Abstract
Measles is a highly contagious disease currently responsible for over one million childhood deaths, particularly in the developing world. Since alpha/beta interferons (IFNs) are pivotal players both in nonspecific antiviral immunity and in specific cellular responses, their induction or suppression by measles virus (MV) could influence the outcome of a viral infection. In this study we compare the IFN induction and sensitivity of laboratory-passaged attenuated MV strains Edmonston and Moraten with those of recent wild-type viruses isolated and passaged solely on human peripheral blood mononuclear cells (PBMC) or on the B958 marmoset B-cell line. We report that two PBMC-grown wild-type measles isolates and two B958-grown strains of MV induce 10- to 80-fold-lower production of IFN by phytohemagglutinin-stimulated peripheral blood lymphocytes (PBL) compared to Edmonston and Moraten strains of measles. Preinfection of PBL with these non-IFN-inducing MV isolates prevents Edmonston-induced but not double-stranded-RNA-induced IFN production. This suggests that the wild-type viruses can actively inhibit Edmonston-induced IFN synthesis and that this is not occurring by double-stranded RNA. Furthermore, the wild-type MV is more sensitive than Edmonston MV to the effect of IFN. MV is thus able to suppress the synthesis of the earliest mediator of antiviral immunity, IFN-α/β. This could have important implications in the virulence and spread of MV.
Measles is a highly contagious disease responsible for many childhood deaths, particularly in the developing world. Despite the generation of a vigorous immune response against measles virus (MV), immunity to other pathogens is depressed. This transient generalized immunosuppression allows the establishment of opportunistic infections and leads to many of the complications associated with measles (reviewed in reference 14). Indirect evidence suggests that the mortality and morbidity of measles is correlated with the extent of viral replication. MV infection in previously vaccinated individuals who demonstrate weak or partial immunity is systematically milder than in cases of nonvaccinated individuals (7, 36). Early control of MV replication may thus determine the severity of the disease.
The principal actors in the early nonspecific immune response are alpha/beta interferon (IFN-α/β) induction, complement activation, natural killer cell (NK) and macrophage activation, and IFN-γ and interleukin-12 (IL-12) production. Although MV infection of cell lines in vitro has been shown to induce IFN (47), the results concerning wild-type MV infection in vivo are conflicting and inconclusive. Active IFN-α/β has been documented in vivo after natural infection by MV in one study and shown to be absent in another (6, 39, 45). Levels of serum IFN and of the IFN-inducible 2′-5′ oligoadenylate-synthetase (2-5A) gene transcript have been shown to rise after MV immunization with the live attenuated vaccine (45). With regard to other innate defense mechanisms, MV does not appear to hamper either complement activation in vitro or IFN-γ production in vivo (16, 40). However, MV has been shown to depress IL-12 synthesis in vitro and to dampen NK cell activity in vivo (15, 18, 37). We wanted to study the effect of MV on the IFN-α/β response since IFN-α/β, along with IL-12, are pivotal players in limiting early virus spread as well as in the activation and priming events of antigen-presenting cells.
IFN-α/β induces the expression of a number of cellular genes such as 2-5A, double-stranded RNA-dependent protein kinase (PKR) and Mx, which confer antiviral properties to the cell (17, 46). In addition to the antiviral function, IFN-α/β have potent effects in regulating the specific immune response (41). They are thought to enhance differentiation of dendritic antigen-presenting cells and to contribute to prolonging T-lymphocyte lifespan (22, 26). Viruses have thus evolved mechanisms to counter the antiviral effects of IFN or, in some cases, to suppress its production.
Resistance to the antiviral effects of IFN is mediated by active inhibition of IFN-inducible gene function. IFN-resistant and -sensitive strains of MV can be isolated by cell culture, and it has been suggested that IFN-resistant strains of MV can contribute to the establishment of persistent infection of the central nervous system (CNS) (4). This is relevant to the rare cases of persistent MV infection of the CNS giving rise to subacute sclerosing panencephalitis (SSPE), a fatal disease. It is not known which MV products contribute to IFN resistance, but studies in the closely related Sendai virus have shown that the nonstructural C protein counteracts the IFN-mediated antiviral state (12).
Virus infection must trigger IFN synthesis prior to the induction of the antiviral state. Studies with viruses such as Sendai or vesicular stomatitis virus (VSV) have shown that different strains of the same virus can induce highly variable quantities of IFN-α/β. These studies have shown that low-IFN-inducing viruses can actively suppress the IFN production of the high-IFN-inducing strains (25, 28).
In this study we compare the IFN-α/β induction and sensitivity of the laboratory-passaged attenuated Edmonston (MV-Ed) and Moraten (vaccine strain) MVs with those of recent wild-type viruses isolated and passaged solely on human peripheral blood mononuclear cells (PBMC) or on the B958 marmoset B-cell line. We report that two PBMC-grown wild-type MV isolates and two B958-grown strains of MV induce significantly lower production of IFN by phytohemagglutinin (PHA)-stimulated peripheral blood lymphocytes (PBL) compared to the MV-Ed laboratory strain of measles. Furthermore, our evidence indicates that these wt MV strains are more sensitive to the effects of IFN and actively inhibit IFN synthesis.
MATERIALS AND METHODS
Lymphocyte preparations, culture conditions, and MV preparations.
