Abstract
By reviewing the relevant literature in the field of T cell and allergic rhinitis, we determined the development status, study hotspots, and research frontiers viewpoints of this field to provide a reference for researchers and clinical workers. Methods: Web of Science Core Collection (WoSCC) was applied to obtain the studies related to T cells and allergic rhinitis (AR) from 2003 to 2023, and the information extracted from these studies was analyzed using CiteSpace 6.1.
R6 and VOSviewer 1.6.18. Results: In total, 1585 articles were collected from WoSCC, with the time set between 2003 and 2023. Overall, a growing number of articles are being published annually. The countries and institutions with the maximum publications volume are China (370, 23.34 %) and Sun Yat-sen University (34, 2.15 %). The biggest contributor to the field was Durham, Stephen R. from the UK (22, 1.39 %). The Journal of Allergy and Clinical Immunology published the most related papers in the field (88, 5.54 %). Immunotherapy, Th cells, and inflammation were found to be the research hotspots in this area of T cells and allergic rhinitis in recent years. Pathway, model, Regulatory T cells (Treg cells), regulatory B cells, immunoglobulin E,and innate lymphoid cells were the current research hotspots in this field. Conclusion: The field of T cell and allergic rhinitis is developing rapidly, and many countries significantly contributed to this field. Most researchers in this field mainly focused on immunotherapy, Th cell, and inflammation. Pathway, model, Treg cell, regulatory B cell, immunoglobulin E,and innate lymphoid cells were the main subject of current research, and future development is expected to occur in this field.
Keywords: Bibliometrics, Visualization, T cell, Allergic rhinitis, CiteSpace, VOSviewer
1. Introduction
Allergic rhinitis (AR), a non-communicable inflammation-associated symptoms, results from the presence of immunoglobulin E in the nasal mucosa. It involves the participation of various immune cells and cytokines [1]. The AR commonly manifest as nasal congestion, sneezing, nasal itching, and runny nose. Many epidemiological surveys conducted globally showed that AR has gained a significant increase of prevalence in recent years, affecting about 10–20 % of the worldwide population [2]. The incidence of AR among adults in China is 17.6 % [3] with an annual increase observed to date. It is a serious respiratory chronic inflammatory disorder that significantly affects the quality of life among patients and their financial conditions [4]. Extreme complexity is one of the hallmarks of AR's pathogenesis, and many cells and cytokines participate in its pathological process. Many researchers still argue that the T cell imbalance theory took a significance in AR, i.e., Th 2 and Th 17 cells become hyperactive, and Th 1 and Treg cells get suppressed [5,6]. IL-13 and IL-4 secreted by Th 2 cells are important inflammatory factors in AR [7]. Th 17 cells belong to the unique subset of Th cells. IL-17 with proinflammatory effect was secreted by Th 17 cells, which can regulate the development of AR by activating proinflammatory factors and regulating Th 2 cytokines [8]. In contrast, Th 1 cells can enhance phagocyte-mediated anti-infection immunity, and their secreted IL-2, INF- γ, TGF-β1, and TNF-α are important anti-inflammatory factors [9]. Treg cells not only release inhibitory factors such as IL-10 and TGF- β, butalso inhibit the mobility of effector T cells (Th 1, Th 2, and Th 17 cells) and other inflammatory cells. Based on this mechanism, Treg cells function in the anti-inflammatory process and induce immune tolerance in AR [10].
Bibliometrics seems a branch of informetrics. In bibliometrics, quantitative models is conducted based on published scientific studies to make clear the emerging trends and information structure in relevant studying fields [11]. Specifically, relevant bibliometric data can be retrieved from databases, and then, the influences of various authors, countries, institutions, and periodicals can be evaluated and visualized using visualization tools to foretell potential study hotspots by understanding changes of the research direction over time [12,13]. The commonly used visualization tools include CiteSpace and VOSviewer [14,15]. Information visualization tools, bibliometric methods, and data mining technologies were integrated into an interactive visualization system to require the data and information from studies and promote cognition of knowledge structures and emerging trends [16,17]. By analyzing many documents, the knowledge structure, research trends, and research hotspots can be presented as a network map.
Although many researchers have used bibliometrics to study AR in recent years, no relevant bibliometric research related to T cells in the field of AR is available. Thus, to replenish this knowledge vacancy, we conducted this research via WoSCC and retrieved the general information on papers, references, institutions, countries, authors, periodicals, and keywords. Then, we utilized CiteSpace 6.1.R6 and VOSviewer 1.6.18 to elucidate the advanced study, hot topics, and frontier informations of T cells in AR. Our findings might be considered as a novel reference for future clinical personnel and researchers.
2. Methods
2.1. Data collection
WoSCC is a vital literature collection database platform and the most trusted global citation database [18]. To improve the significance, accuracy and reproducibility of the results, we selected the Social Sciences Citation Index and Sciences Citation Index Expanded databases of WoSCC as the data source from 2003 to 2023. We included original articles and review articles. We used the following search query: (TS = (“T-lymphocyte”) OR TS = (“T Lymphocytes”) OR TS = (“T-Lymphocyte”) OR TS = (“Thymus-Dependent Lymphocytes”) OR TS = (“Lymphocyte, Thymus-Dependent”) OR TS = (“Lymphocytes, Thymus-Dependent”) OR TS = (“Thymus Dependent Lymphocytes”) OR TS = (“Thymus-Dependent Lymphocyte”) OR TS = (“T-Cells”) OR TS = (“T-Cell”) OR TS = (“T Cell”) OR TS = (“Cell, T”) OR TS = (“Cells, T″) OR TS = (“T Cells”) OR TS = (“T Lymphocyte”) OR TS = (“Lymphocyte, T”) OR TS = (“Lymphocytes, T″)) AND TS = (“allergic rhinitis”) [12], and Language type = English. In total, 1585 articles were retrieved, and the data were downloaded on February 26, 2024. The search procedure are meticulously presented in Fig. 1.
Fig. 1.
A flowchart illustrating the search strategy used in this study.
2.2. Search strategy
We conducted the CiteSpace 6.1.R6 and VOSviewer 1.6.18 for performing a network visualization of the authors, title, abstracts, keywords, institutions, countries, periodicals, and references of the 1585 articles based on bibliometric analysis. The selection criteria included the number of occurrences or the most cited topics in each section, which were analyzed annually. Each topic could contain multiple nodes. The period was set from 2003 to 2023, and the default settings of the software were used for the remaining parameters and values. The results are presented as a chart of visual data analysis with a cluster network of nodes and links. The nodes represented one item, such as the author, keywords, and literature. The node size was directly proportional to their frequency; the links represented the cooperation, co-occurrence, and co-citation relationship between nodes. Nodes of various colors indicated clusters. Additionally, we analyzed the 2023 journal citation report (JCR), containing its impact factor, category quartiles, etc.
3. Results
3.1. Growth trends of the literature
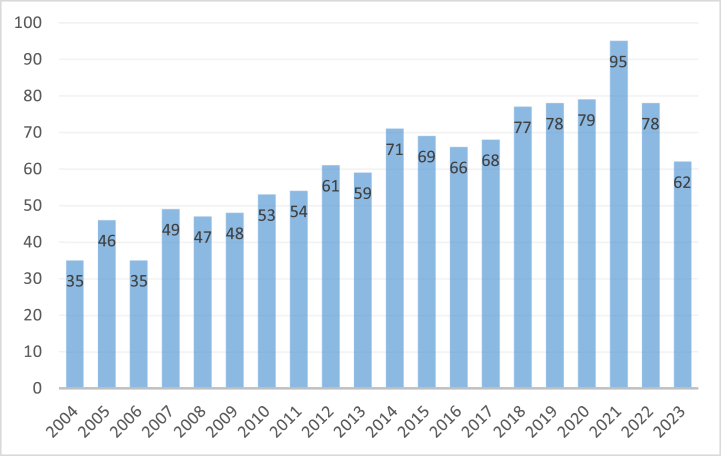
Derek John de Solla Price stated that science, like social activities, is determined by a powerful regular pattern [19,20]. The change of documents numbers over time can partly reflect the development of the regular pattern and the characteristics of the discipline. It can accurately reflect the past, present, and future development trends of the discipline [21]. From January 1, 2003 to December 31, 2023, 1585 T cells and AR articles were included in the WoScc database (Fig. 2). The annual quantities of publications were the largest in 2021 (95; 6.00 %) and the lowest in 2003 (25; 1.58 %). After reaching its peak in 2021, the field basically maintained stability in the following years, indicating that it continued to attract many researchers.At the same time, we also found that the number of publications in 2022 and 2023 has relatively decreased, but a short-term decrease in the number of publications does not necessarily mean a decrease in the field itself. Perhaps due to the lagging collection and reporting of publication data, and the shift in researchers' focus towards producing fewer but higher quality publications, this may lead to an overall decrease in quantity. Therefore, the decline in 2022 and 2023 does not necessarily mean a decrease in the field itself. Among them, published numbers in 2021 was 3.8 times higher than that in 2003. In 2014, the research on T cells and AR reached a relatively stable period, with an average annual publication volume of about 70 or more. In the last 20 years, 1259 original research papers, accounting for 79.43 %, and 326 reviews, accounting for 20.56 %, were published. Original research papers show the latest research in the field, whereas review papers allow readers to quickly obtain the latest comprehensive information.
Fig. 2.
Trends in the growth of publications from 2003 to 2023.
