Abstract
Background
Type 1 diabetes mellitus (T1DM) may be associated with various autoimmune diseases, but the causal relationship between T1DM and autoimmune skin diseases is not yet clear.
Methods
The summary statistical data on T1DM and nine autoimmune skin diseases in European populations were extracted for mendelian randomization (MR) analysis. Subsequently, the analysis was replicated in East Asian populations. In the MR estimation, inverse variance-weighted (IVW), MR-Egger, weighted median, simple mode, and weighted mode methods were utilized. Outliers were excluded using MR-PRESSO, and horizontal pleiotropy was assessed with MR-Egger. Additionally, a multivariable MR analysis was conducted to investigate whether T1DM has an independent effect on autoimmune skin diseases after adjusting for potential confounders.
Results
In Europe, the MR estimated based on IVW method indicated a causal association between genetically determined T1DM and systemic lupus erythematosus (SLE) (OR = 1.38, 95%CI: 1.26−1.50, p<0.01), rheumatoid arthritis (RA) (OR = 1.15, 95%CI: 1.05−1.25, p<0.01), as well as multiple sclerosis (MS) (OR = 1.17, 95%CI: 1.01−1.36, p = 0.04), but there is no association between T1DM and atopic dermatitis (AD), vitiligo, lichen planus (LP), hidradenitis suppurativa (HS), alopecia areata (AA) and systemic sclerosis (SS). After adjusting for time spent watching television, body mass index, type 2 diabetes mellitus, and body fat percentage, we found a causal relationship between T1DM and SLE (OR = 1.29, 95%CI: 1.16–1.44, p < 0.01), RA (OR = 1.28, 95%CI: 1.20–1.38 p < 0.01) and MS (OR = 1.11, 95%CI: 1.04–1.18, p < 0.01). Then, no genetic causal association was found between TIDM and SLE, and AD in East Asia. These results didn't exhibit horizontal pleiotropy, and “leave-one-out” analysis demonstrated result stability.
Conclusion
Our MR research indicates a causal relationship between T1DM and SLE, RA, and MS in Europe. However, no causal relationship between T1DM and SLE has been observed in East Asia. Therefore, it is important to regularly monitor relevant immunological markers of SLE, RA, and MS in T1DM patients and take preventive measures.
Keywords: Type 1 diabetes mellitus, Autoimmune skin diseases, Mendelian randomization
1. Introduction
Autoimmune diseases are a series of fatal diseases caused by the loss of immune tolerance, resulting in excessive activation of immune cells to attack healthy tissues and organs, which seriously threaten human health [1]. The skin, as the largest organ in the human body, is impacted by many autoimmune diseases [2,3]. Vitiligo, alopecia areata and pemphigoid are some of the major autoimmune diseases affecting the skin system [4]. Until now, autoimmune skin diseases are diagnosed still based on clinical manifestations and serum specific antibodies or histological biopsy [5]. Unfortunately, lacking specific diagnostic antibodies and biopsy with invasive procedures makes early diagnosis of autoimmune skin diseases difficult [6]. At present, corticosteroids and immunosuppressant are widely used to relieve symptoms of autoimmune skin diseases, but they can bring many serious side effects [7]. With the deepening of drug research, emerging biologics are used for treatment, but the high cost is prohibitive for patients [8]. Therefore, early prevention of autoimmune skin diseases is necessary.
To prevent the occurrence and development of autoimmune skin diseases, scholars have devoted themselves to exploring its risk factors. The association between various autoimmune diseases is receiving increasing attention [9,10]. Type 1 diabetes mellitus (T1DM) a chronic hyperglycemic marked by insufficient insulin secretion, resulting from the autoimmune assault on the insulin-producing beta cells located in the pancreas [11]. A cohort study including 5895 participants found an increased risk of hives in T1DM children [12]. DiConstanzo et al. analyzed the association of psoriasis with TIDM [13,14], However, solid evidence for a causal relationship between T1DM and autoimmune skin diseases is absent due to uncontrolled confounders and reverse causal bias in observational studies.
To analyze the causal association between T1DM and autoimmune skin diseases for prevention and management better, mendelian randomization (MR) was used to clarify this issue and understand the unique effects of T1D on autoimmune skin diseases from a genetic perspective.
2. Methods
2.1. Study design
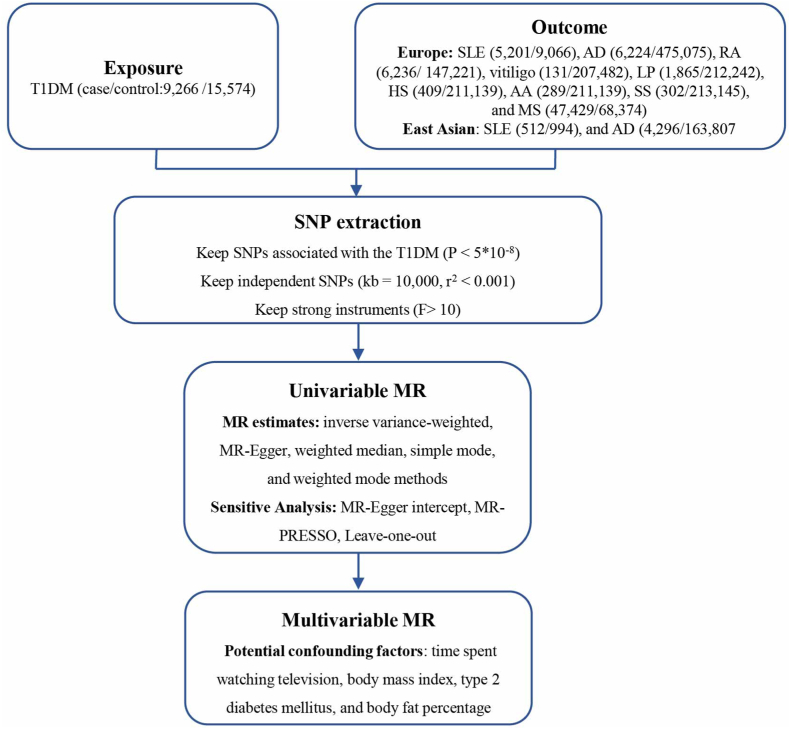
MR is a valuable approach for evaluating causality in observational epidemiology, offering a relatively expedited and less complex alternative to randomized controlled trials while effectively mitigating certain limitations inherent in conventional epidemiological studies. Based on data from genome-wide association analysis studies (GWAS), we conducted this two-sample MR analysis to explore the potential causal effects of T1DM on nine autoimmune skin diseases, including systemic lupus erythematosus (SLE), atopic dermatitis (AD), rheumatoid arthritis (RA), multiple sclerosis (MS), vitiligo, lichen planus (LP), hidradenitis suppurativa (HS), alopecia areata (AA) and systemic sclerosis (SS) (Fig. 1).
