Abstract
Background
The exosome is a critical component of the intercellular communication., playing a vital role in regulating cell function. These small vesicles contain proteins, mRNAs, miRNAs, and lncRNAs, surrounded by lipid bilayer substances. Most cells in the human body can produce exosomes, released into various body fluids such as urine, blood, and cerebrospinal fluid. Bladder cancer is the most common tumor in the urinary system, with high recurrence and metastasis rates. Early diagnosis and treatment are crucial for improving patient outcomes.
Methods
This study employed the PubMed search engine to retrieve publicly accessible data pertaining to urinary exosomes.
Results
We summarize the origins and intricate biological characteristics of urinary exosomes, the introduction of research methodologies used in basic experiments to isolate and analyze these exosomes, the discussion of their applications and progress in the diagnosis and treatment of bladder cancer, and the exploration of the current limitations associated with using urinary exosomes as molecular biomarkers for diagnosing bladder cancer.
Conclusion
Exosomes isolated from urine may be used as molecular biomarkers for early detection of bladder cancer.
Keywords: Bladder cancer diagnosis, Urine, Exosomes, Biomarker, Liquid biopsy
1. Introduction
Bladder cancer is the ninth most common cancer worldwide [1]. Surveys indicate that ∼75 % of bladder cancers are non-muscle invasive, while the remaining 25 % are muscle-invasive. Of high-risk non-muscle invasive cases, ∼50 % eventually experience tumor recurrence [2], and 10–30 % progress to muscle-invasive bladder cancer [3]. Early diagnosis and regular follow-up are crucial for improving the prognosis of patients with bladder cancer [[4], [5], [6]].
The primary tools for diagnosing and monitoring bladder cancer currently involve invasive methods such as transurethral cystoscopy and biopsy. Urine exfoliative cytology, a non-invasive test, is specific (86–99 %) but exhibits low sensitivity (48–70 %), limiting its utility in uroepithelial carcinoma [7]. Consequently, there is a clinical need for more convenient and practical tests for diagnosing and monitoring bladder cancer.
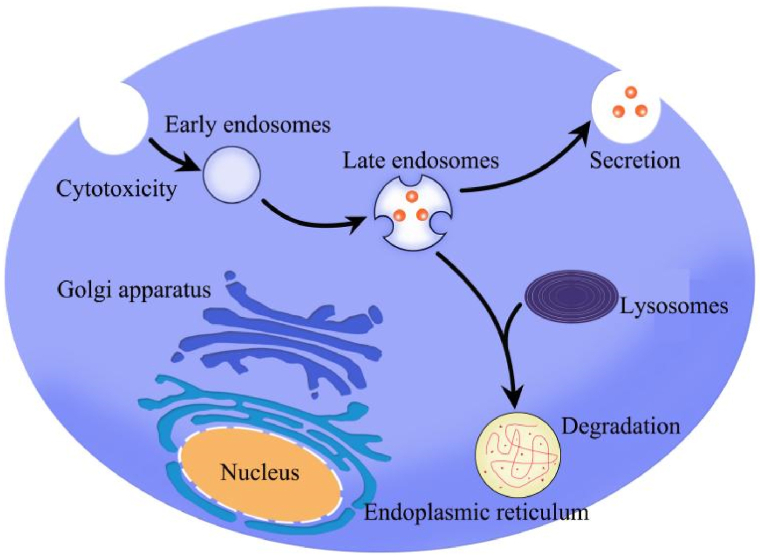
Extracellular vesicles (EVs) constitute a heterogeneous group of membrane-encapsulated nanovesicles secreted by nearly all eukaryotic cells [8,9]. Exosomes, a subtype of EVs, were first identified in the supernatant of sheep erythrocytes cultured in vitro in the 1980s [10,11]. The biological role of exosomes was initially reported in 1996 [12]. The process of exosome formation is part of the endosomal pathway, where the plasma membrane invaginates to create endocytic vesicles. Multiple endocytic vesicles fuse to form early endosomes, which may mature and either return to the plasma membrane or fuse with lysosomes for degradation. As endosomes progress from early to late stages, intraluminal vesicles (ILVs) form, resulting in the creation of multivesicular bodies (MVBs). These MVBs releaseThe internal vesicles, or exosomes, are then released from cells through fusion with the cellular plasma membrane [13].
In the tumor microenvironment (TME), EVs are generated and released by donor cells, such as cancer cells, to influence the phenotype of nearby and distant recipient cells. Additionally, EVs carry donor cell-specific proteins, lipids, and RNA [14,15]. Recognition and interaction between EVs and recipient cells are mediated by specific surface molecules on EVs or recipient cells, including acetyl heparan sulfate proteoglycans [16], integrins [17], phosphatidylserine, T-cell immunoglobulins, and proteins with mucin structural domains [18].