3.2. Distribution of countries/regions
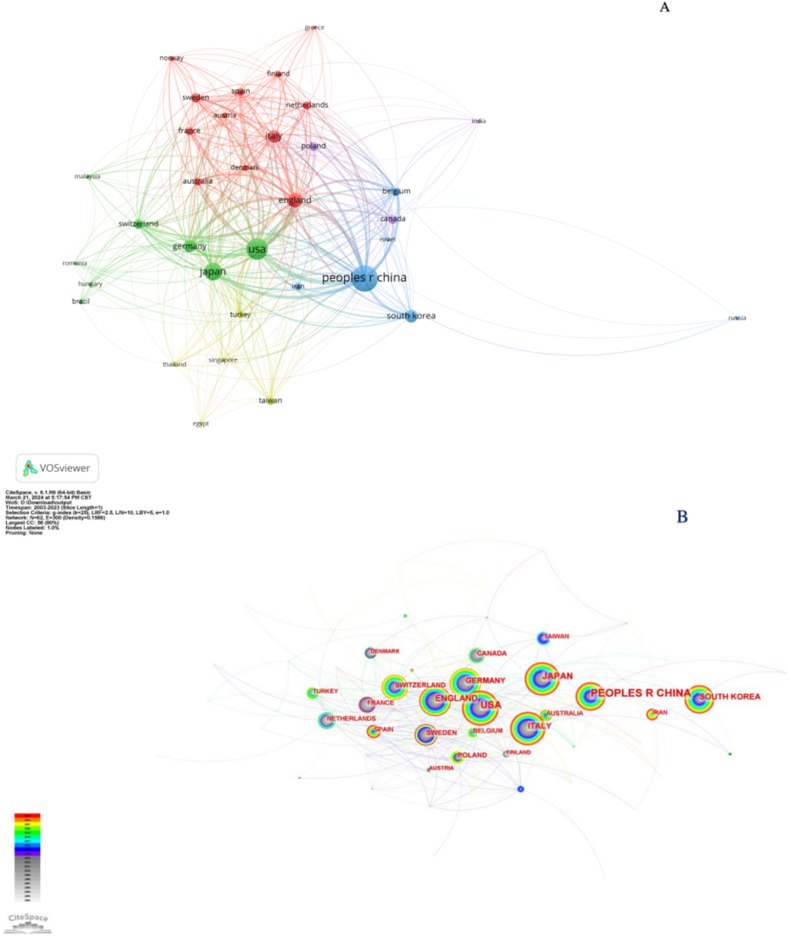
Using VOSviewer, we found that 62 countries have conducted collaborative studies. When the minimum number of publications was set to 5, 34 countries were identified. Based on the country/regional cooperative network analysis, VOSviewer divided the countries into different clusters. Each node in the graph represented the information of a country, the nodes of different colors represented different clusters, and the line represented the cooperative relationship between nodes.
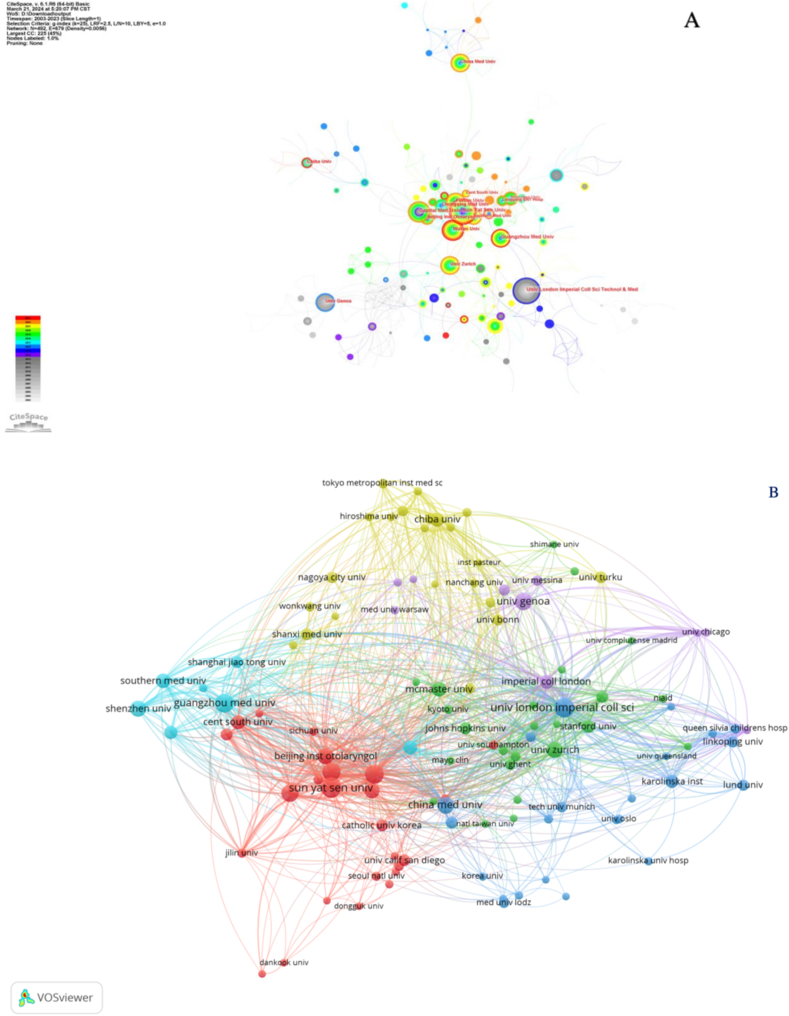
The cooperative plots of the countries/regions in the T cell and AR research are shown in Fig. 3. As reflected in Fig. 3A, China mainly cooperated with the United States, Japan, and South Korea; Britain collaborated with Sweden, Australia, and France; Germany often cooperated with Switzerland, Poland, and Spain; Italy collaborated with Russia, the Netherlands, and Austria. Although a large node represented China, international cooperation by China was lesser than that shown by developed countries like the United States. A visual graph of the CiteSpace network countries is shown in Fig. 3B. Purple outer circles identified prominent nodes, and the larger nodes represented important countries focusing on T cells and AR.
Fig. 3.
Cooperation map of countries/regions associated with research on T cells and AR. (A) A visual map of the VOSviewer network. (B) A visual map of the CiteSpace network.
The 10 countries that made the most contributions in this field are presented in Table 1. The country with the most published articles was China (380, 23.97 %), followed by the United States (222, 14.00 %), Japan (147, 8.89 %), the United Kingdom (97, 6.12 %), Korea (81, 5.10 %), Italy (76, 4.79 %), Germany (71, 4.48 %), Sweden (44, 2.77 %), Switzerland (43, 2.71 %), and Canada (37, 2.33 %). The sum of studies from China, the United States, and Japan accounted for more than half among the top 10 countries, and about 23.97 % of the total published studies came from China. Our findings indicated that these countries were interested in the field of T cells and AR. Average Citation/Publication can reflect the importance of the results in a research field. The UK (78.42), Germany (64.21), and Switzerland (61.14) had the most of average citations/publications, indicating that the results of T cells and AR in these three countries were more informative. Centrality, also called betweenness centrality, is a key indicator of influence, The United States (0.37) ranked the first in regard to centrality, followed by Germany (0.24) and Switzerland (0.15), which indicated that these countries had a strong influence under this field. Although China had the largest quantities of literature contributions, the Average Citation/Publication and centrality were relatively low.
Table 1.
Most important countries in terms of contribution to research on T cells and AR.
| Rank | Country | Citations | Documents | Centrality | Average Citation/Publication |
|---|---|---|---|---|---|
| 1 | USA | 10124 | 221 | 0.37 | 45.81 |
| 2 | England | 7528 | 96 | 0.16 | 78.42 |
| 3 | Germany | 4495 | 70 | 0.24 | 64.21 |
| 4 | Peoples R China | 4041 | 370 | 0.13 | 10.92 |
| 5 | Japan | 3405 | 147 | 0.07 | 23.16 |
| 6 | Switzerland | 2568 | 42 | 0.15 | 61.14 |
| 7 | Italy | 2243 | 76 | 0.10 | 29.51 |
| 8 | Canada | 1526 | 37 | 0.00 | 41.24 |
| 9 | Sweden | 1190 | 43 | 0.03 | 27.67 |
| 10 | South Korea | 1190 | 80 | 0.01 | 14.88 |
3.3. Contributions of the authors and the institutions
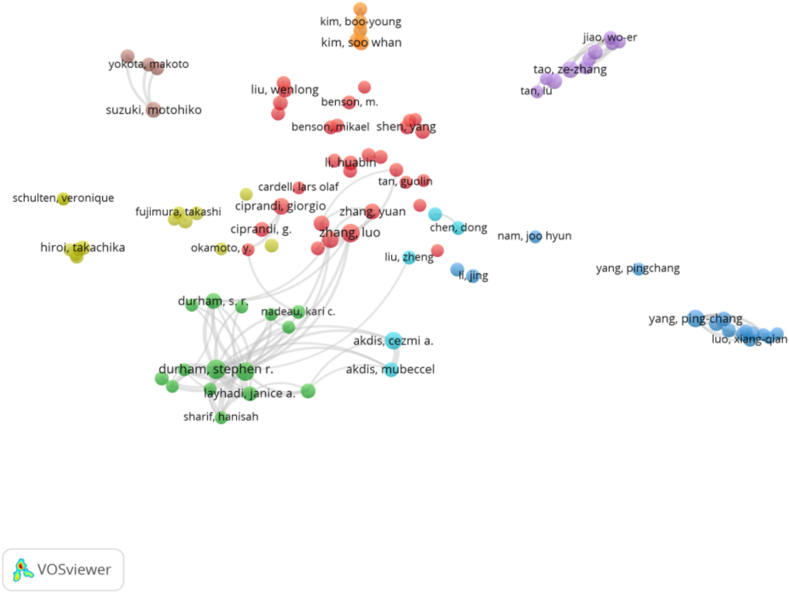
We used VOSviewer to visualize the author cooperative network graph. In the past 20 years, 5998 authors had published articles in the field of T cells and AR, and according to the George Price formula we found 200 core authors with more than four articles published in the field [20]. We plotted an author cooperative network graph, where each circle represented an author and different color clusters represented an author cooperative network.
As shown in Fig. 4, seven cooperative clusters were formed for the authors with more than five articles. These authors included Luo Zhang, Ze-Chang Tao, Akdis Cezmi A, Hua-Bin Li, Ping-Chang Yang, Jing Li, and Durham, Stephen R. We found that Chinese researchers had a good cooperative relationship, but they rarely cooperated with foreign researchers.
Fig. 4.
Joint mapping of the productive authors who published studies on T cells and AR from 2003 to 2023.