PBMC were isolated by Ficoll-Hypaque centrifugation from normal healthy donors. Adherent cells were eliminated by 2 h of adherence to tissue-culture-treated plastic. PBL were cultured in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Lymphocytes were cultured at a concentration of 106 cells/ml.
The MV Edmonston (MV-Ed; American Type Culture Collection, Rockville, Md.) and Moraten strains (kindly provided by Alexandra Valsamakis, Johns Hopkins School of Medicine, Baltimore, Md.) were passaged and plaqued on mycoplasma-free Vero cells. Wild-type viruses were isolated in measles outbreaks from 1991 to 1994 either on PBMC in the United States (JW and IV) or on B958 cells in Spain (Bcl94 and FV93) (9, 35). JW-, IV-, Bcl94-, and FV93-Vero strains were obtained by blindly passaging the parental wild-type viruses on Vero cells and collecting the virus after 10 passages (23). Since the parental wild-type isolates do not produce plaques on Vero cells, the standard plaque assay could not be used to determine the titers of these viruses. Titers of all viruses were thus determined by serial dilutions on PBMC stimulated with 10 μg of PHA (Difco) per ml. After 4 days of infection, cells were lysed in 0.1% sodium dodecyl sulfate (SDS) and applied to Nytran filters (Schleicher & Schuell) before being probed with a 32P-radiolabeled DNA probe for the measles N gene. The 50% tissue culture infective dose (TCID50) was calculated on triplicate wells as previously described (9). Supernatants of viruses were used for infections.
Flow cytometry for surface staining and for cell proliferation.
Antibody I41 to measles hemagglutinin was kindly provided by Ewa Bjorling at the Karolinska Institute, Stockholm, Sweden. In order to label cells for flow cytometry, cells were incubated for 30 min with primary antibody in phosphate-buffered saline containing 1% FBS and 0.05% sodium azide. Cells were washed twice and incubated with a secondary antibody conjugated to phycoerythrin. After 30 min of incubation, cells were washed and fixed in 1% formaldehyde prior to analysis on a FACScan (Becton Dickinson).
To measure cell proliferation by fluorescence decrease of carboxyfluorescein succidmyl ester (CFSE; Molecular Probes, Eugene, Oreg.), lymphocytes were labeled with CFSE prior to infection. Labeling was carried out for 10 min at 37°C at a concentration of 2 × 106 cells/ml in culture medium containing 50 μM CFSE. Cells were washed twice to remove unincorporated dye and cultured with PHA and IL-2.
Analysis of MV-induced IFN production.
Labeled cells were infected with various MV isolates at a multiplicity of infection (MOI) of 0.003 TCID50/cell. For the Vero cell-grown isolates, the equivalent MOI in PFU was 0.8 PFU/cell. The cells were incubated with the virus stock for 3 h at 37°C, pelleted, and resuspended at 106 cells/ml in culture medium containing 5 μg of PHA (U.S. Biochemicals) and 50 U of IL-2 (National Cancer Institute, Biologicals, Bethesda, Md.) per ml. At 72 h postinfection, supernatants were harvested and ultracentrifuged at 110,000 × g for 45 min to remove free virus. The virus-free supernatant was assayed for IFN activity by a cytopathic effect (CPE) assay using encephalomyocarditis virus (ECMV) (49). The cells were analyzed for proliferation and surface expression of MV-hemagglutinin by flow cytometry.
IFN treatments and assessing replication sensitivity to IFN.
Human PBL were plated at 2 × 105 cells/well in round-bottom 96-well plates and incubated with various concentrations of IFN-α/β (Sigma) diluted in complete culture medium. After 24 h, cells were pelleted and infected with virus at an MOI of 0.003 TCID50/cell. The cells were incubated with the virus stock for 3 h at 37°C, pelleted, and resuspended in culture medium containing 5 μg of PHA (U.S. Biochemicals) and 50 U of IL-2 per ml. Four days postinfection, cells were assayed for proliferation and hemagglutinin expression by flow cytometry. Nucleoprotein expression was assayed by dot blotting the cells onto Nytran membranes (Schleicher & Schuell), probing with a 32P-labeled nucleoprotein probe, and quantitation on a phosphorimager. The blots were reprobed with a cyclophilin probe and quantitated as a control of equal RNA content.
Coinfections and poly(I-C) treatments.
PBL were infected at an MOI of 0.004 TCID50/cell for 2 h. Cells were washed and resuspended in complete culture medium supplemented with 5 μg of PHA (U.S. Biochemicals) and 50 U of IL-2 per ml. At 72 h postinfection, the cells were infected with Edmonston at an MOI of 0.002 TCID50/cell (equivalent to an MOI of 0.5 PFU/cell) for 2 h or treated with 200 μg of poly(I-C) (Sigma) per ml. Supernatants were harvested 48 h after the Edmonston infection and 24 h after poly(I-C) treatment.
Western blotting.