Urinary exosomes primarily originate from the kidney, urinary tract epithelium, and the male genital tract (99.96 %). These vesicles are primarily composed of cholesterol and sphingolipids, containing intact membrane proteins, especially glycoproteins, peripheral membrane proteins, cytoplasmic and nuclear proteins, as well as intracellular components such as long non-coding RNAs (lncRNAs), micro RNAs (miRNAs), messenger RNAs (mRNAs), and metabolites. Recent investigations into the genetic origin of urinary exosomes at the organ and cellular levels reveal that the bladder, endothelial cells, basal cells, monocytes, and dendritic cells play integral roles in the formation of these exosomes [19].
Recognized as novel molecular markers, urinary exosomes hold substantial research potential and could play a crucial role in the early detection of bladder cancer. This paper aims to review the biological properties of urinary exosomes, explore research methods in basic experiments, and assess their applications and progress in the diagnosis and treatment of bladder cancer. Additionally, it will discuss the current possibilities and research challenges associated with urinary exosomes as potential tumor markers.
2. Methods
This study utilized the PubMed search engine to retrieve publicly accessible data related to urinary exosomes. The keywords "exosomes" and "bladder cancer" were employed along with "urinary biomarkers" to gather and synthesize English-language articles published before March 2023. The present review aims to comprehensively summarize all narrative and systematic reviews published within the past ten years concerning urinary exosomes in the context of bladder cancer. Additionally, this review endeavors to identify obstacles encountered by urinary exosomes in the diagnosis and treatment of bladder cancer and explore their potential utility as a therapeutic modality.
The review excluded letters, editorials, research protocols, case reports, brief letters, and articles published in languages other than English from the review process.
3. The concept of exosomes
3.1. Basic characteristics of exosomes
Exosomes, ranging in size from 30 to 150 nm, represent the smallest subtype of EVs (30–1000 nm) secreted by living cells [20]. Classification of EVs, based on size, biogenesis, biological properties, and release mechanisms, reveals three main subgroups: endosomal-derived exosomes (<200 nm diameter), plasma membrane-derived microvesicles (>200 nm diameter), and apoptotic vesicles (>500 nm diameter) [20]. Studies on EVs primarily focus on exosomes and microvesicles secreted by living cells, both sharing overlapping biophysical properties such as size, biomarkers, and content (see Fig. 1) [21].
Fig. 1.
Secretory processes of exosomes.
In this review, emphasis is placed on exosomes and microvesicles when discussing EVs. Tumor-derived EVs, particularly exosomes, are implicated in various stages of cancer development and treatment resistance, including pre-metastatic niche formation [22], metastasis [23], and immunosuppression [24]. Notably, numerous studies underscore the significance of exosomes in early tumor diagnosis, progression, and treatment [[25], [26], [27]]. Urine, as a readily obtainable bodily fluid specimen, has emerged as a novel subject for investigating exosomes.
3.2. Extraction and identification of exosomes
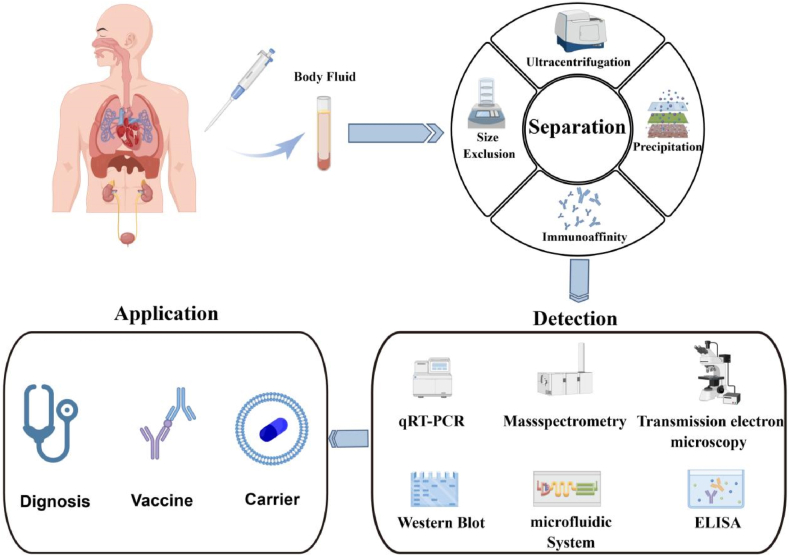
Significant advancements have been made in methods for exploring urinary exosome biomarkers (Fig. 2). To present a more accurate in vivo representation of exosomes, each acquisition step, including pre-treatment, ultracentrifugation, quantitative composition, normalization, and data analysis, must be standardized.
Fig. 2.
Sequential steps in the application process for studying urinary exosomes.
3.3. Storage and pretreatment
Recent studies indicate that the recovery of exosomes from frozen samples stored at −80 °C is superior to that from samples stored at −20 °C. Thawing samples may lead to exosome loss, necessitating extensive vortexing to maximize recovery. Pretreatment with the detergent CHAPS before isolation aims to minimize the interfering effect of Tamm-Horsfall protein (THP) [28].
