Abstract
Periodontal disease is a chronic inflammatory condition that results in the destruction of the teeth supporting tissues, eventually leading to the loss of teeth and reduced quality of life. In severe cases, periodontal disease can limit proper nutritional intake, cause acute pain and infection, and cause a withdrawal from social situations due to esthetic and phonetic concerns. Similar to other chronic inflammatory conditions, periodontal disease increases in prevalence with age. Research into what drives periodontal disease pathogenesis in older adults is contributing to our general understanding of age-related chronic inflammation. This review will present periodontal disease as an age-related chronic inflammatory disease and as an effective geroscience model to study mechanisms of age-related inflammatory dysregulation. The current understanding of the cellular and molecular mechanisms that drive inflammatory dysregulation as a function of age will be discussed with a focus on the major pathogenic immune cells in periodontal disease, which include neutrophils, macrophages, and T cells. Research in the aging biology field has shown that the age-related changes in these immune cells result in the cells becoming less effective in the clearance of microbial pathogens, expansion of pathogenic subpopulations, or an increase in pro-inflammatory cytokine secretions. Such changes can be pathogenic and contribute to inflammatory dysregulation that is associated with a myriad of age-related disease including periodontal disease. An improved understanding is needed to develop better interventions that target the molecules or pathways that are perturbed with age in order to improve treatment of chronic inflammatory conditions, including periodontal disease, in older adult populations.
Keywords: Inflammation, Periodontal disease, Aging, Chronic disease
Introduction
Periodontal disease is a microbially-associated, host-mediated chronic inflammatory condition that affects the tissues that support teeth, including bone, connective tissue, and the surrounding oral mucosa. Bacterial biofilms accumulate on tooth surfaces and initiate an inflammatory response [1]. A prolonged and heightened inflammatory response results in tissue destruction via activation of matrix metalloproteinases and osteoclasts [2]. The clinical hallmark of periodontal disease in humans are signs of inflammation, which present as swollen, red, ulcerated, and bleeding gingival tissue, and the characteristic loss of bone and soft tissue support around the dentition. In the oral cavity, bacteria regularly colonize the tooth surface. A basal inflammatory response to the bacteria is necessary to regulate colony overgrowth and overt pathogenicity [3]. Frequent and adequate removal of the microbial biofilms can limit the inflammatory response and prevent loss of tissue support. However, a dysregulation of the immune system can result in a pathologic inflammatory response to the microbial stimuli which may include a heightened and prolonged response or one that does not resolve in a timely manner upon removal of the stimuli [4]. Such a pathological response contributes to increased tissue destruction and an increase in periodontal disease severity. Understanding the host immune response to the oral pathogens and how the response becomes dysregulated in disease is a major focus in the periodontal research field.
Similar to other chronic inflammatory disease, the prevalence of periodontal disease increases with age [5, 6]. The increased prevalence has been repeatedly demonstrated across multiple national and international population studies [7–11] (Table 1). Age appears to be a risk factor for periodontal disease. In a longitudinal study of a large cohort (n = 2256), tooth loss (end stage of periodontal disease) was used as a biomarker to accurately predict biological age [12]. Moreover, models of biological age that incorporated no oral health data could accurately predict risk of tooth loss over a 10-year period based on the calculated age of the individual [13]. Together, these studies support periodontal disease as an age-related disease.
Table 1.
Population-based studies reporting increased periodontal disease prevalence associated with increasing age
| Study | Population/region studied | Sample size | Year | Periodontal disease prevalence by age |
|---|---|---|---|---|
| NHANES [7] | USA | 7066 | 2009–2012 |
Severe diagnosis Age: 30–34: 2.2% Age: 65 + years: 11% |
| NHANES [9] | USA | 9689 | 1988–1994 |
Severe diagnosis Age: 30–34: 1.4% Age: 50–54: 4.6% Age: 75–79: 3.3% Moderate diagnosis Age: 30–34: 3.8% Age: 50–54: 10.6% Age: 75–79: 19.9% Mild diagnosis Age: 30–34: 17.0% Age: 50–54: 23.6% Age: 75–79: 29.5% No periodontal disease Age: 30–34: 77.8% Age: 50–54: 61.1% Age: 75–79: 47.3% |
| SHIP-Trend [8] | Germany | 4420 | 2008–2012 |
Mean CAL Age: 30–34: 1.32 mm Age: 75 + : 2.32 mm Mean No. Missing teeth Age: 30–34: 1.3 Age: 75 + years: 9.3 |
| Nippon Dental University [11] | Japan | 582 | 2010–2013 |
Severe diagnosis Age: 20–34: 1.0% Age: 45–54: 16.4% Age: 65–74: 48.0% Moderate diagnosis Age: 20–34: 21.9% Age: 45–54: 21.3% Age: 65–74: 20.3% Mild diagnosis Age: 20–34: 28.1% Age: 45–54: 41.0% Age: 65–74: 17.5% No periodontal disease Age: 20–34: 50.0% Age: 45–54: 21.3% Age: 65–74: 14.1% |
| Gian Sagar Dental College and Hospital [10] | India | 1680 | 2014 |
Severe diagnosis Age: 20–29: 4.3% Age: 61 + : 45.6% No periodontal disease Age: 20–29: 13.3% Age: 61 + : 1.3% |
NHANES National Health and Nutrition Examination Survey, SHIP-Trend study of health in Pomerania, CAL clinical attachment loss (clinical measure used to determine disease severity)
Consequences of periodontal disease in older adults are multivariate. The loss of support of the dentition and the loss of teeth in end-stage disease results in decreased chewing capacity that can limit proper nutritional intake [14]. Furthermore, a withdrawal from social situations and a resulting decline in psychological health has been reported due to insecurities about the esthetic appearance resulting from missing teeth and difficulty eating in public [15–17]. In addition, periodontal disease has been shown to be associated with other chronic inflammatory conditions that also increase in prevalence in older adults [18]. Epidemiological studies have demonstrated an association of periodontal disease with cardiovascular disease [19], Alzheimer’s disease [20, 21], type 2 diabetes [22], and obesity [23, 24]. More than 50 systemic disease and conditions have been further investigated for the causal or casual comorbid relationship with periodontal disease [25, 26].
The cooccurrence of periodontal disease with other chronic inflammatory systemic disease suggests an underlying relationship or shared risk factor among these comorbid conditions. A dysregulated inflammatory response is central to periodontal disease pathogenesis as well as other age-related chronic inflammatory conditions [27]. Therefore, understanding how age contributes to periodontal disease may provide further insight into how mechanisms of aging generally contribute to other chronic inflammatory diseases in older adults.
This review will demonstrate how periodontal disease can serve as a geroscience model to investigate the biologic mechanisms that link aging and chronic inflammatory diseases. Known age-related perturbations to the inflammatory response and how they have been shown to contribute to the pathogenesis of periodontal disease will be discussed below and are summarized in Fig. 1.
