Abstract
Background
Oral lichen planus (OLP) is a T cell-mediated autoimmune disease that affects the oral mucosa. Although Helicobacter pylori has been documented in subgingival and supragingival plaques and saliva, little is currently known about the relationship between Helicobacter pylori and OLP, warranting further research.
Methods
PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI), and Web of Science databases were thoroughly searched for relevant articles published from inception until May 23, 2023.
Results
Due to high heterogeneity among the included studies (Tau2 = 2.16; = 40.33, df = 6; I2 = 85 %), we employed a random-effects model (REM). The forest plot revealed a significant correlation between H. pylori infection and OLP, with an odds ratio (OR) of 4.69 (95 % CI: 1.36 to 16.19; P < 0.01). Sensitivity analysis showed that the pooled ORs ranged from 3.69 (95 % CI: 1.01 to 13.44; P = 0.05) to 6.77 (95 % CI: 2.65–17.30; P < 0.001), and no single study significantly influenced the results when removed individually. Additionally, subgroup analysis was conducted to investigate the relationship between H. pylori infection and OLP and explore the sources of heterogeneity. Finally, Begg's test (P = 0.24) and Egger's test (P = 0.35) were performed on the included studies, and the results indicated no significant publication bias.
Conclusion
Our meta-analysis suggests a close association between H. pylori infection and OLP. Nevertheless, further research is warranted to validate these results in the future.
1. Background
Oral lichen planus (OLP) is a T cell-mediated autoimmune disease that affects the oral mucosa [1]. Although it has been established that immune, psychological stress and inflammatory factors play crucial roles in its development, the potential involvement of microbial agents and viral infections in the malignant transformation of OLP remains an area of interest and investigation [1]. The incidence of OLP varies, ranging from 0.5 % to 2 % of the population, with variations influenced by the geographic location, and it tends to be more common in middle-aged women [2]. The World Health Organization has also recognized OLP as an oral potentially malignant disorder (OPMD) [3]. Clinically, OLP can be classified into three forms: reticular, atrophic, and erosive [4]. Furthermore, it can be categorized as non-erosive or erosive, with the latter being considered a risk factor for OLP to turn malignant [5]. OLP typically follows a persistent course, and various conditions, including HCV infection and Sjogren's syndrome, have been linked to OLP, with the most severe being the development of oral squamous cell carcinoma [5].
Indeed, understanding the potential role of microbial agents and virus infection in the malignant transformation of OLP is crucial. The connection between microbial agents and viral infections with OLP arises from several findings. It is widely thought that candida over-infection, frequently observed in OLP lesions, may actively contribute to carcinogenesis by producing carcinogens and inducing chronic inflammation. HPV infection has also been detected at a higher rate in OLP cases, potentially through the activation of oncogene proteins and the deactivation of oncosuppressor genes. Additionally, associations have been found between OLP and HCV, EBV, and HSV-1 infections, as well as H. pylori infection. Candida over-infection can actively contribute to carcinogenesis by infecting OLP lesions during initial diagnosis and chronic immunosuppressive therapy [6]. Oral candidiasis can not only mask or worsen the clinical characteristics of OLP but may also give rise to symptoms like oral burning or pain linked to OLP [6]. It has been shown that candida can produce carcinogens like N-nitrosamine and induce chronic inflammation that may increase the risk of neoplastic progression [7]. Candida is also a common side effect of corticosteroid therapy. Besides, it has been reported that the rate of HPV detection in OLP was twice as high as in normal oral mucosa. In this respect, HPV DNA was detected in 11 % of the 1929 normal oral mucosa samples tested [8] through scrapings or biopsies [8]. The correlation between the activation of oncogene proteins and the deactivation of oncosuppressor genes, induction of cyclins, prevention of apoptosis, and resistance to cellular senescence is widely acknowledged as the main factor contributing to high-risk HPV infections. It is widely thought that there is a synergistic relationship between HPV16/18 and HSP90 in OLP, which may affect the biological behavior of OLP. Besides, HSP90 proteins participate in the malignant transformation of OLP [9]. It also should be noted the possible relationship between HCV infection and OLP. In 1991, it was initially reported that HCV could be linked to OLP, a mere two years following the discovery of HCV [10]. On the other hand, OLP cases possibly related to HCV were published in 1994 [11]. A meta-analysis indicated the association between HCV infection and OLP and emphasized the importance of HCV screening in OLP patients [12]. Importantly, EBV [13] and HSV-1 infections [14] have been associated with OLP. But, he association between H. pylori infection and OLP was insufficiency. During the past years, studies have reported the association between H. pylori infection and OLP. A study documented the DNA of H. pylori in 23 % of patients with OLP [15]. Over the years, many studies have suggested the possibility of oral cavities serving as reservoirs for H. pylori [16]. This bacterium has been found in both subgingival and supragingival plaques and in saliva. In contrast, a study did not detect the presence of H. pylori in any of the OLP cases [16]. Given the potential role of microbial agents and viral infections in the malignant transformation of OLP, conducting further research to fully understand these associations becomes crucial. Therefore, the current study aims to investigate the potential association between H. pylori infection and oral lichen planus. By conducting this study, novel insights can be gained regarding the potential role of H. pylori and its relationship with OLP. This research will deepen our understanding of the pathogenesis of OLP and contribute to the development of preventive and therapeutic strategies for this patient population.