3.4. Isolation
Exosomes possess the unique ability to be enriched from biofluids using surface markers, allowing the isolation of subpopulations with tumor-specific or tumor-rich surface marker proteins [29]. Various methods exist for exosome isolation, such as ultracentrifugation, density gradient centrifugation, filtering centrifugation, as well as magnetic beads and immunoaffinity. It is crucial to note that these techniques are not necessarily interchangeable; each method has its own set of advantages and disadvantages. Further comparative studies are still required (see Table 1 for a simplified summary).
Table 1.
Comparison of the advantages and disadvantages of different separation techniques.
| Separation technology | Advantages | Disadvantages |
|---|---|---|
| Ultracentrifugation | A well-developed and widely used technique | Inadequate purity of samples |
| Density gradient centrifugation | High purity of samples | High time costs |
| Filtering centrifugation | Simple operation process | Inadequate purity of samples |
| Magnetic beads and immunoaffinity | High specificity | Low efficiency |
| Size exclusion chromatography | Good uniformity | High cost |
| Size-based microfluidics | Precise and efficient | Technical complexity |
| Commercial kits | Convenient and fast | No uniform standard |
3.5. Quantification
The prevailing method for exosome quantification currently involves protein blotting with ELISA to determine proteins proportional to the number of exosomes, such as TSG-101. Simultaneously, the direct counting of exosomes using fluoroscopic electron microscopy is becoming increasingly sophisticated, albeit with a cumbersome yet highly accurate process.
In recent years, direct counting methods have made significant advances, and several techniques have emerged that can substantially reduce handling time and improve accuracy. Examples include nanosight nanoparticle tracking analysis, tunable resistive pulse sensing, surface plasmon resonance, etc. We believe that technological innovation will further promote the study of urine exosomes and be better used in revealing the mechanism of tumor development and helping clinical diagnosis and treatment.
3.6. Biological functions of exosomes in bladder cancer
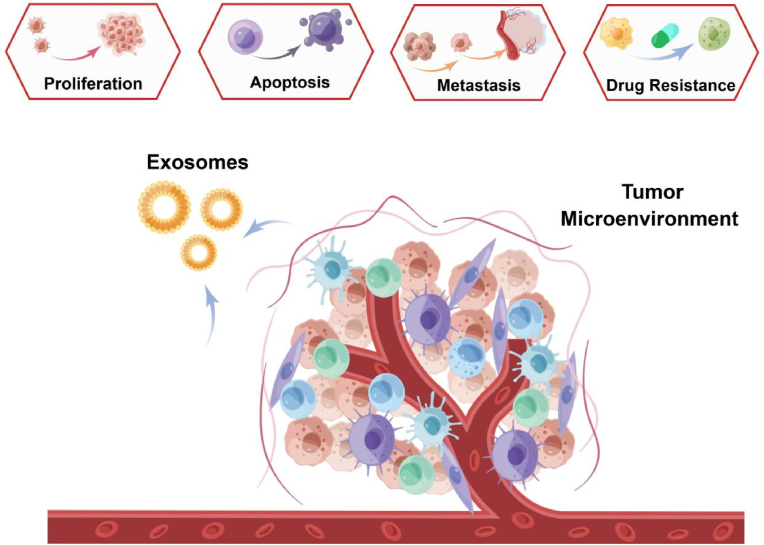
As increasingly exemplified in research, exosomes play a crucial role in tumorigenesis, tumor growth, and metastasis [30]. Recently, tumor-derived exosomes (TEXs) have been recognized as crucial signaling mediators regulating the TME. They regulate extracellular communication not only with cancer cells but also with stromal cells [31], altering and reprogramming receptor cells by interacting with host immune cells [32,33], epithelial cells [34], and tumor cells [35,36] to promote tumor proliferation and metastasis [30]. Another study showed that moderately malignant pancreatic (PC) cell lines could bind exosomes from highly malignant PC cell lines and enhance the proliferation of the former. In addition, upregulation of exosomal zinc transport protein 4 may be associated with enhanced proliferation of PC cancer cells [37]. Tumor-secreted exosomal lncRNA LNMAT2 is involved in lymphangiogenesis and lymph node metastasis of bladder cancer in a VEGF-C non-dependent manner [38]. In diagnosing tumors, exosomes can serve as prospective biomarkers, with exosomal RNA and proteins being the most studied at the forefront. Tumor-associated EV proteins can be a reliable biomarker for early cancer detection and cancer-type determination [39].
4. Advantages of urinary exosomes in bladder cancer
4.1. Organizational and structural advantages
Unlike other neoplasms, the bladder functions as a reservoir for urine, allowing for the direct release of exosomes from bladder cancer cells into the urinary system. Consequently, the presence of exosomes in urine provides a viable approach for directly monitoring alterations in bladder cancer cells [40].
4.2. Stable information preservation advantages
Non-invasive tests using soluble biomarkers in biofluids are more readily available but are limited by sample and biological complexity (e.g., urinary metabolites prone to degradation, etc.) and are usually less informative [41]. In contrast, exosomes are rich in information due to their encapsulation by a lipid bilayer derived from the plasma membrane. Thus, they provide an opportunity for preserving tissue information more effectively [42].