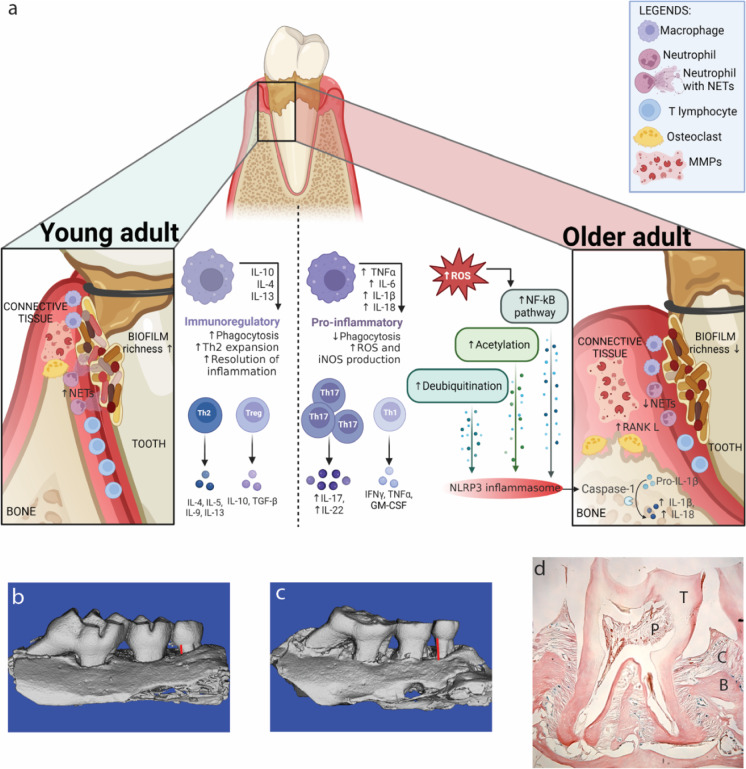
Fig. 1.
a Schematic of a tooth and supporting tissues affected by periodontal disease. Bacterial plaque accumulates on tooth surface and initiates a cellular immune response that activates downstream MMP and osteoclast activity that destroy the supporting bone and connective tissue. The cellular response, cytokine expression, and regulation of inflammatory pathways differ in periodontal disease between young (left) and older (right) adults. Images rendered using micro-CT of the three maxillary molar teeth of mice in b health and c with periodontal disease. Red lines indicate differences in bone loss that can be quantified as a measure of disease severity. d Representative histological section of a mouse molar tooth that depicts the supporting bone (B) connective tissue (C), the tooth (T), and the pulp of the tooth (P). Part a is created with Biorender.com
Aging animal models to study periodontal disease
Studying periodontal disease in animal models has been accomplished through experimental interventions that induce disease or by studying non-experimentally induced, or spontaneous, periodontal disease that occurs in animals and increases in prevalence as a function of age.
Animal models of spontaneous periodontal disease
Animal models that spontaneously develop similar human diseases or at least some pathological characteristic of the disease of interest, as a function of age are commonly studied in the aging biology field. For example, osteoarthritis has been shown to spontaneously occur in guinea pigs, dogs, horses, and inbred strains of mice [28, 29]. Pathological features of macular degeneration are present in older macaque and rhesus monkeys [30]. The characteristic pathological hallmarks of Alzheimer’s disease and Parkinson’s disease have been reported in the brains of apes, old world monkeys, and dogs [31]. Spontaneously occurring cardiovascular disease has been shown to develop in dogs, cats, and rats [32, 33]. Studying spontaneously occurring disease in animal models has its limitations, including a prolonged time for disease to be present, heterogenous disease presentations, and pathological features that are not representative of those present in human disease. In addition, the confounding genetic variables associated with inbred strains may contribute to differential disease susceptibility.
Of interest for this manuscript, an age-related increase in periodontal disease is demonstrated across numerous animal models. The pathological characteristics of spontaneous periodontal disease in animals is representative of the disease presentation in humans, which includes a quantifiable loss of soft and hard tissue support of the dentition, pathogenic microbial changes, and a measurable local and systemic immune response. A wide variety of species have been shown to be affected by spontaneous periodontal disease. Non-human primates, including rhesus monkeys, cynomolgus monkeys, and baboons, demonstrate a natural susceptibility to periodontal disease [34]. Spontaneous periodontal disease in the miniature pig has been well characterized with early local inflammation of the gingiva evident by 6 months of age and the progression to severe periodontal disease by 16 months [35]. Dogs also demonstrate spontaneous periodontal disease. Periodontal disease progresses spontaneously in dogs with increased age frequently associated with tooth loss [36, 37]. The beagle dog has been the most extensively studied model; however, significant differences in disease severity are present across different dog breeds [36]. Rats also demonstrate spontaneous periodontal disease. The rice rat has been shown to be highly susceptible to disease with early onset of gingival inflammation and progression to severe disease by 3 months of age [36]. The murine model has been extensively used to study spontaneously occurring periodontal disease as a function of age. An age-related increase in periodontal disease is well characterized in the C57BL/6 [38, 39] and BALB/cByJ mice [40]. Disease severity progresses constantly, throughout the lifetime of mice, as similarly demonstrated in human populations [40, 41].
The age-related increase in periodontal disease prevalence in humans is modeled well across a variety of animals, making periodontal disease a useful and adaptable model in aging biology. Implementation of cutting edge biomolecular and immunological research techniques are consistently applied to these research models to better understand chronic inflammation in age-related disease pathogenesis.
Experimental periodontal disease models
Periodontal disease can be experimentally modeled in animals via surgical or microbial interventions to induce the desired pathological features. The most common model of inducing experimental periodontal disease is the ligature induction method (Fig. 1). In this model, a suture, either sterile or inoculated with a periodontal pathogen, is tied around the molar of the animal. The suture remains in place for a period of time to act as a nidus of bacterial accumulation and initiates a local inflammatory response affecting the supporting tissues around the teeth [42]. The result of the induced inflammatory response is destruction of the surrounding bone, connective tissue, and gingival epithelium which resembles the tissue destruction observed in periodontal disease in humans [42]. Disease severity can be quantified by measuring the extent of tissue destruction around affected teeth. Micro-CT imaging is a commonly used technique to quantify bone loss around the teeth, and histological techniques are utilized to measure bone and soft tissue changes. The number of teeth lost can also be used as a measure of end-stage disease. In addition, the immunological response locally within the tissues can be profiled using an array of modern immunological assays and techniques.
The ligature induction method has been most widely applied to mouse models; however, other animal models have similarly been used, including rats [43], dogs [44], macaques [45], and pigs [46]. Experimental periodontal disease models have also been tested in young and old animal models to demonstrate a differential response as a function of age. Studies have shown that old mice demonstrate an increase in disease severity as a result of the ligature induction method, compared to young [47]. Other studies have shown quantitative and qualitative difference in the immune response in old mice, compared to young using the ligature induction model [39, 47].