Fig. 1.
Study overview. Notes: SLE, systemic lupus erythematosus; AD, atopic dermatitis; RA, rheumatoid arthritis; MS, multiple sclerosis; LP, lichen planus; HS, hidradenitis suppurativa; AA, alopecia areata; SS, systemic sclerosis; MR, mendelian randomization.
Performing MR Analysis must satisfy three fundamental assumptions: (1) the instrumental variables (IVs) need to be strongly linked to T1DM. (2) IVs have no correlation with potential confounder. (3) the IVs don't affect autoimmune skin diseases independently of TIDM.
2.2. The source of exposure and outcome
All information of the summary data used in this study is summarized in Table 1. The study relied on previously published public database, all original studies received ethical approval.
Table 1.
Detailed information for the GWAS data.
| Exposure | GWAS ID | Data Source | Sample Size | Cases/Controls | Population | Year |
|---|---|---|---|---|---|---|
| T1DM |
GCST010681 |
GWAS Catalog |
24840 |
9266/15,574 |
European |
2020 |
|
Outcomes |
GWAS ID |
Data Source |
Sample Size |
Cases/Controls |
Population |
Year |
| SLE | GCST003156 | GWAS Catalog | 14,267 | 5201/9066 | European | 2015 |
| GCST90014238 | GWAS Catalog | 1506 | 512/994 | East Asian | 2021 | |
| AD | GCST90018784 | GWAS Catalog | 481,299 | 6224/475,075 | European | 2021 |
| GCST90018564 | GWAS Catalog | 168,103 | 4296/163,807 | East Asian | 2021 | |
| RA | finn-b-M13_RHEUMA | FinnGen | 153,457 | 6236/147,221 | European | 2021 |
| MS | ieu-b-18 | IEU OpenGWAS | 115,803 | 47,429/68,374 | European | 2019 |
| Vitiligo | finn-b-L12_VITILIGO | FinnGen | 207,613 | 131/207,482 | European | 2021 |
| LP | finn-b-L12_LICHENPLANUS | FinnGen | 214,107 | 1865/212,242 | European | 2021 |
| HS | finn-b-L12_HIDRADENITISSUP | FinnGen | 211,548 | 409/211,139 | European | 2021 |
| AA | finn-b-L12_ALOPECAREATA | FinnGen | 211,428 | 289/211,139 | European | 2021 |
| SS | finn-b-M13_SYSTSLCE | FinnGen | 213,447 | 302/213,145 | European | 2021 |
Notes: SLE, systemic lupus erythematosus; AD, atopic dermatitis; RA, rheumatoid arthritis; MS, multiple sclerosis; LP, lichen planus; HS, hidradenitis suppurativa; AA, alopecia areata; SS, systemic sclerosis.
TIMD The summary statistical data including 9266 cases and 15,574 controls, extracted from the GWAS Catalog (https://www.ebi.ac.uk/gwas/home), was the largest scale and latest GWAS study we researched for T1DM [15].
Autoimmune skin diseases Analyzing the potential association between T1DM and different types of autoimmune skin diseases, including SLE, AD, RA, MS, vitiligo, LP, HS, AA and SS, was the primary aim of this study. To get more comprehensive information, the largest available GWAS study we searched was chosen to conduct MR analysis. To identify the causal relationship in the European population, the summary data of SLE [16] (5,201cases and 9066 controls) and AD [17] (6224 cases and 475,075 controls) were obtained from GWAS Catalog. In addition, the data of RA (6236 cases and 147,221 controls), vitiligo (131 cases and 207,482 controls), LP (1865 cases and 212,242 controls), HS (409 cases and 211,139 controls), AA (289 cases and 211,139 controls) and SS (302 cases and 213,145 controls) were retrieved from FinnGen Biobank [18]. And the dataset of MS (47,429 cases and 68,374 controls) came from IEU OpenGWAS. To explore the causal link in East Asian populations, by searching different databases, the statistical data of SLE [19] (512 cases and 994 controls), and AD [17] (4296 cases and 163,807 controls) were searched from GWAS Catalog.
2.3. The selection of IVs
These SNPs highly correlated with exposure (P < 5 × 10−8) [20] and without linkage disequilibrium (r2 < 0.001 and clump window >10,000 kb) were screened as IVs. In addition, to decrease bias caused by weak IVs, F-statistic of IVs were calculated, and it was considered appropriate only if the F statistic was greater than 10 [21]. Based on the same allele, the data of SNPs-exposure and SNPs-outcome was integrated, and the palindromic SNPs with intermediate allele frequencies were removed.