Infected or IFN-treated cells were lysed in TNE (Tris, 10 mM; NaCl, 100 mM; EDTA, 1 mM; NP-40, 0.5%) 48 h postinfection for HeLa cells and 72 h postinfection for human PBL. The cells were lysed at 107 cells/ml, and the equivalent of 2 × 105 cells was boiled in lysis buffer (Tris-HCl, 100 mM; SDS, 4%; glycerol, 30%; dithiothreitol, 250 mM) and loaded onto an 8% acrylamide gel and subjected to SDS-polyacrylamide gel electrophoresis. The proteins were electrotransferred to polyvinylidene difluoride membranes (Millipore) and subjected to Western blotting with a specific antibody to STAT-1α/β (Pharmingen) and a rabbit anti-mouse secondary peroxidase-conjugated antibody (Gibco). Enhanced chemiluminescence was carried out with the Supersignal Chemiluminescence System (Pierce) and exposed to Kodak Biomax film.
RESULTS
Attenuated strains of MV induce significantly more IFN than do wild-type strains.
Experiments were designed to determine the relative IFN induction potential of different strains of attenuated and wild-type MV strains after infection of primary human PBL. PBL from different donors were infected at an MOI of 0.003 TCID50 with the various MV strains. Following a 4-day incubation with PHA, biologically active IFN present in virus-free supernatants was quantified. The IFN activity was assayed by determining CPE reduction of ECMV infection and standardized to international units. IFN-α/β was neutralized with anti-IFN-γ antibodies. Table 1 shows that the attenuated Vero cell-grown Edmonston strain of MV and the currently used vaccine strain Moraten induced a high level of IFN production. In contrast, PBMC-grown wild-type isolates JW and IV, as well as B958-grown isolates, induced 10- to 20-fold less IFN than did the Edmonston and Moraten strains. Furthermore, the Vero cell-adapted isolates of IV, JW, FV93, and Bcl94 viruses induced 10- to 20-fold-higher quantities of IFN than did their respective parental viruses (Table 1). A certain degree of virus replication appeared to be necessary for IFN induction since UV-inactivated MV-Ed induced little IFN (313 to 500 U/ml) and MV-Ed-infected nonstimulated PBMC, in which MV replicates poorly, produced less IFN than PHA-stimulated cells (data not shown). There was a high variability in the capacity and efficiency of PBL from different donors to secrete IFN. The two experiments presented are representative of the magnitude of variability observed on PBL from five different healthy donors. All infections led to similar levels of surface hemagglutinin expression on all lymphocyte types (B and T), and >75% of the cells were infected by all of the viruses (data not shown).
TABLE 1.
Wild-type MVs (strains IV, JW, Bcl94, and FV93) induce 10- to 80-fold less IFN-α/β than do the attenuated Edmonston, Moraten, or Vero cell-adapted IV, JW, Bcl, and FV virus strainsa
| Virus strain | IU of IFN/ml of supernatant
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| NI | 250 | 120 |
| Edmonston | 2,000 | 4,000 |
| Moraten | 5,000 | 10,000 |
| JW | 120 | 120 |
| IV | 120 | 250 |
| Bcl94 | 120 | 120 |
| FV93 | 120 | 250 |
| IV-Vero | 10,000 | 1,250 |
| JW-Vero | 2,500 | 10,000 |
| Bcl-Vero | 1,250 | 2,000 |
| FV-Vero | 1,250 | 2,000 |
Virus-free supernatants from cells infected with different MV strains were assayed for the biological activity of IFN-α/β. JW and IV are wild-type MV isolates passaged solely on human PBMC, while Bcl94 and FV93 were isolated and passaged on the B958 marmoset cell line. The Edmonston and Moraten MV strains are highly attenuated and were passaged on the monkey Vero cell line. IV-, JW-, Bcl-, and FV-Vero are the wild-type viruses passaged 10 times on Vero cells. NI, noninfected cells.
These results suggest that attenuation and/or passage of wild-type MV on Vero cells transforms the viruses from a non-IFN-inducing phenotype to an IFN-inducing phenotype.
Wild-type virus replication is more sensitive to IFN effects than is Edmonston virus.
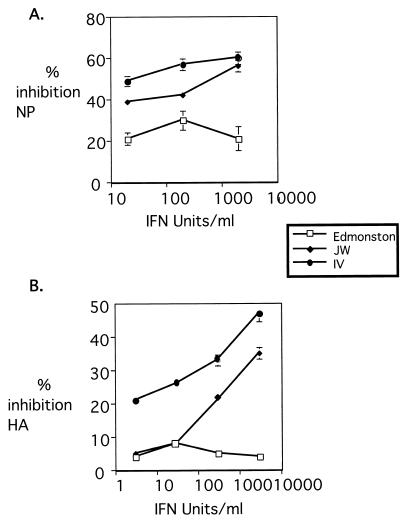
In order to determine whether the replication of Edmonston and wild-type MV differed in their sensitivity to IFN, MV expression levels were analyzed after treatment of PBL with exogenous IFN prior to infection. PBL were treated with increasing quantities of IFN for 24 h, followed by infection with Edmonston or wild-type MV viruses. After 3 to 4 days, hemagglutinin expression was determined by flow cytometry, and NP expression was determined by an RNA dot blot using a radiolabeled DNA probe specific for measles NP. The replication of Edmonston was relatively insensitive to the effects of IFN, whereas the replication of wild-type MV strains JW and IV was reduced after IFN treatment (Fig. 1). Both NP and hemagglutinin expression were decreased by 40 to 50% (Fig. 1).
FIG. 1.