Another method to experimentally induce periodontal disease is based on infection with pathogenic bacteria known to be associated with human periodontal disease, such as Porphyromonas gingivalis and Fusobacterium nucleatum, by means of oral gavage. In this model, bacterial pathogens are prepared in a liquid suspension and administered to the oral cavity of the animal. Repeated administrations of the bacterial suspension are made over several weeks to months. The pathogenic bacteria colonize the tooth surfaces and induce local inflammation and destruction of the tissues surrounding the teeth. Compared to the ligature method, there are multiple weaknesses of the oral gavage model, which include an extended duration of repeated applications, minimal tissue destruction that is difficult to quantify, and inconsistent results within groups [48].
Chronic inflammation in periodontal disease and aging
The inflammatory dysregulation that occurs with increased age is well appreciated. The term inflammaging has been used to describe the age-related systemic elevation of pro-inflammatory mediators that contribute to the pathogenesis of many of the diseases that increase in prevalence with age [49]. While multiple cellular and molecular perturbations have been shown to contribute to the inflammaging phenomenon, a complete understanding of the mechanisms that contribute to inflammaging are not fully understood. Given that inflammatory dysregulation is central to the pathogenesis of periodontal disease, inflammaging may be a key contributor to the age-related increase in periodontal disease prevalence. To better understand how inflammaging may contribute to the pathogenesis of periodontal disease, the following sections will discuss the known age-related changes that affect the regulation of cytokines and cellular activity.
Cytokine dysregulation
Inflammaging has been generally quantified by an increase in circulating levels of IL-6, CRP, and TNFα [50]. This age-related increase in pro-inflammatory mediators appears consistently even when systemic disease and other risk factors are controlled. Dysregulation of IL-6 and TNFα activity have been well demonstrated in the pathogenesis of periodontal disease. Levels of IL-6 within the periodontal tissue are shown to increase with the onset of disease and contribute to destruction of the surrounding tissues through downstream activation of matrix metalloproteinases and osteoclasts [51]. Experimental periodontal disease models in mice have shown increased systemic level of IL-6 after induction of disease [52]. An increase in systemic IL-6 was shown to be associated with increased periodontal disease severity in humans [53]. Similar findings of increased systemic IL-6 expression have been shown to be associated with other age-related diseases and conditions, including frailty [54]. In addition, polymorphism of the IL-6 gene (rs1800795) was shown to be associated with periodontal disease severity [53, 55]. The same polymorphism is associated with other age-related disease including atherosclerosis and Alzheimer’s disease [56, 57]. Interestingly, clinical treatment of periodontal disease has been shown to reduce the systemic levels of IL-6 in humans [58]. Similar to IL-6, levels of TNFα in the periodontium increase during periodontal disease [59]. Systemic levels of TNFα are a marker for frailty in older adults and have also been shown to also be associated with increased severity of periodontal disease [60, 61]. The involvement of TNFα with chronic inflammatory and age-related disease has made it attractive as a therapeutic target. Animal studies have shown that TNFα targeting drugs reduce the tissue destruction and disease severity when administered in periodontal disease models [62, 63].
Additional cytokines are involved in the pathogenesis of periodontal disease (Fig. 1) and have similarly been associated with inflammaging. IL-1β is upregulated in response to microbial invasion in periodontal disease, with higher levels of IL-1β associated with increased severity of periodontal disease and levels of IL-1β decreasing after periodontal treatment [64, 65]. IL-1β has been shown to propagate the inflammatory response by inducing T helper cell expansion and promoting matrix metalloproteinase and osteoclast activity during periodontal disease [65, 66]. An age-related increase in IL-1β has been repeatedly shown across diverse cells and tissues with a demonstrated contribution to age-related pathologies, including type 2 diabetes [67], atherosclerosis [68], and deterioration of the hematopoietic stem cell niche [69]. IL-18 is also a part of the IL-1 cytokine family and is capable of activating T helper 1 cells and natural killer cells [70]. Upregulation of IL-18 was observed in response to periodontal pathogens, and increased levels of IL-18 were demonstrated locally and systemically in patients with periodontal disease [71–73]. An age-related increase in serum level of IL-18 is well demonstrated [74, 75]. In older adult populations, a significant increase in serum IL-18 was associated with decrease in physical performance measures [76]. Additionally, age-related pathologies were also associated with increased levels of IL-18, including Alzheimer’s disease [77], type 2 diabetes [78], and ischemic heart disease [79]. As discussed further in the T cell section below, IL-17 is a pathological inflammatory cytokine that is well supported for its role in periodontal disease along with other age-related conditions, including osteoarthritis [80], atherosclerosis [81], and neurodegenerative diseases [82].
Mechanisms of age-related cytokine dysregulation: NfKB and NLRP3 pathways
While the mechanisms contributing to increased cytokine expression are not fully understood, evidence suggests an age-related increase in NF-κβ signaling significantly contributes to the upregulation of proinflammatory mediators [83]. NF-κβ is a transcription factor that induces the expression of numerous pro-inflammatory genes that are expressed in a variety of cell types. NF-κβ activation occurs in response to changes in redox state and oxidative stress in the local environment. Aging is associated with increased oxidative stress and increased generation of reactive oxygen species (ROS) within tissues [84, 85]. This increased oxidative stress can drive NF-κβ activation and the resulting increased pro-inflammatory cytokine expression in an age-dependent manner. In a study analyzing microarray data of tissue samples of older subjects, the cis-regulatory elements most strongly associated with the age-related increase in cytokine gene expression within the tissues were shown to be the NF-κβ transcription factor [86]. Biochemical pathways known to promote aging, such as insulin/IGF-1 and mammalian target of rapamycin (mTOR), activate NF-κβ [87, 88]. Interestingly, inhibition of these pathways and the resulting downregulation of NF-κβ result in increased longevity in experimental models [89].
Another pathway that regulates cytokine activity involves the formation of the nucleotide-binding domain, leucine-rich repeat-family and pyrin domain-containing protein 3 (NLRP3) inflammasome protein complex. NLRP3 activity has been shown to be affected by increased age resulting in increased inflammatory cytokine expression [90]. NLRP3 is an intracellular sensor that recognizes danger- and pathogen-associated molecular patterns (DAMPS, PAMPS) and forms a protein complex with apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1. Activation of the inflammasome results in the release of IL-1β and IL-18 [91]. Dysregulated cytokine production by the NLRP3 inflammasome has been associated with multiple age-related diseases [92]. Interestingly, mice with genetic depletion of NLRP3 do not demonstrate age-related pathologic changes to the same extent as the wild type controls and demonstrate increased lifespan [93]. Age-related changes appear to affect transcriptional, post-transcriptional, and post-translational modifications, or priming, of the NLRP3 gene and protein in the formation and activation of the inflammasome. Increased transcription of NLRP3 is driven by NF-κβ-dependent pathways, which are activated by age-related inflammatory mediators [94]. Post-translational modifications that activate NLRP3 include deubiquitination of the inflammasome by deubiquitinating enzymes activated downstream of TLR4 and MyD88 [95]. A general increase in deubiquitination across the proteome has been associated with increased age [96]. Post-translational protein acetylation is also implicated in the regulation of NLRP3. Acetylation has been shown to activate the inflammasome, and such activation was upregulated by age-related inflammatory mediators [97].