Eight researchers who have published the most articles since 2003 are presented in Table 2. These authors included Durham Stephen R. from Imperial College London, London, England, followed by Luo Zhang from Capital Medical University, Beijing Tongren Hospital, Department of Otolaryngology Head & Neck Surgery, Beijing, Peoples Republic of China, and Shamji, Mohamed H. from Imperial College London, Immunomodulat & Tolerance Grp, Sect Inflammat Repair & Dev, Allergy & Clin Immunol, Natl Heart & Lung Institute, London, England. Durham Stephen R had the highest average Citation/Publication (80.00), i.e., the research results of Durham Stephen R were relatively more accepted, and their articles were highly cited. Durham Stephen R mainly focused on the mechanism and biomarker prediction of allergen immunotherapy and immunotherapy. Shamji, Mohamed H. (52.80) and Akdis, Cezmi A. (43.13) had the second largest average Citation/Publication. The centrality of all researchers was below 0.1, which indicated that the researchers studying T cells and AR were not very collaborative and need to increase their cooperation with each other.
Table 2.
Most important authors in terms of contribution to research on T cells and AR.
| Rank | Author | Affiliations | Citations | Documents | Centrality | Average Citation/Publication |
|---|---|---|---|---|---|---|
| 1 | durham, stephen r. | Imperial Coll London, London, England | 1760 | 22 | 0.00 | 80.00 |
| 2 | shamji, mohamed h. | Imperial Coll London, Immunomodulat & Tolerance Grp, Sect Inflammat Repair & Dev, Allergy & Clin Immunol, Natl Heart & Lung Inst, London, England | 1056 | 20 | 0.00 | 52.80 |
| 3 | akdis, cezmi a. | Univ Zurich, Swiss Inst Allergy & Asthma Res SIAF, Davos, Switzerland | 690 | 16 | 0.00 | 43.13 |
| 4 | zhang,luo | Capital Med Univ, Beijing TongRen Hosp, Dept Otolaryngol Head & Neck Surg, Beijing, Peoples R China | 331 | 24 | 0.01 | 13.80 |
| 5. | wang, chengshuo | Capital Med Univ, Beijing TongRen Hosp, Dept Otolaryngol Head & Neck Surg, Beijing 100730, Peoples R China | 285 | 13 | 0.00 | 21.92 |
| 6 | ciprandi, giorgio | Casa Cura Villa Montallegro, Genoa, Italy | 267 | 14 | 0.00 | 19.07 |
| 7 | yang.ping-chang | Zhengzhou Univ, Hosp 5, Dept Gastroenterol, Zhengzhou, Henan, Peoples R China | 172 | 16 | 0.00 | 10.75 |
| 8 | tao,ze-zhang | Wuhan Univ, Renmin Hosp, Dept Otolaryngol Head & Neck Surg, Wuhan, Peoples R Chin | 148 | 13 | 0.00 | 11.38 |
From the VOSviewer visualization institution graph, we identified 1458 institutions, of which 111 published more than five articles in the field of T cells and AR. Eleven clusters were formed (Fig. 5A), each representing the close cooperation between the constituent institutions.
Fig. 5.
Cooperation map of institutions that published studies on T cells and AR. (A) A visual map of the VOSviewer network. (B) A visual map of the CiteSpace network.
The parameters of CiteSpace were configured with the following parameters: time slice: 2003–2023, years per slice: 1, term source: entire selection, node type: institution, and selection criteria: top N = 50. The default settings were used for the remaining parameters. A visual graph of the CiteSpace network institution is shown in Fig. 5B. Based on several connections, we identified relatively close connections among the different institutions. Purple outer circles identified prominent nodes, and the larger nodes represented important research institutions focusing on T cells and AR.
Articles on T cells and AR were mainly published from hospitals and universities (Table 3). Sun Yat-sen University (34) published the largest number of research papers, followed by Guangzhou Medical University (32) and Imperial College London (31). Imperial College London had the highest Average Citation/Publication (105.65). The University of Zurich followed closely, with an Average Citation/Publication of 44.10. Among the top 12 institutions related to T cells and AR research, only Imperial College London had a centrality greater than 0.10 (0.11), which indicated the need to further strengthen the cooperation between institutions.
Table 3.
Most important institutions in terms of contribution to research on T cells and AR.
| Rank | Organization | Citations | Documents | Centrality | country | Average Citation/Publication |
|---|---|---|---|---|---|---|
| 1 | Imperial College London | 3275 | 31 | 0.11 | England | 105.65 |
| 2 | University Zurich | 926 | 21 | 0.05 | switzerland | 44.10 |
| 3 | Sun Yat Sen University | 639 | 34 | 0.07 | Peoples r china | 18.79 |
| 4 | Capital Medical University | 633 | 29 | 0.06 | Peoples r china | 21.83 |
| 5 | University genoa | 518 | 25 | 0.04 | italy | 20.72 |
| 6 | Fudan University | 507 | 27 | 0.04 | Peoples r china | 18.78 |
| 7 | Guangzhou Medical University | 485 | 32 | 0.06 | Peoples r china | 15.17 |
| 8 | Beijing institution otolaryngol | 471 | 22 | 0.04 | Peoples r china | 21.41 |
| 9 | Wuhan University | 401 | 27 | 0.02 | Peoples r china | 14.85 |
| 10 | China Medical University | 236 | 22 | 0.06 | Peoples r china | 10.73 |
| 11 | Chongqing Medical University | 207 | 20 | 0.00 | Peoples r china | 10.35 |
| 12 | ShenZhen University | 166 | 23 | 0.02 | Peoples r china | 7.22 |
3.4. Distribution of journals
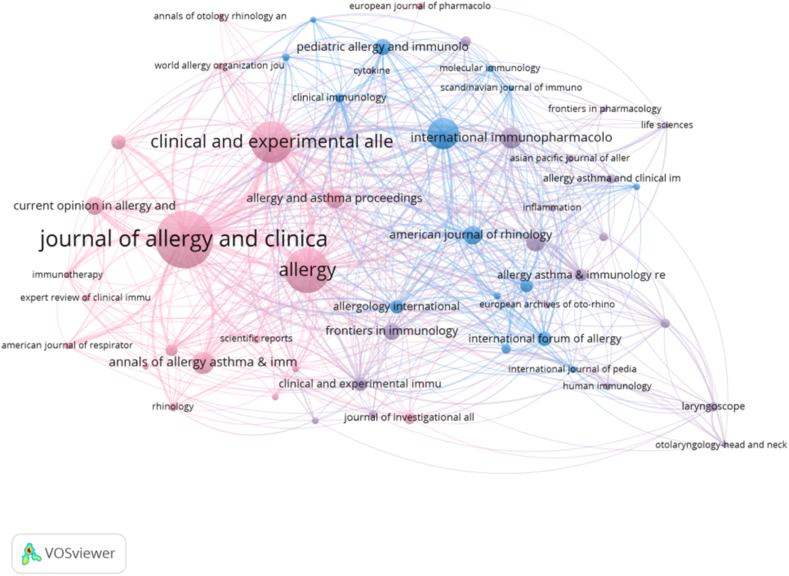
We used VOSvivewer to visualize the journal cooperative network graph (Fig. 6). In the past 20 years, articles on T cells and AR were published in 375 journals, of which 56 published more than 5 articles in this field. Each node in the figure represented a journal and clusters of different colors represented a journal cooperative net, forming six clusters in total.
Fig. 6.
Joint mapping of productive journals that published studies on T cells and AR from 2003 to 2023.
The Journal of Allergy and Clinical Immunology had the largest number of published papers in the field of T cells and AR (Table 4), with 88 published papers. It was followed by Allergy (66), Clinical and Experimental Allergy (58), International Archives of Allergy and Immunology (42), International Immunopharmacology (26), Annals of Allergy, Asthma, & Immunology (25),American Journal of Rhinology & Allergy (24), Plos One(21), Allergy & Asthma Proceedings (21), and Current Opinion in Allergy and Clinical Immunology (21). Journal Citation Report quartile 1 (Q1) included Journal of Allergy and Clinical Immunology, Allergy, Clinical and Experimental Allergy, International Immunopharmacology, Plos One, and American Journal of Rhinology & Allergy; Q2 included Clinical and Experimental Allergy, International Archives of Allergy and Immunology, Annals of Allergy, Asthma, & Immunology, International Immunopharmacology, and Allergy and Asthma Proceedings; Q3 contained International Archives of Allergy and Immunology, American Journal of Rhinology & Allergy, Current Opinion in Allergy and Clinical Immunology, and Allergy and Asthma Proceedings. The most cited journal in the co-citation analysis was the Journal of Allergy and Clinical Immunology (9,115) (Table 5), followed by Allergy (4,061), Clinical and Experimental Allergy (3,378), Journal of Immunology (739), International Archives of Allergy and Immunology (645), Journal of Experimental Medicine (1120), American Journal of Respiratory and Clinical Care Medicine (1112), Nature Immunology (959), Immunity (878), and Proceedings of the National Academy of Sciences of the United States of America (853).
Table 4.
The top 10 journals that published studies on T cells and AR.
| Rank | Source | Documents | IF (2023) | JCR |
|---|---|---|---|---|
| 1 | Journal of allergy and clinical immunology | 88 | 14.2 | Q1 |
| 2 | allergy | 66 | 12.4 | Q1 |
| 3 | Clinical and experimental allergy | 58 | 6.1 | Q1/Q2 |
| 4 | International archives of allergy and immunology | 42 | 2.8 | Q2/Q3 |
| 5 | International immunopharmacology | 26 | 5.6 | Q1/Q2 |
| 6 | Annals of allergy and asthma&immunology | 25 | 5.9 | Q2 |
| 7 | American journal of rhinology&allergy | 24 | 2.6 | Q1/Q3 |
| 8 | Plos one | 21 | 3.7 | Q1/Q3 |
| 9 | allergy and asthma proceedings | 21 | 2.8 | Q2/Q3 |
| 10 | Current Opinion in Allergy and Clinical Immunology | 21 | 2.8 | Q3/Q4 |
Table 5.