4.3. Non-invasive diagnostic advantages
While tissue biopsy remains a common method for cancer diagnosis, the small tissues extracted do not represent tumor heterogeneity or monitor dynamic tumor progression. This invasive method may increase the likelihood of metastasis, leading to poor survival and prognosis [43]. Urine, due to its minimal invasiveness, enables liquid biopsy collection of biological fluid specimens such as blood and urine, attracting significant attention and creating more opportunities for cancer diagnosis and real-time surveillance [44].
Therefore, the collection of urine exosomes offers a convenient, secure, and consistent method, providing a more effective avenue for further exploration of exosomes in the context of bladder cancer.
5. Molecular mechanism and application of urinary exosomes in bladder cancer
5.1. Promoting the development of bladder cancer
Urinary exosomes have been demonstrated to play a crucial role in the development of bladder cancer (Fig. 3). Sun et al. [45] showed that HOTAIR promotes bladder cancer proliferation, migration, and invasion by downregulating miR-205 levels. Additionally, Berrondo et al. [46] found that HOTAIR and certain tumor-related lncRNAs were enriched in T2∼T4 muscle-infiltrating bladder cancer in urinary exosomes. Inhibition of HOTAIR in bladder cancer cell lines resulted in reduced migration and invasion in vitro. CARLA et al. [47] discovered that urinary exosomes from patients with bladder cancer contained epithelial growth factor-like repetitive disc-like structure I-like domain protein 3, whose levels correlated with tumor grade and promoted endothelial cell angiogenesis and migration in the bladder cancer TME. Hao et al. conducted a comprehensive analysis of urinary exosome miRNA profiles from patients with bladder cancer. They determined that compared to healthy controls, miR-93-5p levels were altered, and further investigations revealed that miR-93-5p promotes bladder tumor proliferation, migration, and invasion [48]. Chen et al. screened circular RNA (circRNA) expression profiles using a circRNA microarray in paired urothelial carcinoma of the bladder (UCB) and normal tissues. They studied the clinical significance of an upregulated circRNA, circPRMT5, in a large cohort of patients with UCB. Subsequently, they explored the functions and underlying mechanisms of circPRMT5 in UCB cell epithelial-mesenchymal transition and found that circPRMT5 was upregulated in serum and urinary exosomes from these patients, and significantly correlated with tumor metastasis [49].
Fig. 3.
Biological functions of tumor-derived exosomes.
5.2. Potential biomarkers for the diagnosis of bladder cancer
Urinary exosomes encompass cellular materials of varying sizes, incorporating large molecules like proteins and small molecules (Table 2), such as mRNA, miRNA, and lncRNA. These constituents hold promise as potential molecular biological markers [[50], [51], [52]]. In the context of bladder cancer, both tissue and plasma exosomes exhibited a significant reduction in PTEN pseudogene 1 (PTENP1). This reduction may enhance PTEN levels by serving as a competing endogenous RNA (ceRNA) for miR-17, thereby inhibiting cancer progression. These findings suggest that exosomal PTENP1 stands as a promising biomarker for the clinical detection and prognostic assessment of bladder cancer [53].
Table 2.
The clinical significance and performance of exosomal cargoes as bladder cancer biomarkers.
| Type | Urine exosome | Function |
|---|---|---|
| Affects the biological function of bladder cancer | HOTAIR&miR-93-5p | Regulation of proliferation, migration and invasion |
| EDIL-3 | Promoted endothelial cell angiogenesis and migration | |
| circPRMT5 | Promoted metastasis of urothelial carcinoma | |
| Protein molecular markers | PTENP1 | promising biomarker for clinical detection |
| TACSTD2 | Promising molecular diagnostic for bladder cancer | |
| TPP1, TMPRSS2, FOLR1, RALB, and RAB35 | Protein markers with diagnostic significance | |
| RNA molecular markers | HOTAIR, HOXA-AS2 and MALAT1 | Low expression between normal and tumor exosomes |
| LncRNAs- HYMAI, LINC00477, LOC100506688, and OTX2-AS1 | High expression between normal and tumor exosomes | |
| ARID1A, PIK3CA, FGFR3, HRAS, KMT2D, RB1, TP53, KDM6A, and STAG2 | Urinary exosomes with different effects on postoperative tumor recurrence |
5.3. Protein molecular markers
Chen et al. conducted a study involving comparative and targeted proteomic analyses of urinary microparticles from nine hernia (control) and patients with bladder cancer. They identified a total of 107 differentially expressed proteins [54]. Precise quantification of differences in the concentrations of 29 proteins (41 signature peptides) was achieved by LC−MRM/MS in 48 urine samples from bladder cancer, hernia, and urinary tract infection/hematuria cases. Concentrations of 24 proteins exhibited significant changes (p < 0.05) between bladder cancer (n = 28) and hernia (n = 12), with area-under-the-curve values ranging from 0.702 to 0.896. Finally, the researchers quantified tumor-associated calcium-signal transducer 2 (TACSTD2) in raw urine specimens (n = 221) using a commercial ELISA and confirmed its potential value for diagnosing bladder cancer. This study underscores a robust association of TACSTD2 with bladder cancer and highlights the potential of human urinary microparticles in the non-invasive diagnosis of bladder cancer.
