Abstract
Induction of apoptotic cell death generally requires the participation of cysteine proteases belonging to the caspase family. However, and similar to most cell types, mouse fibroblasts are normally resistant to tumor necrosis factor alpha (TNF-α)-induced apoptosis. Surprisingly, TNF-α treatment of vaccinia virus-infected mouse fibroblasts resulted in necrotic-like cell death, which was significantly reduced in cells infected with a vaccinia virus mutant lacking the caspase inhibitor B13R. Furthermore, TNF-α also induced necrotic-like cell death of fibroblasts in the presence of peptidyl caspase inhibitors. In both cases, necrosis was accompanied by generation of superoxide species. Caspase inhibitors also sensitized fibroblasts to killing by double-stranded RNA and gamma interferon. In all cases, cell death was efficiently blocked by antioxidants or mitochondrial respiratory chain inhibitors. These results define a new mitochondrion-dependent mechanism which may be important in the killing of cells infected with viruses encoding caspase inhibitors.
Tumor necrosis factor alpha (TNF-α) is a pleiotropic cytokine which was originally identified as a consequence of its ability to cause tumor cell killing (5). Later studies demonstrated a key role for this cytokine in inflammation and in responses to infectious agents including bacteria and many types of viruses (38). Two TNF-α receptors called TNFR1 and TNFR2 have been identified (10, 22). Both receptors belong to a large family of related molecules (TNFR superfamily) which also includes the death-inducing Fas protein (30). The cytotoxic effects of TNF-α are generally mediated by TNFR1, which, like Fas but unlike TNFR2, contains a “death domain.” Recent studies have shown that TNFR1 and Fas bind related downstream signaling proteins that function as mediators of cell death signals (1, 29). However, different from Fas, TNFR1 cytotoxicity to most cell types is evident only if RNA or protein synthesis is inhibited, indicating the existence of survival mechanisms dependent on de novo RNA and protein synthesis (38). Such a survival mechanism is dependent on the NF-κB transcription factor and activation of survival genes that are regulated by this factor (2, 23, 37, 41).
Induction of cell death by members of the TNFR superfamily typically occurs by apoptosis (1, 29). A unique feature of apoptotic cells is that they retain cell membrane integrity even after they have disintegrated into characteristic apoptotic bodies (43). Apoptotic cells and bodies are phagocytosed by macrophages, thus preventing an inflammatory reaction that can result from cell lysis (31, 32). In contrast, cells dying by necrosis can readily lose membrane integrity, which can lead to inflammation (43). Induction of apoptotic cell death by TNFR1 requires the activation of cysteine proteases belonging to the caspase family. In particular, activation of caspase 8 is a critical step in induction of TNF-induced apoptosis (6, 28). Consistent with the pivotal role of caspases in apoptotic cell death, TNF-α-induced apoptosis was shown to be blocked by caspase inhibitors in tumor cell lines such as HeLa and MCF7 (27, 35).
TNF-α also possesses significant antiviral activity that can be manifested by either direct killing of infected cells or induction of a state of resistance in uninfected cells, which may prevent further infection (26, 42). These antiviral properties are also shared by the gamma interferon (IFN-γ) and double-stranded RNA (dsRNA) signaling pathways (4, 14). The physiological significance of these mechanisms is evident by the fact that many viruses encode proteins capable of inhibiting the TNF-α, dsRNA, and IFN-γ signaling pathways (17, 21). Many viruses also encode proteins which can inhibit caspase proteases and may thus prevent or delay apoptotic cell death by TNF-α or by other inducers of apoptosis (15). Here we report a new mechanism by which TNF-α may kill virus-infected cells by induction of necrotic-like cell death. This mechanism is dependent on the presence of caspase inhibitors in viruses and is efficiently duplicated in uninfected cells by peptidyl caspase inhibitors. We also show that induction of necrotic-like cell death by TNF-α and caspase inhibitors appears to be dependent on the production of superoxides by the mitochondrial respiratory chain. Similarly to TNF-α, dsRNA and IFN-γ also induced cytotoxicity to caspase inhibitor-treated cells. These results provide evidence for a novel function of caspase inhibitors in inducing cell death by agents which by themselves are noncytotoxic. Such a mechanism may be important in killing of cells infected with viruses encoding caspase inhibitors.
MATERIALS AND METHODS
Cells, viruses, and materials.
Fibroblasts were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM) containing l-glutamate (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and calf serum (10%). Mouse 3T3 fibroblasts were derived as described previously (2). Western reserve (WR) strain wild-type vaccinia virus and B13R mutant and revertant viruses were a generous gift from G. L. Smith (Oxford University, Oxford, United Kingdom) (19). DMEM plus 2.5% fetal bovine serum was used for viral infections. Human TNF-α and mouse IFN-γ was obtained from R&D Systems and used at a final concentration of 10 ng/ml each in all experiments if not specified otherwise. dsRNA [poly(I-C)] was obtained from Sigma and used at a final concentration of 100 μg/ml. Caspase inhibitors (Enzyme System Products) were dissolved in dimethyl sulfoxide at 20 mM and used at 100 μM. The mitochondrial inhibitors amytal, thenoyltrifluoroacetone (TTFA), and the antioxidant butylated hydroxyanisole (BHA) were obtained from Sigma. Actinomycin D and cycloheximide were used 2 and 10 μg/ml, respectively. The nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) and the superoxide-specific dye dihydroethidium (DHE) were obtained from Molecular Probes.