2.4. Statistical analysis
“MR-PRESSO” R package were used to check outliers, when P value < 0.05, this SNP was outliers and removed. Next, “TwoSampleMR” R package was utilized to perform the MR analysis [22]. To clarify the causal relationship between TIDM and different autoimmune skin diseases, the random effect IVW method, recognized as a relatively reliable method, was used as the main analysis [23]. What's more, MR-Egger, weighted median, simple mode and weighted mode were as additional methods for MR analysis. When a causal relationship was found in the univariable MR analysis, to determine whether the observed significant effect of T1DM on autoimmune skin diseases was a direct or indirect impact, we would perform multivariable MR analysis with IVW approach to evaluate the effect of T1DM on autoimmune skin diseases adjusting for time spent watching television (TV), body mass index (BMI), type 2 diabetes mellitus (T2DM), and body fat percentage (BFP) accounting for the traditional risk factors of the autoimmune skin diseases.
The IVW method was a relatively reliable method to performed MR analysis, and p value < 0.05 usually indicated the existence of a causal relationship. To reduce the probability of false positives, q-value procedure was applied to perform false discovery rate (FDR) correction. The q-value <0.1 was considered to be statistically significant [24], and a suggestive association was considered when P < 0.05 but q ≥ 0.1 [25]. Then, the Cochrane's Q value based on the IVW method was used to evaluate the heterogeneity of the results, p value < 0.05 indicated that there was statistically significant heterogeneity [26]. Horizontal pleiotropy means that the effect of genetic variation on outcomes is not entirely through exposure factors, and it violates the assumptions of IVs independence and exclusivity. Therefore, the MR-Egger method was used to check horizontal pleiotropy, P value > 0.05 indicates no horizontal pleiotropy [27]. In addition, we performed “leave-one-out” test to evaluate the reliability and stability of MR results.
3. Results
3.1. Selection of genetic instrument
After filtrating for high associations and remove linkage imbalance, we obtained forty-four SNPs as potential IVs. The F statistic was calculated based on the formula [28]: , and [28,29], to evaluate the strength of the IVs. Then, we were pleasantly surprised to find that F statistics for 44 SNPs were all more than 10, indicating that all SNPs associated with T1DM were all eligible (Supplementary Table 1).
3.2. The causality of T1DM with autoimmune skin diseases was revealed in Europe
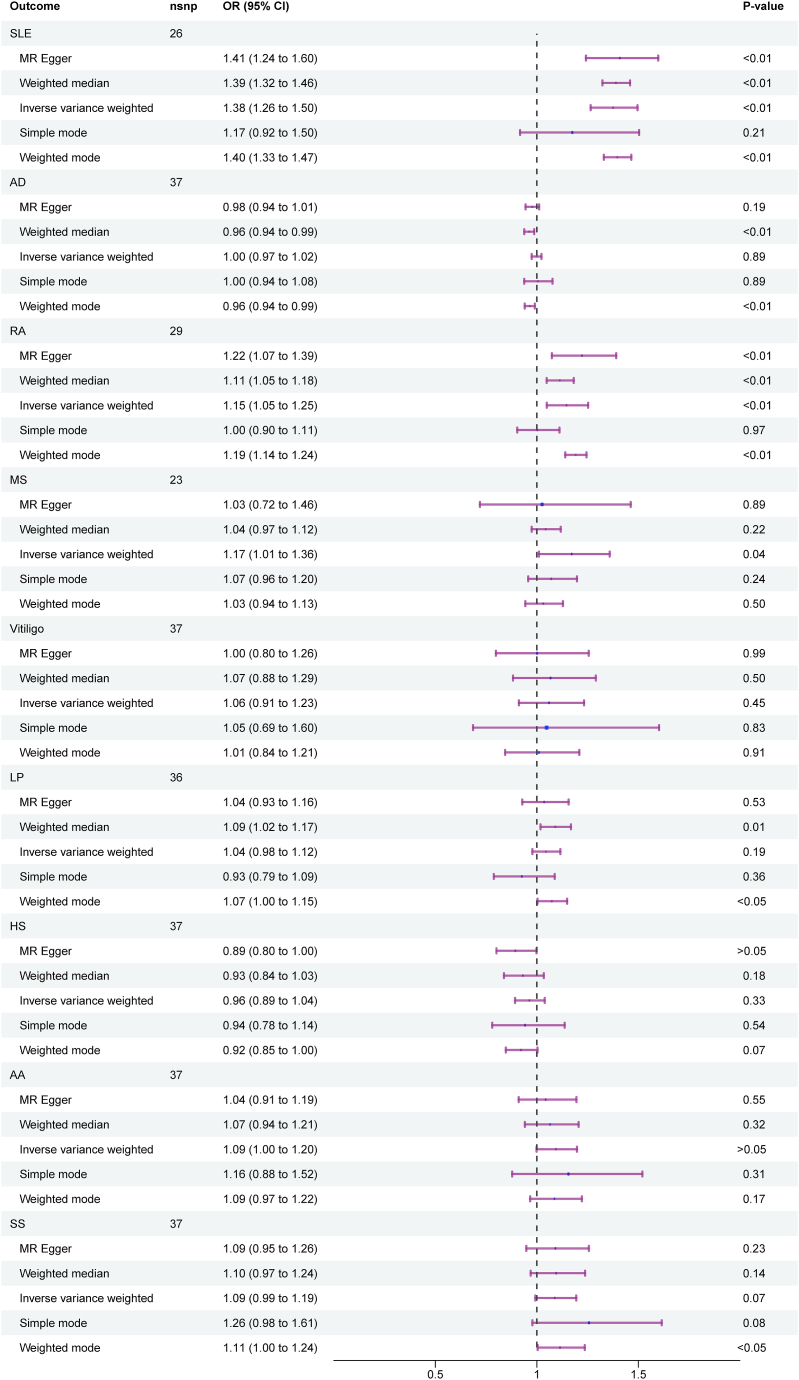
In Europe, the MR estimated based on IVW method indicated a causal association between genetically determined T1DM and SLE (OR = 1.38, 95%CI: 1.26−1.50, p<0.01), RA (OR = 1.15, 95%CI: 1.05−1.25, p<0.01), as well as MS (OR = 1.17, 95%CI: 1.01−1.36, p = 0.04), but there was no association between TIDM and AD (OR = 1.00, 95%CI: 0.97−1.02, p = 0.89), vitiligo (OR = 1.06, 95%CI: 0.91−1.23, p = 0.45), LP (OR = 1.04, 95%CI: 0.98−1.12, p = 0.19), HS (OR = 0.96, 95%CI: 0.89−1.04, p = 0.33), AA (OR = 1.09, 95%CI: 1.00−1.20, p > 0.05), and SS (OR = 1.09, 95%CI: 0.99−1.19, p = 0.07) (Fig. 2). To obtain more rigorous results, we applied FDR correction to our positive analysis. The corrected results still indicated the presence of potential causal links between T1DM and both SLE (q<0.01) and RA(q<0.01), but the connection between T1DM and MS should be interpreted with caution (p = 0.04, q = 0.18).
