.3758/s13428-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam JW, Schmitter-Edgecombe M. The Role of Processing Speed in the Brief Visuospatial Memory Test – Revised. Clin Neuropsychol. 2013;27:962–972. doi: 10.1080/13854046.2013.797500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melrose RJ, Zahniser E, Wilkins SS, Veliz J, Hasratian AS, Sultzer DL, et al. Prefrontal working memory activity predicts episodic memory performance: a neuroimaging study. Behav Brain Res. 2020;379:112307. doi: 10.1016/j.bbr.2019.112307. [DOI] [PubMed] [Google Scholar]
- 58.Zink DN, Miller JB, Caldwell JZK, Bird C, Banks SJ. The relationship between neuropsychological tests of visuospatial function and lobar cortical thickness. J Clin Exp Neuropsychol. 2018;40:518–527. doi: 10.1080/13803395.2017.1384799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardcastle C, Hausman HK, Kraft JN, Albizu A, Evangelista ND, Boutzoukas EM, et al. Higher-order resting state network association with the useful field of view task in older adults. GeroScience. 2022;44:131–145. doi: 10.1007/s11357-021-00441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon EJ, Cho SS, Bang SA, Park HS, Kim YK, Lee WW, et al. Neural correlate of verbal episodic memory: encoding, retrieval, and retrieval strategy 2007;241.
- 61.Andrews G, Halford GS, Shum DHK, Maujean A, Chappell M, Birney DP. Verbal learning and memory following stroke. Brain Inj. 2014;28:442–447. doi: 10.3109/02699052.2014.888758. [DOI] [PubMed] [Google Scholar]
- 62.Faraza S, Waldenmaier J, Dyrba M, Wolf D, Fischer FU, Knaepen K, et al. Dorsolateral prefrontal functional connectivity predicts working memory training gains. Front Aging Neurosci. 2021;13:1–11. 10.3389/fnagi.2021.592261. [DOI] [PMC free article] [PubMed]
- 63.Mondini S, Madella I, Zangrossi A, Bigolin A, Tomasi C, Michieletto M, et al. Cognitive reserve in dementia: implications for cognitive training. Front Aging Neurosci. 2016;8:1–7. 10.3389/fnagi.2016.00084. [DOI] [PMC free article] [PubMed]
- 64.Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement Transl Res Clin Interv. 2018;4:510–520. doi: 10.1016/j.trci.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majoka MA, Schimming C. Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clin Ther. 2021;43:922–929. doi: 10.1016/j.clinthera.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Rossetti HC, Lacritz LH, Hynan LS, Cullum CM, Van Wright A, Weiner MF. Montreal cognitive assessment performance among community-dwelling African Americans. Arch Clin Neuropsychol. 2017;32:238–244. doi: 10.1093/arclin/acw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brailean A, Comijs HC, Aartsen MJ, Prince M, Prina AM, Beekman A, et al. Late-life depression symptom dimensions and cognitive functioning in the Longitudinal Aging Study Amsterdam (LASA) J Affect Disord. 2016;201:171–178. doi: 10.1016/j.jad.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Motter JN, Pimontel MA, Rindskopf D, Devanand DP, Doraiswamy PM, Sneed JR. Computerized cognitive training and functional recovery in major depressive disorder: a meta-analysis. J Affect Disord. 2016;189:184–191. doi: 10.1016/j.jad.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 70.Pocklington C. Depression in older adults. Br J Med Pract. 2017;10:a1007. [Google Scholar]
- 71.Jenkins L, Myerson J, Joerding JA, Hale S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol Aging. 2000;15:157. doi: 10.1037/0882-7974.15.1.157. [DOI] [PubMed] [Google Scholar]
- 72.Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR. Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimers Dement Diagn Assess Dis Monit. 2018;10:66–74. doi: 10.1016/j.dadm.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicholson JS, Hudak EM, Phillips CB, Chanti-Ketterl M, O’Brien JL, Ross LA, et al. The Preventing Alzheimer’s with Cognitive Training (PACT) randomized clinical trial. Contemp Clin Trials. 2022;123:106978. doi: 10.1016/j.cct.2022.106978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are managed under the data sharing agreement established with NIA and the parent R01 clinical trial Data Safety and Monitoring Board (DSMB) in the context of a phase III clinical trial (ACT study, R01AG054077). All trial data will be made publicly available 2 years after completion of the parent clinical trial, per NIA and DSMB agreement. Requests for baseline data can be submitted to the ACT Publication and Presentation (P&P) Committee and will require submission of a data use, authorship, and analytic plan for review by the P&P committee (ajwoods@phhp.ufl.edu).