Vaccinia virus infection of mouse fibroblasts.
3T3 fibroblasts (2 × 105) were plated on each well of a six-well plate 1 day before infection. Viruses were then used to infect cells at 2 PFU per cell for 16 h, after which the cells were left untreated or treated with TNF-α (1 ng/ml) for 3 h.
Analysis of cell death. (i) Cell morphology.
For morphological observations, cells in six-well plates were washed with phosphate-buffered saline (PBS) twice before being treated with trypan blue (Gibco-BRL) for 1 min. The cells were washed with PBS once and then examined under a 20× objective. Representative fields were photographed. Cells which excluded trypan blue and showed membrane blebbing and apoptotic bodies were considered apoptotic, while cells that were trypan blue permeable and exhibited a “balloon-like” morphology were considered necrotic.
(ii) Nucleus morphology.
Cells in plates were rinsed with PBS, fixed with 3.7% formaldehyde, and permeabilized with 0.2% Triton X-100 for 5 min. They were then washed and incubated with DAPI labeling solution (2 μg/ml in PBS) for 5 min and examined under a fluorescence microscope.
(iii) Cell viability experiments.
Approximately 2 × 105 cells were plated on each well of a six-well plate 1 day before the experiments. Caspase inhibitors or mitochondrial inhibitors were added to the indicated concentrations 1 h before the addition of TNF-α or TNF-α plus actinomycin D. After the indicated periods, the cells were trypsinized and viable cells were counted by trypan blue exclusion. Four independent readings within a single experiment were used to calculate the standard deviation.
Detection of superoxide production.
Cells were trypsinized after treatments and resuspended in DMEM plus 10 μM DHE at 106/ml. The cells were incubated at 37°C for 30 min and then washed and resuspended in PBS before being subjected to fluorescence-activated cell sorter analysis.
EMSA, affinity blots, and Western blots.
Electrophoretic mobility shift assay (EMSA) was carried out as described previously (3). Affinity blotting was performed essentially as described previously (9). Briefly, approximately 5 × 106 cells were harvested after the treatments indicated. The cells were then washed once with PBS and pelleted, and the pellet was snap-frozen on dry ice. An equal volume of 1 μM biot-VAD-fmk in MDB buffer (50 mM NaCl, 2 mM MgCl2, 5 mM EGTA, 10 mM HEPES, 1 mM dithiothreitol [pH 7]) was added to the cell pellet, and the cells were lysed by three cycles of freezing and thawing. The lysates were incubated at 37°C for 15 min and centrifuged. Then 20-μg samples of protein lysates from the supernatant were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membrane was blocked for 30 min with TBST (20 mM Tris, 150 mM NaCl, 0.05% Tween 20) supplemented with 2% nonfat dry milk (NFDM) and then incubated for 1 h in avidin-Neutralite (Molecular Probes) at 1 μg/ml in TBST supplemented with 1% NFDM. The membrane was then washed and incubated in biotinylated horseradish peroxidase (Molecular Probes) at 25 ng/ml in TBST for 1 h. The labeled protein was visualized by enhanced chemiluminescence (Amersham). For Western blots, whole-cell extracts were made by boiling the cell pellet in SDS-PAGE sample buffer. The lysates were then sonicated before being subjected to separation by SDS-PAGE. The C2.10 poly(ADP-ribose) polymerase (PARP) antibody (Enzyme System Products) was used at a 1:10,000 dilution in subsequent steps.
RESULTS
The caspase inhibitor B13R sensitizes vaccinia virus-infected fibroblasts to TNF-α cytotoxicity.
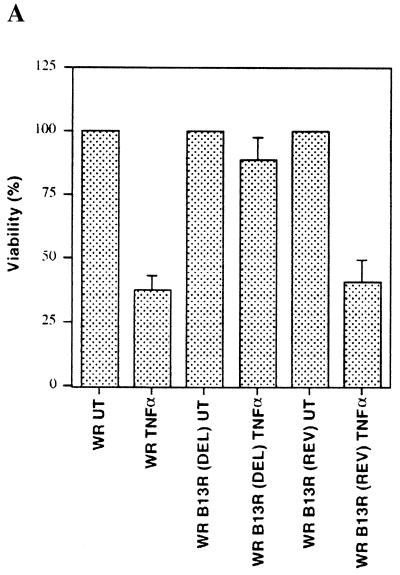
Virus-encoded caspase inhibitors are thought to protect infected cells from apoptotic cell death and may thus represent a strategy to escape from host defense mechanisms (15). Based on these results, we wanted to determine whether TNF-α-induced cell death in susceptible cell types was inhibited upon infection with viruses encoding caspase inhibitors. We tested this by using vaccinia virus, which encodes two proteins that are highly similar to the cowpox virus caspase inhibitor CrmA. These putative caspase inhibitors are B13R (92% identical to CrmA) and B22R (19, 33). We first tested the effect of vaccinia virus infection on a TNF-α-resistant 3T3 fibroblast line derived from mouse embryonic fibroblasts (RelA+/+ 3T3, subsequently referred to as 3T3 fibroblasts) (2). These 3T3 fibroblasts were first infected with vaccinia virus for 16 h, after which TNF-α was added for an additional 3 h. Unexpectedly, infection of these cells with vaccinia virus greatly sensitized them to TNF-α-induced killing (Fig. 1A). More surprisingly, infection of 3T3 fibroblasts with a B13R mutant virus significantly attenuated TNF-α-induced cytotoxicity, while a B13R revertant virus was as cytotoxic as the wild-type virus (Fig. 1A). These results suggest that the putative caspase inhibitor B13R can enhance TNF-α-induced cytotoxicity.