Fig. 2.
Forest plots between T1DM and autoimmune skin diseases in Europe.
There was no heterogeneity in the MR analysis of T1DM on vitiligo (PIVW = 0.07), HS (PIVW = 0.35), AA (PIVW = 0.32), and SS (PIVW = 0.28), while heterogeneity was observed in the MR analysis of T1DM on SLE (PIVW < 0.01), AD (PIVW < 0.01), RA (PIVW < 0.01), MS (PIVW < 0.01), and LP (PIVW < 0.01) (Table 2). Scatter plots of TIMD and different autoimmune skin diseases risk association for the IVs were showed in Supplementary Fig. 1, in which different colored lines represented the slopes of the different methods analyses. And there was not horizontal pleiotropy in the MR analysis of T1DM on SLE (Intercept = −1.28e−2, PMR-Egger = 0.62), AD (Intercept = 1.21e−2, PMR-Egger = 0.10), RA (Intercept = −3.69e−2, PMR-Egger = 0.20), MS (Intercept = 3.63e−2, PMR-Egger = 0.43), vitiligo (Intercept = 3.26e−2, PMR-Egger = 0.51), LP (Intercept = 3.64e−3, PMR-Egger = 0.85), HS (Intercept = 4.19e−2, PMR-Egger = 0.08), AA (Intercept = 2.67e−2, PMR-Egger = 0.36), and SS (Intercept = −1.49e−3, PMR-Egger = 0.96) (Table 2). Furthermore, the "leave one method" analysis revealed that a single SNP didn't affected our results, indicating that our results were reliable and stable Supplementary Fig. 2.
Table 2.
Heterogeneity test and pleiotropy test of type 1 diabetes mellitus and autoimmune skin diseases.
| Exposure | Outcome | Population | NSNP | Pleiotropy Test (MR-Egger) |
Heterogeneity Test (IVW) |
Outlier Test (MR-PRESSO) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Egger Intercept | se | p-value | Q | p-value | Outliers | ||||
| TIDM | SLE | European | 26 | −1.28e−2 | 2.55e−2 | 0.62 | 120.33 | <0.01 | rs1131017, rs192324744, rs231971, rs34954, rs4566101 |
| East Asian | 21 | 6.36e−3 | 3.88e−2 | 0.87 | 25.86 | 0.17 | NA | ||
| AD | European | 37 | 1.21e−2 | 7.08e−3 | 0.10 | 74.23 | <0.01 | rs12722495, rs689,rs8056814 | |
| East Asian | 26 | 1.64e−2 | 1.20e−2 | 0.18 | 42.62 | 0.02 | rs62410259, rs6909461 | ||
| RA | European | 29 | −3.69e-2 | 2.78e−2 | 0.20 | 426.84 | <0.01 | rs1027769, rs1131017, rs202520, rs2111485, rs34296259, rs59680223, rs6679677, rs6719660, rs741172 | |
| MS | European | 23 | 3.63e−2 | 4.49e−2 | 0.43 | 334.29 | <0.01 | rs10911399, rs194749, rs202520, rs34954, rs4566101, rs59680223 | |
| Vitiligo | European | 37 | 3.26e−2 | 4.49e−2 | 0.51 | 49.06 | 0.07 | NA | |
| LP | European | 36 | 3.64e−3 | 1.92e−2 | 0.85 | 76.27 | <0.01 | rs9273363 | |
| HS | European | 37 | 4.19e−2 | 2.36e−2 | 0.08 | 38.59 | 0.35 | NA | |
| AA | European | 37 | 2.67e−2 | 2.91e−2 | 0.36 | 39.42 | 0.32 | NA | |
| SS | European | 37 | −1.49e−3 | 2.97e−2 | 0.96 | 40.48 | 0.28 | NA | |
Notes: SLE, systemic lupus erythematosus; AD, atopic dermatitis; RA, rheumatoid arthritis; MS, multiple sclerosis; LP, lichen planus; HS, hidradenitis suppurativa; AA, alopecia areata; SS, systemic sclerosis.
3.3. The causality of T1DM with SLE, and AD was revealed in East Asia
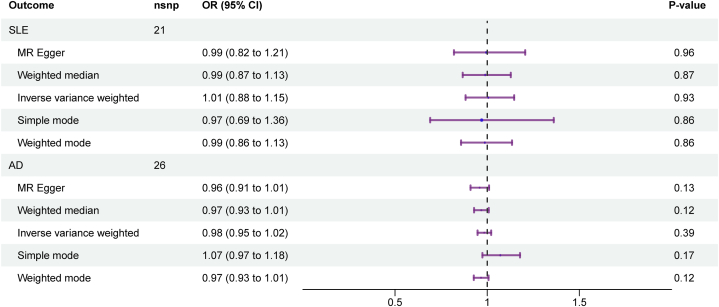
To reconfirm whether the causal relationships were consistent across different population, by further searching, we obtained GWAS data for SLE, and AD in East Asian. And we performed MR analysis to check the causative link between TIDM and aforementioned autoimmune skin diseases. Consistent with the results in the European population, no genetic causal association was found between TIDM and AD (OR = 0.98, 95%CI:0.95−1.02, p = 0.39), while contrary to the results in the European population, a causal relationship between T1DM1 and SLE was still not observed in the East Asian population (OR = 1.01, 95%CI: 0.88−1.15, p = 0.93) (Fig. 3).