Lin et al. executed a comparative proteomic analysis of urinary exosomes in 10 healthy controls and 10 age-matched patients with bladder cancer. They compared the EV proteome with the overall urine proteome. Through MS-based label-free proteomics, they identified a total of 1222 proteins, with 56 proteins exhibiting significantly increased levels in the urinary EV of patients with bladder cancer [55]. This study suggests that urinary EV holds the potential to serve as a source of enrichment for bladder cancer protein biomarkers.
6. RNA molecular markers
6.1. mRNA
In a study by Perez [56], microarray technology was employed to analyze urine samples from five patients with bladder cancer and six patients without cancer before cystoscopy. The results revealed the differential presence of four genes—FOXO3, GALNT1, ARHGEF39, and LASS2—in urine between patients with and without cancer. Previous research has implicated these genes in tumor progression and metastasis. Meanwhile, Wen et al. identified and assessed the diagnostic performance of urinary exosomal CA9 mRNA for bladder cancer by isolating urinary exosome samples from 168 patients with bladder cancer and 90 control subjects.
6.2. LncRNA
Berrondo et al. [46] conducted a study on exosomes extracted from urine collected from eight patients with muscle-invasive bladder cancer and five healthy controls using qRT-PCR. They identified several lncRNAs with differential expression, including HOTAIR, HOXA-AS2, MALAT1, and mRNAs such as SOX2. In addition, they examined lncRNAs from 10 bladder cancer samples and 7 normal samples using RNA-seq and qRT-PCR, finding significantly higher levels of lncRNAs—HYMAI, LINC00477, LOC100506688, and OTX2-AS1—in the exosomes of tumor samples compared to normal samples. These lncRNAs show potential as molecular biological markers for muscle-infiltrating bladder cancer. However, their validation requires a larger cohort of patients with bladder cancer and comparison with urinary exosomes from patients with non-muscle-infiltrating bladder cancer.
Furthermore, a diagnostic method based on the detection of a combination of three urinary exosome-derived lncRNAs (SPRY4-IT1, MALAT1, and PCAT-1) was used for bladder cancer diagnosis [38]. This panel exhibited superior performance compared to urine cytology, with an area under the curve (AUC) value of 0.813 versus 0.619. Additionally, urinary exosomal PCAT-1 emerged as an independent prognostic factor for assessing recurrence-free survival in non-muscle-invasive bladder cancer [57].
6.3. MicroRNA
Andreu et al. [58] employed qRT-PCR to analyze first-day morning urine samples collected preoperatively from 34 patients with bladder cancer (18 high-grade and 16 low-grade uroepithelial carcinomas) and 9 healthy volunteers. In patients with high-grade bladder cancer, only miR-375 showed a significant reduction compared to controls, while miR-146a exhibited significant upregulation in the exosomes of patients with low-grade bladder cancer. Matsuzaki et al. [59] used miRNA microarrays to analyze urine samples from six patients with uroepithelial cancer from admission to surgery versus three healthy volunteers. Their findings revealed that the 36 patients with bladder cancer in urine exosome samples had higher expression of five miRNAs than the 24 controls. Notably, miR-21-5p emerged as the most effective molecular biological marker for detecting uroepithelial cancer, with an AUC area of 0.900 and sensitivities and specificities of 75.0 % and 95.8 %, respectively. However, further validation in a larger sample size is required to confirm these conclusions.
7. Potential therapeutic tool
Apart from their role as potential molecular biological markers, the distinctive biological characteristics of exosomes contribute to their capacity to target biological information, positioning them as specific carriers for targeted therapy of tumors by homing in on cancer cells. The advantage of urinary exosomes lies in their easily accessible properties. We believe that in more in-depth studies of urinary exosomes, it is possible to discover new diagnostic markers and avoid patients undergoing painful biopsy procedures.
7.1. Intervention in the normal function of cancer cells
Exosomes have proven effective as carriers for drug and functional RNA delivery in cancer therapy due to their ability for cellular uptake and stable transfer of therapeutic miRNAs and proteins. In comparison to liposomal nanomaterials, metallic nanomaterials, and polymeric nanomaterials, exosomes overcome disadvantages such as poor bioavailability, minimizing non-targeted cytotoxicity, and reducing immunogenicity [60]. The presence of transmembrane and membrane-anchored proteins in exosomes enhances endocytosis, facilitating the transfer of their contents. Franzen et al. [61] demonstrated the feasibility of using exosomes as delivery vehicles for bladder cancer cells through receptor-mediated endocytosis, laying the groundwork for targeted delivery to bladder tumor cells. While non-targeted delivery has been used in bladder perfusion therapy for bladder cancer, a drawback is the potential adverse effects on normal epithelial cells surrounding tumor cells. Carrie et al. [62] observed that bladder cancer cells exhibited a significantly higher uptake of exosomes (≥50-fold) compared to normal urothelial cells. Additionally, Malam et al. [63] demonstrated that loading therapeutic nanoparticles into liposomes encapsulating exosomes increased drug concentration in the target tissue, reducing toxic effects on normal tissues. Ou et al. found high expression levels of TAMs-derived exosomal miR-193a-5p are directly correlated to angiogenesis and tumor deterioration [64].