FIG. 1.
Caspase inhibitors sensitize mouse fibroblasts to TNF-α-mediated cytotoxicity. (A) 3T3 fibroblasts were infected with either WR strain wild-type vaccinia virus or B13R deletion (DEL) or B13R revertant (REV) vaccinia virus before being treated with TNF-α (1 ng/ml) for 3 h. Viable cells after treatment are shown as a percentage of viable untreated cells (UT). (B) 3T3 fibroblasts were treated with TNF-α (□), z-VAD-fmk (◊), or TNF-α plus z-VAD-fmk (○) for 3 or 6 h. (C) 3T3 fibroblasts were treated with TNF-α alone or with z-VAD-fmk, BD-fmk, or z-FA-fmk for 6 h.
Peptidyl caspase inhibitors can sensitize multiple cell types to TNF-α-cytotoxicity.
We then tested whether the broad-specificity peptidyl caspase inhibitor Cbz-Val-Ala-Asp(OMe)-fmk (z-VAD-fmk), which covalently associates with activated caspases, could also enhance TNF-α killing of 3T3 fibroblasts. 3T3 fibroblasts readily lost viability after TNF-α treatment in the presence of z-VAD-fmk, while TNF-α or z-VAD-fmk alone had no significant effect (Fig. 1B). A different caspase inhibitor, Boc-Asp(OMe)-fmk (BD-fmk), also sensitized these cells to TNF-α (Fig. 1C). Importantly, a cysteine protease inhibitor, Cbz-Phe-Ala-fmk (z-FA-fmk), which does not have a critical aspartate residue and is thus not specific for caspases, showed no significant effect on cell viability after TNF-α treatment (Fig. 1C). These results demonstrate that both virus-encoded and peptidyl caspase inhibitors can enhance the killing of cells that are normally resistant to TNF-α.
We then tested the effect of TNF-α–z-VAD-fmk treatment on other mouse and human cell types. Mouse BALB/c 3T3 mouse fibroblasts also underwent cell death in the presence of TNF-α plus z-VAD-fmk (data not shown). Similarly, vaccinia virus-infected BALB/c fibroblasts were readily killed in the presence of TNF-α, while cell death was significantly attenuated when these cells were infected with B13R mutant virus (data not shown). Similar results were also obtained in human promonocytic U937 cells treated with TNF-α plus z-VAD-fmk (data not shown). Thus, both fibroblast and monocytic cell types can be sensitized to TNF-α killing by caspase inhibitors.
Effect of caspase inhibitors on caspase activation and TNF-α-induced apoptosis.
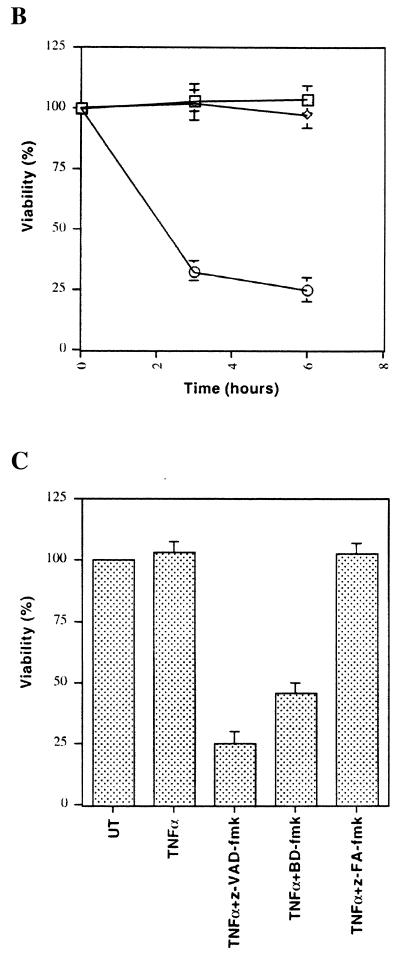
Like most cell types, fibroblasts are resistant to TNF-α but may be sensitized to TNF-α-induced apoptosis in the presence of RNA or protein synthesis inhibitors (38). As expected, significant cell death was observed in 3T3 fibroblasts after treatment with TNF-α and the RNA synthesis inhibitor actinomycin D (Fig. 2A) or with TNF-α and the protein synthesis inhibitor cycloheximide (data not shown). Dying cells displayed typical changes associated with apoptotic cell death, such as cytoplasmic shrinkage, membrane blebbing, and appearance of apoptotic bodies (Fig. 2A). An important feature of apoptotic cell death is retention of cell membrane integrity, sometimes even after disintegration of cells into apoptotic bodies (43). 3T3 fibroblasts undergoing cell death were impermeable to trypan blue, indicating that membrane integrity was retained (Fig. 2A). These results suggest that TNF-α induces apoptotic cell death of fibroblasts in the presence of macromolecule synthesis inhibitors (also see below).
FIG. 2.