Fig. 3.
Forest plots between T1DM and SLE, and AD in East Asia.
Using the IVW approach, we found a statistically significant heterogeneity between T1DM and the risk of AD (PIVW = 0.02), while the statistically heterogeneity between T1DM and the risk of SLE (PIVW = 0.17) was not confirmed (Table 2). In addition, we did not observe the presence of horizontal pleiotropy (SLE, Intercept = 6.36e−3, PMR-Egger = 0.87; AD, Intercept = 1.64e−2, PMR-Egger = 0.18) (Table 2). Similar to the results in the European population, the “Leave-one-out” plot suggested that none of the SNPs dominated the potential causal association between T1DM and SLE, and AD in East Asia (Supplementary Fig. 3). In addition, the scatter plots of TIMD and SLE, and AD were showed in Supplementary Fig. 4.
3.4. Multivariable MR
Previous studies have confirmed the correlation between the body mass index (BMI) and the incidence of autoimmune diseases [30,31]. Body fat percentage (BFP) might be associated with lupus inflammation [32]. T2DM drove autoimmune activation by an innate immune response [33,34]. It was well-known that time spent watching television was closely related to BMI, BFP, and type 2 diabetes [35]. Based on these, to explore the potential complex causal mechanisms for T1DM and SLE, RA and MS, further multivariable MR analysis were conducted after adjusting for BMI, TV, BFP and T2DM, respectively. The results indicated that the potential causal relationships between T1DM and both SLE and RA persist regardless of adjustments for TV, BMI, BFP, or T2DM. While the causal link between T1DM and MS is nullified only after adjusting for BFP, this association remains intact when adjustments are made separately for TV, BMI, and T2DM (Table 3). In addition, we found a causal relationship between T1DM and SLE (OR = 1.29, 95%CI: 1.16–1.44, p < 0.01), RA (OR = 1.28, 95%CI: 1.20–1.38, p < 0.01) and MS (OR = 1.11, 95%CI: 1.04–1.18, p < 0.01) after simultaneously adjusting for BMI, T2DM, TV and BFP (Table 3).
Table 3.
The results of multivariable MR.
| Exposure | Outcome | nsnp | pval | OR | 95%CI |
|---|---|---|---|---|---|
| T1DM adjusting for TV | SLE | 28 | <0.01 | 1.37 | 1.28–1.46 |
| T1DM adjusting for T2DM | 17 | <0.01 | 1.30 | 1.17–1.45 | |
| T1DM adjusting for BMI | 18 | <0.01 | 1.41 | 1.33–1.49 | |
| T1DM adjusting for BFP | 24 | <0.01 | 1.39 | 1.29–1.50 | |
| T1DM adjusting for TV, T2DM, BMI, BFP | 10 | <0.01 | 1.29 | 1.16–1.44 | |
| T1DM adjusting for TV | RA | 34 | <0.01 | 1.18 | 1.11–1.26 |
| T1DM adjusting for T2DM | 20 | <0.01 | 1.26 | 1.18–1.35 | |
| T1DM adjusting for BMI | 19 | <0.01 | 1.25 | 1.18–1.33 | |
| T1DM adjusting for BFP | 28 | <0.01 | 1.20 | 1.10–1.31 | |
| T1DM adjusting for TV, T2DM, BMI, BFP | 13 | <0.01 | 1.28 | 1.20–1.38 | |
| T1DM adjusting for TV | MS | 27 | <0.01 | 1.15 | 1.05–1.26 |
| T1DM adjusting for T2DM | 17 | <0.01 | 1.09 | 1.03–1.16 | |
| T1DM adjusting for BMI | 18 | 0.02 | 1.11 | 1.02–1.21 | |
| T1DM adjusting for BFP | 24 | 0.17 | 1.10 | 0.97–1.25 | |
| T1DM adjusting for TV, T2DM, BMI, BFP | 11 | 0.04 | 1.11 | 1.04–1.18 |
Notes: SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; MS, multiple sclerosis; TV, time spent watching television; BMI, body mass index; T2DM, type 2 diabetes mellitus, BFP, body fat percentage.
4. Discussion
In this study, we conducted a MR analysis from a genetic perspective to investigate the causal relationship between T1DM and various autoimmune skin diseases. Our results indicated that in European, T1DM may be a risk factor for SLE, RA, as well as MS, but it was no statistically significant correlation with AD, vitiligo, LP, HS, AA, and SS. After further multivariable MR analysis, it was found that the causal relationship between T1DM and SLE, RA and MS remained. In addition, we searched available GWAS data for autoimmune skin diseases in East Asian to verify the causal relationship between T1DM and autoimmune skin diseases in European populations. Unfortunately, we only obtained GWAS data for SLE and AD in East Asian. The results of MR Analysis suggested that a potential association between T1DM and SLE and AD has not been found in East Asian populations.