2. Methods
2.1. Search strategy
PubMed, Embase, Chinese National Knowledge Infrastructure (CNKI), and Web of Science databases were thoroughly searched for relevant articles from inception up to May 23, 2023, with the following search words: "Helicobacter pylori", "H. pylori", "OLP", "oral lichen planus", "lichen planus", and "lichen planus, oral". The individual search results were combined using relevant Boolean operators ("OR" and "AND") to obtain a final set of studies. The search strategy was designed and implemented by a medical librarian. Prior to conducting the search, the search terms were peer-reviewed by another medical librarian based on the PRESS criteria. Search results were restricted to human studies and the English language. Regarding cases where chosen study arms or patient subgroups within a study were eligible, the article was included in the analysis. This meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.2. Study selection
Two investigators screened all titles and abstracts using the eligibility criteria according to the PICO (population, intervention, comparator, and outcome) framework. Publications that met the inclusion criteria in the title/abstract phase were retrieved, and the same investigators assessed their eligibility based on their corresponding full-text publication(s). During this process, relevant systematic reviews were identified and used to cross-reference the search strategy to identify any missed publications. In case of disagreements between the investigators, a third reviewer provided arbitration. The aim was to ensure a comprehensive evaluation of all eligible studies. Additional related references cited in the retrieved articles were also explored.
2.3. Inclusion and data extraction
This study had no national limitations. The inclusion criteria were as follows: (1) Case-control studies, cohort studies, and cross-sectional studies that investigated the association between OLP and H. pylori infection; (2) The case group included patients with well-defined OLP who had not used antibiotics, proton pump inhibitors, or antacids in the preceding 2 weeks, had not used corticosteroids in the last month, had no significant concurrent oral diseases, no involvement of other body parts with OLP, no severe heart, kidney, liver, or immune system disorders, no mental abnormalities, and were not pregnant or lactating; (3) The control group consisted of healthy individuals who did not have OLP or any other diseases. Articles and studies meeting any of the following criteria were excluded from this study: (1) Case-control studies that lacked blinding; (2) Studies without a control group; in cases of duplicate publications, the one with the largest sample size was retained; (3) Research with incomplete data or where necessary data could not be inferred from the provided information; (4) Reviews, letters, comments, or social commentary literature, and so on; (5) Literature that does not meet the inclusion criteria is excluded. The following data were extracted from the included articles: first author's name, publication year, sample size, country, and age.
2.4. Risk of bias assessment and subgroup analysis
The Newcastle-Ottawa Scale (NOS), which assesses three quality parameters, namely selection, comparability, and outcomes, was utilized to evaluate the quality of each study. The scale comprises eight specific items and provides final scores that range from poor quality (0) to high quality (9). Studies with a final score below 5 points were considered at high risk of bias. Subgroup analysis was conducted to manage the clinical heterogeneity based on the following categories: Metabolic Syndrome (MetS) subgroup, along with the China subgroup, Iran subgroup, Hp-IgG subgroup, 13C-UBT kit subgroup, Age >47 years in OLP subgroup, and Number <40 in OLP subgroup.