TNF-α induces apoptosis in the presence of macromolecule synthesis inhibitors. (A) Mouse 3T3 fibroblasts were treated with TNF-α plus actinomycin D (2 μg/ml) for 3 h, after which trypan blue was added. Apoptotic cells are indicated by arrows. (B) Cell lysates from untreated (UT) or TNF-α–actinomycin D-treated (TA) cells were made in the presence of biot-VAD-fmk (1 μM), biot-VAD-fmk and z-VAD-fmk, or biot-VAD-fmk and z-FA-fmk, and lysates (20 μg of protein) were examined by a biotin-avidin affinity blot. The activated putative caspase P1 is indicated. (C) Whole-cell lysates from cells treated as in panel B were analyzed by a Western blot assay with a PARP-specific antibody. The 116-kDa precursor and the 85-kDa processed form are indicated. (D) Mock-infected or vaccinia virus-infected 3T3 fibroblasts were left untreated (UT) or treated with TNF-α plus cycloheximide (TC) for 3 h, after which P1 was detected as in panel B. (E) 3T3 fibroblasts were treated with TNF-α plus actinomycin D (□), z-VAD-fmk (◊), or TNF-α plus actinomycin D and z-VAD-fmk (○) for 3 or 6 h. Viable cells remaining after treatment are shown as a percentage of viable untreated cells. DEL, B13R deletion mutant; REV, B13R revertant.
Activation of caspase proteases is generally required for induction of apoptosis (7). To test whether z-VAD-fmk was capable of associating with caspases activated during apoptosis induced by TNF-α plus actinomycin D, we used biotinylated Val-Ala-Asp(OMe)-fmk (biot-VAD-fmk) (9). TNF-α–actinomycin D treatment of fibroblasts resulted in increased binding to biot-VAD-fmk of a protein (P1) which corresponds in molecular mass to the activated form of caspase proteases (approximately 18 kDa) (7) (Fig. 2B). The ability of P1 to associate with a caspase inhibitor and its molecular mass suggest that it represents a caspase protease activated by TNF-α–actinomycin D treatment (also see Discussion). As expected, z-VAD-fmk strongly competes with biot-VAD-fmk for binding to P1 while z-FA-fmk competes to a significantly lesser extent (Fig. 2B). Furthermore, the caspase substrate PARP was also processed after TNF-α–actinomycin D treatment (Fig. 2C). Importantly, z-VAD-fmk, but not z-FA-fmk, inhibited PARP cleavage, suggesting that z-VAD-fmk can inhibit caspase activation in vivo (Fig. 2C). Similar to z-VAD-fmk treatment, vaccinia virus infection completely inhibited P1 activation in 3T3 fibroblasts induced by TNF-α–cycloheximide treatment (Fig. 2D). In contrast, activation of P1 occurred in cells infected with B13R mutant virus but not B13R revertant virus following TNF-α–cycloheximide treatment (Fig. 2D). However, P1 activation in B13R mutant virus-infected cells was significantly lower than in uninfected cells, perhaps as a consequence of the presence of the B22R putative caspase inhibitor. In conclusion, these results indicate that both z-VAD-fmk and B13R can function as caspase inhibitors during apoptosis.
Treatment of cells with caspase inhibitors can prevent apoptosis by death-inducing agents (7). We therefore tested the effect of z-VAD-fmk on apoptosis induced by TNF-α plus actinomycin D in 3T3 fibroblasts. However, z-VAD-fmk significantly enhanced cell death induced by TNF-α plus actinomycin D (Fig. 2E). These results thus demonstrate that z-VAD-fmk enhances the cytotoxicity of TNF-α plus actinomycin D even though it inhibits caspase activation.
TNF-α–caspase inhibitor treatment results in necrotic-like cell death.
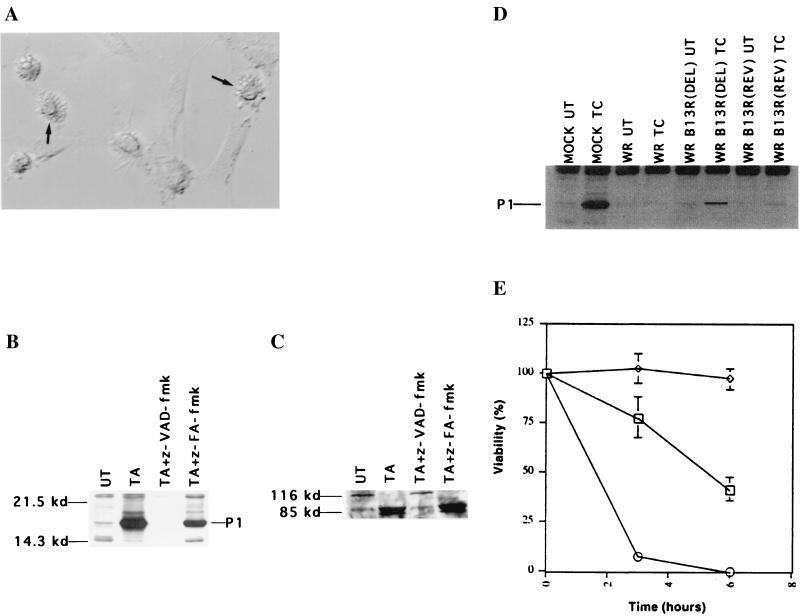
Recent studies have demonstrated an important role for the NF-κB transcription factor in protecting cells from TNF-α-induced apoptosis (2, 23, 37, 41). To investigate whether TNF-α–caspase inhibitor cytotoxicity was a result of inhibition of NF-κB activation, an EMSA analysis was performed. No significant difference was seen in NF-κB activation by TNF-α in the presence of z-VAD-fmk (Fig. 3A). Thus, TNF-α–caspase inhibitor cytotoxicity was not due to inhibition of NF-κB.