The most effective method for preventing and reducing the occurrence of autoimmune skin diseases is targeted control of the risk factors [36]. Therefore, it is essential to proficiently recognize unconventional risk factors to enhance the prevention and management of autoimmune skin conditions. According to previous research, T1DM is one of the risk factors for autoimmune diseases [[37], [38], [39]]. Consistent with our findings, a causal relationship between T1DM and SLE has been demonstrated in a recent study [40]. At the same time, our study supplemented the exploration of the relationship between T1DM and SLE in the East Asian population. Surprisingly, no causal relationship was found in the East Asian population, indicating that geography and race may change the causal association. Furthermore, studies reported a threefold to twentyfold higher prevalence of MS in T1DM patients [41,42], but the exact connection behind this co-occurrence was not fully illustrated. Our study addressed this gap and identified T1DM as a potential cause of MS. Delving into the mechanism, it may be related to environmental and genetic mutations, such as vitamin D deficiency [43], air pollution [44], HLA haplotype [45]. Besides, a previous meta-analysis showed that RA could increase the risk of diabetes, both T1DM and T2DM [46]. Although the concurrence of RA in T1DM patients is not prominent [47], our analysis revealed that even after adjusting for T2DM, T1DM remains a risk factor for RA. The causal association observed between T1DM and autoimmune skin diseases such as SLE, RA, and MS among European populations may be attributed to shared immunome mechanisms [48]. Specifically, T1DM resulted from a complex interplay of genetic susceptibility, environment, and the autoimmune responses of B cells and T cells against beta cells and their products [49]. Similarly, the immunological dysfunction mediated by T cells and B cells, along with cytokines they secrete was common pathogenesis of diseases like SLE, RA, and MS [[50], [51], [52]]. Previous studies have estimated that 33–36 % of individuals exhibit markers of nonislet autoimmunity at the onset of T1DM or shortly thereafter [53], which supported our finding.
Currently, research on the association between T1DM and AD is mostly conducted in children and remains inconclusive. A retrospective cohort study from Taiwan found that the incidence of AD in the T1DM group was 1.4-fold higher than that in the non-T1DM group [54]. However, another study revealed that the prevalence of atopic diseases in T1DM patients was similar to that in the general population [55]. A case-control study indicated that in children who subsequently developed T1DM, the occurrence of AD was notably lower than in the control group prior to the onset of T1DM. Nevertheless, following the onset of T1DM, there was no disparity in the incidence of AD between the group with T1DM and the group without T1DM [56]. Our research from a genetic perspective clarified that, in both European and East Asian populations, no potential causal link has been found between T1DM and AD. This may be due to AD being more associated with Th2 immune responses [57], whereas T1DM primarily involves Th1 responses [58]. Similarly, no causal links were found between T1DM and vitiligo, LP, HS, AA, as well as SS. The reason may be attributed to differences in genetic backgrounds, immune response mechanisms, target tissues, and underlying pathological processes between these diseases and T1DM. Firstly, The pathogenesis of LP was mainly related to CD8+ T cells, which attacked the basal cell layer of the skin expressing specific antigens, leading to the disruption of intercellular junctions and apoptosis of epithelial cells [59]. Although CD8+ T cells also play a significant role in T1DM, CD4+ T cells and other immune-regulating cells, such as regulatory T cells and B cells, were equally indispensable in the disease's pathology. In addition, HS was a chronic inflammatory skin condition involving the abnormal activity of Th17 cells and inflammatory cytokines such as IL-1β, IL-17, IL-12/23, and TNF-α [60], which was distinct from the autoimmune destruction of pancreatic beta cells seen in T1DM. AA and SS primarily targeted the hair follicles and exocrine glands, respectively, the differences in target tissues and their tissue specificity may help explain why their associations with T1DM are not supported in genetic causality analyses.
While our study is based on MR analysis methods, which mitigate certain limitations of traditional observational studies, there are still some limitations in our research. Firstly, we have only established a causal relationship between T1DM and autoimmune skin diseases, but specific mechanisms require further investigation. Next, GWAS data used for this study did not encompass all global populations, so our findings should be interpreted cautiously. Finally, there is heterogeneity in some of the results, but we have addressed outliers using MR-PRESSO and confirmed the reliability of the results through “Leave-one-out” method. Therefore, this slight heterogeneity should not significantly affect the reliability of our findings.
5. Conclusion
In conclusion, our MR research indicates a causal relationship between T1DM and SLE, RA, and MS in European populations, with no evidence of a causal link between T1D and AD, vitiligo, LP, HS, AA, and SS. Therefore, it is important to regularly monitor relevant immunological markers of SLE, RA, and MS in T1DM patients and take preventive measures.
Funding statement
This study was funded by the National Natural Science Foundation of China (No. 82160596), Joint special fund of Applied Fundamental Research of Kunming Medical University granted by Science and Technology Office of Yunnan (202301AY070001-135), Yunnan Basic Research Project (202301AT070138), and Yunnan Provincial Department of Education Gut Microbiota Transplantation Engineering Research Center.
Data availability statement
The datasets presented in this study were obtained from IEU OpenGWAS (https://gwas.mrcieu.ac.uk/), GWAS Catalog (https://www.ebi.ac.uk/gwas/home), and FinnGen (https://www.finngen.fi/fi). Further inquiries can be obtained by contacting the author.
Ethics statement
This study was based on publicly available GWAS data and all original studies have been approved by their ethics committees.
CRediT authorship contribution statement
Jie Liu: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yinde Xu: Data curation. Yuanju Liu: Data curation. Yun Zhu: Writing – review & editing, Formal analysis, Conceptualization. Xiaolan Li: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We sincerely thank all the researchers and participants who provide public available GWAS data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32781.
Contributor Information
Yun Zhu, Email: zhuyun8377@163.com.