The top 10 co-cited journals that published studies on T cells and AR.
| Rank | Source | Citations | IF (2023) | JCR | Centrality |
|---|---|---|---|---|---|
| 1 | Journal of Allergy and Clinical Immunology | 9115 | 14.2 | Q1 | 0.00 |
| 2 | Allergy | 4061 | 12.4 | Q1 | 0.00 |
| 3 | Journal of Immunology | 3378 | 4.4 | Q2 | 0.00 |
| 4 | Clinical and Experimental Allergy | 2488 | 6.1 | Q1/Q2 | 0.00 |
| 5 | Journal of Experimental Medicine | 1120 | 15.3 | Q1 | 0.00 |
| 6 | American Journal of Respiratory and Clinical Care Medicine | 1112 | 24.7 | Q1 | 0.00 |
| 7 | International Archives of Allergy and Immunology | 1012 | 2.8 | Q2/Q3 | 0.00 |
| 8 | Nature Immunology | 959 | 30.5 | Q1 | 0.01 |
| 9 | Immunity | 878 | 32.4 | Q1 | 0.01 |
| 10 | Proceedings of the National Academy of Sciences of the United States of America | 853 | 11.1 | Q1 | 0.00 |
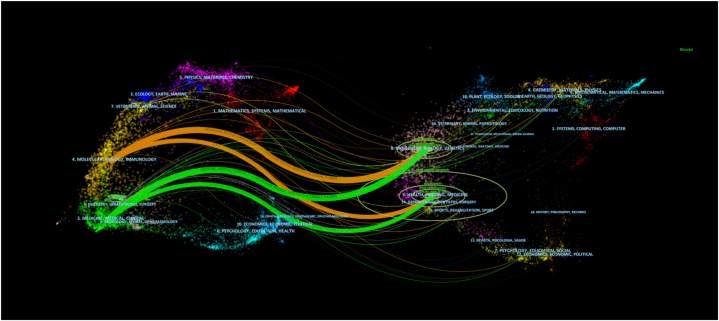
The dual-map overlay of journals showed the distribution of the motifs (Fig. 7). Journal labels represent the disciplines it covers, and color-coded routes show the relationships between citations. We identified the four most important routes. The two orange cited routes indicated that studies published in molecular/biology/genetic journals were cited in molecular/biology/immunology. The two green citation routes indicated that molecular/biology/genetic journals were cited in medicine/medical/clinical fields.
Fig. 7.
Dual-map overlay of the journals that published studies on T cells and AR.
3.5. Analysis of keywords and burst words
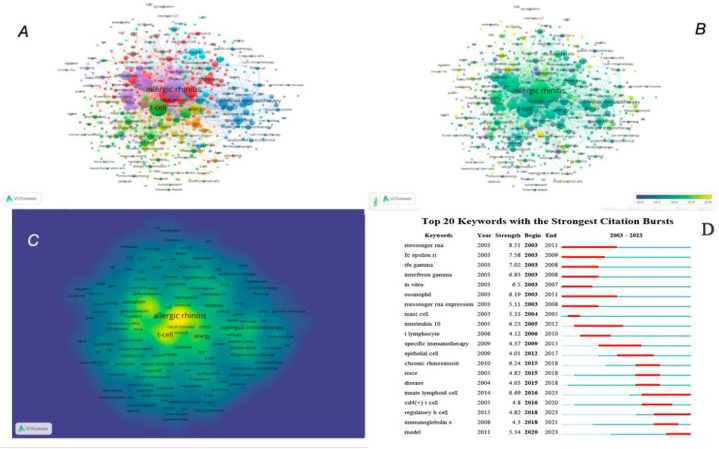
By conducting a visual analysis of the keywords of T cell research in AR using VOSviewer, we found that the distribution characteristics of the keywords reflected the overall content of the discipline or field, the development context and development trend within a period, and the focus and hot spots of research to a certain extent. Each node in the graph represented one keyword, and each link represented the co-occurrence relationship between keywords. The occurrence frequency of keywords was determined by the size of the nodes. In total, 4681 keywords were retrieved, among which 192 keywords had an occurrence frequency greater than 12. In the keyword clustering network, all keywords were placed in six clusters (Fig. 8A). Among them, the core word for the yellow cluster was allergic rhinitis, and the related keywords were expression, inflammation, activation, cell, and cytokine. The core word for the blue cluster was T-cell, and the related keywords were asthma, atopic dermatitis, children, prevalence, and atopy. The core word for the green cluster was regulatory T-cells, and the related keywords were immunotherapy, grass-pollen immunotherapy, mechanisms, allergy, and sublingual immunotherapy. The core word for the red cluster was airway inflammation, and the related keywords were dendritic cells, Th2 cells, mast cells, and innate lymphoid cells. The core word for the purple cluster was rhinitis, and the related keywords were seasonal allergic rhinitis, chronic rhinosinusitis, mucosa, therapy, and quality of life. The core word for the cyan cluster was Th 17, and the related keywords were IL-17, Th 1, Th 2, Treg, and blood mononuclear cells.
Fig. 8.
Co-occurrence analysis of global research on T cells and AR based on the WoSCC database from 2003 to 2023. (A) Mapping of keywords in the research field. (B) The distribution of keywords according to the chronological order of appearance. (C) The distribution of keywords according to the mean frequency of appearance. (D) Keywords with the strongest citation bursts in T cells and AR research.
In the keyword tag view (Fig. 8B), the intensity of the yellow indicated how close the keyword was to the current trend. As shown in the figure, Treg, Th 17, pathway, microRNA, differentiation, mechanisms, immunotherapy, and innate lymphoid cells were the recent research hotspots.
In the keyword density view (Fig. 8C), density represented the frequency of different keywords. Yellow is more closely associated with higher densities. The hotspots of research across this field were mainly found in high-density areas.
According to the keyword co-citation network, we performed outbreak keyword retrieval (Fig. 8D). Blue and red constitute a straight line as the timeline. The red section in the figure meant the burst period, where the length implied the start and end years, as well as, the duration. By extracting outbreak words we identified the changing trend of keywords with time, which indicated the direction of development and research hotspot in the research field. To focus on the research trends of T cells and AR, we analyzed the keywords from 2003 to 2023; messenger RNA (8.51) had the strongest citation burst, followed by high-affinity IgE receptors (Fc epsilon RI, FcεRI) (7.58), Interferon-gamma (IFN-γ) (7.02), innate lymphoid cell 6.69), and in vitro (6.5). As shown in Fig. 8D, model (2020–2023), regulatory B cell (2018–2023), and innate lymphoid cell (2016–2023) were emerging research hotspots.
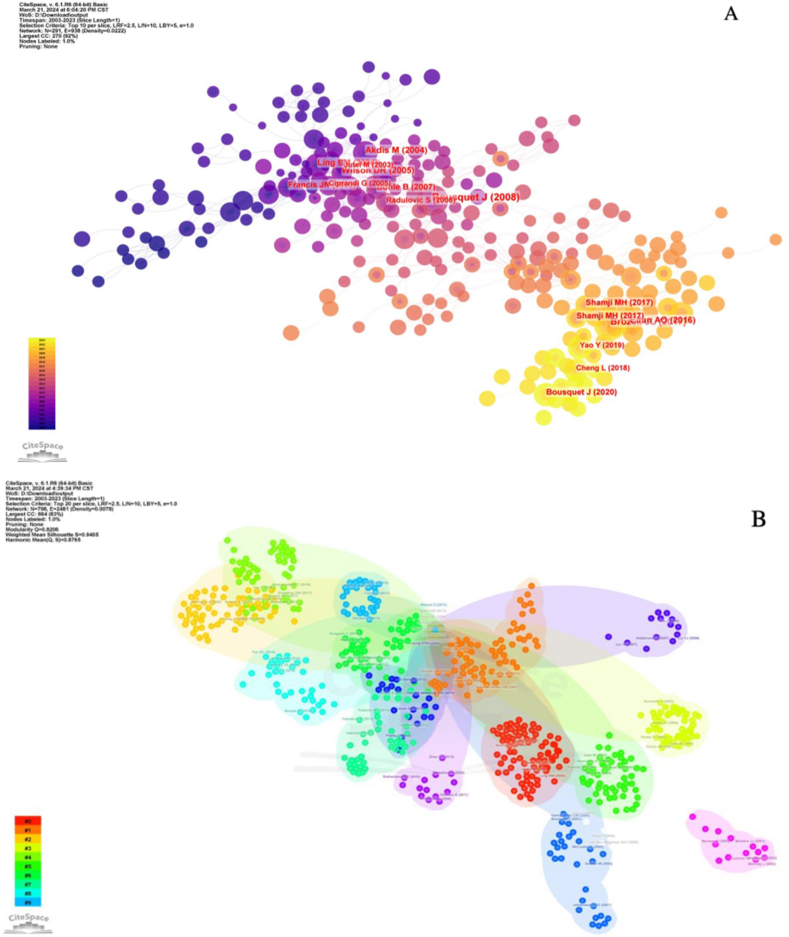
3.6. Reference analysis
In this study, 43,366 articles cited in the 1585 articles were analyzed with the following CiteSpace parameters: time slice (2003–2023), years per slice (1), term source (entire selection), node type (reference), and selection criteria (top N = 50). The default settings were used for the remaining parameters, yielding a network with 1022 nodes and 3998 connections (Fig. 9A). We plotted a cluster network graph based on the results (Fig. 9B). In total, 14 clusters were obtained, which facilitated the discovery of T cells and AR. Additionally, the top 10 widely cited references were identified by the co-citation network (Table 6). Bousquet J Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2) LEN and AllerGen published in Allergy was the most cited (3,223). Excluding similar articles, immune responses were featured with a balance between allergen-specific T regulatory 1 and T helper 2 cells in healthy and allergic individuals. Mubeccel Akdis published articles in the Journal of Experimental Medicine (809) on IL-10 and TGF-beta, indicating that the regulation of immune responses by T cell to mucosal allergens in specific immunotherapy depends on their cooperation. Jan L Brozek published articles in the Journal of Allergy and Clinical Immunology (702).
Fig. 9.
(A) The reference co-citation network related to T cells and AR. (B) The reference co-citation network related to T cells and AR.
Table 6.