Mitochondrial dysfunction is a characteristic hallmark of aging and has been shown to contribute to pathologic NLRP3 and NF-κβ activity. Mitochondria produce a significant amount of ROS. Mitochondrial DNA damage by increased ROS production has been well described as contributing to many pathologic aging phenotypes by disrupting normal protein synthesis and function [98]. Mitochondrial derived ROS (H2O2) was shown to promote thioredoxin-interacting protein (TXNIP) interaction with the NLRP3 protein and the subsequent activation of the inflammasome [99]. In addition, free mitochondrial DNA is released as a result of cellular necrosis and loss of cell membrane integrity and acts as a DAMP in the promotion of the inflammatory response. The free mitochondrial DNA was shown to bind Toll-like receptor 9 and activate NF-κβ to promote the transcription of NLRP3 and other inflammatory cytokines [100]. Free mitochondrial DNA was also shown to directly bind the NLRP3 protein and promote the formation of the inflammasome protein complex [101].
Age-related cellular changes contribute to chronic inflammation
The following sections describe the cellular changes that are known to contribute to age-related inflammatory dysregulation and to the pathology of periodontal disease and other age-related diseases and conditions. The immune cells discussed below are highlighted due to their well demonstrated contributions to periodontal disease pathogenesis. However, a more diverse and complex cellular immune response is present during periodontal disease.
Cellular senescence
Cellular senescence describes an arrest of the normal cell cycle where cells no longer proliferate but remain resistant to apoptosis [102]. Senescent cells accumulate in tissue with increased age, and some develop the senesce-associated secretory phenotype (SASP), which describes the secretion of proinflammatory cytokines and chemokines [103, 104]. The SASP compounds can result in tissue dysfunction and may be a mechanism that contributes to the pathogenesis of many age-related diseases. Senescent cells and their associated SASP have previously been implicated in the pathogenesis of periodontal disease. Senescent cells have been identified via the expression of the marker p16Ink4a within the bone that supports the dentition [105]. They have also been identified in higher numbers within the periodontal ligament cells surrounding teeth of older adults, compared to the younger ones [106]. The cytokines secreted by senescent cells include IL-6 and TNFα, which have been implicated in periodontal disease, as discussed previously. Targeting senescence cells via senolytics has proven effective in reducing frailty and other age-related disease [107–109]. However, no study has examined the effect of senolytics in periodontal disease to date.
Neutrophils
In periodontal disease, neutrophils respond early to invading oral microorganisms and migrate to the periodontal tissues and gingival crevice [110]. Neutrophils phagocytize bacteria and produce reactive oxygen species to kill invading microorganisms. However, the production of the reactive oxygen species, when exacerbated, can be destructive to the local tissues. Thus, proper regulation of neutrophil activity is critical to balance an effective innate immune response while minimizing damage to host tissue [110].
Known age-related changes to neutrophils may contribute to the periodontal disease pathogenesis. The total number of circulating neutrophils and their progenitor cells do not appear to be affected by increased age [111]. However, key antimicrobial functions of neutrophils seem to be affected by age, becoming reduced in old subjects when compared to the young. Phagocytosis of bacteria by neutrophils is reduced in old subjects, compared to the young [112]. In addition, formation of neutrophil extracellular traps (NETs) is an antimicrobial strategy utilized by neutrophils, where decondensed DNA structure is released extracellularly and acts to physically entrap and kill invading bacteria [113]. Similar to phagocytosis, NET formation appears to decline in neutrophils from older subjects, compared to the young [114]. In mouse models, higher invasion of bacterial pathogens was associated with increased age and decreased NET formation [115]. The above describes age-related decrease in the antimicrobial properties of neutrophils that would result in the inadequate clearance of the bacteria, resulting in a sustained inflammatory response that could contribute to the pathogenesis of periodontal disease in older adults.
Macrophage
The macrophage is also an early responder to microbial stimuli and tissue injury in periodontal disease. Stimuli from invading bacteria and injured tissue recruit circulating monocytes locally to the periodontium, where they differentiate into macrophages [116]. Early responding macrophages demonstrate a pro-inflammatory phenotype with secretion of metabolic enzymes, such as inducible nitric oxide synthase (iNOS), and cytokines (TNFα, IL-1β, and IL-6) that act to propagate the inflammatory response [117]. Macrophages also acts as important phagocytic cells to clear bacterial pathogens and infected cells from the tissue [116]. At later stages, macrophages change phenotype and act to resolve inflammation and promote healing of damaged tissues through secretion of an anti-inflammatory cytokine profile (IL-10, TGF-β) [117]. It is not clear to what extent an individual macrophage cell can change its phenotype between pro- and anti-inflammatory phenotypes or if the change in phenotypes is a function of temporal differences in the signaling that affect macrophage recruitment and differentiation. An increase in pro-inflammatory macrophage phenotypes over anti-inflammatory phenotypes in the tissue was associated with greater disease severity [118]. Conversely, a higher ratio of anti-inflammatory macrophage phenotypes was associated with periodontal health.
An age-related perturbation of macrophage phenotypes may contribute to periodontal disease pathogenesis and severity. In experimental induction models using old and young mice, inhibiting macrophages improved resolution from periodontal disease in old mice but had no effect in young mice [119]. In trying to understand the age-related differences in macrophage function, many of the in vitro studies evaluating cytokine secretion and phagocytic activity of old and young macrophages demonstrate heterogeneous and conflicting results [120–123]. However, advancement of immunological techniques has allowed for improved and unbiased analysis of the age-related changes in macrophages. Bulk RNAseq of macrophages isolated from bone after injury in old and young mice demonstrated that the macrophages from old mice were transcriptionally distinct. The transcriptional profile of the macrophages from old mice demonstrated increased expression of pro-inflammatory cytokines and chemokines [124]. This transcriptional change appeared to be detrimental in old mice, as the study demonstrated that inhibiting macrophage recruitment to the site of injury improved healing outcome in old mice. In another study, single cell RNAseq analysis of alveolar lung macrophages demonstrated an increased pro-inflammatory gene signature in the macrophages from old mice, compared to the young [125]. Similarly, macrophages from skeletal muscle of old mice demonstrated increased pro-inflammatory markers, compared to the young, as measured via single cell RNAseq [126]. It remains unclear if these age-related phenotypic changes in macrophages are a result of intrinsic changes to the macrophage or a result of changes to the local environment affecting cell activity.