Xiaolan Li, Email: prolixl@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Cao F., He Y.-S., Sang N., et al. Age-standardized incidence, prevalence, and mortality rates of autoimmune diseases in women of childbearing age from 1990 to 2019. Autoimmun. Rev. 2023;22 doi: 10.1016/j.autrev.2023.103450. [DOI] [PubMed] [Google Scholar]
- 2.Gleave A., Granville D.J. Granzyme B in autoimmune skin disease. Biomolecules. 2023;13 doi: 10.3390/biom13020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetter T., de Graaf D.M., Claus I., et al. Aberrant inflammasome activation as a driving force of human autoimmune skin disease. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1190388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dopytalska K., Czaplicka A., Szymańska E., et al. The essential role of microRNAs in inflammatory and autoimmune skin diseases-A review. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24119130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiorean R., Mahler M., Sitaru C. Molecular diagnosis of autoimmune skin diseases. Romanian Journal of Morphology and Embryology = Revue Roumaine de Morphologie Et Embryologie. 2014;55:1019–1033. [PubMed] [Google Scholar]
- 6.Chai K., Zhu R., Luo F., et al. Updated role of high-frequency ultrasound in assessing dermatological manifestations in autoimmune skin diseases. Acta Derm. Venereol. 2022;102 doi: 10.2340/actadv.v102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pruessmann J.N., Langan E.A., Rupp J., et al. Challenge of hepatitis B testing following intravenous immunoglobulin therapy in patients with autoimmune skin diseases. J. Dermatol. 2022;49:1049–1051. doi: 10.1111/1346-8138.16500. [DOI] [PubMed] [Google Scholar]
- 8.Ly S., Nedosekin D., Wong H.K. Review of an anti-CD20 monoclonal antibody for the treatment of autoimmune diseases of the skin. Am. J. Clin. Dermatol. 2023;24:247–273. doi: 10.1007/s40257-022-00751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T., Feng Y., Wang C., et al. Causal relationships between autoimmune diseases and celiac disease: a Mendelian randomization analysis. Biotechnol. Genet. Eng. Rev. 2023:1–16. doi: 10.1080/02648725.2023.2215039. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W., Cai J., Li Z., et al. Association of atopic dermatitis with autoimmune diseases: a bidirectional and multivariable two-sample mendelian randomization study. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1132719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redondo M.J., Morgan N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2023;19:542–554. doi: 10.1038/s41574-023-00853-0. [DOI] [PubMed] [Google Scholar]
- 12.Lin S.-Y., Lin C.-L., Lin C.-C., et al. Risk of urticaria in children with type 1 diabetes mellitus: a nationwide cohort study. Int. J. Environ. Res. Publ. Health. 2019;17 doi: 10.3390/ijerph17010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caroppo F., Galderisi A., Moretti C., et al. Prevalence of psoriasis in a cohort of children and adolescents with type 1 diabetes. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2021;35:e589–e591. doi: 10.1111/jdv.17318. [DOI] [PubMed] [Google Scholar]
- 14.Di Costanzo L., Fattorusso V., Mozzillo E., et al. Psoriasis in children with type 1 diabetes: a new comorbidity to be considered? Acta Diabetol. 2017;54:803–804. doi: 10.1007/s00592-017-1000-3. [DOI] [PubMed] [Google Scholar]
- 15.Forgetta V., Manousaki D., Istomine R., et al. Rare genetic variants of large effect influence risk of type 1 diabetes. Diabetes. 2020;69:784–795. doi: 10.2337/db19-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentham J., Morris D.L., Graham D.S.C., et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 2015;47:1457–1464. doi: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaue S., Kanai M., Tanigawa Y., et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021;53:1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PubMed] [Google Scholar]
- 18.Kurki M.I., Karjalainen J., Palta P., et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–518. doi: 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song Q., Lei Y., Shao L., et al. Genome-wide association study on Northern Chinese identifies KLF2, DOT1L and STAB2 associated with systemic lupus erythematosus. Rheumatology. 2021;60:4407–4417. doi: 10.1093/rheumatology/keab016. [DOI] [PubMed] [Google Scholar]
- 20.Richardson T.G., Sanderson E., Palmer T.M., et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S., Thompson S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 22.Hemani G., Zheng J., Elsworth B., et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey J.D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P., Wang H., Guo L., et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med. 2022;20:443. doi: 10.1186/s12916-022-02657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanderson E., Davey Smith G., Windmeijer F., et al. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 2019;48:713–727. doi: 10.1093/ije/dyy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitriou N., Dimou N., Tsilidis K.K., et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat. Commun. 2020;11:597. doi: 10.1038/s41467-020-14389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshikawa M., Asaba K. Educational attainment decreases the risk of COVID-19 severity in the European population: a two-sample mendelian randomization study. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.673451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huo J., Xu Y., Yu J., et al. Causal association between body mass index and autoimmune thyroiditis: evidence from Mendelian randomization. Eur. J. Med. Res. 2023;28:526. doi: 10.1186/s40001-023-01480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Zhu J., Zhao W., et al. The causal effect of obesity on the risk of 15 autoimmune diseases: a mendelian randomization study. Obes. Facts. 2023;16:598–605. doi: 10.1159/000534468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sola-Rodríguez S., Vargas-Hitos J.A., Gavilán-Carrera B., et al. Physical fitness attenuates the impact of higher body mass and adiposity on inflammation in women with systemic lupus erythematosus. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.729672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velloso L.A., Eizirik D.L., Cnop M. Type 2 diabetes mellitus--an autoimmune disease? Nat. Rev. Endocrinol. 2013;9:750–755. doi: 10.1038/nrendo.2013.131. [DOI] [PubMed] [Google Scholar]