The top 10 co-citation references related to T cells and AR.
| Rank | Cited reference | Author | Journal | Year | Citations |
|---|---|---|---|---|---|
| 1 | Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) | Bousquet j | Allergy | 2008 | 3223 |
| 2 | Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision | Jan L Brozek | J Allergy Clin Immunol | 2010 | 1077 |
| 3 | Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells | Mübeccel Akdis | J Exp Med | 2004 | 809 |
| 4 | Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision | Jan L Brozek | J Allergy Clin Immunol | 2017 | 772 |
| 5 | IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy | Marek Jutel | Eur J Immunol | 2003 | 702 |
| 6 | Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis | D R Wilson | Allergy | 2005 | 517 |
| 7 | Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity | Kayhan T Nouri-Aria | J Immunol | 2004 | 436 |
| 8 | Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy | James N Francis | J Allergy Clin Immunol | 2003 | 421 |
| 9 | Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation | Barbara Bohle | J Allergy Clin Immunol | 2007 | 319 |
| 10 | Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa | Suzana Radulovic | J Allergy Clin Immunol | 2008 | 226 |
Allergic Rhinitis and its Impact on Asthma (ARIA) is a review article published in Allergy in 2008 by Bousquet et al. [22]. It was updated in 2010 and 2017, and is among the top 10 cited articles; all three versions of this article are highly cited. The article summarizes the pathogenesis of T cells in allergic rhinitis and suggests that in the production of immunoglobulin E, T cells interact with B cells, mast cells, and basophils in a complex manner. The simultaneous upregulation of Th 2 cells and the downregulation of Th 1 cells can drive immunoglobulin E (IgE) synthesis, as well as, the recruitment, activization, and performing functions between helper cells(eosinophils, basophils, and mast cells). The review also summarizes the hygiene hypothesis that the balance between Th 1, Th 2, and Treg cells can affect the development of allergy. The environment of an individual in their early life can initiate the development of the immune system toward Th 1 (non-allergic), while a sterile circumstance promotes the progress of allergy. Akdis et al. reported a study in the Journal of Experimental Biology (2004) in which they revealed that healthy and allergic individuals respond in a balanced way to allergens through allergen-specific T regulatory 1 and T helper 2 cells. They suggested that developing allergies might be controlled by balance between allergen-specific Tr 1 cells and Th 2 cells [23]. Jutel et al. published an article in the European Journal of Immunology (2003) in which they showed that IL-10 and TGF-β cooperated against mucosal allergens in both normal immunity and specific immunotherapy. In normal immunity to house dust mite (HDM) and birch pollen, they found allergen-specific peripheral T cells with inhibitory effects on dermatophagoides protein 1(Der p 1) and birch pollen allergen. They found that proliferative T cells were involved in immune response, and Th 1 (IFN- +) and Th 2 (IL-5, IL-13) cell responses were inhibited, but the IL-10 and TGF-β secretion increased in allergen-specific T cells. Additionally, SIT, IL-10, and TGF-β induced the immunosuppression of mucosal allergen production by T cells. SIT also inhibited antigen-specific CD4 + CD25 + T cells in individuals with allergies. To summarize, the results of that article showed that the T cell response referring to regulator or suppressor deviated during SIT and normal immunity, which has a crucial link to the immune response [24].
4. Discussion
AR is non-communicable occurring in the nasal mainly mucosa mediated by IgE. Although many researchers have investigated the pathological and therapeutic mechanisms of AR, the therapeutic effect of AR in clinical practice is still not satisfactory, and many researchers argue that the T cell imbalance theory functioned in AR. Therefore, in this investigation, using the quantitative analysis software CiteSpace and VOSviewer, we analyzed research on T cells and AR and summarized the findings and current status of the research field.
We retrieved 1585 articles from the WoSCC database. More and more studies in the field were found pulished every year, which indicated that this field received more attention with time. Our results showed that research on T cells and AR increased every year over the past 20 years around the world. It also showed that more researchers consider T cells to be important in AR.
We conducted statistical analyses by quantifying publications of countries and institutions to identify studies on T cells and AR, countries and research institutions that influenced the research field, and determine their partnerships to indicate the direction of cooperation between the countries and institutions. As exhibited in Table 1, China, the United States, and Japan were the core countries where researchers studied T cells and AR. Chinese researchers published the peak volume of articles, followed by the United States and Japan. This indicated that researchers from these countries were relatively more interested in research on T cells and AR. Studies from the UK, Germany, and Switzerland had the highest Average Citation/Publication, which indicated that the research on T cells and AR in these countries were relatively more informative and these countries had a greater influence on the research field. Several countries exhibited the higher literature contributions, yet their average citation/publication and centrality were relatively low. This suggests that improvements are needed in the quality of research on T cells and AR in these nations.
Over the past decades, 200 core authors have conducted four or more studies about T cells and AR. As shown in the author collaboration graph in Fig. 4, most collaborations occurred among Luo Zhang, Ze-Chang Tao, Akdis Cezmi A, Hua-Bin Li, Ping-Chang Yang, Jing Li, and Durham, Stephen R. Although cooperation among Chinese researchers was strong, they rarely cooperated with international researchers. As indicated in Table 2, author having the extreme volume of published articles was Durham, Stephen R from Imperial College London, London, England, and he also published with the largest amounts of citations. Thus, the research conducted by Durham, Stephen R was relatively more informative and recognized; his studies mainly focused on allergen immunotherapy, the mechanism of immunotherapy, and biomarker prediction of immunotherapy. Akdis Cezmi A from the University of Zurich, The Swiss Institute of Allergy & Asthma Research (SIAF), Davos, Switzerland was the author with the highest h-index. Researchers can use the h-index to evaluate their academic output in terms of the quantity and quality. The studies conducted by this researcher were mainly related to the role of various T cells and their cytokines in allergic diseases. Among the top eight authors, four were from China, but all of their average citation/publication levels and h-index were low. Most authors who made prominent contributions to T cells and AR field were from developed countries, for example, the United Kingdom and Switzerland. Hence, it is a need to strengthen communication and cooperation with researchers globally to improve T cell and AR related research in developing countries.
The institutional distribution cooperation graph (Fig. 5) roughly shows the power distribution in the field of T cells and AR and provides a basis for cooperation and communication among relevant research institutions. Among the top 12 institutions, nine were from China; Sun Yat-sen University had the most published research papers in the field. Imperial College, University of London had the highest average citation/publication and centrality among all the research institutions analyzed in this study, which indicated that the results were more informative in the field of T cells and AR, and those studies had a greater influence on the research field. Articles in the field of T cells and AR were mainly published in leading journals, such as Allergy and Immunology. Among the 10 highly co-cited journals, seven were at the JCR Q1 range. These articles contributed greatly to the development of ears, nose, and throat (ENT) and immunology. The dual-journal chart showed that the studies in the field of T cells and AR shifted from basic research (molecular, biology, and immunology) to clinical research (pharmacy and clinical), which greatly benefitted patients with allergic rhinitis.
The top 10 articles focused on immune response, immunotherapy, and inflammation, mainly because AR occurs due to the participation of immune cells in chronic inflammatory disease. Mechanisms of congenital and adaptive immune inflammation occurred in various phases of the disease, where T cells are crucial in the process of immune response that accompanies AR. The most cited reference was the article Allergic Rhinitis and its Impact on Asthma (ARIA) published by Bousquet et al., who proposed the definition, immune mechanism, and diagnostic criteria of allergic rhinitis. Clinical treatment for allergic rhinitis reached a milestone with this discovery. Articles on immune response and inflammatory mechanisms of AR featured in the top 10 most cited articles in recent years.
Keywordsare the label of a paper. By analyzing the keywords, readers can catch on the topics quickly and get information on the study hotspots and frontier ideas under the discipline. After eliminating the relatively unimportant keywords, immunotherapy (immunotherapy), Th cell (Th cell), and inflammation (inflammation) were considered as the research cores in the field of T cell-related allergic rhinitis in recent years. The outbreak of keywords is a significantly higher frequency of some keywords over a set timeframe. Such an outbreak might also reveal new findings in the field and reflect the development trend in a specific field and elucidate the current study hotspots. We found that the top five keywords with strong explosive power were model (2020–2023), regulatory B cell (2018–2023), and innate lymphoid cell (2016–2023) in recent years. Meanwhile, it is noted that pathway, Treg cell, immunoglobuin e also possess significant burst strength.
4.1. Pathway
AR is a global health issue, and a cure for the disease needs to be found urgently. Many researchers have investigated the signaling pathways related to the pathogenesis of AR inflammation, such as the nuclear factor-κB pathway, the myeloid differentiation factor 88 pathway, the mitogen-activated protein kinases (MAPK) pathway, the extracellular-signal-regulated kinases (ERK-DNMT) pathway, the high mobility group box protein B1/TLR4 pathway, the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, and the Notch pathway [25,26]. Among them, the nuclear factor κB (NF-κB) pathway is the most representative signaling pathway associated with AR. NF-κB facilitates the induction of pro-inflammatory response and the generation of Th 2 cytokines, which can promote the development of AR [27]. Dong et al. reported that the expression of TLR4, NF-κB, p65, and other cytokines (IL-4, IL-5, and IL-13) is high in the serum of AR patients. They speculated that the TLR4/NF- κB pathway laid a vital impact in AR [28]. Studies have also shown that PM2.5 can exacerbate oxidative stress via the Nrf2/NF-κB signaling pathway in inflammatory response based on the OVA-induced AR mouse model [29]. These studies also showed that NF-κB can participate in the development of AR through various signaling pathways.
Myeloid differentiation factor 88 (MYD88) often acts as a “bridging molecule” forming a link between TLRs and downstream genes. MYD88 along with TLRs can quickly accumulate in the body and phosphorylate the inhibitory κB kinase complex and mitogen-activated protein kinases, thus activating the NF-κB and MAPK genes. These can promote the synthesis and release of related inflammatory cytokines and induce the immune response to exogenous pathogens [30]. By overexpressing miR-224–5p in AR mice, Wu et al. found nasal lavage and serum of AR mice were lack of inflammatory cells, and the treatment suppressed NLR 3 inflammasome and TLR4/MyD88/NF-κB pathway in AR mice. They concluded that miR-224–5p probably alleviates AR by negatively regulating the TLR4/MyD88/NF-κB pathway [31]. Additionally, the TLRs/MyD88 pathway can activate dendritic cells (DCs) and promote their rapid maturation, possibly using TLR ligands to transmit negative signals to activate DCs to suppress Th2 cell-mediated immune responses [32]. Because of the key bridging role of MYD88, the TLR/MYD88 pathway might be considered to be an important signaling pathway for AR therapy.