2.5. Statistical analysis
Review Manager 5.3 software was utilized for conducting the meta-analysis, and the quality of each study was assessed using the Newcastle-Ottawa scale. Since all study outcomes were dichotomous, odds ratios (OR) with 95 % confidence intervals (CI) were employed. To systematically manage and organize the selected articles from each database, we imported them into EndNote X20 (Clarivate Analytics, USA) to facilitate referencing in a scientific and orderly manner. Heterogeneity was assessed using the I2 index, where I2 values of 75–100 %, 50–75 %, 25–50 %, and less than 25 % indicated high, medium, moderate, and low heterogeneity levels and homogeneity, respectively. A Cochrane Q statistic with a p-value less than 0.10 was considered statistically significant. If the I2 value exceeded 50 %, the random-effects model (REM) was applied; conversely, if the I2 value was below 50 %, the fixed-effects model (FEM) was used. The OR was employed to assess the relationship between OLP and H. pylori infection. Sensitivity analysis was performed by iteratively excluding individual studies to assess their impact on the overall analysis. To evaluate publication bias, Begg's and Egger's tests, along with funnel plots of hazard ratios against standard error, were employed. The funnel plot visually displayed biases and study effects, with each point on the plot representing the log odds ratio from each study and dashed lines indicating the expected 95 % confidence intervals around the summary estimate. In the meta-analysis, all p-values were two-sided, and a significance level of less than 0.05 was considered statistically significant.
3. Results
3.1. Article features
Out of forty initially identified articles, thirty-three remained after duplicates were excluded. Among these, thirteen studies were further excluded during the screening process, leaving twenty articles for full-text review. After this comprehensive assessment, eleven articles were excluded, resulting in the inclusion of nine articles [[15], [16], [17], [18], [19], [20], [21], [22], [23]] for the meta-analysis. Fig. 1 provides a visual representation of the literature retrieval process. Table 1 summarizes the main characteristics of the nine selected studies on oral lichen planus, spanning from 2000 to 2021. These studies encompassed 314 OLP patients and 273 control patients. Notably, two studies [15,19] employed PCR for detecting H. pylori infection, three studies [18,22,23] used Hp-IgG, two studies [17,20] utilized the 13C-UBT kit, and two studies [16,21] applied immunohistochemistry for the same purpose. Four studies were conducted in China [17,[21], [22], [23]], two in Iran [18,20], and two in Europe [15,19]. All of them were case-control studies, with participants in the OLP group being over 40 years old. The Newcastle-Ottawa Scale scores for all articles were 5 or higher, indicating their high quality.
Fig. 1.
Selection process for studies included in the meta-analysis.
Table 1.
Characters of the included studies.
| Author, year | Country | OLP |
Control |
Test method | Age (years)a |
NOS |
Inclusion criteria | |||
|---|---|---|---|---|---|---|---|---|---|---|
| H. pylori positive | N (Total) | H. pylori positive | N (Total) | OLP | Control | |||||
| Kazanowska-Dygdała, 2016 [15] | Poland | 17 | 70 | 0 | 40 | PCR | 53.4 | 51.1 | 6 | OLP group without any gastrointestinal problems. According to the clinical classification of OLP by the modified WHO |
| Taghavi, 2010 [20] | Iran | 24 | 30 | 20 | 30 | 13C-UBT kit | 40 ± 12 | 37 ± 12 | 6 | Patients with history of using antibiotics, H2 inhibitor agents or Omeperazole during the past 15 days, and bismuth during the past 1 month, and patients with concurrent oral and skin lichen planus lesions were excluded from this study. |
| Pourshahidi, 2012 [18] | Iran | 21 | 41 | 54 | 82 | Hp-IgG | 45.17 ± 14.61 | 41.18 ± 11.52 | 5 | OLP was diagnosed based on clinical examination (Jontell and Holmstrup, 2008) |
| Hulimavu, 2014 [16] | India | 0 | 50 | 0 | 10 | Immunohistochemistry | 42.89 ± 15.79 | NA | 7 | Endoscopic biopsies of patients with peptic ulcer as positive controls |
| Riggio, 2000 [19] | Scotland | 0 | 20 | 0 | 13 | PCR | 45 | 43.2 | 6 | OLP samples from the buccal and labial mucosa and floor of the oral cavity, and normal tissue from gingiva during removal of wisdom teeth. |
| Li, 2021 [17] | China | 21 | 30 | 11 | 21 | 13C-UBT kit | 46.71 ± 2.618 | 48.30 ± 3.211 | 8 | According to the clinical classification of OLP by the WHO (2005) |
| Qiu, 2015 [22] | China | 39 | 60 | 7 | 60 | Hp-IgG | 49 | NA | 7 | Suffering from severe infectious or systemic diseases was excluded. |
| Yao, 2014 [21] | China | 11 | 23 | 0 | 10 | Immunohistochemistry | 47 | NA | 5 | According to the clinical classification of OLP by the WHO |
| Lan, 2017 [23] | China | 40 | 60 | 4 | 30 | Hp-IgG | 54.53 ± 6.58 | 54.53 ± 6.58 | 7 | Pathological diagnosis |
NA, not applicable; NOS, Newcastle-Ottawa Scale.