The replication of wild-type JW and IV viruses is more sensitive to IFN than is that of Edmonston. Human PBL were incubated overnight with various concentrations of IFN-α/β. Cells were infected with Edmonston, IV, or JW viruses, and replication was assayed at day 4 postinfection by quantification of NP RNA relative to cyclophilin RNA by dot blot and phosphorimaging (A) and of surface expression of hemagglutinin by flow cytometry (B).
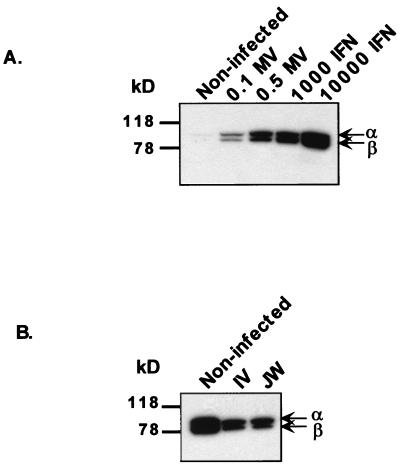
In order to determine whether Edmonston and wild-type MVs could block the IFN signal from being transduced in target IFN-α/β receptor-bearing cells, the levels of the signal transduction intermediates STAT-1α and STAT-1β were assessed after MV infection. These proteins have been shown to be upregulated by IFN treatment (21, 30). When HeLa cells were infected at an MOI of 0.1 or 0.5 PFU with Edmonston MV, the expression of STAT-1α and STAT-1β proteins increased to a level comparable to that observed after IFN treatment (Fig. 2A). The upregulation of STAT-1 was dependent on the virus dose. This finding is consistent with a greater percentage of cells being infected at a 0.5 MOI than at a 0.1 MOI. Since wild-type IV and JW MVs do not infect HeLa cells and do not themselves induce significant levels of IFN, exogenous IFN-α was added to PBL cultures in order to assess the capacity of wild-type MV to block IFN signaling. Preinfection of PBL with IV or JW led to slightly decreased levels of STAT-1α and -β proteins after IFN treatment compared to IFN-treated uninfected cells (Fig. 2B). The slight differences suggest that MV is not significantly blocking IFN signal transduction. Events downstream of induction of the STAT-1 gene are thus likely to account for the above-described resistance of MV-Ed to IFN effects.
FIG. 2.
MV-Ed does not inhibit IFN-inducible STAT-1 factor-mediated upregulation. (A) HeLa cells were infected at an MOI of 0.1 or 0.5 PFU of Edmonston or were treated with 1,000 or 10,000 U of IFN-α/β per ml. At 48 h postinfection, cells were lysed. (B) Human PBL were preinfected with IV or JW MV at an MOI of 0.004 TCID50. At 72 h postinfection, 1,000 U of IFN per ml was added to the culture, and cells were lysed 24 h later. Lysates were probed by Western blotting with an antibody recognizing both STAT-1α (91 kDa) and STAT-1β (84 kDa). Enhanced chemiluminescence was carried out as described in the text. This figure is representative of several experiments.
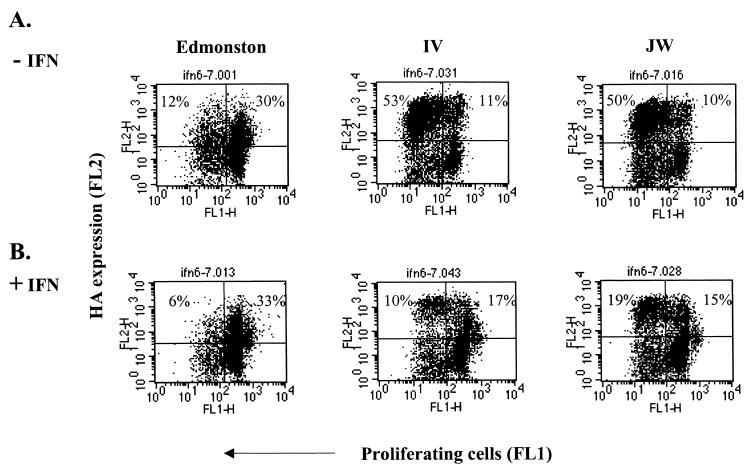
Wild-type MV replicates less efficiently in IFN-treated nonproliferating cells than in proliferating cells.
The inhibitory effect of IFN on the replication of wild-type measles could be due to the antiviral effects or to the antiproliferative effect of IFN on host cells. We thus looked at the distribution of MV-infected cells among the proliferating and nonproliferating cells. Cells were labeled with CFSE to monitor proliferation. IFN treatment and infection were carried out as described above. At 4 days postinfection cells were stained with anti-hemagglutinin antibody and analyzed by flow cytometry. In untreated cells, the majority of cells infected by wild-type virus were proliferating cells (Fig. 3A). After IFN treatment, IFN blocked efficient cell division as expected and there were fewer dividing cells and fewer cells infected with wild-type MV (Fig. 3B). However, in the case of Edmonston infection, IFN treatment reduced the number of proliferating cells, but the proportion of Edmonston-infected cells remained unchanged. Therefore, in IFN-treated cells, Edmonston could compensate for the lack of dividing cells by infecting more nonproliferating cells. Although these cells were not multiplying, they were activated as previously determined by surface expression of activation markers CD69 and CD71 (33). These results suggest that the replication of wild-type MV is more restricted than that of Edmonston in activated nondividing cells.
FIG. 3.