T cells
After an initial acute phase, the transition to chronic inflammation in periodontal disease is characterized by T cell recruitment and differentiation. This adaptive immune response is well described in the pathogenesis of periodontal disease. The most abundant T cell population found in the gingival tissue around teeth is CD4 + T helper cells [127]. Generally, the Th1 subset is characterized by a pro-inflammatory phenotype, and the Th2 subset is characterized by an anti-inflammatory phenotype [128]. The Th1 response in periodontal disease has been shown to produce cytokines that promote osteolytic processes, resulting in the characteristic bone loss around the affected teeth [129]. The Th2 response in periodontal disease has been shown to promote B cell expansion and production of antibodies against oral pathogens [130]. While both Th1 and Th2 subsets are present within the periodontium in health and disease, a shift towards an increased ratio of Th1 cells is observed in the disease [129].
Regulatory T cells (Tregs) and memory T helper 17 cells (Th17) are an additional pair of T cell subset that has complimentary activity in health and periodontal disease. Tregs demonstrate homeostatic and anti-inflammatory roles with characteristic production of IL-10 and TGF-β [131]. Tregs activity in the periodontium has been shown to downregulate the osteolytic processes in periodontal disease [132]. Th17 cells propagate the inflammatory response through their characteristic production of IL-17. The expansion of Th17 population and the associated increase in IL-17 production is characteristic of the pathogenic response in periodontal disease, with IL-17 shown to promote osteolytic activity [133]. Furthermore, inhibition of Th17 cell expansion resulted in decreased bone loss and periodontal disease severity in animal model [132].
Dynamic changes in T cell expansion and activity have been demonstrated as a function of age. Immunosenescence describes an age-related diminished resistance to infection that is largely a result of an involution of the thymus in older adults and a resulting decrease in the production of naïve T cells [134]. This decreased antigen-specific immunity has been implicated in the general susceptibility to infection observed in older adults and would likely confer a similar susceptibility to infection by oral pathogens in the pathogenesis of periodontal disease. In addition, the differentiation and expansion of Th17 cells and an associated increase in IL-17 production have been shown to increase in aging animals and humans [135–137]. Within the periodontium of old mice, increased expansion of Th17 cells was observed, compared to the young [138]. This age-related increase was demonstrated in healthy tissue without the induction of disease, suggesting the tissue may be primed for an elevated and more pathogenic IL-17 response in older mice.
Targeting inflammatory mediators in the treatment of periodontal disease
Chronic inflammatory and autoimmune conditions have benefited from therapies that target the inflammatory cytokines that have been shown to be involved in the disease pathogenesis. While no targeted anti-cytokine therapy has been developed specifically for periodontal disease, host inflammatory modulation during periodontal disease has been demonstrated. Non-steroidal anti-inflammatory drugs (NSAIDs) are well understood to block cyclooxygenase and the broad downstream inflammatory pathways [139]. In clinical and experimental studies of periodontal disease, NSAIDs have been shown to downregulate inflammatory cytokines and improve periodontal clinical parameter [140]. However, taken together, the evidence is conflicting to the benefit of NSAIDs, and along with the known side effects of long-term use of NSAIDs, the clinical effectiveness is limited.
Given the role of TNF-α in periodontal disease, research has investigated the effect of anti-TNF-α therapeutics on periodontal health. TNF inhibitors, including etanercept, adalimumab, and infliximab, have been used routinely for the treatment of rheumatoid arthritis, and the application of such therapeutics has been expanding to other chronic inflammatory conditions [141]. Studies have investigated the effect of the TNF inhibitors on periodontal health in RA patient with and without periodontal disease [141]. Treatment generally improved clinical measures of gingival inflammation in the short term. In longer term follow-up studies (6 and 9 months), subjects with periodontal disease demonstrated improved clinical measures of inflammation and more definitive healing of the tissues supporting the teeth (increased clinical attachment level) [142, 143]. However, most studies are limited to evaluation of periodontal conditions in patients who were prescribed TNF inhibitors for treatment of RA, and no randomized controlled clinical trial has evaluated the effect of TNF inhibitors on periodontal disease to date.
Influence of the aging microbiome
This review has focused on pathologic changes to the host inflammatory response as a function of age. In periodontal disease, this inflammatory response is initiated by the oral bacteria that accumulate on the tooth surfaces. Therefore, it is reasonable to investigate whether the oral bacteria are affected by the age of the host and illicit a more pathogenic inflammatory response.
A contributing factor to age-related inflammatory dysregulation has been shown to be weakened tissue barriers. Skin epidermis, gut epithelium, and the blood–brain barrier all demonstrate an age-related increase in permeability and a resulting increase in microbial invasion [144–146]. This increased permeability was shown to be associated with local pathology and contribute to age-related systemic inflammation. The inflammatory dysregulation may be driven by increased microbial infiltration across the dysfunctional tissue barriers that have been suggested to chronically stimulate a low-grad inflammatory response [144, 147]. During periodontal disease, the gingival epithelium becomes inflamed, ulcerated, and has permeability to local microbes. Thus, epithelial permeability in chronic periodontal disease may similarly drive systemic inflammation, as it occurs with other dysfunctional tissue barriers.
With the advancement of microbiome research and sequencing techniques in recent years, the importance of understanding the human oral microbiome has grown to improve our understanding of human health, diseases, and aging. Periodontal pathogenic bacteria are known to have an impact far beyond the oral cavity, influencing systemic processes throughout the body and being linked to a variety of diseases including cardiovascular conditions [148], stroke [149], Alzheimer’s disease [150], and rheumatoid arthritis [151]. Biological mechanisms include direct translocation of oral bacteria to various parts of the body and locally produced proinflammatory cytokines, which cause systemic inflammation [18]. Some systemic conditions, on the other hand, such as obesity, diabetes, and metabolic syndrome, can lead to changes in the oral microbiome [152, 153]. The proposed mechanism in these cases is related to immune cell malfunction, cytokine imbalances, and increased heme levels in glycosylated hemoglobin, which promotes the growth of proteolytic species [154, 155].
When comparing healthy young and healthy older adults in this regard, it is observed that the phylogenies of the prevalent bacteria remain largely constant as healthy humans age [156]. Small changes in microbiome richness and diversity can be observed, with older adults having lower richness and diversity when compared to the young cohort. In terms of composition, the phylum of Bacteroidetes is more abundant in the young cohort, and the family of Micrococcaceae is more abundant in the older group, with a small increase of the genus Rothia, which has previously been associated with pneumonia in older adults [157].There is evidence that chronic health disorders, smoking, and the presence of yeasts in the oral cavity are the most influential factors related to oral microbiome changes through aging [158]. Furthermore, lifestyle choices, social circumstances, and dental pH levels all influence the composition of the oral microbiome [158]. These confounding variables may explain discrepancies in the literature regarding microbiome changes in older adults.