Data expressed by Mean or Mean ± SD.
3.2. Pooled analysis
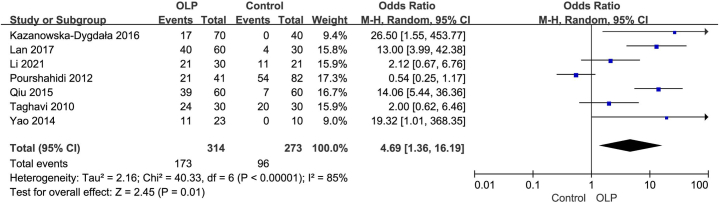
As two studies [16,19] did not yield any H. pylori-positive cases in both the OLP and control groups, they were excluded from the pooled meta-analysis. Given the observed high heterogeneity among the remaining studies (Tau [2] = 2.16; Chi [2] = 40.33, df = 6; I2 = 85 %), the REM was employed. The forest plot indicated a significant correlation between H. pylori infection and OLP, with an OR of 4.69 (95 % CI: 1.36 to 16.19; P < 0.01), as shown in Fig. 2. Sensitivity analysis revealed that the pooled ORs ranged from 3.69 (95 % CI: 1.01 to 13.44; P = 0.05) to 6.77 (95 % CI: 2.65–17.30; P < 0.001), with no single study exerting undue influence when removed individually. Furthermore, subgroup analysis was performed to investigate the relationship between H. pylori infection and OLP and identify sources of heterogeneity. Additionally, Begg's test (P = 0.24) and Egger's test (P = 0.35) were conducted among the included studies, and the funnel plot displayed a symmetric distribution, suggesting no publication bias, as depicted in Fig. 3.
Fig. 2.
The forest plot outcome indicated that H. pylori infection was closely correlated with the OLP.
Fig. 3.
The funnel plot showed a symmetric distribution of included studies.
3.3. Subgroup analysis
China subgroup: Within the China subgroup, moderate heterogeneity was observed among the studies (Tau [2] = 0.63; Chi [2] = 7.39, df = 3; I2 = 59 %). Using the REM, the OR was determined to be 8.21 (95 % CI: 2.90 to 23.26; P < 0.001). Interestingly, removing Li's study reduced the I2 from 59 % to 0 %, indicating that Li's study may have been the source of the heterogeneity(Fig. 4).
Fig. 4.
The forest plot outcome of China subgroup.
Iran subgroup: Heterogeneity among the studies in the Iran subgroup was moderate (Tau [2] = 0.03; Chi [2] = 3.67, df = 1; I2 = 73 %). Utilizing the REM, the analysis yielded an OR of −0.01 (95 % CI: −0.29 to 0.26; P = 0.92), indicating no significant difference between OLP and the control group (Fig. 5).
Fig. 5.
The forest plot outcome of Iran subgroup.
Hp-IgG subgroup: In this subgroup, high heterogeneity was observed (Chi [2] = 35.29, df = 2; I2 = 94 %). The REM was employed, resulting in an OR of 4.52 (95 % CI: 0.45 to 45.44; P = 0.2). When Pourshahidi's study was removed, the I2 decreased from 94 % to 0 %, suggesting that Pourshahidi's study may have contributed to the observed heterogeneity (Fig. 6).
Fig. 6.
The forest plot outcome of Hp-IgG subgroup.
13C-UBT kit subgroup: No heterogeneity was detected among the studies in this subgroup (Chi [2] = 0, df = 1; I2 = 0 %). Consequently, the FEM was applied, yielding an OR of 2.06 (95 % CI: 0.90 to 4.70; P = 0.09). This suggests that the 13C-UBT kit subgroup may not have been a source of heterogeneity (Fig. 7).
Fig. 7.
The forest plot outcome of 13C-UBT kit subgroup.
Age >47 years in OLP subgroup: No heterogeneity was observed among the studies in this subgroup (Chi [2] = 0.27, df = 3; I2 = 0 %). The FEM was applied, and the OR was 15.23 (95 % CI: 7.47 to 31.05; P < 0.001). This finding suggests that the age >47 years in the OLP subgroup may not have contributed to the observed heterogeneity (Fig. 8).
Fig. 8.
The forest plot outcome of Age >47 years in OLP subgroup.
Number < 40 in OLP subgroup: Similarly, there was no heterogeneity among the studies in this subgroup (Chi [2] = 2.19, df = 2; I2 = 9 %). The FEM was used, yielding an OR of 2.81 (95 % CI: 1.31 to 6.00; P = 0.008), indicating that the number <40 in the OLP subgroup may not have been the source of heterogeneity (Fig. 9).