Wild-type IV and JW strains replicate less efficiently than does Edmonston in the nondividing population of IFN-treated cells. Human PBL were labeled with CFSE and incubated overnight with 2,000 U of IFN-α/β per ml. Cells were then infected with Edmonston, IV, or JW viruses. Cell proliferation and surface expression of hemagglutinin were quantitated by dual-color flow cytometry. Proliferation in the absence of IFN (A) or in the presence of IFN (B) is plotted against surface hemagglutinin expression. This figure is representative of several experiments.
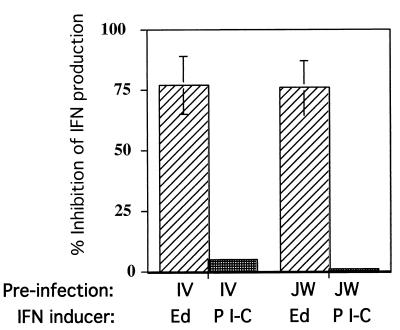
Preinfection with wild-type MV partially suppresses Edmonston-induced but not poly(I-C)-induced IFN production.
In order to determine whether the low-IFN-inducing phenotype of wild-type MV was an active process, we preinfected cells with wild-type viruses. PBL were infected with JW or IV viruses for 72 h prior to coinfection with Edmonston. Quantitation of IFN from supernatants harvested after the second infection showed that preinfection of PBL with IV or JW inhibited approximately 75 to 80% of the IFN production induced by Edmonston alone (Fig. 4). This could not be due to downregulation of the major MV receptor CD46 leading to an inability of MV-Ed to infect target cells since neither JW nor IV downmodulate CD46 (data not shown) nor do they have the marker for downmodulation (amino acid Y at position 481 of the hemagglutinin protein) (23).
FIG. 4.
Preinfection of human PBL with IV or JW strains of MV inhibits IFN production induced by Edmonston MV but not by poly(I-C) (P I-C). Human PBL were infected with IV or JW at an MOI of 0.004 TCID50. At 72 h postinfection, cells were superinfected with Edmonston at an MOI of 0.002 TCID50 (0.5 PFU) or treated with 200 μg of poly(I-C) per ml. Supernatants were harvested and assayed for IFN-α/β biological activity 24 h later for poly(I-C)-treated samples and 48 h later for Edmonston-infected samples. The percent inhibition was calculated by using the amount of IFN induced by Edmonston infection or poly(I-C) alone as the maximum amount of IFN (denominator) compared to the amount of IFN induced after preinfection and addition of MV-Ed or poly(I-C) (numerator). The experiment represents the average obtained with PBL from three donors.
In order to determine whether IV or JW could inhibit double-stranded RNA induction of IFN, cells were preinfected as in the previous experiment and treated with 200 μg of poly(I-C) per ml. The supernatants were then assayed for IFN activity 24 h later. Neither IV nor JW infection prevented poly(I-C)-induced IFN production (Fig. 4). All poly(I-C)-treated samples produced similar quantities of IFN regardless of the pretreatment they had received. As a control, poly(I-C) incubated 24 h in medium alone and processed with other supernatants did not interfere with ECMV infection in the biological assay for IFN (data not shown).
Therefore, wild-type MV can interfere with MV-Ed induced but not with double-stranded-RNA-induced IFN production. However, the inhibition of Ed-induced IFN was not total. As is the case for VSV, this may be due to the presence, within the heterogeneous virus pool, of some subpopulations of variants exhibiting the IFN-suppressive phenotype and others the IFN-inducing phenotype (11, 24).
DISCUSSION
This study demonstrates that a series of wild-type viruses do not induce production of significant quantities of IFN-α/β compared to the attenuated Vero cell-grown Edmonston and Moraten strains of MV. These wild-type MVs can actively suppress IFN production induced by MV-Ed infection. Furthermore, 10 passages of the wild-type MVs on Vero cells is sufficient to transform their phenotype from that of an IFN suppressor to that of an IFN inducer. The replication of these wild-type viruses is more sensitive to IFN treatment than is MV-Ed and particularly to the IFN-induced growth arrest of target cells.
Many viruses have developed mechanisms to neutralize the antiviral effects of the IFN system, thus potentially increasing the early spread of virus. In particular, viruses have been shown to counter the intracellular cascade of events triggered by IFN binding to the IFN receptor. Strategies include blocking IFN receptor signal by interfering with the JAK–STAT-1 pathway and/or preventing the expression or activation of IFN-inducible gene products such as 2-5A and PKR induced after signaling through the IFN receptor (46). Our results suggest that MV-Ed does not interfere with early STAT-1 protein induction or homeostasis but rather with an event further downstream in the IFN response. MV-Ed infection induces production of IFN and successful upregulation of the transcription activator STAT-1α/β. This is in contrast to two MV-related paramyxoviruses, Sendai virus and simian virus 5 (SV5), which have been shown to abrogate STAT-1 expression via two different mechanisms. Sendai virus prevents upregulation of the STAT-1, whereas SV5 promotes the degradation of the STAT-1 protein (8, 12). This indicates that, within the Paramyxoviridae family (within this family, Sendai virus and SV5 are subclassified in the paramyxovirus genus), multiple IFN escape strategies exist (50). Further research may reveal various mechanisms employed within the morbillivirus genus of which MV is a member.