FIG. 3.
TNF-α–caspase inhibitor treatment induces necrotic-like cell death. (A) 3T3 fibroblasts were pretreated with z-VAD-fmk for 1 h or left untreated (UT) before the addition of TNF-α for 1 h. Nuclear extracts were made to test NF-κB activation. The arrow indicates the mobility of the NF-κB–DNA complex. (B) TNF-α–actinomycin D-treated (top) or TNF-α–z-VAD-fmk-treated (bottom) cells were stained with DAPI. Nuclear morphology is indicated by arrows. (C) 3T3 fibroblasts were treated with TNF-α plus z-VAD-fmk for 3 h, and trypan blue was added. Arrows indicate typical necrotic cells.
We then tested whether TNF-α–caspase inhibitor induced cell death was accompanied by morphological transformations similar to those seen during apoptotic cell death (43). TNF-α plus actinomycin D treatment of 3T3 fibroblasts resulted in chromatin condensation and nuclear fragmentation characteristic of apoptosis (Fig. 3B). In contrast, TNF-α–z-VAD-fmk-treated cells did not reveal such changes in nuclear morphology (Fig. 3B). Furthermore, DNA cleavage occurred in apoptotic but not in TNF-α–z-VAD-fmk-treated cells (data not shown). Importantly, TNF-α–z-VAD-fmk-treated 3T3 fibroblasts appeared very different from the TNF-α–actinomycin D-treated cells. The first visible effect in these dying cells was a moderate degree of cellular edema, which progressed to significant cellular swelling so that eventually the cells assumed a “balloon-like” morphology (Fig. 3C). Such effects are typically associated with cells undergoing necrotic death (43). Perhaps the most physiologically relevant difference between apoptotic and necrotic cell death is that apoptotic cells retain membrane integrity while necrotic cells do not (43). Unlike apoptotic cells, TNF-α–z-VAD-fmk-treated fibroblasts lost membrane integrity rapidly and before detachment from the culture dish (Fig. 3C). In addition, TNF-α–z-VAD-fmk treatment of BALB/c 3T3 fibroblasts and U937 cells or TNF-α treatment of vaccinia virus-infected 3T3 fibroblasts also resulted in similar morphological transformations and cell lysis (data not shown). These results indicate that TNF-α–caspase inhibitor-treated cells do not undergo cell death by apoptosis but, rather, by a mechanism that appears similar to necrosis.
Superoxide production by mitochondrial respiratory-chain complexes during necrotic cell death.
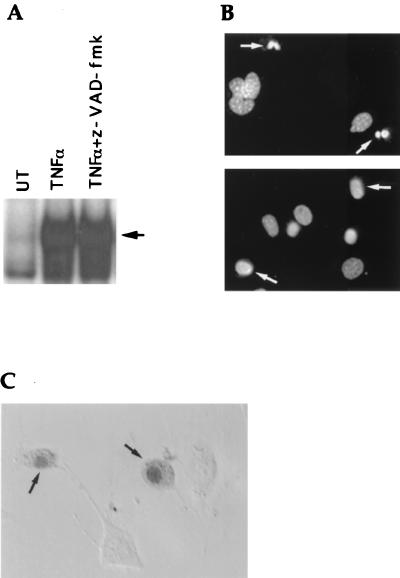
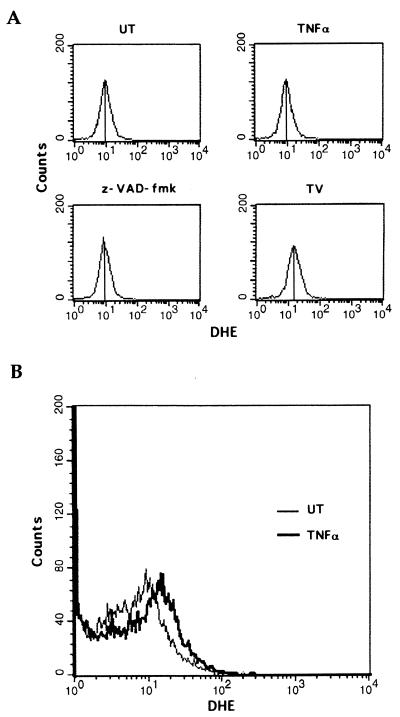
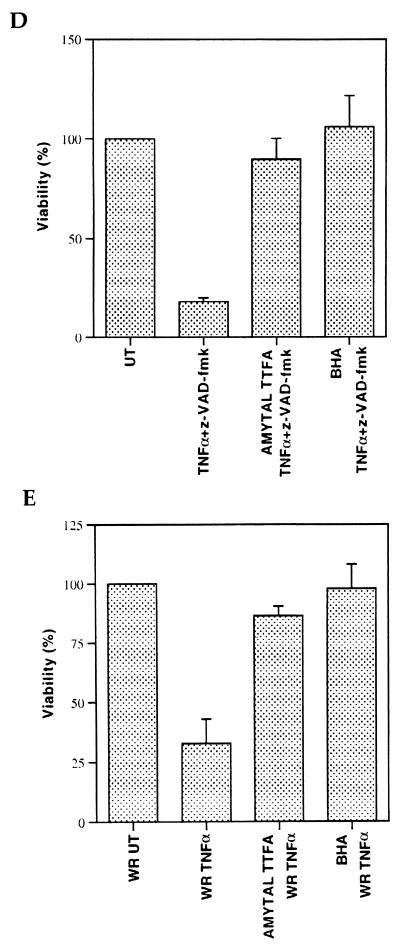
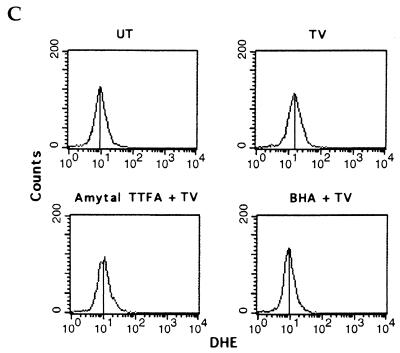
Reactive oxygen species (ROSs) have previously been implicated in the induction of necrotic cell death (11). To investigate whether ROSs were generated after treatment with TNF-α plus caspase inhibitors, we first used DHE, a probe that specifically binds superoxides (45). TNF-α or z-VAD-fmk alone did not significantly affect superoxide levels in 3T3 fibroblasts, while combined treatment with TNF-α plus z-VAD-fmk significantly increased superoxide levels (Fig. 4A). Similarly, TNF-α treatment of vaccinia virus-infected 3T3 fibroblasts was also accompanied by the production of superoxides (Fig. 4B). In contrast, production of the peroxide ROSs was