Fig. 9.
The forest plot outcome of Number<40 in OLP subgroup.
NOS score >5 subgroup: Within this subgroup, moderate heterogeneity was present (Chi [2] = 12.37, df = 4; I2 = 68 %). The REM was applied, and the OR was 6.24 (95 % CI: 2.28 to 17.12; P = 0.0004) (Fig. 10).
Fig. 10.
The forest plot outcome of NOS score > 5 subgroup.
4. Discussion
Our meta-analysis revealed a strong association between H. pylori infection and OLP, highlighting the need to initiate early interventions for patients who test positive for H. pylori. Besides, we acknowledge that the heterogeneity among the studies included in our analysis was substantial. To address this, we conducted subgroup analyses to identify potential sources of heterogeneity. Notably, sensitivity analysis demonstrated that the overall findings remained consistent when individual studies were excluded or when switching between random-effects and fixed-effects models, emphasizing the reliability of our study results.
H. Pylori is typically an opportunistic pathogen with no pathogenic effects under normal conditions. However, it can lead to disease when the host's resistance is compromised or the immune system is weakened. The precise pathogenesis of OLP triggered by H. pylori remains unclear. Some researchers suggest that the primary mode of H. pylori transmission is oral. Even after H. pylori has been eradicated from the gastric tract, dental plaque and saliva may serve as potential sources of reinfection [24]. H. pylori can directly damage oral mucosal cells, induce the release of cytokines and oxygen free radicals, inflame oral soft tissue, and accelerate programmed cell death compared to cell proliferation [25]. This imbalance in the oral bacterial ecosystem can lead to a proliferation of H. pylori. Roughly 50–70 % of H. pylori strains produce two cytotoxins, VacA, which causes vacuolation and degeneration of gastric mucosa epithelial cells, and CagA, a surface protein linked to increased colonization of gastroduodenal mucosa and the advancement of gastroduodenal diseases may trigger a definite T-lymphocyte response and could be a potential antigen candidate for the development of a vaccine. Given that the oral mucosa and gastric mucosa are closely related in anatomy and physiology, and their structure, function, they exhibit physiology and pathology have many similarities, they may have common antigen components. The pathogenesis of OLP is believed to be related to the local immune response mediated by T cells [26], but the reasons for the local immune response are still unclear. The anti oral mucosal antibody that causes oral mucosal damage interacts with the anti gastric mucosal antibody that causes gastric mucosal damage and has partial cross immunity [27], so it is believed that when gastric mucosa is infected with H. pylori and H. pylori antigen can stimulate itself to produce antibodies, and then attack oral mucosal tissue with the same antigen to make it pathological [28].
Our study revealed significant heterogeneity among the included studies, which led us to perform subgroup analyses to identify the potential sources. One crucial factor contributing to heterogeneity is the variability in methods used to detect H. pylori infection across different studies. For instance, we found that the subgroup using the 13C-UBT kit appeared to be a source of heterogeneity. While the urea breath test (UBT) is considered the best noninvasive diagnostic tool for detecting H. pylori due to its high accuracy and ease of use, it may yield false-positive or false-negative results due to urease activity inhibition. For reliable results, using a stool antigen test (SAT) with bioluminescent enzyme immunoassay is highly recommended due to its exceptional sensitivity [29]. Similarly, enzyme immunoassays based on polyclonal antibodies (HpSA) offer a valid method for detecting H. pylori antigens in stool specimens [30]. Serological testing methods can reflect an individual's H. pylori infection status over an extended period and are not influenced by recent medication or localized gastric lesions. However, it should be noted that while positive H. pylori antibodies indicate prior exposure, they cannot definitively confirm a current infection, and the absence of H. pylori antibodies doesn't rule out the possibility of an initial infection [31]. It is now understood that various factors contribute to the variability in the Polymerase Chain Reaction (PCR) method when detecting H. pylori. Although PCR is considered highly accurate, it is not reliable due to the potential for false-positive