There are four types of MAPK, including the ERK, c-Jun N-terminal kinase (JNK), p38MAPK, and MAPK/ERK5. MAPK pathways associated with the pathogenesis of AR are the p38MAPK pathway, the JNK pathway, and the ERK pathway [26]. The protein kinase p38MAPK contribute to inflammatory cell proliferation and differentiation during the regulation of inflammatory response. The activated p38MAPK can participate in the migration and activation of mast cells, monocytes, neutrophils, and eosinophils. It help the generation of pro-inflammatory factors, regulate the transcription of cytokines, and participate in the development of AR [33]. The levels of inflammation-related factors IL-6, p38MAPK, and TNF-α were found to increase significantly in monocytes supplemented with the p38MAPK agonist Postmortem interval (PMI), whereas adding the p38MAPK-related specific inhibitor SB203580 effectively decreased the generation and secretion of IL-6 and TNF-α in AR. These findings indicated that the p38MAPK pathway can regulate monocyte activation and gene expression of inflammatory factors in AR [34]. A previous study found that specific inhibitor of p38 MAPK upregulated OMP expression levels in the model mice with AR by reducing the proportion of apoptotic OSN and significantly enhanced the function of olfactory sensory system in the mice model with AR [35]. Therefore, further studies should investigate whether the p38MAPK signaling pathway plays other unknown roles in AR. JNK renovated and remodeled the nasal mucosa in AR. Some studies have shown that JNK was highly expressed in the nasal cavity of AR rats, and its phosphorylation contribute to the remodeling of the nasal mucosa. The administration of IFN-γsignificantly reduced the expression of JNK, the level of IL-1β upstream of the JNK signaling pathway and the phosphorylation of JNK, which in turn reversed the remodeling of the AR nasal mucosa and alleviated the symptoms of AR; thus, this strategy might be applied for treating AR [36]. Few investigations revealed the role of the JNK pathway in AR, and further studies on this topic are still required. Some studies have also found that activated ERK-DNMT pathway in CD4+T cells causes an asymmetric ratio of Th1/Th2, skewing the ratio toward Th2. It also leads to DNA methylation on promoter region of the IFN-γ gene, thus, aggravating AR; under the inhibition of the ERK-DNMT pathway [37].
High mobility group box B1 (HMGB1), as a nuclear protein, expressed in various cells and participates in DNA replication and transcription. The binding of HMGB1 to TLR can promote the expression and assignment of inflammatory factors under AR inflammatory response [38,39]. Genetic analysis of HMGB1/TLR4 signaling in cultured human nasal epithelial cells showed that proteins expression involved in HMGB1/TLR4 signaling was significantly higher [40].
Interleukin 33 (IL-33) is deemed as a member of the IL-1 family, and tumorigenicity 2 (ST2) is a member of the IL-1 receptor superfamily that acts as a specific receptor for IL-33. When IL-33 interacts with ST2, it promote the subsequent signaling pathways to accelerate the aggregation of various inflammatory cells, including acidophils, alkalophils, and mast cells in the nasal mucosa. It also accelerates the generation of cytokines such as IL-4, IL-5, and IL-13, leading to AR [41].
Toll-like receptors (TLRs) are the upstream genes of many signaling pathways, which influence the incidence of AR. TLRs can initiate immune responses through different signaling pathways, containing TLR/NF-κB, TLRs/MYD88, TLR/MAPK, IL-33/ST2, and HMGB1/TLR 4 pathways, thus, promoting AR [26].
The JAK-STAT signaling pathway is a tyrosine kinase composed of the Janus kinase family and serves as the signal transducer and activator for the transcription [42]. Some AR-related studies have demonstrated that JAK-STAT signaling pathway not only functions in the inflammatory response but also participates in cell processes of proliferation, differentiation, apoptosis, as well as immune regulation [43]. Shi et al. found that blocking the JAK/STAT pathway inhibited the Th2 inflammatory cell response induced by TSLP-DC in AR, while the JAK/STAT pathway inhibitor CYT387 significantly broke down TSLP-DCs to promote the development of differentiated naive CD4 (+) T cells into IL-4-expressing Th2 cells [44]. Their findings suggested that further investigating the inhibitors of the JAK-STAT signaling pathway might reveal new therapeutic agents for treating AR.
The notch signaling pathway is active in diverse tissues and immune cells, and it is a highly conserved signal transduction pathway [45]. Many current studies have demonstrated that the Notch signaling pathway regulated AR. Specifically, the pathway plays an immune regulatory role by regulating the activation of DCs, production of IgE, abnormal differentiation of T lymphocyte subsets, aggregation of inflammatory cells, and degranulation of basophils and mast cells. It also leads to a disrupted balance in the ratio of the Th1/Th2 and Th17/Treg cells [46]. Notch signaling can bidirectionally regulate the Foxp3 promoter through RBP-J and Hes1-dependent mechanisms, thus, participating in Treg differentiation and functional development. The downregulated Foxp 3 expression in Notch signaling can increase the expression of Notch1, Jagged1, and NICD, inhibit Treg cells differentiation, and facilitate the progress of AR [47]. A study also indicated that the inhibited Notch pathway reduced the allergic symptoms and IgE levels in serum, and prevented the imbalance in Th1/Th2; thus, improving the symptoms, which further indicated that blocking the Notch pathway might be an effective therapy direction for AR [48]. The Notch signaling pathway includes four Notch receptors, five Notch ligands, and intracellular effector molecules [49]. Different receptors and ligands expressed in the pathway and the contact signals between different receptors and different ligands might be the way to affect the differentiation and function of T lymphocytes. Thus, the specific role of different receptor ligands might be further studied.
Although several studies have investigated the signaling pathways related to the pathogenesis of AR, it is not fully understood because it involves various factors. Hence, the association of various signal pathways with the formation of AR need to be further investigated to offer new molecular targets and strategies for treating AR.
4.2. Model
The second research frontier might be the model. Empirical studies on AR are based on the AR model. Thus, establishing a stable and reliable AR model is the key to effective empirical studies. The existing AR models are diverse and can be divided into two categories, including animal models and cell models. However, the selection of species, mold drugs, basic sensitization, excitation methods, positive drugs, and detection indicators can have a significant effect on the final results [50]. Therefore, the development of stable and reliable AR models is crucial in AR.
4.3. Treg cells
Activation of the immune system and CD4 + effector Th cell subsets strongly influences the development of AR. The CD4 + effector Th1, Th2, Th17, and Treg cells consisted of cell subgroups. The theory of balance alteration of Th1/Th2 cells in allergic diathesis was recently considered as a “procrustean paradigm” as it was unexplainable for many preclinical findings. Treg cells are necessary for T-cell homeostasis as well as maintaining peripheral tolerance to allergens [51]. In AR, Treg cells restrains the proliferative activation response of T cells, macrophages, dendritic cells, and B cells [52]. In vitro studies have indicated that activated CD4 + CD25 + Treg cells took inhibitory effects by direct cell-to-cell contact, secreting inhibitory cytokines, interacting with T cells, and modifying or killing antigen-presenting cells [53]. In studies on allergic diseases, the occurrence of diseases is often accompanied by a decreased Treg cells and a weakening of their function. For example, in a study on 45 patients with AR, it found lower expression of Foxp 3 in the peripheral blood, a significantly lower proportion of Treg cells, and skewed toward Th 17 between Th17/Treg ratio [54]. In a study, 22 AR patients and nine healthy individuals were selected to receive double-blind, placebo-controlled pollen allergen immunotherapy for two years. In this study, the inferior turbinate mucosa of the patients was collected before and after treatment, the quantitative alteration of Treg cells was analyzed via immunohistochemistry, and quantitative difference of Treg cells before and after administering the placebo was evaluated. In patients administered immunotherapy, the amounts of Treg cells aggrandized significantly after treatment, and it was positively correlated with the efficacy of treatment. These results suggested that a certain amounts of Treg cells is essential for maintaining peripheral tolerance to allergens [55]. A study in which mesenchymal stem cells were used for AR treatment found that upregulating Treg cells can improve the symptoms of AR in animals, suggesting that a certain quantitative of Treg cells is useful to inhibit the occurrence and progress of AR [56]. However, the treatment of mesenchymal stem cells for AR is in the animal experimental stage, and clinical trials need to be conducted. Treg cells in AR was confirmed by several studies, and the amplification and activation of Treg cells were also found to be controlled by various molecules and signaling pathways, such as cytokines and co-stimulatory molecules. For example, Treg cells in AR can be differentiated and activated by miR-146a by targeting STAT5b, thus, alleviating the progression of AR [57]. Therefore, ways to improve the signaling pathway of Treg cells in AR need to be determined to elucidate the mechanism of AR therapy.
4.4. Regulatory B cells
The fourth research topic involves regulatory B cells (Bregs), which are immunosuppressive cells that regulate immune responses by participating in various mechanisms and target different types of immune cells, including DC, macrophages, and lymphocytes. Their most prominent effector functions include the production of potent immunosuppressive cytokines, like IL-10, TGF-β, and IL-35, and the inhibition of target cells based on a cellular contact-dependent mechanism [58]. In AR, Bregs mainly exert their effects through their mediated allergen tolerance mechanism, specifically by inhibiting the antigen-presenting function and regulating the response of helper T cells [59]. Several studies have shown that the percentage of Bregs and Tregs decreases in wild-type mice with a prolonged intranasal challenge of ovalbumin (OVA) [60]. Kim et al. showed that Breg levels in AR patients were lower compared to those in healthy people and increased after immunotherapy. Also, the recovery of nasal mucosal immune function improved as the increased proportion of Breg cells [61]. The mechanism of AR progress is very complex, and studying Breg cells might help in comprehensively understanding AR. However, only a few studies have investigated the regulatory mechanism of Breg in AR, which focused on the immune tolerance characteristics of Breg cells. Additionally, although some researches have indicated that blocking the PD-1/PD-L1 pathway can promote the apoptosis of CD19 (+) CD25 (+) Breg cells, which was deemed as a potentially valuable pathogenic factor of AR [62]. Also, Bregs-specific transcription factors need to be identified, which might be the next step in the treatment of AR.
4.5. Immunoglobulin E
The fifth research frontier might be IgE. Most researchers agree the mediated role of IgE in AR. New therapeutic strategies for IgE might reduce the immune response of allergen stimulation, promote the clinical symptoms of AR, and improve the efficacy and safety of treatment. For example, a study evaluated the SCIT + dupilumab in comparison with SCIT alone in AR patients and revealed that tolerance to SCIT after 16-week treatment by SCIT + dupilumab was higher than that after treatment with SCIT alone [63]. The role of IgE should be further elucidated to understand the development, use, and effects of new therapeutic agents, which might promote new strategies for the management of severe AR.