Conclusion
The aging process is a risk factor for multiple chronic inflammatory conditions, including periodontal disease. This review has demonstrated how a geroscience approach can be used to understand the basic molecular and cellular mechanisms of periodontal disease pathogenesis. In improving our understanding of periodontal disease, we may be able elucidate some of the basic mechanisms that contribute to chronic inflammation in aging and arrive at therapeutic targets that can be utilized across multiple age-related disease.
Funding
This work was supported by the National Institutes of Health (NIDCR) grant number K08DE029505.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 2.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79(8 Suppl):1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 3.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cekici A, et al. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000. 2014;64(1):57–80. [DOI] [PMC free article] [PubMed]
- 5.Eke PI, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dental Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 6.Lopez R, Smith PC, Gostemeyer G, Schwendicke F. Ageing, dental caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl. 18):S145–S152. doi: 10.1111/jcpe.12683. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billings M, et al. Age-dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Periodontol. 2018;89(Suppl 1):S140–S158. doi: 10.1002/JPER.17-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70(1):13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Sekhon TS, Grewal S, Gambhir RS. Periodontal health status and treatment needs of the rural population of India: a cross-sectional study. J Nat Sci Biol Med. 2015;6(1):111–115. doi: 10.4103/0976-9668.149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekino S, et al. Current status of periodontal disease in adults in Takahagi, Japan: a cross-sectional study. BMC Oral Health. 2020;20(1):60. doi: 10.1186/s12903-020-1046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meisel P, et al. Construction of a biological age score to predict tooth loss over 10 years. J Dent Res. 2019;98(10):1096–1102. doi: 10.1177/0022034519861037. [DOI] [PubMed] [Google Scholar]
- 13.Meisel P, Nauck M, Kocher T. Individual predisposition and the intricate interplay between systemic biomarkers and periodontal risk in a general population. J Periodontol. 2021;92(6):844–853. doi: 10.1002/JPER.20-0591. [DOI] [PubMed] [Google Scholar]
- 14.Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: basic biologic considerations. Periodontol 2000. 2021;87(1):143–156. doi: 10.1111/prd.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrovolas S, et al. Population prevalence of edentulism and its association with depression and self-rated health. Sci Rep. 2016;6:37083. doi: 10.1038/srep37083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama Y, et al. Causal effect of tooth loss on depression: evidence from a population-wide natural experiment in the USA. Epidemiol Psychiatr Sci. 2021;30:e38. doi: 10.1017/S2045796021000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott BJ, et al. A transcultural perspective on the emotional effect of tooth loss in complete denture wearers. Int J Prosthodont. 2001;14(5):461–465. [PubMed] [Google Scholar]
- 18.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11):2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 20.Singhrao SK, et al. Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer’s disease. J Alzheimers Dis. 2014;42(3):723–737. doi: 10.3233/JAD-140387. [DOI] [PubMed] [Google Scholar]
- 21.Kamer AR, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015;36(2):627–633. doi: 10.1016/j.neurobiolaging.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30(3):182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 23.Morita I, et al. Five-year incidence of periodontal disease is related to body mass index. J Dent Res. 2011;90(2):199–202. doi: 10.1177/0022034510382548. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez M, et al. Prospective associations between measures of adiposity and periodontal disease. Obesity (Silver Spring) 2012;20(8):1718–1725. doi: 10.1038/oby.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baima G, et al. Periodontitis and accelerated biological aging: a geroscience approach. J Dent Res. 2022;101(2):125–132. doi: 10.1177/00220345211037977. [DOI] [PubMed] [Google Scholar]
- 26.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21(7):426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebersole JL, et al. Aging, inflammation, immunity and periodontal disease. Periodontology 2000. 2016;72(1):54–75. [DOI] [PubMed]
- 28.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1(4):363–376. [PubMed] [Google Scholar]
- 29.McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52(5):803–818. doi: 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher EL, et al. Studying age-related macular degeneration using animal models. Optom Vis Sci. 2014;91(8):878–886. doi: 10.1097/OPX.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youssef SA, et al. Pathology of the aging brain in domestic and laboratory animals, and animal models of human neurodegenerative diseases. Vet Pathol. 2016;53(2):327–348. doi: 10.1177/0300985815623997. [DOI] [PubMed] [Google Scholar]
- 32.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39(1):89–105. doi: 10.1016/S0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 33.Freeman LM, Rush JE. Nutrition and cardiomyopathy: lessons from spontaneous animal models. Curr Heart Fail Rep. 2007;4(2):84–90. doi: 10.1007/s11897-007-0005-6. [DOI] [PubMed] [Google Scholar]
- 34.Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993;64(6):497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, et al. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007;13(6):530–537. doi: 10.1111/j.1601-0825.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 36.Oz HS, Puleo DA. Animal models for periodontal disease. J Biomed Biotechnol. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page RC, Schroeder HE. Spontaneous chronic periodontitis in adult dogs. A clinical and histopathological survey. J Periodontol. 1981;52(2):60–73. doi: 10.1902/jop.1981.52.2.60. [DOI] [PubMed] [Google Scholar]
- 38.An JY, et al. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience. 2017;39(4):457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark D, et al. The contribution of macrophages in old mice to periodontal disease. J Dent Res. 2021;100(12):1397–1404. doi: 10.1177/00220345211009463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang S, et al. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45(4):574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hajishengallis G. Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci. 2014;56(1):30–37. doi: 10.1016/j.job.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394(1–2):49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Molon RS, et al. Characterization of ligature-induced experimental periodontitis. Microsc Res Tech. 2018;81(12):1412–1421. doi: 10.1002/jemt.23101. [DOI] [PubMed] [Google Scholar]
- 44.Kim SE, et al. A modified method for inducing periodontitis in dogs using a silk-wire twisted ligature. J Vet Sci. 2012;13(2):193–197. doi: 10.4142/jvs.2012.13.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol. 1994;65(12):1158–1168. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- 46.Yang M, et al. Alveolar bone loss and mineralization in the pig with experimental periodontal disease. Heliyon. 2018;4(3):e00589. doi: 10.1016/j.heliyon.2018.e00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aung KT, et al. Aging-affected MSC functions and severity of periodontal tissue destruction in a ligature-induced mouse periodontitis Model. Int J Mol Sci. 2020;21(21):8103. doi: 10.3390/ijms21218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Molon RS, et al. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin Oral Investig. 2016;20(6):1203–1216. doi: 10.1007/s00784-015-1607-0. [DOI] [PubMed] [Google Scholar]
- 49.Franceschi C, et al. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90. [DOI] [PubMed]
- 50.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu Q, et al. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Physiol Biochem. 2017;41(4):1360–1369. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda Y, et al. Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues. J Periodontal Res. 2016;51(5):639–646. doi: 10.1111/jre.12344. [DOI] [PubMed] [Google Scholar]