results, which can stem from identifying cDNA from organisms other than H. pylori [32]. PCR tests for H. pylori can exhibit a wide range of cross-reactivity, sometimes leading to positive results in the presence of other microorganisms sharing similar sequences [33]. H. pylori can be detected using diverse methods, including immunohistochemistry (IHC). Notably, in a study by Ito et al., the detection of H. pylori DNA in histologic sections was achieved using reverse transcriptase PCR, with results compared to those obtained via IHC. The study demonstrated that while IHC displayed specificity, it was less sensitive than PCR [34]. Additionally, using different reagents with varying sensitivities and the scarcity of research cases can influence the overall results. Furthermore, age and the number of samples contribute to the observed heterogeneity. Existing evidence from a meta-analysis suggests that the prevalence of OLP significantly and progressively increases from the age of 40, possibly linked to racial differences. However, due to the limited number of studies in our analysis, it is premature to dismiss the relationship between H. pylori infection and the occurrence of OLP in different countries. Further comprehensive research is required to yield higher-quality evidence. H. pylori infection can cause oral diseases. Research has found a correlation between H. pylori infection and an increased risk of oral cancer, and it is necessary to conduct large-scale studies to confirm this, as well as early detection and clearance of H. pylori. H. pylori infection as an essential etiological factor in the development of recurrent aphthous stomatitis and other oral mucosal lesions with ulcations. Our study found that the association between H. pylori and OLP can provide a basis for developing strategies to prevent OLP. For example, changing dietary habits or treating H. pylori infection in the stomach may reduce the risk of developing OLP. Our study marks the first attempt to validate the association between H. pylori infection and OLP through a meta-analysis. The potential link between these conditions suggests a need for heightened awareness among healthcare providers regarding the role of H. pylori in the pathogenesis or exacerbation of OLP. This awareness could lead to improved diagnostic strategies, including the consideration of H. pylori testing in patients with OLP to guide treatment decisions. Implementing targeted screening protocols and incorporating H. pylori treatment into the management plan for affected patients may help improve clinical outcomes and quality of care. However, certain limitations must be considered. Firstly, the insufficient data prevented the exploration of clinicopathological factors. Secondly, the different susceptibilities and measurement methods for H. pylori could introduce bias into the results. Finally, the relatively small number of articles and patients included in the analysis precluded precise subgroup and meta-regression analyses to identify the sources of heterogeneity. Heterogeneity among studies, which may impact the interpretation of results and the generalizability of findings. Therefore, further large-scale research is warranted to strengthen these findings.
5. Conclusion
Our meta-analysis highlights a robust correlation between H. pylori infection and OLP. The results of subgroup analysis are also valid. Nonetheless, it is crucial to emphasize the need for further research to validate these results. Future studies should concentrate on delving deeper into this association to provide more definitive evidence, potentially leading to enhanced prevention and treatment strategies. Identifying and addressing H. pylori infections in patients with OLP may not only alleviate the symptoms of OLP but also contribute to the overall management and prognosis of the condition. Thus, it underscores the significance of considering H. pylori infection as a potential contributing factor in patients with OLP, emphasizing the necessity for collaboration between gastroenterologists and oral medicine specialists in diagnosing and managing this patient population.
Ethics approval and consent to participate
This study not need institutional review board or ethics committee approval.
Data availability statement
No data was used for the research described in the article.
Funding
The research was funding by Science Fund for Distinguished Young Scholars of Hubei (No.2024AFA097), National Natural Science Foundation of China (No.82370972), and the Science Foundation of Union Hospital (No. 2021xhyn090).