Successful application of this technique in the clinic holds the promise of significant reductions in dose requirements and adverse side effects for patients.
7.2. Strengthening the autoimmune system as a cancer vaccine
Exosomes as a cancer vaccine has emerged as a hot research topic. Current studies indicate that TEXs fulfil the conditions for antigen presentation in an acellular vaccine, offering advantages over conventional vaccines, including ease of preservation and a lower incidence of side effects. Zhang et al. [65] found that exosomes isolated from IL-12-anchored modified kidney cancer cells exhibited superior antitumor effects in in vitro experiments compared to TEXs and IL-12 alone. While these studies provide a rationale for using exosomes as cancer vaccines for bladder cancer, a drawback lies in the fact that exosomes derived from tumor cells may express specific immunosuppressive molecules, such as FasL or TGFβ, potentially reducing their immunogenicity and attenuating the specific immune response. Wang et al. engineered breast cancer-derived exosomes to form an in situ DC vaccine (HELA-Exos), leading to potent tumor inhibition in a poorly immunogenic triple negative breast cancer (TNBC) mouse xenograft model and patient-derived tumor organoids [66].
8. Current limitations and perspectives of urinary exosome research
Given its aggressive and fast-growing nature, the early diagnosis of bladder cancer is critical for a patient's prognosis. Traditional diagnostic methods, including clinical evaluation, imaging, urinalysis, and cystoscopy, are costly, limiting their widespread use for bladder cancer screening. Despite efforts to replace these invasive procedures with less costly and invasive diagnostic methods, such as urine biomarkers, the currently available tests exhibit low sensitivity and specificity, hindering their ability to replace cystoscopy for diagnosis. Urine exosomes, with their stability in information preservation and consistent sample characteristics, hold promise as potential liquid biopsies for bladder cancer.
However, urinary exosomes, despite being a focal point in current research, are still in their early stages as molecular biology markers and face several challenges before clinical utilization. The standardization of urinary exosome concentrations remains unresolved, introducing variations that complicate sample comparisons, even from the same patient at different times. Methods for directly measuring exosome counts are not widely available, necessitating improvements in isolation and extraction techniques. Additionally, molecular biology markers need to strike a balance between specificity and convenience. While exosomes offer higher specificity than other soluble molecules like proteins and RNAs, improvements in isolation methods are required for wider accessibility and clinical use in basic and clinical research.
In conclusion, urinary exosomes possess significant research value as cutting-edge molecular biology markers. With a substantial body of basic and clinical studies, there is potential for their gradual clinical application in the field of urological tumors. This holds the promise of introducing safer, more convenient, and more accurate diagnostic measures, contributing to advancements in the diagnosis and monitoring of bladder cancer.
Data availability statement
The data and materials in the current study are available from the corresponding author on reasonable request (drliu7087797@163.com).
Funding information
This work was supported by funding from the Natural Science Foundation of China (grant 82202255).
CRediT authorship contribution statement
Ji Liu: Formal analysis. Zhang Zhijin: Writing – original draft. Wentao Zhang: Writing – original draft. Maskey Niraj: Investigation. Fuhan Yang: Validation. Guo Changcheng: Writing – original draft. Liliang Shen: Methodology. Tianyuan Xu: Methodology, Investigation. Shenghua Liu: Supervision. Zhang Junfeng: Validation. Shiyu Mao: Data curation. Wei Li: Writing – review & editing. Xudong Yao: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Shiyu Mao, Email: maoshiyu1144@sina.com.
Wei Li, Email: weili06@tongji.edu.cn.
Xudong Yao, Email: yaoxudong1967@163.com.