not affected after the treatment with TNFα plus z-VAD-fmk (data not shown), indicating the generation of superoxides but not peroxides during necrosis. Mitochondrial respiratory-chain complexes are an important source of intracellular superoxides (36). We therefore tested the possible involvement of superoxides generated by mitochondrial respiratory-chain complexes in the induction of necrotic cell death by TNF-α plus caspase inhibitors. Remarkably, TNF-α–z-VAD-fmk-induced superoxide production and necrosis were both significantly blocked by either mitochondrial complex I and II inhibitors (amytal and TTFA) or by the anti-oxidant BHA (Fig. 4C and D). Similarly, TNF-α-induced killing of vaccinia virus-infected 3T3 fibroblasts was also significantly blocked by complex I and II inhibitors or BHA (Fig. 4E). These results indicate a unique role for mitochondrial respiratory-chain complexes in TNF-α-induced necrotic cell death that appears to be dependent on their ability to generate superoxide species.
FIG. 4.
TNF-α–caspase inhibitor treatment induces the production of superoxides. (A) 3T3 fibroblasts were either left untreated (UT) or treated with TNF-α, z-VAD-fmk, or TNF-α plus z-VAD-fmk (TV) for 3 h. Intracellular superoxide levels were detected by DHE binding. (B) WR strain wild-type vaccinia virus-infected cells were either left untreated (UT) or treated with TNF-α (1 ng/ml) for 3 h, after which intracellular superoxide levels were detected by DHE binding. (C) 3T3 fibroblasts were left untreated (UT) or treated with TNF-α plus z-VAD-fmk (TV) for 3 h in the absence or presence of mitochondrial complex I and II inhibitors (amytal and TTFA) or the antioxidant BHA. Superoxide levels were detected as in panel A. (D) 3T3 fibroblasts were treated with TNF-α plus z-VAD-fmk for 6 h in the absence or presence of amytal and TTFA or BHA. Cell viability is shown as a percentage of untreated cells (UT). (E) 3T3 fibroblasts were infected with WR strain wild-type vaccinia virus. They were then treated with TNF-α (1 ng/ml) in the absence or presence of amytal and TTFA or BHA for 3 h.
Caspase inhibitors can also sensitize fibroblasts to dsRNA- and IFN-γ-induced cytotoxicity.
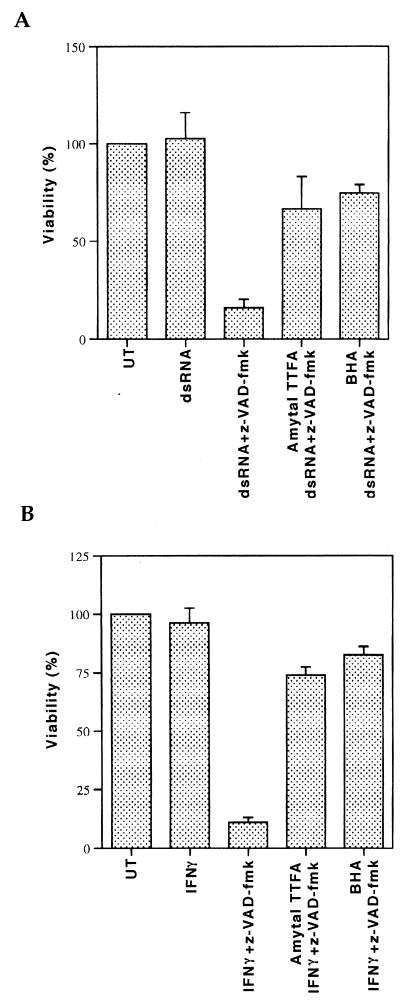
We then tested whether killing by other cytotoxic agents could also be potentiated in the presence of caspase inhibitors. However, killing by two well-known inducers of apoptosis, staurosporine and ceramide, was not potentiated in the presence of z-VAD-fmk in 3T3 fibroblasts (data not shown). We then tested whether treatment with dsRNA or IFN-γ, two cytotoxic agents that play an important role in the antiviral response (4, 14), could induce the killing of 3T3 fibroblasts in the presence of caspase inhibitors. Neither dsRNA nor IFN-γ treatment had any cytotoxic effect on 3T3 fibroblasts (Fig. 5). However, in the presence of z-VAD-fmk, dsRNA and IFN-γ induced dramatic necrotic-like killing (Fig. 5). In both cases, BHA or mitochondrial respiratory-chain complex inhibitors significantly blocked cell death (Fig. 5). These results demonstrate that caspase inhibitors can sensitize fibroblasts to cytotoxicity induced by multiple agents.