- 53.Moreira PR, et al. Interleukin-6 expression and gene polymorphism are associated with severity of periodontal disease in a sample of Brazilian individuals. Clin Exp Immunol. 2007;148(1):119–126. doi: 10.1111/j.1365-2249.2007.03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 55.Babel N, et al. Analysis of tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-10, IL-6, and interferon-gamma gene polymorphisms in patients with chronic periodontitis. J Periodontol. 2006;77(12):1978–1983. doi: 10.1902/jop.2006.050315. [DOI] [PubMed] [Google Scholar]
- 56.Jenny NS, et al. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22(12):2066–2071. doi: 10.1161/01.ATV.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 57.Licastro F, et al. Interleukin-6 gene alleles affect the risk of Alzheimer’s disease and levels of the cytokine in blood and brain. Neurobiol Aging. 2003;24(7):921–926. doi: 10.1016/S0197-4580(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 58.Shimada Y, et al. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81(8):1118–1123. doi: 10.1902/jop.2010.090741. [DOI] [PubMed] [Google Scholar]
- 59.Madureira DF, et al. Tumor necrosis factor-alpha in gingival crevicular fluid as a diagnostic marker for periodontal diseases: a systematic review. J Evid Based Dent Pract. 2018;18(4):315–331. doi: 10.1016/j.jebdp.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Michaud M, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Gorska R, et al. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30(12):1046–1052. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 62.Di Paola R, et al. Effects of etanercept, a tumour necrosis factor-alpha antagonist, in an experimental model of periodontitis in rats. Br J Pharmacol. 2007;150(3):286–297. doi: 10.1038/sj.bjp.0706979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oates TW, Graves DT, Cochran DL. Clinical, radiographic and biochemical assessment of IL-1/TNF-alpha antagonist inhibition of bone loss in experimental periodontitis. J Clin Periodontol. 2002;29(2):137–143. doi: 10.1034/j.1600-051x.2002.290208.x. [DOI] [PubMed] [Google Scholar]
- 64.Reis C, et al. Clinical improvement following therapy for periodontitis: association with a decrease in IL-1 and IL-6. Exp Ther Med. 2014;8(1):323–327. doi: 10.3892/etm.2014.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74(3):391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106(17):7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boni-Schnetzler M, et al. IL-1beta promotes the age-associated decline of beta cell function. iScience. 2021;24(11):103250. doi: 10.1016/j.isci.2021.103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111(2):245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell CA, et al. Stromal niche inflammation mediated by IL-1 signalling is a targetable driver of haematopoietic ageing. Nat Cell Biol. 2023;25(1):30–41. doi: 10.1038/s41556-022-01053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okamura H, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez-Hernandez PE, et al. IL-12 and IL-18 levels in serum and gingival tissue in aggressive and chronic periodontitis. Oral Dis. 2011;17(5):522–529. doi: 10.1111/j.1601-0825.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 72.Figueredo CM, et al. Increased interleukin-18 in gingival crevicular fluid from periodontitis patients. Oral Microbiol Immunol. 2008;23(2):173–176. doi: 10.1111/j.1399-302X.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 73.Yee M, et al. Porphyromonas gingivalis stimulates IL-18 secretion in human monocytic THP-1 cells. Microbes Infect. 2012;14(9):684–689. doi: 10.1016/j.micinf.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Gangemi S, et al. Increased circulating Interleukin-18 levels in centenarians with no signs of vascular disease: another paradox of longevity? Exp Gerontol. 2003;38(6):669–672. doi: 10.1016/S0531-5565(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 75.Ferrucci L, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frayling TM, et al. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J Gerontol A Biol Sci Med Sci. 2007;62(1):73–78. doi: 10.1093/gerona/62.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ojala J, et al. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30(2):198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Aso Y, et al. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care. 2003;26(9):2622–2627. doi: 10.2337/diacare.26.9.2622. [DOI] [PubMed] [Google Scholar]
- 79.Mallat Z, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104(14):1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 80.Faust HJ, et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest. 2020;130(10):5493–5507. doi: 10.1172/JCI134091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, et al. Th17/IL-17 induces endothelial cell senescence via activation of NF-kappaB/p53/Rb signaling pathway. Lab Invest. 2021;101(11):1418–1426. doi: 10.1038/s41374-021-00629-y. [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Liu X, Zhong Y. Interleukin-17A: The key cytokine in neurodegenerative diseases. Front Aging Neurosci. 2020;12:566922. doi: 10.3389/fnagi.2020.566922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung HY, et al. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8(3–4):572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 84.Shields HJ, Traa A, Van Raamsdonk JM. Beneficial and detrimental effects of reactive oxygen species on lifespan: a comprehensive review of comparative and experimental studies. Front Cell Dev Biol. 2021;9:628157. doi: 10.3389/fcell.2021.628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999;18(45):6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 86.Adler AS, et al. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21(24):3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dan HC, et al. Akt-dependent regulation of NF-kappaB is controlled by mTOR and raptor in association with IKK. Genes Dev. 2008;22(11):1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madrid LV, et al. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276(22):18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 89.Tilstra JS, et al. NF-kappaB in aging and disease. Aging Dis. 2011;2(6):449–465. [PMC free article] [PubMed] [Google Scholar]
- 90.Gritsenko A, et al. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 2020;55:15–25. doi: 10.1016/j.cytogfr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Latz E, Duewell P. NLRP3 inflammasome activation in inflammaging. Semin Immunol. 2018;40:61–73. doi: 10.1016/j.smim.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Youm YH, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salminen A, et al. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7(2):83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287(43):36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koyuncu S, et al. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature. 2021;596(7871):285–290. doi: 10.1038/s41586-021-03781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He M, et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 2020;31(3):580–591 e5. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sevini F, et al. mtDNA mutations in human aging and longevity: controversies and new perspectives opened by high-throughput technologies. Exp Gerontol. 2014;56:234–244. doi: 10.1016/j.exger.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 99.Zhou R, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 100.Liu T, et al. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimada K, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuilman T, et al. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coppe JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Acosta JC, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aquino-Martinez R, et al. LPS-induced premature osteocyte senescence: implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020;132:115220. [DOI] [PMC free article] [PubMed]