4.6. Innate lymphoid cells
Meanwhile, it is noted that innate lymphoid cells (ILCs) possess significant burst strength in 2016–2023. As recent key effector cells from the innate immune system, ILCs, with three subtypes (ILC1-3), correspond functionally to Th1, Th2, and Th17. These cells participated in the mucosal immune formation, the development of lymphocyt, repairing tissue, and protecting epithelial barrier, help infection combination, inflammation regulation, and immune homeostasis [64]. ILC1s, including NK cells and ILC1 cells, depend on the T-box transcription factor and generate large amounts of IFN-γ and tumor necrosis factor-α. ILC2s, a reliant on the transcription factor GATA3, produce Th2-type cytokines and other effector molecules, containing IL-4, IL-5, IL-9, IL-13, and Vascular endothelial growth factor, driving type 2 immune response development. ILC3s is independent to the transcription factor RORTt, which can produce cytokines IL-17 and IL-22, similar to Th17 [65]. Doherty et al. observed an increased ILC2 in the peripheral blood of patients after 4 h [66]. Concurrently, AR patients affected by pollen exhibited increased ILC2 in peripheral blood during pollen season and a significant decrease after subcutaneous immunotherapy [67], suggesting the involvement of ILC2 in the onset and progression of AR. Another study demonstrated that KLRG1(+) ILC2s, generated by IL-33 and retinoic acid in AR patients, had lower IL-10 (+) KLRG1(+) in comparison with healthy subjects, indicating their role in attenuating Th response and maintaining epithelial integrity. In this study, the ability of KLR1(+) ILC2s to produce in patients undergoing immunotherapy was restored [68], indicating the varying roles of different ILC2 types, warranting further research. Many studies have confirmed the regulation of ILC2 by signaling pathways. For instance, MSC-sEV can inhibit mDC's activation effect on ILC2 in AR patients. After co-culture with sEV-mDCs, ILC2's GATA3 levels decrease, and introducing a PGE2 receptor antagonist into the co-culture can change the negative effect of sEV-mDCs on ILC2 [69]. Therefore, it was proposed that PGE2 also took immunomodulatory effect of sEV-mDCs on ILC2s. However, the specific mechanism of ILC2 in AR seems vague, and future study on different regulatory mechanisms may aid in discovering new therapeutic targets for AR. Research predominantly focuses on ILC2, with limited studies on ILC1 and ILC3. Exploring ILC1 and ILC3 mechanisms in AR could be the next step. The respiratory tract surface is lined with epithelial cells, forming the innate immune system alongside innate immune cells and molecules. As a primary physical barrier against external antigens, its role is crucial. Epithelial barrier dysfunction is a common AR feature, causing immune cell activation; for example, ILC2 responds to airway epithelial-derived alarmins (thymic stromal lymphopoietin, IL-33) and secretes more type 2 cytokines [70]. Furthermore, research has shown that histone deacetylase activation by type 2 immune response significantly affects the leakage barrier, and inhibiting histone deacetylase activity could correct the barrier defect in an allergic asthma with rhinitis-related mouse model [71]. Future research might also explore interactions between tissue barriers, innate immune cells or molecules in AR.
5. Limitations
First, to conduct bibliometric analysis with high-quality, the analysis was on basise of articles available in the WoSCC database; hence, articles present only in other databases were missed. Second, the absence of chapters, books, magazines, letters, and non-English language literature might have biased our analysis. Third, bibliometric citation metrics depend on time, indicating potential less cited articles than earlier ones in recent times. Fourth, the sole topic search term used in this article is allergic rhinitis. While this term is commonly utilized, the potential for the omission of relevant articles still exists due to the use of synonymous terms. Although these shortcomings might slightly affect the integrated results, the main trendency presented in this field cannot be altered. In conclusion, our investigation provided a reference for comprehending the study topics, hot ideas, and developmental trends of T cells and AR.
6. Conclusion
To summarize, in this study, we used the bibliometric method to comprehensively estimate the present development status and future development direction of T cells and AR. The amounts of publications in the field of T cells and AR were steadily growing during the latest decades. This research mainly demonstrates the inflammatory or immune mechanisms, Th cell, and related molecules of AR. Pathway, model, Treg cell, regulatory B cell, immunoglobulin E and ILCs are the hotspots of research in this field in recent years. Our findings might provide a comprehensive review of this field and serve as theoretical support for subsequent research.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The original contributions presented in the study are included in the article/supplementary material. And the original data can be downloaded in WoS (https://www.webofscience.com).Further inquiries can be directed to the corresponding authors.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding and acknowledgments
This study was supported by SichuanScience and Technology Program (2022YFS0629, 2023YFS0090).
Ethics statement
No ethical approval was required as this study did not involve human participants or laboratory animals.
CRediT authorship contribution statement
Shuang Liu: Writing – original draft. Xiaoyan Hu: Writing – review & editing. Jing Zhang: Writing – original draft. Liangge Lv: Writing – original draft. Yuxiao He: Writing – original draft. Liang Jiang: Writing – review & editing, Conceptualization. Gang Qin: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery,Chinese Medical Association Chinese guideline for diagnosis and treatment of allergic rhinitis (2022, revision) Chin. J. Otorhinolaryngol. Head Neck Surg. 2022;57(2):106–129. doi: 10.3760/cma.j.cn115330-20211228-00828. [DOI] [PubMed] [Google Scholar]
- 2.Brozek J.L., Bousquet J., Baena-Cagnani C.E., Bonini S., Canonica G.W., Casale T.B., et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J. Allergy Clin. Immunol. 2010;126(3):466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L., Chen J., Fu Q., He S., Li H., Liu Z., et al. Chinese society of allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2018;10(4):300–353. doi: 10.4168/aair.2018.10.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X.D., Zheng M., Lou H.F., Wang C.S., Zhang Y., Bo M.Y., et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui-hui Peng, Jian-hang Leng, Jun-ya Shen, Zhuo Guang-chao, Chen Li. Research of imbalance of Th17/Treg and Th1/Th2 in the pathogenesis of allergic rhinitis. Chin. J. Health Lab. Technol. 2020;30(18):2234–2237. [Google Scholar]
- 6.Li C.N. ShanXi Medical University; 2021. The Level and Significance of Peripheral Blood CD4+T Cell Subsets in Allergic Rhinitis. [Google Scholar]
- 7.Perkins C., Wills-Karp M., Finkelman F.D. IL-4 induces IL-13-independent allergic airway inflammation. J. Allergy Clin. Immunol. 2006;118(2):410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Cheng, Hong Suling, Hu Guohua. The expression of Treg/Th17 cells related transcription factors and cytokines in PBMCs and plasma in patients with allergic rhinitis. Journal clinical Otorhinolaryngol Head Neck surgery. 2012;26(5):209–211. doi: 10.13201/j.issn.1001-1781.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski J.A., Borish L. Novel cytokines and cytokine-producing T cells in allergic disorders. Allergy Asthma Proc. 2011;32(2):83–94. doi: 10.2500/aap.2011.32.3428. [DOI] [PubMed] [Google Scholar]
- 10.Jiao W.E., Sun L., Xu S., Deng Y.Q., Qiao Y.L., Xi Y., et al. Notch2 suppresses the development of allergic rhinitis by promoting FOXP3 expression and Treg cell differentiation. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119922. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Zhou S., Liu Y., Li Y., Sun X. Bibliometric analysis of the inflammatory mechanism in aortic disease. Rev. Cardiovasc. Med. 2022;23(2):67. doi: 10.31083/j.rcm2302067. [DOI] [PubMed] [Google Scholar]
- 12.Wei N., Xu Y., Li Y., Shi J., Zhang X., You Y., et al. A bibliometric analysis of T cell and atherosclerosis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.948314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W., Li Q., Peng F., Huang D. Worldwide Meniere's disease research: a bibliometric analysis of the published literature between 2002 and 2021. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.1030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song L., Zhang J., Ma D., Fan Y., Lai R., Tian W., et al. A bibliometric and knowledge-map analysis of macrophage polarization in atherosclerosis from 2001 to 2021. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.910444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Zhang T., Jin Y., Ma Y., Xian Z., Zeng M., et al. Emerging trends and research foci in allergic rhinitis immunotherapy from 2002 to 2021: a bibliometric and visualized study. Am J Transl Res. 2022;14(7):4457–4476. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong D., Li Y., Huang Y., Hong X., Li J., Jin R. Molecular mechanisms of exercise on cancer: a bibliometrics study and visualization analysis via CiteSpace. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.797902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S., Sun Y.P., Gao X.L., Sui Y. Knowledge domain and emerging trends in Alzheimer's disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. 2019;14(9):1643–1650. doi: 10.4103/1673-5374.255995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Y.F., Ren S.H., Shao B., Qin H., Wang H.D., Li G.M., et al. The intellectual base and research fronts of IL-37: a bibliometric review of the literature from WoSCC. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.931783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price D.J. de S., et al. Yale University Press; 1961. Science since Babylon[M] [Google Scholar]
- 20.Price D.J. de S., et al. Columbia University Press; 1963. Little Science, Big science[M] p. 301. [Google Scholar]
- 21.Ni-bo Liu, wei-ling Yuan, Zhang Bo, Yang Dong, Yuan-yuan Li. Omestic research progress in allergic rhinitis based on the bibliometric analysis. J Med Postgra. 2013;26(12):1307–1309. [Google Scholar]
- 22.Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Togias A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World health organization, GA(2)len and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 23.Akdis M., Verhagen J., Taylor A., Karamloo F., Karagiannidis C., Crameri R., et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199(11):1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jutel M., Akdis M., Budak F., Aebischer-Casaulta C., Wrzyszcz M., Blaser K., et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 2003;33(5):1205–1214. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 25.Yi-cheng Li, Jun Xiong. Research progress of pro-inflammatory and non-pro-inflammatory signaling pathways in allergic rhinitis. Chinese Manipulation and Rehabilitation Medicine. 2022;13(19):48–52+7. [Google Scholar]