Many studies have analyzed viral IFN resistance mechanisms; however, little attention has been focussed on the viral infections which target molecular events upstream of IFN secretion. Studies of Sendai virus and of VSV indicate that, in addition to blocking the downstream effects of IFN initiated by STAT-1, some virus strains actively suppress IFN synthesis (25, 28). Our results show that the non-IFN-inducing phenotype of wild-type PBMC-grown and B958-grown MV is dominant over the IFN-inducing phenotype of Edmonston. This indicates that these viruses, like Sendai virus and VSV, can interfere with the pathway leading to IFN production. A virus which prevents IFN production would have more time to spread before activation of the specific immune response and thus be potentially more virulent. The observation that wild-type MV prevents IFN induction by MV-Ed but not by poly(I-C) suggests that MV-Ed induction of IFN occurs by a pathway not involving double-stranded RNA. It has been shown for other viruses (e.g., human immunodeficiency virus, herpes simplex virus, cytomegalovirus) that viral protein-cell interactions alone can induce IFN (1, 13, 42). Thus, one or more MV proteins may be necessary for IFN induction by Edmonston, but preinfection by a noninducing MV would prevent successful induction. Alternatively, since CD46 engagement has recently been shown to transduce a signal leading to IFN production (19), CD46 binding affinity may influence the level of IFN produced. The exact mechanisms by which MV and other IFN-suppressing viruses affect the regulation of IFN-α/β synthesis are unknown.
Most studies on MVs have been carried out with Edmonston or other strains grown on the Vero cell line, which is defective in its response to IFN. Indeed, earlier studies, carried out with Vero cell-grown MV isolates by A. Billiau and collaborators, had suggested that virulent measles strains induced less IFN than did attenuated strains (47, 48). The isolates termed “virulent” in the early studies were adapted to Vero cells and so were most likely more attenuated than natural MVs. However, that study did set a precedent for the hypothesis that the virulence of MV may be associated with the ability to prevent cellular IFN-α/β induction. Adaptation of MVs occurs as a consequence of passage on Vero cells (38, 43). Thus, a non-IFN-inducing virus growing on Vero cells would no longer have a selective advantage in preventing IFN synthesis and could likely be outcompeted by other variants in the population. Our results indicate that 10 passages of non-IFN-inducing IV, JW, Bcl94, and FV93 MV strains on Vero cells leads to an increase in IFN induction on PBL compared to that of the parental-PBMC-grown MV. However, the phenotype change for all of the viruses may not be complete since there was variability from donor to donor. The number of Vero cells required to achieve a completely IFN inducing phenotype undoubtedly varies according to the virus strain and according to the number of mutations necessary to change the phenotype. This is illustrated by recent evidence showing that 10 passages of IV, JW, Bcl94, and FV93 on Vero cells leads to a change of the amino acid in the hemagglutinin molecule responsible for CD46 affinity and downmodulation (N to Y at amino acid 481). However, this phenotype conversion was not absolute, since only IV and JW changed after 10 passages, whereas Bcl94 and FV93 did not (23).
Mouse models of viral infection have clearly shown that disrupting the IFN-α/β response undermines the immune response and leads to massive viral replication. Infection of mice deficient in the IFN-α/β receptor (IFNRI−/−) with VSV, lymphocytic choriomeningitis virus, or vaccinia leads to a 103- to 105-fold increase in virus titer (32). However, in the absence of IFN-α/β, IL-12 can compensate to boost the cellular response and induce IFN-γ secretion (5). For certain viruses, the infection can thus be controlled, but it can give rise to a persistent infection (32). Viruses, such as hepatitis C virus or human immunodeficiency virus, known to cause chronic infections in humans, have mechanisms both for blocking the response to IFN (3, 44) and for affecting IL-12 synthesis (27). Although MV rarely persists in its host, MV infection in vitro has been shown to depress IL-12 production in both macrophages and dendritic cells (10, 18). Macrophages, dendritic cells, epithelial cells, and NK cells provide the initial sources of IFN-α/β, IL-12, and IFN-γ. MV may have established a redundancy of mechanisms to slow the innate immune response to allow early dissemination. The degree to which IFN-α/β induction and IL-12 synthesis are disrupted by MV may determine the virulence of a particular strain. Such virulent measles strains could thus replicate more efficiently and gain access more rapidly to the bone marrow and, on rare occasions, to the CNS. Infection of bone marrow cells may be important in the severity of measles, and some reports have suggested that MV is present in the bone marrow osteoclasts of Paget's disease patients (20, 31, 34). The CNS is persistently infected by MV in the rare cases which lead to SSPE. The question as to whether the potential to inhibit IFN-α/β and IL-12 determines virulence and/or the potential to infect the bone marrow and possibly the CNS remains open.
These hypotheses are based on in vitro studies. Further studies in existing monkey models (2, 29) may aid in determining whether suppression of IFN-α/β is an indicator of virulence. If the pathogenesis of human infection in vivo mirrors the in vitro observations presented here and elsewhere, MV may be able to increase virulence by suppressing the synthesis of the earliest mediators of antiviral immunity, IFN-α/β and IL-12. We are pursuing this hypothesis by isolating strains of wild-type MV from severe and mild cases of measles and testing them for their capacity to inhibit IFN-α/β and IL-12 production in vitro.