CRediT authorship contribution statement
Zaiyu Zhang: Writing – review & editing, Writing – original draft, Software, Methodology. Xiaohui Yi: Investigation, Conceptualization. Yumei Ding: Software, Resources, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
References
- 1.Fu H., Yang J., Shen Z., Zhang Y., Kuang S., Li L., Lin Z., Shi X. Antibacterial, wet adhesive, and healing-promoting nanosheets for the treatment of oral ulcers. Biomater. Sci. 2023 May 2;11(9):3214–3226. doi: 10.1039/d2bm02063g. [DOI] [PubMed] [Google Scholar]
- 2.Bardellini E., Amadori F., Flocchini P., Bonadeo S., Majorana A. Clinicopathological features and malignant transformation of oral lichen planus: a 12-years retrospective study. Acta Odontol. Scand. 2013 May-Jul;71(3–4):834–840. doi: 10.3109/00016357.2012.734407. [DOI] [PubMed] [Google Scholar]
- 3.El-Howati A., Thornhill M.H., Colley H.E., Murdoch C. Immune mechanisms in oral lichen planus. Oral Dis. 2023 May;29(4):1400–1415. doi: 10.1111/odi.14142. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G., Zhang J., Ren X.W., Hu J.Y., Du G.F., Xu X.Y. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J. Clin. Immunol. 2012 Aug;32(4):794–801. doi: 10.1007/s10875-012-9683-2. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani M., Troiano G., Cordaro M., Corsalini M., Gioco G., Lo Muzio L., Pignatelli P., Lajolo C. Rate of malignant transformation of oral lichen planus: a systematic review. Oral Dis. 2019 Apr;25(3):693–709. doi: 10.1111/odi.12885. [DOI] [PubMed] [Google Scholar]
- 6.Bombeccari G.P., Giannì A.B., Spadari F. Oral Candida colonization and oral lichen planus. Oral Dis. 2017 Oct;23(7):1009–1010. doi: 10.1111/odi.12681. [DOI] [PubMed] [Google Scholar]
- 7.Krogh P., Hald B., Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis. 1987 Oct;8(10):1543–1548. doi: 10.1093/carcin/8.10.1543. [DOI] [PubMed] [Google Scholar]
- 8.Syrjänen S.M. Basic concepts and practical applications of recombinant DNA techniques in detection of human papillomavirus (HPV) infection. Review article. APMIS. 1990 Feb;98(2):95–110. doi: 10.1111/j.1699-0463.1990.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 9.Bar J.K., Cierpikowski P., Lis-Nawara A., Duc P., Hałoń A., Radwan-Oczko M. Comparison of p53, HSP90, E-cadherin and HPV in oral lichen planus and oral squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2021 Dec;41(6):514–522. doi: 10.14639/0392-100X-N1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokni M., Rybojad M., Puppin D., Jr., et al. Lichen planus and hepatitis C virus. J. Am. Acad. Dermatol. 1991;24:792. doi: 10.1016/s0190-9622(08)80376-3. [DOI] [PubMed] [Google Scholar]
- 11.Gandolfo S., Carbone M., Carrozzo M., Gallo V. Oral lichen planus and hepatitis C virus (HCV) infection: is there a relationship? A report of 10 cases. J. Oral Pathol. Med. 1994;23:119–122. doi: 10.1111/j.1600-0714.1994.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 12.Alaizari N.A., Al-Maweri S.A., Al-Shamiri H.M., Tarakji B., Shugaa-Addin B. Hepatitis C virus infections in oral lichen planus: a systematic review and meta-analysis. Aust. Dent. J. 2016 Sep;61(3):282–287. doi: 10.1111/adj.12382. [DOI] [PubMed] [Google Scholar]
- 13.Raybaud H., Olivieri C.V., Lupi-Pegurier L., Pagnotta S., Marsault R., Cardot-Leccia N., Doglio A. Epstein-barr virus–infected plasma cells infiltrate erosive oral lichen planus. J. Dent. Res. 2018;97:1494–1500. doi: 10.1177/0022034518788282. [DOI] [PubMed] [Google Scholar]
- 14.Cox M., Maitlan N., Scully C. Human herpes simplex-1 and papillomavirus type 16 homologous DNA sequences in normal, potentially malignant and malignant oral mucosa. Eur. J. Cancer B Oral Oncol. 1993;29:215–219. doi: 10.1016/0964-1955(93)90025-a. [DOI] [PubMed] [Google Scholar]
- 15.Kazanowska-Dygdała M., Duś I., Radwan-Oczko M. The presence of Helicobacter pylori in oral cavities of patients with leukoplakia and oral lichen planus. J. Appl. Oral Sci. 2016 Jan-Feb;24(1):18–23. doi: 10.1590/1678-775720150203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hulimavu S.R., Mohanty L., Tondikulam N.V., Shenoy S., Jamadar S., Bhadranna A. No evidence for Helicobacter pylori in oral lichen planus. J. Oral Pathol. Med. 2014 Sep;43(8):576–578. doi: 10.1111/jop.12194. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Zhang Y., Yang Z., Li J., Li Y., Li H., Li W., Jia J., Ge S., Sun Y. Helicobacter pylori infection is correlated with the incidence of erosive oral lichen planus and the alteration of the oral microbiome composition. BMC Microbiol. 2021 Apr 20;21(1):122. doi: 10.1186/s12866-021-02188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pourshahidi S., Fakhri F., Ebrahimi H., Fakhraei B., Alipour A., Ghapanchi J., Farjadian S. Lack of association between Helicobacter pylori infection and oral lichen planus. Asian Pac J Cancer Prev. 2012;13(5):1745–1747. doi: 10.7314/apjcp.2012.13.5.1745. [DOI] [PubMed] [Google Scholar]