References
- 1.Yang Y., Okada S., Sakurai M. Adenosine-to-inosine RNA editing in neurological development and disease. RNA Biol. 2021;18(7):999–1013. doi: 10.1080/15476286.2020.1867797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enhanced visualization methods for first transurethral resection of bladder tumour in suspected non-muscle-invasive bladder cancer: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(12):1–123. [PMC free article] [PubMed] [Google Scholar]
- 3.Rieken M., Shariat S.F., Kluth L., Crivelli J.J., Abufaraj M., Foerster B., et al. Comparison of the EORTC tables and the EAU categories for risk stratification of patients with nonmuscle-invasive bladder cancer. Urol. Oncol. 2018;36(1):8.e17–8.e24. doi: 10.1016/j.urolonc.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasteiro A.M., E S.E.L., Oliveira P.A., Gil da Costa R.M. Molecular markers in urinary bladder cancer: applications for diagnosis, prognosis and therapy. Vet Sci. 2022;9(3) doi: 10.3390/vetsci9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria F., Droller M.J., Lotan Y., Gontero P., D'Andrea D., Gust K.M., et al. An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J. Urol. 2018;36(12):1981–1995. doi: 10.1007/s00345-018-2380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lotan Y., OʼSullivan P., Raman J.D., Shariat S.F., Kavalieris L., Frampton C., et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol. Oncol. 2017;35(8):531.e15. doi: 10.1016/j.urolonc.2017.03.008. e22. [DOI] [PubMed] [Google Scholar]
- 7.Krabbe L.M., Woldu S.L., Shariat S.F., Lotan Y. Improving diagnostic molecular tests to monitor urothelial carcinoma recurrence. Expert Rev. Mol. Diagn. 2016;16(11):1189–1199. doi: 10.1080/14737159.2016.1244006. [DOI] [PubMed] [Google Scholar]
- 8.Minciacchi V.R., Freeman M.R., Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng M., Liu X., Xu G. Extracellular vesicles as messengers in atherosclerosis. J Cardiovasc Transl Res. 2020;13(2):121–130. doi: 10.1007/s12265-019-09923-z. [DOI] [PubMed] [Google Scholar]
- 10.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15(10):617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 11.Shehzad A., Islam S.U., Shahzad R., Khan S., Lee Y.S. Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol. Ther. 2021;223 doi: 10.1016/j.pharmthera.2021.107806. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y., Zhang Y., Gong H., Luo S., Cui Y. The role of exosomes and their applications in cancer. Int. J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Q.F., Li W.J., Hu K.S., Gao J., Zhai W.L., Yang J.H., et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol. Cancer. 2022;21(1):207. doi: 10.1186/s12943-022-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian X., Shen H., Li Z., Wang T., Wang S. Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment. J. Hematol. Oncol. 2019;12(1):84. doi: 10.1186/s13045-019-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22(5):560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milutinović B., Goč S., Mitić N., Kosanović M., Janković M. Surface glycans contribute to differences between seminal prostasomes from normozoospermic and oligozoospermic men. Ups. J. Med. Sci. 2019;124(2):111–118. doi: 10.1080/03009734.2019.1592266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wortzel I., Dror S., Kenific C.M., Lyden D. Exosome-mediated metastasis: communication from a distance. Dev. Cell. 2019;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Silva A.M., Lázaro-Ibáñez E., Gunnarsson A., Dhande A., Daaboul G., Peacock B., et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles. 2021;10(10) doi: 10.1002/jev2.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Q., Cheng L., Deng C., Huang L., Li J., Wang Y., et al. The genetic source tracking of human urinary exosomes. Proc Natl Acad Sci U S A. 2021;118(43) doi: 10.1073/pnas.2108876118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hade M.D., Suire C.N., Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10(8) doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan Y., Du L., Wang L., Jiang X., Zhang S., Li J., et al. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol. Cancer. 2018;17(1):142. doi: 10.1186/s12943-018-0893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian W., Lei N., Zhou J., Chen M., Guo R., Qin B., et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022;13(1):64. doi: 10.1038/s41419-022-04510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doeppner T.R., Herz J., Görgens A., Schlechter J., Ludwig A.K., Radtke S., et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Canc. 2019;1871(2):455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021;19(1):47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kok V.C., Yu C.C. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int. J. Nanomed. 2020;15:8019–8036. doi: 10.2147/IJN.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Droste M., Tertel T., Jeruschke S., Dittrich R., Kontopoulou E., Walkenfort B., et al. Single extracellular vesicle analysis performed by imaging flow cytometry and nanoparticle tracking analysis evaluate the accuracy of urinary extracellular vesicle preparation techniques differently. Int. J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu W., Hurley J., Roberts D., Chakrabortty S.K., Enderle D., Noerholm M., et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 2021;32(4):466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Invest. 2016;126(4):1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5(1):242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haderk F., Schulz R., Iskar M., Cid L.L., Worst T., Willmund K.V., et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2(13) doi: 10.1126/sciimmunol.aah5509. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M., Fritsche J., Roszik J., Williams L.J., Peng X., Chiu Y., et al. RNA editing derived epitopes function as cancer antigens to elicit immune responses. Nat. Commun. 2018;9(1):3919. doi: 10.1038/s41467-018-06405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Gu Y., Han Y., Zhang Q., Jiang Z., Zhang X., et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30(2):243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto A., Takahashi Y., Nishikawa M., Sano K., Morishita M., Charoenviriyakul C., et al. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci. 2017;108(9):1803–1810. doi: 10.1111/cas.