- 26.Gulibairemu-Yusuyin. Yan Mao, Liu Yan, He Jin-hua, Gu Zhengyi, Muhetaer-Abudurehemu Research progress in TLRs-mediated inflammatory signaling pathways and pathogenesis of allergic rhinitis. Med. Recapitulate. 2020;26(1):50–53+8. [Google Scholar]
- 27.Li Li, Xiao-Bing Zhang. Relationship between nuclear factor ?b signaling pathway and allergic rhinitis. Med. Recapitulate. 2019;25(7):1266–1271. [Google Scholar]
- 28.Dong J., Xu O., Wang J., Shan C., Ren X. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol. Immunotoxicol. 2021;43(3):319–327. doi: 10.1080/08923973.2021.1905659. [DOI] [PubMed] [Google Scholar]
- 29.Piao C.H., Fan Y., Nguyen T.V., Shin H.S., Kim H.T., Song C.H., et al. PM(2.5) exacerbates oxidative stress and inflammatory response through the Nrf2/NF-κB signaling pathway in OVA-induced allergic rhinitis mouse model. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deguine J., Barton G.M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Wu L., Zhang L., Xu H., Wang M., Wang L., et al. Overexpression of miR-224–5p alleviates allergic rhinitis in mice via the TLR4/MyD88/NF-κB pathway. Exp. Anim. 2021;70(4):440–449. doi: 10.1538/expanim.20-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh J.Z., Kurche J.S., Burchill M.A., Kedl R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118(11):3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 34.Jian-ping Lan, Xian-hai Zeng, Xiao-xia Zeng, Wen-li Chen. The regulation of the expression of IL-6 and TNF-α by P38MAPK of monocyte in allergic rhinitis. Mod. Hosp. 2009;9(8):22–24. [Google Scholar]
- 35.Gao X., Li N., Zhang J. SB203580, a p38MAPK inhibitor, attenuates olfactory dysfunction by inhibiting OSN apoptosis in AR mice (activation and involvement of the p38 mitogen-activated protein kinase in olfactory sensory neuronal apoptosis of OVA-induced allergic rhinitis) Brain Behav. 2019;9(6) doi: 10.1002/brb3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q., Chen Y.L., Ma Y.Y., Zhang Y.D., Sun C.W., You C.P. [Effects of inhibiting the phosphorylation of JNK by absorbed INF-γon the remodeling of nasal mucosa in allergic rhinitis rats] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30(13):1034–1037. doi: 10.13201/j.issn.1001-1781.2016.13.007. [DOI] [PubMed] [Google Scholar]
- 37.Ai S., Lin Y., Zheng J., Zhuang X. Xingbi gel ameliorates allergic rhinitis by regulating IFN-γ gene promoter methylation in CD4+ T cells via the ERK-DNMT pathway. Front Surg. 2020;7 doi: 10.3389/fsurg.2020.619053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dvoriantchikova G., Hernandez E., Grant J., Santos A.R., Yang H., Ivanov D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Invest. Ophthalmol. Vis. Sci. 2011;52(10):7187–7194. doi: 10.1167/iovs.11-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen G., Ward M.F., Sama A.E., Wang H. Extracellular HMGB1 as a proinflammatory cytokine. J. Interferon Cytokine Res. 2004;24(6):329–333. doi: 10.1089/107999004323142187. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S., Kouzaki H., Kato T., Tojima I., Shimizu T. HMGB1-TLR4 signaling contributes to the secretion of interleukin 6 and interleukin 8 by nasal epithelial cells. American journal of rhinology & allergy. 2016;30(3):167–172. doi: 10.2500/ajra.2016.30.4300. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Wei, Wang Tian, Xiao-yi Chu, Li-li Wang, Tang Li. Research progress on pathological mechanism of seasonal allergic rhinitis and its Chinese medicine therapeutic mechanism. Chinese Journal of Drug Evaluation. 2022;39(5):399–405. [Google Scholar]
- 42.Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Targeted Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell M.D., Fitzsimons C., Smith P.A. JAK/STAT inhibitors and other small molecule cytokine antagonists for the treatment of allergic disease. Ann. Allergy Asthma Immunol. 2018;120(4):367–375. doi: 10.1016/j.anai.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Shi Z., Jiang W., Wang M., Wang X., Li X., Chen X., et al. Inhibition of JAK/STAT pathway restrains TSLP-activated dendritic cells mediated inflammatory T helper type 2 cell response in allergic rhinitis. Mol. Cell. Biochem. 2017;430(1–2):161–169. doi: 10.1007/s11010-017-2963-7. [DOI] [PubMed] [Google Scholar]
- 45.Shang Y., Smith S., Hu X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell. 2016;7(3):159–174. doi: 10.1007/s13238-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wo-er Jiao, Chen Shi-ming. The research progress of function and mechanism of Notch signaling pathway in allergic rhinitis. Chinese Journal of Difficult and Complicated Cases. 2018;17(8):860–864. [Google Scholar]
- 47.Shi L., Ma Y., Zheng C., Zhang Q. The effect of blocking Notch signaling by γ-secretase inhibitor on allergic rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2017;98:32–38. doi: 10.1016/j.ijporl.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Zheng G., Liu L., Zhu K., Wei J. [Expression and significance of Notch receptors in the mouse model of allergic rhinitis] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28(20):1585–1589. [PubMed] [Google Scholar]
- 49.Wang H., Zang C., Liu X.S., Aster J.C. The role of Notch receptors in transcriptional regulation. J. Cell. Physiol. 2015;230(5):982–988. doi: 10.1002/jcp.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Rui, Xiu-min Li, Ming-san Miao. Animal model analysis of Parkinson's disease based on clinical characteristics of traditional Chinese and western medicine. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(4):204–207. [Google Scholar]
- 51.Moitra S., Datta A., Mondal S., Hazra I., Faruk S.M.O., Das P.K., et al. Modulation of regulatory T cells by intranasal allergen immunotherapy in an experimental rat model of airway allergy. Int. Immunopharm. 2017;47:9–19. doi: 10.1016/j.intimp.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Dikiy S., Rudensky A.Y. Principles of regulatory T cell function. Immunity. 2023;56(2):240–255. doi: 10.1016/j.immuni.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Jayasekera N.P., Toma T.P., Williams A., Rajakulasingam K. Mechanisms of immunotherapy in allergic rhinitis. Biomed. Pharmacother. 2007;61(1):29–33. doi: 10.1016/j.biopha.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Li Bao-wei, Wu Wei, Xu Min-min Clinical significance of detection of CD4 + CD25 + regulatory T cells in peripheral blood of patients with allergic rhinitis. Hebei Medical Journal. 2012;6(6):27–28. [Google Scholar]
- 55.Radulovic S., Jacobson M.R., Durham S.R., Nouri-Aria K.T. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008;121(6):1467–1472. doi: 10.1016/j.jaci.2008.03.013. 72.e1. [DOI] [PubMed] [Google Scholar]
- 56.Wei X.M., Gao X., Yu C.J. [Research progress in therapeutic action of mesenchymal stem cell in allergic rhinitis] Zhonghua er bi yan hou tou jing wai ke za zhi. 2018;53(10):789–793. doi: 10.3760/cma.j.issn.1673-0860.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Yang Y., Guo J., Cui L., Yang L., Li Y., et al. miR-146a enhances regulatory T-cell differentiation and function in allergic rhinitis by targeting STAT5b. Allergy. 2022;77(2):550–558. doi: 10.1111/all.15163. [DOI] [PubMed] [Google Scholar]
- 58.Rosser E.C., Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Fan K., Jin L., Yu S. Roles of regulatory B cells in the pathogenesis of allergic rhinitis. Allergol. Immunopathol. 2022;50(5):7–15. doi: 10.15586/aei.v50i5.615. [DOI] [PubMed] [Google Scholar]
- 60.Wang M., Gu Z., Yang J., Zhao H., Cao Z. Changes among TGF-β1(+) Breg cells and helper T cell subsets in a murine model of allergic rhinitis with prolonged OVA challenge. Int. Immunopharm. 2019;69:347–357. doi: 10.1016/j.intimp.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Kim A.S., Doherty T.A., Karta M.R., Das S., Baum R., Rosenthal P., et al. Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J. Allergy Clin. Immunol. 2016;138(4):1192. doi: 10.1016/j.jaci.2016.03.017. 5.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z., Tan F. The blockade of PD-1/PD-L1 pathway promotes the apoptosis of CD19(+) CD25(+) Bregs and suppresses the secretion of IL-10 in patients with allergic rhinitis. Scand. J. Immunol. 2020;91(2) doi: 10.1111/sji.12836. [DOI] [PubMed] [Google Scholar]
- 63.Corren J., Saini S.S., Gagnon R., Moss M.H., Sussman G., Jacobs J., et al. Short-term subcutaneous allergy immunotherapy and dupilumab are well tolerated in allergic rhinitis: a randomized trial. J. Asthma Allergy. 2021;14:1045–1063. doi: 10.2147/JAA.S318892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Y., Liu Y. [Current status of research on group 2 innate lymphocytes in allergic rhinitis] Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34(4):376–380. doi: 10.13201/j.issn.2096-7993.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang G., et al. Observation on the efficacy of sublingual immunotherapy with dust mite allergen for perennial allergic rhinitis and the mechanism of action on ILCs with ILC1s and ILC2s and ILC3s. Medicine (Baltim.) 2022;101(48) doi: 10.1097/MD.0000000000032019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doherty T.A., et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J. Allergy Clin. Immunol. 2014;133(4):1203–1205. doi: 10.1016/j.jaci.2013.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lao-Araya M., et al. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J. Allergy Clin. Immunol. 2014;134(5):1193. doi: 10.1016/j.jaci.2014.07.029. 5.e4. [DOI] [PubMed] [Google Scholar]
- 68.Golebski K., et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54(2):291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Liu X.Q., et al. Dendritic cells mediated by small extracellular vesicles derived from MSCs attenuated the ILC2 activity via PGE2 in patients with allergic rhinitis. Stem Cell Res. Ther. 2023;14(1):180. doi: 10.1186/s13287-023-03408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang Y.A., Strohm A., Doherty T. Modulating ILC2 function for treatment of type 2 airway diseases. Curr. Trends Immunol. 2022;23:85–90. [PMC free article] [PubMed] [Google Scholar]
- 71.Sugita K., et al. Outside-in hypothesis revisited: the role of microbial, epithelial, and immune interactions. Ann. Allergy Asthma Immunol. 2020;125(5):517–527. doi: 10.1016/j.anai.2020.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The original contributions presented in the study are included in the article/supplementary material. And the original data can be downloaded in WoS (https://www.webofscience.com).Further inquiries can be directed to the corresponding authors.