- 19.Riggio M.P., Lennon A., Wray D. Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J. Oral Pathol. Med. 2000 Nov;29(10):507–513. doi: 10.1034/j.1600-0714.2000.291005.x. [DOI] [PubMed] [Google Scholar]
- 20.Taghavi Zenouz A., Mehdipour M., Jafari Heydarlou M., Gholizadeh N. Relationship between lichen planus and Helicobacter pylori infection. J. Dent. Res. Dent. Clin. Dent. Prospects. 2010 Winter;4(1):17–20. doi: 10.5681/joddd.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiaowu Yao, Lin Minxiao, Chen Shisheng, et al. Preliminary exploration of the correlation between Helicobacter pylori infection and oral lichen planus and squamous cell carcinoma. Chinese Journal of Stomatological Research (Electronic Edition) 2014;8(5):364–369. [Google Scholar]
- 22.Qiu Yongle, Jia Nan, Shi Tingting, et al. Study on the relationship between Helicobacter pylori and its related toxins and oral lichen planus. Beijing Stomatology. 2015;23(6):309–311. [Google Scholar]
- 23.Lan Yulian. A study on the relationship between Helicobacter pylori infection, anxiety and depression, and the onset and carcinogenesis of oral lichen planus. Journal of Hunan Normal University (Medical Edition) 2017;14(3):48–51. [Google Scholar]
- 24.Al Asqah M., Al Hamoudi N., Anil S., Al Jebreen A., Al-Hamoudi W.K. Is the presence of Helicobacter pylori in dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can. J. Gastroenterol. 2009;23:177–179. doi: 10.1155/2009/950527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albanidou-Farmaki E., Giannoulis L., Markopoulos A., Fotiades S., Aggouridaki X., Farmakis K., Papanayotou P. Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis. 2005 Jan;11(1):22–26. doi: 10.1111/j.1601-0825.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M., Wang L., Zhou C., Wang J., Cheng J., Fan Y.E. Coli LPS/TLR4/NF-κB signaling pathway regulates Th17/treg balance mediating inflammatory responses in oral lichen planus. Inflammation. 2023 Jun;46(3):1077–1090. doi: 10.1007/s10753-023-01793-7. [DOI] [PubMed] [Google Scholar]
- 27.Mager D.L. Bacteria and cancer: cause, coincidence or cure? A review. J. Transl. Med. 2006 Mar 28;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haziri A., Juniku-Shkololli A., Gashi Z., Berisha D., Haziri A. Helicobacter pylori infection and precancerous lesions of the stomach. Med. Arh. 2010;64(4):248–249. [PubMed] [Google Scholar]
- 29.Kajihara Y., Shimoyama T., Mizuki I. Evaluation of the effects of a proton pump inhibitor on Helicobacter pylori stool antigen testing. Helicobacter. 2023 Jun;28(3) doi: 10.1111/hel.12961. [DOI] [PubMed] [Google Scholar]
- 30.Manes G., Zanetti M.V., Piccirillo M.M., Lombardi G., Balzano A., Pieramico O. Accuracy of a new monoclonal stool antigen test in post-eradication assessment of Helicobacter pylori infection: comparison with the polyclonal stool antigen test and urea breath test. Dig. Liver Dis. 2005 Oct;37(10):751–755. doi: 10.1016/j.dld.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Hu J., Mei H., Su N.Y., Sun W.J., Zhang D.K., Fan L.L., He P., Pan J., Wang X.W., Zou P.Y., Liu Y.X., Guo Y., Lan C.H. Eradication rates of Helicobacter pylori in treatment-naive patients following 14-day vonoprazan-amoxicillin dual therapy: a multicenter randomized controlled trial in China. Helicobacter. 2023 May 9 doi: 10.1111/hel.12970. [DOI] [PubMed] [Google Scholar]
- 32.Dowsett S.A., Kowolik M.J. Oral Helicobacter pylori: can we stomach it? Crit. Rev. Oral Biol. Med. 2003;14 doi: 10.1177/154411130301400307. 226-33. Can we stomach it? Crit Rev Oral Biol Med . 2003;14: Can we stomach it? Crit Rev Oral Biol Med 2003;14. [DOI] [PubMed] [Google Scholar]
- 33.El-Zaatari F.A., Nguyen A.M., Genta R.M., Klein P.D., Graham D.Y., El-Zaatari F.A., Nguyen A.M., Genta R.M., Klein P.D. Graham DYDetermination of Helicobacter pylori status by reverse transcription-polymerase chain reactionComparison with urea breathe test. Dig. Dis. Sci. 1995;40:109–113. doi: 10.1007/BF02063952. [DOI] [PubMed] [Google Scholar]
- 34.Ito T., Kobayashi D., Uchida K., Takemura T., Nagaoka S., Kobayashi I., et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab. Invest. 2008;88:664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.