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purushothaman A., Bandari S.K., Liu J., Mobley J.A., Brown E.E., Sanderson R.D. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J. Biol. Chem. 2016;291(4):1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin H., Liu P., Wu Y., Meng X., Wu M., Han J., et al. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109(9):2946–2956. doi: 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C., Luo Y., He W., Zhao Y., Kong Y., Liu H., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Invest. 2020;130(1):404–421. doi: 10.1172/JCI130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044. doi: 10.1016/j.cell.2020.07.009. 61.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferro M., La Civita E., Liotti A., Cennamo M., Tortora F., Buonerba C., et al. Liquid biopsy biomarkers in urine: a route towards molecular diagnosis and personalized medicine of bladder cancer. J Pers Med. 2021;11(3) doi: 10.3390/jpm11030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panigrahi A.R., Srinivas L., Panda J. Exosomes: insights and therapeutic applications in cancer. Transl Oncol. 2022;21 doi: 10.1016/j.tranon.2022.101439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M., Hu S., Liu L., Dang P., Liu Y., Sun Z., et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124. doi: 10.1038/s41392-023-01382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sala M., Ros M., Saltel F. A complex and evolutive character: two face aspects of ECM in tumor progression. Front. Oncol. 2020;10:1620. doi: 10.3389/fonc.2020.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X.X., Zhang L., Lu Y. Advances in the molecular pathogenesis and cell therapy of stress urinary incontinence. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun R., Sundahl N., Hecht M., Putz F., Lancia A., Rouyar A., et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berrondo C., Flax J., Kucherov V., Siebert A., Osinski T., Rosenberg A., et al. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckham C.J., Olsen J., Yin P.N., Wu C.H., Ting H.J., Hagen F.K., et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 2014;192(2):583–592. doi: 10.1016/j.juro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Lin H., Shi X., Li H., Hui J., Liu R., Chen Z., et al. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer. 2021;21(1):1293. doi: 10.1186/s12885-021-08926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X., Chen R.X., Wei W.S., Li Y.H., Feng Z.H., Tan L., et al. PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin. Cancer Res. 2018;24(24):6319–6330. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]
- 50.Wang C., Li Z., Liu Y., Yuan L. Exosomes in atherosclerosis: performers, bystanders, biomarkers, and therapeutic targets. Theranostics. 2021;11(8):3996–4010. doi: 10.7150/thno.56035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin J., Li J., Huang B., Liu J., Chen X., Chen X.M., et al. Exosomes: novel biomarkers for clinical diagnosis. Sci. World J. 2015;2015 doi: 10.1155/2015/657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu D., Li Y., Wang M., Gu J., Xu W., Cai H., et al. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer. 2022;21(1):56. doi: 10.1186/s12943-022-01509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng R., Du M., Wang X., Xu W., Liang J., Wang W., et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer. 2018;17(1):143. doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C.L., Lai Y.F., Tang P., Chien K.Y., Yu J.S., Tsai C.H., et al. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J. Proteome Res. 2012;11(12):5611–5629. doi: 10.1021/pr3008732. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., McKinney K.Q., Pavlopoulos A.J., Niu M., Kang J.W., Oh J.W., et al. Altered proteome of extracellular vesicles derived from bladder cancer patients urine. Mol Cells. 2018;41(3):179–187. doi: 10.14348/molcells.2018.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez A., Loizaga A., Arceo R., Lacasa I., Rabade A., Zorroza K., et al. A pilot study on the potential of RNA-associated to urinary vesicles as a suitable non-invasive source for diagnostic purposes in bladder cancer. Cancers. 2014;6(1):179–192. doi: 10.3390/cancers6010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez M., Bajo-Santos C., Hessvik N.P., Lorenz S., Fromm B., Berge V., et al. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer. 2017;16(1):156. doi: 10.1186/s12943-017-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreu Z., Otta Oshiro R., Redruello A., López-Martín S., Gutiérrez-Vázquez C., Morato E., et al. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci. 2017;98:70–79. doi: 10.1016/j.ejps.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Matsuzaki K., Fujita K., Jingushi K., Kawashima A., Ujike T., Nagahara A., et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget. 2017;8(15):24668–24678. doi: 10.18632/oncotarget.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franzen C.A., Simms P.E., Van Huis A.F., Foreman K.E., Kuo P.C., Gupta G.N. Characterization of uptake and internalization of exosomes by bladder cancer cells. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/619829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greco K.A., Franzen C.A., Foreman K.E., Flanigan R.C., Kuo P.C., Gupta G.N. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology. 2016;91:241.e1–241.e7. doi: 10.1016/j.urology.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 63.Malam Y., Loizidou M., Seifalian A.M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Zhou M., He X., Mei C., Ou C. Exosome derived from tumor-associated macrophages: biogenesis, functions, and therapeutic implications in human cancers. Biomark. Res. 2023 Nov 19;11(1):100. doi: 10.1186/s40364-023-00538-w. PMID: 37981718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y., Luo C.L., He B.C., Zhang J.M., Cheng G., Wu X.H. Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int. J. Oncol. 2010;36(1):133–140. [PubMed] [Google Scholar]
- 66.Huang L., Rong Y., Tang X., Yi K., Qi P., Hou J., Liu W., He Y., Gao X., Yuan C., Wang F. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer. 2022 Feb 11;21(1):45. doi: 10.1186/s12943-022-01515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials in the current study are available from the corresponding author on reasonable request (drliu7087797@163.com).