FIG. 5.
Caspase inhibitors sensitize mouse fibroblasts to dsRNA-induced or IFN-γ-induced cytotoxicity. (A) 3T3 fibroblasts were left untreated (UT) or treated with dsRNA or with dsRNA plus z-VAD-fmk for 6 h in the absence or presence of mitochondrial complex I and II inhibitors (amytal and TTFA) or the antioxidant BHA. Viable cells after treatment are shown as a percentage of viable untreated cells. (B) 3T3 fibroblasts were left untreated (UT) or treated with IFN-γ or with IFN-γ plus z-VAD-fmk for 6 h in the absence or presence of amytal and TTFA or BHA.
DISCUSSION
The results presented here provide evidence for a novel function of caspase inhibitors in induction of necrotic-like cell death. Thus, both the vaccinia virus-encoded B13R protein and peptidyl caspase inhibitors can mediate necrotic killing of cells by TNF-α. Previous studies have demonstrated a key role for NF-κB in inhibiting TNF-α-induced apoptosis. Here we show that the caspase inhibitor z-VAD-fmk allows necrotic killing of fibroblasts by TNF-α but does not inhibit the activation of NF-κB. Thus, TNF-α cytotoxicity can be mediated by at least two mechanisms: inhibition of NF-κB results in death by apoptosis, while inhibition of caspase proteases results in death by necrosis. Our results demonstrate that TNF-α-induced necrotic killing can be used to eliminate cells infected with viruses encoding caspase inhibitors. Thus, the very presence of virus-encoded caspase inhibitors may render infected cells susceptible to TNF-α-induced necrotic cell death.
We have also shown that necrotic killing appears to be dependent on the generation of superoxides. Interestingly, superoxide production was not elevated during TNF-α-induced apoptosis of fibroblasts (data not shown), suggesting that superoxide production accompanies necrotic but not apoptotic cell death. In addition, TNF-α and z-VAD-fmk did not induce superoxide production in cells which are resistant to necrotic killing, such as HeLa (data not shown). In fact, vaccinia virus infection of HeLa cells can render them resistant to killing by TNF-α plus cycloheximide in a B13R-dependent manner (references 8 and 19 and data not shown). These results suggest that induction of necrosis by TNF-α and caspase inhibitors may occur only when superoxide generation takes place. Our results also demonstrate the involvement of the mitochondrial respiratory chain in superoxide production and induction of necrotic cell death. Thus, mitochondria may also play an important role in the regulation of necrotic cell death, in addition to their established function during apoptosis (12). Interestingly, recent studies have shown that caspase proteases are also present in mitochondria (25, 34). It is intriguing to speculate that inhibition of these caspases may be responsible for increased production of ROSs by the mitochondrial respiratory chain and induction of necrotic killing by TNF-α.
Previous studies have demonstrated a key role for caspase proteases in the induction of apoptotic cell death. However, our results provide evidence for a novel function of caspase proteases in inhibition of necrotic cell death by TNF-α. Although caspases involved in inhibiting necrosis are not known, we have recently identified caspase 3 as a major caspase activated in RelA−/− 3T3 fibroblasts treated with TNF-α or RelA+/+ 3T3 fibroblasts treated with TNF-α plus actinomycin D (corresponding to P1 [unpublished results]). However, since both z-VAD-fmk and B13R (based on similarity to CrmA) are capable of interaction with many distinct caspase proteases (including caspase 3), their necrotic effects could be mediated by inhibition of multiple caspases. These may also include constitutively active caspases present at low levels in nonapoptotic cells, such as TNF-α-treated 3T3 fibroblasts.
A recent study has also demonstrated increased necrosis in L929 cells after caspase inhibition (39). However, L929 cells also undergo necrosis in the presence of TNF-α alone (13), while our results indicate that inhibition of necrosis by caspases is likely to be a more general phenomenon which can efficiently kill cells that are normally resistant to TNF-α. In this regard, our results are also distinct from those of previous studies, which have shown a switch from apoptosis to necrosis in the presence of caspase inhibitors but without affecting total killing of cells (16, 44). During the preparation of this paper, Khwaja and Tatton also reported that NIH 3T3 and U937 cells undergo necrotic killing in the presence of TNF-α and peptidyl caspase inhibitors (20). These findings, and the results presented here, demonstrate a role for caspase inhibitors in killing of cells that are normally resistant to TNF-α.