ACKNOWLEDGMENTS
We thank Don Forthal at University of California at Irvine for the original IV and JW isolates and Rafael Fernandez-Muñoz at the University of Madrid for the original FV93 and Bcl94 isolates. We also thank John Patterson for helpful discussions.
This work was supported by NIH grants AI39466 (M.B.A.O.) and AI41514 (M.M.) and WHO grant V21/181/119 (D.N.).
REFERENCES
- 1.Ankel H, Capobianchi M R, Castilletti C, Dianzani F. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology. 1994;205:34–43. doi: 10.1006/viro.1994.1617. [DOI] [PubMed] [Google Scholar]
- 2.Auwaerter P G, Rota P A, Elkins W R, Adams R J, DeLozier T, Shi Y, Bellini W J, Murphy B R, Griffin D E. Measles virus infection in Rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J Infect Dis. 1999;180:950–958. doi: 10.1086/314993. [DOI] [PubMed] [Google Scholar]
- 3.Brand S R, Kobayashi R, Mathews M B. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced virally activated protein kinase PKR. J Biol Chem. 1997;272:8388–8395. doi: 10.1074/jbc.272.13.8388. [DOI] [PubMed] [Google Scholar]
- 4.Carrigan D R, Knox K K. Identification of interferon-resistant subpopulations in several strains of measles virus: positive selection by growth of the virus in brain tissue. J Virol. 1990;64:1606–1615. doi: 10.1128/jvi.64.4.1606-1615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousens L P, Peterson R, Hsu S, Dorner J D, Ahmed R, Biron C A. Two roads diverged: interferon alpha/beta and interleukin 12-mediated pathways in promoting T cell interferon-gamma. J Exp Med. 1999;189:1315–1327. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crespi M, Struther J K, Smith A N, Lyons S F. Interferon status after measles virus infection. S Afr Med J. 1988;73:711–712. [PubMed] [Google Scholar]
- 7.Desgrees du Lou A, Pison G, Aaby P. Role of immunizations in the recent decline in childhood mortality and the changes in the female/male mortality ratio in rural Senegal. Am J Epidemiol. 1995;142:643–652. doi: 10.1093/oxfordjournals.aje.a117688. [DOI] [PubMed] [Google Scholar]
- 8.Didcock L, Young D F, Goodbourn S, Randall R E. The V protein of Simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forthal D N, Aarnaes S, Blanding J, Maza L, Tilles J. Degree and length of viremia in adults with measles. J Infect Dis. 1992;166:421–424. doi: 10.1093/infdis/166.2.421. [DOI] [PubMed] [Google Scholar]
- 10.Fugier-Vivier I, Servet-Delprat C, RIvailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaccione C, Marcus P I. Interferon induction by viruses. XVIII. Vesicular stomatitis virus-New Jersey: a single infectious particle can both induce and suppress interferon production. J Interferon Res. 1989;9:603–614. doi: 10.1089/jir.1989.9.603. [DOI] [PubMed] [Google Scholar]
- 12.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobl A E, Funa K, Alm G V. Different induction patterns of mRNA for IFN-alpha and -beta in human mononuclear leukocytes after in vitro stimulation with herpes simplex virus-infected fibroblasts and Sendai virus. J Immunol. 1988;140:3605–3609. [PubMed] [Google Scholar]
- 14.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D N, Howley P M, editors. Fields virology. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1267–1312. [Google Scholar]
- 15.Griffin D E, Ward B J, Jauregui E, Johnson R T, Vaisberg A. Natural killer cell activity during measles. Clin Exp Immunol. 1990;81:218–224. doi: 10.1111/j.1365-2249.1990.tb03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin D E, Ward B J, Jauregui E, Johnson T, Vaisberg A. Immune activation during measles: interferon-γ and neopterin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J Infect Dis. 1990;161:449–453. doi: 10.1093/infdis/161.3.449. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs A, Lindenmann J. Virus interference. 1. The interferon. Proc R Soc Lond Biol. 1957;147:258–267. [PubMed] [Google Scholar]
- 18.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 19.Katayama Y, Hirano A, Wong T C. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating production of alpha/beta interferon. J Virol. 2000;74:1252–1257. doi: 10.1128/jvi.74.3.1252-1257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurihara N, Reddy S V, Menaa C, Anderson D, Roodman G D. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Investig. 2000;105:607–614. doi: 10.1172/JCI8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehtonen A, Matikainen S, Julkunen I. Interferons up regulate Stat1, Stat2 and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol. 1997;159:794–803. [PubMed] [Google Scholar]
- 22.Luft T, Pang K C, Thomas E, Hertzog P, Hart D N J, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 23.Manchester M, Eto D S, Valsamakis A, Liton P B, Fernandez-Munoz R, Rota P A, Bellini W J, Forthal D N, Oldstone M B. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus P I, Gaccione C. Interferon induction by viruses. XIX. Vesicular stomatitis virus—New Jersey: high multiplicity passages generate interferon-inducing, defective-interfering particles. Virology. 1989;171:630–633. doi: 10.1016/0042-6822(89)90637-5. [DOI] [PubMed] [Google Scholar]