- 34.Hemminki K., Liu X., Försti A., et al. Subsequent type 2 diabetes in patients with autoimmune disease. Sci. Rep. 2015;5 doi: 10.1038/srep13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rincón-Pabón D., Urazán-Hernández Y., González-Santamaría J. Association between the time spent watching television and the sociodemographic characteristics with the presence of overweight and obesity in Colombian adolescents (secondary analysis of the ENSIN 2010) PLoS One. 2019;14 doi: 10.1371/journal.pone.0216455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter L.M., McGonagle D., Vital E.M., et al. Applying early intervention strategies to autoimmune skin diseases. Is the window of opportunity preclinical? A dermato-rheumatology perspective. J. Invest. Dermatol. 2022;142:944–950. doi: 10.1016/j.jid.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 37.James J.A., Chen H., Young K.A., et al. Latent autoimmunity across disease-specific boundaries in at-risk first-degree relatives of SLE and RA patients. EBioMedicine. 2019;42:76–85. doi: 10.1016/j.ebiom.2019.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida C., Venade G., Duarte D., et al. Type 1 diabetes mellitus and multiple sclerosis: an association to consider. Cureus. 2022;14 doi: 10.7759/cureus.30762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.4 Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S46–S59. doi: 10.2337/dc22-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Si S., Li J., et al. Association between type 1 diabetes and systemic lupus erythematosus: a Mendelian randomization study. Clin. Rheumatol. 2024;43:41–48. doi: 10.1007/s10067-023-06800-8. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen N.M., Westergaard T., Frisch M., et al. Type 1 diabetes and multiple sclerosis: a Danish population-based cohort study. Arch. Neurol. 2006;63:1001–1004. doi: 10.1001/archneur.63.7.1001. [DOI] [PubMed] [Google Scholar]
- 42.Dorman J.S., Steenkiste A.R., Burke J.P., et al. Type 1 diabetes and multiple sclerosis: together at last. Diabetes Care. 2003;26:3192–3193. doi: 10.2337/diacare.26.11.3192. [DOI] [PubMed] [Google Scholar]
- 43.Johnson C.R., Thacher T.D. Vitamin D: immune function, inflammation, infections and auto-immunity. Paediatr. Int. Child Health. 2023;43:29–39. doi: 10.1080/20469047.2023.2171759. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C.-N., Xu Z., Wu G.-C., et al. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019;18:607–614. doi: 10.1016/j.autrev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Lernmark A. Multiple sclerosis and type 1 diabetes: an unlikely alliance. Lancet (London, England) 2002;359:1450–1451. doi: 10.1016/S0140-6736(02)08464-7. [DOI] [PubMed] [Google Scholar]
- 46.Jiang P., Li H., Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin. Exp. Rheumatol. 2015;33:115–121. [PubMed] [Google Scholar]
- 47.Kota S.K., Meher L.K., Jammula S., et al. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metabol. Syndr. 2012;6:70–76. doi: 10.1016/j.dsx.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Zeng L., Yang K., He Q., et al. Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials. BMC Med. 2024;22:110. doi: 10.1186/s12916-024-03303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaberi-Douraki M., Liu S.W.S., Pietropaolo M., et al. Autoimmune responses in T1DM: quantitative methods to understand onset, progression, and prevention of disease. Pediatr. Diabetes. 2014;15:162–174. doi: 10.1111/pedi.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taubmann J., Müller F., Yalcin Mutlu M., et al. CD19 chimeric antigen receptor T cell treatment: unraveling the role of B cells in systemic lupus erythematosus. Arthritis Rheumatol. 2024;76:497–504. doi: 10.1002/art.42784. [DOI] [PubMed] [Google Scholar]
- 51.Jelcic I., Al Nimer F., Wang J., et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell. 2018;175 doi: 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marks K.E., Rao D.A. T peripheral helper cells in autoimmune diseases. Immunol. Rev. 2022;307:191–202. doi: 10.1111/imr.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hughes J.W., Bao Y.K., Salam M., et al. Late-onset T1DM and older age predict risk of additional autoimmune disease. Diabetes Care. 2019;42:32–38. doi: 10.2337/dc18-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C.H., Wei C.C., Lin C.L., et al. Childhood type 1 diabetes may increase the risk of atopic dermatitis. Br. J. Dermatol. 2016;174:88–94. doi: 10.1111/bjd.14166. [DOI] [PubMed] [Google Scholar]
- 55.Gazit V., Tasher D., Hanukoglu A., et al. Atopy in children and adolescents with insulin-dependent diabetes mellitus. Isr. Med. Assoc. J. : Isr. Med. Assoc. J. 2008;10:858–861. [PubMed] [Google Scholar]
- 56.Olesen A.B., Juul S., Birkebaek N., et al. Association between atopic dermatitis and insulin-dependent diabetes mellitus: a case-control study. Lancet (London, England) 2001;357:1749–1752. doi: 10.1016/S0140-6736(00)04896-0. [DOI] [PubMed] [Google Scholar]
- 57.He H., Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am. J. Clin. Dermatol. 2019;20:181–192. doi: 10.1007/s40257-018-0413-2. [DOI] [PubMed] [Google Scholar]
- 58.Wang C.-H., Lu W.-L., Chiang S.-L., et al. T cells mediate kidney tubular injury via impaired PDHA1 and autophagy in type 1 diabetes. J. Clin. Endocrinol. Metabol. 2022;107:2556–2570. doi: 10.1210/clinem/dgac378. [DOI] [PubMed] [Google Scholar]
- 59.Qing M., Yang D., Shang Q., et al. CD8+ tissue-resident memory T cells induce oral lichen planus erosion via cytokine network. Elife. 2023;12 doi: 10.7554/eLife.83981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aarts P., Dudink K., Vossen A.R.J.V., et al. Clinical implementation of biologics and small molecules in the treatment of hidradenitis suppurativa. Drugs. 2021;81:1397–1410. doi: 10.1007/s40265-021-01566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study were obtained from IEU OpenGWAS (https://gwas.mrcieu.ac.uk/), GWAS Catalog (https://www.ebi.ac.uk/gwas/home), and FinnGen (https://www.finngen.fi/fi). Further inquiries can be obtained by contacting the author.