We also show that caspase inhibitors can mediate cytotoxicity in dsRNA- and IFN-γ-treated cells. Similar to TNF-α, dsRNA and IFN-γ cytotoxicity was also significantly inhibited by antioxidant or mitochondrial respiratory-chain inhibitor treatment. These results suggest that a similar cytotoxic pathway is induced by TNF-α, dsRNA, and IFN-γ in the presence of caspase inhibitors. In this respect, it is interesting that viruses have devised strategies to subvert signaling by all three of these agents (17, 21). Thus, inhibition of these signaling pathways may not only inhibit the activation of antiviral gene expression (e.g., alpha/beta IFNs, PKR, adenylate synthase) but may also inhibit the killing of virus-infected cells. Similar to TNF-α, dsRNA, and IFN-γ, the Fas death receptor is an important mediator of host defense. More specifically, Fas expression on virus-infected cells mediates cytotoxicity through interaction with Fas ligand-expressing cytotoxic T lymphocytes (24). Two studies have recently demonstrated the induction of necrotic killing by the Fas death receptor (18, 40). Vercammen et al. have shown that Fas induces the necrotic killing of L929 cells in the presence of caspase inhibitors (40), while Kawahara et al. have demonstrated the induction of necrosis by Fas in cells devoid of caspase 8 (18). These studies and the results presented here suggest that caspase inhibitor-mediated killing may be a general mechanism shared by multiple antiviral agents. Such killing may play an important role in host defense strategies against viral infections.
ACKNOWLEDGMENTS
We gratefully acknowledge G. L. Smith for providing vaccinia virus mutants. We also thank J. Manley and Carol Prives for comments on the manuscript, P. Bruzzo for technical assistance, and J. Shanley for help with photography.
This work was supported in part by NIH grant R01 CA074892 to A.A.B.
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 3.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 4.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 5.Carswell E A, Old L J, Kassel R L, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinnaiyan A M, Tepper C G, Seldin M F, O'Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 7.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 8.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J. 1997;16:2271–2281. doi: 10.1093/emboj/16.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin R G, Anderson D, Jerzy R, Davis T, Brannan C I, Copeland N G, Jenkins N A, Smith C A. Molecular cloning and expression of the type 1 and type 2 murine receptors for tumor necrosis factor. Mol Cell Biol. 1991;11:3020–3026. doi: 10.1128/mcb.11.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 13.Grooten J, Goossens V, Vanhaesebroeck B, Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor- induced cytotoxicity. Cytokine. 1993;5:546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 14.Haines D S, Strauss K I, Gillespie D H. Cellular response to double-stranded RNA. J Cell Biochem. 1991;46:9–20. doi: 10.1002/jcb.240460104. [DOI] [PubMed] [Google Scholar]
- 15.Hardwick J M. Viral interference with apoptosis. Semin Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch T, Marchetti P, Susin S A, Dallaporta B, Zamzami N, Marzo I, Geuskens M, Kroemer G. The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene. 1997;15:1573–1581. doi: 10.1038/sj.onc.1201324. [DOI] [PubMed] [Google Scholar]
- 17.Ho C K, Shuman S. Physical and functional characterization of the double-stranded RNA binding protein encoded by the vaccinia virus E3 gene. Virology. 1996;217:272–284. doi: 10.1006/viro.1996.0114. [DOI] [PubMed] [Google Scholar]
- 18.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith G L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta- converting enzyme and protects virus-infected cells from TNF- and Fas- mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 20.Khwaja A, Tatton L. Resistance to the cytotoxic effects of tumor necrosis factor alpha can be overcome by inhibition of a FADD/caspase-dependent signaling pathway. J Biol Chem. 1999;274:36817–36823. doi: 10.1074/jbc.274.51.36817. [DOI] [PubMed] [Google Scholar]
- 21.Krajcsi P, Wold W S. Inhibition of tumor necrosis factor and interferon triggered responses by DNA viruses. Semin Cell Dev Biol. 1998;9:351–358. doi: 10.1006/scdb.1998.0244. [DOI] [PubMed] [Google Scholar]
- 22.Lewis M, Tartaglia L A, Lee A, Bennett G L, Rice G C, Wong G H, Chen E Y, Goeddel D V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 24.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 25.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestan J, Digel W, Mittnacht S, Hillen H, Blohm D, Moller A, Jacobsen H, Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature. 1986;323:816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- 27.Miura M, Friedlander R M, Yuan J. Tumor necrosis factor-induced apoptosis is mediated by a CrmA-sensitive cell death pathway. Proc Natl Acad Sci USA. 1995;92:8318–8322. doi: 10.1073/pnas.92.18.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzio M, Chinnaiyan A M, Kischkel F C, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signalling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 31.Platt N, da Silva R P, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 32.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 33.Smith G L, Howard S T, Chan Y S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989;70:2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- 34.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost M C, Alzari P M, Kroemer G. Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 36.Turrens J F. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 37.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 38.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 39.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 42.Wong G H, Goeddel D V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature. 1986;323:819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 43.Wyllie A H, Kerr J F, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 44.Xiang J, Chao D T, Korsmeyer S J. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]