- 25.Marcus P I, Rodriguez L L, Sekellick M J. Interferon induction as a quasispecies marker of vesicular stomatitis virus populations. J Virol. 1998;72:542–549. doi: 10.1128/jvi.72.1.542-549.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–529. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall J D, Chehimi J, Gri G, Kostman J R, Montaner L F, Trinchieri G. The interleukin-12 mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–1011. [PubMed] [Google Scholar]
- 28.Mattana P, Viscomi G C. Variations in the interferon-inducing capacity of Sendai virus subpopulations. J Interferon Cytokine Res. 1998;18:399–405. doi: 10.1089/jir.1998.18.399. [DOI] [PubMed] [Google Scholar]
- 29.McChesney M B, Miller C J, Rota P A, Zhu Y D, Antipa L, Lerche N W, Ahmed R, Bellini W J. Experimental measles I: pathogenesis in the normal and the immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- 30.Meraz M Q W, Sheehan K C, Back E A, Rodig S J, Dighe A S, Kaplan D H, Riley J, Greenlund K A C, Campbell D, Carver-Moore K, DuBois R N, Aguet C R M, Schreiber R D. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 31.Mills B G, Frausto A, Singer F R, Ohsaki Y, Demulder A, Roodman G D. Multinucleated cells formed in vitro from Paget's bone marrow express viral antigens. Bone. 1994;15:443–448. doi: 10.1016/8756-3282(94)90823-0. [DOI] [PubMed] [Google Scholar]
- 32.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 33.Naniche D, Reed S I, Oldstone M B. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J Virol. 1999;73:1894–1901. doi: 10.1128/jvi.73.3.1894-1901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy S V, Singer F R, Mallette L, Roodman G D. Detection of measles virus nucleocapsid transcripts in circulating blood cells from patients with Paget disease. J Bone Miner Res. 1996;11:1602–1607. doi: 10.1002/jbmr.5650111103. [DOI] [PubMed] [Google Scholar]
- 35.Rima B K, Earle J A P, Baczko K, ter Meulen V, Liebert U G, Carstens C, Carabana J, Caballero B, Celma M L, R. F-M. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J Gen Virol. 1997;78:97–106. doi: 10.1099/0022-1317-78-1-97. [DOI] [PubMed] [Google Scholar]
- 36.Samb B, Aaby P, Whittle H C, et al. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr Infect Dis. 1995;14:203–209. doi: 10.1097/00006454-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Schnorr J J, Dunster L M, Nanan R, Schneider-Schaulies J, Schneider-Schaulies S, ter Meulen V. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur J Immunol. 1995;25:976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 38.Shibahara K, Hotta H, Katayama Y, Homma M. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cell cultures. J Gen Virol. 1994;75:3511–3516. doi: 10.1099/0022-1317-75-12-3511. [DOI] [PubMed] [Google Scholar]
- 39.Shiozawa S, Yoshikawa N, Iijima K, Negishi K. A sensitive radioimmunoassay for circulating alpha-interferon in the plasma of healthy children and patients with measles virus infection. Clin Exp Immunol. 1988;73:366–369. [PMC free article] [PubMed] [Google Scholar]
- 40.Sissons J G P, Schreiber R D, Perrin L H, Cooper N R, Muller-Eberhard H J, Oldstone M B A. Lysis of measles virus-infected cells by the purified cytolytic alternative complement pathway and antibody. J Exp Med. 1979;150:415–454. doi: 10.1084/jem.150.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 42.Starr S E, Dalton B, Garrabrant T, Paucker K, Plotkin S A. Lymphocyte blastogenesis and interferon production in adult human leukocyte cultures stimulated with cytomegalovirus antigens. Infect Immun. 1980;30:17–22. doi: 10.1128/iai.30.1.17-22.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda M, Kato A, Kobune F, Sakata H, Li Y, Shioda T, Sakai Y, Asakawa M, Nagai Y. Measles virus attenuation associated with transcriptional impediment and a few amino acid changes in the polymerase and accessory proteins. J Virol. 1998;72:8690–8696. doi: 10.1128/jvi.72.11.8690-8696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor D R, Shi S T, Romano P R, Barber G N, Lai M M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 45.Tilles J F, Balkwill F, Davilla J. 2′,5′-Oligoadenylate synthetase and interferon in peripheral blood after rubella measles or mumps live virus vaccine. Proc Soc Exp Biol Med. 1987;186:70–74. doi: 10.3181/00379727-186-42586. [DOI] [PubMed] [Google Scholar]
- 46.Vilcek J, Sen G C. Interferons and other cytokines. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 375–399. [Google Scholar]
- 47.Volckaert-Vervliet G, Billiau A. Induction of interferon in human lymphoblastoid cells by Sendai and measles viruses. J Gen Virol. 1977;37:199–203. doi: 10.1099/0022-1317-37-1-199. [DOI] [PubMed] [Google Scholar]
- 48.Volckaert-Vervliet G, Heremans H, De Ley M, Billiau A. Interferon induction and action in human lymphoblastoid cells infected with measles virus. J Gen Virol. 1978;41:459–466. doi: 10.1099/0022-1317-41-3-459. [DOI] [PubMed] [Google Scholar]
- 49.Yeh T J, McBride P T, Overall J C, Jr, Green J A. Automated, quantitative cytopathic effect reduction assay for interferon. J Clin Microbiol. 1982;16:413–415. doi: 10.1128/jcm.16.2.413-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young D F, Didcock L, Goodbourn S, Randall R E. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology. 2000;269:383–390. doi: 10.1006/viro.2000.0240. [DOI] [PubMed] [Google Scholar]