- 106.Wu RX, et al. Age-related decline in the matrix contents and functional properties of human periodontal ligament stem cell sheets. Acta Biomaterialia. 2015;22:70–82. doi: 10.1016/j.actbio.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 107.Xu M, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farr JN, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dental Res. 2003;82(2):82–90. [DOI] [PubMed]
- 111.Chatta GS, et al. Hematopoietic progenitors and aging: alterations in granulocytic precursors and responsiveness to recombinant human G-CSF, GM-CSF, and IL-3. J Gerontol. 1993;48(5):M207–12. [DOI] [PubMed]
- 112.Wenisch C, et al. Effect of age on human neutrophil function. J Leukocyte Biol. 2000;67(1):40-45. [DOI] [PubMed]
- 113.Wang J, et al. The role of neutrophil extracellular traps in periodontitis. Front Cell Infect Microbiol. 2021;11:639144.. [DOI] [PMC free article] [PubMed]
- 114.Hazeldine J, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690-8. [DOI] [PMC free article] [PubMed]
- 115.Tseng CW, et al. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PloS One. 2012;7(7):e41454. [DOI] [PMC free article] [PubMed]
- 116.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu T, et al. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol. 2016;87(9):1092–102. doi: 10.1902/jop.2016.160081. [DOI] [PubMed] [Google Scholar]
- 118.Zhou LN, et al. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25(1):265–273. doi: 10.1111/odi.12983. [DOI] [PubMed] [Google Scholar]
- 119.Clark D, et al. The contribution of macrophages in old mice to periodontal disease. J Dental Res. 2021;100(12):1397–1404. doi: 10.1177/00220345211009463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mariani E, et al. RANTES and MIP-1α production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37(2–3):219–26. doi: 10.1016/S0531-5565(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 121.Nyugen J, et al. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30(6):806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lynch AM, et al. The impact of glial activation in the aging brain. Aging Dis. 2010;1(3):262–278. [PMC free article] [PubMed] [Google Scholar]
- 123.Aprahamian T, et al. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152(3):448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clark D, et al. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell. 2020;19(3):e13112. [DOI] [PMC free article] [PubMed]
- 125.Lafuse WP, et al. Identification of an increased alveolar macrophage subpopulation in old mice that displays unique inflammatory characteristics and is permissive to Mycobacterium tuberculosis infection. J Immunol. 2019;203(8):2252–2264. doi: 10.4049/jimmunol.1900495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krasniewski LK, et al. Single-cell analysis of skeletal muscle macrophages reveals age-associated functional subpopulations. Elife. 2022;11:e77974. [DOI] [PMC free article] [PubMed]
- 127.Williams DW, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184(15):4090–4104.e15. [DOI] [PMC free article] [PubMed]
- 128.Constant SL, Bottomly K. Induction of TH1 and TH2 CD4+ T cell responses: the alternative approaches. Ann Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 129.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12(2):125–135. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 130.Garlet GP. Critical reviews in oral biology & medicine: destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dental Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 131.Alvarez C, et al. Regulatory T lymphocytes in periodontitis: a translational view. Mediators Inflamm. 2018;2018:7806912. [DOI] [PMC free article] [PubMed]
- 132.Dutzan N, et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10(463):eaat0797. [DOI] [PMC free article] [PubMed]
- 133.Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 2017;23(7):854–865. doi: 10.1111/odi.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Minato N, Hattori M, Hamazaki Y. Physiology and pathology of t-cell aging. Int Immunol. 2020;32(4):223–231. doi: 10.1093/intimm/dxaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhadricha H, et al. Increased frequency of Th17 cells and IL-17 levels are associated with low bone mineral density in postmenopausal women. Sci Rep. 2021;11(1):16155. doi: 10.1038/s41598-021-95640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JS, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140(1):84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lim MA, et al. Increased Th17 differentiation in aged mice is significantly associated with high IL-1beta level and low IL-2 expression. Exp Gerontol. 2014;49:55–62. doi: 10.1016/j.exger.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 138.Dutzan N, et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 2017;46(1):133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bacchi S, et al. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11(1):52–64. doi: 10.2174/187152312803476255. [DOI] [PubMed] [Google Scholar]
- 140.Ren J, et al. The role of non-steroidal anti-inflammatory drugs as adjuncts to periodontal treatment and in periodontal regeneration. J Transl Med. 2023;21(1):149. doi: 10.1186/s12967-023-03990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zamri F, de Vries TJ. Use of TNF inhibitors in rheumatoid arthritis and implications for the periodontal status: for the benefit of both? Front Immunol. 2020;11:591365. doi: 10.3389/fimmu.2020.591365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pers JO, et al. Anti-TNF-alpha immunotherapy is associated with increased gingival inflammation without clinical attachment loss in subjects with rheumatoid arthritis. J Periodontol. 2008;79(9):1645–1651. doi: 10.1902/jop.2008.070616. [DOI] [PubMed] [Google Scholar]
- 143.Fabri GM, et al. Periodontitis response to anti-TNF therapy in ankylosing spondylitis. J Clin Rheumatol. 2015;21(7):341–345. doi: 10.1097/RHU.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 144.Hu L, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137(6):1277–1285. doi: 10.1016/j.jid.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hussain B, Fang C, Chang J. Blood-brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front Neurosci. 2021;15:688090. doi: 10.3389/fnins.2021.688090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim KA, et al. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 148.Teles R, Wang CY. Mechanisms involved in the association between periodontal diseases and cardiovascular disease. Oral Dis. 2011;17(5):450–461. doi: 10.1111/j.1601-0825.2010.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cho HJ, et al. Severe periodontal disease increases acute myocardial infarction and stroke: a 10-year retrospective follow-up study. J Dent Res. 2021;100(7):706–713. doi: 10.1177/0022034520986097. [DOI] [PubMed] [Google Scholar]
- 150.Hu X, et al. Periodontal disease and the risk of Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. Psychogeriatrics. 2021;21(5):813–825. doi: 10.1111/psyg.12743. [DOI] [PubMed] [Google Scholar]
- 151.Gonzalez-Febles J, Sanz M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol. 2021;87(1):181–203. doi: 10.1111/prd.12385. [DOI] [PubMed] [Google Scholar]
- 152.Negrini TC, et al. Interplay among the oral microbiome, oral cavity conditions, the host immune response, diabetes mellitus, and its associated-risk factors-an overview. Front Oral Health. 2021;2:697428. doi: 10.3389/froh.2021.697428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nibali L, et al. Clinical review: association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(3):913–920. doi: 10.1210/jc.2012-3552. [DOI] [PubMed] [Google Scholar]
- 154.Sanz M, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 155.Smiga M, et al. Glycation of host proteins increases pathogenic potential of Porphyromonas gingivalis. Int J Mol Sci. 2021;22(21):12084. doi: 10.3390/ijms222112084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou X, et al. Exploratory studies of oral and fecal microbiome in healthy human aging. Front Aging. 2022;3:1002405. doi: 10.3389/fragi.2022.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Steenhuijsen Piters WA, et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J. 2016;10(1):97–108. doi: 10.1038/ismej.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Willis JR, et al. Citizen-science reveals changes in the oral microbiome in Spain through age and lifestyle factors. NPJ Biofilms Microbiomes. 2022;8(1):38. doi: 10.1038/s41522-022-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]